Abstract
Buckwheat is a rapidly growing cover crop with the potential to improve soil quality and health. In this study, the impact of buckwheat plant material (BPM) as a soil amendment on seedling emergence and suppression of damping-off and root rot of radish (Rhizoctonia solani) and cucumber (Pythium ultimum) was investigated in pot assays. Fresh BPM grown in a greenhouse potting mix for 4 weeks was chopped in small 1–2 cm long pieces and incorporated into field soil containing 0–10% of R. solani or P. ultimum inoculum produced on sterilized rye seed. The infested soil was then incubated for 0–8 weeks prior to planting radish or cucumber seed. The effects of BPM on plant growth and disease development were determined 2 weeks after planting. There was no disease protection if radish and cucumber seeds were planted within 1–2 weeks after soil incorporation of BPM. Disease protection was evident when planting was delayed for 3 weeks after BPM amendment to soil. BPM soil amendment provided protection of radish plants from Rhizoctonia damping-off and protection of cucumber plants from Pythium damping-off and root rot. BPM amendments had no effect on promoting plant growth but slightly increased soil pH. In amended soil receiving 4% and 10% of BPM planted with radish or cucumber seeds 0, 3 and 8 weeks later, total populations of indigenous fungi were 0.66–2.01 log units higher and bacteria were 0.53–0.92 log units higher compared with the populations in the non-amended soils. Buckwheat soil amendment offers a potential option to improve plant and soil health.
Résumé
Le sarrasin est une plante de couverture à croissance rapide qui offre la possibilité d’améliorer la qualité et la santé du sol. Dans cette étude, l’effet du matériel végétal provenant du sarrasin (MVS) comme amendement du sol quant à l’émergence des semis et à la suppression de la fonte des semis et du pourridié chez le radis [Rhizoctonia solani] et le concombre [Pythium ultimum] a été étudié au cours d’essais en pots. Du MVS frais cultivé dans un terreau pour culture en serre pendant quatre semaines a été coupé en petits morceaux de 1 à 2 cm et incorporé à de la terre contenant de 0 à 10% d’un inoculum de R. solani ou de P. ultimum produit sur de la graine de seigle stérilisée. Le sol infesté a par la suite été incubé jusqu’à huit semaines avant d’y semer les graines de radis ou de concombre. Les effets du MVS sur la croissance des plants et le développement des maladies ont été constatés deux semaines après avoir semé les graines. Il n’y avait aucune protection contre les maladies si les graines de radis et de concombres avaient été semées de une à deux semaines après l’incorporation du MVS au sol. La protection contre les maladies s’est manifestée lorsqu’on attendait trois semaines après avoir amendé le sol avant de semer les graines. L’amendement à base de MVS a protégé les plants de radis de la fonte des semis causée par Rhizoctonia et ceux de concombres de la fonte des semis et du pourridié causé par Pythium. Pour ce qui est de promouvoir la croissance des plants, le MVS n’a eu aucun effet, mais il a contribué à légèrement élever le pH du sol. Dans les sols ayant été amendés avec 4% et 10% de MVS et dans lesquels on a semé des graines de radis et de concombre, zéro, trois et huit semaines plus tard, les populations totales de champignons indigènes étaient de 0.66 à 2.01 unités logarithmiques plus élevées et celles de bactéries, de 0.53 à 0.92 unité logarithmique plus élevée, comparativement aux populations des sols non amendés. L’amendement à base de sarrasin constitue une option possible en ce qui a trait à l’amélioration de la santé des plants et du sol.
Introduction
Buckwheat (Fagopyrum esculentum Moench) is a fast-growing, short-season cover crop with the potential to improve soil health. Buckwheat seedlings emerge within 3–5 days after seeding, while the crop sets flowers in 4–6 weeks and then forms seed within 10–12 weeks after planting. Due to its fast growth and short life cycle, buckwheat can be an ideal cover or rotation crop in both organic and conventional systems. In addition, it has potential for the control of weeds as the crop is known for its weed-suppressive abilities during its growth (Creamer & Baldwin, Citation2000; Kumar et al., Citation2011). For example, soil incorporation of fresh buckwheat residues was demonstrated to reduce and delay the emergence of redroot pigweed (Amaranthus retroflexus L.) and lambsquarters (Chenopodium album L.) (Haramoto & Gallandt, Citation2005), Powell amaranth (Kumar et al., Citation2008), hairy galinsoga (Kumar et al., Citation2009b) and Canada thistle (Bicksler & Masiunas, Citation2009). Similarly, the use of buckwheat pellets made from plant shoots reduces the dry weight and density of weeds in paddy rice (Oryza sativa L.) (Xuan & Tsuzuki, Citation2004). This suppression for some weed species (e.g. Capsella bursapastoris) was the result of immobilization of nitrogen (Kumar et al., Citation2008), whereas for Powell amaranth, allelochemicals concentrated in shoot tissue appear to have played an important role (Kumar et al., Citation2009a). Similar to mustard cover crops, chemicals isolated from buckwheat have inhibitory effects on weeds (Iqbal et al., Citation2002, Citation2003; Xuan & Tsuzuki, Citation2004; Golisz et al., Citation2007).
