?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Wheat stem base disease (WSD) is an important disease complex which can be caused by various pathogens with variable life cycles, ecological requirements and sensitivities to fungicides. Agronomic practices are considered important tools to control this disease complex and influence the spectrum of fungal communities in infected wheat stems. The aim of the present study was to determine the impact of crop rotation schemes and soil tillage methods on the development of WSD and to elucidate the spectrum of causal agents of this disease. The development of WSD was assessed in a two-factorial (soil tillage practice and crop rotation) experiment. The incidence of WSD was evaluated at the BBCH growth stages 83–85. Causal agents of WSD and other fungi were identified using molecular methods. The soil tillage method did not influence the development of WSD, but the impact of crop rotation was significant, and the cropping system where oilseed rape, barley and faba beans were included decreased disease levels. Members of the genera Oculimacula and Fusarium were the most prevalent fungi associated with WSD. Moreover, fungi from other taxonomic groups were detected, with Phaeosphaeria spp. being prevalent. An increasing occurrence of Microdochium spp. was also observed. In conclusion, agronomic practices influenced the level of WSD, but no distinct impact on the fungal spectrum was identified. Further investigation is required to clarify the roles that pathogens and fungi from other taxonomic groups play in the fungal–wheat relationship.
Résumé
La maladie qui s’attaque à la tige du blé (MBT) résulte d’un important ensemble de maladies qui peuvent être causées par divers agents pathogènes dont les cycles de vie, les exigences écologiques et les sensibilités aux fongicides varient. Les pratiques agronomiques sont considérées comme des outils essentiels pour combattre cet ensemble de maladies et agir sur la gamme de communautés fongiques qui infectent les tiges du blé. Le but de cette étude était de déterminer l’influence de la rotation des cultures et du travail des sols sur le développement de la MBT et d’en élucider la gamme d’agents causaux. Le développement de la MBT a été évalué selon un plan expérimental à deux facteurs (travail des sols et rotation des cultures). L’incidence de la MBT a été évaluée aux stades de croissance BBCH 83–85. Les agents causaux de la MBT ainsi que d’autres champignons ont été identifiés à l’aide de diverses méthodes moléculaires. Le travail des sols n’a pas influencé le développement de la MBT, mais les effets de la rotation des cultures étaient notables, et le système cultural combinant colza oléagineux, orge et féverole a réduit le taux d’incidence de la maladie. Les champignons des genres Oculimacula et Fusarium étaient les plus couramment associés à la MBT. En outre, des champignons appartenant à d’autres groupes taxinomiques ont été détectés, Phaeosphaeria spp. étant les plus répandus. On a également observé une occurrence accrue de Microdochium spp. Finalement, les pratiques agronomiques ont influencé le taux d’incidence de la MBT, mais on n’a observé aucune portée particulière sur la gamme des communautés fongiques. Des recherches supplémentaires sont nécessaires pour préciser les rôles que jouent les agents pathogènes et les champignons des autres groupes taxinomiques dans la relation champignon-blé.
Introduction
Wheat stem base disease (WSD) is an important disease complex, caused by various pathogens that are characterized by variable life cycles, ecological requirements and fungicide sensitivities. The disease is difficult to control with fungicides, and most associated research has been devoted to particular pathogens and their interactions with wheat and the environment. The situation in the field is more complex, however, because a single plant can be affected by more than one pathogen.
Agronomic practices (appropriate soil tillage methods and crop rotation) have been described as important tools that can reduce the impact of WSD. However, the results from trials conducted in different countries have provided contradictory results. Generally, reduced soil tillage is considered an increasing factor for the development of Fusarium foot rot, which is caused by Fusarium spp. (especially F. culmorum and F. graminearum). Nevertheless, Sharma-Poudyal et al. (Citation2017) did not identify significant differences between the amounts of Fusarium spp. in conventionally tilled fields and non-tilled fields. This result likely was related to different species compositions and variable agrometeorological conditions. For example, the inoculum density of F. culmorum was greater in a non-till cropping system during wet years, but the opposite results were obtained during drier years (Smiley et al. Citation2016).
The market situation has influenced the diversity of crops in Latvia, and short rotations (only wheat and oilseed rape) have become popular during the last 20 years. Unfortunately, these cropping systems promote the development of stem base diseases. The results of a study conducted by Wenda-Piesik et al. (Citation2016) confirmed the effect of crop rotation with wheat as a pre-crop on the development of stem base disease, because wheat increased the occurrence of take-all and Fusarium foot rot. Incidences of wheat eyespot and Fusarium foot rot were significantly higher in cropping systems where wheat was cultivated every second year compared with crop rotations where the proportion of wheat was 33% or less (Winter et al. Citation2014). In general, Oculimacula spp. were found more frequently in wheat monocultures, but some exceptions (particular years and varieties) were observed (Palicová et al. Citation2018). Nevertheless, there are also contrary findings, and Bainard et al. (Citation2017) revealed that the use of pulses as pre-crops significantly increased the level of F. avenaceum, which is one of the most widespread causal agents of wheat stem base diseases. Consequently, classical cropping systems with crop rotations do not provide sufficient control of this group of diseases.
