Abstract
Hot pepper (Capsicum chinense Jacq.) is an economically important commercial crop cultivated in Trinidad for domestic consumption and for export in its fresh and processed forms. Collar rot and wilting symptoms have been observed in major hot pepper producing areas of Trinidad over several years, resulting in severe crop losses. Isolations were made from infected plants using corn meal agar (CMA) amended with antibiotics. The isolated organism was identified as Phytophthora capsici based on morphological and microscopic observations. Inoculation of zoospores of P. capsici onto healthy seedlings confirmed the typical expression of symptoms of collar rot and wilting in hot peppers under greenhouse conditions. PCR amplification and nucleotide sequencing of the internal transcribed spacer (ITS) region of rDNA using the primers ITS4 and ITS6 and of the mitochondrial cytochrome c oxidase gene using primers FMPhy-8b and FMPhy-10b confirmed the pathogen as P. capsici. To the best of our knowledge, this is the first report identifying and confirming P. capsici affecting hot peppers in Trinidad.
Résumé
Le piment fort (Capsicum chinense Jacq.) est une culture commerciale économiquement importante à Trinité. Il est cultivé pour être consommé localement et être exporté, frais et transformé. Depuis plusieurs années, des symptômes de la pourriture du collet et de la flétrissure ont été observés dans les principales régions productrices de Trinité, engendrant d’importantes pertes de rendement. Des isolements ont été effectués, à partir de plants infectés, sur de la gélose à la farine de maïs amendée avec des antibiotiques. En se basant sur des observations morphologiques et microscopiques, l’organisme isolé a été identifié en tant que Phytophthora capsici. En serre, l’inoculation de semis sains avec des zoospores de P. capsici a confirmé l’expression typique de la pourriture du collet et de la flétrissure chez le piment fort. L’amplification par PCR et le séquençage des nucléotides de la région de l’espaceur transcrit interne (ITS) de l’ADNr avec les amorces ITS4 et ITS6, ainsi que du gène du cytochrome c oxydase avec les amorces FMPhy-8b et FMPhy-10b, ont confirmé que l’agent pathogène était bien P. capsici. À notre connaissance, il s’agit du premier rapport confirmant l’identité P. capsici en tant qu’agent s’attaquant au piment fort à Trinité.
Introduction
Hot pepper (Capsicum chinense Jacq.) is one of the most important commercial crops cultivated in the Caribbean region. The Trinidad ‘Moruga Scorpion’ hot pepper is considered to be one of the hottest peppers in the world, with more than 2 million Scoville heat units (Bosland et al., Citation2012). The crop has great value in local and foreign markets. Furthermore, the regionally renowned ‘Scotch Bonnet’ and ‘Moruga Red’ cultivars are preferred by consumers in the USA, UK and Canada due to their shape, pungency and flavour profile (Singh et al., Citation2006).
However, several factors affect the productivity of hot peppers despite the crop having an ecological advantage in the Caribbean. In Trinidad, one of the main challenges has been the prevalence of collar rot and wilting symptoms believed to be caused by Phytophthora species, resulting in up to 40% reported crop loss regardless of pesticide application. The disease is known to affect plants from the seedling to the harvest stage. The symptoms include black discolouration and constriction at the collar region and disintegration of the basal part of the stem followed by wilting and defoliation. Nevertheless, to date and to the best of our knowledge, there has been no study on the isolation of the causal agent, confirmation of pathogenicity or characterization of isolates associated with hot peppers in Trinidad. Therefore, the objectives of this study were to: (i) isolate and identify the causal agent associated with collar rot and wilting symptoms in hot peppers; (ii) confirm the disease association through pathogenicity tests; and (iii) characterize the isolates affecting hot peppers using morphological and molecular studies.
Materials and methods
Sample collection and isolation of pathogen
Two hot pepper plants ‘Moruga Red’ showing typical symptoms of collar rot were collected from each of two farmers’ fields located in Valencia and Maloney, Trinidad, in December 2017 during the rainy season. Both fields were ~0.45 hectares in size and the first 30 cm of the soil was comprised of fine sand with a subsurface clay horizon.