The benefit of buckwheat crop rotation also extends to the control of insect pests. Results from a field study in Hawaii showed inhibition of the development of wireworm larvae due to the cultivation of buckwheat that led to a reduction of the wireworm population in the subsequent crop (Valenzuela & Smith, Citation2002). Preliminary field studies from Prince Edward Island, Canada with buckwheat as a rotation crop with potatoes also demonstrated a reduction in wireworm populations (Christine Noronha, personal communication). The deleterious impact of buckwheat on weeds and wireworms may be due to the direct effects of buckwheat metabolites or the indirect effects of microbial activity in the soil triggered by buckwheat crop residue or simply by nutrient immobilization. The effects of buckwheat rotation or cover crop on plant pathogens and their activities are not well known or understood. In general, the use of non-host cover, rotation crops or green manures in pathogen-infested fields can break pathogen cycles by reducing inoculum levels, thereby providing disease relief in the subsequent crops. This has been described in a number of host–pathogen systems (Larkin, Citation2013, Citation2015; Himmelstein et al., Citation2016).
Rhizoctonia solani Kühn and Pythium ultimum Trow are two common soil and root pathogens that can cause serious crop losses in both greenhouse and field production systems. These pathogens cause a number of disease symptoms such as blights, damping-offs, crown and root rots, fruit rots and wilts in a number of hosts including cucumber (Cucumis sativus L.) and radish (Raphanus sativus L.) (Howard et al., Citation1994). Additionally, these pathogens can survive in soil for many years in the absence of host crops, and can cause significant early crop losses by infecting newly emerging seedlings of horticultural and vegetable crops. There is an increasing desire to improve environmental stewardship within the agricultural sector which may reduce the number of chemical fungicides utilized in the future. Therefore, it is essential to supplement existing disease-control strategies to manage R. solani, P. ultimum and most other soil and root pathogens. Non-chemical, sustainable management options for these and other soil-borne pathogens are in great demand, especially in organic production systems, to reduce losses from diseases. For instance, soil incorporation of buckwheat and other cover crops could serve as an integrated management strategy for both organic and conventional production systems.
The aims of this study were to: (i) assess the impact of buckwheat plant material (BPM) as a soil amendment on plant emergence and suppression of seedling damping-off and root rot of radish caused by Rhizoctonia solani and seedling damping-off and root rot of cucumber caused by Pythium ultimum; and (ii) measure the effect of BPM amendment on plant dry weights, soil pH and indigenous soil microbial populations.
Materials and methods
Plant material and soils
Buckwheat plants were grown in a greenhouse potting mix in 10-cm diameter pots. The greenhouse potting mix was prepared by mixing peat moss (85 L), perlite (10 kg), sand (20 L), dolomitic limestone (1 kg) and a starter fertilizer [2-3-6] (1 kg) in a cement mixer. Each pot received 4–5 seeds and plants were grown for 4 weeks. Plant shoots were excised near the soil line and then chopped in small pieces (1–2 cm) using a Waring blender, henceforth called buckwheat plant material (BPM), and used as a soil amendment in growth room plant bioassays as described later. A nutrient analysis of BPM is given in . Untreated cucumber (Cucumis sativus L. ‘Straight Eight’) and radish (Raphanus sativus L. ‘Raxe’) seed used in this study were obtained from William Dam Seeds Ltd, Flamborough, Ontario. A heavy soil (organic matter 3.4% and pH 6.4) used in growth room plant bioassays was collected from Kentville Research and Development Centre Farm. The soil was air dried for a week by spreading it on a greenhouse bench and was used in growth room plant bioassays.
Pathogen cultures and preparation of inoculum
An isolate of Pythium ultimum (isolate Pu14-01 L) was originally isolated from beans and stored at −80°C in a freezer. Its pathogenicity to cucumber seedlings was confirmed in preliminary growth room pot assays. The culture of R. solani (isolate Rs14-01 L; anastomosis group AG4) used in this study was originally isolated from infected potato plants, and was pathogenic to radish seedlings (Abbasi et al., Citation2004). Both pathogens were recovered from their respective infected hosts and maintained on potato dextrose agar (PDA, Difco) for use in growth room disease bioassays. Pathogenicity of the R. solani isolate was again confirmed in preliminary growth room pot assays. The inoculum of P. ultimum and R. solani was produced on sterilized moist rye seed in still culture. Moist seeds were placed in a 250-mL flask and sterilized by autoclaving for 1 h on 2 consecutive days. Cooled flasks were inoculated with 6–8 plugs (4-mm diameter) obtained from the edge of freshly growing P. ultimum or R. solani colonies on PDA. The inoculated flasks were kept in an incubator at 24°C for 2 weeks or until the pathogen had completely colonized the rye seed in the flask. The rye seed inoculum was air-dried overnight in a laminar flow hood with sterile air, cut in tiny pieces with a sterile scalpel, and used in growth room disease bioassays as described below. Various quantities (0, 0.25, 0.5, 1.0 and 2.0 g inoculum kg−1 soil) of the rye seed inocula of P. ultimum or R. solani were added to a potting mix in preliminary growth room disease bioassays in order to determine the amounts required to give more than 80% disease incidence within 2 weeks after sowing cucumber or radish seed.
