Abstract
Postharvest diseases pose serious threats to the pome fruit industry worldwide. Grey mould, Mucor rot and blue mould decay on apples, caused by Botrytis cinerea, Mucor piriformis and Penicillium expansum, respectively, are the most economically important postharvest fungal pathogens in apple storage in British Columbia, Canada. The biocontrol capability of Pseudomonas fluorescens isolate 4–6 alone, or in combination with calcium chloride (CaCl2), sodium bicarbonate (SBC) or salicylic acid (SA), against B. cinerea, M. piriformis and P. expansum was evaluated in vitro and on ‘Ambrosia’ apple in commercial cold storage at 1°C. The combination of the antagonist with the chemical additives generally did not enhance the level of control of the pathogens in vitro. The combination of isolate 4–6 with SBC did provide levels of control of B. cinerea, M. piriformis and P. expansum comparable to the commercial fungicide Scholar® on ‘Ambrosia’ apples after 15 weeks in cold storage. The biocontrol capability of P. fluorescens isolates 1–112, 2–28 and 4–6 to control B. cinerea, M. piriformis and P. expansum was also evaluated on ‘Ambrosia’ apples in commercial controlled atmosphere (1.5% CO2 + 1.2% O2) storage at 1°C. All three isolates of P. fluorescens significantly (P < 0.05) inhibited the development of grey mould and blue mould on ‘Ambrosia’ apples after CA storage, and provided levels of control comparable to BioSave® (containing Pseudomonas syringae ESC-10). These results suggest that P. fluorescens has the potential to control common postharvest fungal pathogens during commercial storage, and by combining the antagonist with SBC, decay can be reduced to a commercially acceptable level.
Résumé
Les maladies postérieures à la récolte constituent une sérieuse menace pour la pomiculture, et ce, partout dans le monde. La pourriture grise, la pourriture à mucor et la moisissure bleue, causées chez la pomme par Botrytis cinerea, Mucor piriformis et Penicillium expansum, respectivement, sont les agents pathogènes fongiques les plus importants sur le plan économique quant à l’entreposage des pommes en Colombie-Britannique, au Canada. La capacité de lutte biologique de l’isolat 4–6 de Pseudomonas fluorescens, utilisé seul ou en combinaison avec le chlorure de calcium (CaCl2), le bicarbonate de sodium (BCS) ou l’acide salicylique (AS), contre B. cinerea, M. piriformis et P. expansum, a été évalué in vitro et sur le cultivar de pomme ‘Ambrosia’ dans un entrepôt frigorifique à 1°C. La combinaison de l’antagoniste avec les additifs chimiques n’a généralement pas amélioré son degré d’efficacité contre les agents pathogènes lors des évaluations in vitro. La combinaison de l’isolat 4–6 avec le BCS a affiché un taux d’efficacité contre B. cinerea, M. piriformis et P. expansum comparable à celui du fongicide commercial Scholar® sur les pommes ‘Ambrosia’, et ce, après 15 semaines passées en entrepôt frigorifique. La capacité de lutte biologique des isolats 1–112, 2–28 et 4–6 de P. fluorescens contre B. cinerea, M. piriformis et P. expansum a également été évaluée sur les pommes ‘Ambrosia’ entreposées en atmosphère contrôlée (AC) (1.5% CO2 + 1.2% O2) à 1°C. Les trois isolats de P. fluorescens ont significativement inhibé (P < 0.05) le développement de la pourriture grise et de la moisissure bleue sur les pommes ‘Ambrosia’ après entreposage en AC, et ont affiché des taux d’efficacité analogues à ceux de BioSave® (contenant Pseudomonas syringae ESC-10). Ces résultats suggèrent que P. fluorescens peut inhiber l’action des agents pathogènes fongiques courants postérieurs à la récolte durant l’entreposage commercial des pommes, et, en combinant l’antagoniste avec le BSC, la pourriture peut être réduite à un degré commercialement acceptable.
Introduction
Millions of dollars are lost annually to postharvest diseases of fruits and vegetables (Cappellini and Ceponis Citation1984). Postharvest diseases can be a limiting factor for long-term storage of many varieties of apples, resulting in yield losses ranging from 5 to 25% (Stockwell and Stack Citation2007). Three major postharvest fungal pathogens, Botrytis cinerea, Mucor piriformis and Penicillium expansum, commonly infect and cause rot on apples in storage in British Columbia, Canada. On pome fruit, B. cinerea, M. piriformis and P. expansum are the causal agents of grey mould, Mucor rot and blue mould, respectively. Chemical fungicides such as Mertect® (thiabendazole) and Scholar® (fludioxonil) have been applied to tree fruits to reduce postharvest loss; however, pathogen resistance is emerging (Errampalli et al. Citation2006; Jurick et al. Citation2017). Public pressure to reduce fungicide use and the demand for produce that is free of synthetic fungicides, has led to research for safer alternatives, such as biological control agents (BCAs) (Janisiewicz and Korsten Citation2002). Biological control of postharvest decay has made significant advances over the past three decades, with nine microbial antagonists being developed into commercial products (Wisniewski et al. Citation2016). The commercial product BioSave® 10LP (JET Harvest Solutions, Longwood, FL) containing saprophytic Pseudomonas syringae ESC-10 (Janisiewicz and Jeffers Citation1997) was registered for use in Canada in 2011 by the Pest Management Regulatory Agency (PMRA) and its use throughout North America has been continually increasing (Janisiewicz et al. Citation2008). In the USA, BioSave® was initially only registered for postharvest application on apples, pears and citrus fruit, but registration has been extended to use on cherries, potatoes and sweet potatoes (Buckner Citation2005; Stockwell and Stack Citation2007).