Five small sections of 1–3 cm length from the stems of plants showing advanced lesions were used for isolation of the pathogen. The affected tissues were cut and surface-sterilized in 1% NaOCl for 2 min followed by washing three times with sterilized distilled water. The surface-sterilized samples were placed onto Petri plates containing corn meal agar (CMA, Oxoid Ltd, UK) and amended with pimaricin, ampicillin and rifampicin (Sigma-Aldrich, USA) at 0.4, 2.5, 1.0 mL per L. The plates were incubated in the dark at 25 ± 1°C for 3 days. From the two diseased plants collected from each location, only one from each area had observable pathogen growth. Subsequently, pure cultures of the two resulting isolates were obtained by transferring hyphal tips from the CMA plates to potato dextrose agar (PDA, BD Difco™, USA) plates. The morphologically distinct isolates that resembled Phytophthora were named MACO1 and MAL2. The isolates were maintained on PDA slants at 25 ± 1°C for further studies.
Morphological characters of Phytophthora isolates
The growth rates of isolates MACO1 and MAL2 were assessed on different media. Mycelial discs, 5 mm in diameter, were placed in the centre of Petri plates containing CMA, PDA and V8A (V8 juice agar, HiMedia, India). The plates were incubated at 25 ± 1°C for 5 days. Mycelial colony diameters of 5 replicates were measured at 3 and 5 days after plating onto different media for each isolate.
A study was also carried out to observe the sexual and asexual reproductive structures of isolates MACO1 and MAL2. The production of asexual reproductive structures was induced by adding 5 mL of sterilized distilled water to 5-day-old isolates on CMA, after which they were placed under fluorescent lights at 23 ± 1°C for 2 days. On the second day, the plates were incubated at 4°C to study the zoospore characteristics. Conversely, the production of oospores was assessed by pairing the two isolates 30 mm apart on CMA plates at 25 ± 1°C for 20 days in continuous darkness. The size and shape of the spores were observed under a light microscope (Olympus BX51, Japan) at 40× and 100× magnifications with CellSens imaging analysis software. A minimum of 30 spores were observed.
PCR amplification and nucleotide sequencing of rDNA and mitochondrial COX gene of Phytophthora isolates
Genomic DNA was extracted from mycelia of the two isolates grown in potato dextrose broth (PDB, BD Difco™, USA) using a MoBio Ultraclean® Microbial DNA isolation kit (Carlsbad, CA, USA) following the user’s manual. Both the internal transcribed spacer (ITS) region of the ribosomal DNA and the mitochondrial cytochrome c oxidase (COX1 and COX2) gene were amplified using primer pairs ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) and ITS6 (5ʹ-GAAGGTGAAGTCG TAACAAGG-3ʹ) and FMPhy-8b (5ʹ-AAAAGAGAAGGTGTTTTTTATGGA-3ʹ) and FMPhy-10b (5ʹ-GCAAAAGCACTAAAAATTAAATATAA-3ʹ), respectively (White et al., Citation1990; Cooke et al., Citation2000; Grünwald et al., Citation2011). The PCR reaction contained 1 μL template DNA (100 ng μL−1), 1 μL (4.5 pMol) of respective primers, 5μL Red eye master mix which contains 1 μL dNTPs (10 mM dNTP stock), 5 μL PCR buffer (Tris-HCl buffer), 5 μL MgCl2 (25 mM stock), 2.5 μL gelatin (1%) and 0.5 μL Taq polymerase (5 units μL−1). Nuclease-free water was used to make the total reaction volume up to 50 μL. In order to ensure the reproducibility of the reaction, appropriate negative controls (without DNA template) were used. PCR tubes were placed in a thermocycler (Applied Biosystems, GeneAmp® PCR System 9700, Singapore) for DNA amplification. The amplification protocol for the ITS primer pair consisted of an initial denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 1 min, and a final elongation step of 72°C for 10 min. Whereas, the amplification protocol for the Phytophthora genus-specific COX gene primer pair entailed an initial denaturation of 95°C for 3 min, followed by 35 cycles of 95°C for 1 min, 55°C for 1 min and 72°C for 1 min, and a final elongation step of 72°C for 5 min. PCR products were separated on a 1% agarose gel stained with SYBR safe and photographed using a gel documentation system (BioDoc-It Imaging Systems, USA). The PCR products were purified and sequenced at Macrogen Inc., South Korea. The nucleotide sequence data were analysed using the Basic Local Alignment Search Tool (BLAST) and sequences were subsequently deposited in GenBank of NCBI.
