Abstract
Chinese pearl barley (Coix chinensis Tod.) is grown worldwide as a cereal food and medicinal plant. During 2016 and 2017, a severe leaf blight outbreak was observed in Nanjing prefecture, Fujian Province, China. The disease outbreak affected almost 100% of the Chinese pearl barley plants under field conditions. The pathogen was isolated from infected leaves showing typical symptoms of dark brown spots surrounded by yellow halos, and was identified as Curvularia coicis Castellani based on morphological characteristics and sequencing of the ribosomal DNA internal transcribed spacer (rDNA-ITS). The optimal temperature for mycelial growth of C. coicis on potato dextrose agar (PDA) ranged from 25°C to 28°C. Pathogenicity assays were performed using Chinese pearl barley ‘Longyi 1' at the seven- to eight-leaf stage. Overall, C. coicis had a high virulence on these inoculated plants, even in the absence of wounds, and the pathogen was successfully reisolated. The results from this study identified the causal organism of leaf blight on Chinese pearl barley.
Résumé
L’orge perlé chinois (Coix chinensis Tod.) est cultivé partout dans le monde comme produit céréalier et plante médicinale. En 2016 et 2017, une grave épidémie de brûlure helminthosporienne a été observée dans la préfecture de Nanjing de la province du Fujian, en Chine. L’épidémie a touché près de 100% des plants d’orge perlé cultivés en champ. L’agent pathogène a été isolé à partir de feuilles infectées affichant les taches brunes typiques entourées de halos jaunes et a été identifié en tant que Curvularia coicis Castellani, et ce, en se basant sur les caractéristiques morphologiques et le séquençage de l’espaceur transcrit interne de l’ADN ribosomique (ITS-ADNr). La température optimale de croissance du mycélium de C. coicis sur de la gélose dextrosée à la pomme de terre a varié de 25°C à 28°C. Des tests de pathogénicité ont été effectués sur le cultivar d’orge perlé chinois ‘Longyi 1ʹ, au stade de sept à huit feuilles. En général, la virulence de C. coicis à l’égard de ces plants inoculés était élevée, même chez les plants ne portant aucune blessure; et l’agent pathogène a été de nouveau isolé avec succès. Les résultats de cette étude ont permis d’identifier l’organisme causant la brûlure helminthosporienne chez l’orge perlé chinois.
Introduction
Chinese pearl barley (Coix chinensis Tod.), also known as adlay or Job’s tear, is frequently used in traditional Chinese medicine. It has also long been regarded as a nutritious food source, and contains significant amounts of amino acids, minerals, vitamins and dietary fibre (Lin et al., Citation2009; Liu et al., Citation2015a, Citation2015b; Li et al., Citation2018). Due to its profitability, the cultivated acreage of Chinese pearl barley has increased in the provinces of Guizhou, Yunnan and Guangxi in China, as well as in Fujian Province (Li et al., Citation2017). In Fujian Province, China, Chinese pearl barley is cultivated on more than 7000 hectares, with a total yield of over 20 000 tons (Lin et al., Citation2016).
Many fungi have been reported to cause foliar diseases on Chinese pearl barley. Powdery mildew, caused by Erysiphe graminis (DC Speer), is a disease that seriously affects commercial production (Wu et al., Citation2009). Leaf blight, caused by Cercospora species, causes water-soaked lesions with a pale-yellow halo during the initial stage of infection, and brown lesions with a yellow halo during the later stages of infection (Wu et al., Citation2009). Smut, caused by Ustilago coicis Bref., can also affect Chinese pearl barley leaves, causing single or conglomerate purple strumae (Xu, Citation2014). These diseases can result in serious yield losses of Chinese pearl barley in China (Zhang et al., Citation2016).
During 2016 and 2017, typical symptoms of leaf blight were observed on Chinese pearl barley leaves in Meilin town, Nanjing prefecture, Fujian Province, China. Disease incidence was estimated as more than 90% in each of eight fields. Symptoms included water-soaked spots or small yellow lesions that later became dark brown surrounded by yellow halos. These symptoms were inconsistent with the diseases previously reported on Chinese pearl barley. To determine the causal agent of leaf blight, we conducted a morphological, pathological and molecular-based study to identify the pathogen on Chinese pearl barley.
