 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cercospora leaf spot (CLS), caused by Cercospora beticola, is the most detrimental disease of sugar beet in temperate climates. In the Great Lakes region, CLS was well managed by pyraclostrobin-based programmes using the decision support tool BEETcast™. Due to C. beticola resistance to pyraclostrobin in the region, other groups of fungicides were evaluated. Field experiments were conducted at six sites from 2013 to 2015 in Pain Court (PC) and Ridgetown (RT), Ontario. BEETcast™ application schedules for prothioconazole and mancozeb as well as carrier volume (115 and 235 L ha−1) were compared with label-based (calendar) applications. When disease intensity was high, the conservative calendar application schedule reduced the standardized area under the disease progress curve (sAUDPC) by an average of 84% compared with all BEETcast™ schedules, and the BEETcast™ 50/35 schedule (235 L ha−1) reduced sAUDPC by 48% compared with the 55/50 schedules. However, there were fewer differences among application schedules at sites with low or moderate disease intensity. BEETcast™ application schedules reduced the number of fungicide applications by 34–55%. All application schedules increased sucrose (%) and recoverable white sugar (RWS) to an equivalent level, but none of the schedules increased profit margin compared with the non-treated control. Using a carrier volume of 235 L ha−1 reduced sAUDPC by 28% compared with 115 L ha−1 at one site when disease severity exceeded 95% in non-treated control plots. Thus, management recommendations should consider that CLS severity is reduced by application schedules using a shortened interval and appropriate carrier volume, but this does not necessarily result in higher beet or sucrose yield or increases in profit margin.
Résumé
La cercosporose, causée par Cercospora beticola, est la maladie qui nuit le plus à la betterave à sucre cultivée sous des climats tempérés. Dans la région des Grands Lacs, la cercosporose était bien gérée grâce aux programmes recommandant l’utilisation de la pyraclostrobine et basés sur l’outil d’aide à la prise de décision BEETcastMD. À cause de la résistance de C. beticola à la pyraclostrobine dans la région, d’autres groupes de fongicides ont été évalués. De 2013 à 2015, des expériences ont été menées sur le terrain à six sites, à Pain Court (PC) et Ridgetown (RT), en Ontario. Les calendriers d’application de prothioconazole et de mancozèbe découlant de BEETcastMD, de même que le volume porteur (115 et 235 L ha−1), ont été comparés à ceux des étiquettes. Quand l’intensité de la maladie était élevée, le calendrier d’application modérée a réduit l’aire normalisée sous la courbe de progression de la maladie (AnUDPC) de 84% en moyenne, comparativement à tous les calendriers de BEETcastMD, et le calendrier BEETcastMD 50/35 (235 L ha−1) a réduit de 48% l’AnUDPC, comparativement aux calendriers 55/50. Toutefois, aux sites où l’intensité de la maladie était faible ou modérée, les différences entre les calendriers d’application étaient moindres. Les calendriers d’application de BEETcastMD ont permis de réduire le nombre d’applications de fongicides de 34 à 55%. Tous les calendriers d’application ont permis d’augmenter la quantité de sucrose et de sucre blanc extractible à un taux équivalent, mais aucun calendrier n’a contribué à accroître la marge de profit, et ce, comparativement aux témoins non traités. À un des sites, quand la gravité de la maladie excédait 95% dans des parcelles témoins non traitées, un volume porteur de 235 L ha−1 a réduit l’AnUDPC de 28%, comparativement à celui de 115 L ha−1. Ainsi, les recommandations de gestion devraient prendre en compte que la gravité de la cercosporose est réduite par des calendriers d’application recommandant un intervalle écourté et un volume porteur approprié, mais que cela ne garantit pas nécessairement un rendement plus élevé en betterave ou en sucrose ni un accroissement de la marge de profit.
Introduction
Sugar beet is a major source of sucrose in temperate countries. Approximately 4050 ha of sugar beet are grown in Ontario every year, with an average annual farm gate value of almost $19 million from 2010–2014 (W. Martin, Michigan Sugar Company, personal communication). Cercospora leaf spot (CLS), caused by a hemibiotrophic fungus Cercospora beticola Sacc., is the primary foliar disease of sugar beet (Beta vulgaris L. ssp. vulgaris) worldwide (Weiland & Koch, Citation2004; Jacobsen & Franc, Citation2009; Khan et al., Citation2009). Cercospora leaf spot presents as necrotic purple or red-brown round spot lesions, coalescing into leaf necrosis that limits photosynthetic ability and reduces sucrose production and root growth (Weiland & Koch, Citation2004; Harveson, Citation2013). Disease-induced leaf regrowth occurs as CLS severity increases. This further decreases root yield, sugar yield and sugar purity as energy is redirected from sugar production and root growth to foliar growth (Shane & Teng, Citation1992; Jacobsen & Franc, Citation2009). CLS epidemics in 2015 caused yield loss of up to 11 T ha−1 and sugar losses of up to 19% in the Great Lakes sugar beet production region (Stewart, Citation2016).
Foliar fungicides are important for the management of CLS and several active ingredients are registered on sugar beet in Canada (OMAFRA, Citation2017). These include the group M1 (inorganic) fungicides copper hydroxide and copper octanoate; M3 (dithiocarbamates) fungicides mancozeb and metiram; group 1 (methyl-benzimidazole carbamates, MBC) fungicide thiophanate-methyl; group 3 (demethylation inhibitors, DMI) fungicides difenoconazole, metconazole, prothioconazole and tetraconazole; and group 11 (quinone outside inhibitors/strobilurin, QoI) fungicides pyraclostrobin (OMAFRA, Citation2017). However, C. beticola resistance to MBC and QoI fungicides is present in Ontario and their use is no longer recommended in the region (LeBoeuf et al., Citation2012; Trueman et al., Citation2013).
