Abstract
Recent advances in genomics have led to a greater understanding of blackleg disease of canola, caused by the fungus Leptosphaeria maculans. Genome sequences are available for several L. maculans isolates and different Brassica species, and several resistance and avirulence genes, which have a ‘gene for gene’ relationship, have been characterised. Although several pairs of complementary resistance and avirulence genes have been sequenced, the molecular basis of their recognition is unknown. Mechanisms responsible for conferring virulence include amino acid substitutions, gene deletion and Repeat Induced Point mutations of avirulence genes. In some cases, a single avirulence gene is recognised by two resistance genes, and virulence towards each resistance gene is conferred by separate mutations. Furthermore, some avirulence-resistance gene interactions are epistatic over others; for instance, the presence of AvrLm7 (avirulent towards Rlm7) masks the AvrLm3-Rlm3 interaction resulting in isolates phenotypically appearing virulent towards Rlm3 despite having the avirulent form of the AvrLm3 allele. Several high throughput methods, including the ascospore shower technique, are used to characterise cultivar specificity, frequency of avirulence genes, and to detect fungicide sensitivity of fungal populations from stubble. The frequency of virulence in field populations can change rapidly under selection pressure when cultivars with the same resistance genes are sown in subsequent seasons. If there is a high risk of breakdown of a particular source of resistance, canola growers can be advised to sow a different cultivar, thus avoiding an epidemic.
Résumé
Les progrès récents en génomique ont engendré une meilleure compréhension de la jambe noire du canola, causée par le champignon Leptosphaeria maculans. Des séquences du génome de plusieurs isolats de L. maculans sont disponibles, et plusieurs gènes de virulence et d’avirulence, qui affichent une relation « gène pour gène », ont été caractérisés. Bien que plusieurs paires de gènes de résistance et d’avirulence complémentaires aient été séquencées, le fondement moléculaire de leur reconnaissance est inconnu. Les mécanismes responsables de la virulence incluent les substitutions d’acides aminés, la suppression de gènes et les mutations ponctuelles induites par des répétitions des gènes d’avirulence. Dans certains cas, un gène d’avirulence unique est reconnu par deux gènes de résistance et la virulence à l’égard de chaque gène de résistance est conférée par des mutations distinctes. De plus, certaines interactions impliquant des gènes d’avirulence et des gènes de résistance sont épistatiques par rapport à d’autres. Par exemple, AvrLm7 (avirulent à l’égard de Rlm7) masque l’interaction AvrLm3-Rlm3, ce qui engendre des isolats qui semblent phénotypiquement virulents à l’égard de Rlm3, bien qu’ils affichent la forme avirulente de l’allèle AvrLm3. Certaines méthodes à haut débit, y compris la méthode de l’averse d’ascospores, sont utilisées pour caractériser la spécificité des cultivars et la fréquence des gènes d’avirulence ainsi que pour détecter la sensibilité des populations fongiques du chaume aux fongicides. La fréquence de virulence dans les populations au champ peut changer rapidement sous la pression de la sélection lorsque des cultivars possédant les mêmes gènes de résistance sont semés au cours de saisons subséquentes. S’il y a risque de dégradation d’une source particulière de résistance, il est possible de suggérer aux producteurs de canola de semer un cultivar différent, évitant ainsi une épidémie.
Introduction
Brassica napus (canola, rapeseed) is one of the most economically important oilseed crops worldwide, with approximately 70 million metric tonnes (mmt) produced annually (USDA Citation2019) (). Canada is the world’s largest producer, making up 20–30% of the total world tonnage, whilst countries from the European Union have the highest yields (). Over the past five years, Canada has produced on average 18.8 mmt, with a peak of 21.3 mmt in 2017/2018 whilst China’s production has been declining with 11.6 mmt produced in 2013/2014 and only 5.0 mmt in 2017/2018. A major constraint to canola production is blackleg disease, caused by the ascomycete fungus, Leptosphaeria maculans. Blackleg has been reported in all canola-growing regions except China (Fitt et al. Citation2008; Zhang et al. Citation2014). Currently, China only has the closely related species, L. biglobosa ‘brassicae’, which although it can cause yield losses of up to 10–30%, is not as devastating as L. maculans (Liu et al. Citation2014; Cai et al. Citation2018). In Australia, Canada and the UK, annual yield losses associated with blackleg disease caused by L. maculans are 10–20% (West et al. Citation2001; Fitt et al. Citation2006; Van de Wouw et al. Citation2016; Zhang and Fernando Citation2018), but can be much higher in some regions in some years. Epidemics causing significant levels of disease occurred in regions of France in 2000 and in Lower Eyre Peninsula, Australia, in 2003 (Rouxel et al. Citation2003; Sprague et al. Citation2006a, Citation2006b). These epidemics in Australia have resulted in up to 90% yield losses (Sprague et al. Citation2006b).
Table 1. Canola/rapeseed (Brassica napus) production (million metric tonnes), area sown (million hectares) and yield (tonnes/hectare) over the past five years for specific countries.
The disease process begins with the release of sexual spores (ascospores) from stubble (crop debris) left from the previous season following a rainfall event. These ascospores land on the cotyledons or leaves of seedlings, germinate and then invade via wounds or the stomatal aperture. The fungus then colonises the intercellular spaces, causing necrotic cotyledon and leaf lesions before entering a biotrophic phase whilst growing down the petiole and into the stem. The necrotic cotyledon and leaf lesions then produce asexual pycnidiospores, which are dispersed via rain-splash and can give rise to secondary infection events. At plant maturity, the fungus causes blackening of the stem and cankering, thus restricting the nutrient flow up the plant, and in severe situations, completely killing the plant (Hammond et al. Citation1985; Hammond and Lewis Citation1987; Chen and Howlett Citation1996).
Recently, additional symptoms responsible for yield loss have been reported in Australia. These symptoms, referred to as upper canopy infection, include infection of the lateral branches, the upper parts of the main stem, and individual flowers and pods (Sprague et al. Citation2018). These symptoms are observed in crops that have been sown in April rather than in May/June, to accommodate low rainfall during spring. The earlier flowering of these crops has resulted in the reproductive parts of the plants, i.e., flowers and pods, being present during ascospore shower events in late July and August, a time when later sown plants would still have been in a vegetative stage of development.
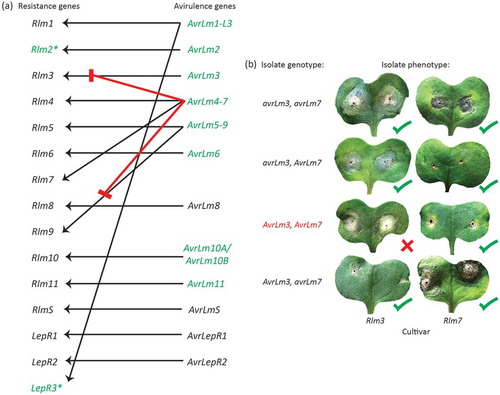
The impact of blackleg disease is minimised through the combination of cultural, chemical and genetic control strategies (Fitt et al. Citation2006; Van de Wouw et al. Citation2016; Zhang and Fernando Citation2018). Cultural strategies to minimise blackleg include rotation of canola with other crop species and avoidance of L. maculans-infested stubble. Fungicides are applied as fertilizer-amended seed dressing and/or by spraying (Van de Wouw et al. Citation2016). Genetic resistance is a major tool in the fight against blackleg disease. There are two types of resistance: quantitative (minor gene, polygenic, adult plant) and qualitative (major gene) resistance (Delourme et al. Citation2006). Quantitative resistance is poorly understood but is thought to involve numerous minor genes, all contributing a small amount towards the overall resistant phenotype (Delourme et al. Citation2006; Stuthmann et al. Citation2007). Generally, quantitative resistance is assessed at maturity, as it is expressed in the stem, leading to the term adult plant resistance. Although little is known about quantitative resistance in the L. maculans – Brassica interaction, the combination of quantitative resistance and major-gene resistance can lead to more durable disease control in the field (Brun et al. Citation2010; Delourme et al. Citation2014). Qualitative or major-gene resistance is much better understood. It occurs in a gene-for-gene interaction whereby for each resistance gene in the Brassica host, there is a corresponding avirulence gene in L. maculans (Balesdent et al. Citation2005) (). These avirulence genes belong to a larger class of genes, known as effectors, which are a general class of genes involved in disease progression. The avirulence effector genes, although involved in the specific recognition of the corresponding resistance genes, presumably also play a general role in disease progression. Major-gene resistance is usually assessed at the seedling stage of development, using cotyledon assays (), however, it has also been shown phenotypically to be expressed in true leaves, branches and pods (Balesdent et al. Citation2005; Marcroft et al. Citation2012; Elliott et al. Citation2016; Sprague et al. Citation2018).