Determining the effects of BPM soil amendment on plant emergence and disease suppression
The effects of the BPM soil amendment applied as a pre-plant treatment on plant emergence and disease suppression were assessed in growth room pot assays in non-infested and pathogen (R. solani or P. ultimum) infested field soil using radish–Rhizoctonia and cucumber–Pythium model systems as described previously (Abbasi et al., Citation2004) with some modifications. The air-dried field soil was weighed in plastic bags and infested with the freshly prepared rye seed inoculum of P. ultimum (1.0 g inoculum kg−1 soil) or R. solani (0.25 g inoculum kg−1 soil). The inoculum was mixed thoroughly by shaking the contents of the bags vigorously. After 24 h, the infested and non-infested soils were then amended with various amounts (0, 4 and 10% weight/weight soil for initial experiments; or 0, 2 and 5% for subsequent experiments) of freshly chopped BPM in plastic bags and the soil was also moistened with sterile distilled water (SDW; 100 mL kg−1 soil). The infested and non-infested soils not amended with BPM served as controls. The plastic bags containing the soils were incubated at 22°C in a growth cabinet in the dark for 0, 3 and 8 weeks (initial experiments) or 1, 2 and 3 weeks (subsequent experiments). Immediately prior to planting, the contents of each bag were remixed and added to the 10-cm plastic pots, and 18–20 radish or 8–10 cucumber seeds were planted in each of three replicate pots per treatment. Pots were kept in a growth room (16 h of fluorescent light at 22°C and 6 h of darkness at 20°C) and arranged in a completely randomized design. Plants were watered (~100 mL per pot) daily to maintain appropriate moisture (~60% field capacity) level for growth and disease development. Radish seedlings were rated 2 weeks after planting for pre- and post-emergence damping-off using a 1–5 scale: 1, asymptomatic; 2, small (< 5 mm) root/stem lesion; 3, large (> 5 mm) root/stem lesion; 4, post-emergence damping-off; and 5, pre-emergence damping-off. Cucumber seedlings were rated 2 weeks after planting for Pythium damping-off and root rot severity using a 1–5 scale: 1, asymptomatic; 2, < 20% discolouration (yellowing) of root or stem near soil line; 3, > 20% discolouration (severe yellowing) of root or stem near soil line; 4, plant death; and 5, seedling not emerged. Data were also expressed as the percentage of asymptomatic seedlings. After rating, plant dry weights were determined by drying the plant tops overnight in an oven at 65°C. All the experiments were repeated at least once.
Determining the effects of BPM amendment on soil microbial populations and pH
The populations of total culturable bacteria and fungi were assessed in the non-infested and infested soil amended with 0, 4 and 10% BPM and incubated for 0, 3 and 8 weeks (initial experiments) prior to planting to determine the effects of BPM on plant growth and seedling damping-off and root rot. After rating plants for growth and disease assessments were made, soil samples were removed from each pot, and analysed for the population of indigenous culturable bacteria and fungi by plating dilutions of soil–water mixtures on different growth media. Soil (1 g) was suspended in 99 mL sterile saline water (8.5 g NaCl L−1) in a 250-mL sterile Erlenmeyer flask, and the mixture was shaken for 1 h on an orbital rotary shaker (200 rpm) at 22°C. The resulting suspension was then serially diluted by placing 1 mL suspension in 9 mL sterile saline solution in 20-mL tubes. Ten µL of suspension from 10−5 and 10−6 dilutions was plated onto tryptic soy agar (TSA) plates for the cultivation of bacteria. For the cultivation of fungi, 10 µL of the suspension from 10−3 and 10−4 dilutions were plated on water agar (WA) plus chloramphenicol (0.1 g L−1) and streptomycin sulphate (0.07 g L−1) or rose bengal (RB) plus chloramphenicol (0.08 g L−1) plates. Two replicate plates per medium were used for each dilution. Plates were kept at room temperature (20 ± 2°C) and the developing colonies were counted daily for 5–6 days. The microbial densities of bacteria and fungi were estimated and expressed as the number of colony-forming units (CFU) g−1 of soil.
The soil–water mixture used for preparing the serial dilutions for enumeration of microbial populations was also used for taking pH measurements. The pH of the suspension was determined at room temperature (20 ± 2°C) using a benchtop ultrabasic UB-10 pH/mV meter (Denver Instrument, Arvada, Colorado, USA).