Group 3 fungicides were the most effective fungicides for CLS management in 2013 (Trueman & Burlakoti, Citation2014). For example, among the eight fungicide groups evaluated in Ontario, the DMI fungicide difenoconazole was the most effective product, resulting in 2361 and 3359 kg ha−1 more sugar production than the non-treated control using a BEETcast™ 50/35 and 14 day calendar schedule (Trueman & Burlakoti, Citation2014). Application of the group M3 fungicide mancozeb resulted in 258 and 483 kg ha−1 more sugar production than the non-treated control when applied using a BEETcast™ 50/35 and 14 day calendar schedule. Disease severity was up to 38% and 34% lower than the non-treated control using mancozeb or difenoconazole, respectively (Trueman & Burlakoti, Citation2014). The relatively high efficacy of DMI fungicides compared with mancozeb amplifies the importance of resistance management for DMI fungicides, as mixing partners that are less effective at managing the pathogen are also less effective at reducing selection pressure to more effective fungicides (van den Bosch et al., Citation2014).
Fungicide efficacy is influenced by coverage and retention on crop foliage, which is affected by the volume of water used in the fungicide application (referred to hereafter as carrier volume) (Armstrong-Cho et al., Citation2008; Gossen et al., Citation2008; Secor et al., Citation2010). Armstrong-Cho et al. (Citation2008) assessed the efficacy of fungicide applications applied with different carrier volumes (100, 200 and 300 L ha−1) against Ascochyta blight of chickpea in Saskatchewan. Fungicide treatments with larger carrier volumes (200 and 300 L ha−1) resulted in improved canopy coverage and lower disease severity than for treatments applied with low carrier volume (100 L ha−1) (Armstrong-Cho et al., Citation2008). Similar results are reported for powdery mildew in strawberry (Cross et al., Citation2000). Out of convenience, growers may prefer to apply fungicides using carrier volumes that are appropriate for herbicides (115 L ha−1) rather than for fungicides (W. Martin, Michigan Sugar Company, personal communication), but carrier volume of at least 187 L ha−1 is recommended to manage CLS (Secor et al., Citation2010).
Fungicides are often applied using a disease forecasting system or decision support tool because of the potential of these tools to aid in reducing fungicide use and maintain fungicide effectiveness (Carisse et al., Citation2010). In the Great Lakes region, the BEETcast™ system has been in use since 2004 (Windels, Citation2010). BEETcast™ uses leaf wetness duration and temperature data to generate a daily disease severity value (DSV) between 0 and 4, where ‘0' indicates unfavourable conditions and ‘4' indicates favourable conditions for CLS development. Daily DSVs accumulate to a pre-determined threshold, which triggers a fungicide application (Windels, Citation2010). Current BEETcast™ DSV thresholds were developed and optimized for use with pyraclostrobin. These DSV thresholds may not be appropriate for DMI and dithiocarbamate-based fungicide programmes because their mode of action and efficacy differ from pyraclostrobin (OMAFRA, Citation2017). Thresholds applicable to prothioconazole- and mancozeb-based management programmes have not been evaluated in the Great Lakes region.
Prior to beginning the current study, insensitivity to DMI fungicides was reported in Europe (Karaoglandis et al., Citation2000; Trkulja et al., Citation2015) and the Red River Valley growing region in North Dakota and Minnesota in the USA (Secor et al., Citation2010; Bolton et al., Citation2012). Concern about potential for development of DMI insensitivity by C. beticola in Canada triggered the evaluation of disease management tactics for CLS that were also reported to delay the evolution of resistance. These included the use of appropriate carrier volumes, mode of action rotations, and judicious use of fungicides (Brent & Hollomon, Citation2007). The objectives of this research were to: (i) evaluate thresholds of calendar and BEETcast™-based fungicide application schedules using prothioconazole and mancozeb fungicides to manage CLS in sugar beet; and (ii) assess the effect of carrier volume in the efficacy of these fungicides.
Materials and methods
Field experiments were conducted at two sites in the Ontario sugar beet production region from 2013–2015 to compare the performance of application schedules using prothioconazole and mancozeb applied according to the BEETcast™ model or label-recommended calendar intervals. Evaluations included root yield, sugar purity and content, recoverable white sucrose, disease severity and schedule cost. For disease severity, the CLS epidemic progress was depicted and then expressed as standardized daily area under the disease progress curve (sAUDPC).
Field site setup
The experiments were conducted in commercial sugar beet fields in Pain Court, Ontario (42°24ʹ17.2′′N, 82°20ʹ22.1′′W) and research fields at the University of Guelph, Ridgetown Campus, Ridgetown, Ontario (42°26ʹ55.9′′N, 81°53ʹ05.3′′W) from 2013–2015. At the Pain Court (PC) site, sugar beet cultivar ‘HM-173RR’ (CLS resistance rating: good) (Michigan Sugar Company, Citation2014) was initially seeded on 23 April 2013. The experiment was reseeded on 17 May 2013 due to frost damage. Cultivar ‘C-RR059' (CLS resistance rating: good) (Michigan Sugar Company, Citation2014) was seeded on 17 April 2014 and 15 April 2015. At the Ridgetown (RT) site, cultivar ‘C-RR074NT’ (CLS resistance rating: poor) (Michigan Sugar Company, Citation2014) was seeded on 3 May 2013, 12 May 2014, and 29 April 2015. At all sites, rows were spaced 75 cm apart and seeded at a rate of 10 seeds m−1. Plots consisted of two rows each 7 m long with one sprayed guard row separating each plot.