Fig. 1 (Colour online) Interaction of Leptosphaeria maculans avirulence genes and Brassica resistance genes. (a) Fifteen resistance genes have been identified; two of which have been cloned (green). Eight of the corresponding avirulence genes have been cloned. Of these, AvrLm1-L3, AvrLm4-7 and AvrLm5-9 have dual specificity, recognised by two different resistance genes. The AvrLm10-Rlm10 interaction involves two avirulence genes (AvrLm10A/AvrLm10B). (b) The AvrLm4-7 gene is epistatic over AvrLm3 and AvrLm9 (represented by red lines in (a)). When the avirulent allele of AvrLm7 is present, isolates will be virulent towards Rlm3 (b) or Rlm9, regardless of the genotype at the AvrLm3 and AvrLm9 locus, respectively.
Although major-gene resistance can minimise blackleg disease, when cultivars with major-gene resistance are used on a wide scale, strong selection pressure is put on the pathogen population resulting in an increase in the frequency of virulent individuals, and resistance can be overcome, as occurred in the epidemics mentioned above. This is a particular problem in Australia, where disease pressure is very high. This is partly because, unlike the situation in Canada and Europe, canola is grown in a temperate climate over at least six months. Thus, plants are exposed to ascospore showers for long periods of time, allowing multiple infection events to occur.
The epidemics in the early 2000s triggered an increased focus on understanding this disease. This knowledge was summarised in this journal (Howlett Citation2004). More recently Zhang and Fernando (Citation2018) and Van de Wouw et al. (Citation2016) have reviewed the impact of blackleg on the canola industries in Canada and Australia, respectively. Here we describe the application of recent technologies and their impact on understanding the L. maculans-Brassica interaction, particularly avirulence and resistance genes, and consequently on disease management strategies. We focus on the research carried out on the fungus, which is more amenable to genetic analysis than the plant. We highlight management strategies carried out in Australia and describe how these strategies are being taken up in Canada (Cornelsen et al. Citation2019).
Molecular genetics and genomics
The amenability of both L. maculans and host Brassica species to molecular analysis, including transformation, genome sequencing and transcriptomic techniques has led to an explosion in knowledge about the genetics and molecular biology underlying this fungal-plant interaction over the last 15 years. Below we outline characteristics of the recognition process between fungus and host that have been elucidated using these technologies.
Identification of resistance and avirulence genes using genetic and genomic techniques
The complexity and the amphidiploid nature of the B. napus genome has made identification and cloning of resistance genes challenging. Several have been fine-mapped (Rlm1, Rlm2, Rlm3, Rlm4, Rlm5, Rlm6, Rlm7, Rlm8, Rlm9, Rlm10, Rlm11, RlmS (BLMR1.2), LepR1, LepR2, LepR3 (BLMR1.1), and LepR4) (Yu et al. Citation2005, Citation2008, Citation2013; Delourme et al. Citation2006; Long et al. Citation2011; Larkan et al. Citation2013, Citation2015), but only two (LepR3 and Rlm2) have been cloned (Larkan et al. Citation2013, Citation2015; Yu et al. Citation2013) (). The cloning was aided by analysis of the B. rapa genome, which became available in 2011 (Wang et al. Citation2011). Genes LepR3 and Rlm2 encode leucine-rich repeat receptor-like proteins (LRR-RLP) and are in fact alleles of the same gene. They share structural similarity to the well-characterised Cf-9 resistance protein from tomato, whereby a signal peptide is followed by multiple LRR domains and a cytoplasmic C-terminal region (Larkan et al. Citation2013, Citation2015).
In contrast to the plant, L. maculans has been much easier to manipulate in the laboratory, partly due to its much smaller and less complex genome, which is haploid, as well as its rapid life cycle. The ability to carry out sexual crossing and transformation in the laboratory enabled the map-based cloning of several avirulence genes (AvrLm1, AvrLm4-7 and AvrLm6) (Gout et al. Citation2006; Fudal et al. Citation2007; Parlange et al. Citation2009). The cloning of these genes was a very significant achievement given that that closest markers were a long way physically from the individual genes, which had flanking repetitive DNA. Since the genome sequence has become available (Rouxel et al. Citation2011), several more avirulence genes have been identified and cloned (AvrLm2, AvrLm3, AvrLm5-9, AvrLm10, AvrLm11) (Balesdent et al. Citation2013; Van de Wouw et al. Citation2014b; Ghanbarnia et al. Citation2015, Citation2018; Plissonneau et al. Citation2016, Citation2017b; Petit-Houdent et al. Citation2019).
The genome sequence revealed a compartmentalised structure with avirulence genes embedded in AT-rich repeat regions, a location that enables genes to be readily lost, gained or mutated, particularly by Repeat-Induced Point (RIP) mutations (see below). These regions are gene-poor (one gene per 30 kb) and riddled with degenerated transposable elements (Rouxel et al. Citation2011). Although these AT-rich regions contain only 3.5% of the total gene content, they harbour 20% of the candidate effector genes, including all 11 avirulence genes characterised to date. Another feature of the genome is GC-rich regions that are gene-rich (one gene per 2.4 kb on average) and lack repetitive elements. The presence of effectors in the AT-rich repetitive regions explains why it is very difficult to mutate these genes using homologous recombination techniques. Gene silencing, rather than mutation, has been used to confirm the identity of avirulence genes in several cases. For instance, RNA interference (RNAi) gene silencing reduced the expression of candidate AvrLm3, AvrLm6, AvrLm10A/B genes and switched the phenotype of each isolate from avirulent to virulent (Fudal et al. Citation2007; Plissonneau et al. Citation2017a; Petit-Houdent et al. Citation2019). The other identification approach has been to complement virulent isolates with the wild-type avirulent allele, and test for the gain of avirulence on cultivars containing the corresponding resistance gene.
Three main mechanisms are responsible for conferring virulence: amino acid substitutions, whole gene deletion (including loss of the entire dispensable mini-chromosome) and RIP mutations, a genome defence mechanism that occurs during sexual crossing and generates G to A and C to T transitions within repetitive DNA sequences. This latter mechanism is thought to be aimed at inactivating transposable elements through the generation of stop codons (). For several of the Avr-R interactions (AvrLm2:Rlm2, AvrLm3:Rlm3, AvrLm4-7:Rlm4, AvrLm5-9:Rlm5, AvrLm5-9:Rlm9), non-synonymous substitutions within the avirulence gene, leading to either amino acid substitutions or premature stop codons, confer virulence towards their corresponding resistance gene (). For AvrLm3, three non-synonymous mutations, leading to two amino acid substitutions, are present in virulent individuals, and it is yet to be determined whether all three are required for virulence (Plissonneau et al. Citation2017a). For AvrLm1 and AvrLm6, complete gene deletion is responsible for virulence towards Rlm1/LepR3 and Rlm6, respectively; furthermore, for AvrLm1, the breakpoints associated with the deletion are highly conserved in isolates collected from across the globe (Gout et al. Citation2007). AvrLm11 is located on a dispensable mini-chromosome and virulence has been associated with complete loss of the chromosome (Balesdent et al. Citation2013). Repeat-induced point (RIP) mutations are a major mechanism of virulence for AvrLm7, and a minor mechanism of virulence for AvrLm1, AvrLm2 and AvrLm6 (Gout et al. Citation2007; Van de Wouw et al. Citation2010a; Daverdin et al. Citation2012). While RIP is speculated to occur widely in fungi, it has only been demonstrated in a handful of species, and it is assumed that the process is identical to what is seen in Neurospora crassa, where duplicated DNA sequences are mutated by RIP only during the pre-meiotic stage of the sexual cycle (Selker et al. Citation1987; Selker Citation1990). It has recently been shown that L. maculans differs from N. crassa in that multiple rounds of RIP occur at different stages within a single sexual cycle (Van de Wouw et al. Citation2019), generating greater diversity in the resultant progeny. Widespread RIP of transposable elements within the AT-rich repetitive regions of the L. maculans has occurred (Rouxel et al. Citation2011; Grandaubert et al. Citation2014). The close proximity of single copy avirulence genes to the RIP-targeted transposable elements, leads to leakage of RIP into the avirulence genes (Van de Wouw et al. Citation2010a). The discovery of large AT-rich regions in which avirulence genes are embedded can explain in part why disease resistance could be overcome so readily in the field, as such regions are unstable and/or subject to the RIP process, which can inactivate adjacent non-duplicated genes.
Table 2. Avirulence genes of Leptosphaeria maculans, mechanisms for virulence and technologies used to monitor their frequencies.