Preparation of inoculum
Zoospores were induced by adding 5 mL of sterile distilled water to 5-day-old cultures on CMA, after which they were placed under fluorescent lights at 23 ± 1°C for 2 days. By the second day, sporangial structures were observed and the plates were further incubated under refrigerated conditions (4°C) for 30 min to induce zoospore release. Zoospore concentration was adjusted to 7 × 103 spores mL−1 using a hemocytometer.
Inoculation onto hot pepper seedlings
One hot pepper ‘Moruga Red’ seedling was grown in each of 30 styrofoam cups (13.0 × 9.0 × 6.0 cm) containing sterilized potting mix (The Green formula®, Lambert Peat Moss, Canada). The seedlings were grown in a gable roof greenhouse exposed to 12 h of daylight. The zoospore suspensions (7 × 103 spores mL−1) prepared from isolates MACO nd MAL2 were used to inoculate 6-week-old seedlings by pouring 5 mL into the pot 2 cm away from the base of the plant. Control seedlings received 5 mL of sterilized distilled water. Both inoculated and uninoculated seedlings (10 of each) were grown under the same environmental conditions in the greenhouse and monitored daily for disease symptoms. The experiment was repeated twice and re-isolations were made from advancing lesions of inoculated seedlings. No pathogen was isolated from uninoculated seedlings using the method described above.
Results and discussion
Morphological characters of Phytophthora isolates
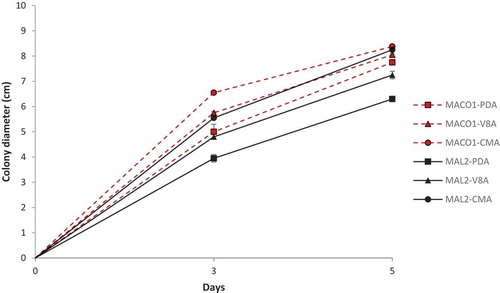
The study on the growth rate of mycelium on different media showed variations between isolates MACO1 and MAL2. Isolate MACO1 grew faster on all media tested in comparison to MAL2. The growth rate was highest on CMA followed by V8A and PDA for both isolates (). The isolates showed sparse aerial mycelium with a slight radiate pattern on CMA and on V8A, an appressed mycelium with a chrysanthemum pattern. However, on PDA, there was dense and cottony mycelium with a rosaceous pattern (). The hyphae of both isolates were aseptate forming long cells with many nuclei.
Fig. 1 (Colour online) Mycelial growth rate of P. capsici isolates MACO1 and MAL2 on different media. Error bars indicate the standard error (SE) of five replicates.
The sporangia of MACO1 and MAL2 isolates were variable in shape, mostly ovoid, limoniform and globose with papillae. The length and width of the sporangia of MACO1 ranged from 32.1–51.3 µm and 23.3–37.1 µm, respectively; whereas for MAL2, the length ranged from 29.6–55.4 µm and width varied from 23.3–34.4 µm (n = 30). The zoospores released from sporangia were spherical and their diameter ranged from 5.84–10.2 µm for MACO1 and 6.12–11.3 µm for MAL2 (n = 30). Furthermore, the morphological characteristics and measurements were similar to strains deposited in Q-bank’s comprehensive databases on quarantine plant pests and diseases (Leonian, Citation1922; Stamps, Citation1985) identified as P. capsici. When the isolates were paired with themselves, no oogonia and antheridia were observed. However, after pairing both isolates together on CMA at 25 ± 1°C for 20 days, a spherical oogonium, 29.2 µm in diameter was observed along with an ellipsoid antheridium 14.8 µm long and 16.9 µm wide (). Therefore, the data suggest the possible existence of both mating types (A1 and A2) of P. capsici in Trinidad.
Phytophthora capsici is heterothallic and when both mating types are present (A1 and A2), dormant oospores can be produced that can persist in the soil for at least a year (Granke et al., Citation2012). However, each individual mating type can reproduce asexually under ideal conditions and infect the plant (Sanogo & Ji, Citation2013). Moreover, the high level of genetic diversity and ability to develop resistance against selective fungicides such as mefenoxam and metalaxyl has made this pathogen difficult to control (Parra & Ristaino, Citation2001). Therefore, characterization of virulence in isolates and determination of fungicide resistance would be useful in developing management strategies for this disease in Trinidad.
