Abstract
Fusarium wilt of watermelon, caused by Fusarium oxysporum f. sp. niveum (FON), is a devastating soil-borne disease in watermelon production. Race 1 and race 2 of the pathogen are widely distributed in different watermelon producing regions. To investigate whether the root-knot nematodes break resistance of watermelon genotypes with resistance to FON race 1 and race 2, four greenhouse experiments were conducted on two watermelon cultivars ‘Calhoun Gray’ and ‘Fascination’ that are resistant to race 1, and an accession PI 296 341-FR that is resistant to race 2. The treatments included seedlings of ‘Calhoun Gray’ and ‘Fascination’ inoculated with Meloidogyne incognita (1000 J2/plant) or FON race 1 alone, nematodes 5 days before FON, FON 5 days before nematodes, nematodes and FON simultaneously, and non-inoculated control. PI 296 341-FR was inoculated with FON race 2, with or without M. incognita inoculation. The presence of M. incognita enhanced the susceptibility of all watermelon genotypes to Fusarium wilt. Co-inoculation with M. incognita led to an early development of wilt symptoms and increased disease severity. Galls were observed on roots of all nematode-inoculated plants, and sequential inoculation of FON followed by nematodes resulted in numerically lower galling indices compared with other inoculation methods. Whereas inoculation of the nematodes alone did not reduce plant growth, growth suppression was evident when seedlings were inoculated with both pathogens. The results indicated that M. incognita could enhance susceptibility of resistant watermelon genotypes to respective FON races, and host resistance alone is not sufficient for managing Fusarium wilt on watermelon in soils infested with root-knot nematodes.
Résumé
En ce qui a trait à la production de la pastèque, la fusariose, causée par Fusarium oxysporum f. sp. niveum (FON), est une maladie terricole dévastatrice. Les races 1 et 2 de l’agent pathogène sont largement disséminées dans les diverses régions productrices. Pour vérifier si les nématodes à galles affaiblissaient la résistance des génotypes de pastèques aux races 1 et 2 de FON, quatre expériences ont été menées en serre avec deux cultivars de pastèque résistants à la race 1, ‘Calhoun Gray’ et ‘Fascination’, et une accession (PI 296 341-FR) résistante à la race 2. Les traitements comprenaient l’inoculation de semis de ‘Calhoun Gray’ et de ‘Fascination’ avec Meloidogyne incognita (1 000 J2/plant) ou seulement la race 1 de FON, avec nématodes cinq jours avant FON, avec FON cinq jours avant les nématodes, avec nématodes et FON simultanément, et un témoin non inoculé. PI 296 341-FR a été inoculé avec la race 2 de FON, avec ou sans M. incognita. Ce dernier a accru la susceptibilité de tous les génotypes de pastèque à l’égard de la fusariose. L’inoculation mixte avec M. incognita a engendré l’apparition hâtive des symptômes de la fusariose et en a accru la gravité. Des galles ont été observées sur les racines de tous les plants inoculés avec les nématodes, et l’inoculation séquentielle de FON suivie par celle de nématodes a engendré des indices de galles numériquement inférieurs comparativement aux autres traitements. Alors que l’inoculation de nématodes seuls n’a pas réduit la croissance des plants, la suppression de la croissance était évidente lorsque les semis étaient inoculés avec les deux agents pathogènes. Les résultats ont indiqué que M. incognita pouvait accroître la susceptibilité des génotypes de pastèques résistants aux races respectives de FON et que la résistance de l’hôte seule ne suffisait pas pour combattre la fusariose chez la pastèque lorsque les sols étaient infestés de nématode à galles.
Introduction
Fusarium oxysporum f. sp. niveum (FON), the causal agent of Fusarium wilt, is a destructive soil-borne pathogen that is well established in major watermelon (Citrullus lanatus L.) growing regions in the USA (Zhou & Everts, Citation2003; Bruton et al., Citation2008; Kleczewski & Egel, Citation2011; Martyn, Citation2014; Amaradasa et al., Citation2018). Fusarium wilt is one of the most economically important diseases of watermelon worldwide and has been reported on almost all continents (Kleczewski & Egel, Citation2011; Egel & Martyn, Citation2013). FON can survive as a saprophyte in soil and plant debris for long periods due to the ability to form thick-walled chlamydospores (Martyn, Citation1996). It can infect watermelon plants and induce disease at any growth stage (Beattie & Doolittle, Citation1951), resulting in considerable losses in both yield and quality of the crop. Infection occurring in young seedlings results in stunting or damping-off. In older seedlings, the blockage of the xylem vessels due to FON infection first causes yellowing of older leaves, brown discolouration of vascular tissue and temporary wilting of individual vines at the hottest times of the day. As disease progresses, wilting becomes permanent and plants may die (Holliday, Citation1970).