The impact of cropping systems on the development of pathogens is complex and poorly understood. The findings of Sommermann et al. (Citation2018) indicated that the soil mycobiome (including pathogens) of wheat fields was dependent on the pre-crop but the influence of soil tillage was only marginal. Smiley et al. (Citation2016) highlighted the importance of long-term experiments, because the results differed depending on the duration of the experiments. In Latvia, the development of wheat crown rot in relation to crop rotation and soil tillage methods has been studied since 2009. Previous investigations confirmed that non-cereal pre-crops significantly decreased crown rot levels in wheat, whereas the impact of the soil tillage method depended on the year (Bankina et al. Citation2013; Paulovska et al. Citation2017). The aim of the present study was to examine the impact of varying crop rotation schemes (continuous wheat; wheat and oilseed rape; oilseed rape, barley, faba beans, wheat) and soil tillage methods on the development of WSD in winter wheat and to clarify the spectrum of the causal agents of this disease in Latvia.
Materials and methods
Design of the experiment
This study is the continuation of previous investigations conducted in association with a broad many-sided field experiment that was established at the Study and Research Farm ‘Pēterlauki’ of the Latvia University of Life Sciences and Technologies (LLU) (56° 30.658°N, 23° 41.580°E) in the autumn of 2008. The soil was fertile and appropriate for wheat cultivation, and it was characterized as follows: cambic calcisol, silty clay loam, and neutral (pH 7.3) with high phosphorus and potassium contents (148 and 295 mg kg−1, respectively).
The development of winter wheat crown rot was assessed using the following two-factorial (soil tillage method and crop rotation) experiment: (A) soil tillage system (A1, traditional soil tillage with ploughing at a depth of 22–24 cm (TT); A2, reduced soil tillage with disc harrowing up to a depth of 10 cm (RT)); (B) crop rotation (B1, continuous wheat (W–W); B2, oilseed rape and wheat (OR–W); B3, crop rotation (CR) where oilseed rape, barley and faba beans were included). Trials were arranged using a split-plot design with two replications (area of each plot = 0.25 ha; altogether 24 plots), and four sub-replications (each replication was divided into four sections) were established within each plot. The main factor was soil tillage; subfactor – crop rotation. There were 12 wheat plots every year. In the present study, data obtained between 2012 and 2017 were analysed. Data from 2014 were excluded because the plots were re-sown with spring wheat because of the exceptionally poor overwintering of winter wheat.
The ‘Zentos’ cultivar was used each year, and agronomic measures were applied uniformly based on the agronomic practice requirements associated with intensive wheat production conditions. Seeds were treated with fungicides (75 g L−1 fludioxonil and 25 g L−1 cyproconazole; dose of 1.5 L t−1). Fungicides used to treat stem base diseases were not used, but foliar fungicides (84 g L−1 epoxiconazole and 150 g L−1 fenpropimorph; dose of 1.5 L ha−1) were sprayed across all wheat plots at the heading stage (BBCH 55–59) each year. The incidence of WSD was evaluated shortly before harvesting at the 83–85 BBCH growth stage. Plants were collected from two adjacent rows (each 5 cm long) of each replicate in five randomly chosen places, representing ~300 stems from each wheat plot. The disease was assessed visually, and the incidence (%) of crown rot was then calculated.
Assessment of disease and the determination of causal agents
From each plot, a total of 100 randomly chosen stems with symptoms of wheat crown rot were prepared for the identification of disease causal agents. The areas of infected wheat stem bases (~2 mm long) were surface sterilized with a 1% solution of sodium hypochlorite for 3 min, rinsed three times in sterile distilled water, and placed onto potato-dextrose agar (PDA) enriched with streptomycin (100 ppm L−1) and penicillin (100 ppm L−1). Plates were incubated at 20°C for seven days, and the fungi from each colony were then transferred to pure PDA plates before isolates were obtained. The isolates were divided into morphologically identical groups (i.e. colour and texture of mycelium, pigmentation of medium, and morphological characteristics of spores), and two samples from each group were analysed using molecular genetic methods.