Materials and methods
Sample collection and fungus isolation
Sixty-three leaf samples showing symptoms of leaf blight were collected from eight fields in Meilin town, Nanjing prefecture, Fujian Province in 2017. Lesions (4 mm × 5 mm) were surface-sterilized in 75% (v/v) ethyl alcohol (Sinopharm Chemical Reagent Co. Ltd, China) for 2 min and in 0.1% (w/v) mercuric chloride (Sinopharm Chemical Reagent Co. Ltd, China) for 90 s. Following sterilization, leaf segments were rinsed three times in distilled water, placed on potato dextrose agar (PDA; 200 g fresh potato extract, 20 g dextrose, 18 g agar, in 1 L distilled water), and incubated at 28°C in the dark for 3 days. A plug (6 mm in diameter) with fresh mycelia from the initial sample plate of each isolate was inoculated at the centre of the Petri dishes (9 cm in diameter) containing 20 mL PDA. The plates were incubated at 28°C in the dark for 7 days. Pure isolates were obtained through single-spore isolation following previously described procedures (Fang, Citation1998). Finally, a total of 89 single-spore isolates with identical morphology on PDA were obtained. These isolates were maintained on PDA slants at 4°C. Three isolates (NJYM1714, NJYM1725 and NJYM1732) were randomly selected for further identification.
Morphological identification
To determine the optimal temperature for mycelial growth of the fungus, the three single-spore isolates were cultured on fresh PDA plates at 28°C for 5 days. A 6 mm plug with fresh mycelia from the margin of 5-day-old colonies of each isolate was inoculated at the centre of 9 cm Petri dishes containing 20 mL PDA. The inoculated plates were incubated in the dark at 4, 12, 16, 20, 25, 28, 35 and 40°C, respectively. Each temperature gradient contained three replicate plates, and the entire assay was performed three times. Two perpendicular measurements of the colony diameters were recorded on each plate (subtracting the 6 mm plug diameter) 7 days post inoculation and averaged for analysis. Statistical analyses were performed using DPS v7.05 software (Hangzhou Reifeng Information Technology Ltd, Hangzhou, China). Differences in the respective colony diameter for each temperature gradient and isolate were analysed using analysis of variance (ANOVA, with F and P values for treatment effects reported) with mean separation using Duncan’s new multiple-range test (P < 0.05). All of the data analysed using ANOVA were square-root transformed prior to analysis.
The three randomly selected isolates were cultured on fresh PDA plates at 28°C for seven days. Morphological characteristics of the mycelium, pycnidia and conidia, including shape, colour, size, and presence or absence of septation, were observed and recorded using an OLYMPUS microscope under 400× magnification (Olympus Corporation, Japan). For each isolate, more than 100 measurements were recorded for each structure.
Pathogenicity assay
To conduct the pathogenicity assays, Chinese pearl barley seeds were sown in black polypropylene flowerpots (two seeds per pot) containing mixtures of sphagnum peat moss (Pindstrup Mosebrug A/S, Ryomgaard, Denmark) and loamy soil (1:2 w/w) in a greenhouse. Pathogenicity tests were performed using a conidial suspension (105 conidia ml–1) on ‘Longyi 1' Chinese pearl barley (Fujian Longyan Longjin Crop Varieties Research Institute) at the seven- to eight-leaf stage. To obtain the conidial suspension, a 6 mm plug containing fresh mycelia from each fungal colony was transferred into a 9 cm Petri dish containing 20 mL PDA and grown at 28°C in the dark for 10 days. Conidia of each isolate were washed from the PDA plates with sterilized water and filtered through double-layer gauze (Xuzhou Weicai Hygiene of Material Factory Co., Ltd, China). The concentration of conidia in each isolate was adjusted to 105 conidia mL−1 and was sprayed on 10 plants until run-off. Control plants were sprayed with distilled water. The inoculated plants were incubated at 25–28°C in a greenhouse (12 h light per day, and relative humidity (RH) > 90%) until symptoms became apparent, 20–30 days after inoculation (Dai et al., Citation2018). Fungal isolates were re-isolated from any resulting lesions. The characteristics of these cultures on PDA plates, and morphological characteristics of these isolates were also compared with those of the three randomly selected isolates mentioned above. The pathogenicity assay was conducted three times.
Molecular identification
To perform molecular identification, genomic DNA was extracted from the mycelia of the three isolates using the modified CTAB method through a mini-preparation procedure as described by Neuhauser et al. (Citation2009). DNA samples were suspended in 30 μL TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and stored at −20°C in a refrigerator.
