Abstract
In the late winter of 2016, an unusual bacterial disease was suspected of causing severe wet rot (tip over) of banana plants grown in various greenhouses in the Mersin province of Turkey. The symptoms of the disease included small round or irregular areas of wet rot, collar rotting, yellowing of the leaves, sucker tip over, and plant death. Infected pseudo-stem samples were collected for isolation of the putative pathogen. The pathogen was identified as Pectobacterium carotovorum subsp. carotovorum (Pcc) after subjecting all isolates to conventional nested PCR with EXPCCF/EXPCCR and INPCCR/INPCCF primer sets, which produced amplification fragments of 550 and 400 bp in the first and the second PCR reactions, respectively. BLAST analysis of amplified PCR products of pel gene and 16S rRNA and the phylogenetic relationships among the various Pcc strains showed high similarities, which ranged from 99–100% with those retrieved from the NCBI GenBank nucleotide database. Real-time PCR was conducted using a locked nucleic acid (LNA) probe specific to Pcc. The pathogenicity tests of all the Pcc isolates were confirmed on banana cultivar ‘Grand Naine’ and potato slices by inoculation with bacterial suspension and subsequent re-isolation of the pathogen from the infected plants. To our knowledge, this is the first time Pcc has been detected, identified and implicated as the causal agent of wet rot disease of banana plants in Turkey.
Résumé
Vers la fin de l’hiver de 2016, une maladie bactérienne inusitée était soupçonnée de causer une grave pourriture aqueuse (renversement) chez les bananiers cultivés en serre dans la province de Mersin, en Turquie. Les symptômes de la maladie incluaient de petites zones rondes ou de forme irrégulière de pourriture aqueuse, la nécrose du collet, le jaunissement des feuilles, le renversement des drageons et la mort du plant. Des échantillons de pseudo-tiges infectées ont été collectés afin d’en isoler l’agent pathogène putatif. L’agent a été identifié en tant que Pectobacterium carotovorum subsp. carotovorum (Pcc) après que tous les isolats ont été soumis à une PCR nichée avec les jeux d’amorces EXPCCF/EXPCCR et INPCCR/INPCCF, qui ont produit des fragments d’amplification de 550 et de 400 bp lors de la première et de la deuxième réaction de la PCR, respectivement. L’analyse BLAST des produits amplifiés de la PCR du gène pel et de l’ARNr 16S ainsi que les relations phylogénétiques entre les différentes souches de Pcc ont affiché des similarités frappantes qui variaient de 99 à 100% par rapport à la base de données des nucléotides de la GenBank du NCBI. Une PCR quantitative a été effectuée avec une sonde d’acide nucléique bloqué spécifique de Pcc. Les tests de pathogénicité de tous les isolats de Pcc ont été confirmés sur le cultivar de banane ‘Grand Naine’ et des tranches de pommes de terre inoculés avec une suspension bactérienne ainsi qu’avec un isolement subséquent de l’agent pathogène prélevé sur les plants infectés. À notre connaissance, c’est la première fois que Pcc a été détecté, identifié et confirmé en tant qu’agent causal de la pourriture aqueuse chez les bananiers en Turquie.
Introduction
Banana (Musa sp.) constitutes one of the main fruit plants that serve as a source of food for millions of people in tropical and temperate regions. The world production of banana reported by the FAO in 2014 was estimated to be 114 130 151 tonnes, with a harvested area of 5 393 811 ha. Turkey is one of the many banana-producing countries of the world, and produced 251 994 tonnes in an area of 5350 ha in 2014 (FAO, Citation2014). With the use of greenhouses specially designed for banana production supported by the Turkish Ministry of Agriculture, production increased by 13% to reach 306 000 tonnes in 2016. Banana production in open fields in Turkey dates back as far as the 1930s, but production in protected environments or greenhouses started in the 1980s in Mersin province, specifically in the Anamur districts, and gained substantial popularity in the 1990s (Gubbuk & Pekmezci, Citation2001). Banana production is mostly carried out in the Turkish provinces with favourable tropical microclimates, such as Antalya (27% total production) and Mersin (72% total production) in the Mediterranean region (Turkstat, Citation2016). The cultivation of this economically important fruit crop is faced with numerous challenges, including insect pests and diseases caused by pathogens (Gold et al., Citation2002; Ploetz et al., Citation2003).
Tip-over (soft rot) disease of banana is one of the major threats to the production of bananas, and causes serious economic losses both in monetary terms and yield in many parts of the world. Soft rot is caused by Pectobacterium spp., including Pectobacterium carotovorum subsp. carotovorum (Pcc) (Gardan et al., Citation2003), which is a Gram-negative plant pathogen in the Enterobacteriaceae family. Other important pathogens in this family known to cause soft rot diseases of plants include P. atrosepticum, P. carotovorum subsp. brasiliense, P. wasabiae and Dickeya chrysanthemi (Czajkowski et al., Citation2009; Rahmanifar et al., Citation2012). Pectobacterium spp. can also cause soft rot diseases on other crops such as potatoes, lettuce, onions, tomatoes, carrots, irises and cucumbers (Garba et al., Citation2014). Soft rot is often found together with other pre-diseases or injuries and becomes the most prominent disease after harvest, and during transit and storage of plant products (van der Wolf et al., Citation2013). Among the Pectobacterium spp., Pcc is considered the most diverse, with a wide host range and dispersal across many different locations in the world, and is known to cause serious economic losses (Toth et al., Citation2003; Agrios, Citation2006).