All DNA extractions as well as sequencing analyses of internal transcribed spacer (ITS) and translation elongation factor 1-alpha (TEF) gene regions were performed as previously described in Bankina et al. (Citation2013, Citation2017). Briefly, samples were homogenized, and the supernatant phase was treated with phenol and chloroform. DNA purification was conducted using a NucleoMag 96 Plant Kit (Macherey-Nagel, Germany). ITS and TEF regions were amplified using Phire Plant Direct PCR Master Mix (Thermo Fisher Scientific, USA) and ITS4, ITS5, EF1T-Fw and EF2T-Rs primers. The resulting fragments were treated enzymatically with exonuclease I (Thermo Fisher Scientific, USA) and shrimp alkaline phosphatase (Thermo Fisher Scientific, USA), and sequencing products were generated using BigDye Terminator v3.1 Cycle Sequencing Reaction Mixture (Applied Biosystems, USA). The sequencing products were subsequently analysed on a 3130XL Genetic Analyzer (Applied Biosystems, USA).
The relative density, which indicates the percentage of a particular species/genera out of all isolates, was calculated for all fungal genera/species. The influence of factors (i.e. year, crop rotation and soil tillage) associated with crown rot was evaluated using the following model:
where Yijkl represents the number of observations of dependent variable ‘crown rot’ incidence (%); YRi represents the fixed factor ‘year’ (I = 1…5); CRj represents the fixed factor ‘crop rotation’ (j = 1…3); STk represents the fixed factor ‘soil tillage’ (k = 1…2); CR × STjk represents the interaction effect of crop rotation and soil tillage; and eijkl represents the model error. Factor means were compared using the least significant difference (LSD) test. Regarding statistical significance, alpha levels of 0.1 and 0.05 were used, and data analyses were performed with SPSS15.
Meteorological conditions
Between 2012 and 2017, the average annual growing season temperature varied from 13°C to 16.5°C. In 2012, the vegetation season began in the middle of April, and it was characterized by a relatively cool May–June with generous precipitation throughout the entire season. In May 2013, the weather was warm, and the entire 2013 growing season was the warmest and driest observed during the study period. From April to August 2013, there were only five to nine rainy days per month. April 2015 was cool, and abundant precipitation continued throughout May. Between April and August 2015, the weather on average was as dry as that of the same period in 2013, but the 2013 period was warmer. Moreover, the number of rainy days per month varied between 12 in August to 31 in May 2015. The growing period in 2016 began with rainy and cool weather in April, followed by warm and arid weather in May. There were 18 rainy days in April, but only four rainy days were recorded in May. In June, July and August, there were 3, 16 and 20 rainy days, respectively, and this season was noted as the second warmest and rainiest observed during the study period. The vegetation in 2017 experienced late recovery because of the cold April and dry May. During the growing season in 2017, the number of rainy days varied from seven days in both May and August to 18 days in July, representing the coolest and one of the driest seasons recorded during the entire observation period.
Results
Development of disease depending on year, soil tillage method and crop rotation
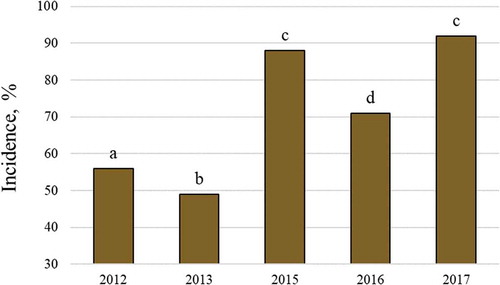
The incidence of WSD was influenced significantly by yearly agroecological conditions (P < 0.001) (). The highest disease levels were observed in 2015 and 2017, and the lowest level was noted in 2013.
Fig. 1 (Colour online) Yearly incidence of wheat stem base disease. Different letters denote statistical differences at α = 0.05.
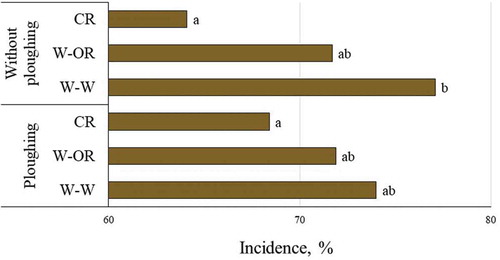
The soil tillage method did not influence the development of stem base rot, but the impact of crop rotation was significant (P = 0.006). Continuous wheat sowings promoted disease development. Short crop rotation did not decrease stem base rot when only wheat and oilseed rape were included, compared with a full crop rotation (oilseed rape, barley, faba beans and wheat). Although the interaction between crop rotation and soil tillage method was not significant, the importance of crop rotation was expressed more clearly under reduced soil tillage conditions (). Crop rotation decreased the level of WSD regardless of the soil tillage method, but incidence of WSD was significantly higher in continuous wheat sowings only under reduced soil tillage conditions, compared with the cropping system where oilseed rape was included.