The nuclear ribosomal DNA internal transcribed spacer (rDNA-ITS) regions were amplified with the universal primers ITS5/ITS4 (White et al., Citation1990). PCR reactions were performed in a total volume of 50 μL containing 25 μL Premix Taq (Takara Biomedical Technology Co., Ltd, Dalian, China), 10 nmol primers, and 100 ng DNA templates. PCR amplification was conducted using a C1000TM Thermal Cycler (Bio-Rad Laboratories, Life Science Research, Hercules, CA) under the following conditions: 94°C for 5 min, 35 cycles of 94°C for 30 s, 56°C for 40 s, and 72°C for 45 s, followed by 72°C for 10 min. PCR amplification products were separated by 1.0% agarose gel electrophoresis (Biowest Inc., Barcelona, Spain) at 120 V for 20 min, stained with ethidium bromide (Shanghai Sangon Biotech, China), and visualized using a BioDoc-ItTM Imaging System (UVP, Upland, CA, USA). The sizes of the amplified fragments were estimated using a 2 kb DNA ladder (Takara Biomedical Technology Co., Ltd, Dalian, China). The PCR products were sequenced by Shanghai Sangon Biotech (China). The multiple sequences of the three isolates were aligned with those available in the GenBank database (http://www.ncbi.nlm.nih.gov) using the DNAMAN 5.0 program (Lynnon Biosoft, Quebec, Canada), and phylogenetic analysis was performed using MEGA 4 with the Neighbour-joining method (Tamura et al., Citation2007). Colletotrichum gloeosporioides Penz. was used as an out-group.
Results and discussion
Morphological identification
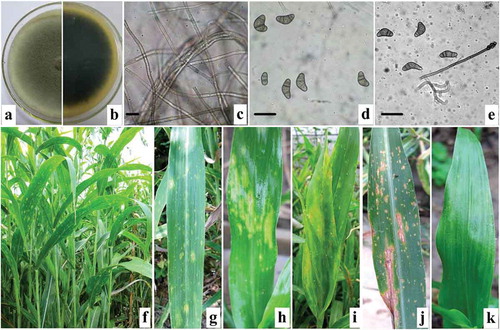
Mycelial growth on PDA occurred over the temperature range 12–35°C, and the optimal temperatures were 25°C and 28°C (). The colonies of the three isolates were grey on PDA and appeared dark underneath (). The 7-day-old mycelia were branched, hyaline, septate, and 8.0 to 14.8 µm (avg. 12.8 µm) in diameter (). The mature conidia were slightly curved, blunt round on the two sides of the cell, pale brown to brown, non-transparent, had 3–4 septations, and were 20.5 to 32.8 μm (avg. 28.6 µm) long and 8.5 to 17.5 μm (avg. 13.3 µm) wide (). Conidiophores were straight, single, or fascicular, brown to dark brown, and 127.5–485.0 μm long (). The morphological characteristics of the three isolates were consistent with Curvularia coicis Castellani (teleomorph: Cochliobolus nisikadoi (Tsuda, Ueyama & Nishihara) Alcorn) () (Alcorn, Citation1983; Sivanesan, Citation1987).
Table 1. Effect of temperature on mycelial growth of three Curvularia coicis isolates from Fujian Province, China.
Table 2. Morphological characteristics of the three pathogenic isolates obtained from blighted leaves of Chinese pearl barley, and Curvularia coicis, Curvularia spicifera, Curvularia lunata and Curvularia eragrostidis previously described in the literature.
Fig. 1 (Colour online) Morphological characteristics of Curvularia coicis and symptoms of leaf blight on naturally infected and inoculated Chinese pearl barley leaves. a–b, top (a) and reverse (b) view of colony characteristics of the fungal pathogen on PDA 7 days post inoculation. c, hyphae. d, mature conidia. e, conidiophore and conidia. f–g, symptoms of natural infection with blight on leaves. h–j, symptoms of blight on leaves inoculated with conidial suspensions of C. coicis under greenhouse conditions 20–30 days post inoculation. k, control leaves sprayed with distilled water, note that leaves did not display any symptoms of disease. Bars = 30 μm.
Pathogenicity assay
Results indicated that all inoculated leaves showed identical blight symptoms (-j) as those observed initially on naturally infected leaves () in the field at 20–30 days after inoculation. No symptoms were observed on the leaves inoculated with distilled water (). Fungal isolates were re-isolated from all the lesions using the methods described above. The cultures of these isolates exhibited identical characteristics as the initial isolates on PDA, thus fulfilling Koch’s postulates. Identical results were obtained from the three repeated experiments.