This study was initiated as a result of numerous reports by banana farmers in Mersin province regarding the frequent occurrence of wet rot disease in banana plants grown in greenhouses, which they erroneously believed was caused by fungi, frost injury and continuous use of fertilizers and pesticides. The majority of greenhouses used by the farmers in this province are constructed from plastic materials that maintain high relative humidity, which could favour both plant and disease development.
There have been several reports of Pcc causing wet rot or soft rot diseases of other plants in Turkey (Aksoy et al., Citation2017; Ustun & Arslan, Citation2016; Dadasoglu & Kotan, Citation2017), but this pathogen has never been implicated in causing wet rot in banana plants. Characterization of the causal agent of banana wet rot in Turkey is paramount for its management. Thus, the main objective of this study was to isolate and characterize the Pcc population that causes banana wet rot using molecular identification techniques.
Materials and methods
Sample collection, isolation and identification
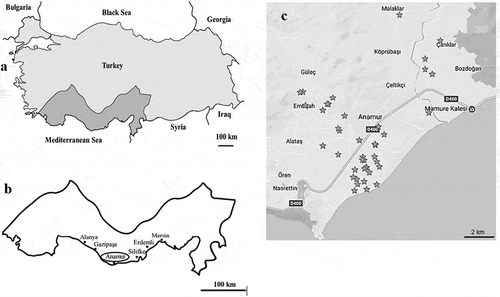
The pseudo-stems of 52 banana plants showing wet rot disease symptoms were collected from 73 plastic greenhouses in several districts of Mersin province in the late winter of 2016. The GPS coordinates of the locations where the samples were collected are shown in ,b,c, and Supplementary Table 1. The disease incidence from the locations where the disease was observed was calculated as described by Bansal et al. (Citation1994), using the formula: Disease Incidence (DI) (%) = (Number of diseased plants/Total number of plants) × 100. The disease symptom scores were obtained using the scale described by Madden et al. (Citation2007), i.e. p. 0) No disease; 1) Trace to 5% infection; 2) 5 to 15% infection; 3) 15 to 35% infection; 4) 35 to 67.5% infection; 5) 67.5 to 100% infection.
Table 1. Primers, probe and PCR conditions for the identification of P. c. subsp. carotovorum in this study.
Fig. 1 Distribution of Pectobacterium carotovorum subsp. carotovorum (Pcc) in the Mediterranean coast of Turkey. (a) Map of Turkey, (b) Banana-producing areas in Turkey, (c) Survey areas in Anamur province. The asterisks indicate the specific locations of the wet rot disease detected.
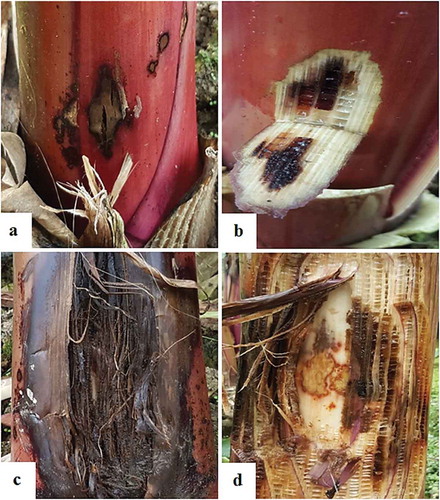
From infected plants, small sections of infected suckers together with adjacent healthy tissues were cut and surface-sterilized in 70% ethanol under aseptic conditions. The symptoms of the disease occurred at both collar level (10 cm) and on the upper part (170 cm) of the plant suckers. The upper layers of the suckers were cut off aseptically, and with the aid of a flamed loop, aliquots of internal sap were obtained from infected tissues and streaked onto nutrient agar medium (NA) following standard isolation procedures (Schaad et al., Citation2001). The Petri dishes were incubated at 27°C for 3 days for observation of representative colonies. The colonies from mixed colony populations were further purified by re-streaking single desired colonies on fresh NA. The purified colonies were transferred to −80°C storage media (Nutrient Broth with 30% glycerol) for further use.
Pathogenicity test
The pathogenicity of 41 isolates was carried out with tobacco (Nicotiana tabacum ‘Samsun’) plants. The freshly cultured and diluted bacterial suspensions (108 cfu mL−1) (OD600, A:0.3) were injected into intercellular spaces of tobacco leaves using a sterile injector with a 26 G-0.45 × 13 mm-gauge needle. The inoculated plants were kept in a growth chamber at 25–28°C with 12-h-light/12-h-dark cycle with 80% humidity and observed for development of a hypersensitive reaction during next 24 h.
The pathogenicity of the isolates was carried out on healthy 4-month-old ‘Grand Naine’ banana plants. The banana pseudo-stems were surface-sterilized with 70% ethanol. Small holes were created on the stems using a 0.6 mm cork borer device. The intercellular spaces of the pseudo-stems were injected using sterilized pipette tips with 100 µL freshly grown bacterial suspension adjusted to 106, 107 or108 cfu mL−1. Three test plants were used for each of the bacterial concentrations, replicated three times. The inoculated portions were wrapped with Scotch tape for 12 h and then removed. The plants were kept in a growth chamber at 28°C and monitored for symptom development for 4 weeks. The experiment was conducted twice. Re-isolations were made from lesions on the pseudostem of the all inoculated seedlings.
Pectolytic activity test
In vitro testing of the isolates on potato (Solanum tuberosum ‘Agria’) was carried out by disinfecting the surfaces of tubers with 70% ethanol and cutting them aseptically into ~8 mm thick slices. The slices were placed on moist Whatman filter paper in Petri dishes. Bacterial suspensions (50 µL) containing ~108 cfu mL−1 were streaked on the surfaces of the slices using a flamed inoculation loop. The Petri plates were wrapped and incubated at 25–27°C for three days for monitoring and documentation of tissue maceration and degradation as described by Schaad et al. (Citation2001) and Mikicinski et al. (Citation2010).