Statistical analysis
The data on soil bacterial and fungal populations were transformed to the logarithmic scale before subjecting to analysis of variance. The experimental designs for Rhizoctonia–radish and Pythium–cucumber were two separate setups and both were a split-plot design. The random effects were Experiments (2)/Replicates (3) × main plot (2)/sub-plot. The fixed effects were pathogen and no pathogen (2) × amendment rate (3) × incubation time (3). The pathogen was on the main plot with the amendment rate and incubation time on the sub-plots. Large residuals were removed before the final analysis of variance (ANOVA) using Genstat for Windows 18th Edition (VSN International, Hemel Hempstead, UK). A mixed model ANOVA (Genstat) was used for analysis of the data sets including all interactions. Polynomial contrasts for linear and quadratic response were used to determine differences between amendment rates and incubation times.
Results
Effect on plant emergence and suppression of Rhizoctonia damping-off and root rot of radish
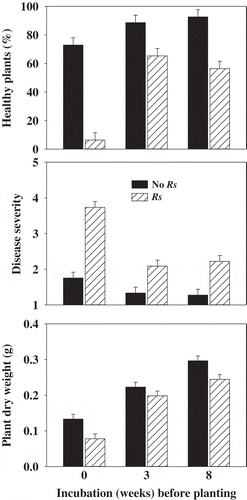
In the initial experiments, the fixed effects such as pathogen (R. solani soil inoculation) and incubation time (0, 3 and 8 weeks after BPM amendment and prior to planting radish seed) showed significant (P < 0.001) effects, and amendment rate (0, 4 and 10% BPM) showed non-significant effects on the percentage of disease-free healthy seedlings, damping-off severity and plant dry weights. Three-way interaction (pathogen × amendment rate × incubation time) was non-significant, but two-way interactions (pathogen × incubation time and amendment rate × incubation time) were significant. In the two-way interaction (pathogen × incubation time), radish plants produced in non-infested soil amended with 0, 4 and 10% BPM and incubated for 0, 3 and 8 weeks prior to planting showed no significant differences in the percentage of disease-free healthy plants, the ratings of disease severity and plant dry weights after 2 weeks of growth (). In the R. solani-infested soil, there was no protection of radish plants from Rhizoctonia damping-off if seeds were planted immediately (week 0) after incorporating BPM in the infested soil. Plants grown for 2 weeks in the infested non-amended control or infested BPM-amended soil on average resulted in low percentage (6.2%) of disease-free healthy seedlings, high disease severity (3.74) and low plant dry weights (0.08 g) (). Disease protection of radish plants was observed when planting was delayed for 3 weeks after the BPM amendment in the infested soil; an average of 65.3% plants were healthy in the R. solani-infested and BPM-amended and non-amended soil with a mean disease severity of 2.09 and plant dry weights of 0.20 g after 2 weeks of growth (). When planting was delayed for 8 weeks after the BPM amendment in the infested soil, no further disease protection was observed with the BPM amendment ().
Fig. 1 Effect of Rhizoctonia solani-inoculation and incubation time prior to planting radish seed in a buckwheat-amended and non-amended field soil on disease-free healthy plants, damping-off severity, and dry weight of radish plants. Means are the average of two experiments and three replicates per experiment and error bars are standard error of mean. Plants were grown for 2 weeks before assessing for damping-off incidence and severity.
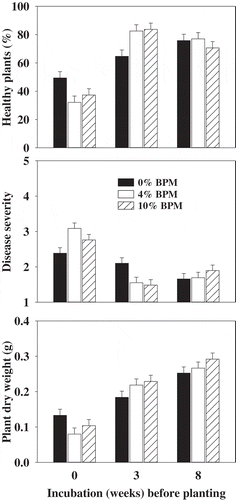
BPM amendment × incubation time also showed significant effects on the percentage of disease-free healthy seedlings (P < 0.001), damping-off severity (P < 0.001) and plant dry weights (P < 0.078). In the immediate planting (week 0), 4 and 10% BPM soil amendment showed no significant effects on plant growth or disease protection (). When planting of radish seed was delayed for 3 weeks after the BPM amendment in the infested and non-infested soil, the percentage of disease-free healthy plants was significantly improved over the non-amended treatments and such plants also showed reduced disease severity ratings (). When planting was delayed for 8 weeks, no further improvement was observed in the percentage of disease-free healthy plants, disease severity ratings or plant dry weights between the plants produced in BPM amended and non-amended soils ().
Fig. 2 Effect of buckwheat plant material (BPM) amendment and incubation time prior to planting radish seed in a non-infested or Rhizoctonia solani-infested field soil on disease-free healthy plants, damping-off severity and dry weight of radish plants. Means are the average of two experiments and three replicates per experiment and error bars are standard error of mean. Plants were grown for 2 weeks before assessing for damping-off incidence and severity.