Molecular identification
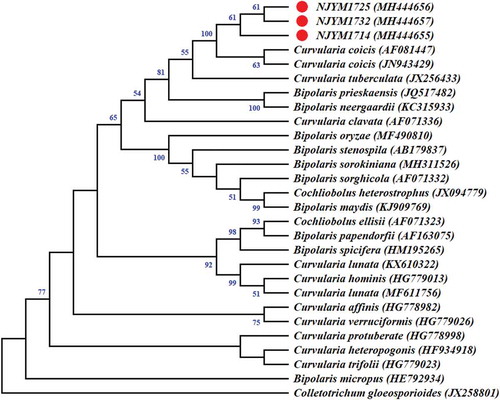
A fragment of ~628-bp in the rDNA-ITS region was obtained from the three isolates tested. The sequences of the three isolates (accession numbers MH444655, MH444656 and MH444657 for isolates NJYM1714, NJYM1725 and NJYM1732, respectively) were submitted to GenBank, and compared with existing sequences of C. coicis and closely related species in the database. The consensus sequences of the rDNA-ITS region from the three isolates showed 99% identity with the ITS sequences of isolates NBRC 100174 (JN943429) and CBS 192.29 (AF081447) of C. coicis, which were isolated in Japan. Results of the phylogenetic analysis according to ITS sequences indicated that the three isolates were most closely related to C. coicis (). Therefore, the causal organism of leaf blight on Chinese pearl barley in Fujian Province in China was identified as C. coicis based on the characteristics of the cultures, morphological characteristics, pathogenicity and molecular profiles.
Fig. 2 (Colour online) Phylogenetic tree inferred from the nuclear ribosomal DNA internal transcribed spacer (rDNA-ITS) sequences of the three Curvularia coicis isolates (NJYM1714, NJYM1725 and NJYM1732) obtained from leaves of Chinese pearl barley, two C. coicis isolates (AF081447 and JN943429), and closely related species isolates retrieved from GenBank through the Neighbour-joining method using MEGA 4. The numbers near the branches represent Neighbour-joining bootstrap values calculated from 1000 replicates. Values below 50% were collapsed. Colletotrichum gloeosporioides Penz. was used as an out-group.
Species of Curvularia and its teleomorph Cochliobolus, to which C. coicis belongs, occur on numerous economically important crops worldwide (Sivanesan, Citation1987; Manamgoda et al., Citation2011, Citation2012). For example, Gao et al. (Citation2012) reported a severe black sheath spot disease caused by Curvularia fallax Boedijin on rice (Oryza sativa L.) in China. Sarkar et al. (Citation2018) described a new record of leaf spot disease caused by C. spicifera (Bainier) Boedijn on pointed gourd (Trichosanthes dioica Roxb.), an economically important vegetable crop in India. Chen et al. (Citation2013) recorded a serious leaf blight disease caused by C. clavata Jain on Curcuma wenyujin Y.H. Chen & C. Ling, a traditional medicinal herb grown in China. Torres et al. (Citation2013) reported that C. gladioli Boerema & Hamers caused a leaf spot disease on Gladiolus grandiflorus Hort. in Brazil. Majeed et al. (Citation2016) reported brown leaf spots on rice caused by C. lunata (Wakkar) Boevijn in Pakistan. Furthermore, C. lunata occasionally causes opportunistic infection on cereal crops (Akram et al., Citation2014; Tong et al., Citation2016), vegetables (Iftikhar et al., Citation2016), and fruits (Verma & Gupta, Citation2010; Abbas et al., Citation2016) under certain conditions. These pathogen species are well known for their destructiveness and persistence under natural conditions (Ahmad et al., Citation2006; Gao et al., Citation2012; Chen et al., Citation2013). For this reason, many previous studies have focused on the diseases caused by Curvularia species on economically important crops.