Although the acquisition of the first genome assembly and annotation of L. maculans was a great breakthrough at the time, it had limitations in that avirulence genes were either annotated incorrectly, such as AvrLm5 (previously named AvrLmJ1) or missing from the genome completely, such as AvrLm3 (Van de Wouw et al. Citation2014b; Plissonneau et al. Citation2016). Recently, an improved genome sequence for L. maculans has been released that halves the percentage of ambiguous calls and improves the quality of the genome assembly (Dutreux et al. Citation2018). This improved genome sequence was achieved through the use of improved sequencing technologies including Oxford Nanopore MinION reads, and RNAseq for gene annotation. Furthermore, additional reference genome sequences are now available for other L. maculans isolates and other Leptosphaeria species (Grandaubert et al. Citation2014; Lowe et al. Citation2014). Similarly, new molecular knowledge has been gained on the host side with the release of genome sequences for B, rapa, B. oleracea, B. juncea, B. nigra and B. napus (Wang et al. Citation2011; Chalhoub et al. Citation2014; Golicz et al. Citation2016; Yang et al. Citation2016; Zhang et al. Citation2018).
Genomic approaches have provided insights beyond what could have been gained from just the discovery of either individual Brassica resistance genes or L. maculans avirulence genes. It is now clear that the Brassica genomes often feature large clusters of genes with putative roles in plant defence. Furthermore, analysis of a pan-genome of 19 different B. oleracea lines revealed that only 81% of genes were present in all lines. Of the 19% of genes present only in some lines, those with a predicted role in disease resistance were the most common (Cheng et al. Citation2016). Thus, reference genome sequences of single lines may be missing regions containing candidate resistance genes. To complicate things further, variation in copy number of genes is now implicated in disease resistance in Brassica species (Neik et al. Citation2017). Hence, mapping to a specific region in the genome may be a first step of a complex process to identify which of a set of candidate resistance genes can detect a specific L. maculans avirulence product. It also means that molecular markers used to track resistance genes within breeding lines have to be extremely close to, or coincident on the gene (i.e., a perfect marker).
Gene expression patterns during infection and down-stream signalling in the plant
The development of RNA-seq technology has enabled an understanding of the timing and sites of expression of both fungal and host genes involved in disease, and host defence responses. In several studies, cultivars of B. napus have been infected with L. maculans, with RNA prepared at several times post-infection and the dual organism RNA-seq transcriptomes analysed. These studies have shown that avirulence genes and several other effector genes are expressed early in infection during the biotrophic growth phase of the fungus. During the later necrotrophic growth phase, a different range of genes including those involved in plant cell wall degradation and the ethylene-inducing Nep1-like peptide are expressed at high levels (Lowe et al. Citation2014; Haddadi et al. Citation2016; Sonah et al. Citation2016; Gervais et al. Citation2017). Interestingly, the compartmentalized genome structure of L. maculans affects gene expression. Avirulence genes and other effector candidate genes predicted to be involved in early colonisation are expressed at high levels soon after infection and are located within the AT-rich regions. Effector candidate genes that are expressed later during infection and putatively involved in systemic colonisation of the host are located within GC-rich regions (Gervais et al. Citation2017). These characteristics, location within AT-rich and high expression during the initial stages of infection, have become molecular characteristics for identifying candidate avirulence genes within the L. maculans genome.
A range of signal transduction pathways are triggered in plants upon invasion by pathogens. Several studies have examined the impact of AvrLm1 on expression of defence-related pathways in B. napus. Sasek et al. (Citation2012) found that when isolates harbouring the AvrLm1 allele were inoculated on B. napus plants harbouring Rlm1, salicylic acid (SA) production, the expression of SA-associated genes and genes involved in ethylene (ET) signalling were all induced. When Ma et al. (Citation2018) transiently expressed AvrLm1 within Arabidopsis Columbia-0 line, SA- and jasmonic acid (JA) signalling pathways were suppressed, whilst ET-signalling pathways were unaffected, compared with the control line (lacking AvrLm1). Reasons for the conflicting results between Ma et al. (Citation2018) and Sasek et al. (Citation2012) are the presence and absence of the cognate resistance gene, Rlm1, respectively, and that one study was done in Arabidopsis whilst the other was done in the Brassica host. Experiments have been carried out with other resistance/avirulence gene pairs. Both SA- and ET-dependent signalling pathways were suppressed when isolates harbouring the AvrLm4-7 allele were inoculated onto B. napus cultivars lacking Rlm4 resistance (Nováková et al. Citation2015). Becker et al. (Citation2017) used RNA sequencing and a streamlined bioinformatics pipeline, to compare transcription profiles in the incompatible interaction between B. napus LepR1 and L. maculans AvrLepR1 and the compatible interaction between B. napus LepR1 and L. maculans avrLepR1. In the incompatible interaction, wall-associated kinases, LRR-NBS receptors, receptor-like kinases (including SOBIR1 – see below) and receptor-like proteins were all expressed at 3 days post-inoculation. Genes involved in SA-mediated signalling, ET-mediated signalling and JA-mediated signalling were up-regulated in the incompatible compared with the compatible interaction. A range of genes predicted to be involved in sulfate assimilation and indole glucosinolate biosynthesis were differentially expressed, with two genes, SULFATE TRANSPORTER 4.1 and APS-KINASE 2, specifically expressed during the incompatible interaction (Becker et al. Citation2017). Since indole glucosinolate biosynthesis and sulfur metabolism are involved in the production of phytoalexins in Brassicas, this suggests that indole-derived phytoalexins may play a role in defence in the AvrLepR1-LepR1 interaction (Becker et al. Citation2017).
Complexity of the avirulence gene – resistance gene interactions
The identity of molecules within the plant that interact with resistance or avirulence proteins is known in only a few cases. Both LepR3 and Rlm2 bind the plant receptor-like kinase, SOBIR1 (described above), to then form a signalling-complex to initiate downstream signalling after inoculation with isolates with the corresponding avirulence proteins (AvrLm1 and AvrLm2) (Larkan et al. Citation2015; Ma and Borhan Citation2015). However, whether LepR3/Rlm2 interact directly with their cognate avirulence protein or via a guard or decoy is unknown. Since no direct interaction has been detected between tomato Cf-9 (which, as discussed above, has strong sequence similarity to the LepR3 and Rlm2 proteins) and its complementary avirulence protein from Cladosporium fulvum, Larkan et al. (Citation2013) speculate that a direct interaction is unlikely, but more studies are needed to determine the nature of the interaction.
Insights into the function of AvrLm1 and AvrLm4 have come from expression studies. The AvrLm1 protein accumulates within the plant apoplast during infection, binds to and then phosphorylates the B. napus Mitogen-Activated Protein Kinase, BnMPK9, resulting in enhanced induced cell-death in plants containing LepR3 (Ma et al. Citation2018). This is the first example of an effector from a fungal plant pathogen targeting the MPK pathway to suppress plant host defence. It should be noted, however, that these experiments were performed in a heterologous system, and therefore may not represent what occurs in the native Brassica interaction.
Whilst less is known about the in-planta function of AvrLm7, its crystal structure has revealed potential motifs essential for its function (Blondeau et al. Citation2015). Bombardment of AvrLm7 protein into B. napus leaves and transient expression studies in Nicotiana benthamiana suggested that AvrLm4-7 protein interacts with its cognate resistance protein, Rlm4 or Rlm7, inside the plant cell and this translocation requires two motifs: RAWG and (R/N)(Y/F)(R/S)E(F/W). Alignments of the L. maculans AvrLm4-7 protein with similar proteins from other species, identified a second motif, (R/N)(Y/F)(R/S)E(F/W), with conserved residues across a range of species. When these conserved residues are mutated, Rlm7 recognition is altered, suggesting that this motif represents a functional epitope of the protein; however, its function is unknown (Blondeau et al. Citation2015).
Characterisation of the avirulence genes from L. maculans highlights that a simple gene-for-gene model is not always appropriate for all L. maculans – B. napus interactions. Three of the cloned avirulence genes are recognised by two different resistance genes, i.e., AvrLm1 confers avirulence towards both Rlm1 and LepR3, AvrLm4-7 confers avirulence towards both Rlm4 and Rlm7, and AvrLm5-9 confers avirulence towards both Rlm5 and Rlm9 (, ). Since AvrLm1 interacts with both Rlm1 and LepR3, we propose that AvrLm1 be renamed AvrLm1-L3 to be consistent with other avirulence genes with dual specificity, i.e., AvrLm4-7 and AvrLm5-9. For the AvrLm10-Rlm10 interaction, two neighbouring genes (AvrLm10A and AvrLm10B) located in reverse orientation within the L. maculans genome, are required for avirulence towards Rlm10 suggesting a ‘two-genes-for-one-gene’ interaction, i.e., both avirulence proteins are recognised by a single resistance protein (Petit-Houdent et al. Citation2019). However, Rlm10 is yet to be fine-mapped and cloned, so an alternative possibility is that two very closely linked resistance genes may be involved.