DNA extraction and PCR assays
Genomic DNA of 41 isolates was extracted based on modified CetylTrimethyl Ammonium Bromide (CTAB) method (Doyle & Doyle, Citation1990). The isolated DNA was dissolved in 50 μL volumes of TE buffer and the concentrations were measured using a spectrophotometer (Nanodrop Thermo ND-1000, Thermo Scientific, MA, USA) at 100 ng µL−1 (OD260), after which the DNA was stored at 4°C for further use.
The bacterial isolates, including reference strains DSM-30 168 (Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH Inhoffenstr, Germany), were subjected to conventional PCR using a gradient thermal cycler (Techne TC-512, Bibby Scientific Ltd, Staffordshire, UK). The pel gene was first amplified using Pcc-specific primer pair EXPCCR/EXPCCF, then by nested PCR using primer pair INPCCR/INPCCF (Kang et al., Citation2003) (). 16S rRNA sequences of Pcc isolates were amplified by using fD1/rP2 primer pair (Weisburg et al., Citation1991). Each PCR reaction was conducted in a final volume of 50.05 µL (for first PCR) and 46.25 µL (for nested PCR) containing 5.0 μL10× Taq buffer with KCl (Thermo Fisher Scientific, Waltham, MA, USA), 3.0 μL 2.5 mM MgCl2, 8.0 μL 100 mM dNTPs, 1 μL of each primer, 0.25 μL 5 U μL−1 Taq DNA polymerase-recombinant (Thermo Fisher Scientific), 27.8 μL sterile dH2O, and 4.0 μL DNA suspension (for first PCR) or 0.20 μL amplified DNA products (for nested PCR).
The PCR cycle conditions are shown in . The amplified PCR products (10 µL) and 100 bp DNA Ladder (Thermo Fisher Scientific) were separated by gel electrophoresis in 1.5% agarose gel in 0.5× TAE (Tris-Base 4.84 g, acetic acid (glacial) 1.02 mL, 0.5 M EDTA (pH: 8.0) 2 mL, dH2O 1000 mL) buffer after staining with ethidium bromide (1 mg L−1) and run at 90 V/cm for 1 h. The gel was then visualized with an ultraviolet (UV) light imaging system (Vilber Lourmat SR 12 575 UV transilluminator, France).
Real-time PCR was conducted in a Cepheid Smart Cycler® II (Cepheid Smart Cycler® Software V.2.0c, Sunnyvale, CA, USA). Each real-time PCR reaction was conducted in a final volume of 32.4 µL containing 3.0 μL 10× Taq buffer with KCl, 7.2 μL 2.5 mM MgCl2, 8.0 μL 100 mM dNTPs, 1 μL of each primer (HBPccL/HBPccR) (BM Labosis-Macro Gene) (Çankaya, Ankara, Turkey) (www.bmlabosis.com), 0.36 μL 5 U μL−1 Taq DNA polymerase-recombinant, 0.3 µL locked nucleic acid (LNA) probe labelled with FAM (Roche Molecular Systems, Inc. CA, USA), 9.54 μL sterile dH2O, and 2.0 μL template DNA or 2.0 μL diseased banana tissue extract. The internal necrotic banana tissues were carefully cut and put into an Eppendorf tube containing 800 µL sterilized distilled water and ground with sterilized plastic pestles until homogeneous extracts were obtained. The real-time PCR cycle conditions are shown in .
The specificity of the LNA probe and the primer sets were tested against species closely related to Pcc, including Pseudomonas cichorii (DSM-50 259) and Dickeya chrysanthemi (DSM-4610). All the Pcc strains were processed using the real-time PCR including reference strains DSM-30 168. All the tests were repeated three times for each bacterial strain.
DNA sequencing and phylogenetic analysis
The amplified PCR products of pel gene (550 and 400 bp) and 16S rRNA (1500 bp) were sequenced by BM Labosis-Macro Gene (Çankaya, Ankara, Turkey) (www.bmlabosis.com). The sequenced products were edited and aligned using Bio-Edit (Hall, Citation1999), and searched for homology with Pcc and related species in NCBI GenBank using the BLASTn algorithm with default parameters (Altschul et al., Citation1990). The phylogram analysis was conducted with MEGA 7.0.21 (Kumar et al., Citation2016) using the Maximum likelihood scheme established on the Kimura 2-parameter models (Kimura, Citation1980). The bootstrap was carried out with 1000 replicates to assess the stability and validity of the various clusters. The DNA sequences used in the analysis are deposited in GenBank with accession numbers MH242500-MH242539 and MH242540-MH242579 for pel and 16S rRNA genes, respectively.
Results
Strains, collection and greenhouse survey
A total of 41 isolates were recovered from 52 infected banana suckers collected from 73 greenhouses in Mersin province, and were used in this study together with two reference strains, DSM-30 168 and HBPcc2 (Supplementary Table 1). The colonies of the isolates on nutrient agar plates were round, creamy, shiny, convex and mucoid colonies after 48 h incubation at 27°C.