In the subsequent experiments, three-way interaction (pathogen × amendment rate × incubation time) showed significant effects on the percentage of disease-free healthy seedlings (P < 0.001), damping-off severity (P < 0.001) and plant dry weights (P < 0.05). Plants produced in the non-infested control soil and non-infested soil amended with 2 and 5% BPM and incubated for 1, 2 and 3 weeks prior to planting showed similar percentages of disease-free healthy seedlings, similar ratings of disease severity, and similar dry weights after 2 weeks of growth (). Also, there was no disease protection if radish seeds were planted within 1–2 weeks after the BPM amendment in the R. solani-infested soil; most of the plants in the infested control or BPM-amended soil were dead or diseased (less than 2% healthy with a mean disease severity of 4.5–5.0) (). Disease protection of radish plants from Rhizoctonia damping-off was observed when planting was delayed for 3 weeks after the BPM amendment in the infested soil. On average, 41–47% plants were healthy with a mean disease severity of 2.3 in the BPM amendment treatments compared with 1% healthy with a disease severity of 3.4 in the infested control (). Plants produced in the infested and BPM-amended soil weighed 24–41% more than the plants grown in the infested and non-amended control soil ().
Table 1. Analysis of buckwheat plant material used as a soil amendment in this study.
Table 2. Effect of buckwheat plant material (BPM) amended to a non-infested or Rhizoctonia solani (Rs)-infested field soil 1, 2 and 3 weeks prior to planting on per cent healthy, damping-off severity and dry weight of radish plantsa.
Effect of plant emergence and suppression of Pythium damping-off and root rot of cucumber
The amendment rate (0, 4 and 10% BPM) and incubation time (0, 3 and 8 weeks after BPM amendment and prior to planting cucumber seed) showed significant (P < 0.001) effects and pathogen (P. ultimum soil inoculation) showed non-significant effects on the percentage of disease-free healthy seedlings, damping-off severity and plant dry weights. Two-way interaction (amendment rate × incubation time) was also significant. As the pathogen fixed effects were non-significant, only the main effects from combined treatments are reported (). BPM soil amendment affected the emergence of cucumber plants in the immediate planting. Plant emergence was improved when planting was delayed for 3 and 8 weeks after the BPM incorporation in the soil. Similar trends were also seen for disease severity and plant dry weights (). High disease severity values and low dry weights in the BPM-amended soils were due to lower plant emergence.
Fig. 3 Effect of buckwheat plant material (BPM) amendment and incubation time prior to planting cucumber seed in a non-infested or Pythium ultimum-infested field soil on disease-free healthy plants, damping-off severity and dry weight of cucumber plants. Means are the average of three experiments and three replicates per experiment and error bars are standard error of mean. Plants were grown for 2 weeks before assessing for damping-off incidence and severity.
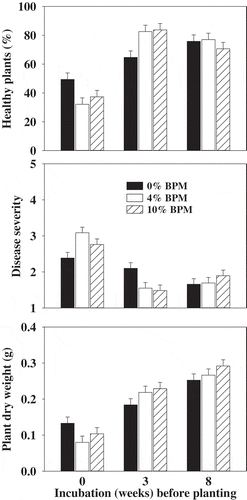
In the subsequent Pythium–cucumber experiments, three-way interaction (pathogen × amendment rate × incubation time) showed significant effects on the percentage of disease-free healthy seedlings (P < 0.001) and damping-off and root rot severity (P < 0.001) but no significant effect on plant dry weights. In non-infested soil, the BPM soil amendment rate and incubation time prior to planting cucumber seed had no significant effect on plant growth. Cucumber plants produced in the 0, 2 and 5% BPM-amended soil incubated for 1, 2 and 3 weeks prior to planting showed no significant differences in the percentages of disease-free healthy plants, the ratings of disease severity and plant dry weights after 2 weeks of growth (). No disease protection was observed when cucumber seeds were planted within 1–2 weeks after the BPM amendment in the infested soil (). When the planting was delayed for 1 week, the majority of the plants grown in the infested and non-amended control soil and in the infested and BPM-amended soils were diseased (1–17% healthy with a mean disease severity of 4.1–5.0) after 2 weeks of growth (). When the planting was delayed for 2 weeks, the majority of the plants grown in the infested and non-amended control soil and in the infested and BPM-amended soils were still diseased (4–7% healthy with a mean disease severity of 4.5–4.7) after 2 weeks of growth (). There was no disease protection of cucumber plants from Pythium damping-off and root rot when planting was delayed for 3 weeks after BPM amendment in the infested soil (). On average, more than 81% of the plants were healthy in the BPM treatments with a mean disease severity of 1.7 compared with 22% healthy and 3.4 mean severity in the control after 2 weeks of growth in the infested soil (). There were no significant treatment differences for plant dry weights ().
Table 3. Effect of buckwheat plant material (BPM) amended to a non-infested or Pythium ultimum (Pu)-infested field soil 1, 2 and 3 weeks prior to planting on per cent healthy, damping-off severity and dry weight of cucumber plantsa.