At present, four physiological races of FON have been described and they are referred to as 0, 1, 2 and 3 (Martyn & Bruton, Citation1989; Martyn & Netzer, Citation1991; Zhou et al., Citation2010; Amaradasa et al., Citation2018). Race 0 only causes wilt in watermelon cultivars with no resistance genes (Martyn, Citation2014). Race 1 is the predominant race throughout commercial watermelon regions worldwide and seedless (triploid) watermelon cultivars are usually susceptible to race 1 (Elmstrom & Hopkins, Citation1981; Martyn & Bruton, Citation1989; Zhou & Everts, Citation2003; Kleczewski & Egel, Citation2011). Race 2 has been reported in several states in the USA and is aggressive on both seeded (diploid) and seedless watermelons (Martyn & Bruton, Citation1989; Zhou & Everts, Citation2003; Bruton et al., Citation2008). Race 3, the most virulent race of FON described to date, was shown to cause wilt on almost all PI 296 341-FR seedlings, though this inbred line was very resistant to FON race 2 (Zhou et al., Citation2010; Amaradasa et al., Citation2018).
Root-knot nematodes, Meloidogyne spp., are obligate plant parasites with a global distribution. They are widespread in soil and attack a variety of economically important crops (Sikora & Fernandez, Citation2005). Most commercially available watermelon cultivars are highly susceptible to M. incognita (Montalvo & Esnard, Citation1994; Davis, Citation2007; Thies et al., Citation2010). According to Lynch & Carpenter (Citation1999), ~20% of watermelon yield can be lost due to root-knot nematode infection. Plants infected by nematodes usually show leaf discolouration, root deformation due to the development of galls, and stunted growth.
It is well established that nematodes can interact additively or synergistically with fungal pathogens when they infect the same plants, resulting in equal or greater plant damage compared with the sum of the individual damage (Back et al., Citation2002; LaMondia & Timper, Citation2016). When feeding on plants, nematodes create wounds, induce host cell death and suppress plant defense responses thereby facilitating the establishment and development of soilborne diseases. Conversely, plants infected with fungi can be more attractive and easily penetrated by the nematodes (LaMondia & Timper, Citation2016). The interaction between nematodes and fungi was first found on cotton by Atkinson (Citation1892). Since then, nematode–fungus interactions have been observed on a wide range of crops (Sayre & Walter Citation1991; Abdel-Momen and Starr Citation1998; Back et al. Citation2002; Al-Hazmi and Al-Nadary Citation2015). On watermelon, Sumner and Johnson (Citation1973) reported that the presence of root-knot nematodes in the soil increased wilt symptoms caused by FON. However, no reports provided evidence about the influence of plant-parasitic nematodes on the severity of watermelon wilt induced by different races of FON. Therefore, the main purpose of this study was to investigate the influence of M. incognita on the severity of disease induced by FON race 1 and race 2 on watermelon genotypes resistant to the different races of FON.
Materials and methods
FON isolates and plant materials
Isolates of FON race 1 and 2 were collected from symptomatic watermelon plants in Georgia, USA, and identified to race by inoculation of a set of watermelon differentials (Petkar & Ji, Citation2017a, Citation2017b). Two watermelon cultivars, ‘Calhoun Gray’ and ‘Fascination’, and a wild accession PI 296 341-FR were used in the study. ‘Calhoun Gray’ and ‘Fascination’ are moderately resistant to FON race 1 (Everts & Hochmuth, Citation2011), and PI 296 341-FR is highly resistant to FON race 2 (Martyn & Netzer, Citation1991).
Preparation of inocula
FON was cultured on potato dextrose agar (PDA) at 25ºC for 7 days. Several mycelial plugs from the edge of the colony were transferred to 500 mL flasks containing potato dextrose broth and incubated on a rotary shaker at 160 rpm at room temperature. After 2 weeks, the liquid culture was poured through two layers of sterile cheesecloth and the concentration of FON conidia was adjusted to 105 spores mL−1 using a hemocytometer.
A population of M. incognita race 3 was obtained from a field in Tifton, GA, USA and maintained on eggplant (Solanum melongena L.) in a greenhouse at 22–30°C. Nematode inoculum was obtained from 4-month-old infested plants by cutting the roots, washing them in tap water, and placing the roots in a mist chamber for 2–7 days for eggs to hatch. Freshly hatched second-stage juveniles (J2) were collected every 2–3 days, and diluted with tap water to 1000 J2 mL−1.