Experiments were arranged in a randomized complete block design with four replicates per treatment. The experiment had a 3 × 2 factorial design with fungicide application schedule at three levels as one factor and carrier volume at two levels as a second factor. A non-treated control was also included. The application schedules included BEETcast™ 55/50 (referred to hereafter as 55/50), BEETcast™ 55/35 (referred to hereafter as 50/35) and a label-based spray programme (referred hereafter as calendar). The carrier volume included 115 L ha−1 (typical herbicide carrier volume) and 235 L ha−1 (typical fungicide carrier volume). Applications were timed based on accumulated DSVs from the BEETcast™ model, or applied on calendar dates according to product label recommendations (). The planned schedule for 50/35 was an initial fungicide application at 50 DSV, with subsequent applications at a 35 DSV interval. For 55/50 an initial fungicide application was made at 55 DSV, with subsequent applications at a 50 DSV interval. For calendar schedules, an initial application at 55 DSV, and subsequent applications 10 days later when the previous application was mancozeb alone or 14 days when the previous application included prothioconazole, which was consistent with product label recommendations (Bayer Crop Science, Citation2014; UPI, Citation2016). For all schedules, the first application was prothioconazole (175.2 g ha−1) (Proline® 480 SC, Bayer Crop Science, Calgary, AB, Canada) and mancozeb (1687.5 g ha−1) (Manzate® Pro-Stick™ DG, United Phosphorous Inc., King of Prussia, PA, USA), the second application was mancozeb, the third application was with prothioconazole plus mancozeb, and any subsequent applications were with mancozeb. Treatments were applied at 275.8 kPa using a hand-held 2.0-m CO2 boom sprayer (Research & Demonstration Sprayers, Opelousa, LA, USA). The total number of fungicide applications made is presented in . Fungicide treatments with 115 L ha−1 carrier volume were applied using AirMix® 110 01 nozzles (Agrotop GmbH, Obertraubling, Germany), and those with 235 L ha−1 carrier volume were applied using TeeJet® AT 110 02VS nozzles (TeeJet Technologies, Springfield, IL, USA).
Table 1. Number of fungicide applications and application dates for fungicide schedules used to manage Cercospora leaf spot of sugar beet at Pain Court (PC) and Ridgetown (RT), Ontario, 2013–2015.
Weather data and BEETcast™ DSV accumulation
Weather data (temperature, relative humidity, leaf wetness, rainfall) was monitored in near-real time by Weather INnovations Consulting LP (Chatham, ON, Canada) as described in Tedford et al. (Citation2018). BEETcast™ DSVs for each site were obtained from the programme website (www.michiganbeets.com).
Disease intensity
Experimental plots were scouted weekly for CLS symptoms beginning in early June. After symptoms were found, the disease severity for 10 randomly selected plants in the first row of each plot was evaluated weekly using the scale described by Tedford et al. (Citation2018). Midpoint per cent leaf area values for each plant were used to calculate an average for each plot, and these values were used to calculate AUDPC using the following equation:
where Yi is mean rating at day Xi and Yi−1 is mean rating at day Xi−1.
The sAUDPC was calculated as AUDPC/number of days (Madden et al., Citation2007).
Yield and sugar measurement
Harvest occurred on 15 Oct 2013, 14 Oct 2014 and 21 Sept 2015 for PC and 9 Oct 2013, 27 Oct 2014 and 5 Oct 2015 for RT. Four metres of each plot were harvested by hand and number and weight of harvested beets was recorded. At the RT 2015 site, treatment 55/50 with 115 L ha−1 carrier volume in the second replication only had 2 m of beets available for harvest due to poor emergence and the presence of Rhizoctonia crown and root rot, and data were adjusted to yield per 4 m of row prior to analysis.
A random subsample of 12–15 roots, representing ~27–72% of the harvested beets depending on root size, was collected during harvest and shipped to the Michigan Sugar Company processing facility in Bay City, MI for sugar analysis. Samples were sliced in half by a rasping circular saw to obtain 1 L of root pulp (brei). The brei was filtered and the juice extracted, frozen and transported to the Michigan Agriculture Research Laboratory (MARL) (B. Groulx, Michigan Sugar Company, personal communication; Van Eerd et al., Citation2012) for sucrose yield and standard quality analysis (Carruthers & Oldfield, Citation1961). The polarimeter method was used to determine sucrose content (Halvorson et al., Citation1978). Methods reported by Last et al. (Citation1976) were used to determine sucrose purity (clear juice purity, CJP) and brei impurity amino-N. Recoverable white sucrose per ton of fresh beets (RWST) was calculated using the following equation: (Van Eerd et al., Citation2012) and converted to metric units. Recoverable white sucrose per acre (RWSA) was calculated using the following equation: RWSA (lb acre−1) = RWST × Total Yield, where: Total Yield (ton acre−1) = (total weight of harvested plot (kg 3 m−2)/0.0003/1000/2.5) × 1.1 (P. May, University of Guelph, personal communication) and converted to metric units.
