Abstract
Characterization and quantification of Fusarium communities and other microorganisms are crucial for understanding Fusarium ecology and management of root diseases of wheat. Fusarium communities in soils and on wheat roots from 12 Nebraska farms under dryland, and irrigated production systems were investigated using dilution plating assays, denaturing gradient gel electrophoresis (DGGE), and subsequent DNA sequence analysis. Results showed that the population densities of Fusarium in soils were significantly higher under dryland than irrigated production systems, and Pythium, Trichoderma, and Pseudomonas spp. in soils and on wheat roots were significantly lower in dryland systems. However, colonization of Fusarium spp. on the root surface, Shannon diversity indices and richness of Fusarium spp. in soils were generally higher in soils under dryland than irrigation systems based on the soils from 12 farms. Also, Shannon diversity indices and richness of Fusarium spp. in soils were significantly higher in soils under non-irrigated compared to adjacent irrigated centre-pivot field. Canonical correspondence analysis (CCA) showed that soil Fusarium communities generally formed two groups with overlap based on dryland and irrigation systems with 12 farm samplings, whereas the Fusarium communities formed two distinct groups in soils under irrigated and non-irrigated section of the centre pivot field. Moreover, dryland and irrigation systems affected several soil chemical properties. In general, soils under long-term irrigation tend to reduce populations and diversities of Fusarium spp. and increase populations and diversities of Pythium spp. compared with dryland systems.
Résumé
La caractérisation et la quantification des communautés de Fusarium et d’autres micro-organismes sont essentielles pour comprendre l’écologie de Fusarium et la gestion des maladies racinaires du blé. Les communautés de Fusarium dans les sols et les racines de blé de 12 exploitations agricoles du Nebraska dans des systèmes de production de terres sèches et irriguées ont été étudiées à l’aide d’analyses par dilution, d’électrophorèse sur gel en gradient dénaturant (DGGE) et d’une analyse ultérieure de la séquence d’ADN. Les résultats ont montré que les densités de population de Fusarium dans les sols étaient significativement plus élevées dans les zones sèches que dans les systèmes de production irrigués, ainsi que dans Pythium, Trichoderma et Pseudomonas spp. dans les sols et sur le blé, les racines étaient significativement plus basses dans les systèmes de terres sèches. Cependant, la colonisation de Fusarium spp. à la surface des racines, les indices de diversité de Shannon et la richesse de Fusarium spp. dans les sols étaient généralement plus élevés dans les sols sous terres sèches que les systèmes d’irrigation basés sur les sols de 12 exploitations. Les indices de diversité de Shannon et la richesse de Fusarium spp. dans les sols étaient significativement plus élevés dans les sols non irrigués par rapport au champ de pivot central irrigué adjacent. Une analyse canonique de la correspondance (CCA) a montré que les communautés de Fusarium du sol formaient généralement deux groupes superposés basés sur des systèmes de terres sèches et d’irrigation avec 12 échantillonnages à la ferme, alors que les communautés de Fusarium formaient deux groupes distincts dans les sols sous une section irriguée et non irriguée du champ central . De plus, les terres arides et les systèmes d’irrigation ont affecté plusieurs propriétés chimiques du sol. En général, les sols irrigués à long terme ont tendance à réduire les populations et la diversité des espèces de Fusarium. et augmenter les populations et la diversité de Pythium spp. par rapport aux systèmes de terres arides.
Introduction
Fusarium species are widely distributed in agricultural soils (Burgess Citation1981; Wakelin et al. Citation2008), and play an important role in ecological functions, such as soil denitrification (Laughlin and Stevens Citation2002), biological inhibition of plant pathogens (Gupta et al. Citation1979; Alabouvette and Couteaudier Citation1992; Larkin et al. Citation1993) and plant growth promotion (Forsyth et al. Citation2006). More importantly, many Fusarium spp. are involved in diseases which cause significant crop losses and health problems for humans and animals due to mycotoxin production (Abbas et al. Citation2012).
In the midwest region of the United States, no-till farming practices have been widely adopted, as this approach conserves soil moisture, lowers labour inputs, and reduces soil and water erosion (Triplett and Warren Citation2008). In no-till and conservation tillage systems, large amounts of plant residue on the soil surface favour Fusarium pathogen survival between crops, as pathogenic Fusarium species can overwinter both on plant debris and in soil (Bateman and Murray Citation2001). Research in the states of Colorado and Wyoming showed that there has been a significant increase in soil Fusarium populations and Fusarium crown rot and common root rot in wheat after the adoption of no-till practices compared to conventional tillage systems (Hill et al. Citation1983).
