Abstract
Stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst), is a destructive disease of wheat in the USA and fungicides have been used to manage the disease. However, it is not clear whether Pst has developed resistance to the commonly used fungicides. In this study, historical and recent Pst isolates were tested for sensitivity to Tilt, a propiconazole demethylation inhibitor fungicide, and Headline, a pyraclostrobin quinone outside inhibitor fungicide. Both Tilt and Headline at their labelled full concentration completely prevented urediniospore germination of all tested isolates. However, differences in germination rate among Pst isolates were observed at reduced fungicide concentrations. In the infection tests of both seedlings and adult plants, the full labelled concentration of either fungicide applied at the same day as inoculation with Pst completely prevented sporulation, but differences in rust sporulation or disease severity were observed among isolates at some reduced fungicide concentrations, or at the same full concentration applied either one day before or 3 or 7 days after inoculation. Thus, the fungicides are still effective for managing stripe rust at the full concentrations, but differences in sensitivity exist among Pst isolates. The results provide the technique and baseline for continually monitoring Pst populations for changes in fungicide sensitivity.
Résumé
La rouille jaune, causée par Puccinia striiformis f. sp. tritici (Pst), est une maladie destructrice du blé aux États-Unis, et l’on utilise des fongicides pour la gérer. Toutefois, nous ne savons pas exactement si Pst a développé une résistance aux fongicides couramment utilisés. Dans cette étude, des isolats anciens et récents ont été testés en fonction de leur sensibilité à Tilt, un fongicide à base de propiconazole inhibiteur de la déméthylation, et Headline, un fongicide à base de pyraclostrobine inhibiteur externe de la quinone. Tilt et Headline utilisés à pleine concentration, selon les recommandations inscrites sur les étiquettes, ont inhibé la germination des urédospores de tous les isolats testés. Par ailleurs, des différences entre les taux de germination chez les isolats de Pst ont été observées à de plus faibles concentrations. Au cours de tests d’infection menés sur des plantules et des plants matures, la pleine concentration recommandée de chacun des fongicides, appliquée le jour même de l’inoculation avec Pst, a complètement enrayé la sporulation, mais des différences quant à la sporulation de la rouille ou à la gravité de la maladie ont été observées chez les isolats à certaines concentrations réduites ou à concentration recommandée appliquée un jour avant ou trois ou sept jours après inoculation. Ainsi, les fongicides sont toujours efficaces pour ce qui est de gérer la rouille jaune lorsqu’utilisés à pleine concentration, mais des différences quant à la sensibilité existent chez les isolats de Pst. Les résultats indiquent la technique à utiliser et constituent une référence quant à la surveillance en continu des populations de Pst relativement aux changements de leur sensibilité aux fongicides.
Introduction
Wheat (Triticum aestivum L.) is one of the most important staple food sources for humans, providing nearly 55% of the carbohydrates and 20% of the food calories consumed globally (Pathak & Shrivastav, Citation2015). Stripe rust, caused by Puccinia striiformis Westend. f. sp. tritici Erikss. (Pst), is one of the most destructive diseases of wheat. Stripe rust has been reported to cause significant yield losses worldwide (Stubbs, Citation1985; Chen, Citation2005; Wellings, Citation2011; Chen & Kang, Citation2017a). In the USA, yield losses greater than 90% have been observed on susceptible varieties in experimental plots (Chen, Citation2014). The disease is managed by growing resistant cultivars and applying fungicides when necessary. When severe stripe rust epidemics occur, millions of dollars are spent on fungicide application to prevent yield loss (Chen, Citation2014; Chen & Kang, Citation2017b). In the US Pacific Northwest (PNW), over 80% of the wheat acreage may be sprayed with fungicides at least once, often twice, or even three times if stripe rust starts before or at the tillering stage and continues developing during the growing season. For example, in 2011 when the stripe rust epidemic was extremely severe, wheat growers in Washington State spent ~$28 million on fungicide applications, which saved ~517,560 metric tons of grain (Chen, Citation2014).
Foliar fungicides can effectively manage stripe rust when applied before rust incidence reaches 5% and again after 3–5 weeks depending upon active ingredient or disease pressure (Chen & Kang, Citation2017b). Much of the earlier research on fungicide use to manage stripe rust in the USA was conducted by Hardison (Citation1963, Citation1975), Powelson & Shaner (Citation1966) and later by Line and colleagues (Line & Rowell, Citation1973; Line, Citation1976, Citation2002; Rakotondradona & Line, Citation1984). The first large-scale, successful use of fungicides to manage stripe rust in North America occurred in 1981 (Line, Citation2002). Fungicides, including new formulations, have been tested every year for management of stripe rust, together with testing wheat cultivars for responses to applications of major fungicides (Chen & Wood, Citation2002, Citation2011; Chen et al., Citation2016). Fungicide efficacy trials and individual cultivar responses to fungicide application are used to develop a cultivar-based, integrated management system for stripe rust (Chen, Citation2014). Currently, more than 40 different fungicide products are registered for stripe rust management, with most of them in the demethylation inhibitor (DMI; triazole) and quinone outside inhibitor (QoI; strobilurin) classes (Chen & Kang, Citation2017b). Among these fungicides, propiconazole, registered as Tilt® and under several other trade names in the DMI class, has been used for more than 30 years (Chen, Citation2014). Pyraclostrobin, registered as Headline® in the QoI class, has been used since the early 2000s. In more recent years, Quilt® or Quilt Xcel® (azoxystrobin + propiconazole) have become popular choices since the two products consist of two modes of action by combining QoI and DMI chemicals.