Economic analysis
The net return for each fungicide application schedule was calculated as: Profit margin ($ ha−1) = gross payment ($ ha−1) – (product application cost + grower trucking cost + fungicide cost). Profit margin calculations assumed trucking and fungicide applications were the only grower costs. The gross payment calculator was obtained from Michigan Sugar Company to determine gross payment. Calculations assumed an early delivery premium for beets harvested prior to large storage pile setup on 21 October each year and a base payment of $50 ton−1 of sucrose (RWST). Fungicide costs were determined by multiplying the mean 2015 list price (mancozeb = $8.00 kg−1, prothioconazole = $172.00 L−1) from three different fungicide dealers located in Kent and Lambton counties: Fungicide cost = product price × product label rate × number of fungicide applications. Fungicide cost per application was $17.00 ha−1 for mancozeb and $63.00 ha−1 for prothioconazole. Application cost ($ ha−1) was calculated as the product of a custom fungicide application in 2015 with 115 L ha−1 or 235 L ha−1 and the number of fungicide applications, where custom fungicide application with 115 L ha−1 = $22.00 ha−1 and custom fungicide application with 235 L ha−1 = $30.00 ha−1 (Molenhuis, Citation2016). This assumes that the additional costs associated with using a higher carrier volume (water use, time and equipment) are accounted for by the higher custom fungicide application cost. Grower trucking cost was calculated as: Grower trucking cost ($ ha−1) = (sucrose yield (ton ac−1) × $6 ton −1)/0.405. In 2015, the Michigan Sugar Company was responsible for paying another $6 ton−1 for trucking but this cost was not included in the profit margin calculations since it was not incurred by the grower. Grower trucking cost was included in the profit margin calculations because a grower with a low root yield and high sucrose yield can have reduced trucking costs. Costs and revenues were calculated assuming parity between USD and CAD for simplicity.
Statistical analysis
Statistical analyses were conducted using SAS v.9.4 (SAS Institute Inc., Cary, NC). Analysis of variance (ANOVA) was performed for all the measured disease and yield variables, using PROC MIXED (P ≤ 0.05). The fixed effects were fungicide treatment and site; replication within site was the random effect. Field experiments from each site and year were considered individually in the statistical analyses. The normality of residuals was tested with the Shapiro–Wilk test, and distribution of error was determined by visual analysis of residual plots (Bowley, Citation1999). Log transformations were performed as necessary to meet assumptions of normality. Outliers were assessed using Lund’s test but only removed if this affected significance (Bowley, Citation1999). Data were pooled across sites when analysis showed no site × treatment interaction. Means were separated using Tukey’s Multiple Mean Comparison (P ≤ 0.05). A contrast analysis was completed to determine effect of water volume on measured variables.
Results
Fungicide applications, BEETcast™ DSV accumulation and disease progress
The calendar programme resulted in the application of seven to nine fungicide applications per season compared with four to five for 50/35 and three to four for 55/50. Use of the BEETcast™ decision support tool resulted in a reduction of two to four and three to five fungicide applications per season for the 50/35 and 55/50 programmes, respectively (). BEETcast™ DSV accumulation began between 1 May and 15 May each year with the exception of RT 2013, which did not have environmental conditions favouring DSV accumulation until late May. The date to reach the 50 DSV threshold (for the first fungicide application) ranged from 23 June to 30 June among site years, which was 16 to 79 days prior to the first observation of symptoms. At the PC site, symptoms were first observed on 12 Aug 2013, 10 Sept 2014, and 23 July 2015. At the RT site, symptoms were first observed on 16 July 2013, 16 July 2014 and 23 July 2015. The 55 DSV threshold was reached from 21 June to 3 July among site years, which was 13 to 32 days prior to the first observation of symptoms. Thus, both BEETcast™ thresholds reduced the numbers of fungicide applications at both sites in all years and the first fungicide application for all programmes occurred before the appearance of CLS symptoms.
Influence of fungicide application schedule and carrier volume on disease progress
Maximum CLS severity ratings peaked in September or October at all sites, with severity in non-treated control plots ranging from 8% at RT 2013 to 96% at RT 2015 (). Fungicide applications following the calendar, 50/35, and 55/50 schedules with 235 L ha−1 carrier volume reduced disease severity on the final assessment date at all sites except for RT 2013, the 50/35 and 55/50 schedules at PC 2014 and PC 2015, and the 55/50 schedule at RT 2015 (). Disease severity on the final assessment in the calendar schedule was lower than 55/50 with the exception of RT 2013 and PC 2014 (). Disease severity in the 50/35 schedule was equivalent to both the calendar and 55/50 schedules with the exception of PC 2013, when it was lower than 55/50, and PC 2015 and RT 2015, when it was higher than the calendar schedule.
Fig. 1 Weekly progression of mean Cercospora leaf spot severity on sugar beet in Pain Court and Ridgetown, Ontario, in 2013, 2014 and 2015 comparing several fungicide spray schedules using a carrier volume of 235 L ha−1. Data were log10 transformed with back transformed means presented, confidence intervals are presented in Table S1. Data points in individual figures with the same letter were not significantly different on the final assessment date at P ≤ 0.05 Tukey’s adjustment. For figures with an ‘*’, differences are discussed in the text. Confidence intervals (95%) are available in Table S1.