Plant inoculation
Watermelon seeds were sown in seedling trays (3.5 × 3.5 cm cells) containing a potting mix (Miracle-Gro, Marysville, OH, USA). The trays were kept in a greenhouse at 26 ± 4°C. For inoculation, 5 mL of FON suspension and/or 1 mL of J2 suspension, at the above-mentioned concentrations, were pipetted onto soil surrounding the stem base of each plant. ‘Calhoun Gray’ and ‘Fascination’ were inoculated with FON race 1, and PI 296 341-FR was inoculated with FON race 2, in separate experiments. The treatments included (a) non-inoculated control (seedlings were treated with sterile water), (b) FON alone, (c) M. incognita (Mi) alone, (d) Mi+FON5 (M. incognita inoculation 5 days before FON), (e) Mi+FON (M. incognita and FON inoculation simultaneously), and (f) FON+Mi5 (FON inoculation 5 days before M. incognita). Watermelon seedlings in all experiments were inoculated at the same age. The earliest nematode inoculation was done when seedlings reached the 2-leaf stage. Seedlings inoculated with nematodes were kept in the seedling trays for at least 24 h to allow thorough contact of nematode with the roots. One day after the last nematode inoculation, seedlings were transplanted to 9-cm plastic pots filled with ~200 g potting mix.
In the experiments with FON race 1, a split-plot design was used with a factorial arrangement. In the experiments with FON race 2, a randomized complete block design was used. In all the experiments, there were four replications and 16 plants for each treatment. Plants were kept in the greenhouse with 14 h photoperiod, and were watered daily with overhead irrigation until the soil was saturated. All experiments were conducted twice under similar conditions.
Evaluation of disease and plant growth
Wilt symptoms developed earlier in the second set of experiments (Trial 2) with FON race 1 or 2, so disease severity was recorded earlier in Trial 2 (starting 2 weeks after inoculation with FON) than in Trial 1 (starting 3 weeks after inoculation with FON). Disease severity was evaluated weekly using a 0–4 scale generated in this study, where 0 = no symptoms; 1 = less than 25% leaves wilted; 2 = 25–50% leaves wilted; 3 = 51–75% leaves wilted; 4 = more than 75% leaves wilted or plants dead. At the end of the experiments, i.e. 4 (Trial 2) or 5 (Trial 1) weeks after inoculation with FON, all seedlings were carefully excavated. Roots were washed free of soil under tap water and blotted dry on paper towels. Since roots of severely infected plants were completely rotten and could not be rated for nematode damage, the degree of root galling was only determined when possible using a 0–10 scale (Aryal et al., Citation2011), where 0 = no galls, 1 = 1–10% of roots galled, 2 = 11–20% of roots galled, etc., with 10 = 91–100% of roots galled. Fresh weights of both roots and shoots were recorded for all plants in the experiments.
Three wilted plants from each treatment were randomly selected for FON isolation. Small sections of lower stem (about 2 × 3 × 8 mm) were surface sterilized with 0.5% NaOCl for 1 min, rinsed with sterile distilled water, and placed on peptone PCNB Agar (Nash & Snyder, Citation1962) plates. Fungal colonies emerging from the plant tissues were purified by hyphal tip subculturing, and genomic DNA extracted from pure cultures was subjected to PCR amplification with FON specific primers Fon-1/Fon-2 (Lin et al., Citation2010) to confirm the identity of the isolates.
Statistical analysis
In the experiments with FON race 1, non-parametric disease severity data were aligned and ranked using ARTool (Wobbrock et al., Citation2011). Factorial ANOVA was then employed on aligned and ranked disease severity data and plant growth data to analyse the differences among treatments and the interactions between cultivar and inoculation method using SPSS 24.0 (SPSSinc, Illinois, USA). Because there was an interaction between watermelon cultivar and inoculation method for disease severity and plant growth (Supplementary Table S2), disease severity and plant growth data from the experiments with FON race 1 were then analysed separately for each cultivar from data in the experiments with FON race 2 described below.
In the experiments with FON race 2, disease severity data were subjected to the non-parametric Kruskal–Wallis test for k independent samples before pair-wise comparisons were conducted for all treatments by Mann–Whitney tests at P ≤ 0.05 using SPSS 24.0. ANOVA was used to analyse plant growth data. Treatment means were compared for significant differences by Fisher’s least significant difference (LSD) test at P ≤ 0.05. Since disease developed earlier in Trial 2 than Trial 1 in both the experiments with FON race 2 and race 1, disease severity was assessed at different times in Trial 1 and Trial 2. So, data from Trial 1 and Trial 2 could not be combined and were analysed separately.
Results
Effect of M. incognita on watermelon resistance to FON race 1
Fusarium wilt developed on plants inoculated with FON in 10–16 days in the experiments. Factorial ANOVA on aligned and ranked disease severity data showed that there was a main effect of inoculation method, with FON inoculation alone resulting in less disease than that of FON and nematode co-inoculation in both trials (Table S2). There was an interaction between cultivar and inoculation method, so results of the two cultivars were then analysed and presented separately.