Fig. 1 Inhibitory effect of P. fluorescens isolates 1–112, 2–28 and 4–6 on mycelial growth of B. cinerea on ¼ TSA-PDA amended with no chemicals (NC) or amended with calcium chloride (CaCl2), sodium bicarbonate (SBC) or salicylic acid (SA) after 72 h incubation at 25°C. Data represent the mean ± standard error from two independent experiments. Means followed by a common letter are not significantly different according to Tukey’s test (P < 0.05). ![]()
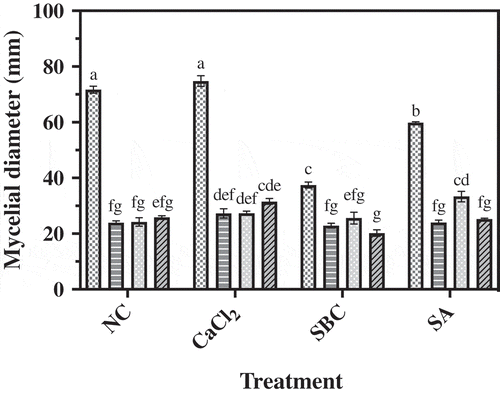
Fig. 2 Inhibitory effect of P. fluorescens isolates 1–112, 2–28 and 4–6 on mycelial growth of M. piriformis on ¼ TSA-PDA amended with no chemicals (NC) or amended with calcium chloride (CaCl2), sodium bicarbonate (SBC) or salicylic acid (SA) after 72 h incubation at 25°C. Data represent the mean of three replicates ± standard error. Means followed by a common letter are not significantly different according to Tukey’s test (P < 0.05). ![]()
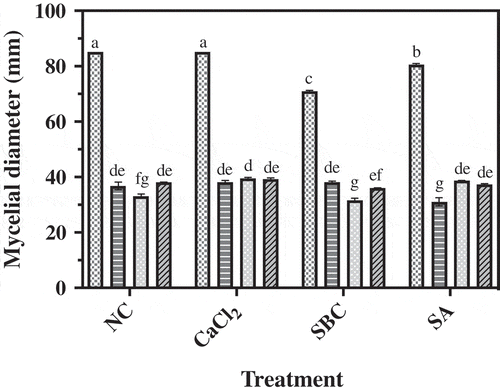
Fig. 3 Inhibitory effect of P. fluorescens isolates 1–112, 2–28 and 4–6 on mycelial growth of P. expansum on ¼ TSA-PDA amended with no chemicals (NC) or amended with calcium chloride (CaCl2), sodium bicarbonate (SBC) or salicylic acid (SA) after 72 h incubation at 25°C. Data represent the mean of three replicates ± standard error. Means followed by a common letter are not significantly different according to Tukey’s test (P < 0.05). ![]()
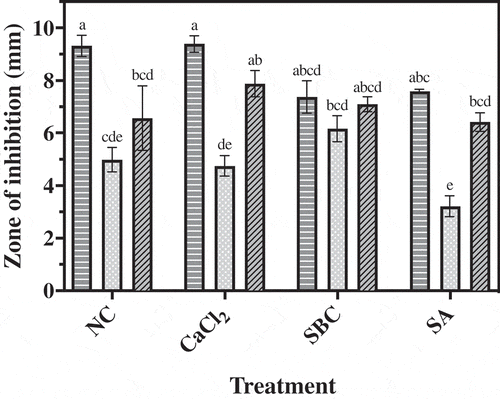
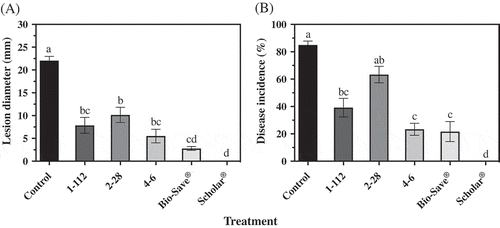
Fig. 4 Grey mould (a) lesion diameter and (b) disease incidence of ‘Ambrosia’ apples after 15 weeks in commercial controlled atmosphere storage at 0°C. Apples treated with B. cinerea were also subjected to treatment with each isolate of P. fluorescens, 1–112, 2–28 or 4–6, or BioSave® or Scholar®. Control apples were treated with the pathogen only. Each bar represents the mean of three replicates of 10 apples each ± standard error. Different letters indicate significant differences according to Tukey’s test (P < 0.05).
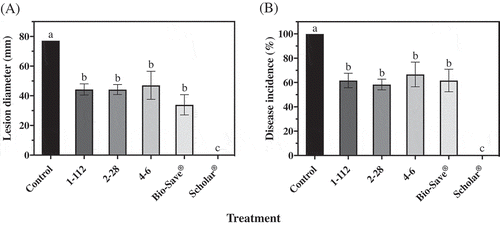
Fig. 5 Mucor rot (a) lesion diameter and (b) disease incidence of ‘Ambrosia’ apples after 15 weeks in commercial controlled atmosphere storage at 0°C. Apples treated with M. piriformis were also subjected to treatment with each isolate of P. fluorescens, 1–112, 2–28 or 4–6, or BioSave® or Scholar®. Control apples were treated with the pathogen only. Each bar represents the mean of three replicates of 10 apples each ± standard error. Different letters indicate significant differences according to Tukey’s test (P < 0.05).
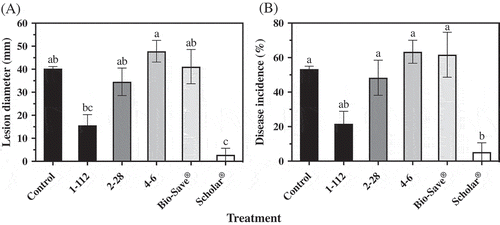
Fig. 6 Blue mould (a) lesion diameter and (b) disease incidence of ‘Ambrosia’ apples after 10 weeks in commercial controlled atmosphere storage at 0°C. Apples treated with P. expansum were also subjected to treatment with each isolate of P. fluorescens, 1–112, 2–28 or 4–6, or BioSave® or Scholar®. Control apples were treated with the pathogen only. Each bar represents the mean of three replicates of 10 apples each ± standard error. Different letters indicate significant differences according to Tukey’s test (P < 0.05).
Initially it was suggested that the potential of microbial antagonists to control postharvest decay was greater than their potential to control preharvest diseases in the field (Janisiewicz and Korsten Citation2002). The basis for this assertion was the fact that the postharvest environment, with its controlled temperature, humidity and atmospheric content, was more conducive to supporting BCAs; however, this has not been the case as postharvest chemicals can interact with antagonists to reduce their efficacy (Droby et al. Citation2016; Wisniewski et al. Citation2016). Yeast BCAs have been shown to be very effective in reducing fruit decays, but their effectiveness may decline under suboptimal conditions (Janisiewicz et al. Citation2008). For example, preharvest treatments of fungicides or salts used for flotation in packing may weaken the antagonist and reduce its survival, resulting in reduced disease control (Janisiewicz et al. Citation1998). In general, BCAs have a narrow spectrum of activity, have shown inconsistent performance under commercial conditions and are generally less effective than chemical fungicides presently on the market (Droby et al. Citation2016; Wisniewski et al. Citation2016). As a result, further research is needed to enhance the efficacy of BCAs, so that they can provide disease control comparable to or superior to the commercially available fungicides.