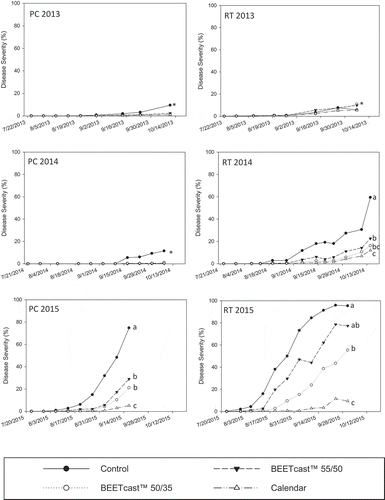
Standardized AUDPC differed among treatments at all sites (). There was no difference among treatments for sAUDPC in 2013, except for 50/35 at 115 L ha−1 carrier volume, which was lower than the non-treated control. Disease severity was very low in non-treated control plots in 2013, where at both sites severity at harvest was <10%. When disease was low to moderate ranging from 11% at PC 2014 to 59% at RT 2014 and 75% at PC 2015, all fungicide application schedules reduced disease severity compared with the non-treated control. The 55/50 programme using 115 L ha−1 carrier volume was less effective in reducing sAUDPC than the calendar programme using either carrier volume, and the 55/50 using 235 L ha−1 carrier volume was less effective than the calendar using 115 L ha−1 carrier volume. Under high disease intensity at RT 2015, only the BEETcast™ 50/35 and calendar programmes had lower disease severity than the non-treated control. Calendar application schedules reduced disease severity to a greater extent than the 50/35 programme at RT 2015, but at other sites there was no difference among the two programmes. Implementing the 50/35 programme reduced two to four fungicide applications per season (). There was no effect of carrier volume on sAUDPC except at RT 2015, which had the highest disease severity among all sites (). At RT 2015, applications using a 235 L ha−1 carrier volume reduced sAUDPC by 28% compared with a 115 L ha−1 carrier volume.
Table 2. Standardized area under disease progress curve (sAUDPC) (per day) for Cercospora leaf spot in sugar beets treated with different fungicide spray schedules and carrier volumes, Pain Court (PC) and Ridgetown (RT), Ontario, 2013–2015.
Influence of fungicide application schedule and carrier volume on beet yield and sucrose yield and quality
Total root yield and sucrose purity were not affected by application schedule (). Sucrose content was 4.2 to 7.2% higher for all fungicide application schedules compared with non-treated controls (16.7% sucrose content). Similarly, RWS was 7.4 to 8.7% higher than measured in non-treated controls (122.5 kg T−1), except for 55/50 with 115 L ha−1 carrier volume, which was not different from the control. RWSH was higher than the non-treated control treatments only for calendar fungicide application programmes. Contrast analysis showed that carrier volume did not affect root or sucrose yields or sucrose quality. Thus, all fungicide application schedules increased sucrose content but programmes that included more fungicide applications tended to have reduced CLS severity, higher RWSH and RWS, but not higher beet yield.
Table 3. Root and sucrose yield from sugar beets harvested from plots treated with different spray schedules and carrier volumes for management of Cercospora leaf spot, Pain Court (PC) and Ridgetown (RT), Ontario, 2013–2015.
Influence of fungicide application schedule and carrier volume on profit margin
Grower profit margin was calculated using gross payment and the costs of product application, trucking and fungicide. Profit margin ranged from $4040 ha−1 in 50/35 with 235 L ha−1 carrier volume to $4384 ha−1 in 55/50 with 115 L ha−1 carrier volume, but these differences were not significant (). Neither the application schedule nor the carrier volume used affected grower profit margin.
Table 4. Cost analysis of different spray schedules from plots treated with different fungicide application timings and carrier volumes for management of Cercospora leaf spot, Pain Court and Ridgetown, Ontario, 2013–2015.
Discussion
This study examined the impact of fungicide carrier volume and calendar and BEETcast™-based fungicide application schedules for management of CLS in sugar beet using the protectant M3 fungicide mancozeb and the group 3 DMI fungicide prothioconazole, which was highly effective against C. beticola in Ontario at the time this study began (Trueman & Burlakoti, Citation2014). This is the first report of the effects of these application schedules and carrier volume on CLS management using solely the representative group 3 DMI fungicide prothioconazole and the contact protectant mancozeb.
Disease severity in response to fungicide application schedule varied among sites, which appeared to influence efficacy. For example, there were few differences in sAUDPC and disease severity was very low (< 10%) in 2013, but the most intensive programme (i.e. calendar) resulted in lower disease severity than the least intensive programme (i.e. 55/50) at PC 2014 and 2015 and RT 2014, where disease severity was low to moderate (11 to 75%). Disease severity was highest at RT 2015 (> 95%), where 55/50 did not reduce sAUDPC compared with the non-treated control, and the moderately intensive programme 50/35 was less effective than the calendar-based application schedule. Thus, more intensive application schedules using prothioconazole and mancozeb provided better management for CLS when disease intensity was high. This is not surprising, since these programmes used shorter application intervals and more fungicide applications per season. The group 3 DMI fungicide tetraconazole reduced CLS when applied with a 14 d interval compared with a 21 d interval under high disease conditions in Minnesota and North Dakota (Khan & Smith, Citation2005). This is also consistent with observations by Pitblado ((Citation2003a, Citation2003b, Citation2003c, Citation2003d, Citation2005) that CLS incidence was reduced using BEETcast™ application schedules with combinations of pyraclostrobin, tetraconazole, thiophanate-methyl, and triphenyltin hydroxide in Michigan. Schedules that included intervals exceeding 55 DSVs in these studies were less effective, indicating that a shorter application interval gives better disease management.