Molecular identification of P. capsici affecting hot pepper plants
The PCR products were amplified for both isolates and the amplicon size of the ITS and Phytophthora genus-specific (COX1 and COX2) primers were ≈914 and 460 bp, respectively (). The rDNA sequences of the ITS region of the MACO1 and MAL2 isolates showed 99% nucleotide similarity with P. capsici strain TARI 9222 (accession no. GU111643) and P. capsici isolate SA (accession no. MG670447) in BLAST, respectively, along with several other high-quality matches, such as P. capsici isolate KN-PC12 from Pakistan (accession no. LT707536) and P. capsici isolate JS8 from China (accession no. MH842162). The rDNA sequences of ITS region of P. capsici isolates MACO1 and MAL2 were submitted to the NCBI database and assigned with the accession nos. MH196529 and MH196530, respectively.
Fig. 2 (Colour online) Morphological characteristics of Phytophthora capsici isolated from hot pepper plants. a, d, colony morphology of isolates MACO1 and MAL2 on PDA after 5 days. b, e, colony morphology of isolates MACO1 and MAL2 on CMA after 3 days. c, f, colony morphology of isolates MACO1 and MAL2 on V8A after 5 days. g, sporangia (Sp) and zoospores (Zs) from isolate MACO1. h, sporangia (Sp) from isolate MAL2. i, antheridium (At) and oogonium (Og) produced from the pairing of isolates MACO1 and MAL2 on CMA for 20 days. Bars: a–f = 1 cm, g–i = 10 µm.
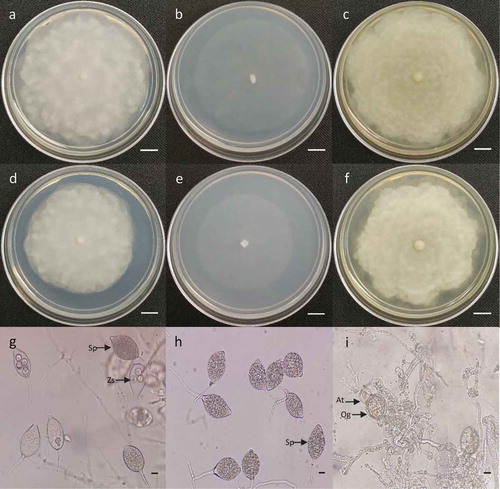
Fig. 3 (Colour online) Gel electrophoresis of PCR products amplified from P. capsici isolates MACO1 and MAL2 using Phytophthora genus-specific (COX1 & COX2) and ITS (ITS4 & 6) primer pairs. Lane 1: 100 bp marker (Promega Corporation, USA), 2: MACO1 (COX gene), 3: MAL2 (COX gene), 4: blank sample, 5: MACO1 (ITS), 6: MAL2 (ITS).
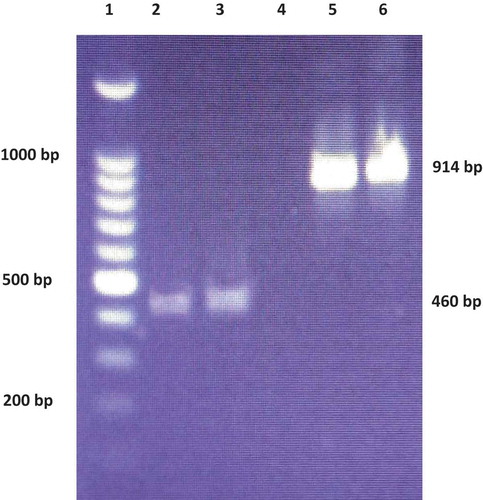
Fig. 4 (Colour online) Hot pepper plants showing wilting and collar rot symptoms of Phytophthora capsici. a, b, symptoms on hot pepper plants in the field. c, healthy seedling; d, diseased seedling at 3 days after inoculation; e, diseased seedling at 6 days after inoculation; f, dead seedling at 10 days after inoculation. Bar = 2 cm.

The BLAST of mitochondrial encoded COX1 and COX2 sequences of both isolates also showed several high-quality matches. However, MACO1 and MAL2 had the highest similarity (99%) to P. capsici isolate P1319 (accession no. GU221958) and P. capsici isolate P10386 (accession no. GU221957). The mitochondrial COX gene sequences of the MACO1 and MAL2 isolates were submitted to the NCBI database and assigned with accession nos. MH507142 and MH507143, respectively.
