?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The peach (Prunus persica) is a fruit that is susceptible to many fungal infections following harvest, leading to significant losses. This study was aimed at evaluating the efficacy of a novel formulation prepared from mint and thyme essential oils and used to protect peaches from postharvest decay. The formulation was prepared by mixing the oils with Arabic gum as a coating material and Tween 20 as an emulsifier. The formulations were tested for their efficacy in inhibiting fungal growth in vitro and suppressing disease development on the fruit under a wide range of temperatures and under cold storage conditions. The results demonstrated the inhibition of growth of Botrytis cinerea, Penicillium expansum and Rhizopus stolonifer following application of the formulations. Six days following application of the formulations, the reduction in growth of the three pathogens was 83.0%, 77.0% and 88.0%, respectively. The formulations succeeded in protecting peaches from postharvest rot under a wide range of temperatures (5–30°C) in vivo. In cold storage (4°C), the formulations protected peaches from the three fungal pathogens for 10 days and significantly hindered disease progress compared with the controls, Arabic gum and Tween 20, for up to 30 days. Use of oil formulations reduced the disease incidence to 25.0–30.0% and lowered disease severity to 26.6–46.7% throughout the time the peach fruit was kept in storage. The findings support the application of the essential oil formulations, Arabic gum and Tween 20 as effective, natural and edible coating materials to preserve peaches free from infections for an extended period.
Résumé
La pêche (Prunus persica) est un fruit sujet à de nombreuses infections à la suite de la récolte, ce qui entraîne de lourdes pertes. Cette étude visait à évaluer l’efficacité d’une nouvelle formulation, préparée à partir d’huiles essentielles de menthe et de thym, pour protéger les pêches de la pourriture se développant après la récolte. La formulation a été préparée en mélangeant les huiles avec de la gomme arabique, comme enduit, et du Tween 20, comme émulsifiant. Les formulations ont été testées en fonction de leur efficacité à inhiber la prolifération fongique in vitro et le développement des maladies sur le fruit pour un vaste éventail de températures ainsi que dans des conditions d’entreposage à froid. À la suite de l’application des formulations, les résultats ont démontré l’inhibition de la croissance de Botrytis cinerea, de Penicillium expansum et de Rhizopus stolonifer. Six jours après l’application, le ralentissement de la croissance des trois agents pathogènes était respectivement de 83,0%, 77,0% et 88,0%. Les formulations ont réussi à protéger les pêches contre la pourriture postrécolte in vivo, et ce, pour une vaste gamme de températures (de 5 à 30°C). En entreposage frigorifique (4°C), les formulations ont protégé les pêches contre les trois agents pathogènes pendant 10 jours et ont significativement entravé la progression de la maladie, comparativement aux témoins, gomme arabique et Tween 20, et ce, jusqu’à 30 jours. L’utilization des formulations à base d’huiles a réduit l’incidence de la maladie à 25,0-30,0% et sa gravité à 26,6-46,7% pendant la durée d’entreposage des pêches. Les résultats soutiennent l’application des formulations d’huiles essentielles, gomme arabique et Tween 20 en tant qu’enduits comestibles efficaces et naturels pour protéger les pêches contre les infections pendant une période prolongée.
Introduction
Peach (Prunus persica L. Batsch) is a sweet stone fruit that is part of the Rosaceae family (Faust and Timon Citation1995). Peach is rich in both bioactive and phenolic compounds that have anti-obesity and anti-inflammatory properties through their ability to reduce ‘bad cholesterol’ or low-density lipoprotein (LDL), a cause of cardiovascular diseases (Kamas et al. Citation2013). Developing countries can experience postharvest losses as high as 50% with losses increasing with variations in climatic conditions and storage methods (Khan et al. Citation2008). The most serious diseases that affect peach quality are postharvest decays that impact the storability of this crop. Grey mould rot caused by Botrytis cinerea, and Rhizopus rot caused by Rhizopus stolonifer are among the most destructive postharvest diseases with the latter being involved in the production of soaked and soft areas on the infected fruit (Fan et al. Citation2000; Parveen et al. Citation2016). Abata et al. (Citation2016) described the signs of B. cinerea infections as starting off as circular and sunken lesions that rapidly spread over the peach surface, subsequently developing grey cottony mycelium associated with the lesions. The growth of the fungus continues to extend to the surface of the healthy portions of the fruit that are often covered by grey and hairy mycelia comprising a mass of black sporangia (Nishijima et al. Citation1990). Penicillium expansum is also a major pathogen implicated in the decay of peaches and is responsible for the loss of a considerable amount of productivity (Liu et al. Citation2005). This fungus forms a blue mould disease on the fruit that can even take hold at temperatures below 0°C, though the decay proceeds slowly at cold storage temperatures. The pathogen may also lead to serious health problems in humans because of the toxic secondary metabolites it produces, such as patulin and citrinin (Neri et al. Citation2006; Yin et al. Citation2017)