The emergence and spread of fungicide resistance often occurs in populations of fungal pathogens following the widespread use of fungicides to manage disease. Strong selection pressure due to repeated use of fungicides is thought to underlie the cause-effect relationship between fungicide use and resistance evolution (Lucas et al., Citation2015). In some cases, the strength of selection can lead to rapid development of resistance within the fungal population even after limited use of the fungicide. Grimmer et al. (Citation2014) listed 66 cases of fungicide resistance in fungi and oomycetes in wheat production systems in Europe, including wheat powdery mildew [Blumeria graminis (DC.) Speer f. sp. tritici] and Septoria tritici blotch [Zymoseptoria tritici (Desm.) Quaedvl. & Crous), syn. Mycosphaerella graminicola (Fückel) Schroeter] pathogens to both DMI and QoI fungicides; eyespot [Oculimacula acuformis (Boerema, R. Pieters & Hamers) Crous & W. Gams) and Oculimacula yallundae (Wallwork & Spooner) Crous & W. Gams] and leaf rust (Puccinia tritici Erikss.) pathogens to DMI fungicides; and the tan spot pathogen [Pyrenophora tritici-repentis (Died.) Drechsler]; and some of the wheat Fusarium head blight and snow mould pathogens [e.g. Microdochium nivale (Fr.) Samuels & Hallett] to QoI fungicides. The number of years between initial utilization of a fungicide in crop production to documented resistance ranged from 2 to 25 years (Grimmer et al., Citation2014). In China, resistance to the cyanoacrylate fungicide JS399-19 in the Fusarium head blight fungal population was reported a few years after the initial use of the fungicide in commercial production systems (Chen et al., Citation2009). In Canada, Akhavan et al. (Citation2017) reported isolates of Pyrenophora teres Drechsler f. sp. teres and Pyrenophora teres f. sp. maculate insensitive to propiconazole and pyraclostrobin, respectively. In the USA, resistance to QoI fungicides has been reported in several pathogens, including the pistachio late blight pathogen (Alternaria alternata (Fries) Keissler) (Avenot et al., Citation2008, Citation2009) and the wheat Septoria tritici blotch pathogen (Z. tritici) (Estep et al., Citation2013, Citation2015). For wheat fungal pathogens, Estep et al. (Citation2013) were the first to report resistance to QoI fungicides in North American populations of Z. tritici. More recently, the same group reported resistance to DMI fungicides in the Z. tritici populations (Estep et al., Citation2015). They reported that Z. tritici mutants resistant to either QoI or DMI fungicides were absent in 1992 but became more frequent in Oregon in 2012. Meyers et al. (Citation2015) studied the sensitivity of B. graminis isolates collected in different regions of the USA to five fungicides (tebuconazole, prothioconazole, pyraclostrobin, picoxystrobin and fluxapyroxad) and observed significant differences in sensitivity to tebuconazole and prothioconazole among the pathogen populations from different regions. In addition, and more specifically within rust fungal populations, fungicide resistance was reported in the wheat leaf rust pathogen as mentioned above, and fungicide resistance has also been reported in the soybean rust pathogen (Phakopsora pachyrhizi Syd.) populations in Brazil (Schmitz et al., Citation2014).
For wheat stripe rust, Bayles et al. (Citation2000) was the first to document fungicide resistance in the Pst population in the UK. They detected resistant isolates to DMI fungicides (triadimenol, epoxiconazole and tebuconazole) and the shift of the Pst population trending to resistance between 1990 and 1997/1998, which was coincident with the increased exposure of the population to this group of fungicides in the 1990s. However, no additional studies have been reported. In the USA, studies have not previously been conducted to determine differences in fungicide sensitivity in the Pst populations. During the last 20 years, the senior authors occasionally heard reports from wheat growers that stripe rust was not well managed following fungicide applications. However, after conferring with growers or reporters, the senior author determined that the unsuccessful cases were most likely related to reduced fungicide concentrations and/or later than optimal timing of application. As some of the DMI and QoI fungicides have become more cost effective, wheat growers in the PNW have tended to apply fungicides mixed with herbicides in the early crop growing season on an annual basis, oftentimes in the absence of stripe rust. Although the early application of fungicides can effectively reduce rust epidemics when the Pst fungus overwinters and disease incidence is high, fungicide applications made in the absence of disease are more generally considered to be a waste of money and may increase the potential selection pressure towards the development of resistance in the Pst population (Chen, Citation2014; Chen & Kang, Citation2017b). The widespread use of fungicides to manage stripe rust has been related to the increased tolerance of non-targeted pathogens, such as Z. tritici in western Oregon (Hayes et al., Citation2016).
The objective of this study was to determine sensitivities of Pst isolates to the commonly used Tilt and Headline fungicides through germination assays and infection tests at seedling and adult-plant stages. The data of fungicide sensitivity can be used as a baseline for continually monitoring Pst populations for changes in fungicide sensitivity over time. The monitoring information should be useful for guiding stripe rust management.
Materials and methods
Fungicides
Throughout this study, Tilt 3.6 EC, containing 41.8% propiconazole (Syngenta Crop Protection, Greensboro, NC) to represent the DMI class and Headline 350 SC, containing 23.6% pyraclostrobin (BASF, Florham Park, NJ) to represent the QoI class, were used in all experiments.
Pst isolates and urediniospore production
A total of 39 Pst isolates were used in this study, including eight historical isolates selected to represent different periods from the 1960s to the early 2010s and 31 isolates collected from fungicide-sprayed experimental plots in 2014 to represent the recent population under direct fungicide application pressure (Supplementary Table 1). All of the isolates were collected in Washington State. For obtaining isolates to represent a local contemporary Pst population under fungicide selection pressure, stripe rust samples were collected from plots sprayed with propiconazole or pyraclostrobin in fungicide efficacy trials conducted near Pullman, Washington in 2014.
Table 1. Effects on urediniospore germination rates by Puccinia striiformis f. sp. tritici isolates collected between 1968 and 2013, concentrations of Tilt or Headline fungicide and interaction determined using the Mixed Procedure.
Spring wheat variety ‘Avocet S’ (AvS), which is susceptible to most Pst races, was used to increase inoculum of each isolate. Approximately 10 seeds were planted in each plastic pot (7 × 7 × 7 cm) with a potting mixture obtained by mixing 24 L of peat moss, 8 L of perlite, 12 L of sand, 12 L of commercial potting soil, 16 L of vermiculite and 250 g Osmocote. Seedlings at the two-leaf stage (~10 days after planting) grown in a rust-free greenhouse (diurnal temperature cycle gradually changing from 10°C at 2:00 am to 25°C at 2:00 pm with a 16 h light/8 h dark cycle) were uniformly dusted with a mixture of urediniospores of a single Pst isolate and talc at a ratio of ~ 1 mg: 20 mg.
The initial inoculum for each of the historical isolates (Supplementary Table 1) consisted of urediniospores stored in liquid nitrogen, which were revived by submerging the glass vial or foil bag in hot water at 50–55°C for 2 min before being used for inoculations.
The initial inoculum for each of the 2014 isolates consisted of a piece of leaf bearing uredinia. Before inoculating plants, the segments of leaf samples bearing uredinia were incubated on moist filter papers in Petri dishes at 4°C overnight. Urediniospores on the leaf segments were picked and brushed onto seedling leaves using a painting brush. After inoculation, plants were placed in a dew chamber at 10°C for 24 h in the dark and then transferred to a growth chamber operating at 16:8 h light:dark with diurnal temperatures gradually changing from 4°C at 2:00 am to 20°C at 2:00 pm.