Numerous studies have been conducted to evaluate agricultural practices influencing soil Fusarium communities (Fernández et al. Citation2008; Silvestro et al. Citation2013). Nesci et al. (Citation2006) observed that Fusarium communities were significantly different in soils under tillage versus no-till production systems. Steinkellner and Langer (Citation2004) found that soil Fusarium communities were affected by sampling year, tillage and cultivated crops. Wakelin et al. (Citation2008) investigated the effect of stubble and nitrogen management, crop type, incorporation of residue originating from different plants and seed-applied fungicides on soil Fusarium communities with denaturing gradient gel electrophoresis (DGGE) analysis. Results showed that Fusarium communities were strongly associated with different agricultural practices. To date, the populations and communities of Fusarium spp. have not been systematically investigated under long-term dryland and irrigated no-till systems. Long-term dryland or irrigated production practices may lead to a shift in diversity and frequency of communities of Fusarum spp. in soil. Understanding how dryland and irrigation practices affect these communities in no-till systems and their interaction with other soil microbes is an important step towards effective soilborne disease management and sustainable agriculture.
The populations and compositions of Fusarium spp. have been mainly studied by dilution plating assay (Leslie and Summerell Citation2006); however, quantifications based on dilution plating might be biased due to different growth rates for different Fusarium spp. Moreover, traditional isolation methods were hindered by many factors, including competition and antagonism from other organisms (Leslie and Summerell Citation2006). In recent years, molecular techniques have been used to detect the variation of Fusarium communities and species identification, such as DGGE and DNA sequence analysis (Yergeau et al. Citation2005, Citation2006; Wakelin et al. Citation2008). For example, the diversity of Fusarium spp. was examined in asparagus plant tissues with Fusarium-specific PCR primers using DGGE analysis (Yergeau et al. Citation2005). Fusarium communities in soils with different crop residues were explored using Fusarium-specific PCR primers under different management practices (Wakelin et al. Citation2008). In addition, metagenomics using next-generation sequencing might be a promising approach for Fusarium community analysis in soil, since this technique was widely used in understanding soil microbial communities and their functional attributes in recent years (Fierer et al. Citation2012). Molecular techniques provide advantages in understanding soil microbial ecology than regular media culturing since they could identify both cultural and uncultured microorganisms in agricultural soil.
Soil management practices influence nutrient cycling, which might further contribute to variations in populations of soil Fusarium spp. Jones et al. (Citation1989), Wakelin et al. (Citation2007), and
Yergeau et al. (Citation2006) demonstrated that shifts of Fusarium communities in soil were associated with the levels of soil phosphorus and calcium. Potassium positively impacted the richness and diversity of Fusarium spp., and favoured Fusarium-caused plant diseases (Yergeau et al. Citation2006). Lower quantities, richness and diversity of Fusarium spp. were observed in less disturbed soil, which might be related to high levels of soil nitrogen and extractable acidity (Wakelin et al. Citation2007). Moreover, high levels of soil nitrogen were documented to reduce soil populations of F. oxysporum (Wang et al. Citation1999). Addition of organic material with high levels of nitrogen and carbon were demonstrated to reduced populations of F. oxysporum in soil (Wu et al. Citation2008), and higher levels of nitrogen were found to greatly reduce soil Fusarium spp. populations with different fertilization schemes (Rezacova et al. Citation2005).
Effective soilborne disease management depends on understanding Fusarium spp. ecology and the interaction between Fusarium spp. and other soil microbes. This knowledge will benefit the development of strategies for durable disease management and sustainable crop production systems. The objectives of this research were to: (1) characterize and quantify the populations and communities of Fusarium spp. in wheat fields under long-term dryland and irrigated systems using traditional (dilution plating) and molecular tools (DGGE and DNA sequence analysis); and (2) understand the major soil physical, chemical, and biological factors influencing the Fusarium populations using multivariate statistical analysis.
Materials and methods
Fields evaluated
Twelve representative wheat farms with a long-term history of either dryland or irrigated production under no-till cultivation in central and western Nebraska, USA, were selected for this survey (). Seven farms were under dryland, non-irrigated production and five farms were under irrigated production for a minimum of 10 continuous years prior to sampling. The soil type of each field is shown in .
Table 1. The 12 locations sampled from dryland and irrigation production systems and the cropping history in central and western Nebraska USA.