The most commonly grown and infected banana cultivars from which the samples were collected included ‘Grand Naine’, ‘Overgrown’ (Azman), ‘Dwarf overgrown’ (Bodur Azman) and ‘Şimşek’. The symptoms included small round or irregular wet rot that started from the outer part of the suckers and progressed to the inner layers, with collar rotting, a foul smell, swollen and split suckers, exudates, yellowing of the leaves, pseudo-stem tip over, with the breakage of the upper part of the plant normally occurring from the rotted portion, mostly at the collar level, and in some cases, ~1–2 cm from the plant base, and subsequent plant death (, ). The disease incidences in the locations sampled were: Orta Köy (30%), Taş Köprü (42%), Kalınören (54%), Emirşah (32%), Tekeli (70%), Çarıklar (20%), Kemer Mevkii (25%), Koca Dut Mah. (35%), Fatih Mah. (75%), Güzel Yurt Mah. (27%), Yıldırım Beyazit Mah. (28%), Sultan Alaaddin Mah. (23%), Bahçeli Evler Mah. (33%) and Malaklar Mah. (21%). Pcc was isolated from all banana cultivars.
Pathogenicity test
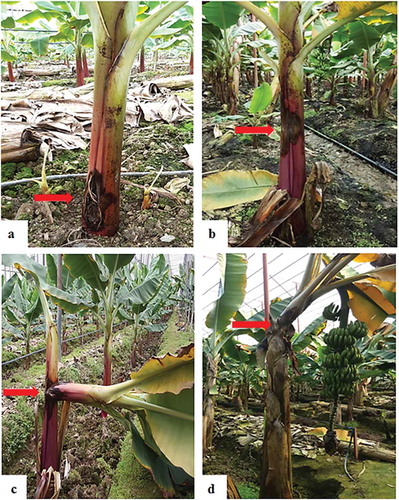
All the 41 bacterial isolates injected into the intracellular spaces of N. tabacum ‘Samsun’ formed a desiccated brown necrotic area after 24 h. The pathogenicity of all isolates on a 4-month-old banana cultivar produced characteristic symptoms typical of disease caused by Pcc. The symptoms were the same as observed in the various greenhouses sampled ().
Fig. 4 (Colour online) Pathogenicity of Pcc on banana plants (Grand Naine): (a) healthy plant inoculated with water, (b) 2 weeks after inoculation, (c) 3 weeks after inoculation, (d) 4 weeks after inoculation. The plants were inoculated with different bacterial concentrations (cfu mL−1): 1 = 106, 2 = 107, 3 = 108 (e) death of the pseudostem.
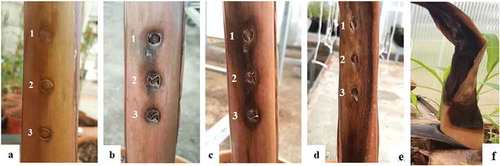
The pathogen also caused tissue maceration and degradation on potato slices inoculated in vitro (Supplementary Table 1). There was no variation in the pathogenicity tests based on different bacterial concentrations used for both banana and potato slices. The healthy banana suckers and potato slices inoculated with sterile ddH2O as a negative control did not produce any symptoms ( and Supplementary table). The pathogen was consistently re-isolated from the inoculated banana plants in the two separate experiments, thereby satisfying Koch’s postulates and establishing Pcc as the causal agent of the wet rot disease in the banana plants in this study.
PCR assays
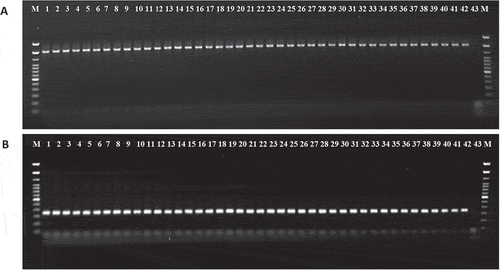
Genomic DNA from the isolates recovered from the infected banana plants collected from Mersin province as well as the reference strains (DSM-30 168) and (HBPcc2) produced the expected amplification fragments of 1500 bp in 16S rRNA PCR reaction using fD1/rP2 primer pair (), and 400 bp in second nested PCR reactions using INPCCR/INPCCF primer pairs ().
Fig. 5 Agarose gel (1.5%) of the PCR products of Pcc strains on banana with primer pairs (a) fD1/rP2 based on 16S rDNA, (b) INPCCF/INPCCR based on pel gene. M – 100 bp DNA ladder; Lanes: 1–41; Pcc 1 – Pcc 41, Lane: 42; Positive control (DSM-30 168), Lane: 43; Negative control, respectively.
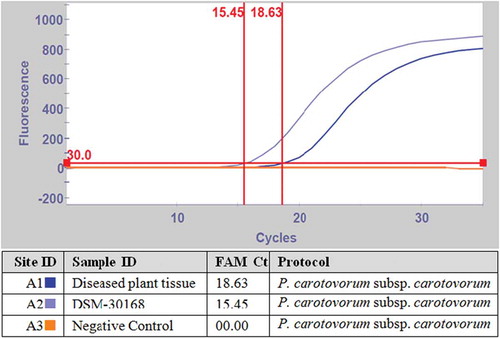
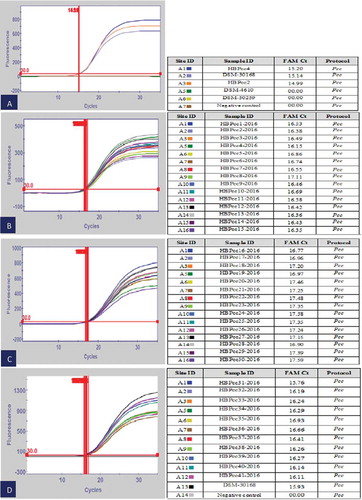
The detection of the Pcc isolate (HBPcc4), including the reference strains DSM-30 168 and HBPcc2 by real-time PCR using our previously designed LNA probe specific to Pcc (Baki & Basım, Citation2016) yielded fluorescent signals with different threshold cycle (Ct) values. The specificity of the LNA probe tested against closely related strains (P. cichorii (DSM-50 259) and D. chrysanthemi (DSM-4610)) to Pcc failed to yield any amplification peaks and as such had no detectable fluorescence, thereby producing 0.0 Ct values. The negative control using sterile dH2O also did not produce any signal and recorded 0.0 values (). All the Pcc strains were detected using the real-time PCR including reference strains DSM-30 168 (,c,d). The real-time PCR assay results confirmed the presence of Pcc in the analysed infected banana samples collected from various greenhouses in Mersin province of Turkey. The Pcc pathogen was sensitively and selectively detected directly from diseased banana tissue by real-time PCR without bacterial isolation, with mean Ct values of 15.45 and 18.63 for the reference strain DSM-30 168 and diseased banana plant, respectively. No detectable fluorescence was observed for negative control treated with dH2O ().