Fig. 2 (Colour online) Incidence of wheat stem base disease with regard to crop sequence and soil tillage conditions (2012–2017 average, with the exception of 2014). W-W, continuous wheat; OR-W, oilseed rape, wheat, wheat; CR, oilseed rape, barley, faba beans and wheat. Different letters denote statistical differences at α = 0.05.
Fungal spectrum in the infected wheat stems
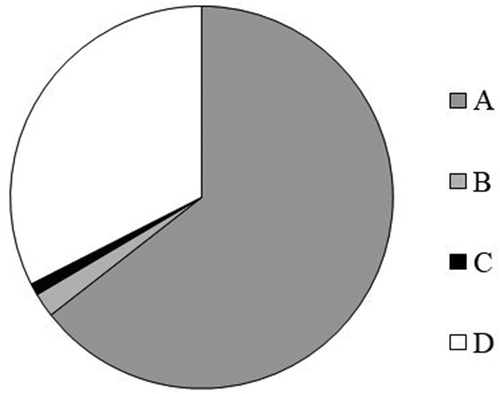
During the experiment, a total of 4881 fungal isolates were obtained and identified at the genus or species level. No distinct impacts of agronomic practices on the fungal spectrum were identified. The majority of the isolates were recognized as causal agents of WSD (), while other isolates belonged to other fungal guilds (i.e., functional groups).
Fig. 3 Guilds of fungi isolated with visible symptoms of stem base disease in wheat. A, causal agents of stem base diseases; B, causal agents of other diseases; C, saprotrophs; D, other fungi.
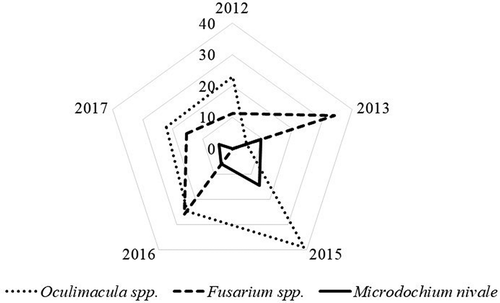
Pyrenophora tritici-repentis (causal agent of tan spot) and Parastagonospora nodorum (causal agent of glume blotch) were found in 2% of cases. A few of the fungi (1%) belonged to the genera Alternaria, Botrytis, Penicillium and Aspergillus, which can live as saprotrophs in plants. Endophytes and some fungi that occur incidentally in wheat plants represented 35% of all isolates. Fungi from the genus Phaeosphaeria (likely P. pontiformis) were the most common. Other genera, including Chaetomium, Nigrospora, Arthrinium and Peniophora, were found only in some cases. Oculimacula spp. and Fusarium spp. were among the fungi considered causal agents of SWD, and Microdochium nivale was also frequently found (). Other causal agents were only occasionally identified, and Bipolaris sorokiniana was the most widespread in this group. Pythium spp. and Rhizoctonia solani were rarer and were detected only in some samples. The dominant species of Fusarium were F. avenaceum (36%), F. culmorum (24%) and F. tricinctum (19%). Fusarium graminearum was rarely detected, and F. oxysporum, F. acuminatum, F. sporotrichioides and F. equiseti were detected only in some cases.
Discussion
Market situations and economic considerations often determine the composition of cropping systems. Wheat, especially winter wheat, is one of the most important crops in Latvia, where continuous wheat sowing or short crop rotations (only wheat and oilseed rape) are becoming increasingly popular. A similar situation has been observed in other parts of Europe, for example, crop rotations that included not only cereals and maize but also oilseed rape and sugar beet represented only 10% of arable land in Germany (Steinmann and Dobers Citation2013).
Concerning the development of WSD and agronomic practices, contrasting results have been found. In general, the impact of agronomic practices has been evaluated in relation to particular pathogens. However, the assessment process is complicated, because disease symptoms can be atypical. Palicová et al. (Citation2018) noted variance in symptoms depending on different conditions, including the level of cultivar resistance. Our previous investigations indicated that it was impossible to separately assess the diseases caused by particular pathogens (Bankina et al. Citation2013), so we evaluated the incidence of WSD as a disease complex.
A high incidence of WSD has been observed in different wheat production regions. For instance, Moya-Elizondo et al. (Citation2015) reported a 72.2% incidence of WSD in Chile. In our investigations, disease incidence varied significantly between years, but it was generally high. It is not possible to find precise reasons for different disease levels depending on the year, but some regularities have been found. In the present study, the highest levels of WSD were observed in 2015 and 2017, when the greatest number of rainy days were recorded. Nonetheless, the amount of total precipitation was similar during all years with the exception of 2012.