Soil sampling
Composite soil samples were obtained in June 2012 by sampling approximately 10 kg of soil using a 2.5 cm soil auger to a depth of 20 cm from each of 16 areas at each farm (). The distance between each sampled area was approximately 20 m (). Soil samples were kept separate by location within each farm and transported in coolers on ice. They were stored at 4°C until the time of analysis. Samples for DGGE analysis were stored for 1 week in a − 20°C freezer; microbial cultural assays were done within 48 to 72 hr. Additional soil samples were collected from a centre pivot irrigated field in Chase county (; and c). This farm was equipped a centre pivot irrigation system whereby a circular area within the field would receive irrigation, while areas outside of this circle would not have received irrigation, and thus can be considered as being under both irrigation and dryland production. In the farm, extra soil samples were collected from inside of the area irrigated with the centre pivot (irrigated section) and outside of the area without centre pivot (non-irrigated section). The sampling method is presented in . Since soil physical properties are similar for this field (inside and outside of the area with centre pivot), it is useful for comparisons between irrigated and non-irrigated areas regarding soil chemical and biological properties including microbial populations and communities. The distance between each sampled area in the centre pivot irrigated field was approximately 20 m ().
Plant, root and soil analyses
Four mature wheat plants at growth stage 11 (www2.ca.uky.edu/agcomm/pubs/AGR/AGR224/AGR224.pdf) (the cultivars of wheat were ‘NE10589ʹ (Dryland-1), ‘SY-Wolf’ (Dryland-2), ‘Winterhawk’ (Dryland-3), ‘NE10589ʹ (Dryland-4), ‘SY Monument’ (Dryland-5), ‘SY-Wolf’ (Dryland-6), ‘NE10589ʹ (Dryland-7), ‘SY Monument’ (Irrigation-1), ‘Winterhawk’ (Irrigation-2), ‘SY-Wolf’ (Irrigation-3), ‘SY-Wolf’ (Irrigation-4), and ‘NE10589ʹ (Irrigation-5)) were collected from within each sampled location for each field and were used for measuring fungal colonization on root surface ( and c). Soil physical and chemical properties were characterized for each of 16 samples from each farm. The detailed protocols used are described by Liu et al. (Citation2018). For microbial analysis, for each location within a field, a 10 g subsample from the bulked location sample was suspended in 90 mL (10−1 dilution) of sterile 0.25% water agar (Difco Laboratories, Detroit, MI). Serial soil dilutions (up to 10-fold) were spread on different selective media, depending on the particular microorganisms being quantified (Liu et al. Citation2018). Triplicate plates for each medium were used for each sample. Detailed protocols for quantifying Fusarium spp., Pythium spp., Rhizoctonia spp., Trichoderma spp., Pseudomonas spp., total fungi, and total bacterial populations were described by Liu et al. (Citation2018).
Colony numbers of fungi on wheat root surfaces
Four plants were collected in each subsample, and one fibrous root was arbitrarily chosen from each of the plants (5 mm length) to quantify fungal colonization represented as colony numbers on the root surfaces after washing with distilled water and placing on selective media. The incidence of Fusarium, Pythium, Rhizoctonia, and Trichoderma from the root segments were determined based on the protocol described by Liu et al. (Citation2018).
Species diversity and microbial identification in soils using DGGE
DNA analysis using a DGGE technique was used to determine fungal species. The DGGE procedure was as described previously by Liu et al. (Citation2018). DNA was extracted from soil samples (0.5 g) using a power soil DNA isolation kit (MO BIO Laboratories, Inc., Carlsbad, CA). Fusarium primers used for DGGE and detailed PCR protocol are described by Liu et al. (Citation2018).
Major DGGE bands were excised, re-amplified, and cloned (TOPO TA Cloning Kit, Invitrogen, Carlsbad, CA) for DNA sequence analysis. The band sequences were then compared with the GenBank database using a Blast search to determine species identity (Altschul et al. Citation1997). The detailed calculation for Shannon–Weaver index, richness, and evenness based on DGGE bands were described by Liu et al. (Citation2018).
Statistical analysis for soil physical, chemical and biological parameters
The Statistical Analysis Systems software (PC-SAS 8.0; SAS Institute, Cary, NC, USA) was used to analyze all data. A generalized linear model procedure (Proc GLM) was used and an analysis of variance (ANOVA) was performed for soil physical, chemical and biological properties including soil microbial populations based on dilution plating and diversity indices. The dilution plating numbers (cfu/g) were converted to log scale before performing the statistical analyses. F-test was used to compare the data from irrigated and dryland farms, and P < 0.05 was used to denote statistical significance. The relationship of Fusarium communities, soil physical, chemical, and microbial communities based on the dilution plating and DGGE were also subjected to CCA analysis using the Multivariate Statistical Package (MVSP) (http://www.kovcomp.co.uk/mvsp). Detailed CCA analysis was followed as described by Liu et al. (Citation2018).