Although more intensive application schedules tended to result in a greater reduction in disease, this study identified no effect of application schedule on beet yield or sugar purity. All application schedules improved sucrose content and all application schedules using 235 L ha−1 carrier volume improved RWS in an equivalent manner compared with applying no fungicide, suggesting even though the 55/50 schedule sometimes had higher levels of CLS than more intensive programmes, management was adequate to limit sucrose losses. A CLS field trial in Michigan also yielded equivalent sucrose (%) and RWS among different fungicide application schedules (Michigan Sugarbeet Research and Education Advisory Council, Citation2017). This result contrasts with research in North Dakota in susceptible sugar beets ‘Beta 3800', which found that any fungicide treatment (tetraconazole and pyraclostrobin) and application schedule (calendar or Shane and Teng schedule) combination increased root yield and RWSH compared with the control (Khan et al., Citation2007). Similarly, tetraconazole applications every 14 d increased root and sucrose yield compared with a 21 d schedule in Minnesota and North Dakota (Khan & Smith, Citation2005). It is possible that differences in disease level could be responsible for the yield differences among studies. The impact of CLS on sucrose yield may also be genotype dependent. Research to evaluate potential interactions among fungicide application schedules and sugar beet genotypes with variable CLS resistance is currently underway in Ontario.
Interestingly, profit margin did not increase compared to the non-treated control using any application schedule. This was true even for the RT 2015 site where disease severity in non-treated control plots reached 95% four days before harvest. Thus, the relative increase in sucrose yield attributed to fungicides did not compensate for the increase in input cost. However, profit margin can change over time with changes in fungicide and application costs and maintaining a predictable yield level is important for sugar processing plants. The impact of CLS on beet yield, sugar yield and quality, and profit margin may depend on reaching a certain disease threshold within a specified period before harvest that justifies the fungicide expense. However, ceasing fungicide applications early may increase the amount of overwintering inoculum and thus increase disease potential in the following year, and does not consider negative effects of CLS on beet storability (Jacobsen & Franc, Citation2009).
Implementation of the 50/35 and 55/50 application schedules resulted in a 34% and 55% mean reduction in the number of fungicide applications compared with the calendar application schedule with no difference in sucrose yield or content. Although profit margin was unaffected by application schedule, reducing fungicide use has other benefits including reductions in human exposure and off-target effects of pesticides. For example, Canada’s Pest Management Regulatory Agency (PMRA) recently announced the phase-out of almost all uses of mancozeb because of unacceptable risks to human health (Health Canada, Citation2018), although this decision is now again under review (Davidson, Citation2018). However, there may also be long-term risks for disease management when fungicide use is reduced. Since all application schedules required at least three applications, and prothioconazole was applied in the first and third applications, the implementation of BEETcast™ using prothioconazole and mancozeb may not contribute to delaying the evolution of DMI insensitive C. beticola populations. This is because these programmes result in a reduction in applications of the protectant fungicide mancozeb, but not prothioconazole, thereby increasing overall selection pressure on prothioconazole. Furthermore, BEETcast™ generally results in extended application intervals. Extended application intervals are associated with acceleration of fungicide resistance in the apple scab pathosystem in the USA (Beckerman et al., Citation2015), but this has not been explored for the CLS pathosystem. Thus, the decision to implement a calendar or BEETcast™ based application schedule should consider multiple factors including economics, the effectiveness and off-target effects of available fungicides, and long-term management of CLS.
Carrier volume only affected disease level at RT 2015, which had the highest disease severity. At that site, using a carrier volume of 235 L ha−1 reduced sAUDPC by 28% compared with 115 L ha−1. Increased carrier volume was also most important under high disease intensity in chickpea in Saskatchewan (Armstrong-Cho et al., Citation2008) and strawberry in the UK (Cross et al., Citation2000). However, when disease severity is low or moderate, carrier volume is a less important factor because complete fungicide coverage is not as necessary to manage disease (Armstrong-Cho et al., Citation2008). High carrier volumes may offer other benefits such as better plant coverage, which is important for protectant fungicides such as mancozeb (Secor et al., Citation2010). Methods to improve the efficacy of broad-spectrum fungicides such as mancozeb are needed to reduce selection pressure on more effective fungicides such as prothioconazole. Thus, appropriate carrier volumes for fungicides should be included in best management strategies for CLS. Since we only observed an effect of carrier volume at one site with high disease intensity, it may be beneficial to further evaluate the impact of additional carrier volumes under high disease intensity to understand the impact on CLS management.
This study provides evidence that prothioconazole and mancozeb effectively managed CLS during the study period. Application schedules using the BEETcast™ system were effective at reducing fungicide use and limiting sucrose yield losses, but the 55/50 programme did not reduce disease levels compared with the non-treated control when disease intensity was high. Additionally, use of an appropriate carrier volume improves management under high disease conditions. The recent discovery of prothioconazole insensitive field isolates of C. beticola in Ontario (Trueman et al., Citation2017) is of concern and demonstrates that management of CLS in Ontario must continue to evolve. Growers should continue practices of crop rotation and tillage to manage overwintering inoculum, and research must continue to facilitate adoption of resistant cultivars and better understand evolution of fungicide resistance as part of a sustainable CLS management strategy.
Supplemental Table 1
Download MS Word (17.6 KB)Acknowledgements
The technical assistance of Phyllis May is greatly appreciated.