Ploughing did not decrease the incidence of WSD in this study. Our previous investigations showed only minor impacts of soil tillage on the disease progress (Bankina et al. Citation2013), but, generally, the influence of soil tillage system was dependent on the pathogen spectrum. According to Matusinsky et al. (Citation2009), the soil tillage method did not influence the incidence of stem base disease caused by Oculimacula spp., and Majchrzak et al. (Citation2016) did not find a decrease in Oculimacula spp. in ploughed fields. Similar results were found with regard to Fusarium spp. (Majchrzak et al. Citation2016). Annual ploughing can bury plant debris and reduce the amount of inoculum, but ploughing can also return partially decomposed debris buried during previous years to the soil surface (Jenkyn et al. Citation2010).
There is no doubt that crop rotation influences the fungal communities in soil and wheat roots (including causal agents of stem base diseases); however, this impact is contradictory. Although crop rotation has been described as an effective tool to control diseases, Colbach et al. (Citation1996) did not observe crop rotation effects on the severity of foot rot: the incidence of disease was not dependent on the proportion of wheat in crop rotation, and no difference existed between the 2nd, 3rd, 4th and 10th wheat crop. The utility of crop rotation also depends on the pathogen spectrum, which differs significantly depending on the region, year, meteorological conditions and particular field. Wenda-Piesik et al. (Citation2016) reported that Gaeumannomyces graminis and Fusarium spp. were the dominant pathogens in Poland, but G. graminis was seldom identified in our investigation. The occurrence of Fusarium spp. was not dependent on crop succession in France (Colbach et al. Citation1996), and similar findings were later confirmed. Specifically, crop rotation only slightly reduced F. culmorum infections, while other factors (e.g. rate of nitrogen fertilization and meteorological conditions) mitigated the impact of pre-crops (Davis et al. Citation2009). Therefore, the effects of continuous wheat sowings or short crop rotations can be mitigated by ploughing, or the effects of the soil tillage method can be reduced by crop rotation. Moreover, in our study, the effects of crop rotation were clearer in fields with reduced tillage.
Wheat crown rot can be caused by different pathogens, and a complex infection can occur in a single plant, making it difficult or even impossible to visually distinguish the causal agents. Mycological and molecular genetic methods were used to identify pathogens and other fungi in this study. Typical causal agents of stem base diseases represented 64.4% of all isolates, and smaller amounts of other pathogens (e.g. P. tritici-repentis) were detected. Furthermore, an unexpectedly high proportion of fungi from different ecological niches were isolated from wheat stems with root rot symptoms. Similar results were obtained in studies conducted in Canada, with fungi from the same genera (Alternaria, Acremonium, Arthrinium, Nigrospora and Phaeosphaeria) detected (Fernandez et al. Citation2014). Phaeosphaeria (most likely P. pontiformis) was the dominant fungus among non-pathogenic fungi, and Moya-Elizondo et al. (Citation2011, Citation2015) frequently detected P. pontiformis in infected wheat stems. There are very few reports regarding this fungus, and it is commonly described as an endophyte. Therefore, further studies are required to clarify the interactions between this fungus, wheat, and causal agents of stem base diseases.
Fungi from the genera Oculimacula and Fusarium were the main causal agents of WSD that were detected not only in our study but also in experiments conducted in Poland and Germany (Gala et al. Citation2014; Winter et al. Citation2014). Investigations in Lithuania supported the harmful nature of Oculimacula spp., in that a significant reduction in the number of grains per ear and in grain weight per ear were observed (Ramanauskiené et al. Citation2014). Our study demonstrated that the proportions of Fusarium spp. and Oculimacula spp. depended on the year, and this could be explained by the meteorological conditions. No specific agronomic measures that might have influenced the development of either pathogen were found. For instance, the cool and wet spring of 2015 could have promoted the development of Oculimacula spp., and a similar conclusion was made by Matusinsky et al. (Citation2009). In our study, the highest occurrence of Oculimacula spp. was regularly found during years with the lowest occurrence of Fusarium spp. This observation is in agreement with the findings of Colbach et al. (Citation1996) and Gala et al. (Citation2014), who also noted a negative relationship between the occurrence of Fusarium spp. and Pseudocercosporella herpotrichoides (previous name of Oculimacula spp.).