Results
Soil physical properties
Average soil bulk densities in farms with dryland production system were 1.07 g/cm3, and 1.31 g/cm3 in farms with irrigated systems (). Average bulk densities of dryland and irrigated sections of the centre pivot irrigated field were 1.08 and 1.17 g/cm3, respectively (). Average soil water contents in farms with dryland production systems were 3.12%, and those with irrigation were 5.31% (). Average water contents of non-irrigated dryland and irrigated sections in the centre pivot irrigated field were 1.94% and 6.23%, respectively ().
Table 2. Soil physical and chemical parameters in farms with dryland and irrigation systems.
Table 3. Soil physical and chemical parameters under non-irrigated and irrigated sections of the centre pivot irrigated field.
Soil chemical properties
Levels of soil nitrogen in farms with dryland and irrigated production were 19.10 and 47.69 ppm, respectively (P = 0.05), while concentrations of potassium in farms with dryland, and irrigated production were 484.07 and 381.24 ppm, respectively (P = 0.05). The remaining soil parameters, such as pH, organic matter, phosphate, sulphur, calcium, magnesium, sodium, zinc, iron, manganese, boron, copper, cation exchange capacity, and base saturation, did not show significant differences between fields under dryland and irrigated production systems (). For the centre pivot irrigated field, levels of nitrogen, sodium, and zinc were significantly higher at 60.93 ppm, 37.25 ppm, and 1.73 ppm for the irrigated portion of the field, compared with 24.20 ppm, 19.0 ppm, and 0.90 ppm, respectively, for the irrigated sections of same field (). Levels of iron and manganese were significantly higher at 36.60 ppm, and 46.0 ppm for the non-irrigated section, compared with 27.33 ppm, and 18.8 ppm, respectively, in the irrigated section of the same farm ().
Microbial populations based on the dilution plating assay
Population densities of Fusarium spp. were significantly (P= 0.05) higher in soils from dryland than irrigated systems based on 12 farms () and the centre pivot irrigated farm (). Population densities of Fusarium averaged 1.16 × 104 cfu/g soil in dryland fields, and 6.75 × 103 cfu/g soil in irrigated fields (). Population densities of Fusarium averaged 1.17 × 105 cfu/g soil in the dryland section of the centre pivot irrigated farm, and 1.82 × 104 cfu/g soil in irrigated sections of the same field (). However, population densities of Pythium, Trichoderma and Pseudomonas were significantly higher in soils from irrigated than non-irrigated fields () as well as for the centre pivot irrigated field ().
Table 4. Population density of microorganisms (CFU g/dry soil) in soils and on wheat roots, as well as Fusarium diversity, richness and evenness based on DGGE from the farms with dryland and irrigation production systems.
Table 5. Population density of microorganisms (CFU g/dry soil) in soils and on wheat roots, as well as Fusarium diversity, richness and evenness based on DGGE under non-irrigated and irrigated sections of the center pivot irrigated field.
Population densities of thermophiles, total fungi and bacteria were not significantly different in soils under dryland versus irrigated production based on the 12 farms (). However, population densities of bacteria were significantly higher in irrigated versus non-irrigated sections of the centre pivot irrigated field. In addition, thermophile populations were significantly higher in non-irrigated than irrigated sections of the centre pivot irrigated field (). Population densities of fungi and Rhizoctonia were not significantly different in soils under non-irrigated compared with irrigated production systems based on the 12 farms () and the centre pivot irrigated field ().
Colony numbers of pathogenic and beneficial fungi on wheat root surfaces
Average colony numbers of Fusarium spp. on wheat roots were generally higher in soils from dryland than irrigated fields based on 12 farms and the centre pivot irrigated field ( and ). Colony numbers of Fusarium averaged 16/root segment in dryland and 14 in irrigated systems based on 12 farms (). Colony numbers of Fusarium averaged 2.96/root segment in non-irrigated and 2.75 in irrigated sections of the centre pivot irrigated field (). However, the colony numbers of Pythium and Trichoderma on root surfaces were significantly higher in irrigated than dryland fields based on 12 farms (), and in irrigated than non-irrigated sections of the centre pivot irrigated field (). Colony numbers of Rhizoctonia were not significantly different between dryland and irrigated fields based on 12 farms and the centre pivot irrigated field ( and ).