Supplementary material
Supplementary data can be accessed online here: https://doi.org/10.1080/07060661.2018.1561518
Additional information
Funding
References
- Armstrong-Cho C, Wolf T, Chongo G, Gan Y, Hogg T, Lafond G, Johnson E, Banniza S. 2008. The effect of carrier volume on ascochyta blight (Ascochyta rabiei) control in chickpea. Crop Prot. 27:1020–1030.
- Bayer Crop Science. 2014. Proline 480 SC Foliar Fungicide. [ accessed 2018 June 7]. http://pr-rp.hc-sc.gc.ca/1_1/view_label?p_ukid=101683512.
- Beckerman JL, Sundin GW, Rosenberger DA. 2015. Do some IPM concepts contribute to the development of fungicide resistance? Lessons learned from the apple scab pathosystem in the United States. Pest Manag Sci. 71:331–342.
- Bolton MD, Rivera-Varas V, del Río Mendoza LE, Khan MFR, Secor GA. 2012. Efficacy of variable tetraconazole rates against Cercospora beticola isolates with differing in vitro sensitivities to DMI fungicides. Plant Dis. 96:1749–1756.
- Bowley, S. R. 1999. A hitchhiker’s guide to statistics in plant biology. Guelph (ON): Any Old Subject Books.
- Brent KJ and Hollomon DW. 2007. Fungicide resistance in crop pathogens: how can it be managed. 2nd ed. FRAC Monograph No.1. Brussels, Belgium: Croplife International; [ accessed 2016 March 14]. http://www.frac.info/docs/default-source/publications/monographs/monograph-1.pdf?sfvrsn=8.
- Carisse O, Tremblay D-M, Jobin T, Walker AS. 2010. Disease decision support systems: their impact on disease management and durability of fungicide effectiveness. Carisse, O, editor. Fungicides. [place unknown]: IntechOpen; [accessed 2018 September 23]. https://www.intechopen.com/books/fungicides/disease-decision-support-systems-their-impact-on-disease-management-and-durability-of-fungicide-effe.
- Carruthers LG and Oldfield LFT. 1961. Methods for the assessment of beet quality. Int Sugar J. 63:71–74.
- Cross JV, Berrie AM, Murray RA. 2000. Effect of droplet size and spray volume on deposits and efficacy of strawberry spraying. Asp Appl Biol. 57:313–320.
- Davidson K. 2018. Mancozeb and metiram update. The Grower. [ accessed 2018 September 21]. http://thegrower.org/news/mancozeb-and-metiram-update.
- Gossen BD, Peng G, Wolf TM, McDonald MR. 2008. Improving spray retention to enhance the efficacy of foliar-applied disease- and pest-management products in field and row crops. Can J Plant Pathol. 30:505–516.
- Halvorson A, Hartman GP, Cole DF, Haby VA, Baldridge DE. 1978. Effect of N fertilization on sugarbeet crown tissue production and processing quality. Agron J. 70:876–880.
- Harveson R. 2013. Cercospora leaf spot of sugarbeet. NebGuide. University of Nebraska-Lincoln Extension. [ accessed 2014 June 1]. http://ianrpubs.unl.edu/live/g1753/build/g1753.pdf.
- Health Canada. 2018. Re-evaluation decision RVD2018-21 Mancozeb and its associated end-use products. Ottawa (ON): Pest Management Regulatory Agency, Health Canada.
- Jacobsen BJ and Franc GD. 2009. Foliar disease casused by fungi and Oomycetes. In: Harveson RM, Hanson LE, Hein GL, editors. Compendium of beet diseases and pests. 2nd ed. St.Paul (MN): American Phytopathological Society Press. Cercospora leaf spot; p. 7–10.
- Karaoglandis GS, Ioannidis PM, and Thanassoulopoulos CC. 2000. Reduced sensitivity of Cercospora beticola isolates to sterol demythylation-inhibiting fungicides. Plant Pathol. 49:567–572.
- Khan J, del Río LE, Nelson R, Khan MFR. 2007. Improving the cercospora leaf spot management model for sugar beet in Minnesota and North Dakota. Plant Dis. 91:1105–1108.
- Khan J, Qi A, Khan MFR. 2009. Fluctuations in number of Cercospora beticola conidia in relationship to environment and disease severity in sugar beet. Phytopathology. 99:796–801.
- Khan MFR and Smith LJ. 2005. Evaluating fungicides for controlling cercopora leaf spot on sugar beet. Crop Prot. 24:79–86.
- Last PJ, Draycott AP, Hull R. 1976. The influence of level of topping and other cultural practices on sugar beet yield and quality. Inter Sugar J. 78:167–170.
- LeBoeuf J, Trueman C, Martin W, Clark G. 2012. Cercospora leaf spot resistance management for Ontario 2012. [ accessed 2014 June 16]. http://www.michigansugar.com/reach_updates/2012%20Cercospora%20Spray%20Program%20Ontario.pdf.
- Madden VM, Hughes G, van den Bosch F. 2007. The study of plant disease epidemics. St. Paul (MN): American Phytopathological Society Press.
- Michigan Sugar Company. 2014. Growers’ guide for producing quality sugarbeets. Michigan sugar company corporate agricultural office. Bay City, MI: Michigan Sugar Company. [ accessed 2014 June 1]. http://www.michigansugar.com/wp-content/uploads/2014/02/Growers-Guide-2014.pdf
- Michigan Sugarbeet Research and Education Advisory Council. 2017. Evaluate fungicide application timings (BEETcast™), p 10–13. In: 2017 research results. Bay City, MI: Michigan Sugarbeet Research and Education Advisory Council; [ accessed 2018 June 12]. https://www.michigansugar.com/wp-content/uploads/2018/02/Research-Results-2017.pdf.