Effect on soil pH and soil microbial populations of bacteria and fungi
In the initial BPM-radish experiments, two-way and three-way interactions between the fixed effects were non-significant for soil pH and soil microbial populations of indigenous bacteria and fungi. Therefore, only significant main effects were reported. The soil amendment of 4 and 10% BPM showed a significantly higher pH over the non-amended soils (). In the 4 and 10% BPM-amended soil, the populations of total culturable bacteria were 0.55–0.69 log units higher and total culturable fungi were 0.66–0.91 log units higher compared with the populations in the non-amended soils (). Growth observation on plates showed no predominance of a single bacterial or fungal species on any of the culture media. An incubation period following the BPM incorporation in the soil before planting radish seed also showed significant effects on soil pH and soil microbial populations of total culturable bacteria and fungi (). The combined soil pH of the control and BPM amendment treatments were higher in soil samples from the 8-week incubation period than the 0 and 3 week incubation periods (). There was no significant effect of incubation period on bacterial numbers but fungal numbers were increased by 0.54 log units after 3 weeks of incubation and 0.31 log units after 8 weeks of incubation ().
Table 4. Main effects of buckwheat plant material (BPM) amendment in a non-infested or Rhizoctonia solani-infested field soil incubated for 0, 3 and 8 weeks prior to planting radish on soil pH and soil microbial populations of indigenous bacteria and fungia.
Table 5. Main effects of incubation time prior to planting radish seed in a non-infested or Rhizoctonia solani-infested field soil amended with buckwheat plant material (BPM) on soil pH and soil microbial populations of indigenous bacteria and fungia.
In the BPM-cucumber experiments, two-way interaction (amendment rate × incubation time) was also significant for soil pH and soil microbial populations of indigenous bacteria and fungi. Pathogen fixed effects were non-significant so only main effects from combined treatments were reported. The soil pH in the 4 and 10% BPM amended soil was significantly higher than in the non-amended soils in all three plantings, i.e. after 0, 3 and 8 weeks of incubation (). In the 4 and 10% BPM-amended soil, the populations of total culturable bacteria were 0.53–0.92 log units higher and total culturable fungi were 0.75–2.01 log units higher compared with the populations in the non-amended soils ().
Table 6. Main effects of buckwheat plant material (BPM) amendment and incubation time (0, 3 and 8 weeks) prior to planting cucumber seed in a non-infested or Pythium ultimum-infested field soil on soil pH and soil microbial populations of indigenous bacteria and fungia.
Discussion
The need for safer and alternative technologies for the management of plant diseases caused by soil-borne pathogens is increasing. The lack of effective management strategies for soil and root diseases continues to be a major issue faced by growers and producers. Restricted use of soil chemicals and fumigants is still permitted in conventional crop production systems; however, their use is steadily diminishing due to increasing concerns over negative effects of these chemicals on human health and the environment. Disease management in organic production systems is more challenging due to a lack of available registered products for managing a wide range of disease problems in such settings. The use of cover crops to retain soil nutrients, suppress weeds, and limit soil pathogens is a good alternative approach to be exploited for disease management. A short-duration cover crop such as buckwheat provides an ideal option to improve plant and soil health, as demonstrated in this study.
In the present study, plant emergence was improved through disease reduction when BPM was used as a soil amendment prior to planting in a pathogen-infested field soil. This was consistently observed within a radish–Rhizoctonia system and further confirmed within a cucumber–Pythium system. A pre-plant BPM amendment provided protection of radish plants from seedling damping-off caused by R. solani, and protection of cucumber plants from damping-off and root rot caused by P. ultimum. Disease protection, as characterized by a high percentage of healthy plants and low disease severity ratings, was observed only when planting was delayed for at least 3 weeks after BPM incorporation in the infested soil. This suggests that an incubation period after the BPM amendment in the infested soil was necessary for the disease suppressive effect to occur. The use of plant-based products and other materials from the agriculture-related industry as soil amendments previously provided disease suppression in delayed plantings (Abbasi et al., Citation2004, Citation2007). These studies showed increased damping-off control as the incubation time after the amendment incorporation in the infested soil was increased. This type of disease control may be general and related to increased microbial activity in the amended soil. In a very early study, Davey & Papavizas (Citation1959) compared several green or immature (1% rate) and dry or mature (2% rate) plant residues as soil amendments for protection against Rhizoctonia disease of snap beans caused by R. solani in three consecutive plantings. They showed no protection of snap beans from R. solani infections with 1% green buckwheat residue as an amendment to a R. solani-infested loamy sand soil; however, 2% dry buckwheat residue reduced infection index in the first two of the three plantings compared with the non-amended control. Wiggins & Kinkel (Citation2005) compared buckwheat green manure as a soil amendment with fallow control for suppression of Phytophthora root rot of alfalfa, and reported no effect on alfalfa stand count and no reduction of root rot severity. Buckwheat in their study was grown in a Phytophthora-infested field soil in pots for 7 weeks and then incorporated into the same pots and alfalfa was planted 20 days later. Both of these earlier studies may have missed the critical time period needed after amendment incorporation and before planting, to attain maximum disease suppression as shown in the present study, and therefore did not experience the same level of disease protection or increased stand counts. In addition, the amendment rate may also have had an effect on disease suppression.