The plants inoculated with each sample in a single plastic pot (7 × 7 × 7 cm) were isolated using a cylindrical booth formed with a transparent plastic sheet (30 cm wide × 40 cm tall) to prevent cross-contamination. Generally, 16 days after inoculation, urediniospores were collected using a vacuum spore collector approximately once every 2 days. Urediniospores were stored in a desiccator at 4°C for later use. For a germination or inoculation test, urediniospores collected one day before were used so that different isolates were tested using spores with similar viabilities.
Germination tests
Fungicide solutions were made by diluting commercial product with distilled water. The commercial product of Tilt 3.6 EC is labelled as 4.0 fl oz per acre for the full concentration used in the USA, which was converted to 1.56 mL L−1 using 20 L water solution for ground application. Similarly, Headline 350 SC is labelled as 9.0 fl oz/A for the full concentration, which was converted to 3.52 mL L−1 water solution. For easy comparison of various concentrations, these full concentrations were presented as concentration 1.0× for their respective fungicides.
For the first germination experiment with the eight historical isolates (Supplementary table 1), nine Tilt concentrations were used (0.0×, 0.01×, 0.02×, 0.04×, 0.08×, 0.10×, 0.15×, 0.20× and 1.0×) and 10 Headline concentrations (0.0×, 0.00075×, 0.00100×, 0.00150×, 0.00200×, 0.00300×, 0.00400×, 0.00600×, 0.00750× and 1.0×). Based on preliminary test results, 170 μL of a Tilt or Headline solution in water at each defined concentration was added to a piece of autoclaved cheesecloth (1 cm × 1 cm) on a glass slide kept in a Petri dish in the germination assay. Fresh urediniospores mixed with talc at 1:20 were uniformly spread on the cheese cloth by gently tapping a cotton swab carrying spore/talc mixture (~3,000 to 4,000 spores) and incubated at 10°C in the dark for 24 h. After counting germinated spores at 3, 6, 9, 12, 15, 18, 21 and 24 h after incubation in a preliminary test, 12 h after incubation was determined as the optimal time because no additional germination occurred after 12 h and used for recording germination rates in the subsequent spore germination experiments. Percentage of germinated spores was recorded by counting 10 microscopic fields (100×) on each cheesecloth slide using a ZEISS Axioskop 40 microscope (ZEISS, Jena, Germany). A spore was scored as germinated if the germ tube reached at least twice the length of the spore. For each isolate at each concentration, three replications in different Petri dishes were used. The experiment was arranged in a complete randomized block design.
In the second germination experiment with the 31 isolates collected in 2014 (Supplementary Table 1), five Tilt concentrations (0.0×, 0.03×, 0.10×, 0.15× and 1.0×) and four Headline concentrations (0.0×, 0.0003×, 0.003× and 0.03×) were used. The experiment was conducted in the same way as described above.
Seedling inoculation tests
In these tests, five isolates (1968-31, 1972-7-5, 1991-2, 2002-168 and 2013-242-1) selected from the historical isolates based on the germination tests were used (Supplementary table 1). Seedlings of AvS at the two-leaf stage were inoculated with fresh urediniospores from each isolate as outlined above. Six concentrations of Tilt (0.0×, 0.125×, 0.25×, 0.5×, 1.0× and 2.0× relative to the full concentration (1.56 mL L−1) as 1.0×); and for Headline (0.0×, 0.002×, 0.003×, 0.006×, 0.5× and 1.0× relative to the full concentration (3.52 mL L−1) as 1.0×) were used. Five-millilitre solution for each concentration of Tilt or Headline was sprayed on 15 seedlings in three pots (7 × 7 × 7 cm), which produced tiny drops uniformly covering all leaves without run-off. The following three fungicide timing treatments were used for both fungicides: (1) fungicide was applied on plants 1 day before Pst inoculation (1 DBI); (2) fungicide was applied on the same day immediately after inoculation (0 DAI); and fungicide was applied 3 days after inoculation (3 DAI). The number of uredinia and disease severity (percentage of the leaf areas infected) were recorded. Uredinia were enumerated from 10 randomly chosen microscope fields (10×) with the aid of a ZEISS Stemi 2000-C stereoscope (ZEISS, Jena, Germany), and severity was based on visual estimation of the percentage of leaf tissue exhibiting symptoms (0–100%). The experiment was conducted three times, with five plants for each fungicide concentration and timing.
Adult-plant inoculation tests
Plants of AvS at the boot stage grown in the greenhouse were inoculated with Pst as outlined above and treated with a specified concentration of Tilt or Headline. The same five Pst isolates (1968-31, 1972-7-5, 1991-2, 2002-168 and 2013-242-1) used in the seedling tests were used in this experiment (Supplementary table 1). Similarly, the concentrations for Tilt were 0.0×, 0.125×, 0.25×, 0.5×, 1.0× and 2.0× relative to the full concentration (1.56 mL L−1) as 1.0×; and for Headline 0.0×, 0.002×, 0.003×, 0.006×, 0.5× and 1.0×, relative to the labelled full rate (3.52 mL L−1) as 1.0×. Ten-millilitre solution of a specific fungicide concentration was sprayed onto 15 plants, each grown in a plastic tube of 5 cm in diameter and 20 cm in length, to ensure every flag leaf was covered uniformly by tiny drops without run-off. The three timing treatments as described above were used for both fungicides, plus a 7 DAI (fungicide was applied 7 days after Pst inoculation) treatment of Tilt. Similar to the seedling experiments, data on the number of uredinia and disease severity on flag leaves were recorded 20 days after inoculation. The experiment for each chemical was conducted three times, with five plants for each application timing and concentration.
Data analyses
The PROC MIXED procedure was used in the SAS for windows (Version 9.2, SAS Institute, Cary, NC) to determine the effects of the different fungicide concentrations and Pst isolates on germination, and of the different isolates, fungicide application timings, and concentrations on rust sporulation and disease severity in the seedling and adult-plant tests. For the germination tests, effects of isolate, fungicide rate, and the interaction between isolate and rate were determined; and in the plant tests, the effects of isolate, fungicide rate, application timing, and interactions between any two factors and the three-factor interaction were determined. Analysis of variance was performed for each concentration using the PROC ANOVA procedure and the Tukey test was used to separate the means of the isolates in the SAS program. Fungicide Tilt and Headline were analysed separately.