Inoculation onto hot pepper seedlings
Following artificial inoculation of zoospores onto healthy seedlings, the isolates caused disease symptoms in hot pepper plants. The first visible symptoms were observed 3 days after inoculation and included dark brown lesions above the soil level extending upwards on the stem. Wilting and extended lesion development were observed on all inoculated plants by day 6, and all inoculated plants died by the 10th day (). The uninoculated seedlings did not show any disease symptoms. The isolates MACO1 and MAL2 were re-isolated from the stem of the infected seedlings and showed similar morphological characteristics as described above, whereas no pathogen was isolated from the uninoculated controls, thus confirming Koch’s postulates. The oomycete pathogen P. capsici has been reported in a number of locations worldwide, such as South Africa, USA and Peru (Lamour et al., Citation2012) and has a wide host range and affects vegetable crops such as tomatoes, peppers and cucurbits (Tian & Babadoost, Citation2004). Hausbeck & Lamour (Citation2004) reported that P. capsici caused brown to black lesions on the collar region just above the soil level of pepper plants and discolouration of roots. In addition, root infection and damping-off in pepper seedlings was observed, whereas mature plants showed signs of wilting, stunted growth and ultimately died (Hausbeck & Lamour, Citation2004). The results from this study, along with morphological and molecular characterization, confirm that the isolates from hot pepper were P. capsici. To the best of our knowledge, the current study is the first report on the identification and confirmation of P. capsici associated with collar rot infection in hot pepper plants cultivated in Trinidad. The occurrence of this disease could drastically affect hot pepper production in the Caribbean islands, resulting in severe economic losses to growers. The pathogen’s broad host range and its ability to develop resistance against fungicides requires developing early disease diagnosis and integrated control strategies. More research on the diagnosis, characterization of the pathogen and development of effective management strategies are warranted in the Caribbean islands.
Acknowledgements
The authors are grateful to the Research and Development Impact Fund (UWI-TT RDI) of The University of the West Indies, St. Augustine campus for sponsoring this research under the project “Promoting agriculturally important microorganisms (AIMS) to address the challenges in food safety and food security in the Caribbean”.
Additional information
Funding
References
- Bosland PW, Coon D, Reeves G. 2012. Trinidad moruga scorpion pepper is the world’s hottest measured chile pepper at more than two million scoville heat units. HortTechnology. 22(4):534–538.
- Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet Biol. 30(1):17–32.
- Granke LL, Quesada-Ocampo L, Lamour K, Hausbeck MK. 2012. Advances in research on Phytophthora capsici on vegetable crops in the United States. Plant Dis. 96(11):1588–1600.
- Grünwald NJ, Martin FN, Larsen MM, Sullivan CM, Press CM, Coffey MD, Hansen EM, Parke JL. 2011. Phytophthora-ID.org: a sequence-based Phytophthora identification tool. Plant Dis. 95(3):337–342.
- Hausbeck MK, Lamour KH. 2004. Phytophthora capsici on vegetable crops: research progress and management challenges. Plant Dis. 88(12):1292–1303.
- Lamour KH, Stam R, Jupe J, Huitema E. 2012. The oomycete broad‐host‐range pathogen Phytophthora capsici. Mol Plant Pathol. 13(4):329–337.
- Leonian LH. 1922. Stem and fruit blight of peppers caused by Phytophthora capsici species nov. Phytopathol. 12(9):401–408.
- Parra G, Ristaino JB. 2001. Resistance to mefenoxam and metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper. Plant Dis. 85(10):1069–1075.
- Sanogo S, Ji P. 2013. Water management in relation to control of Phytophthora capsici in vegetable crops. Agric Water Manag. 129:113–119.
- Singh RH, Rankine LB, Seepersad G 2006. The CARICOM regional transformation programme for agriculture: Market Intelligence Report - hot peppers. St. Augustine (Trinidad): Caribbean Agricultural Research and Development Institute (CARDI) Publications. p. 75.
- Stamps DJ. 1985. Phytophthora capsici. CMI Descriptions of Pathogenic Fungi and Bacteria. UK. No. 836.
- Tian D, Babadoost M. 2004. Host range of Phytophthora capsici from pumpkin and pathogenicity of isolates. Plant Dis. 88(5):485–489.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. 1st ed. ed. San Diego (SD): Academic Press; p. 315–322.