On ‘Calhoun Gray’, inoculation with FON alone caused significantly lower disease severity compared with the treatments with sequential and concomitant inoculation of nematodes and FON (Fig. 1a). The treatments inoculated with both nematodes and FON had similar levels of Fusarium wilt, with disease severity index ranging from 2.13 to 2.69 in Trial 1 and from 3.13 to 3.50 in Trial 2. On ‘Fascination’, wilt symptoms were also more severe in treatments inoculated with both nematodes and FON compared with FON inoculation alone in the first trial (). Disease severity was higher with mean ratings ranging from 3.25 to 3.69 in treatments with combined inoculation of FON and nematodes. In the second trial, however, disease severity on plants inoculated with FON alone was high (3.63), and no significant difference was observed among FON-inoculated treatments, regardless of the presence or absence of nematodes.
Fig. 1 Influence of Meloidogyne incognita (Mi) on the severity of wilt disease caused by Fusarium oxysporum f. sp. niveum (FON) race 1 in watermelon ‘Calhoun Gray’ (a) and ‘Fascination’ (b). Mi+FON5: nematode inoculation followed by FON 5 days later; Mi+FON: nematodes and FON inoculated simultaneously; FON+Mi5: FON inoculation followed by nematodes 5 days later. Error bars are standard errors of the means of four replications. Disease severity recorded at the end of each trial was statistically analysed. Treatments marked with different letters are significantly different at P ≤ 0.05.
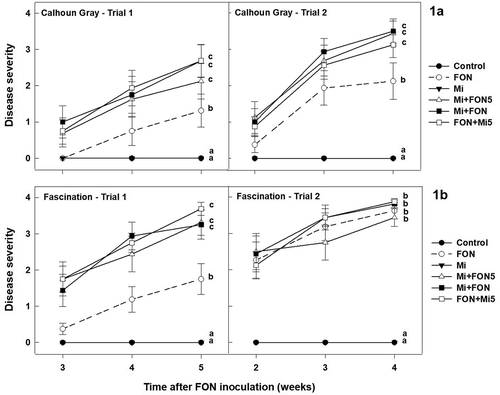
In all trials, no wilt symptoms were observed on plants treated with sterile water or M. incognita alone. FON was only isolated from seedlings inoculated with FON and the identity was confirmed by morphological characteristics and PCR analysis with FON-specific primers.
Root galling induced by M. incognita on watermelon resistant to FON race 1
On ‘Calhoun Gray’, root galling was observed in all nematode-inoculated treatments with galling indices from 1.17 to 3.36 in the first trial and 1.00 to 1.44 in the second trial (Table S1). Inoculation with FON 5 days prior to nematodes resulted in relatively less galling as compared with nematode inoculation alone, nematodes and FON at the same time, and nematode inoculation 5 days before FON. Nematode inoculation appeared to induce numerically more galls on roots of ‘Fascination’ than on ‘Calhoun Gray’ (Table S1). No root galling was observed on plants inoculated with FON alone. In other treatments, root galling indices varied from 2.33 to 3.50 (Trial 1) and 2.67 to 3.20 (Trial 2). Because root galling could not be determined for seedlings severely infected by FON, root galling was not assessed for all seedlings and root galling indices could not be analysed statistically.
Influence of M. incognita and FON race 1 on plant growth
On ‘Calhoun Gray’, inoculation of FON, alone or in combination with nematodes, did not suppress the growth of watermelon significantly in the first trial. However, plant growth was significantly reduced in all FON-inoculated treatments in the second trial. The greatest reduction of fresh shoot weight was observed in Mi+FON5 and Mi+FON treatments, whereas FON inoculation, with or without nematodes, resulted in significantly lower fresh root weight compared with the non-inoculated control (). Interestingly, a stimulation of plant growth was observed when plants were inoculated with nematodes alone. Plants treated with nematodes alone were larger and weighed more than non-inoculated plants, although the difference in fresh root weight between nematode treatment and non-inoculated treatment was not statistically significant. A significant reduction of plant biomass of ‘Fascination’ was also observed in all treatments inoculated with FON compared with the control. Growth suppression was not observed in ‘Fascination’ seedlings inoculated with nematodes alone ().
Table 1. Effect of Meloidogyne incognita (Mi) and Fusarium oxysporum f. sp. niveum (FON) race 1 on the growth of watermelon ‘Calhoun Gray’ and ‘Fascination’.
Effect of M. incognita on watermelon resistance to FON race 2
Inoculating PI 296 341-FR seedlings with FON and nematodes, together or sequentially, significantly increased Fusarium wilt severity compared with inoculation with FON alone (). Fusarium wilt severity of plants inoculated with FON alone was 1.19 (Trial 1) and 1.25 (Trial 2) at the end of the experiments. When seedlings were co-inoculated with FON and nematodes, Fusarium wilt severity was more than doubled in both trials.