One approach to enhance the efficacy of BCAs that has shown promise is the application of BCAs in combination with generally recognized as safe (GRAS) compounds, such as calcium chloride (CaCl2) and sodium bicarbonate (SBC). In the fresh produce industry, CaCl2 is a widely used preservative and firming agent (Martín-Diana et al. Citation2007). Many researchers have shown that calcium plays an important role in control of postharvest diseases of fruits (Conway et al. Citation1992) and can enhance the efficacy of some BCAs (Wisniewski et al. Citation1995). Yu et al. (Citation2012) reported that treating pears with Cryptococcus laurentii in combination with CaCl2 provided greater control of grey mould and blue mould in comparison to either treatment alone. Not only has calcium been shown to enhance the activity of BCAs, but it may also allow reduced amounts of both products to be applied without compromising control of decay (Janisiewicz et al. Citation1998). Sodium bicarbonate is a commonly used food additive that is readily available, inexpensive and has little risk of phytotoxicity when applied at low concentrations (1–4%) (Palou et al. Citation2001). The combination of SBC with the commercial product AspireTM (Ecogen, Inc. Langhorne, PA), containing Cryptococcus oleophila, resulted in superior control of postharvest fungal pathogens compared with either treatment alone (Droby et al. Citation2003).
Another approach to enhancing the level of control provided by BCAs is applying them in combination with natural inducers of resistance such as salicylic acid (SA). The role of SA in local plant defences and in systemic acquired resistance, as a signal molecule, has been well documented (Yu and Zheng Citation2006). In the absence of the pathogen, exogenous application of SA has been shown to activate resistance in plants (Durrant and Dong Citation2004). SA not only plays a role in induction of disease resistance, but it has also been shown to have direct antimicrobial activity in the postharvest system of fruits (Terry and Joyce Citation2004). Combined treatment of the biological control yeast C. laurentii with SA resulted in improved control of P. expansum and B. cinerea infections in pear fruit (Yu et al. Citation2007). The combination of BCAs with physical and chemical treatments has been reviewed by Janisiewicz and Conway (Citation2010).
Controlled atmosphere storage, where O2 levels are decreased to 1–4% and CO2 levels are increased to 2–5%, has been found to be effective in delaying the onset of storage disorders (Smock Citation1979). Although CA storage has been shown to significantly reduce physiological disorders such as softening, bitter pit and internal breakdown, its effects on decay development are variable (Conway et al. Citation2007). While many studies have investigated the ability of microbial antagonists to control postharvest decay in cold storage, very few studies have looked at the ability of BCAs to inhibit disease in commercial CA storage. In semi-commercial trials, Pantoea agglomerans provided control of blue mould and grey mould on ‘Golden Delicious’ apples in low O2 storage comparable to the fungicide imazalil (Nunes et al. Citation2002).
In our earlier studies, Pseudomonas fluorescens 1–112, 2–28 and 4–6, isolated from the rhizosphere of pulse crops in Saskatchewan, Canada (Hynes et al. Citation2008) showed inhibitory activity against B. cinerea, M. piriformis and P. expansum in commercial cold storage (Nelson et al. Citation2011; Wallace et al. Citation2016, Citation2017, Citation2018a, Citation2018b). The objectives of the present study were to: (i) investigate the potential of three isolates of P. fluorescens, 1–112, 2–28 and 4–6, alone or in combination with CaCl2, SBC or SA to inhibit mycelial growth of B. cinerea, M. piriformis and P. expansum in vitro; (ii) evaluate the potential of P. fluorescens isolate 4–6 alone or in combination with CaCl2, SBC or SA to control grey mould, Mucor rot and blue mould on ‘Ambrosia’ apples during commercial cold storage at 0°C and compare them to two commercial products, Scholar® and BioSave® and (iii) assess the ability of three isolates of P. fluorescens, 1–112, 2–28 and 4–6, to control grey mould, Mucor rot and blue mould on ‘Ambrosia’ apples during commercial CA storage at 0°C and compare them to commercial products.
Materials and methods
Antagonists
The bacterial isolates, P. fluorescens 1–112, 2–28 and 4–6, were obtained from the roots of chickpea, lentil and pea in Saskatchewan, Canada, respectively (Hynes et al. Citation2008). Bacteria were stored at −80°C in 20% glycerol. Bacterial isolates were maintained, short-term, on half strength tryptic soy agar (½ TSA: 15 g tryptic soy broth (TSB), 15 g agar in 1 L of water) at 4°C. For experiments, isolated colonies of each bacterial isolate were transferred to ½ TSB (15 g TSB in 1 L of water) and incubated at 20°C for 30 h on a rotary shaker set at 185 rpm. A spectrophotometer was used to measure the optical density (OD) of the cultures at 600 nm. Cell concentrations were determined using standard calibration curves and cultures were diluted in sterile distilled water to the desired concentration depending on the experiment.
Pathogens
Botrytis cinerea Pers.:Fr strain 27, M. piriformis Fischer strain 563 and P. expansum Link strain 1790 were obtained from Dr P. Sholberg, Agriculture and Agri-Food Canada, Summerland Research and Development Centre, Summerland, British Columbia and maintained on half-strength potato dextrose agar (½ PDA: 12 g potato dextrose broth (PDB), 15 g agar in 1 L of water) at 4°C. Inoculum of each fungal pathogen was prepared according to the method used by Errampalli (Citation2004). The spore concentration of each pathogen was determined with a Petroff–Hauser counting chamber and spore inocula were adjusted to the appropriate concentration with sterile distilled water.
Fruit
Apple (Malus domestica Borkh.) fruit ‘Ambrosia’ were harvested in orchards in the Okanagan Valley, British Columbia, Canada and kindly provided by the British Columbia Tree Fruits Cooperative (BCTFC) for this study. ‘Ambrosia’ apples were chosen for this study as they were previously shown to be highly susceptible to postharvest decay in comparison to other apple cultivars (Wallace et al. Citation2018b). Fruit were selected for their uniform size and absence of physical damage and stored at 0°C, for no longer than 1 month, prior to the experiments.