To add even more complexity, some avirulence interactions are epistatic over others, whereby the presence of specific avirulence alleles masks and prevents other Avirulence-Resistance gene interactions. The AvrLm4-7 gene, as mentioned above, confers avirulence towards both Rlm4 and Rlm7. A single amino acid substitution (G120 > R) is responsible for virulence towards Rlm4, whilst maintaining the avirulence function towards Rlm7 (). Conversely, as well as RIP mutations, which are a major mechanism giving rise to virulence towards Rlm7, partial or complete deletion of the entire gene, one or two base pair deletions within the coding sequence and single nucleotide polymorphism (SNPs) are also responsible (Daverdin et al. Citation2012; Plissonneau et al. Citation2017a). Furthermore, these mutations also confer virulence towards Rlm4; no isolates have been detected that are virulent towards Rlm7 and avirulent towards Rlm4, i.e., an AvrLm4 avrLm7 genotype (Parlange et al. Citation2009; Daverdin et al. Citation2012; Plissonneau et al. Citation2016). The presence of AvrLm7 (avirulent towards Rlm7) masks the AvrLm3-Rlm3 interaction resulting in isolates phenotypically appearing virulent towards Rlm3 despite having the avirulent form of the AvrLm3 allele (Plissonneau et al. Citation2016) (). However, in genotypic avrLm7 isolates (virulent towards Rlm7), the AvrLm3-Rlm3 interaction is no longer masked and isolates show the expected avirulent reaction towards Rlm3. Additionally, AvrLm7 also masks the AvrLm9-Rlm9 interaction. All AvrLm7 isolates appear virulent towards Rlm9, despite their underlying genotype (Ghanbarnia et al. Citation2018). Interestingly however, the AvrLm5-Rlm5 interaction is not masked by AvrLm7, despite a single avirulence gene (AvrLm5-9) conferring avirulence towards both Rlm5 and Rlm9. An explanation for this masking effect of AvrLm3 and AvrLm9 but not of AvrLm5, is that Rlm3, Rlm4, Rlm7 and Rlm9 are all alleles of the same resistance gene, whilst Rlm5 is a separate gene. The genes Rlm3, Rlm4, Rlm7 and Rlm9 are all tightly linked on chromosome A7 in B. napus, whilst Rlm5 is a B. juncea resistance gene that maps to a region homologous to chromosome A10 in B. napus (Balesdent et al. Citation2002, Citation2005; Delourme et al. Citation2006). There is precedence for this scenario as Rlm2 and LepR3 are alleles of the same gene.
Development of molecular markers for resistance and avirulence genes
The mapping and cloning of both resistance genes and avirulence genes allows molecular markers to be developed and used to select for resistance genes in breeding lines and to monitor changes in virulence in fungal populations, respectively. However, the use of molecular markers for the two cloned resistance genes, LepR3 and Rlm2, to track these genes within commercial cultivars has not been reported as of yet. In contrast, molecular markers have been established for ten of the cloned avirulence genes and are used both for research and commercial purposes (). For analyses of individual isolates, these molecular markers include presence/absence PCRs for deletion alleles and restriction fragment length polymorphisms (RFLPs), tetra-primer amplification refractory mutation system-PCR markers (ARMS-PCR), Kompetitive-allele specific PCR (KASP) markers, and markers based on high-resolution mapping for non-synonymous polymorphisms (Van de Wouw et al. Citation2010a, Citation2018; Daverdin et al. Citation2012; Carpezat et al. Citation2014; Zou et al. Citation2018). Alternatively, the frequency of avirulence alleles in entire fungal populations on stubble can be analysed by molecular markers (Van de Wouw et al. Citation2010b; Van de Wouw and Howlett Citation2012; Kaczmarek et al. Citation2014). This high throughput technology is discussed in the next section.
Differential isolate sets and high throughput inoculation and disease assessment technologies
To complement the molecular advances described above, some sophisticated technologies have been developed to characterise L. maculans isolates and Brassica cultivars in the glasshouse and in the field.
Development of differential sets of L. maculans isolates
The identification of the gene-for-gene interaction between L. maculans and Brassica species has led to the development of differential isolate sets that are used to characterise resistance genes in commercial cultivars. These comprise well-characterised isolates with known avirulence/virulence alleles. Each isolate is inoculated onto seedlings of the cultivar of interest and the pattern of resistance/susceptibility is used to infer the underlying genotype of the cultivar.
The cloning of particular avirulence genes has enabled the development of Differential Addition Isolates (DAIs), isolates that have been generated through transformation and only differ by a single avirulence gene (Van de Wouw et al. Citation2014a). These DAIs can be used to determine if the corresponding resistance gene is present or absent, based on reactions on a particular cultivar. These isolates are particularly useful when unusual phenotypic patterns are detected using the standard set of differential isolates. For example, cultivars Hyola50 and GT-Mustang were classified as an ‘unknown’ source of resistance using the standard set of differential isolates. However, the use of DAIs showed that Hyola50 contains Rlm1 (in addition to an unknown source of resistance) and GT-Mustang contains Rlm4 and Rlm6 (Van de Wouw et al. Citation2014a, Citation2014c, Citation2016). A limitation of DAIs is that the isolate being transformed with the avirulence gene must be virulent on the cultivar being tested.
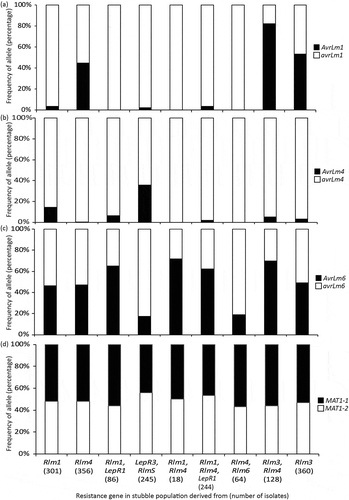
Differential isolates are now used routinely to screen all Australian and Canadian cultivars and advanced breeding lines (Marcroft et al. Citation2012; Van de Wouw et al. Citation2016; Zhang et al. Citation2016; Cornelsen et al. Citation2019). To simplify nomenclature of the suite of resistance genes in cultivars for agronomists and growers, a single letter system corresponding to each gene is used (). The systems used in Australia and Canada vary slightly in that the letters represent the common resistance genes that are present in cultivars in the respective countries (Zhang and Fernando Citation2018). These slight differences have arisen due to Australia implementing the groups earlier, before the current understanding of Avr-R genes. For example, AvrLm4-7 recognises two R gene receptors, yet in the Australian system they are allocated two different R groups, Groups B and H, whilst in the Canadian system this is acknowledged by giving them the same R group, E1 and E2. As discussed above, knowledge of the resistance gene groupings enables farmers to choose cultivars that will minimise blackleg disease. With the advances in the understanding of Avr-R gene interactions, there is potential to tweak these management systems further. For instance, with the epistatic interaction of AvrLm7 with AvrLm3 and AvrLm9, it would be expected that if Group H Australian cultivars (Rlm7) were grown widely and resistance overcome, the AvrLm3 and AvrLm9 alleles will then be expressed and therefore cultivars with Rlm3 and Rlm9 genes would become effective again. This would suggest that rotation of Group H and Group C, for example, might be effective when/after the resistance conferred by Group H breaks down. Furthermore, analysis of avirulence allele frequencies from isolates collected from stubble of cultivars with differing resistance genes, suggests that not all rotation strategies are equally effective (). Isolates collected and genotyped by Van de Wouw et al. (Citation2018) and analysed by the year collected, were reanalysed by classifying the isolates in terms of the resistance gene grouping of the stubble from which they were cultured. The frequency of AvrLm1-L3 was much higher in isolates collected from cultivars harbouring Rlm3 and Rlm4, than in isolates collected from cultivars with either Rlm3 or Rlm4 (>80% and 40–50%, respectively) (). The frequency of AvrLm4 was 15% from Rlm1 cultivars, but almost 40% in isolates collected from LepR3, RlmS cultivars (). Conversely, the frequency of AvrLm6 is lower (18%) in isolates collected from LepR3, RlmS cultivars compared with isolates collected from Rlm1, LepR1 cultivars (). These preliminary data suggest that different combinations of resistance genes may lead to differences in allele frequencies, and that not all resistance genes can be rotated to affect disease resistance. Stacking of resistance genes will have an impact on the success of rotation, as isolates with multiple virulences will be selected for in the fungal population. However, these isolates decrease in frequency upon removal of the corresponding resistance genes, presumably because they have a fitness penalty (Bousset et al. Citation2018). With rotation of resistance genes already being implemented as a management option in Australia (Van de Wouw et al. Citation2016) and currently being developed in Canada (Zhang and Fernando Citation2018; Cornelsen et al. Citation2019), it is essential to have an understanding of how resistance genes should be rotated to have the greatest impact on disease control.