Foliar diseases caused by Curvularia coicis have been recorded on many economically important crops and other gramineous plants, including corn (Zea mays L.), wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), tiger grass (Thysanolaena maxima (Roxb.) Kuntze) and cockspur grass (Echinochloa crusgalli (L.) Beauv) (Sivanesan, Citation1987; Manamgoda et al., Citation2012; Ahmadpour et al., Citation2013). Kim et al. (Citation1997) previously isolated and identified C. coicis as the causal agent of leaf blight on Coix lacryma-jobi (Roman) Stapf in South Korea. Meanwhile, they confirmed that Cochliobolus nisikadoi is the sexual phase of C. coicis, using in vitro cross assay methods (Kim et al., Citation1998). Ahmadpour et al. (Citation2013) also reported leaf spot caused by C. coicis on C. lacryma-jobi in Iran. In China, C. coicis has been reported as a biocontrol agent, and is used for controlling E. crusgalli in rice fields (Geng et al., Citation2008). However, in Fujian Province, C. coicis infected Chinese pearl barley leaves, and caused a destructive leaf blight disease, with an incidence of 90–100% under field conditions. The culture and morphological characteristics of the pathogen in this study were similar to those previously reported by Sivanesan (Citation1987) and Kim et al. (Citation1997). Nevertheless, the conidiophore lengths of C. coicis in this study were much shorter than those described by Ahmadpour et al. (Citation2013) but were similar to those previously reported by Deng & Zhang (Citation2002). It is worth noting that the comparison analysis of rDNA-ITS sequences revealed 99% identity with the ITS sequences of the Japanese isolates NBRC 100174 (JN943429) and CBS 192.29 (AF081447) of C. coicis. These results confirm C. coicis as the causal agent of leaf blight on Chinese pearl barley. To our knowledge, this is the first report of leaf blight caused by C. coicis on Chinese pearl barley in Fujian Province, China.
The occurrence of leaf blight on Chinese pearl barley in Nanjing, Fujian Province during 2016–2017 resulted in an occurrence of infection in more than 90% of the leaves, suggesting that this disease could cause a significant reduction in yield and quality of Chinese pearl barley in China. As such, accurate identification of the causal organism of diseases on Chinese pearl barley cultivated in new regions is indispensable for the management of these diseases, and for guaranteeing the security of Chinese pearl barley production.
Additional information
Funding
References
- Abbas MF, Naz F, Tariq A, Mumtaz A, Irshad G, Rauf CA. 2016. First report of Curvularia lunata causing leaf spots on loquat from Pakistan. J Plant Pathol. 98:374.
- Ahmad I, Iram S, Cullum J. 2006. Genetic variability and aggressiveness in Curvularia lunata associated with rice-wheat cropping areas of Pakistan. Pak J Bot. 38:475–485.
- Ahmadpour A, Pordel A, Heidarian Z, Javan-Nikkhah M. 2013. Bipolaris coicis causing adlay leaf blight in Iran. Australas Plant Dis Notes. 8:137–139.
- Akram W, Anjum T, Ahmad A, Moeen R. 2014. First report of Curvularia lunata causing leaf spots on Sorghum bicolor from Pakistan. Plant Dis. 98:1007.
- Alcorn JL. 1983. Generic concepts in Drechslera, Bipolaris and Exserohilum. Mycotaxon. 17:1–86.
- Chen XY, Feng JD, Su Z, Sui C, Huang X. 2013. First report of Curvularia leaf blight on Curcuma wenyujin caused by Curvularia clavata in China. Plant Dis. 97:138.
- Dai YL, Gan L, Ruan HC, Shi NN, Du YX, Chen FR, Yang XJ. 2018. A PCR method to detect mating types of Cochliobolus heterostrophus. Can J Plant Pathol. 40:358–367.
- Deng H, Zhang TY. 2002. Taxonomic studies of Bipolaris (hyphomycetes) from China Ι. Mycosystema. 21:327–333.
- Fang ZD. 1998. Plant pathology research methods. Beijing: China Agriculture Press.
- Ferreira APS, Pinho DB, Machado AR, Pereira OL. 2014. First report of Curvularia eragrostidis causing postharvest rot on pineapple in Brazil. Plant Dis. 98:1277.
- Gao BD, Huang W, Xia H. 2012. A new rice disease, black sheath spot, caused by Curvularia fallax in China. Plant Dis. 96:1224.
- Geng RM, Fu Y, Zhang WM, Zhang JP, Yu LQ. 2008. Efficacy and safety of Bipolaris sorokiniana and Bipolaris coicis for the control of Echinochloa crusgalli in paddy field. Chin J Rice Sci. 22:307–312.
- Iftikhar S, Shahid AA, Nawaz K, Ali SW. 2016. First report of Curvularia lunata causing fruit rot of tomato (Lycopersicum esculentum) in Pakistan. Plant Dis. 100:1013.