BPM soil amendment also showed the potential to increase soil populations of indigenous bacteria and fungi in the amended soil. The populations of total culturable bacteria were 0.55–0.69 log units higher and total culturable fungi 0.66–0.91 log units higher in the BPM-amended soil from radish experiments. Similarly, the populations of bacteria (0.53–0.92 log units higher) and fungi (0.75–2.01 log units higher) were also higher in the BPM-amended soil from cucumber experiments. Increased soil microbial activity due to soil amendments generally provides disease suppression and possibly may contain biocontrol and other beneficial soil organisms (Abbasi et al., Citation2008). It is possible that this increased microbial activity in the BPM-amended soil may have had a biocontrol effect against these damping-off pathogens. Further studies are needed to investigate the nature of this disease control using a microbiome approach. The soil incorporation of green plant residues has been shown to increase the microbial population density and diversity of bacteria and fungi with antagonistic activity against pathogens in amended soil (Perez et al., Citation2008). In the present study, buckwheat green plant residue or BPM increased the total number of culturable bacteria in amended soil compared with non-amended soil starting from the immediate planting after the amendment incorporation. The bacterial numbers remained consistently higher in the BPM-amended soil in 3 and 8 weeks plantings after the amendment incorporation. The population of total fungi was higher after 3 weeks and remained one log unit higher after 8 weeks of incubation. Davey & Papavizas (Citation1959) also reported a similar stimulative effect of buckwheat plant residue on fungi and bacteria where they used 1% immature or green plant residue as a soil amendment.
A delayed protection of radish and cucumber plants from seedling diseases suggests a biological basis of disease control. This 3-week incubation period also coincided with higher numbers of both total bacteria and fungi induced in the BPM-amended soil. It is likely that these induced changes in microbial activity in the soil may have contributed to disease suppression. This delayed disease suppression may well be correlated to the decomposition of fresh or green plant material in the soil, which can affect soil suppressiveness (Grünwald et al., Citation2000b). BPM soil amendment did not provide immediate protection of radish or cucumber seedlings from damping-off and root rot disease, indicating that BPM had no direct impact on plant pathogens. In fact, extracts of BPM did not show any in vitro antimicrobial activity against R. solani, P. ultimum or several other pathogens in plate assays (data not shown). The green or immature plant residue contains a low C:N ratio (Lewis & Papavizas, Citation1977), and therefore can increase germination of fungal propagules and may be associated with a low level of disease suppression (Grünwald et al., Citation2000a), as seen in early plantings in the present study. Lewis & Papavizas (Citation1977) also reported no significant reduction of Fusarium root rot severity index in beans caused by Fusarium solani f. sp. phaseoli with 1% green buckwheat residue used as a soil amendment 3 weeks before planting. However, the effect of the decomposition state of plant residues in the soil and disease suppression can be variable depending on the pathogen and plant tissue (Papavizas & Davey, Citation1960; Grünwald et al., Citation2000a).
Buckwheat plants grown in a greenhouse potting mix and used as a soil amendment showed no negative effects on growth of radish plants. Radish plants produced in a field soil amended with up to 10% BPM grew normally and showed no signs of phytotoxic effects. However, BPM soil amendment affected emergence of cucumber seeds in immediate plantings. BPM soil amendment also had no effect on plant growth promotion. In the non-infested field soil, plants produced in the BPM-amended and non-amended control soil had similar dry weights. Based on the low percentage of total nitrogen in BPM (0.72%; available N may be substantially less), a plant growth promotion effect was not anticipated. It may be interesting to compare the nutrient contents of buckwheat plants produced in a greenhouse potting mix with those of field-grown plants. BPM soil amendment also did not cause any major or drastic shifts in soil pH. Due to its low C:N ratio as compared with grass species (Kumar et al., Citation2009b), it is possible that BPM may have been rapidly decomposed or degraded in the soil. This rapid decomposition may have additional benefits for growers in terms of application in the field.
In summary, soil incorporation of BPM prior to planting can provide suppression of seedling damping-off and root rot diseases of certain direct-seeded crops. However, in order to achieve maximum disease protection, planting should be delayed for at least 3 weeks after the amendment incorporation in the infested soil. It appears that BPM shows no direct toxicity to pathogens and provides disease suppression by enhancing microbial activity in the amended soil.
Acknowledgements
Technical assistance of the following co-op or summer students is gratefully acknowledged: Alexa Busef, Emma MacDougall, Meghan Chisholm, Brock Burgess, Alya Ahmed-Praught, Brendan Ward, Taylor Shaw and Jacqueline Bradbury.
Additional information
Funding
References
- Abbasi PA, Conn KL, Lazarovits G. 2004. Suppression of Rhizoctonia and Pythium damping-off of radish and cucumber seedling by addition of fish emulsion to peat mix or soil. Can J Plant Pathol. 26:177–187.