Species diversity based on DGGE
Shannon diversity indices and richness of Fusarium spp. were 1.04 and 14 in soils under dryland, and 0.88 and 9.6 in fields under irrigation, respectively (). Shannon diversity indices and richness of Fusarium spp. were 1.14 and 16 in the non-irrigated sections, and 0.90 and 9.75 in the irrigated sections of the centre pivot irrigated field, respectively (). Evenness was similar in soils with non-irrigated and irrigated production systems () and in dryland and irrigated sections of the centre pivot irrigated field (). In addition, all the indices were not significantly different in soils under dryland and irrigation ( and ).
Species identification in soils based on DGGE
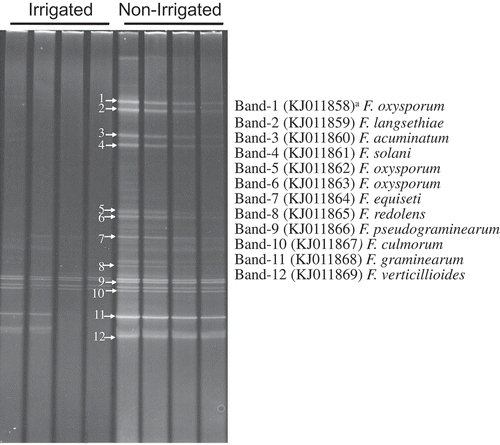
There was no clear separation for Fusarium species composition in soils from dryland and irrigation based on 12 farms using DGGE analysis. F. proliferatum was only found in dryland systems, F. merismoides was greater in dryland than irrigated fields, the remaining Fusarium species were found in both dryland and irrigated farms, although the quantity of each Fusarium spp. was different in each farm. For the contrasted sections of non-irrigated and irrigation in the centre pivot irrigated field, treatments selected for specific Fusarium spp. The major Fusarium species identified included F. oxysporum, F. graminearum, F. acuminatum, F. langsethiae, F. equiseti, F. redolens, F. pseudograminearum, F. culmorum, F. verticillioides and F. solani based on DGGE profiles (). F. oxysporum, F. langsethiae, F. acuminatum, and F. solani were only found in the non-irrigated section. However, F. equiseti, F. redolens, F. pseudograminearum, F. culmorum, F. graminearum, and F. verticillioides were found in both dryland and irrigated sections of the centre pivot irrigated field ().
CCA analysis
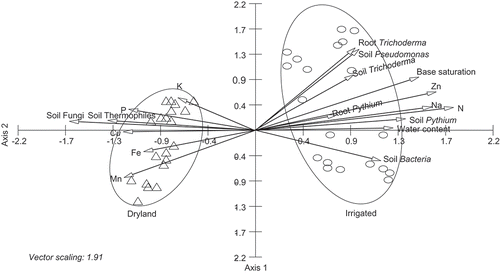
In the CCA biplots, Fusarium communities in soil samples generally formed two groups based on dryland and irrigation and the groupings overlapped (). Fusarium communities in soil samples formed distinct groups based on non-irrigated and irrigation in the centre pivot irrigated field (). CCA permits direct analysis of Fusarium communities in relation to specific soil physical, chemical and biological parameters represented by vectors. Based on 12 farm samples, dryland production system was characterized by high levels of potassium, high populations of soil fungi and soil thermophiles, and irrigated production system was characterized by high levels of soil water content, nitrogen, high populations of soil and root Pythium, soil and root Trichoderma, soil Pseudomonas, and soil bacteria (). Based on the centre pivot irrigated field, non-irrigated section was characterized by high levels of phosphate, potassium, iron, manganese, high populations of soil fungi and soil thermophiles, and irrigated section was characterized by high levels of water content, nitrogen, sodium, zinc, base saturation, high populations of soil and root Pythium, soil and root Trichoderma, soil Pseudomonas, and other soil bacteria ().
Fig. 2 CCA ordination biplot of Fusarium communities with soil physical, chemical and soil microbial populations in soils from the farms with dryland (Δ) and irrigation (ο) systems based on twelve farms. Vectors with each of the axes represent different physical, chemical and soil microbial parameters.
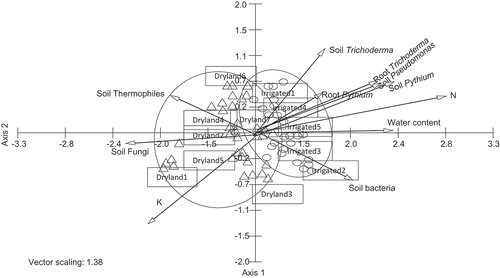
Fig. 4 CCA ordination biplot of Fusarium communities with soil physical, chemical and soil microbial populations in soils with non-irrigated (Δ) and irrigated (ο) sections of the center pivot irrigated farm. Vectors with each of the axes represent different physical, chemical and soil microbial parameters.