Table 3. Classification of resistance genes of Australian and Canadian commercial cultivars of Brassica napus.
Fig. 2 Changes in avirulence allele frequencies for AvrLm1 (a), AvrLm4 (b) and AvrLm6 (c) of Leptosphaeria maculans isolates collected from cultivars with different resistance genes in Australia. The frequency of the avirulence alleles differs depending on genotype of the cultivars from which the isolates were collected. The mating-type (d) allele was used as a control as the presence of resistance genes does not alter the frequency of the two mating types in fungal populations.
Analysing virulence in fungal populations from stubble and in the field
Several high throughput methods have been developed to rapidly characterise fungal populations. To quantify frequency of virulence in populations, stubble with mature sexual fruiting bodies (pseudothecia) from different geographic regions and/or different cultivars is placed in an ascospore liberator and thousands to millions of ascospores are captured onto tape, genomic DNA extracted and the frequency of avirulence in the population determined using molecular markers (Van de Wouw et al. Citation2010b; Van de Wouw and Howlett Citation2012; Kaczmarek et al. Citation2014). These population-specific molecular markers include quantitative PCR for deletion alleles and pyrosequencing for non-synonymous polymorphisms.
Recently commercial services have been established in Canada whereby growers can submit stubble and the frequency of virulent isolates in their field is determined using Kompetitive-allele specific PCR (KASP) markers for all cloned available avirulence genes, except AvrLm10A and B (Cornelsen et al. Citation2019). One caveat to this approach is correct interpretation of frequencies of AvrLm3 and AvrLm9 due to epistatic interactions with AvrLm7; isolates genotyped as AvrLm3 and AvrLm9 will be virulent (phenotypically avrLm3, avrLm9) if AvrLm7 is also present.
To complement these lab-based assays, monitoring sites (36) have been established across Australia whereby cultivars representing the various resistance groups are sown and monitored for disease. Each year, advice is provided to plant breeders, agronomists and farmers regarding the effectiveness of each of the resistance groups at each of the monitoring sites. These data are also used to provide warnings to growers regarding potential crop failures. In 2011, an unusually high level of disease was detected in a Group D cultivar that had been widely sown for several years on the Eyre Peninsula, South Australia. A warning was released to growers to avoid sowing cultivars harbouring this resistance group in 2012 as resistance was being overcome. This warning was heeded by growers and resulted in an estimated saving of $AUD13 million (Van de Wouw et al. Citation2014c).
Analysing quantitative resistance in Brassica cultivars
As mentioned earlier, two types of resistance exist for the L. maculans – Brassica pathosystem; qualitative (discussed above) and quantitative gene resistance. Traditional methods of assessing quantitative resistance are time-consuming, as individual plants are inoculated and symptoms screened at maturity, either in the glasshouse or under natural conditions in the field. A high-throughput screening technique, namely the ascospore-shower technique, has been developed to screen for quantitative resistance (Marcroft et al. Citation2012). Stubble with pseudothecia is collected and evenly distributed on mesh, above seedlings. The stubble is moistened, triggering the release of ascospores, which land on seedlings underneath, allowing natural infection. The plants are then transplanted, grown to maturity and the amount of internal infection in plants cut at the crown is assessed. Thus, stubble can be sourced from any geographical location and screened in the laboratory. Furthermore, unlike field-based experiments, environmental conditions such as drought and frost do not have an impact on the experiment. Additional screening methods using inoculations with ascospores or conidia and assessing young plants in controlled environments, have also been explored by Huang et al. (Citation2014). However, a limitation of screening for quantitative resistance by any technique is that if the material being screened has effective major gene resistance, a resistant phenotype will be identified regardless of whether quantitative resistance is present or not.
Analysis of mapping populations of Brassicas segregating for quantitative resistance, using the ascospore shower technique and in the field under natural inoculum, resulted in the identification of a 13 Kb region hosting a major QTL (Raman et al. Citation2016, Citation2018). This finding highlights the effectiveness of the technology.
Detecting fungicide resistance in L. maculans
The ascospore shower technique has been modified to screen for fungicide resistance in L. maculans populations. Traditionally, fungicide resistance studies involved screening small numbers of isolates on fungicide-amended media and measuring differences in radial growth. Few in vitro studies have examined the response of Leptosphaeria species to fungicides (Eckert et al. Citation2010; Huang et al. Citation2011; Liu et al. Citation2013; Fraser et al. Citation2017). These studies have looked at limited numbers of isolates (112 across the UK and 117 across Alberta, Canada) and only two different fungicides (Huang et al. Citation2011; Fraser et al. Citation2017) with no resistance being detected. We developed a high throughput assay by collecting stubbles from various locations across Australia and then placing them above seedlings grown from seeds that had been treated with various fungicides (Van de Wouw et al. Citation2017). The advantage of this screen is two-fold; firstly, many thousands of isolates from each location are screened and therefore fungicide resistance, even at low frequencies, can be detected. Secondly, it is biologically relevant to what occurs in the field unlike an in vitro assay. Using this method, fungicide resistance was detected for the first time to an azole fungicide, fluquinconazole, in approximately 15% of Australian populations (Van de Wouw et al. Citation2017). Although fungicide resistance has been detected in these glasshouse-based experiments, whether this leads to reduced efficacy of these fungicides in the field is unknown. As the development of these screening techniques are relatively recent, no such studies have been reported in other countries, so the extent of fungicide resistance elsewhere remains unclear.
The future: staying ahead of blackleg
The technologies described above are being used to ‘stay ahead’ of the ever-evolving blackleg pathogen and prevent major yield losses in the field. In terms of genomics, many new resources are becoming available, including more and better quality genome sequences. Gene mutation technologies have also improved. As discussed, the presence of AT-rich regions in L. maculans has made it very challenging to carry out gene mutation by homologous recombination. Recently, the CRISPR-Cas9 system has been developed in L. maculans to create targeted knock-out mutants (Idnurm et al. Citation2017; Fernando Citation2018). Fernando (Citation2018) has used CRISPR/Cas9 to knock-out AvrLm4-7. CRISPR/Cas9 technologies have also been tested in Brassica species, but no genes involved in disease have been targeted to date (Lawrenson et al. Citation2015; Yang et al. Citation2017; Murovec et al. Citation2018).
The identification of avirulence genes with dual recognition, e.g., AvrLm1-L3 recognising both Rlm1 and LepR3, raises the possibility of other such interactions within this pathosystem. For instance, Rlm6 has been identified in a range of Australian B. napus cultivars based on phenotyping with differential isolates or DAIs that vary for the AvrLm6 allele. However, Rlm6 is a B. juncea-derived resistance gene (Chévre et al. Citation1997; Delourme et al. Citation2006) and, therefore, AvrLm6 may be recognising a yet uncharacterised B. napus resistance gene, in addition to the B. juncea Rlm6.
The cloning of the avirulence and resistance genes is leading to the standardising of nomenclature by the research community. Avirulence genes that are recognised by two resistance genes are now named to reflect the dual specificity; for example, AvrLm5-9 recognised by both Rlm5 and Rlm9, and AvrLm4-7 recognised by both Rlm4 and Rlm7. Likewise, as previously mentioned, since AvrLm1 interacts with both Rlm1 and LepR3, we propose that the nomenclature for this gene be changed to AvrLm1-L3.
On the host side, there has been much confusion about the resistance genes present in ‘sylvestris’ cultivars such as Surpass400, whereby they have been designated Rlm1, RlmS, LepR3, BLMR1.1 and BLMR1.2 (Van de Wouw et al. Citation2009; Long et al. Citation2011; Larkan et al. Citation2013). The cloning of LepR3 revealed that Rlm1 was not present in these cultivars, and instead AvrLm1 was interacting with LepR3, and that BLMR1.1 is the same gene as LepR3 (Larkan et al. Citation2013). It is proposed that RlmS and BLMR1.2 are likely to be the same gene, however, neither gene has been identified to date.