Apples were placed in high-density polyethylene mesh bags (53.3 cm (length) × 30.5 cm (height); Hubert Company, Toronto, Ontario, Canada) and then surface disinfected with 6% bleach (containing 8.25% sodium hypochlorite) and 0.01% Tween 20 for 4 min, rinsed with tap water for 4 min, and dried before wounding. The fruit were wounded (2 × 2 × 7 mm), in duplicate, on opposite sides (midway between the stem and calyx end) with a sterile nail.
Biological control activity of P. fluorescens alone or in combination with CaCl2, SBC and SA in vitro
To assess the effect of P. fluorescens alone or in combination with CaCl2, SBC or SA on the mycelial growth of B. cinerea, M. piriformis and P. expansum, dual culture inhibition assays were performed. Agar plugs (6 mm in diameter) containing mycelia from the 7-day-old cultures of B. cinerea or M. piriformis were placed in the centre of Petri dishes containing quarter-strength tryptic soy agar-potato dextrose agar (¼ TSA-PDA: 7.5 g TSB, 6 g PDB, 15 g agar in 1 L of water) medium amended with no chemicals (NC) or amended with CaCl2 (Sigma, 5.0 g L−1), SBC (Fisher, 1.0 g L−1) or SA (Sigma, 0.1 g L−1). The antagonists, P. fluorescens isolates 1–112, 2–28 or 4–6, were then streaked 2 cm away on either side of the fungal plug. A fungal lawn of P. expansum was created on ¼ TSA-PDA, medium amended with NC or amended with CaCl2, SBC or SA by spreading 100 μL of 106 conidia mL−1 with a sterile glass rod. Concomitantly, the three different isolates of P. fluorescens (10 μL of a solution of 108 CFU mL−1) were inoculated onto sterile 6-mm filter discs (VWR 415 filter paper). The inoculated filter discs were allowed to air dry in a biosafety cabinet before being aseptically transferred onto the middle of the fungal lawn. Penicillium expansum positive controls consisted of filter discs inoculated with 10 μL of sterile water. The fungal lawn assay was chosen to assess the inhibitory activity of the isolates against P. expansum in vitro because, unlike the other two fungi, P. expansum did not form a uniform colony on the medium. Cultures were incubated at 25°C until the pathogen covered the positive control plates, containing only the fungi. Once the control plates were covered, the mycelial diameter of B. cinerea and M. piriformis, or the inhibition zone of P. expansum, was measured. Each treatment contained five replicates and the experiment was performed twice.
Biological control activity of P. fluorescens alone or in combination with CaCl2, SBC and SA on apples in cold storage
After ‘Ambrosia’ apples were bagged, disinfected and wounded they were then inoculated by submerging the bag of apples into a solution of CaCl2 (1.0% w/v), SBC (0.5% w/v) or SA (0.01% w/v) for one min. Treated apples were drained for one min, followed by drenching for one min in 1 × 108 CFU ml−1 of P. fluorescens 4–6, allowed to drain for one min, followed by drenching for one min in 1 × 104 spores mL−1 of B. cinerea, M. piriformis or P. expansum. Some fruit were treated, by drenching in CaCl2, SBC or SA and successive inoculation with the antagonist, P. fluorescens isolate 4–6, prior to inoculation with spores of the pathogen. Other fruit were exposed to one single treatment, by drenching with P. fluorescens isolate 4–6, CaCl2, SBC, SA, BioSave® (JetHarvest Solutions, Longwood, FL, USA) or Scholar® 50 WG (a.i. fludioxonil, Syngenta, Guelph, ON), prior to inoculation with spores of the pathogen. The drenching method of inoculation was chosen for this study as it closely mimics how fungicides would be applied in a commercial setting. Positive controls consisted of apples treated only with spores of the pathogen. Negative controls consisted of apples treated with P. fluorescens isolate 4–6, CaCl2, SBC or SA alone, or the bacterium in combination with one of the chemicals. The lesion diameters and disease incidence were determined after 15 weeks in 0°C commercial cold storage at the BCTFC in Winfield, BC, Canada. Each treatment contained three replicates of 10 fruit each and each experiment was performed at least twice.
Biological control activity of P. fluorescens on apples in CA storage
‘Ambrosia’ apples were inoculated by submerging the bag of fruit into 1 × 108 CFU mL−1 of P. fluorescens 1–112, 2–28 or 4–6 for one min, allowed to drain for one min, followed by drenching for one min in 1 × 104 conidia mL−1 of B. cinerea, M. piriformis or P. expansum. Similarly, apples were drenched in commercial products, BioSave® or Scholar® 50 WG, as per manufacturer’s instructions, allowed to drain for one min, followed by drenching for one min in 1 × 104 conidia mL−1 of B. cinerea, M. piriformis or P. expansum. Positive controls consisted of apples treated with spores of the pathogen only. Negative controls consisted of apples treated with P. fluorescens isolate 1–112, 2–28 or 4–6 only. The lesion diameters and disease incidence were determined after 10–15 weeks of incubation in 0°C commercial CA storage (1.5% CO2 and 1.2% O2) at the BCTFC in Winfield, BC, Canada. Each treatment contained three replicates of 10 fruit each and each experiment was performed twice.
Data analysis
All statistical analyses were performed with Statistical Package for Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA). To test the effects of the treatment, the data were analysed using the GLM ANOVA procedures. Terms in the model were treatment (biological or chemical), replicate and experiment. Means separation was performed using Tukey’s test. Differences at P < 0.05 were considered significant. Disease incidence data were subjected to arcsine-square root transformations. All experiments were performed at least twice and when the treatment means were statistically (P > 0.05) similar, the data were pooled and analysed together. Biocontrol experiments on apples in commercial storage were repeated in separate years and differences between experiments were likely due to environmental variation in apple physiology between years. In the case of controlled atmosphere storage trials, storage time varied from year to year as this was a commercial facility and room opening was under the control of the industry, not the researcher.
Results
Biological control activity of P. fluorescens alone or in combination with CaCl2, SBC and SA in vitro
All three isolates of P. fluorescens inhibited the mycelial growth of B. cinerea in vitro by more than 60%, on average, when cultured on ¼ TSA-PDA not amended with any chemicals, in comparison to the NC control treatment (). When the culture medium was supplemented with CaCl2 or SBC, isolates 1–112, 2–28 and 4–6 provided comparable levels of inhibition of B. cinerea. When the fungus (control) was grown on medium containing 0.1% (1.0 g L−1) SBC, the mean mycelial diameter was 37.2 mm, while in the absence of SBC, the mean mycelial diameter of the fungus (NC control) was 71.7 mm. The combination of isolates 1–112 and 4–6 with SA provided greater inhibition of B. cinerea than isolate 2–28 plus SA. In comparison to the NC control, the mycelial growth of the fungus was inhibited by 16.5% on the SA control (antagonist absent). Both chemical additives, SBC and SA alone provided direct inhibition of B. cinerea in vitro in comparison to the NC control.