Discussion
Dryland wheat production generally tends to have more diversity and abundance of soil Fusarium spp. compared with irrigated production systems. However, the colonization of Fusarium spp. on wheat roots was not significantly different in soils from both dryland and irrigated systems, indicating that while dryland and irrigation practices could affect the Fusarium populations in soil, they may not be significant enough to influence the pathogen colonization on wheat roots. Fields under dryland production generally tended to have increased populations of soil fungi and thermophiles, while irrigated fields had increased populations of soil and root fungi such as Pythium, Trichoderma, Pseudomonas, and other soil bacteria. This agrees with earlier findings, in which soils with high moisture enhanced the populations of soil Trichoderma spp., Pseudomonas, and total numbers of bacteria (Garbeva et al. Citation2004; Harvey et al. Citation2006). The beneficial microbes in soils under higher water content were more suppressive to Fusarium spp. than those with lower water content, since populations of Fusarum spp. were low in soil containing high populations of Trichoderma spp. (Papavizas Citation1985) and Pseudomonas spp. (Parke et al. Citation1986; Sivan and Chet Citation1989), and the germination of F. oxysporum, F. solani, and F. graminearum was inhibited by fluorescent Pseudomonas in soil as well (Elad and Baker Citation1985). Likewise, irrigation was demonstrated to increase the activities and populations of specific microbes including Pseudomonas and Trichoderma spp. (Ruppel and Makswitat Citation1999); the increased activity of these beneficial microbes could inhibit the activities of Fusarium spp. in soil (Parke et al. Citation1986; Wakelin et al. Citation2008).
There is another explanation for the abundance of Fusarium in dryland soil. Earlier research showed that F. culmorum infections of below-ground parts of wheat are more severe in drier soils, since some Fusarium are adapted to growing at very low water potential and escape microbial competition (Cook and Papendick Citation1970). Moreover, crown rot caused by a complex of F. pseudograminearum, F. culmorum, F. avenaceum, Bipolaris sorokiniana, and Microdochium nivale, can cause the greatest losses during seasons of lowest precipitation (Smiley et al. Citation2005), which indicate some Fusarium spp. can survive at low water potential in dried soil and also explain the dominance of Fusarium in dryland production system.
Long-term dryland or irrigation based on 12 farms sampled did not generally select for specific Fusarium spp., although Fusarium communities were roughly separated into two overlapping groups affected by the different production systems. F. proliferatum was only found in dryland systems, and F. merismoides was found more in dryland than irrigated farms, whereas the remaining of Fusarium spp. were found in both dryland and irrigation systems. These results suggest that Fusarium composition might be influenced by multiple factors such as rotation crops, soil physical, chemical and biological factors and annual precipitation. Early research demonstrated that Fusarium species composition, including F. equiseti, F. flocciferum and F. oxysporum, were not related to weather conditions and soil moisture (Bateman and Murray Citation2001). However, based on the data from the centre pivot irrigatedfield, F. oxysporum, F. langsethiae, F. acuminatum, and F. solani were only found in the non-irrigated section, while F. equiseti, F. redolens, F. pseudograminearum, F. culmorum, F. graminearum, and F. verticillioides were found in both non-irrigated and irrigated sections. Therefore, further research should be focused on how long-term dryland and irrigation affect individual pathogenic Fusarium species using designed experiments within the same field so as to minimize confounding effects due to rotation, weather, and soil conditions.
The shift of Fusarium populations under dryland and irrigation systems might be associated with differences in soil chemical properties. This research showed that Fusarium populations were positively correlated with high levels of phosphate, potassium, copper, and manganese, and negatively correlated with nitrogen, base saturation, sodium, and zinc. These results agree with earlier reports that potassium was positively related to richness and diversity and population structure of Fusarium species in soil (Yergeau et al. Citation2006; Wakelin et al. Citation2007, Citation2008). In addition, the increasing level of soil nitrogen reduced population densities of F. oxysporum (Wang et al. Citation1999). Other researchers also showed that incorporation of organic nitrogen and carbon reduced the populations of Fusarium spp. in soil (Wu et al. Citation2008), and high nitrogen fertilizer applications significantly suppressed the abundance of soil Fusarium populations with different fertilization schemes (Rezacova et al. Citation2005).
Multivariate statistical analyses generally separated Fusarium communities into two overlapping groups based on dryland and irrigated production systems in 12 farms evaluated. This overlapping might be due to the interactions among rotation crops, weather conditions, soil physical, chemical and biological properties. However, Fusarium communities were separated into two distinct groups based on non-irrigated and irrigation in the center pivot irrigated field, which might be due to the contrasted differences in soil water content, soil chemical parameters and beneficial microbial populations in the sections of dryland and irrigation.