Fig. 2 Influence of Meloidogyne incognita (Mi) on the severity of wilt disease caused by Fusarium oxysporum f. sp. niveum (FON) race 2 in watermelon accession PI 296 341-FR. Mi+FON5: nematode inoculation followed by FON 5 days later; Mi+FON: nematodes and FON inoculated simultaneously; FON+Mi5: FON inoculation followed by nematode 5 days later. Error bars are standard errors of the means of four replications. Disease severity recorded at the end of each trial was statistically analysed. Treatments marked with different letters are significantly different at P ≤ 0.05.
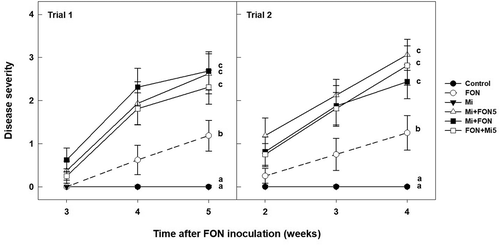
Root galling induced by M. incognita on watermelon resistant to FON race 2
Meloidogyne incognita inoculation resulted in gall formation on the roots of PI 296 341-FR, irrespective of the presence of FON (Table S1). Root-galling indices ranged from 0.67 to 1.83 in the first trial and 1.00 to 1.81 in the second trial. In both trials, galling indices tended to be lowest on plants inoculated with FON followed by nematodes, among all nematode inoculation treatments, but root galling was not rated for seedlings severely infected by FON and root galling indices could not be analysed statistically.
Influence of M. incognita and FON race 2 on plant growth
A significant root weight reduction was observed due to FON inoculation, with or without the presence of nematodes, as compared with the non-inoculated control (). Co-inoculation with nematodes and FON also led to the reduced growth of the above-ground plant part in the second trial. Lowest fresh shoot weight was noted in the sequential inoculation with nematodes prior to FON, followed by inoculation with FON prior to nematodes, and concomitant inoculation of nematodes and FON. Plants inoculated with nematodes alone grew well and the foliage remained healthy during the tests ().
Table 2. Effect of Meloidogyne incognita (Mi) and Fusarium oxysporum f. sp. niveum (FON) race 2 on the growth of watermelon accession PI 296 341-FR.
Discussion
Watermelon is an important crop grown worldwide and Fusarium wilt is a destructive disease affecting its production. In this study, the influence of root-knot nematodes on the severity of Fusarium wilt of watermelon was evaluated. Generally, the three watermelon genotypes tested were significantly more susceptible to Fusarium wilt when the plants were inoculated with root-knot nematodes and FON. Greater wilt damage was observed on seedlings inoculated with both pathogens, either sequentially or simultaneously, compared with inoculation with FON alone. Additionally, the presence of nematodes resulted in an earlier onset of Fusarium wilt and further growth suppression of watermelon plants. Our results are in line with previous findings which indicate that root-knot nematodes can break watermelon resistance to soil-borne pathogens. Since the first report on the effect of M. incognita on the pathogenicity of Fusarium wilt in cotton (Atkinson, Citation1892), synergistic interaction between Meloidogyne spp. and the soil-borne fungus F. oxysporum was also observed on other crops such as watermelon (Sumner & Johnson, Citation1973), tomato (Abawi & Barker, Citation1984), summer squash (Caperton et al., Citation1986), alfalfa (Griffin, Citation1986), papaya (Khan & Husain, Citation1991), bean (France & Abawi, Citation1994), and chickpea (Castillo et al., Citation2003). In 2004, Naji & Abu-Gharbieh reported the increase in wilt susceptibility of wilt resistant muskmelon cultivars inoculated with F. oxysporum f. sp. melonis plus M. javanica or M. incognita. More recently, the presence of M. javanica in soil was found to be involved in the loss of Fusarium wilt resistance in tomato (Lobna et al., Citation2016).
‘Fascination’, a high yielding watermelon cultivar that was reported to be moderately resistant to FON race 1 (Everts & Hochmuth, Citation2011), appeared to be more susceptible to Fusarium wilt than ‘Calhoun Gray’ when they were inoculated with FON race 1. Even in the absence of nematodes, most seedlings of ‘Fascination’ were severely infected by FON race 1 in the second trial with a disease severity of 3.63 (0–4 scale). With such a high disease severity by inoculation with FON alone, the effect of nematodes on Fusarium wilt development could not be precisely evaluated on ‘Fascination’. This indicated that the resistance of ‘Fascination’ to FON race 1 may not be consistent under certain inoculum pressure, and supplementary disease control tactics are needed in fields with high levels of FON infestation.