Results
Germination of the historical isolates
Based on preliminary tests, maximum urediniospore germination occurred at 12 h after incubation; therefore, all analyses of urediniospore germination were based on the data obtained at this time point. In the Tilt experiment, differences in germination were observed among the isolates (P < 0.0001) and fungicide concentrations (P < 0.0001); and there was an interaction between isolate and fungicide concentration (P < 0.0001) (). Therefore, the isolates were compared separately by their germination rates at different concentrations (). In the water control (no Tilt or concentration 0), the means of germination rates were not significantly different (P = 0.1747). At the concentration of 1.0× (1.56 mL L−1), no urediniospores germinated, indicating that the full concentration was able to completely inhibit urediniospore germination. However, differences were observed in germination rate among the eight historical Pst isolates at seven concentrations (0.01×, 0.02×, 0.04×, 0.08×, 0.10×, 0.15× and 0.20×). For example, isolate 1968-31 had ≥96.2% germination at Tilt concentrations of 0.01×, 0.02× and 0.04×; 82.8% at 0.08×; 75.2% at 0.10×; 2.7% at 0.15×; and 0.0 at 0.20×. In contrast, more than 96% urediniospores of isolate 2013-242-1 germinated at the seven concentrations from 0.01× to 0.15×, but the rate was reduced to 16.9% at 0.20×. Similarly, 8.6% urediniospores of isolate 1972-7-5 were germinated. Similar to 1968-31, the remaining five isolates were unable to germinate at 0.20×. These results showed that the full concentration of Tilt was able to completely inhibit urediniospore germination of various isolates, but sensitivity of isolates varied at reduced rates.
Table 2. Mean values of urediniospore germination rates in the treatments of Tilt at various concentrations for eight Puccinia striiformis f. sp. tritici (Pst) isolates collected from 1968 to 2013 in Washington State.
Similarly, in the Headline experiment, the effects of isolates, concentrations and their interaction on germination rate were all significant (P < 0.0001) (). No differences in germination rate were observed among the Pst isolates at the fungicide-free treatment; and no germination occurred for any isolate at both concentrations 0.0075× and 1.0× (). In contrast, differences were observed in Pst germination rates at the remaining seven fungicide concentrations (P < 0.0001–0.0166). For example, at concentration 0.00075×, germination of isolate 1968-31 was 87.8% whereas isolate 2013-246 had 99.9% germination. At concentration 0.006×, isolate 1972-7-5 had 3.1% and 1985-118 had 0.5% germination, whereas all other isolates completely failed to germinate. These results showed that differences in sensitivity to Headline existed among Pst isolates at much reduced concentrations.
Table 3. Mean values of urediniospore germination rates in the treatments of Headline at various concentrations for eight Puccinia striiformis f. sp. tritici (Pst) isolates collected from 1968 to 2013 in Washington State.
Germination tests of the 2014 isolates
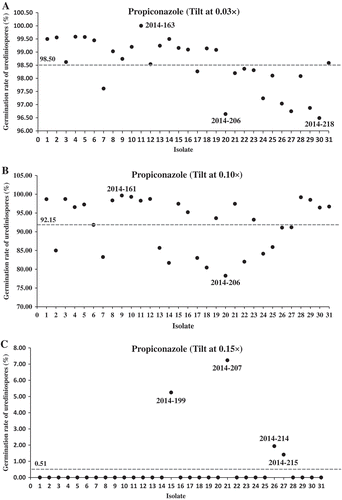
The 31 isolates collected from fungicide-sprayed field plots were tested for urediniospore germination. Similar to the test results of the historical isolates, differences were observed among the fungicide concentrations (P < 0.0001) and the isolates (P < 0.0001), and there was an isolate × concentration interaction (P < 0.0001) for both Tilt and Headline experiments (). In the tests with Tilt, differences were observed among the isolates tested at concentrations of 0.03×, 0.10× and 0.15× (). The germination rates ranged from 96.5% (2014-218) to 100% (2014-163) at concentration 0.03× () and from 78.3% (2014-206) to 99.7% (2014-161) at concentration 0.10× (). At concentration 0.15×, four isolates (2014-199, 2014-207, 2014-214 and 2014-215) had a low level of germination (1.1% to 7.2%), whereas all other isolates completely failed to germinate (). At the full concentration of 1.0× (1.56 mL L−1), urediniospore germination of all isolates was completely inhibited.
Table 4. Effects on urediniospore germination rates by Puccinia striiformis f. sp. tritici isolates collected in 2014, concentrations of Tilt or Headline fungicide and their interaction determined using the Mixed Procedure.
Fig. 1 (Colour online) Scatterplots of urediniospore germination rates of Puccinia striiformis f. sp. tritici isolates collected in 2014 tested with Tilt at concentrations 0.03× (a), 0.10× (b), and 0.15× (c) relative to the full concentration of Tilt (1.56 mL L−1) treated as 1.00×. Refer to for all isolate names. The dash line denotes the mean germination rate of the specific fungicide concentration treatment.
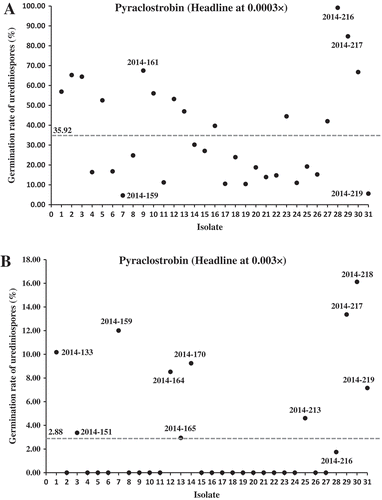
Similarly, differences in sensitivity to Headline were detected among the 31 2014 isolates at relative concentrations 0.0003× and 0.003× (). When tested at concentration 0.0003×, the germination rates ranged from 4.7% (2014-159) to 99.1% (2014-216) (). At concentration 0.003×, 11 isolates had urediniospore germination rates of 1.7% to 16.1%, whereas the other 20 isolates completely failed to germinate (). At concentration 0.03× and the full concentration 1.0× (3.52 mL L−1), all of the isolates completely failed to germinate.
Fig. 2 (Colour online) Scatterplots of urediniospore germination rates of Puccinia striiformis f. sp. tritici isolates collected in 2014 tested with Headline at concentrations 0.0003× (a) and 0.003× (b) relative to the full concentration of Headline (3.52 mL L−1) treated as 1.0×. Refer to for the names of all isolates.
Inoculation tests at the seedling and adult-plant stages
The effects of Pst isolate, fungicide application timing and concentration, as well as all possible two-factor interactions and the three-factor interaction, on the number of uredinia were significant in the seedling and adult-plant tests with both Tilt and Headline ().