Early works suggest that Fusarium communities have a strong relationship with different crop rotations (Steinkellner and Langer Citation2004; Fernández et al. Citation2008; Silvestro et al. Citation2013). To minimize the influence to Fusarium populations by crop rotation, the farms selected for this study were mainly chosen from wheat and corn rotation systems, and the populations and communities of Fusarium spp. from those farms did not show significant differences owing to rotation crops (data not shown). Moreover, no significant differences were observed in Fusarium diversity between farms under non-irrigated and irrigated production. In contrast, there were significant differences in Fusarium diversity between irrigated and dryland sections of the centre pivot field. Therefore, large composite samples from an individual farm might reduce diversity differences in the Fusarium community.
In general, soils under long-term dryland production might have increased the populations and communities of Fusarium spp. compared with irrigated systems. The underlying mechanism for lower Fusarium populations in irrigated systems might be due to the higher microbial diversities and activities and high levels of nutrients such as nitrogen in soil, as well as the tolerance to low water potential for some Fusarium. In addition, higher levels of soil nitrogen were observed under long-term irrigation than dryland production for the 12 farms and the single centre pivot field. Higher nitrogen levels under irrigation likely reflect higher application rates of nitrogen as yield targets would be increased under irrigation versus dryland production. The current study suggests that cultural and molecular analyses can be used to characterize populations and communities of Fusarium under long-term dryland and irrigation under no-till and minimum tillage system. However, subsequent research with next-generation sequence analysis, a metagenomics approach, may be a more powerful tool for investigation and characterization of microbial communities than other molecular approaches (Fierer et al. Citation2012).
Acknowledgements
This research was supported by a grant from the University of Nebraska Foundation. The authors thank Dr. James Steadman for aiding to obtain the grant, Dr. Bob Harveson for collecting three sets of soil and wheat root samples from western Nebraska, and Mr. Bob Klein for locating the farms for soil and wheat sampling, and the anonymous wheat growers in central and western Nebraska for allowing sampling of their fields.
Additional information
Funding
References
- Abbas HK, Mascagni HJ Jr, Bruns HA, Shier WT, Damann KE. 2012. Effect of planting density, irrigation regimes, and maize hybrids with varying ear size on yield, and aflatoxin and fumonisin contamination levels. Am J Plant Sci. 3:1341–1354.
- Alabouvette C, Couteaudier Y. 1992. Biocontrol of Fusarium wilts with nonpathogenic fusaria. In: Tjamos EC, Papavizas GC, Cook RJ, editors. Biological control of plant diseases. New York (NY, USA): Plenum Press; p. 415–425.
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402.
- Bateman GL, Murray G. 2001. Seasonal variations in populations of Fusarium species in wheat-field soil. Appl Soil Ecol. 18:117–128.
- Burgess LW. 1981. General ecology of the Fusaria. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium: disease, biology, and taxonomy. University Park (PA, USA): Penn. Univ. Press; p. 225–235.
- Cook RJ, Papendick RI. 1970. Soil water potential as a factor in the ecology of Fusarium roseum f. sp. cerealis culmorum. Plant Soil. 32:131–145.
- Elad Y, Baker R. 1985. The role of competition for iron and carbon in suppression of chlamydospore germination of Fusarium spp. by Pseudomonas spp. Phytopathology. 75:1053–1059.
- Fernández MR, Huber D, Basnyat P, Zentner RP. 2008. Impact of agronomic practices on populations of Fusarium and other fungi in cereal and noncereal crop residues on the Canadian Prairies. Soil Till Res. 100:60–71.
- Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL, Owens S, Gilbert JA, Wall DH, Caporaso JG. 2012. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Nat Acad Sci USA. 109:21390–21395.
- Forsyth LM, Smith LJ, Aitken EAB. 2006. Identification and characterization of non-pathogenic Fusarium oxysporum capable of increasing and decreasing Fusarium wilt severity. Mycol Res. 110:929–935.
- Garbeva P, van Veen JA, van Elsas JD. 2004. Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Ann Rev Phytopathol. 42:243–270.
- Gupta RC, Upadhyay RS, Rai B. 1979. Hyphal parasitism and chlamydospore formation by Fusarium oxysporum on Rhizoctonia solani. Mycopathol. 67:147–152.
- Harvey PR, Warren RA, Wakelin S. 2006. Emerging soilborne constraints to irrigated maize: a Pythium–Fusarium root diseases complex. In: Humphreys E, O’Keeffe K, Hutchings N, Gill R, editors. Water to gold, proceedings of the maize association of Australia 6th Triennial conference. Darlington Point (New South Wales); pp. 133–140.