All three isolates of P. fluorescens alone or in combination with SBC, CaCl2 or SA, inhibited M. piriformis in vitro (). Isolate 2–28 alone provided greater inhibition of the pathogen than isolates 1–112 or 4–6 alone (NC). All three isolates inhibited the growth of M. piriformis by more than 50%, but the level of inhibition was not enhanced when the isolates were combined with CaCl2 or SBC. When combined with SA, the antagonistic activity of isolate 1–112 was enhanced in comparison to treatment with isolate 1–112 alone (NC). The biological control activity of isolates 2–28 and 4–6 was not enhanced when they were combined with any of the chemicals tested. In vitro, SBC and SA alone provided direct inhibition of M. piriformis in comparison to the NC control.
All three isolates alone or in combination with one of the chemicals provided inhibition of P. expansum in vitro (). Isolate 1–112 alone provided greater inhibition of P. expansum than isolates 2–28 and 4–6 alone. None of the chemical additives tested enhanced the biological control activity of P. fluorescens against P. expansum. When combined with the additive SBC, all three isolates provided comparable levels of control of P. expansum. Isolates 1–112 and 4–6 in combination with SA provided superior levels of control in comparison to isolate 2–28 in combination with SA. In general, isolates 1–112 and 4–6 showed the strongest inhibitory activity against P. expansum in vitro.
Biological control activity of P. fluorescens alone or in combination with CaCl2, SBC and SA on apples in cold storage
All treatments tested, except BioSave®, significantly reduced the sizes of the lesions caused by B. cinerea on ‘Ambrosia’ apples, in comparison to the control (). The combination of the bacteria with the chemical additives did not enhance the biological control activity of the antagonist against grey mould. Of the treatments of P. fluorescens tested, only isolate 4–6 in combination with SBC significantly reduced Mucor rot decay to a level comparable to BioSave® and Scholar®. Treatment with CaCl2 and SBC alone also significantly reduced the size of the Mucor rot lesion to a level comparable to BioSave® and Scholar®. The biological control activity of isolate 4–6 against P. expansum was not enhanced when the antagonist was combined with SBC, SA or CaCl2, in comparison to the antagonist alone.
Table 1. Effect of P. fluorescens isolate 4–6 alone or in combination with calcium chloride (CaCl2), sodium bicarbonate (SBC) or salicylic acid (SA) in comparison to the registered biological control agent, BioSave® and fungicide, Scholar® on the lesion diameter of B. cinerea, M. piriformis and P. expansum on ‘Ambrosia‘ apples.
There was a significant reduction in the disease incidence of B. cinerea on ‘Ambrosia’ apples by all treatments tested, except BioSave® (). The biological control activity of isolate 4–6 was not enhanced by combining it with CaCl2, SBC or SA. The greatest reduction in the incidence of M. piriformis on ‘Ambrosia’ apples was provided by isolate 4–6 in combination with SBC, and with Scholar® alone. Of the chemical treatments alone, only SBC significantly reduced the incidence of Mucor rot. The best control of blue mould decay was achieved with the fungicide Scholar® and isolate 4–6 in combination with SBC. Only isolate 4–6 in combination with SBC was able to reduce the incidence of P. expansum to a level that was comparable to Scholar®.
Table 2. Effect of P. fluorescens isolate 4–6 alone or in combination with calcium chloride (CaCl2), sodium bicarbonate (SBC) or salicylic acid (SA) in comparison to the registered biological control agent, BioSave® and fungicide, Scholar® on the disease incidence of B. cinerea, M. piriformis and P. expansum on ‘Ambrosia‘ apples.
Biological control activity of P. fluorescens on apples in CA storage
After 15 weeks in commercial CA storage, all treatments tested reduced the size of the lesion and incidence of grey mould on ‘Ambrosia’ apples in comparison to the control (). On average, the three isolates of P. fluorescens reduced the size of the grey mould lesion by 38.9%, in comparison to the control (). Although the best control of B. cinerea was achieved with Scholar®, P. fluorescens isolates 1–112, 2–28 and 4–6 provided levels of decay control that were comparable to the commercial product, BioSave®. All three isolates of the antagonist provided comparable levels of control of grey mould in CA storage.
The only treatment tested that significantly reduced the size of the lesions and incidence of Mucor rot on ‘Ambrosia’ apples after 15 weeks of commercial CA storage was Scholar® (). None of the biological treatments tested was effective at inhibiting the growth of M. piriformis on apples.
After 10 weeks of commercial CA storage, all the treatments tested significantly reduced the size of the blue mould lesion on ‘Ambrosia’ apples. Isolates 1–112, 2–28 and 4–6 reduced the size of the decay lesion by 64.4, 53.9 and 74.9%, respectively (). Of the isolates of P. fluorescens tested, isolates 1–112 and 4–6 were the most effective at reducing the incidence of blue mould and were both comparable to BioSave®. The best control of blue mould on ‘Ambrosia’ apples in CA storage was provided by Scholar®.
Discussion
Based on previous in vivo trials in commercial cold storage (Wallace et al. Citation2016, Citation2017, Citation2018a, Citation2018b), here we examined the effect of CaCl2, SBC and SA on postharvest pathogens, B. cinerea, M. piriformis and P. expansum, to determine if they enhanced the efficacy of P. fluorescens isolate 4–6 on apples. Preliminary studies in vitro showed that all three isolates of P. fluorescens, 1–112, 2–28 and 4–6, alone or in combination with CaCl2, SBC or SA inhibited the mycelial growth of B. cinerea, M. piriformis and P. expansum.