Table 5. Effects on the number of uredinia on seedlings and adult plants of wheat produced by Puccinia striiformis f. sp. tritici isolates, timings and concentrations of Tilt and Headline fungicides, and their interactions determined using the Mixed Procedure.
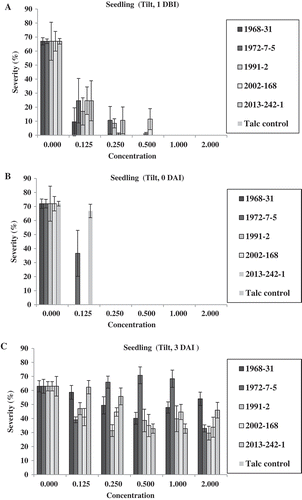
In the seedling experiment of Tilt, there were no significant differences in the number of uredinia among the five isolates tested in the water control (0 chemical). No uredinia were produced at concentrations 1.0× (the full concentration of 1.56 mL L−1) and 2.0× (double the full concentration) applied 1 DBI of the inoculation of Pst. However, at concentration 0.5× (half of the full concentration), two isolates (1991-2 and 2012-168) produced few uredinia, whereas the other three isolates did not produce any uredinia (). At concentrations 0.25× and 0.125×, all five isolates, except isolate 1968-31 at concentration 0.25×, produced uredinia. In general, more uredinia were produced at concentration 0.125× than at 0.25×. The oldest isolate, 1968-31, had the least uredinia, while isolates 1972-7-5 and 2013-242-1 produced more uredinia than the other isolates at concentrations of 0.125× and 0.25×. When applied on the same day (0 DAI) as the Pst inoculation (), no uredinia were produced at any concentration of Tilt, except 0.125×, at which only isolates 1972-7-5 and 2013-242-1 produced uredinia. In contrast, when Tilt was applied 3 DAI (), all of the five isolates produced uredinia, but the numbers of uredinia varied among the isolates and among the concentrations tested. Isolates 1972-7-5 and 2013-242-1 consistently produced more uredinia than the other three isolates (1968-31, 1991-2 and 2002-168). The disease severity data showed a similar trend ().
Fig. 3 (Colour online) Numbers of uredinia on seedlings of Avocet S wheat treated with Tilt at various concentrations relative to the full concentration of Tilt (1.56 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b), and 3 days (3 DAI) (c) after inoculation with Puccinia striiformis f. sp. tritici.
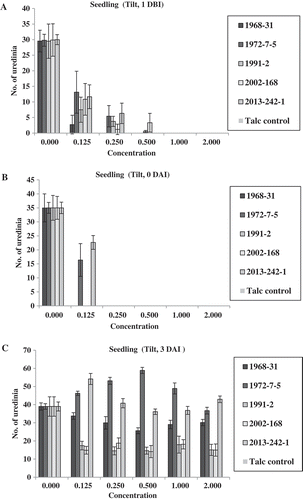
Fig. 4 (Colour online) Disease severity on seedlings of Avocet S wheat treated with Tilt at different concentrations relative to the labelled full concentration of Tilt (1.56 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b) and 3 days (3 DAI) (c) after inoculation with Puccinia striiformis f. sp. tritici isolates.
In the seedling experiment of Headline, no uredinia were produced by any of the isolates at both the full concentration 1.0× (3.52 mL L−1) and the half concentration 0.5× for the treatments 1 DBI and 0 DAI, and at rate 1.0× for the treatment of 3 DAI (). In the same day (0 DAI) treatment, isolate 2002-168 produced the highest number of uredinia, followed by isolate 2013-242-1 at concentrations 0.002×, 0.003× and 0.006× (). At concentration 0.5× applied 3 DAI, three isolates (1991-2, 2002-168 and 2013-242-1) produced few uredinia, whereas isolates 1968-31 and 1972-7-5 did not produce any uredinia (). At the concentrations of 0.002×, 0.003× and 0.006× applied 3 DAI, all isolates produced uredinia, but the numbers of uredinia were significantly different among the isolates for all three treatments at different timings of the fungicide application. In general, the oldest isolate, 1968-31, produced the lowest number of uredinia. In the treatment of 3 DAI, isolate 2013-242-1 tended to produce more uredinia, and was thus less sensitive to Headline. The disease severity data followed a similar trend ().
Fig. 5 (Colour online) Numbers of uredinia on seedlings of Avocet S wheat treated with Headline at various concentrations relative to the full concentration of Headline (3.52 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b), and 3 days (3 DAI) (c) after inoculation with Puccinia striiformis f. sp. tritici.
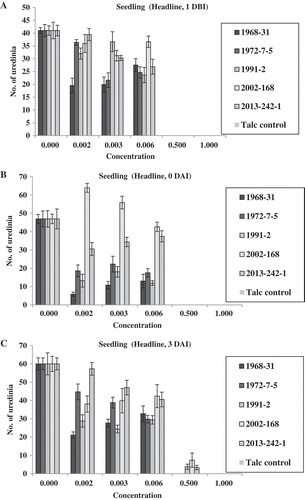
Fig. 6 (Colour online) Disease severity on seedlings of Avocet S wheat treated with Headline at different concentrations relative to the labelled full concentration of Headline (3.52 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b) and 3 days (3 DAI) (c) after inoculation with Puccinia striiformis f. sp. tritici isolates.
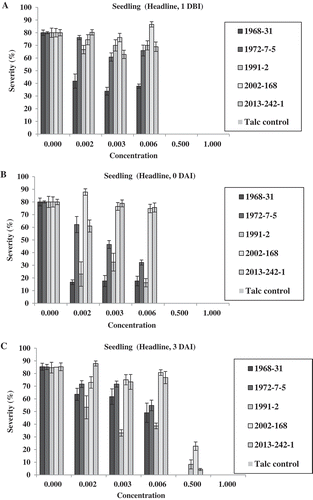
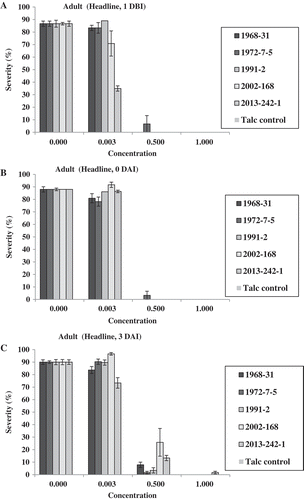
In the adult-plant Tilt experiment, no isolates produced uredinia at both fungicide concentrations of 1.0× and 2.0× for the treatments 1 DBI, 0 DAI and 3 DAI, except a few uredinia were observed for isolate 2013-242-1 at 3 DAI (). At 0.5×, only isolate 1968-31 produced some uredinia at 1 DBI; isolate 2013-242-1 produced some uredinia at 0 DAI; and all of the five isolates produced uredinia at 7 DAI (). At 0.25×, none of the isolates produced uredinia at 1 DBI; isolate 1972-7-5 produced few uredinia at 0 DAI; and all isolates produced uredinia, with isolate 1968-31 producing the least and isolates 1972-7-5 and 2013-242-1 the greatest number of uredinia in the 3 and 7 DAI treatments. Differences in sporulation were observed among the isolates at both 0.25× and 0.5× concentrations applied 3 DAI. In the 7 DAI treatment, all isolates produced uredinia, but the number of uredinia varied among the isolates (). Isolates 1972-7-5 and 2013-242-1 almost always produced more uredinia than other isolates in this treatment. A similar trend was observed based on the disease severity data (). It is worthy to note that at 7 DAI, the disease severity was much higher than were the number of uredinia. This was because disease severity also included necrotic leaf area. The difference between uredinial formation and disease severity indicated the effect of Tilt on reduction of spore production even 7 DAI, which was much less effective than the earlier applications.