To reduce losses in peaches, a suitable strategy must be employed to control development of postharvest diseases. Traditionally, control of postharvest diseases involves use of fungicides and chemical compounds, but their intense application has compromised their efficacy over time due to the development of resistant pathogens (Baraldi et al. Citation2003). Moreover, the use of chemical fungicides as a control strategy risks compromising the well-being of consumers (du Plooy Et Al. Citation2009). Modern green farming technology reduces the use of synthetic pesticides in crop management and encourages the search for safe alternatives as control methods (Brzozowski and Mazourek Citation2018). Biological control agents (BCAs) have emerged as an alternative crop management strategy with many biopesticides developed based on these BCAs (Chan and Tian Citation2005; Xu et al. Citation2013; Pansera et al. Citation2016). However, the use of BCAs to control postharvest diseases of fruit still lacks the required level of success. Primary challenges to using BCAs include the possibility of decay or contamination by the BCA itself. Additionally, application of BCAs can lead to the fruits developing an undesirable appearance which stops consumers from eating them. Therefore, use of natural products with fungicidal properties such as essential oils could play a key role as eco-friendly alternatives for the management of postharvest rots (Abdolahi et al. Citation2010; Elshafie et al. Citation2015; El Ouadi et al. Citation2017).
Application of essential oils in management of postharvest diseases varies depending on their chemical constituents and the strategy of application (Boubaker et al. Citation2016). Many factors affect the efficacy of essential oils including temperature, light and storage time. The varying impact of essential oils is attributed to the fact that these factors can lead to degradation of the essential oil or a change of their integrity (Turek and Stintzing Citation2013). Consequently, these factors are barriers to the use of essential oils in plant disease management. Moreover, the high rate of the oils’ evaporation reduces their efficiency and could make them ineffective, especially for long term applications. Therefore, an appropriate formulation of essential oils is required to reduce their evaporation rate and increase their efficiency in management of plant diseases (Soliman et al. Citation2013).
Arabic gum is a dried exudate of the stems and branches of Acacia trees. It is a water soluble polysaccharide of the hydrocolloid group and composed of arabinogalactan, proteins and several minerals (Motlagh et al. Citation2006). It is used worldwide in the food, beverage and pharmaceutical industry as a versatile additive with many functions such as a protective colloid, coating agent, encapsulating agent, oxidation inhibitor, stabilizer, emulsifier, texturant and food adhesive (El-Kheir et al. Citation2008). Tween (polysorbate) is a non-ionic polymer with hydrophilic and lipophilic groups that are suitable for preparing an oil in water emulsion. The efficient emulsification of Tween 20 compared to other Tweens is attributed to its numerous hydrophilic groups and the short length of its lipophilic groups (Roodbari et al. Citation2016).
The aim of this study was to formulate mint and thyme essential oils by mixing them with Arabic gum and Tween 20 to increase their efficiency in the control of postharvest decay of peaches. The suppressive effect of formulated essential oils and their possible mode of action against Botrytis cinerea, Penicillium expansum and Rhizopus stolonifera in vitro and in vivo were investigated.
Materials and methods
The pathogens
Botrytis cinerea, P. expansum, R. stolonifer were isolated from naturally decayed peaches and grown on potato dextrose agar (PDA) at 27 ± 2°C using the method of Gamagae et al. (Citation2003). Identification keys were used to identify each pathogen based on their micro- and macro-morphological characteristics (Frisvad and Samson Citation2004; Mari et al. Citation2004; Beever and Weeds Citation2007).
Pathogenicity test
The pathogenicity of the three fungi was determined on peach cv. ‘Desert Red’. Healthy fruit were surface sterilized with 1% NaOCl3 for 1 min, washed with sterilized distilled water and left to dry in the open air at room temperature (25 ± 2°C). A cork borer was used to make small holes measuring 0.5 cm in diameter and 0.4 cm in depth on each fruit followed by their individual inoculation with a spore suspension (105 CFU mL−1) of the pathogens (B. cinerea, P. expansum, R. stolonifer). The inoculated fruits were incubated at 27 ± 2°C for 7 d. Ten fruits were used for each treatment and the experiment was repeated twice. At the end of the incubation period, the disease severity was expressed as the decay index of infection by measuring the diameter of the rotten area according to Chastagner and Ogawa (Citation1979).