The enhancement of wilt severity due to nematode co-infection is probably related to physiological and anatomical changes induced by nematode infection in root tissues (LaMondia & Timper, Citation2016). After introduction into soil, root-knot nematodes must locate their host, penetrate the root, migrate intercellularly into the vascular cylinder, establish a permanent feeding site, induce the formation of giant cells and develop root galls (Bird, Citation2004). In response to nematode infection, plants release more exudates and, as a consequence, they attract more microorganisms including soilborne fungal pathogens to their roots (Back et al., Citation2002). Once in contact with the root surface, fungal hyphae can rapidly penetrate the roots through nematode wound sites (Bird, Citation2004). Since nematode infected cells are nutrient-rich, they could promote fungal growth and facilitate disease development (Bird, Citation2004; Meena et al., Citation2016), potentially hastening the expression of wilt symptoms and increasing wilt severity in plants infected by both pathogens as observed in our study.
Watermelons are susceptible to four different races of M. incognita (Sasser & Carter, Citation1982) and obvious symptoms such as stunting, yellowing and reduced plant growth have frequently been observed in nematode-infected plants (Thies, Citation1996; Xing et al., Citation2006). In our experiments, plants inoculated with M. incognita race 3 alone did not show any of those above-ground symptoms and less than 40% of their root systems were galled. The lack of the above-ground symptoms, together with the low degree of root-galling, may be attributed to the low concentration of initial J2 inoculum used since the severity of nematode damage depends on the nematode population density (Barker & Olthof, Citation1976). The use of low inoculum level may also be responsible for the slight increase in plant biomass when seedlings were only inoculated with M. incognita. This is consistent with Starr et al. (Citation1989) who found that cotton growth was stimulated at low initial nematode population levels. Similar findings were also reported by Rocha et al. (Citation2008) when they exposed soybean plants to 100, 200 or 300 J2 of M. javanica. Root-knot nematodes can synthesize cytokinin-like substances while parasitizing plant roots (Bird & Loveys, Citation1980; de Meutter et al., Citation2003). Cytokinins are phytohormones involved in cell division and promotion of plant growth (Vankova, Citation2014). Although the role of nematode-produced cytokinins in plant growth has not been reported, it is possible that the increase in shoot weight observed in plants only infected with nematodes is related to the presence of these substances.
In contrast to nematodes, FON infection suppressed plant growth. Interestingly, in some trials, the greatest growth reduction was recorded in plants co-infected with nematodes. These observations support previous findings about the enhanced adverse effects of combined inoculation of nematodes and fungi on plant biomass (Naji & Abu-Gharbieh, Citation2004; Wang & Roberts, Citation2006; Meena et al., Citation2016). According to Holliday (Citation1970), F. oxysporum can colonize the roots, enter the xylem and block the xylem vessels. Occlusion of the xylem severely reduces water transport in the plant, leading to poor growth, wilting and death of the host crops. Thus, our results suggest that M. incognita inoculation might indirectly contribute to reduced plant growth through accelerating the pathogenesis of FON and the main factor for this growth suppression is the pathological effect of FON.
Together, our findings indicate that nematode infection can disrupt watermelon resistance to FON race 1 and race 2, thereby increasing disease severity and reducing plant growth. Therefore, the use of Fusarium wilt resistant watermelon cultivars alone may not be sufficient to protect plants from the disease when soil is infested with root-knot nematodes. This study points towards the need to develop new watermelon cultivars which are resistant to the infection of both FON and root-knot nematodes, or develop integrated management strategies to incorporate host resistance and other tactics for more effective protection against Fusarium wilt.
Supplemental Tables
Download MS Word (17 KB)Acknowledgements
Technical assistance by Chang Liu is appreciated.
Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2018.1564939.
Additional information
Funding
References
- Abawi GS, Barker KR. 1984. Effects of cultivar, soil temperature and population levels of Meloidogyne incognita on root necrosis and Fusarium wilt of tomatoes. Phytopathology. 74:433–438.
- Abdel-Momen SM, Starr JL. 1998. Meloidogyne javanica-Rhizoctonia solani disease complex of peanut. Fund Appl Nematol. 21:611–616.
- Al-Hazmi AS, Al-Nadary SN. 2015. Interaction between Meloidogyne incognita and Rhizoctonia solani on green beans. Saudi J Biol Sci. 22:570–574.
- Amaradasa BS, Beckham K, Dufault N, Sanchez T, Ertek TS, Iriarte F, Paret M, Ji P. 2018. First report of Fusarium oxysporum f. sp. niveum race 3 causing wilt of watermelon in Florida, U.S.A. Plant Dis. 102:1029.