Fig. 7 (Colour online) Numbers of uredinia on adult plants of Avocet S wheat treated with Tilt at different concentrations relative to the labelled full rate of Tilt (1.56 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b), 3 days (3 DAI) (c), and 7 days (7 DAI) (d) after inoculation with Puccinia striiformis f. sp. tritici.
Fig. 8 (Colour online) Disease severity on adult plants of Avocet S wheat treated with Tilt at different concentrations relative to the labelled full concentration of Tilt (1.56 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b), 3 days (3 DAI) (c) and 7 days (7 DAI) (d) after inoculation with Puccinia striiformis f. sp. tritici isolates.
In the Headline tests, only three application timings (1 DBI and 0 and 3 DAI) were used. No isolates produced uredinia at the full concentration 1.0× (3.52 mL L−1), in any of the timing treatments, except for the few uredinia that were observed in the inoculation of isolate 2013-242-1 (). At the half concentration (0.5×), isolate 1972-7-5 produced some uredinia in the treatments of 1 DBI and 0 DAI, but in the treatment of 3 DAI, all isolates produced uredinia with isolate 1972-7-5 producing the least uredinia. At 0.003×, all isolates produced uredinia, but the number varied. No isolate consistently produced more uredinia than others. Similar results were obtained for disease severity ().
Fig. 9 (Colour online) Numbers of uredinia on adult plants of Avocet S wheat treated with Headline at different concentrations relative to the full concentration of Headline (3.52 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b), and 3 days (3 DAI) (c) after inoculation with Puccinia striiformis f. sp. tritici.
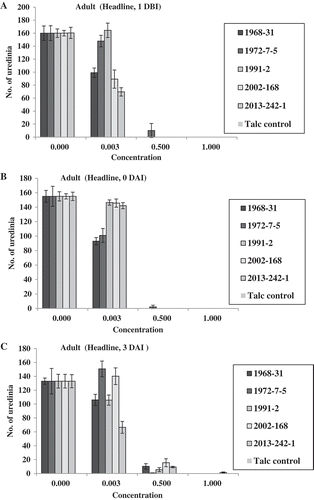
Fig. 10 (Colour online) Disease severity on adult plants of Avocet S wheat treated with Headline at different concentrations relative to the labelled full concentration of Headline (3.52 mL L−1) 1 day before (1 DBI) (a), on the same day (0 DAI) (b) and 3 days (3 DAI) (c) after inoculation with Puccinia striiformis f. sp. tritici.
Discussion
In the present study, we identified differences in sensitivity to Tilt and Headline fungicides among historical and contemporary Pst isolates through urediniospore germination. Different fungicide concentrations also had significant effects in inhibiting urediniospore germination. In the plant tests, we also observed the significant differences among isolates, fungicide concentrations, plus application timings on infection inhibition. Moreover, these factors significantly interacted with each other. This is the first report of differences in sensitivity existing in the Pst population in the USA.
Propiconazole, represented by Tilt, is a member of the DMI group, the largest class of fungicides that inhibits fungal sterol biosynthesis (Morton & Staub, Citation2008). The DMI fungicides have been used the longest for managing stripe rust in the US Pacific Northwest (Line, Citation1993, Citation2002). The first DMI fungicide launched was triadimefon (Bayleton) in 1973. After intensive testing, this fungicide was first registered for application on wheat to reduce stripe rust damage in 1981 (Line, Citation2002). Propiconazole, which was launched in 1979 (Morton & Staub, Citation2008), has been widely used since the mid-1980s (Line, Citation1993) and is still the most widely used fungicide for management of stripe rust (Chen, Citation2014). Pyraclostrobin, represented by Headline, belongs to the QoI group of fungicides that inhibit cytochrome bc1 of fungi (Morton & Staub, Citation2008). Launched in 1996, QoI fungicides are now the second largest class of fungicides used on cereals for managing various foliar fungal pathogens, including Pst. Among QoI fungicides, pyraclostrobin has been used to manage stripe rust since the early 2000s, although it has never been as widely used as propiconazole (Chen & Kang, Citation2017b). In the present study, we tested Tilt and Headline and analysed the data in the same way, but separately. Using their labelled full concentration as references, we found that Pst isolates were more sensitive to Headline than Tilt as the former was able to completely inhibit urediniospore germination at a concentration as low as 0.0075× whereas the latter needed at a concentration between 0.2× and 1.0× to perform a similar function.
Instead of the active ingredients of propiconazole and pyraclostrobin, we used their representative fungicides Tilt and Headlines, respectively. These tests provided the sensitivity information directly useful to the management of stripe rust using these fungicides. However, Tilt and Headline contain only 41.8% propiconazole and 23.6% pyraclostrobin, respectively. Further tests with propiconazole or pyraclostrobin are needed to determine if Pst isolates have different levels of sensitivity to the active ingredients. As each class has many other formulations, the data with the active ingredient can be used as a better reference for determination of Pst sensitivity to the fungicides containing the same active ingredients.
In the present study, we did not find any Pst isolate resistant to either Tilt or Headline at their labelled full concentrations. Thus, both fungicides are still effective against Pst when applied on time and at the full concentration. The results were consistent with the efficacy tests of these fungicides (Chen et al., Citation2014, Citation2016). However, differences in sensitivity among Pst isolates were observed for both fungicides at reduced concentrations, as measured by in vitro germination. In the germination tests, as urediniospores were placed immediately in close contact with fungicide, the minimal concentration required to completely prevent spore germination was much lower than that needed on plants. Therefore, we also conducted fungicide tests on seedlings and adult plants inoculated with a number of Pst isolates. The in planta tests also revealed differences in fungicide sensitivity among isolates. Moreover, the effects of fungicide timing and concentration were significant as well as the interactions among isolate, timing and concentration. These results indicated that fungicides need to be applied on time and at an adequate concentration in order to provide maximum protection.