During the first years of their study, Bankina et al. (Citation2013) rarely detected Oculimacula spp., so the importance of this pathogen was revealed only over a longer period. Both species of Oculimacula (O. yallundae and O. pontiformis) were also found in Latvia. Our results suggest that O. yallundae is a dominant species, but a precise proportion of both species has not yet been established. Therefore, the detection of species proportions should be addressed in future studies. Both species have similar life cycles, cause identical symptoms, and can coexist on the same plant, but their sensitivities to the fungicidal active ingredients differ. This result also was observed in other studies (Palicová et al. Citation2018; Ramanauskiené and Gaurilčikiené Citation2016; Ramanauskiené et al. Citation2016), so additional research is essential.
Fusarium spp. are an important group of pathogens that are causal agents of head scab, and the species are involved in the production of mycotoxins. The spectrum of Fusarium species differs depending on the region and the crop rotation scheme. Fusarium avenaceum and F. culmorum were the most widespread in our study, and this result was independent of crop rotation. Fusarium spp. were also identified as prevalent pathogens in a study conducted in Chile, and the dominant species were the same as those identified in Latvia – F. culmorum and F. avenaceum (Moya-Elizondo et al. Citation2015). Gebremariam et al. (Citation2018) reported that F. culmorum was one of the most widespread Fusarium species that acted as a causal agent of crown rot in Turkey. Furthermore, pathogenicity tests of different Fusarium species indicated that more severe disease symptoms were caused by F. culmorum, and this species was also dominant in Canada (Fernandez et al. Citation2014). Although F. culmorum has been reported commonly as a dominant pathogen in cool-climate regions, the latest studies also examined the prevalence of this fungus in Sardinia (Balmas et al. Citation2015); therefore, our previous assumptions about relationships between temperatures and the incidence of Fusarium species should be revised. Recent findings (Beccari et al. Citation2018) revealed the role of F. culmorum as an additional source of mycotoxin accumulation in grains, even if it had not yet reached ear tissues. It is interesting to note that F. graminearum has only been found in some cases in Germany (Tillmann et al. Citation2017). The same situation was also observed in Latvia in that F. graminearum was detected only occasionally, even with continuous wheat sowings, and no differences among different cropping systems were detected.
The incidence of Microdochium spp. has recently increased. Microdochium nivale is a typical causal agent of snow mould, but it has now been recognized as a pathogen of stems and leaves. A high prevalence of M. nivale has also been recognized in the Czech Republic (Matušinsky et al. Citation2016). In Lithuania, Jonavičiene et al. (Citation2016) highlighted the importance of another species, M. majus. We assumed the occurrence of both species, but a more precise identification is required.
Microdochium bolleyi was isolated relatively frequently, and its prevalence could have been enhanced by the soil type (clay loam) in accordance with the findings of Sieber and Grunig (Citation2006). Although M. bolleyi has been found in soil (Sharma-Poudyal et al. Citation2017), wheat rhizosphere (Navarro-Borrell et al. Citation2017) and infected wheat stems (Vujanovic et al. Citation2012; Fernandez et al. Citation2014), it is not commonly considered to be a pathogen of wheat. In contrast, several researchers characterized M. bolleyi as a ‘minor’ pathogen, because it does not seriously affect the development of plants (Sturz and Bernier Citation1989; Sharma-Poudyal et al. Citation2017). Furthermore, other researchers described this fungus as an effective antagonist to Septoria nodorum (current name Parastagonospora nodorum), Fusarium spp. and Gaeumannomyces graminis (Sieber and Grunig Citation2006). Further investigations are necessary to clarify the role of this fungus in the fungal–wheat plant relationship.
We did not find clear patterns between the fungal spectrum and cropping system. Moreover, different literature sources reported different results, which were sometimes contradictory. The results of several studies have supported the ability of Fusarium spp. to infect different hosts, and this explains the lack of correlation between cropping systems and of the occurrence of these species. The highest frequency of colonization by Fusarium spp. was found in wheat cultivated after sugar beets, but it was not observed in wheat planted after wheat (Tillmann et al. Citation2017). Fusarium tricinctum was frequently found in our investigations, independently of the cropping system, and Navarro-Borrell et al. (Citation2017) found this species in pulses but not in wheat. Recent investigations demonstrated that F. graminearum can survive in different hosts, and this fungus has been isolated from different weeds collected in fields with different crop rotations (Postic et al. Citation2012; Sneideris et al. Citation2018). These results raise doubt regarding the efficacy of crop rotation as a control method for WSD.