Fig. 6 (Colour online) Real-time PCR detection of Pcc isolates using the LNA probe. (a) DSM-30 168 = positive control, HBPcc2 = positive control, DSM-4610 = D. chrysanthemi, DSM-50 259 = P. cichorii, HBPcc4 = Banana Pcc isolate (b) HBPcc-1-HBPcc15 = Pcc banana isolates (c) HBPcc-16-HBPcc30 = Pcc banana isolates. (d) HBPcc-31-HBPcc41 = Pcc banana isolates, DSM-30 168 = positive control.
DNA sequencing and phylogenetic analysis
BLAST analysis of the amplified PCR products of pel gene and 16S rRNA, and the phylogenetic relationships among the various Pcc strains showed high similarities, which ranged from 99–100% with those retrieved from the NCBI GenBank nucleotide database. The phylogram constructed from multiple alignments of the sequences showed a high lineage or evolutionary relationship (60–100% bootstrap values) between the Turkish banana isolates and those strains recovered from the NCBI GenBank nucleotide database. The strains were categorized into various clusters that were in agreement with the phenotypic and PCR tests (, ).
Discussion
The incidence of the wet rot disease observed in the greenhouses sampled in the province of Mersin ranged from 20–75%. The highest disease incidence was observed in the towns of Tekeli and Fatih, which could be attributed to their proximity to the sea, where the relative humidity is very high. The soil texture profiles in these areas also have characteristics for retaining water for a considerable number of days, which could be another possible reason for the high incidence.
All the identification methods used confirmed the putative pathogen causing the banana wet rot to be Pcc. This bacterium is known to be one of the most destructive pathogens with worldwide distribution affecting different plant hosts in the field, and during transit, storage and marketing, thereby leading to much larger economic losses than those caused by other bacterial subspecies (Wells & Moline, Citation1991; Agrios, Citation2006). Although Pcc is a highly successful pathogen of potato, it is also known as an opportunistic pathogen with a wide host range, including tomato, carrot, leafy greens, potato, green pepper, eggplant, garlic, squash and onion. Approximately 64 plant hosts are known to be susceptible to this soft rot disease (Perombelon, Citation2002; Toth et al., Citation2003; Agrios, Citation2006; Garba et al., Citation2014). Latent infection of potato is widespread and the disease develops when host resistance is weakened (Perombelon, Citation2002). The Pcc inoculum is transmitted by water and soil (Helias et al., Citation1998). Potato and tomato production in Mersin province can play an important role for the potential origin of the transmission of Pcc to bananas. Potato is produced on 260 ha with a total 10,580 tonnes, and tomato is produced on 13,246 ha with a total 1,446,691 tonnes in Mersin province (Turkstat, Citation2017).
Although there are reports of Pcc causing soft rot diseases of banana in other parts of the world, this pathogen was not previously known to cause banana wet rot in Turkey, and this is the first report to confirm Pcc as the causal agent. The wet rot disease caused serious losses in Panama, Costa Rica and Guatemala (Wardlaw, Citation1950). A rhizome rot of bananas in Honduras was found to be caused by P. carotovorum (Stover, Citation1959). Bacterial soft rot of banana plants caused by Pcc was detected for the first time at Rosh Hanikra in 1965 and then was reported in other parts of Israel (Zutra & Volcani, Citation1971). ‘Tip-over’ or ‘bacterial head rot’ caused by Pcc was reported in a small piece of land with alluvial soil located close to Yamuna river for the first time on variety Basarai banana in 1968 from India (Edward et al., Citation1973). Erwinia carotovora was isolated from a watery rot of pseudo-stem of plantain in Venezuela (Ordosgoitty et al., Citation1974). A corm rot in banana caused by Pcc was reported in Cuba (Rivera Docando, Citation1978). Soft rot of the rhizome and pseudo-stem of banana (Musa accuminata) caused by Pcc was reported from Brazil (Periara & Nunes, Citation1988). Several researchers in the past have attributed this disease to three Pectobacterium spp., which include Dickeya chrysanthemi (Chattopadhyay & Mukherjee, Citation1986); P. atrosepticum and Pcc (Dickey & Victoria, Citation1980; Choi et al., Citation1988). Reports by Edward et al. (Citation1973) and Lakshmanan & Mohan (Citation1986) from India have indicated the causal agent of banana soft rot to be Pcc, which is in conformity with the findings of this study. Hassanzadeh (Citation1990) reported 10 strains of P. carotovorum isolated from infected banana plants from different locations in Chabahar, Iran, and compared the isolates with those from rotted cucumber fruit. After pathogenicity and biochemical tests, it was concluded that these isolates were an intermediate between D. chrysanthemi and P. carotovorum. In the Republic of Korea, Pcc strains were found to be the most prevalent pathogen associated with different plant hosts in different geographic locations, and were considered the most problematic bacteria causing soft rot diseases (Kang et al., Citation2003). Choi et al. (Citation1988) in their study of seven bacteria that caused banana soft rot identified four isolates as P. cichorii and three isolates as Pcc pathogens.