For Tilt, the relative concentration of 0.2× was able to differentiate isolates based on spore germination rate, although the 0.15× concentration was the best to separate isolates as at this concentration the historical Pst isolates tested had the widest range of germination. With the exception of isolate 1972-7-5, more recent isolates (e.g. 2013-242-1 and 2014-207) appeared to be less sensitive to Tilt. The sensitivity of isolate 1972-7-5 might not represent the population in the early 1970s as Tilt or related chemicals had not been commercially used before that time. Another possibility is that the sensitivity of this isolate may be still within the consensus range of Pst to the chemical prior to use of the fungicide as the concentrations of 0.15× and 0.20× were far less than the labelled full concentration. As only eight isolates were randomly picked to represent the Pst population of 1968–2014 for determining the baseline sensitivity, no conclusions could be drawn about the trend of sensitivity changing over the years. Further studies with a large number of isolates are needed. Because germination and in planta infection tests are time-consuming for Pst, more efficient tests using molecular markers for fungicide sensitivity can be used to test a large number of isolates for addressing the changes of sensitivity in the Pst population.
For Headline, using concentrations of 0.004× and 0.006× separated isolates based on spore germination rate. The 0.002× and 0.003× concentrations appeared to be the best for screening isolates as all isolates had some spores germinated and the germination rates varied greatly. When tested with the 2014 isolates at concentration 0.003×, we determined that 65% of the 31 isolates were completely inhibited, while 35% isolates germinated. The germination tests demonstrated that different sensitivities exist among Pst isolates. When the spore germination rate for pyraclostrobin at concentration 0.003× was compared with that of Tilt at 0.15×, the isolates less sensitive to these chemicals were not the same. Thus, populations that are not well managed by Tilt can be managed by Headline, supporting the use of mixing fungicides or rotating fungicides with different modes of action.
The in planta infection tests showed that the same day (0 DAI) treatment with either Tilt or Headline was better to reduce infection than the 1 DBI and 3 DAI treatments. In the same day treatment, Tilt at concentrations 0.5× and 0.25× completely suppressed Pst infection for all isolates tested in the seedling stage, although some isolates sporulated slightly in the adult-plant stage. Similar results were obtained in the Headline experiments with different concentrations. Based on the in planta tests, the best concentrations for distinguishing isolates with different levels of sensitivity were 0.125× to 0.25× relative to the full concentration 1.0× (1.56 mL L−1) for Tilt and from 0.002× to 0.006× relative to the full concentration 1.0× (3.52 mL L−1) for Headline. These values, together with the EC50 values for germination tests (data not shown), can be used as baseline sensitivity to continually monitor changes in the Pst populations.
The DMI class of fungicides targets the CYP51 gene that is involved in sterol biosynthesis (Steffens et al., Citation1996). QoI fungicides inhibit mitochondrial respiration by binding to the quinone outside (Qo) of the cytochrome bc1 complex, blocking electron transfer and halting ATP synthesis (Fernández-Ortuño et al., Citation2008). Thus, detecting mutations in these genes can be an effective method for identifying fungicide resistant isolates. Identifying mutants of these genes have been used to study fungicide resistance in other fungal pathogens. Schmitz et al. (Citation2014) reported that the DMI fungicide resistance of P. pachyrhizi is related to point mutations in the CYR51 gene. By detecting mutations in this gene, DMI resistant isolates of Z. tritici have been identified in western Oregon (Estep et al., Citation2015; Hayes et al., Citation2016). Blixt et al. (Citation2009) found that the majority of Swedish isolates of Phaeosphaeria nodorum (E. Müll.) Hedjar. possessed the amino acid substitution G143A in the cytochrome b gene, which was associated with loss of sensitivity to QoI fungicides. However, resistance to the DMI fungicide prochloraz was reported not to be related to sequence changes in the CYP51 gene in O. yallundae (syn. Tapesia yallundae Wallwork & Spooner), the wheat eyespot fungus, indicating a different mechanism of fungicide resistance (Wood et al., Citation2001). For Pst, Cook et al. (Citation2017) identified six isolates from New Zealand containing a Y134F mutation within the CYP51 gene. The mutant genotype is homologous to the Y137F mutation characterized in many other fungi including Z. tritici, and known to cause reduction in DMI sensitivity. However, these mutant isolates have not been determined to be resistant to DMI fungicides. We are currently testing thousands of Pst isolates collected from the USA since 1968 for CYP51 mutants. Such results, together with the results of the present study, should provide useful information for determination of the dynamics of fungicide sensitivity in the pathogen population and for more effective management of stripe rust.
Supplementary_Table_1.docx
Download MS Word (17.7 KB)Acknowledgements
We thank Kent Evans for the technical assistance. The China Scholarship Council scholarships to Zhanhai Kang and Xing Li are greatly appreciated.
Supplementary material
Supplemental data for this article can be accessed online here: http://doi.org/10.1080/07060661.2019.1577301.
Additional information
Funding
References
- Akhavan A, Strelkov SE, Askarian H, Kher SV, Fraser M, Kutcher RH, Turkington KT. 2017. Sensitivity of western Canadian Pyrenophora teres f. teres and P. teres f. maculata isolates to propiconazole and pyraclostrobin. Can J Plant Pathol. 39:11–24.
- Avenot H, Morgan DP, Michailides TJ. 2008. Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine® (pyraclostrobin + boscalid) fungicide in Alternaria alternate causing alternaria late blight of pistachios in California. Plant Pathol. 57:135–140.
- Avenot H, Sellam A, Michailides T. 2009. Characterization of mutations in the membrane-anchored subunits AaSDHC and AaSDHD of succinate dehydrogenase from Alternaria alternata isolates conferring field resistance to the fungicide boscalid. Plant Pathol. 58:1134–1143.
- Bayles RA, Stigwood PL, Clarkson JDS. 2000. Shifts in sensitivity of Puccinia striiformis to DMI fungicides in the UK. Acta Phytopathol Et Entomol Hungarica. 35:381–382.
- Blixt E, Djurle A, Yuen J, Olson Å. 2009. Fungicide sensitivity in Swedish isolates of Phaeosphaeria nodorum. Plant Pathol. 58:655–664.