Since alternatives to fungicides, such as BCAs, generally do not possess a broad spectrum of activity and are less effective than chemical treatments, we investigated the combination of P. fluorescens with GRAS compounds, CaCl2 and SBC on ‘Ambrosia’ apples in commercial cold storage. Preharvest treatment with calcium is a routine practice in orchards that are prone to calcium-related disorders such as bitter pit (Biggs Citation1999). Calcium is essential for cell wall stabilization and membrane integrity (Saure Citation2005), and increased levels of calcium have been shown to be effective at reducing disease and disorders, while prolonging the storage life of the fruit (Conway Citation1982). Consequently, calcium may indirectly reduce the activity of postharvest pathogens on fruits and vegetables in storage by making the fruit less susceptible to mechanical injury (Conway et al. Citation1992). Fan and Tian (Citation2001) reported that CaCl2 enhanced the biological control activity of Cryptococcus albidus against B. cinerea and P. expansum on apples. Pseudomonas syringae in combination with heat treatment and calcium also has been shown to significantly reduce the incidence of blue mould on ‘Gala’ apples (Conway et al. Citation1999). Tian et al. (Citation2002a) reported that combining CaCl2 with yeast significantly enhanced the biological control activity of Candida guilliermondii on peaches and Pichia membranefaciens on nectarines. In contrast to previous reports, in this study combining CaCl2 with P. fluorescens isolate 4–6 did not enhance the biological control activity of the bacterium in vitro or on ‘Ambrosia’ apples. Although Janisiewicz et al. (Citation1998) reported that combining the bacterial antagonist P. syringae with CaCl2 resulted in enhanced decay control, these findings are not directly comparable to ours as they used a higher concentration of calcium (4%) and used pressure infiltration to apply the chemical. Future studies should investigate if calcium infiltration is a more effective method than calcium drenching to apply this GRAS compound and reduce postharvest decay on apples. Sodium bicarbonate is inexpensive, readily available and can be applied to fruit with minimal risk of injury. In previous work, a combination of SBC with the biological control product, AspireTM, which contains the yeast C. oleophila, resulted in superior control of grey mould and blue mould, compared with either treatment alone (Droby et al. Citation2003). Control of green mould of oranges by P. syringae strain ESC-10 was improved when its application followed treatment of fruit in heated solutions of SBC (Smilanick et al. Citation1999). More recently, the combination of two yeast antagonists, Metschnikowia pulcherrima and C. laurentii with SBC (2% w/v) was an effective treatment to control P. expansum on ‘Golden Delicious’ apples in commercial CA storage (Janisiewicz et al. Citation2008). Although SBC had direct antifungal activity in vitro in the present study, it is unable to kill spores (Spadaro et al. Citation2004); thus, the application of this chemical alone is not sufficient to control postharvest decay. Preliminary in vitro studies indicated that 0.5% SBC completely inhibited the growth of all three fungal pathogens, without adversely affecting the bacteria (data not shown). As a result, for the commercial storage trials with apples, the concentration of SBC was increased from 0.1%, used in the in vitro experiments (–), to 0.5%. On apples, the combination of SBC with P. fluorescens isolate 4–6 was the only treatment of the antagonist that effectively controlled Mucor rot and was comparable to the commercial product Scholar®. Similarly, the combination of isolate 4–6 with SBC, was the only treatment that provided levels of control against blue mould that were comparable to the synthetic fungicide, Scholar®. Our results also show that treatment with SBC directly inhibited grey mould and Mucor rot on ‘Ambrosia’ apples. A disadvantage of the use of SBC is that its activity is only fungistatic and salt residues must remain on the fruit or at least within the wound, for the treatment to inhibit infection (Smilanick et al. Citation1999). The combination of microbial antagonists with GRAS compounds overcomes significant shortcomings of either of these treatments alone. The use of P. fluorescens in this study has been found to be compatible with food additives, CaCl2 and SBC, and commercial cold storage. However, further research is needed to assess the potential of P. fluorescens isolate 4–6 in combination with GRAS compounds, particularly SBC, to control postharvest pathogens in commercial CA storage.
In order to enhance the activity of P. fluorescens isolate 4–6 against postharvest decay of apples we also investigated applying the bacteria with SA, a chemical inducer of resistance. In a previous report, SA induced fruit resistance to blue mould and grey mould, and markedly enhanced the biological control activity of C. laurentii (Yu et al. Citation2007). Although Quaglia et al. (Citation2011) confirmed the antimicrobial activity of chemical inducers of resistance, acibenzolar-S-methyl, β-aminobutyric acid and methyl jasmonate, the resistance response activated in apple following biological or chemical treatment was ineffective against P. expansum infection. Our findings are in agreement with Yu et al. (Citation2007) who observed little direct inhibitory effect of SA against B. cinerea and P. expansum in vitro. We hypothesized that when SA was combined with P. fluorescens, the antagonist would serve as a primary line of defence against decay, while SA would serve as a secondary line of defence, activating the apple fruit natural resistance. In this study, the antagonistic activity of P. fluorescens was not enhanced by SA. Many postharvest elicitors such as β-aminobutyric acid (Porat et al. Citation2003), chitosan (Molloy et al. Citation2004), sodium silicate (Bi et al. Citation2006) and UV irradiation (de Capdeville et al. Citation2002) have effectively induced disease resistance against postharvest pathogens, but only after they were applied one to several days before inoculation. These reports suggest that in order to enhance the control of postharvest pathogens on apple by P. fluorescens, SA should be applied at least 24 h before the antagonist. As a result, applying SA one minute before P. fluorescens was likely not enough time to induce the natural resistance of the apple before the infection process commenced. On apples, SA had no direct antifungal activity except against the postharvest pathogen B. cinerea.
Controlled atmosphere storage is commonly used by packinghouses to reduce fruit rot, delay senescence and maintain the quality of fresh fruit (Tian et al. Citation2002b). The growth of pathogenic fungi is generally inhibited by low temperature, high CO2 and low O2, but B. cinerea, M. piriformis and P. expansum can cause decay in fruit even at 0°C (Errampalli Citation2014; Spotts Citation2014; Xiao Citation2014). The results of our experiments demonstrated that all three isolates of P. fluorescens were capable of controlling grey mould and blue mould decay on ‘Ambrosia’ apples in commercial CA storage at 0°C. In our previous work, only isolates 1–112 and 4–6 on ‘McIntosh’ and isolate 2–28 on ‘Spartan’ apples provided control of blue mould in commercial cold storage (Wallace et al. Citation2017). Similarly, when the three isolates of P. fluorescens were tested for their ability to control grey mould on ‘Spartan’ apples in commercial cold storage, only isolates 1–112 and 4–6 provided significant levels of decay control (Wallace et al. Citation2018b). These findings suggest that the combination of decreased O2, increased CO2 and low temperature enhances the performance of the bacteria in commercial storage for control of grey mould and blue mould decay of apples. In agreement with our findings, two yeast antagonists, Trichosporon sp. and C. albidus, were more effective at controlling apple decay in CA storage than air storage (Tian et al. Citation2002b). On ‘Golden Delicious’ apples, P. agglomerans has been shown to effectively control P. expansum under seven different CA conditions (Nunes et al. Citation2002). Previous work showed that the bacterial antagonist, P. syringae MA-4 significantly inhibited the growth of P. expansum on ‘Empire’ and ‘Delicious’ apples under conditions similar to commercial air and CA storage (Zhou et al. Citation2001). Further work is needed to assess the population dynamics of P. fluorescens in vivo to determine if their ability to quickly colonize the wound site and multiply rapidly under commercial storage conditions is the basis for their biological control capabilities in vivo.