Conclusions
The advances in understanding the interaction between L. maculans and its Brassica host have largely resulted from the cloning of both resistance and avirulence genes. The striking features of the genome compartmentation in L. maculans highlights that adaptation to resistance genes is a rapid process as avirulence effector genes are housed in plastic genomic regions (Rouxel and Balesdent Citation2017). The once simple gene-for-gene model is evolving as quickly as populations of the blackleg pathogen itself and this highlights the necessity of understanding both the host and pathogen side of the equation. For instance, without the cloning of the AvrLm3 and AvrLm9 genes, the epistatic function of AvrLm7 would have remained unknown. This has implications for management strategies, as mentioned above, and implications for the use of molecular makers for determining virulence allele frequencies in populations.
The impressive progress in the last 15 or so years promises to be maintained as more information is gleaned about this important disease and findings from the laboratory are translated to the field in a ‘genome to paddock’ approach.
Acknowledgements
We thank the Australian Research Council and the Australian Grains and Research Development Council for funding our research and Alex Idnurm for helpful comments on the manuscript.
Additional information
Funding
References
- Balesdent MH, Attard A, Kuhn ML, Rouxel T. 2002. New avirulence genes in the phytopathogenic fungus Leptosphaeria maculans. Phytopathology. 92:1122–1133.
- Balesdent MH, Barbetti MJ, Li H, Sivasithamparam K, Gout L, Rouxel T. 2005. Analysis of Leptosphaeria maculans race structure in a worldwide collection of isolates. Phytopathology. 95:1061–1071.
- Balesdent MH, Fudal I, Ollivier B, Bally P, Grandaubert J, Eber F, Chevre AM, Leflon M, Rouxel T. 2013. The dispensable chromosome of Leptosphaeria maculans shelters an effector gene conferring avirulence towards Brassica rapa. New Phytol. 198:887–898.
- Becker M, Zhang X, Walker P, Wan J, Millar J, Khan D, Granger M, Cavers J, Chan A, Fernando DWG, et al. 2017. Transcriptome analysis of the Brassica napus-Leptosphaeria maculans pathosystem identifies receptor, signalling and structural genes underlying plant resistance. Plant J. 90:573–586.
- Blondeau K, Blaise F, Graille M, Kale SD, Linglin J, Ollivier B, Labarde A, Lazar N, Daverdin G, Balesdent MH, et al. 2015. Crystal structure of the effector AvrLm4-7 of Leptosphaeria maculans reveals insights into its translocation into plant cells and recognition by resistance proteins. Plant J. 83:610–624.
- Bousset L, Sprague SJ, Thrall PH, Barrett LG. 2018. Spatio-temporal connectivity and host resistance influence evolutionary and epidemiological dynamics of the canola pathogen Leptosphaeria maculans. Evolutionary Appl. 11:1354–1370.
- Brun H, Chevre AM, Fitt BD, Powers S, Besnard AL, Ermel M, Huteau V, Marquer B, Eber F, Renard M, et al. 2010. Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 185:285–299.
- Cai X, Huang Y, Jiang D, Fitt B, Li G, Yang L. 2018. Evaluation of oilseed rape seed yield losses caused by Leptosphaeria biglobosa in central China. Eur J Plant Pathol. 150:179–190.
- Carpezat J, Bothorel S, Daverdin G, Balesdent MH, Leflon M. 2014. Use of high resolution melting analysis to genotype the avirulence AvrLm4-7 gene of Leptoaphaeria maculans, a fungal pathogen of Brassica napus. Ann Appl Biol. 164:430–444.
- Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B, et al. 2014. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 345:950–953.
- Chen CY, Howlett BJ. 1996. Rapid necrosis of gaurd cells is associated with the arrest of fungal growth in leaves of Indian mustard (Brassica juncea) inoculated with avirulent isolates of Leptosphaeria maculans. Physiol Mol Plant Pathol. 48:73–81.
- Cheng F, Wu J, Cai C, Fu L, Liang J, Borm T, Zhuang M, Zhang Y, Zhang F, Bonnema G, et al. 2016. Genome requencing and comparative variome analysis in a Brassica rapa and Brassica oleracea collection. Sci Data. 20:160119.
- Chévre AM, Barrett P, Eber F, Dupuy P, Brun H, Tanguy X, Renard M. 1997. Selection of stable Brassica napus-B. juncea recombinant lines resistant to blackleg (Leptosphaeria maculans). 1. Identification of molecular markers, chromosomal and genomic origin of the introgression. Theor Appl Genet. 95:1104–1111.
- Cornelsen J, Jurke C, Rempel C. 2019. Joint meeting of the Canadian Phytopathological Society and the Quebec Society for the protection of plants, 2018. Can J Plant Pathol. 21:138–167.
- Daverdin G, Rouxel T, Gout L, Aubertot JN, Fudal I, Meyer M, Parlange F, Carpezat J, Balesdent MH. 2012. Genome structure and reproductive behaviour influence the evolutionary potential of a fungal phytopathogen. PLoS Pathog. 8:e1003020.
- Delourme R, Bousset L, Ermel M, Duffe P, Besnard AL, Marquer B, Fudal I, Linglin J, Chadoeuf J, Brun H. 2014. Quantitative resistance affects the speed of frequency increase but not the diversity of the virulence alleles overcoming a major resistance gene to Leptosphaeria maculans in oilseed rape. Infect Gen Evol. 27:490–499.
- Delourme R, Chevre AM, Brun H, Rouxel T, Balesdent MH, Dias JS, Salisbury P, Renard M, Rimmer SR. 2006. Major gene and polygenic resistance to Leptosphaeria maculans in oilseed rape (Brassica napus). Eur J Plant Pathol. 114:41–52.
- Dutreux F, Da Silva C, d’Agata L, Couloux A, Gay E, Istace B, Lapalu N, Lemainque A, Linglin J, Noel B, et al. 2018. De novo assembly and annotation of three Leptosphaeria genomes using Oxford Nanopore MinION sequencing. Sci Data. 5:180235.
- Eckert M, Rossall S, Selley A, Fitt B. 2010. Effects of fungicides on in vitro spore germination and mycelial growth of the phytopathogens Leptosphaeria maculans and L. biglobosa (phoma stem canker of oilseed rape). Pest Manag Sci. 66:396–405.
- Elliott VL, Marcroft SJ, Howlett BJ, Van de Wouw AP. 2016. Gene-for-gene resistance is expressed in cotyledons, leaves and pods in the Leptosphaeria maculans-Brassica napus pathosystem but not during stem colonisation. Plant Breed. 135:200–207.
- Fernando DWG. 2018. [updated 2018 Nov 11; accessed 2018 Nov]. http://www.australianoilseeds.com/__data/assets/pdf_file/0010/12601/Session_7-1.50pm_Dilantha_Fernando_AusCanola_2018_Aug_31.pdf.
- Fitt BDL, Brun H, Barbetti MJ, Rimmer SR. 2006. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur J Plant Pathol. 114:3–15.
- Fitt BDL, Hu BC, Li ZQ, Liu SY, Lange RM, Kharbanda PD, Butterworth MH, White RP. 2008. Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathol. 57:652–664.
- Fraser M, Hwang SF, Ahmed HU, Akhavan A, Stammler G, Barton W, Strelkov SE. 2017. Sensitivity of Leptosphaeria maculans to pyraclostrobin in Alberta, Canada. Can J Plant Sci. 97:83–91.
- Fudal I, Ross S, Brun H, Besnard AL, Ermel M, Kuhn ML, Balesdent MH, Rouxel T. 2009. Repeat-induced point mutation (RIP) as an alternative mechanism of evolution toward virulence in Leptosphaeria maculans. Mol Plant Microbe Interact. 22:932–941.
- Fudal I, Ross S, Gout L, Blaise F, Kuhn ML, Eckert MR, Cattolico L, Bernard-Samain S, Balesdent MH, Rouxel T. 2007. Heterochromatin-like regions as ecological niches for avirulence genes in the Leptosphaeria maculans genome: map-based cloning of AvrLm6. Mol Plant Microbe Interact. 20:459–470.
- Gervais J, Plissonneau C, Linglin J, Meyer M, Labadie K, Cruaud C, Fudal I, Rouxel T, Balesdent MH. 2017. Different waves of effector genes with contrasted genomic location are expressed by Leptosphaeria maculans during cotyledon and stem colonization of oilseed rape. Mol Plant Pathol. 18:1113–1126.
- Ghanbarnia K, Fudal I, Larkan NJ, Links MG, Balesdent M, Profotova B, Fernando DWG, Rouxel T, Borhan H. 2015. Rapid identification of the Leptosphaeria maculans avirulence gene AvrLm2 using an intraspecific comparative genomics approach. Mol Plant Pathol. 16:699–709.