- Chen XM. 2005. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can J Plant Pathol. 27:314–337.
- Chen XM. 2014. Integration of cultivar resistance and fungicide application for control of wheat stripe rust. Can J Plant Pathol. 36:311–326.
- Chen XM, Evans CK, Liu YM. 2016. Control of stripe rust of winter wheat with various foliar fungicides, 2015. Plant Dis Manag Rep. 10:CF:022.
- Chen XM, Evans KC, Liu YM. 2014. Control of stripe rust of winter wheat with various foliar fungicides. Plant Dis Manag Rep. 8:CF023.
- Chen XM, Kang ZS. 2017a. Chapter 1 Introduction: history of research, symptoms, taxonomy of the pathogen, host range, distribution, and impact of stripe rust. In: Chen XM, Kang ZS, editors. Stripe rust. Dordrecht: Springer; p. 1–33.
- Chen XM, Kang ZS. 2017b. Chapter 6 Integrated control of stripe rust. In: Chen XM, Kang ZS, editors. Stripe rust. Dordrecht: Springer; p. 559–599.
- Chen XM, Wood DA. 2002. Control of stripe rust of spring wheat with foliar fungicides, 2001. F&N Tests. 57:CF03.
- Chen XM, Wood DA. 2011. Control of stripe rust of winter wheat with foliar fungicides, 2010. Plant Dis Manag Rep. 5:CF004.
- Chen Y, Chen CJ, Zhou MG, Wang JX, Zhang WZ. 2009. Monogenic resistance to a new fungicide, JS399-19, in Gibberella zeae. Plant Pathol. 58:565–570.
- Cook NM, Maintz J, Chng S, Saunders DGO. 2017. New Zealand: the home of Hobbits and fungicide resistant yellow rust. the 29th Fungal Genet. Conf. Program and Abstract Book; Mar 14–18; Asilomar (California, USA). Genetics Society of America. p. 218.
- Estep LK, Torriani SFF, Zala M, Anderson NP, Flowers MD, McDonald BA, Mundt CC, Brunner PC. 2015. Emergence and early evolution of fungicide resistance in North American populations of Zymoseptoria tritici. Plant Pathol. 64:961–971.
- Estep LK, Zala M, Anderson NP, Sackett KE, Flowers M, McDonald BA, Mundt CC. 2013. First report of resistance to QoI fungicides in North American populations of Zymoseptoria tritici, causal agent of Septoria tritici blotch of wheat. Plant Dis. 97:1511.
- Fernández-Ortuño D, Torés JA, de Vicente A, Pérez-García A. 2008. Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. Int Microbiol. 11:1–9.
- Grimmer MK, van Den Bosch F, Powers SJ, Paveley ND. 2014. Fungicide resistance risk assessment based on traits associated with the rate of pathogen evolution. Pest Manag Sci. 71:207–215.
- Hardison JR. 1963. Commercial control of Puccinia striiformis and other rusts in seed crops of Poa pratensis by nickel fungicides. Phytopathology. 53:209–216.
- Hardison JR. 1975. Control of Puccinia striiformis by two new systemic fungicides, Bay MEB 6447 and BAS 31702 F. Plant Dis Rep. 59:652–655.
- Hayes LE, Sackett KE, Anderson NP, Flowers MD, Mundt CC. 2016. Evidence of selection for fungicide resistance in Zymoseptoria tritici populations on wheat in western Oregon. Plant Dis. 100:483–489.
- Line RF. 1976. Chemical control of Puccinia striiformis and Puccinia recondita on wheat in Northwestern United States. Proc. 4th Eur. Mediterr. Cereal Rusts Conf.; Sept 5-10; Interlaken, Switzerland. Wageningen (Netherlands): European and Mediterranean Cereal Rust Foundation. p. 105–108.
- Line RF. 1993. Integrated pest management for wheat: IPM in a wide-ranging system. Plant Dis. 77:303–307.
- Line RF. 2002. Stripe rust of wheat and barley in North America: a retrospective historical review. Annu Rev Phytopathol. 40:75–118.
- Line RF, Rowell JB. 1973. Systemic fungicides for control of rusts and smuts. Proc. 2nd Int. Cong. Plant Pathol.; Sept 5–12; Minneapolis (MN). St Paul (MN): International Society for Plant Pathology. American Phytopathological Society. p. 549.
- Lucas JA, Hawkins NJ, Fraaije BA. 2015. The evolution of fungicide resistance. Adv Appl Microbiol. 90:29–92.
- Meyers E, Parks R, Arellano C, Cowger C. 2015. Evaluating fungicide sensitivity of regional Blumeria graminis f. sp. tritici populations in the United States. Phytopathology. 105(Supplement2):S2.7.
- Morton V, Staub T. 2008. A short history of fungicides. Online, APSnet Features; [ accessed 2018 Jun 10]. http://www.apsnet.org/publications/apsnetfeatures/Pages/Fungicides.asp.
- Pathak V, Shrivastav S. 2015. Biochemical studies on wheat (Triticum aestivum L.). J Pharmacogn Phytochem. 4:171–175.
- Powelson RL, Shaner GE. 1966. An effective chemical seed treatment for systemic control of seedling infection of wheat by stripe rust (Puccinia striiformis). Plant Dis Rep. 50:806–807.
- Rakotondradona R, Line RF. 1984. Control of stripe rust and leaf rust of wheat with seed treatments and effects of treatments on the host. Plant Dis. 68:112–117.
- Schmitz K, Medeiros C, Craig IR, Stammler G. 2014. Sensitivity of Phakopsora pachyrhizi towards quinone-outside-inhibitors and demethylation-inhibitors, and corresponding resistance mechanisms. Pest Manag Sci. 70:378–388.
- Steffens JJ, Pell EJ, Tien M. 1996. Mechanisms of fungicide resistance in phytopathogenic fungi. Curr Opin Biotechnol. 7:348–355.
- Stubbs RW. 1985. Stripe rust. In: Roelfs AP, Bushnell WR, editors. The cereal rusts, Vol. 2, Diseases, distribution, epidemiology and control. Orlando: Academic Press; p. 61–101.
- Wellings CR. 2011. Global status of stripe rust: a review of historical and current threats. Euphytica. 179:129–141.
- Wood HM, Dickinson MJ, Lucas JA, Dyer PS. 2001. Cloning of the CYP51 gene from the eyespot pathogen Tapesia yallundae indicates that resistance to the DMI fungicide prochloraz is not related to sequence changes in the gene encoding the target site enzyme. FEMS Microbiol Lett. 196:183–187.