- Aryal SK, Davis RF, Stevenson KL, Timper P, Ji P. 2011. Induction of systemic acquired resistance by Rotylenchulus reniformis and Meloidogyne incognita in cotton following separate and concomitant inoculations. J Nematol. 43:160–165.
- Atkinson GF. 1892. Some diseases of cotton. Bull Ala Agric Exp Stn. 41:61–65.
- Back MA, Haydock PPJ, Jenkinson P. 2002. Disease complexes involving plant parasitic nematodes and soil borne pathogens. Plant Pathol. 51:683–697.
- Barker KR, Olthof THA. 1976. Relationships between nematode population densities and crop response. Annu Rev Phytopathol. 14:327–353.
- Beattie JH, Doolittle SP. 1951. Watermelons. U.S. Government Printing Office.
- Bird AF, Loveys BR. 1980. The involvement of cytokinins in a host-parasite relationship between the tomato (Lycopersicon esculentum) and a nematode (Meloidogyne javanica). Parasitology. 80:497–505.
- Bird DM. 2004. Signaling between nematodes and plants. Curr Opin Plant Biol. 7:372–376.
- Bruton BD, Fish WW, Langston DB. 2008. First report of Fusarium wilt caused by Fusarium oxysporum f. sp. niveum race 2 in Georgia watermelons. Plant Dis. 92:983.
- Caperton CM, Martyn RD, Starr JL. 1986. Effects of Fusarium inoculum density and root-knot nematodes on wilt resistance in summer squash. Plant Dis. 70:207–209.
- Castillo P, Navas-Cortés JA, Gomar-Tinoco D, Di Vito M, Jiménez-Diaz RM. 2003. Interactions between Meloidogyne artiellia, the cereal and legume root-knot nematode, and Fusarium oxysporum f. sp. ciceris race 5 in chickpea. Phytopathology. 93:1513–1523.
- Davis RF. 2007. Effect of Meloidogyne incognita on watermelon yield. Nematropica. 37:287–293.
- de Meutter J, Tytgat T, Witters E, Gheysen G, van Onckelen H, Gheysen G. 2003. Identification of cytokinins produced by the plant parasitic nematodes Heterodera schachtii and Meloidogyne incognita. Mol Plant Pathol. 4:271–277.
- Egel DS, Martyn RD. 2013. Fusarium wilt of watermelon and other cucurbit crops. St. Paul (MN, USA): Plant Health Instructor. Published online, APS Press.
- Elmstrom GW, Hopkins DL. 1981. Resistance of watermelon cultivars to Fusarium wilt. Plant Dis. 65:825–827.
- Everts KL, Hochmuth ME. 2011. Field evaluation of triploid cultivars for resistance to Fusarium wilt of watermelon in Delaware, 2010. Plant Dis Manage Rep. 5:V175.
- France RA, Abawi GS. 1994. Interaction between Meloidogyne incognita and Fusarium oxysporum f. sp. phaseoli on selected bean genotypes. J Nematol. 26:467–474.
- Griffin GD. 1986. The importance of nematode resistance on the interaction of Meloidogyne hapla and Fusarium oxysporum on alfalfa. Phytopathology. 76:843.
- Holliday P. 1970. Fusarium oxysporum f. sp. niveum. CMI Descript Pathogenic Fungi Bacteria. 219:1–2.
- Khan TA, Husain SI. 1991. Effect of interaction of Meloidogyne incognita and Fusarium solani on the growth of papaya and nematode reproduction. Nematol Mediterr. 19:195–196.
- Kleczewski NM, Egel DS. 2011. A diagnostic guide for Fusarium wilt of watermelon. Plant Health Prog. 12:27. Online. doi:10.1094/PHP-2011-1129-01-DG.
- LaMondia J, Timper P. 2016. Interactions of microfungi and plant-parasitic nematodes. In: Li DW, editor. Biology of microfungi. Switzerland: Springer; p. 573–614.
- Lin YH, Chen KS, Chang JY, Wan YL, Hsu CC, Huang JW, Chang PFL. 2010. Development of the molecular methods for rapid detection and differentiation of Fusarium oxysporum and F. oxysporum f. sp. niveum in Taiwan. New Biotechnol. 27:409–418.
- Lobna H, Hajer R, Naima MB, Najet HR. 2016. Studies on disease complex incidence of Meloidogyne javanica and Fusarium oxysporum f. sp. lycopersici on resistant and susceptible tomato cultivars. J Agr Sci Food Technol. 2:41–48.
- Lynch L, Carpenter J. 1999. The economic impacts of banning methyl bromide: where do we need more research? 1999 Annual Research Conference on Methyl Bromide Alternatives and Emissions Reductions. http://mbao.org/mbrpro99.html.