- Ghanbarnia K, Ma L, Larkan NJ, Haddadi P, Fernando DWG, Borhan MH. 2018. Leptosphaeria maculans AvrLm9: a new player in the game of hide and seek with AvrLm4-7. Mol Plant Pathol. 19:1754–1764.
- Golicz AA, Bayer PE, Barker GC, Edger PP, Kim H, Martinez PA, Chan CKK, Severn-Ellis A, McCombie WR, Parkin IA, et al. 2016. The pangenome of an agronomically important crop plant Brassica oleracea. Nature Comm. 7:13390.
- Gout L, Fudal I, Kuhn ML, Blaise F, Eckert M, Cattolico L, Balesdent MH, Rouxel T. 2006. Lost in the middle of nowhere: the AvrLm1 avirulence gene of the Dothideomycete Leptosphaeria maculans. Mol Microbiol. 60:67–80.
- Gout L, Kuhn ML, Vincenot L, Bernard-Samain S, Cattolico L, Barbetti M, Moreno-Rico O, Balesdent MH, Rouxel T. 2007. Genome structure impacts molecular evolution at the AvrLm1 avirulence locus of the plant pathogen Leptosphaeria maculans. Environ Microbiol. 9:2978–2992.
- Grandaubert J, Lowe RG, Soyer JL, Schoch CL, Van de Wouw AP, Fudal I, Robbertse B, Lapalu N, Links MG, Ollivier B, et al. 2014. Transposable element-assisted evolution and adaptation to host plant within the Leptosphaeria maculans-Leptosphaeria biglobosa species complex of fungal pathogens. BMC Genomics. 15:891.
- Haddadi P, Ma L, Wang H, Borhan MH. 2016. Genome-wide transcriptomic analyses provide insightes into the lifestyle transition and effector repertoire of Leptosphaeria maculans during colonization of Brassica napus seedlings. Mol Plant Pathol. 17:1196–1210.
- Hammond KE, Lewis BG. 1987. Differential responses of oilseed rape leaves to Leptosphaeria maculans. Trans Br Mycol Soc. 88:329–333.
- Hammond KE, Lewis BG, Musa TM. 1985. A systemic pathway in the infection of oilseed rape plants by Leptosphaeria maculans. Plant Pathol. 34:557–565.
- Howlett BJ. 2004. Current knowledge of the Brassica napus-Leptosphaeria maculans interaction: a review. Can J Plant Pathol. 53:468–474.
- Huang YJ, Hood JR, Eckert M, Stonard JF, Cools H, King GJ, Rossall S, Ashworth M, Fitt BDL. 2011. Effects of fungicide on growth of Leptosphaeria maculans and L. biglobosa in relation to development of phoma stem canker on oilseed rape (Brassica napus). Plant Pathol. 60:607–620.
- Huang YJ, Qi A, King GJ, Fitt BD. 2014. Assessing quantitative resistance against Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) in young plants. PLoS One. 9:e84924.
- Idnurm A, Urquhart AS, Vummadi DR, Chang S, Van de Wouw AP, Lõpez-Ruiz FJ. 2017. Spontaneous and CRISPR/Cas9-induced mutation of the osmosensor histidine kinase of the canola pathogen Leptosphaeria maculans. Fungal Biol Biotechnol. 4:12.
- Kaczmarek J, Latunde-Dada AO, Irzykowski W, Cools H, Stonard JF, Brachaczek A, Jedryczka M. 2014. Molecular screening for avirulence alleles AvrLm1 and AvrLm6 in airborne inoculum of Leptosphaeria maculans and winter oilseed rape (Brassica napus) plants from Poland and the UK. J Appl Gen. 55:529–539.
- Larkan NJ, Lydiate DJ, Parkin IA, Nelson MN, Epp DJ, Cowling WA, Rimmer SR, Borhan MH. 2013. The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytol. 197:595–605.
- Larkan NJ, Ma L, Borhan H. 2015. The Brassica napus receptor-like protein RLM2 is encoded by a second allele of the LepR3/Rlm2 blackleg resistance locus. Plant Biotechnol J. 13:983–992.
- Lawrenson T, Shorinola O, Stacey N, Li C, Ostergaard L, Patron N, Uauy C, Harwood W. 2015. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol. 16:258.
- Liu C, Fernando DWG, Gan YT, Kutcher HR, Peng G. 2013. Baseline sensitivity of Leptosphaeria maculans to strobilurin fungicides. Can J Plant Pathol. 35:192–199.
- Liu Z, Akinwunmi O, Latunde-Dada AO, Hall AM, Fitt BDL. 2014. Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. Eur J Plant Pathol. 140:841–857.
- Long Y, Wang Z, Sun Z, Fernando DWG, McVetty PBE, Li G. 2011. Identification of two blackleg resistance genes and fine mapping of one of these two genes in a Brassica napus cultivar ‘Surpass400ʹ. Theor Appl Gen. 122:1223–1231.
- Lowe R, Cassin A, Grandaubert J, Clark BL, Van de Wouw AP, Rouxel T, Howlett BJ. 2014. Genomes and transcriptomes of partners in plant-fungal-interactions between canola (Brassica napus) and two Leptosphaeria species. PLoS One. 9:e103098.
- Ma L, Borhan H. 2015. The receptor-like kinase SOBIR1 interacts with Brassica napus LepR3 and is required for Leptosphaeria maculans AvrLm1-triggered immunity. Front Plant Sci. 6:933.
- Ma L, Djavaheri M, Wang H, Larkan NJ, Haddadi P, Beynon E, Gropp G, Borhan MH. 2018. Leptosphaeria maculans effector protein AvrLm1 modulates plant immunity by enhancing MAP Kinase 9 phosphorylation. iScience. 3:177–191.
- Marcroft SJ, Elliott VL, Cozijnsen AJ, Salisbury PA, Howlett BJ, Van de Wouw AP. 2012. Identifying resistance genes to Leptosphaeria maculans in Australian Brassica napus cultivars based on reactions to isolates with known avirulence genotypes. Crop Pasture Sci. 63:338–350.
- Murovec J, Gucek K, Bohanec B, Avbelj M, Jerala R. 2018. DNA-free genome editing of Brassica oleracia and B. rapa protoplasts using CRISPR-Cas9 Ribonucleoprotein complexes. Front Plant Sci. 9:1594.
- Neik T, Barbetti M, Batley J. 2017. Current status and challenges in identifying disease resistance genes in Brassica napus. Front Plant Sci. 8:1788.
- Nováková M, Sasek V, Trdá L, Krutinová H, Mongin T, Valentová O, Balesdent MH, Rouxel T, Burketová L. 2015. Leptosphaeria maculans effector AvrLm4-7 affects salicylic acid (SA) and ethylene (ET) signalling and hydrogen peroxide (H2O2) accumulation in Brassica napus. Mol Plant Pathol. 17:818–831.
- Parlange F, Daverdin G, Fudal I, Kuhn ML, Balesdent MH, Blaise F, Grezes-Besset B, Rouxel T. 2009. Leptosphaeria maculans avirulence gene AvrLm4-7 confers a dual recognition specificity by the Rlm4 and Rlm7 resistance genes of oilseed rape, and circumvents Rlm4-mediated recognition through a single amino acid change. Mol Microbiol. 71:851–863.
- Petit-Houdent Y, Degrave A, Meyer M, Blaise F, Ollivier B, Marais CL, Jauneau A, Audran C, Rivas S, Veneault-Fourrey C, et al. 2019. A two genes-for-one gene interaction between Leptosphearia maculans and Brassica napus. New Phytol. 223:397–411. doi:10.1111/nph.15762
- Plissonneau C, Blaise F, Ollivier B, Leflon M, Carpezat J, Rouxel T, Balesdent MH. 2017a. Unusual evolutionary mechanisms to escape effector-triggered immunity in the fungal phytopathogen Leptosphaeria maculans. Mol Ecol. 26:2183–2198.
- Plissonneau C, Daverdin G, Ollivier B, Blaise F, Degrave A, Fudal I, Rouxel T, Balesdent M. 2016. A game of hide and seek between avirulence genes AvrLm4-7 and AvrLm3 in Leptosphaeria maculans. New Phytol. 209:1613–1624.
- Plissonneau C, Rouxel T, Chevre AM, Van de Wouw AP, Balesdent MH. 2017b. One gene-one name: the AvrLmJ1 avirulence gene of Leptosphaeria maculans is AvrLm5. Mol Plant Pathol. 19:1012–1016.
- Raman H, Raman R, Coombes N, Song J, Diffey S, Klilian A, Lindbeck K, Barbulescu DM, Batley J, Edwards D, et al. 2016. Genome-wide association study identified new loci for resistance to Leptosphaeria maculans in Canola. Front Plant Sci. 7:1513.