No significant impact of soil tillage on the soil fungal community has been found (Navarro-Borrell et al. Citation2017; Schlatter et al. Citation2017). Similarly, the soil tillage system did not influence the development of Fusarium spp. and Oculimacula spp. in trials performed in Poland (Majchrzak et al. Citation2016). Therefore, it is difficult to find any trends in the occurrence of different soil-borne fungi, likely because of their ability to adapt to changes in meteorological conditions (Moya-Elizondo et al. Citation2015). The level of WSD and the spectrum of causal agents depend on different factors, including meteorological conditions and crop rotation. Nonetheless, further investigations are required to better understand this complicated interaction among plants, fungi and weather.
Acknowledgements
The research was supported by the state research programme 'Agricultural Resources for Sustainable Production of Qualitative and Healthy Foods in Latvia', project No. 1 “SOIL”.
Additional information
Funding
References
- Bainard LD, Navarro-Borrell A, Hamel C, Braun K, Hanson K, Gan Y. 2017. Increasing the frequency of pulses in crop rotations reduces soil fungal diversity and increases the proportion of fungal pathotrophs in a semiarid agroecosystems. Agric Ecosyst Environ. 240:206–214.
- Balmas V, Scherm B, Marcello A, Beyer M, Hoffmann L, Migheli Q, Pasquali M. 2015. Fusarium species and chemotypes associated with fusarium head blight and fusarium root rot on wheat in Sardinia. Plant Pathol. 64:972–979.
- Bankina B, Bimšteine G, Neusa-Luca I, Roga A, Fridmanis D. 2017. What influences the composition of fungi in wheat grains? Acta Agrobotanica. 70:1726.
- Bankina B, Bimšteine G, Ruža A, Priekule I, Paura L, Vaivade I, Fridmanis D. 2013. Winter wheat crown and root rot are affected by soil tillage and crop rotation in Latvia. Acta Agric Scand B Soil Plant Sci. 63:723–730.
- Beccari G, Prodi A, Pisi A, Nipoti P, Onofri A, Nicholson P, Pfohl K, Karlovsky P, Gardiner DM, Covarelli L. 2018. Development of three Fusarium crown rot causal agents and systemic translocation of deoxynivalenol following stem base infection of soft wheat. Plant Pathol. 67:1055–1065.
- Colbach N, Maurin N, Huet P. 1996. Influence of cropping system on foot rot of winter wheat in France. Crop Prot. 15:295–305.
- Davis RA, Huggins DR, Cook JR, Paulitz TC. 2009. Nitrogen and crop rotation effects on fusarium crown rot in no-till spring wheat. Can J Plant Pathol. 31:456–467.
- Fernandez MR, Fox SL, Hucl P, Singh AK, Stevenson FC. 2014. Root rot severity and fungal populations in spring common, durum and spelt wheat, and Kamut grown under organic management in western Canada. Can J Plant Sci. 94:937–946.
- Gala D, Gorczyca A, Oleksy A, Kołodziejczyk M. 2014. Stem-base disease in winter durum and common wheat cultivation in the years 2009–2011. J Plant Prot Res. 54:15–21.
- Gebremariam ES, Sharma-Poudyal D, Paulittz TC, Erginbas-Orakci G, Karakaya A, Dababat AA. 2018. Identity and pathogenicity of Fusarium species associated with crown rot on wheat (Triticum spp.) in Turkey. Eur J Plant Pathol. 150:38–399.
- Jenkyn JF, Gutteridge RJ, Bateman GL, Jalaluddin M. 2010. Effects of crop debris and cultivations on the development of eyespot of wheat caused by Oculimacula spp. Ann Appl Biol. 156:387–399.
- Jonavičiene A, Suproniene S, Semaškiene R. 2016. Microdochium nivale and M. majus as causative agents of seedling blight in spring cereals. Zemdirbyste-Agriculture. 103:36–368.
- Majchrzak L, Sawinska Z, Natywa M, Skrzpczak G, Głowicka-Wołoszym R. 2016. Impact of different tillage systems on soil dehydrogenase activity and spring wheat infection. J Agr Sci Tech. 18:1871–1881.
- Matusinsky P, Mikolasova R, Klem K, Spitzer T. 2009. Eyespot infection risks on wheat with respect to climatic conditions and soil management. J Plant Pathol. 91:93–1001.
- Matušinsky P, Svačinová I, Jonavičienè A, Tvůružek L. 2016. Long-term dynamics of causative agents of stem base diseases in winter wheat and reaction of Czech Oculimacula spp. and Microdochium spp. populations to prochloraz. Eur J Plant Pathol. 148:199–206.
- Moya-Elizondo EA, Arismendi N, Paz Castro M, Doussoulin H. 2015. Distribution and prevalence of crown rot pathogens affecting wheat crops in southern Chile. Chil J Agric Res. 75:78–84.