- Martyn RD. 1996. Fusarium wilts. In: Zitter TA, Hopkins DL, Thomas CE, editors. Compendium of cucurbit diseases. St. Paul (MN): APS Press; p. 11–16.
- Martyn RD. 2014. Fusarium wilt of watermelon: 120 years of research. Hortic Rev. 42:349–442.
- Martyn RD, Bruton BD. 1989. An initial survey of the United States for races of Fusarium oxysporum f. sp. niveum. HortScience. 24:696–698.
- Martyn RD, Netzer D. 1991. Resistance to races 0, 1, and 2 of Fusarium wilt of watermelon in Citrullus sp. PI-296341-FR. HortScience. 26:429–432.
- Meena KS, Ramyabharathi SA, Raguchander T, Jonathan EI. 2016. Interaction of Meloidogyne incognita and Fusarium oxysporum in carnation and physiological changes induced in plants due to the interaction. SAARC J Agri. 14:59–69.
- Montalvo AE, Esnard J. 1994. Reaction of ten cultivars of watermelon (Citrullus lanatus) to a Puerto Rican population of Meloidogyne incognita. J Nematol. 26:640–643.
- Naji I, Abu-Gharbieh W. 2004. Effect of Meloidogyne javanica and M. incognita on resistance of muskmelon cultivars to Fusarium wilt. Phytopathol Mediterr. 43:360–368.
- Nash SM, Snyder WC. 1962. Quantitative estimations by plate counts of propagules of the bean rot Fusarium in field soils. Phytopathology. 52:567–572.
- Petkar A, Ji P. 2017a. Diversity of Fusarium oxysporum f. sp. niveum from watermelon in Georgia. Phytopathology. 107:123(abstr.).
- Petkar A, Ji P. 2017b. Infection courts in watermelon plants leading to seed infestation by Fusarium oxysporum f. sp. niveum. Phytopathology. 107:828–833.
- Rocha FS, Pinheiro JB, Campos HD, Campos VP. 2008. Relação entre populações iniciais de Meloidogyne javanica e Heterodera glycines e do desenvolvimento do sistema radicular da soja. Nematol Bras. 32:161–166.
- Sasser JN, Carter CC. 1982. Root-knot nematodes (Meloidogyne spp.): identification, morphological and physiological variation, host range, ecology, and control. In: Riggs RD, editor. Nematology in the southern region of the United States. Fayetteville: Southern Cooperative Series Bulletin 276, Arkansas Agricultural Experimental Station; p. 21–32.
- Sayre RM, Walter DE. 1991. Factors affecting the efficacy of natural enemies of nematodes. Annu Rev Phytopathol. 29:149–166.
- Sikora RA, Fernandez E. 2005. Nematode parasites of vegetables. In: Luc M, Sikora RA, Bridge J, editors. Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford (UK): CAB International; p. 319–392.
- Starr JL, Jeger MJ, Martyn RD, Schilling K. 1989. Effects of Meloidogyne incognita and Fusarium oxysporum f. sp. vasinfectum on plant mortality and yield of cotton. Phytopathology. 79:640–646.
- Sumner DR, Johnson AW. 1973. Effect of root-knot nematodes on Fusarium wilt of watermelon. Phytopathology. 63:857–861.
- Thies JA. 1996. Diseases caused by nematodes. In: Zitter TA, Hopkins DL, Thomas CE, editors. Compendium of cucurbit diseases. St. Paul (MN): APS Press; p. 56–58.
- Thies JA, Ariss JJ, Hassell RL, Olson S, Kousik CS, Levi A. 2010. Grafting for management of southern root-knot nematodes, Meloidogyne incognita, in watermelon. Plant Dis. 94:1195–1199.
- Vankova R. 2014. Cytokinin regulation of plant growth and stress responses. In: Tran LS, Pal S, editors. Phytohormones: a window to metabolism, signaling and biotechnological applications. New York: Springer; p. 55–79.
- Wang C, Roberts PA. 2006. A Fusarium wilt resistance gene in Gossypium barbadense and its effect on root-knot nematode-wilt disease complex. Phytopathology. 96:727–734.
- Wobbrock JO, Findlater L, Gergle D, Higgins JJ. 2011. The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. Chi. 2011:143–146.
- Xing L, Kruger G, Egel DS, Westphal A. 2006. Quantitative growth response of watermelon to infection by Meloidogyne incognita. J Nematol. 38:301–302.
- Zhou XG, Everts KL. 2003. Races and inoculum density of Fusarium oxysporum f. sp. niveum in commercial watermelon fields in Maryland and Delaware. Plant Dis. 87:692–698.
- Zhou XG, Everts KL, Bruton BD. 2010. Race 3, a new and highly virulent race of Fusarium oxysporum f. sp. niveum causing Fusarium wilt in watermelon. Plant Dis. 94:92–98.