- Raman H, Raman R, Diffey S, Qiu Y, McVittie B, Barbulescu DM, Salisbury P, Marcroft S, Delourme R. 2018. Stable quantitative resistance loci to blackleg disease in canola (Brassica napus L.) over continents. Front Plant Sci. 9:1622.
- Rouxel T, Balesdent MH. 2017. Life, death and rebirth of avirulence effectors in a fungal pathogen of Brassica crops, Leptosphaeria maculans. New Phytol. 214:526–532.
- Rouxel T, Grandaubert J, Hane JK, Hoede C, van de Wouw AP, Couloux A, Dominguez V, Anthouard V, Bally P, Bourras S, et al. 2011. Effector diversification within compartments of the Leptosphaeria maculans genome affected by Repeat-Induced Point mutations. Nat Commun. 2:n202.
- Rouxel T, Penaud A, Pinochet X, Brun H, Gout L, Delourme R, Schmit J, Balesdent MH. 2003. A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. Eur J Plant Pathol. 109:871–881.
- Sasek V, Nováková M, Jindrichová B, Bóka K, Valentová O, Burketová L. 2012. Recognition of avirulence gene AvrLm1 from hemibiotrophic ascomycete Leptopshaeria maculans triggers salicylic acid and ethylene signalling in Brassica napus. Mol Plant Microbe Interact. 25:1238–1250.
- Selker EU. 1990. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Gen. 24:579–613.
- Selker EU, Cambareri EB, Jensen BC, Haack KR. 1987. Rearrangement of duplicated DNA in specialized cells of Neurospora. Cell. 51:741–752.
- Simes J. 2018. [accessed 2018 Nov]. https://www.producer.com/2018/04/new-lab-test-helps-farmers-protect-against-blackleg/.
- Sonah H, Zhang X, Deshmukh R, Borhan H, Fernando DWG, Belanger R. 2016. Comparative transcriptomic analysis of virulence factors in Leptosphaeria maculans during compatible and incompatible interactions with canola. Front Plant Sci. 7:1784.
- Sprague SJ, Balesdent MH, Brun H, Hayden HL, Marcroft SJ, Pinochet X, Rouxel T, Howlett BJ. 2006a. Major gene resistance in Brassica napus (oilseed rape) is overcome by changes in virulence of populations of Leptosphaeria maculans in France and Australia. Eur J Plant Pathol. 114:33–44.
- Sprague SJ, Marcroft SJ, Hayden HL, Howlett BJ. 2006b. Major gene resistance to blackleg in Brassica napus overcome within three years of commercial production in southeastern Australia. Plant Dis. 90:190–198.
- Sprague SJ, Marcroft SJ, Lindbeck KD, Ware AH, Khangura RK, Van de Wouw AP. 2018. Detection, prevalence and severity of upper canopy infection on mature Brassica napus plants caused by Leptosphaeria maculans. Crop Pasture Sci. 69:65–78.
- Stuthmann DD, Leonard JJ, Miller-Garvin J. 2007. Breeding crops for durable resistance to disease. Adv Agron. 95:319–367.
- [USDA] United States Department of Agriculture. 2019. Oilseeds: world markets and trade.
- Van de Wouw AP, Cozijnsen AJ, Hane JK, Brunner PC, McDonald BA, Oliver RP, Howlett BJ. 2010a. Evolution of linked avirulence effectors in Leptosphaeria maculans is affected by genomic environment and exposure to resistance genes in host plants. PLoS Pathog. 6:e1001180.
- Van de Wouw AP, Elliott CE, Howlett BJ. 2014a. Transformation of fungal isolates with avirulence genes provides tools for identification of corresponding resistance genes in the host plant. Eu J Plant Pathol. 140:875–882.
- Van de Wouw AP, Elliott CE, Popa KM, Idnurm A. 2019. Analysis of repeat induced point (RIP) mutations in Leptosphaeria maculans indicates variability in the RIP process between fungal species. Genetics. 211:89–104.
- Van de Wouw AP, Elliott VL, Chang S, López-Ruiz F, Marcroft SJ, Idnurm A. 2017. Identification of isolates of the plant pathogen Leptosphaeria maculans with resistance to the triazole fungicide fluquinconazole using a novel in planta assay. PLoS One. 12:e0188106.
- Van de Wouw AP, Howlett BJ. 2012. Estimating frequencies of virulent isolates in field populations of a plant pathogenic fungus, Leptosphaeria maculans, using high-throughput pyrosequencing. J Appl Microbiol. 113:1145–1153.
- Van de Wouw AP, Howlett BJ, Idnurm A. 2018. Changes in allele frequencies of avirulence genes in the blackleg fungus, Leptosphaeria maculans, over two decades in Australia. Crop Pasture Sci. 69:20–29.
- Van de Wouw AP, Lowe RGT, Elliott CE, Dubois DJ, Howlett BJ. 2014b. An avirulence gene, AvrLmJ1, from the blackleg fungus, Leptosphaeria maculans, confers avirulence to Brassica juncea cultivars. Mol Plant Pathol. 15:523–530.
- Van de Wouw AP, Marcroft SJ, Barbetti MJ, Hua L, Salisbury PA, Gout L, Rouxel T, Howlett BJ, Balesdent MH. 2009. Dual control of avirulence in Leptosphaeria maculans towards a Brassica napus cultivar with ‘sylvestris-derived’ resistance suggests involvement of two resistance genes. Plant Pathol. 58:305–313.
- Van de Wouw AP, Marcroft SJ, Howlett BJ. 2016. Blackleg disease of canola in Australia. Crop Pasture Sci. 67:273–282.
- Van de Wouw AP, Marcroft SJ, Ware A, Lindbeck K, Khangura R, Howlett BJ. 2014c. Breakdown of resistance to the fungal disease, blackleg, is averted in commercial canola (Brassica napus) crops in Australia. Field Crops Res. 166:144–151.
- Van de Wouw AP, Stonard JF, Howlett BJ, West JS, Fitt BD, Atkins SD. 2010b. Determining frequencies of avirulent alleles in airborne Leptosphaeria maculans inoculum using quantitative PCR. Plant Pathol. 59:809–818.
- Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun J, Bancroft I, Cheng F, et al. 2011. The genome of the mesopolyploid crop species Brassica rapa. Nat Gen. 43:1035–1039.
- West JS, Kharbanda PD, Barbetti M, Fitt BDL. 2001. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 50:10–27.
- Yang H, Wu J, Tang T, Liu K, Dai C. 2017. CRISPR/Cas-9-mediated genome editing efficiently creates specific mutations at multiple loci using one sgRNA in Brassica napus. Sci Rep. 7:7289.
- Yang J, Liu D, Wang X, Ji C, Cheng F, Liu B, Hu Z, Chen S, Pental D, Ju Y, et al. 2016. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat Gen. 48:1225–1232.
- Yu F, Gugel RK, Kutcher R, Peng G, Rimmer SR. 2013. Identification and mapping of a novel blackleg resistance locus LepR4 in the progenies from Brassica napus x B. rapa subsp. sylvestris. Theor Appl Genet. 126:307–315.
- Yu F, Lydiate DJ, Rimmer SR. 2005. Identification of two novel genes for blackleg resistance in Brassica napus. Theor Appl Genet. 110:969–979.
- Yu F, Lydiate DJ, Rimmer SR. 2008. Identification and mapping of a third blackleg resistance locus in Brassica napus derived from B. rapa subsp. sylvestris. Genome. 51:64–72.
- Zhang L, Cai X, Wu J, Liu M, Grob S, Cheng F, Liang J, Cai C, Liu Z, Liu B, et al. 2018. Improved Brassica rapa reference genome by single-molecule sequencing and chromosome conformation capture technologies. Hort Res. 5:50.
- Zhang X, Fernando DWG. 2018. Insights into fighting against blackleg disease of Brassica napus in Canada. Crop Pasture Sci. 69:40–47.
- Zhang X, Peng G, Kutcher HR, Balesdent MH, Delourme R, Fernando DWG. 2016. Breakdown of Rlm3 resistance in the Brassica napus-Leptosphaeria maculans pathosystem in western Canada. Eur J Plant Pathol. 145:659–674.
- Zhang X, White RP, Demir E, Jedryczka M, Lange RM, Islam M, Li ZQ, Huang YJ, Hall AM, Zhou G, et al. 2014. Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathol. 63:598–612.
- Zou Z, Liu F, Fernando DWG. 2018. Rapid detection of Leptosphaeria maculans avirulence gene AvrLm4-7 conferring the avirulence/virulence specificity on Brassica napus using a tetra-primer ARMS-PCR. Eur J Plant Pathol. 152:515–520.