In conclusion, the growing public concern over potentially adverse human health and environmental effects associated with the use of chemical fungicides will continue to drive the search for alternative control strategies. Although we hypothesized that integration of P. fluorescens isolate 4–6 with GRAS compounds, CaCl2 and SBC, or a chemical inducer of resistance, SA, would result in enhanced disease control on apples, the combination of the bacterium with low doses of chemicals generally did not increase the decay control activity of the antagonist. However, our findings suggest that P. fluorescens isolate 4–6 in combination with SBC is a promising alternative to control economically important postharvest pathogens, by providing levels of disease control comparable to the synthetic fungicide Scholar®, on apples in commercial cold storage. In commercial CA storage, all three isolates of P. fluorescens provided control of grey mould and blue mould, and isolates 1–112 and 4–6 had efficacy comparable to the commercial product, BioSave®.
Acknowledgements
The authors thank the British Columbia Tree Fruits Cooperative for the donation of the apples, use of their commercial storage facilities and their quality development lab in Winfield, BC; Dr Peter Sholberg from Agriculture and Agri-Food Canada – Summerland Research and Development Centre for providing P. expansum Link strain 1790, B. cinerea Pers.:Fr strain 27, and M. piriformis Fischer strain 563; and Lucie Grant (Jet-Harvest solutions, FL) for kindly providing BioSave®.
Additional information
Funding
References
- Bi Y, Tian SP, Guo YR, Ge YH, Qin GZ. 2006. Sodium silicate reduces postharvest decay on Hami melons: induced resistance and fungistatic effects. Plant Dis. 90:279–283.
- Biggs AR. 1999. Effect of calcium salts on apple bitter rot caused by two Colletotrichum spp. Plant Dis. 83:1001–1005.
- Buckner S. 2005. Good bugs vs. bad; beneficial micro-organisms gain grounds in disease battles. Grower. 38:B12–14.
- Cappellini RA, Ceponis MJ. 1984. Postharvest losses in fresh fruits and vegetables. In: Moline HE, editor. Postharvest pathology of fruits and vegetables: postharvest losses of perishable crops. UC Berkeley: Agric Exp Stn; p. 24–30.
- Conway WS. 1982. Effect of postharvest calcium treatment on decay of delicious apples. Plant Dis. 66:402–403.
- Conway WS, Janisiewicz WJ, Klein JD, Sams CE. 1999. Strategy for combining heat treatment, calcium infiltration, and biological control to reduce postharvest decay of ‘Gala’ apples. HortScience. 34:700–704.
- Conway WS, Janisiewicz WJ, Leverentz B, Saftner RA, Camp MJ. 2007. Control of blue mold of apple by combining controlled atmosphere, an antagonist mixture, and sodium bicarbonate. Postharvest Biol Technol. 45:326–332.
- Conway WS, Sams CE, McGuire RG, Kelman A. 1992. Calcium treatment of apples and potatoes to reduce postharvest decay. Plant Dis. 76:329–334.
- de Capdeville G, Wilson CL, Beer SV, Aist JR. 2002. Alternative disease control agents induce resistance to blue mold in harvest ‘Red Delicious’ apple fruit. Phytopathology. 92:900–908.
- Droby S, Wisniewski M, El Ghaouth A, Wilson C. 2003. Influence of food additives on the control of postharvest rots of apple and peach and efficacy of the yeast-based biocontrol product Aspire. Postharvest Biol Technol. 27:127–135.
- Droby S, Wisniewski M, Teixidó N, Spadaro D, Jijakli MH. 2016. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol Technol. 122:22–29.
- Durrant WE, Dong X. 2004. Systemic acquired resistance. Annu Rev Phytopathol. 42:185–209.
- Errampalli D. 2004. Effect of fludioxonil on germination and growth of Penicillium expansum and decay in apple cvs. Empire and Gala. Crop Prot. 23:811–817.
- Errampalli D. 2014. Penicillium expansum (Blue mold). In: Bautista-Baños S, editor. Postharvest decay control strategies. USA: Elsevier; p. 189–231.
- Errampalli D, Brubacher NR, DeEll JR. 2006. Sensitivity of Penicillium expansum to diphenylamine and thiabendazole and postharvest control of blue mold with fludioxonil in ‘McIntosh’ apples. Postharvest Biol Technol. 39:101–107.
- Fan Q, Tian SP. 2001. Postharvest biological control of grey mold and blue mold on apple by Cryptococcus albidus (Saito) Skinner. Postharvest Biol Technol. 21:341–350.
- Hynes RK, Leung GCY, Hirkala DLM, Nelson LM. 2008. Isolation, selection and characterization of beneficial rhizobacteria from pea, lentil and chickpea grown in western Canada. Can J Microbiol. 54:248–258.
- Janisiewicz WJ, Conway WS. 2010. Combining biological control with physical and chemical treatments to control fruit decays after harvest. Stewart Postharvest Rev. 9:1–16.
- Janisiewicz WJ, Conway WS, Glenn DM, Sams CE. 1998. Integrating biological control and calcium treatment for controlling postharvest decay of apples. HortScience. 33:105–109.
- Janisiewicz WJ, Jeffers SN. 1997. Efficacy of commercial formulation of two biofungicides for control of blue mold and gray mold of apples in cold storage. Crop Prot. 16:629–633.
- Janisiewicz WJ, Korsten L. 2002. Biological control of postharvest disease of fruits. Annu Rev Phytopathol. 40:411–441.