- Moya-Elizondo EA, Rew LJ, Jacobsen BJ, Hogg AC, Dyer AT. 2011. Distribution and prevalence of Fusarium crown rot and common root rot pathogens of wheat in Montana. Plant Dis. 95:1099–1108.
- Navarro-Borrell A, Shi Y, Gan Y, Bainard LD, Germida JJ, Hamel C. 2017. Fungal diversity associated with pulses and its influence on the subsequent wheat crop in the Canadian prairies. Plant Soil. 414:13–31.
- Palicová J, Matušinsky P, Dumalasova V, Hanzalova A, Bižová I. 2018. Resistance of winter wheat cultivars to eyespot and characterisation of causal agents of the disease. Plant Prot Sci. 54:24–30.
- Paulovska L, Bankina B, Roga A, Fridmanis D. 2017. The incidence of wheat crown rot depending on agronomic practices. In: Research for Rural Development –Proceedings of the Annual 23rd International Scientific Conference, Jelgava. p. 13–18.
- Postic J, Cosic J, Vrandecic K, Jurkovic D, Saleh AA, Leslie JF. 2012. Diversity of Fusarium species isolated from weeds and plant debris in Croatia. J Phytopathol. 160:76–81.
- Ramanauskiené J, Gaurilčikiené J. 2016. Incidence of eyespot in winter wheat and quantification of the fungi Oculimacula acuformis and O. yallundae in Lithuania. J Plant Dis Prot. 123:75–81.
- Ramanauskiené J, Gaurilčikiené J, Česnulevičiené R. 2014. Relationship between eyespot severity and productivity of winter wheat in Lithuania. Proc Latvia Univ Agric. 32:41–44.
- Ramanauskiené J, Gaurilčikiené J, Suproniene S. 2016. Effects of fungicides on the occurrence of winter wheat eyespot caused by fungi Oculimacula acuformis and O. yallundae. Crop Prot. 90:90–95.
- Schlatter DC, Schillinger WF, Bary AI, Sharratt B. 2017. Biosolids and conservation tillage: impacts on soil fungal communities in dryland wheat-fallow cropping systems. Soil Biol Biochem. 115:556–567.
- Sharma-Poudyal D, Schlatter D, Yin C, Hulbert S, Paulitz T. 2017. Long-term no-till: a major driver of fungal communities in dryland wheat cropping systems. PLoS One. 12:e0184611.
- Sieber TN, Grunig CR. 2006. Biodiversity of fungal root-endophyte communities and populations, in particular of the dark septate endophyte Phialocephala fortinii s.l. In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes. Soil biology. Vol. 9. Berlin, Heidelberg: Springer; p. 107–132.
- Smiley RW, Machado S, Rhinhart KEL, Reardon CI, Wuesi B. 2016. Rapid quantification of soilborne pathogen communities in wheat-based long-term field experiments. Plant Dis. 100:1692–1708.
- Sneideris D, Ivanauskas A, Suproniene S, Kadziene G, Sakalauskas S. 2018. Genetic diversity of Fusarium graminearum isolated from weeds. Eur J Plant Pathol. doi:10.1007/s10658-018-1543–3
- Sommermann L, Geistlinger J, Wibberg D, Deubel A, Zwanzig J, Babin D, Schlüter A, Schellenberg I. 2018. Fungal community profiles in agricultural soils of a long-term field trial under different tillage, fertilization and crop rotation conditions analysed by high-throughputs ITS-amplicon sequencing. PLoS One. 13(4):e0195345.
- Steinmann HH, Dobers ES. 2013. Spatio-temporal analysis of crop rotations and crop sequence patterns in Northern Germany: potential implications on plant health and crop protection. J Plant Dis Prot. 120:85–94.
- Sturz AV, Bernier SS. 1989. Influence of crop rotations on winter wheat growth and yield in relation to the dynamics of pathogenic crown and root rot fungal complexes. Can J Plant Pathol. 11:114–121.
- Tillmann M, von Tiedemann A, Winter M. 2017. Crop rotation effects on incidence and diversity of Fusarium species colonizing stem bases and grains of winter wheat. J Plant Dis Prot. 124:121–130.
- Vujanovic V, Mavragani D, Hamel C. 2012. Fungal communities associated with durum wheat production system: a characterization by growth stage, plant organ and preceding crop. Crop Prot. 37:26–34.
- Wenda-Piesik A, Lemańscyk G, Pańka D, Piesik D. 2016. Risk assessment posed by diseases in context of integrated management of wheat. J Plant Dis Prot. 123:3–18.
- Winter M, de Mol F, von Tiedemann A. 2014. Cropping systems with maize and oilseed rape for energy production may reduce the risk of stem base diseases in wheat. Field Crops Res. 156:249–257.