Pcc enters plant tissues via natural openings and produces tissue maceration enzymes, such as cellulase and pectinase, which degrade hemicelluloses and cellulose, resulting in the decay of organs and tissues (Oladoye et al., Citation2013). High relative humidity and temperatures of ~30°C increase the rate of tissue decay (Bhat et al., Citation2010). The Pcc pathogen is known to thrive well at temperatures between 27–30°C (Perombelon, Citation2002).
Pcc has a wide host range and therefore requires efficient and sensitive identification and detection methods. Several independent studies on the detection of Pectobacterium sp. using PCR have been published. The intergenic transcribed spacer (ITS) region of Pectobacterium strains that cause soft rot diseases of various plants was analysed as a means of strain identification (Toth et al., Citation2001). Randomly amplified polymorphic DNA PCR (RAPD-PCR) (Parent et al., Citation1996), amplified fragment length polymorphism (AFLP) (Avrova et al., Citation2002) and whole-cell fatty acid analysis (Persson & Sletten, Citation1995) have also been used for the identification of Pcc. Specific DNA primers for single and simultaneous detection of Pectobacterium species and subspecies have also been reported (Helias et al., Citation1998; Kang et al., Citation2003).
There are several other reports of specific detection of Pcc using DNA hybridization and serological techniques (Klopmeyer & Kelman, Citation1988; Ward & De Boer, Citation1990). However, detection of Pectobacterium at the species level has mainly focused on P. atrosepticum and the use of primers designed based on the pectate lyase gene (pel) (Darrasse et al., Citation1994; Ward & De Boer, Citation1994). However, the detection of P. carotovorum at strain and subspecies levels using PCR techniques, including nested PCR, PCR-RFLP analysis, sequencing of housekeeping genes, complete genome sequences has been recently reported (Fleischmann et al., Citation1995; Maiden et al., Citation1998; Hyman et al., Citation2000; Toth et al., Citation2001; Kang et al., Citation2003).
The high sensitivity of the nested PCR allowed accurate detection and identification of the causal agent of banana wet rot, which can provide useful information for control measures for this new threat to banana production in Turkey. Our findings are confirmed by Kang et al. (Citation1998, Citation2003) in their studies on the sensitive detection of Pcc using the same primers developed from URP-PCR profiling-derived polymorphic fragments. A similar study was conducted by Phokum et al. (Citation2006), who carried out a genomic analysis of P. carotovorum isolated from 10 different vegetable cultivars using a fragment amplified from the pel gene of this pathogen, to classify all the isolates as soft rot bacteria belonging to the Pectobacterium genus. The phylogenetic analysis of the isolates also showed a high evolutionary relationship with those strains recovered from the NCBI GenBank database, thereby confirming the true identity of the putative pathogen as Pcc. The Erwinia genus has recently been separated into three phyletic clusters, and Erwinia carotovora subspecies were categorized into the Pectobacterium genus (Hauben et al., Citation1998).
To ensure rapid identification and detection of Pcc, and to facilitate management decisions in practice, the isolates were subjected and confirmed by real-time PCR using our previously designed primers and an LNA probe specific to the Pcc pathogen (Baki & Basım, Citation2016), which confirmed Pcc as the causal agent of the banana wet rot collected from different greenhouses in the Mersin province of Turkey.
Real-time PCR is a highly sensitive system for the detection and identification of a wide range of pathogens, including different plant pathogens of interest (Schaad et al., Citation2003; Alvarez, Citation2004; Gitaitis & Walcott, Citation2007). Real-time PCR is capable of detecting bacterial pathogens directly from infected environmental samples (McManus & Jones, Citation1995; Kang et al., Citation2003). Similar results were demonstrated in this study, where Pcc was detected directly from infected banana tissue by real-time PCR without bacterial isolation. Real-time PCR has great potential for examining plant-borne inoculum in epidemiological efforts to regulate and track transmission thresholds of plant pathogens as a means of enhancing disease management (Gitaitis & Walcott, Citation2007).
Supplemental Table
Download MS Word (21.3 KB)Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2019.1577302
Additional information
Funding
References
- Agrios GN. 2006. Bacterial soft rots. In: Agrios GN, editor. Plant pathology. San Diego: Academic Press; p. 656–662.
- Aksoy HM, Ozturk M, Aktas A. 2017. First report of Pectobacterium carotovorum subsp. carotovorum causing soft rot on white head cabbage in Turkey. J Plant Pathol. 99:810.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215:403–410.
- Alvarez AM. 2004. Integrated approaches for detection of plant pathogenic bacteria and diagnosis of bacterial diseases. Annu Rev Phytopathol. 42:339–366.
- Avrova AO, Hyman LJ, Toth RL, Toth IK. 2002. Application of amplified fragment length polymorphism fingerprinting for taxonomy and identification of the soft rot bacteria Erwinia carotovora and Erwinia chrysanthemi. Appl Environ Microbiol. 68:1499–1508.