- Abbasi PA, Conn KL, Lazarovits G. 2007. Managing soilborne diseases of vegetable crops with a pre-plant soil or substrate amendment of a corn distillation product. Biocont Sci Technol. 17:331–344.
- Abbasi PA, Lazarovits G, Conn KL. 2008. Enhancing biological control of soilborne plant diseases by organic amendments. In: Barka EA, Clément C, editors. Plant-microbe interactions. Kerala: Research Signpost; p. 319–343.
- Bicksler AJ, Masiunas JB. 2009. Canada thistle (Cirsium arvense) suppression with buckwheat or sudangrass cover crops and mowing. Weed Technol. 23:556–563.
- Creamer NG, Baldwin KR. 2000. An evaluation of summer cover crops for use in vegetable production systems in North Carolina. HortScience. 35:600–603.
- Davey CB, Papavizas GC. 1959. Effect of organic soil amendments on the Rhizoctonia disease of snap beans. Agron J. 51:493–496.
- Golisz A, Lata B, Gawronski SW, Fujii Y. 2007. Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol Manag. 7:164–171.
- Grünwald NJ, Hu S, van Bruggen AHC. 2000a. Short-term cover crop decomposition in organic and conventional soils: characterization of soil C, N, microbial and plant pathogen dynamics. Eur J Plant Pathol. 106:37–50.
- Grünwald NJ, Hu S, van Bruggen AHC. 2000b. Short-term cover crop decomposition in organic and conventional soils: soil microbial and nutrient cycling indicator variables associated with different levels of soil suppressiveness to Pythium aphanidermatum. Eur J Plant Pathol. 106:51–60.
- Haramoto ER, Gallandt ER. 2005. Brassica cover cropping: I. Effects on weed and crop establishment. Weed Sci. 53:695–701.
- Himmelstein J, Maul JE, Balci Y, Everts KL. 2016. Factors associated with leguminous green manure incorporation and Fusarium wilt suppression. Plant Dis. 100:1910–1920.
- Howard RJ, Garland JA, Seaman WL. 1994. Diseases and pests of vegetable crops in Canada: an illustrated compendium. Ottawa: Canadian Phytopathological Society and Entomological Society of Canada.
- Iqbal Z, Hiradate S, Noda A, Isojima S, Fuji Y. 2002. Allelopathy of buckwheat: assessment of allelopathic potential of extract of aerial parts of buckwheat and identification of fagomine and other related alkaloids as allelochemicals. Weed Biol Manag. 2:110–115.
- Iqbal Z, Hiradate S, Noda A, Isojima S, Fuji Y. 2003. Allelopathic activity of buckwheat: isolation and characterization of phenolics. Weed Sci. 51:657–662.
- Kumar V, Brainard DC, Bellinder RR. 2008. Suppression of Powell amaranth (Amaranthus powellii), shepherd’s-purse (Capsella bursa-pastoris), and corn chamomile (Anthemis arvensis) by buckwheat residues: role of nitrogen and fungal pathogens. Weed Sci. 56:271–280.
- Kumar V, Brainard DC, Bellinder RR. 2009a. Suppression of Powell amaranth (Amaranthus powellii) by buckwheat residues: role of allelopathy. Weed Sci. 57:66–73.
- Kumar V, Brainard DC, Bellinder RR. 2009b. Effects of spring sown cover crops on establishment and growth of hairy galinsoga (Galinsoga ciliata) and four vegetable crops. HortScience. 44:730–736.
- Kumar V, Brainard DC, Bellinder RR, Hahn RR. 2011. Buckwheat residue effects on emergence and growth of weeds in winter-wheat (Triticum aestivum) cropping systems. Weed Sci. 59:567–573.
- Larkin RP. 2013. Green manures and plant disease management. CAB Rev. 8:1–10.
- Larkin RP. 2015. Soil health paradigms and implications for disease management. Annu Rev Phytopathol. 53:199–221.
- Lewis JA, Papavizas GC. 1977. Effect of plant residues on chlamydospore germination of Fusarium solani f. sp. phaseoli and on Fusarium root rot of bean. Phytopathology. 67:925–929.
- Papavizas GC, Davey CB. 1960. Rhizoctonia disease of bean as affected by decomposing green plant materials and associated microfloras. Phytopathology. 50:516–522.
- Perez C, Dill-Macky R, Kinkel LL. 2008. Management of soil microbial communities to enhance populations of Fusarium graminearum-antagonists in soil. Plant Soil. 302:53–69.
- Valenzuela H, Smith J. 2002. Sustainable agriculture green manure crops: buckwheat. Cooperative extension service. Honolulu (HA): College of Tropical Agriculture and Human Resource, Hawaii University; p. 4.
- Wiggins B, Kinkel L. 2005. Green manures and crop sequences influence alfalfa root rot and pathogen inhibitory activity among soil-borne Streptomycetes. Plant Soil. 268:271–283.
- Xuan TD, Tsuzuki E. 2004. Allelopathic plants. Buckwheat. Allelopathy J. 13:137–148.