- Janisiewicz WJ, Saftner RA, Conway WS, Yoder KS. 2008. Control of blue mold decay of apple during commercial controlled atmosphere storage with yeast antagonists and sodium bicarbonate. Postharvest Biol Technol. 49:374–378.
- Jurick WM, Macarisin O, Gaskins VL, Park E, Yu JJ, Janisiewicz W, Peter KA. 2017. Characterization of postharvest fungicide-resistant Botrytis cinerea isolates from commercially stored apple fruit. Phytopathology. 107:362–368.
- Martín-Diana AB, Rico D, Frías JM, Barat JM, Henehan GTM, Barry-Ryan C. 2007. Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: a review. Trends Food Sci Technol. 18:210–218.
- Molloy C, Cheah L, Koolaard J. 2004. Induced resistance against Scleortinia sclerotiorum in carrots treated with enzymatically hydrolysed chitosan. Postharvest Biol Technol. 33:61–65.
- Nelson LM, Nagel C, Bose-Roberts N, Mantyka D, Hirkala D, Sholberg PL. 2011. Characterization of cold-adapted rhizobacteria for control of postharvest fungal decay of pome fruit. Acta Hort. 905:181–188.
- Nunes C, Usuall J, Teixidó N, Fons E, Viñas I. 2002. Post-harvest biological control by Pantoea agglomerans (CPA-2) on Golden Delicious apples. J Appl Microbiol. 92:247–255.
- Palou L, Smilanick JL, Usall J, Viñas I. 2001. Control of postharvest blue and green molds of oranges by hot water, sodium carbonate, and sodium bicarbonate. Plant Dis. 85:371–376.
- Porat R, Vinokur V, Weiss B, Cohen L, Daus A, Goldschmidt EE, Droby S. 2003. Induction of resistance to Penicillium digitatum in grapefruit by β-aminobutyric acid. Eur J Plant Pathol. 109:901–907.
- Quaglia M, Ederli L, Pasqualini S, Zazzerini A. 2011. Biological control agents and chemical inducers of resistance for postharvest control of Penicillium Link. on apple fruit. Postharvest Biol Technol. 59:307–315.
- Saure MC. 2005. Calcium translocation to fleshy fruit: its mechanism and endogenous control. Sci Hortic. 105:65–89.
- Smilanick JL, Margosan DA, Mlikota F, Usall J, Michael IF. 1999. Control of citrus green mold by carbonate and bicarbonate salts and the influence of commercial postharvest practices on their efficacy. Plant Dis. 83:139–145.
- Smock RM. 1979. Controlled atmosphere storage of fruits. Hort Rev. 1:301–336.
- Spadaro D, Garibaldi A, Gullino ML. 2004. Control of Penicillium expansum and Botrytis cinerea on apple combining a biocontrol agent with hot water dipping and acibenzolar-S-methyl, baking soda, or ethanol application. Postharvest Biol Technol. 33:141–151.
- Spotts RA. 2014. Mucor rot. In: Sutton TB, Aldwinckle HS, Agnello AM, Walgenbach JF, editors. Compendium of Apple and Pear Diseases and Pests. USA: American Phytopathological Society Press; p. 80–81.
- Stockwell VO, Stack JP. 2007. Using Pseudomonas spp. for integrated biological control. Phytopathology. 97:244–249.
- Terry LA, Joyce DC. 2004. Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharvest Biol Technol. 32:1–13.
- Tian SP, Fan Q, Xu Y, Jiang AL. 2002a. Effects of calcium on biocontrol activity of yeast antagonists against the postharvest fungal pathogen Rhizopus stolonifer. Plant Pathol. 51:352–358.
- Tian SP, Fan Q, Xu Y, Liu H. 2002b. Biocontrol efficacy of antagonist yeasts to gray mold and blue mold on apples and pears in controlled atmosphere. Plant Dis. 86:848–853.
- Wallace RL, Hirkala DL, Nelson LM. 2016. Biological control of Botrytis cinerea, Penicillium expansum, and Mucor piriformis on ‘Gala‘ and ‘McIntosh‘ apples using Pseudomonas fluorescens strains. Acta Hort. 1144:113–120.
- Wallace RL, Hirkala DL, Nelson LM. 2017. Postharvest biological control of blue mold of apple by Pseudomonas fluorescens during commercial storage and potential modes of action. Postharvest Biol Technol. 133:1–11.
- Wallace RL, Hirkala DL, Nelson LM. 2018a. Efficacy of Pseudomonas fluorescens for control of Mucor rot of apple during commercial storage and potential modes of action. Can J Microbiol. 64:420–431.
- Wallace RL, Hirkala DL, Nelson LM. 2018b. Mechanisms of action of three isolates of Pseudomonas fluorescens active against postharvest grey mold decay of apple during commercial storage. Biol Control. 117:13–20.
- Wisniewski M, Droby S, Chalutz E, Eilam Y. 1995. Effect of Ca2+ and Mg2+ on Botrytis cinerea and Penicillium expansum in vitro and on the biocontrol activity of Candida oleophila. Plant Pathol. 44:1016–1024.
- Wisniewski M, Droby S, Norelli J, Liu J, Schena L. 2016. Alternative management technologies for postharvest disease control: the journey from simplicity to complexity. Postharvest Biol Technol. 122:3–10.
- Xiao CL. 2014. Gray mold. In: Sutton TB, Aldwinckle HS, Agnello AM, Walgenbach JF, editors. Compendium of Apple and Pear Diseases and Pests. USA: American Phytopathological Society Press; p. 77–78.
- Yu T, Chen J, Chen R, Huang B, Liu D, Zheng X. 2007. Biocontrol of blue and gray mold diseases of pear fruit by integration of antagonistic yeast with salicylic acid. Int J Food Microbiol. 116:339–345.
- Yu T, Yu C, Lu H, Zunun M, Chen F, Zhou T, Sheng K, Zheng X. 2012. Effect of Cryptococcus laurent}ii and calcium chloride on control of Penicillium expansum and Botrytis cinerea infections in pear fruit. Biol Control. 61:169–175.
- Yu T, Zheng XD. 2006. Salicylic acid enhances biocontrol efficacy of the antagonist Cryptococcus laurentii in apple fruit. J Plant Growth Regul. 25:166–174.
- Zhou T, Chu CL, Liu WT, Schaneider E. 2001. Postharvest control of blue mold and grey mold of apples using isolates of Pseudomonas syringae. Can J Plant Pathol. 23:246–252.