- Hill JP, Fernandez JA, McShane MS. 1983. Fungi associated with common root rot of winter wheat in Colorado and Wyoming. Plant Dis. 67:795–797.
- Jones JP, Engelhard AW, Woltz SS. 1989. Management of fusarium wilt of vegetables and ornamentals by macro- and microelement nutrition. In: Engelhard AW, editor. Soilborne plant pathogens: management of diseases with macro- and microelements. American Phytopathological Society; APS Press, St. Paul, Minn. pp. 18–32.
- Larkin RP, Hopkins DL, Martin FN. 1993. Ecology of Fusarium oxysporum f. sp. niveum in soils suppressive and conducive to Fusarium wilt of watermelon. Phytopathology. 83:1105–1116.
- Laughlin RJ, Stevens RJ. 2002. Evidence for fungal dominance of denitrification and codentrification in a grassland soil. Soil Sci Soc Amer J. 66:1540–1548.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell Publishing Ltd.; p. 388.
- Liu B, Shen WS, Wei H, Smith H, Correll JC. 2018. Microbial communities in soils with different population densities of soybean cyst nematodes. Can J Plant Pathol. 40:48–60.
- Nesci A, Barros G, Castillo C, Etcheverry M. 2006. Soil fungal population in preharvest maize ecosystem in different tillage practices in Argentina. Soil Till Res. 91:143–149.
- Papavizas GE. 1985. Trichoderma and Gliocladium: biology, ecology and potential for biocontrol. Ann Rev Phytopathol. 23:23–54.
- Parke JL, Moen R, Rovira AD, Bowen GD. 1986. Soil water flow affects the rhizosphere distribution of a seed-borne biological control agent, Pseudomonas fluorescens. Soil Biol Biochem. 6:583–588.
- Rezacova V, Hanahrselova H, Gamper H, Gryndler M. 2005. Saprobic microfungi under Lolium perenne and Trifolium repens at different fertilization intensities and elevated atmospheric CO2 concentration. Global Change Biol. 11:224–230.
- Ruppel S, Makswitat E. 1999. Effect of nitrogen fertilization and irrigation on soil microbial activities and population dynamics - a field study. J Plant Nutr Soil Sci. 162:75–81.
- Silvestro LB, Stenglein SA, Forjan H, Dinolfo MI, Arambarri AM, Manso L, Moreno MV. 2013. Occurrence and distribution of soil Fusarium species under wheat crop in zero tillage. Span J Agric Res. 11:72–79.
- Sivan A, Chet I. 1989. The possible role of competition between Trichoderma harzianum and Fusarium oxyporum on rhizosphere colonization. Phytopathology. 79:198–203.
- Smiley RW, Gourlie JA, Easley GS, Patterson LM, Whittaker RG. 2005. Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis. 89:595–604.
- Steinkellner S, Langer I. 2004. Impact of tillage on the incidence of Fusarium spp. in soil. Plant Soil. 267:13–22.
- Triplett GB, Warren AD. 2008. No-tillage crop production: A revolution in agriculture! Agro J. 100:S-153–S-165.
- Wakelin SA, Colloff MJ, Harvey PR, Marschner P, Gregg A, Rogers SL. 2007. The effects of stubble retention and nitrogen application on soil microbial community structure and functional gene abundance under irrigated maize. FEMS Microbiol Ecol. 59:661–670.
- Wakelin SA, Warren RA, Kong L, Harvey PR. 2008. Management factors affecting size and structure of soil Fusarium communities under irrigated maize in Australia. Appl Soil Ecol. 39:201–209.
- Wang B, Dale ML, Kochman JK, Allen SJ, Obst NR. 1999. Variations in soil population of Fusarium oxysporum f. sp. vasinfectum as influenced by fertilizer application and growth of different crops. Austral Plant Pathol. 28:174–181.
- Wu T, Chellemi DO, Graham JH, Rosskopf EN. 2008. Assessment of fungal communities in soil and tomato roots subjected to diverse land and crop management systems. Soil Biol Biochem. 40:1967–1970.
- Yergeau E, Filion M, Vujanovic V, St-Arnaud M. 2005. A PCR denaturing gradient gel electrophoresis approach to assess Fusarium diversity in asparagus. J Microbiol Meth. 60:143–154.
- Yergeau E, Sommerville DW, Maheux E, Vujanovic V, Hammel C, Whalen J, St-Arnaud M. 2006. Relationships between Fusarium population structure, soil nutrient status and disease incidence in field grown asparagus. FEMS Microb Ecol. 58:394–403.