- Baki D, Basım H 2016. Identification and detection of Dickeya chrysanthemi, Pectobacterium carotovorum subsp. carotovorum, Pseudomonas cichorii, Pseudomonas corrugata, Pseudomonas fluorescens, Pseudomonas mediterranea and Pseudomonas viridiflava, causal agents of tomato (Solanum lycopersicum L.) pith necrosis disease by Real-Time PCR. 6th Plant Protection Congress with International Participation, (pp. 550). Konya (Turkey).
- Bansal VK, Kharbanda PD, Stringam GR, Thiagarajah MR, Tewari JP. 1994. A comparison of greenhouse and field screening methods for blackleg resistance in doubled haploid lines of Brassica napus. Plant Dis. 78:276–281.
- Bhat R, Rai RV, Karim AA. 2010. Mycotoxins in food and feed: present status and future concerns. Compr Rev Food Sci Food Safety. 9:57–81.
- Chattopadhyay PK, Mukherjee N. 1986. A pseudostem rot of banana due to Erwinia chrysanthemi cv. paradisiaca. Curr Sci. 55:789–790.
- Choi JE, Park JS, Kang HW. 1988. Bacterial soft rot of banana fruit caused by Erwinia carotovora subsp. carotovora and Pseudomonas cichorii. Kor J Plant Pathol. 4:202–206.
- Czajkowski R, Grzegorz G, van der Wolf JM. 2009. Distribution of Dickeya spp. and Pectobacterium carotovorum subsp. carotovorum in naturally infected seed potatoes. Eur J Plant Pathol. 125:263–275.
- Dadaşoğlu F, Kotan R. 2017. Identification and characterization of Pectobacterium carotovorum. J Anim Plant Sci. 27:647–654.
- Darrasse A, Priou S, Kotoujansk A, Bertheau Y. 1994. PCR and restriction fragment length polymorphism of a pel gene as a tool to identify Erwinia carotovora in relation to potato disease. Appl Environ Microbiol. 60:1437–1443.
- Dickey RS, Victoria JI. 1980. Taxonomy and emended description of strains of Erwinia isolated from Musa paradisiaca Linnaeus. Int J Syst Bacteriol. 30:129–134.
- Doyle JJ, Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus. 12:13–15.
- Edward JC, Tripathi SC, Singh KP. 1973. Observations on a tip-over disease of banana in Allahabad. Curr Sci. 42:696–697.
- FAO. 2014. Food and agriculture organization of the united nations. Banana Stat.
- Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb J-F, Dougherty BA, Merrick JM. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 269:496–512.
- Garba H, Muhammad SD, Manga SS 2014. Isolation and identification of bacteria and fungi associated with rots of Citrullus lanatus and Capsicum frutescence in Sokoto markets. International Conference on Advances in Agricultural, Biological & Environmental Sciences (AABES-2014), Dubai (UAE).
- Gardan L, Gouy C, Christen R, Samson R. 2003. Elevation of three subspecies of Pectobacterium carotovorum to species level: pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. Int J Syst Evol Microbiol. 53:381–391.
- Gitaitis R, Walcott R. 2007. The epidemiology and management of seed borne bacterial diseases. Annu Rev Phytopathol. 45:71–97.
- Gold CS, Pinese B, Pena JE. 2002. Pest of banana. In: Pena JE, Sharp JL, Wysocki M, editors. Tropical fruit pests and pollinators: biology, economic importance, natural enemies and control. Wallingford (Oxon, UK): CABI Publishing; p. 13–56.
- Gubbuk H, Pekmezci M. 2001. Determination of genetic variations among banana clones via RAPD markers. Bahçe. 30:71–79.
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98NT. Nucleic Acids Symp Ser. 41:95–98.
- Hassanzadeh N. 1990. Characterization of a new soft rot (Erwinia) to banana in Iran. Iranian J Plant Pathol. 26:5–6.
- Hauben L, Moore ER, Vauterin L, Steenackers M, Mergaert J, Verdonck L, Swings J. 1998. Phylogenetic position of phytopathogens within the Enterobacteriaceae. Syst Appl Microbiol. 21:384–397.
- Helias V, Le Roux AN, Bertheau Y, Andrivon D, Gauther JP, Joan B. 1998. Characterization of Erwinia carotovora subspecies and detection of Erwinia carotovora subsp. atroseptica in potato plants, soil and water extracts with PCR based methods. Eur J Plant Pathol. 104:685–699.
- Hyman LJ, Birch PRJ, Dellagi A, Avrova A, Toth IK. 2000. A competitive PCR-based method for the detection and quantification of Erwinia carotovora subsp. atroseptica on potato tubers. Lett Appl Microbiol. 30:330–335.
- Kang HW, Go SJ, Kwon SW. 1998. Specific detection of Erwinia carotovora subsp. carotovora by DNA probe selected from PCR polymorphic bands. Kor J Plant Pathol. 14:161–170.
- Kang HW, Kwon SW, Go SJ. 2003. PCR-based specific and sensitive detection of Pectobacterium carotovorum spp. carotovorum by primers generated from URP-PCR fingerprinting-derived polymorphic band. Plant Pathol. 52:127–133.
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16:111–120.
- Klopmeyer MJ, Kelman A. 1988. Use of monoclonal antibodies specific for pectate lyase as serological probes in the identification of soft rot Erwinia spp. Phytopathol. 78:1430–1434.
- Kumar S, Stecher G, Tamura K. 2016. Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Lakshmanan P, Mohan S. 1986. Control of tip over disease of Cavendish banana. South Indian Hort. 34:336–337.
- Madden LV, Hughes G, Van den Bosch F. 2007. The study of plant disease epidemics. St. Paul (Minnesota): The American Phytopathological Society, APS Press; p. 421.
- Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, et al. 1998. Multilocus sequence typing: a potable approach to the identification of clones within populations of pathogenic microorganisms. Proc Nat Acad Sci USA. 95:3140–3145.
- McManus PS, Jones AL. 1995. Detection of Erwinia amylovora by nested PCR and PCR-dot-blot and reverse-blot hybridization. Phytopathol. 85:618–623.
- Mikicinski A, Sobiczewski P, Sulikowska M, Puławska J, Treder J. 2010. Pectolytic bacteria associated with soft rot of calla lily (Zantedeschia spp.) tubers. J Phytopathol. 158:201–210.
- Oladoye CO, Olaoye OA, Cornnerton IF. 2013. Isolation and identification of bacteria associated with spoilage of sweet potatoes during postharvest storage. Int Agric Food Sci. 3:10–15.
- Ordosgoitty FA, Santos PR, Haddad GO. 1974. Watery rot of the pseudostem of plantain and its presence in three plantain growing regions of Venezuela. Agron Trophic. 24:247–258.
- Parent JG, Lacroix M, Page D, Vezina L, Vegiard S. 1996. Identification of Erwinia carotovora from soft rot diseased plants by random amplified polymorphic DNA (RAPD) analysis. Plant Dis. 80:494–499.
- Periara LV, Nunes RAS. 1988. Soft rot of the rhizome and pseudostem of banana (Musa accuminata L.). Fitopathol Brasil. 13:70–71.
- Perombelon MCM. 2002. Potato diseases caused by soft rot Erwinia: an overview of pathogenesis. Plant Pathol. 51:1–12.
- Persson P, Sletten A. 1995. Fatty acid analysis for the identification of Erwinia carotovora subsp. atroseptica and Erwinia carotovora subsp. carotovora. Bulletin OEPP/EPPO. 25:151–156.
- Phokum C, Jitareerat P, Photchanachai S, Cheevadhanarak S. 2006. Detection and classification of soft rot Erwinia of vegetables in Thailand by DNA polymerase chain reaction. Acta Hort. 712:917–925.
- Ploetz RC, Thomas JE, Slaubaugh W. 2003. Diseases of banana and plantain. In: Ploetz RC, editor. Diseases of tropical fruit crops. Wallingford (Oxon, UK): CABI Publishing; p. 73–134.
- Rahmanifar B, Hasanzadeh N, Razmi J, Ghasemi A. 2012. Genetic diversity of Iranian potato soft rot bacteria based on polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis. African J Biotech. 11:1314–1320.
- Rivera Docando N. 1978. Comparative study of two new bacterial diseases in banana growing areas of Cuba. Agrotech Cuba. 10:35–44.
- Schaad NW, Frederick RD, Shaw J, Schneider WL, Hickson R, Petrillo MD, Luster DG. 2003. Advances in molecular-based diagnostics in meeting crop biosecurity and phytosanitary issues. Annu Rev Phytopathol. 41:305–324.
- Schaad NW, Jones JB, Chun W. 2001. Laboratory guide for identification of plant pathogenic bacteria. 3rd ed. St. Paul: APS Press; p. 373.
- Stover RH. 1959. Bacterial rhizome rot of bananas. Phytopathol. 49:290–292.
- Toth IK, Avrova AO, Hyman LJ. 2001. Rapid identification and differentiation of the soft rot Erwinias using 16S-23S intergenic transcribed spacer (ITS) - PCR and RFLP analyses. Appl Environ Microbiol. 67:4070–4076.
- Toth IK, Bell KS, Holeva MC, Birch PRJ. 2003. Soft-rot Erwiniae: from genes to genomes. Mol Plant Pathol. 4:17–30.
- Turkstat. 2016. Turkish statistical institute, Banana statistics. [ accessed 2017 Feb 10]. http://www.turkstat.gov.tr/PreTablo.do?alt_id=1001
- Turkstat. 2017. Turkish statistical institute, Banana statistics. [ accessed 2017 Feb 10]. http://biruni.tuik.gov.tr/medas/?kn=92&locale=tr
- Ustun N, Arslan N. 2016. Bacterial stem rot of globe artichoke (Cynara cardunculus var. scolymus) caused by Pectobacterıum carotovorum subsp. carotovorum in Turkey. J Plant Pathol. 98:91–96.
- van der Wolf JM, Nijhuis EH, Kowalewska MJ, Saddler GS, Parkinson N, Elphinstone JG, Pritchard L, Toth IK, Lojkowska E, Potrykus M, et al. 2013. Dickeya solani sp. nov., a pectinolytic plant pathogenic bacterium isolated from potato (Solanum tuberosum). Int J Syst Evol Microbiol. 64:768–774.
- Ward LJ, De Boer SH. 1990. A DNA probe specific for serologically diverse strains of Erwinia carotovora. Phytopathol. 80:665–669.
- Ward LJ, De Boer SH. 1994. Specific detection of Erwinia carotovora subsp. atroseptica with a digoxigenin-labeled DNA probe. Phytopathol. 84:180–186.
- Wardlaw CW. 1950. Banana diseases. VIII, Notes on the various diseases occurring Trinidad. Trop Agric. 11:143–149.
- Weisburg WG, Barns SM, Pelletier DA, Jane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173:697–703.
- Wells JM, Moline HE. 1991. Differentiation of the soft rot Erwinias (the carotovora group) by fatty acid composition. J Phytopathol. 131:22–32.
- Zutra D, Volcani Z. 1971. Bacterial soft rot and wilt of banana plants. Rev Plant Pathol. 50:279.