- Molenhuis J. 2016. Table 8: Survey of custom farmwork rates charged in 2015. Place unkown: OMAFRA. [ accessed 2016 June 3]. http://www.omafra.gov.on.ca/english/busdev/2015customratesa1.htm
- Ontario Ministry of Agriculture, Food, and Rural Affairs. 2017. Vegetable crop protection guide, pub 838. Sugarbeets. Toronto: Queen’s Printer for Ontario.
- Pitblado R. 2003a. Evaluation of BEETcast at Sylvester’s sugar beet farm, Michigan – 2003 – large plot, p. 24–30. In: Insect and disease management research results. Ridgetown: University of Guelph; [ accessed 2018 May 25]. https://atrium.lib.uoguelph.ca/xmlui/handle/10214/6276.
- Pitblado R. 2003b. Evaluation of BEETcast at Sylvester’s sugar beet farm, Michigan – 2003 – small plot, p. 16–23. In: Insect and disease management research results. Ridgetown: University of Guelph; [ accessed 2018 May 25]. https://atrium.lib.uoguelph.ca/xmlui/handle/10214/6276.
- Pitblado R. 2003c. Evaluation of BEETcast at Uebler sugar beet farm, Michigan – 2003 – small plot, p. 31–33. In: Insect and disease management research results. Ridgetown: University of Guelph; [ accessed 2018 May 25]. https://atrium.lib.uoguelph.ca/xmlui/handle/10214/6276.
- Pitblado R. 2003d. Evaluation of BEETcast at Uebler sugar beet farm, Michigan – 2003 – large plot, p. 34–37. In: Insect and disease management research results. Ridgetown: University of Guelph; [ accessed 2018 May 25]. https://atrium.lib.uoguelph.ca/xmlui/handle/10214/6276.
- Pitblado R. 2005. Evaluation of BEETcast in Michigan for the control of Cercospora leaf spot in sugarbeets – 2005, p. 7–10. In: Insect, disease and irrigation management research results. Ridgetown: University of Guelph; [ accessed 2018 May 25]. https://atrium.lib.uoguelph.ca/xmlui/handle/10214/6307.
- Secor GS, Rivera VV, Khan MFR, and Gudmestad NC. 2010. Monitoring fungicide sensitivity of Cercospora beticola of sugar beet for disease management decisions. Plant Dis. 94:1272–1282.
- Shane WW and Teng PS. 1992. Impact of cercospora leaf spot on root weight, sugar yield, and purity of Beta vulgaris. Plant Dis. 76:812–820.
- Stewart J. 2016. When the pressure is high how can we control cercospora leaf spot?, p. 18–19. Perry, J, editor. In: The newsbeet: winter 2015-2016. Vol. 30. Michigan: Michigan Sugar Company; [ accessed 2016 July 13]. http://www.michigansugar.com/wp-content/uploads/2016/03/winter-2015-16.pdf.
- Tedford SL, Burlakoti RR, Schaafsma AW, Trueman CL. 2018. Relationships among airborne Cercospora beticola concentration, weather variables and cercospora leaf spot severity in sugar beet (Beta vulgaris L.). Can J Plant Pathol. 40:1–10.
- Trkulja N, Milosavljević A, Stanisavljević R, Mitrović M, Jović J, Toševski I, Bošcović J. 2015. Occurrence of Cercospora beticola populations resistant to benzimidazoles and demethylation-inhibiting fungicides in Serbia and their impact on disease management. Crop Prot. 75:80–87.
- Trueman CL and Burlakoti RR. 2014. Evaluation of products for management of Cercospora leaf spot in sugarbeet, 2014. Plant Dis Manag Rep. 9:FC009.
- Trueman CL, Hanson LE, Rosenweig N, Jiang QW, and Kirk WW. 2013. First report of QoI insensitive Cercospora beticola on sugar beet in Ontario, Canada. Plant Dis. 97:1255.
- Trueman CL, Hanson LE, Somohano P, Rosenzweig N. 2017. First report of DMI-insensitive Cercospora beticola on sugar beet in Ontario, Canada. New Dis Rep. 36:20.
- United Phosphorus Inc. 2016. Manzate Pro-Stick Fungicide. [ accessed 2018 June 7]. http://pr-rp.hc-sc.gc.ca/1_1/view_label?p_ukid=101683821.
- van den Bosch F, Paveley N, van den Berg F, Hobbelen P, Oliver R. 2014. Mixtures as a fungicide resistance management tactic. Phytopathology. 104:1264–1273.
- Van Eerd LL, Congreves KA, Zandstra JW. 2012. Sugar beet (Beta vulgaris L.) storage quality in large outdoor piles is impacted by pile management but not by nitrogen fertilizer or cultivar. Can J Plant Sci. 92:129–139.
- Weiland J and Koch G. 2004. Pathogen profile: sugarbeet leaf spot disease (Cercospora beticola Sacc.). Mol Plant Pathol. 5:157–166.
- Windels CE. 2010. Cercospora leaf spot prediction models in North America. In: Lartey RT, Weiland JJ, Panella L, Crous PW, Windels CE, editors. Cercospora leaf spot of sugar beet and related species. St. Paul (MN): American Phytopathological Society Press; p. 235–250.