- Kim SK, Kim KW, Hong SS, Park EW, Yang JS, Kim YJ. 1997. Isolation and identification of Bipolaris coicis, causing leaf blight of job’s tears. Kor J Mycol. 25:291–296.
- Kim SK, Yang JS, Kim KW, Park EW, Kang WS. 1998. In vitro formation of Cochliobolus nisikadoi, the perfect state of Bipolaris coicis. Kor J Mycol. 26:287–292.
- Li FY, Shi M, Qin LK. 2017. The blue book of coix seed industry: the development report of Chinese coix seed industry No 1. Beijing: Social Sciences Academic Press.
- Li XD, Pan H, Lu XJ, Wei XY, Lu P, Shi M, Qin LK. 2018. Characteristics and comprehensive assessment of principal nutritional components in adlay landraces. Sci Agric Sinica. 51:835–842.
- Lin LJ, Hsiao ESL, Tseng HS, Chung MC, Chua ACN, Kuo ME, Tzen JTC. 2009. Molecular cloning, mass spectrometric identification, and nutritional evaluation of 10 coixins in adlay (Coix lachryma-jobi L.). J Agric Food Chem. 57:10916–10921.
- Lin ZN, Deng SF, Li CY, Ying CY, Chen MJ. 2016. The development of present situation and countermeasure of the coix industry in Fujian. China Agric Inform. 4:119–120.
- Liu X, Rong YZ, Zhang X, Mao DZ, Yang YJ, Wang ZW. 2015a. Rapid determination of total dietary fiber and minerals in Coix seed by near-infrared spectroscopy technology based on variable selection methods. Food Anal Methods. 8:1607–1617.
- Liu X, Zhang X, Rong YZ, Wu JH, Yang YJ, Wang ZW. 2015b. Rapid determination of fat, protein and amino acid content in Coix seed using near-infrared spectroscopy technique. Food Anal Methods. 8:334–342.
- Majeed RA, Shahid AA, Ashfaq M, Saleem MZ, Haider MS. 2016. First report of Curvularia lunata causing brown leaf spots of rice in Punjab, Pakistan. Plant Dis. 100:219.
- Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD. 2011. Cochliobolus: an overview and current status of species. Fungal Divers. 51:3–42.
- Manamgoda DS, Cai L, Mckenzie EHC, Crous PW, Madrid H, Chukeatirote E, Shivas RG, Tan YP, Hyde KD. 2012. A phylogenetic and taxonomic re-evaluation of the Bipolaris - Cochliobolus - Curvularia complex. Fungal Divers. 56:131–144.
- Neuhauser S, Huber L, Kirchmair M. 2009. A DNA based method to detect the grapevine root-rotting fungus Roesleria subterranea in soil and root samples. Phytopathol Mediterr. 48:553–559.
- Sarkar T, Chakraborty P, Das S, Saha D, Saha A. 2018. Curvularia leaf spot of pointed gourd in India. Can J Plant Pathol. 40:594–600.
- Sivanesan A. 1987. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Wallingford (UK): CAB International. Mycological Papers No. 158.
- Sun JJ, Nguyen TTT, Lee HB. 2015. Phylogenetic status of an unrecorded species of Curvularia, C. spicifera, based on current classification system of Curvularia and Bipolaris group using multi loci. Mycobiology. 43:210–217.
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA 4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 24:1596–1599.
- Tong L, Longzhou L, Jumei H, Lan J. 2016. First report of Curvularia lunata causing leaf spots on sweet sorghum (Sorghum bicolor) in China. Plant Dis. 100:652.
- Torres DP, Silva MA, Pinho DB, Pereira OL, Furtado GQ. 2013. First report of Curvularia gladioli causing a leaf spot on Gladiolus grandiflorus in Brazil. Plant Dis. 97:847.
- Verma VS, Gupta VK. 2010. First report of Curvularia lunata causing root rot of strawberry in India. Plant Dis. 94:477.
- White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; p. 315–322.
- Wu RH, Zhuang KZ, Tang RY, Ding WJ, Sun W, Li L. 2009. Common diseases and insect pests on Coix and their control. Crops. 3:82–84.
- Xu CJ. 2014. Prevention and control for main pests and diseases in Coix. Fujian Agric Sci Technol. 45:37–38.
- Zhang LW, Li SM, Chen X. 2016. Identification and isolation of Coix leaf blight pathogen and screening of biological products. Pestic Sci Admin. 37:45–49.