Essential oil formulations
Pure essential oils of mint (Mentha piperita) and thyme (Thymus vulgaris) were purchased from The Essential Oil Company (Portland, OR, USA). Arabic gum (AG) was obtained from a public market (Abha, Saudi Arabia). Tween 20 was purchased from Sigma-Aldrich Chemicals, Ltd. (Germany). To make 10% Arabic gum (AG) solution, 100 g of AG was dissolved in 1 L of sterilized distilled water and stirred at medium speed using a hotplate magnetic stirrer at 40°C for 1 h (Maqbool et al. Citation2011). The mint essential oil formulation (Mint-T-G) was made by thoroughly mixing mint essential oil (2 mL L−1) and Tween 20 (1 mL L−1) in 10% AG solution. The thyme essential oil formulation (Thyme-T-G) made was by mixing thoroughly thyme essential oil (2 mL L−1) and Tween 20 (1 mL L−1) in 10% AG solution. The pH of both formulations (Mint-T-G and Thyme-T-G) was adjusted to 5.5 with NaOH.
The antifungal activity of the essential oil formulations in vitro
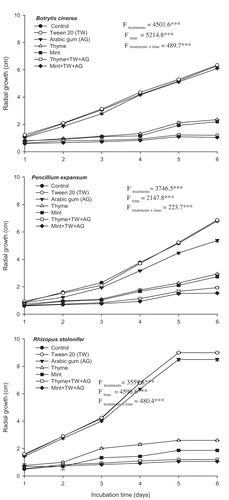
The antifungal activity of Mint-T-G, Thyme-T-G and their individual components (mint essential oil, thyme essential oil, Arabic gum and Tween 20) were tested on PDA medium according to a modified protocol of Tatsadjieu et al. (Citation2009). A melted PDA medium was mixed thoroughly with the essential oil of either mint or thyme (1 mL L−1), Mint-T-G (1 mL L−1), Thyme-T-G (1 mL L−1), Tween 20 (1 mL L−1) or Arabic gum (10%). Then, 15 mL of the medium was poured into Petri dishes (9 cm in diameter), and the plates were inoculated at the centre with a mycelial disc (5 mm diameter) taken from an actively growing fungal colony (4 days old). Control plates that included only PDA were also inoculated using the same procedure. Five plates were used for each treatment, and the plates were incubated at 27 ± 2°C for 6 days, during which the radial growth of the fungal colony was measured daily.
Effect of the essential oil formulations on disease progress in vivo under different temperatures
The method of Maqbool et al. (Citation2011) was followed with modification. Peach fruits were washed with 1% NaOCl3 for 1 min. The peach fruits were subsequently rinsed several times in sterilized water and dried at room temperature (25 ± 2°C). They were dipped in a spore suspension (105 CFU mL−1) of the desired pathogen (B. cinerea, P. expansum and R. stolonifer) for 2 min and left to air dry. Next, fruits for each pathogen were dipped for 2 min in one of the seven preparations: distilled water (control), mint oil, thyme oil, Mint-T-G, Thyme-T-G, Tween 20 (1.0 mL L−1) and AG (10%). In a preliminarily test, application of Tween 20 + AG in one preparation (as a control) did not show any significant change compared with application of either of them singly, so their combined application was excluded. The treated and control fruits were packed in plastic packaging boxes and stored at 30°C, 20°C, and room temperature (min. 5°C and max. 18°C) for 7 d under high relative humidity (80–90%). The three temperatures were selected to enable assessment of the efficiency of the essential oil formulations across a wide range of temperatures to assess their protective impact under different climatic conditions including harvesting, commercialization and storage. Three boxes (five peaches per box) were used for each treatment. Disease incidence and severity were determined after 7 d of incubation. Disease incidence was calculated as the percentage of fruit showing signs of postharvest rot out of the total number of fruit used in each treatment. The disease severity was recorded as described by Sivakumar et al. (Citation2002) using the following scale: 0 = 0% of fruit surface rotten; 1 = 1–5%; 2 = 11–25%; 3 = 26–50%; 4 = 51–75% and 5 = 76–100% rotten. The disease severity percentage was calculated according to the following equation (Waller et al. Citation2002):
Where, n = Number of plants in each category, v = Numerical values of symptoms category (0–5). N = Total number of plants, 5 = Maximum numerical value of symptom category.
Efficiency of the essential oil formulations in controlling the postharvest rot of peach under cold storage (4°C)
The experiment was conducted as described in the previous section, except that the boxes were stored at 4°C for 30 d (time taken for the control fruit to fully rot). Disease incidence, disease severity and weight loss percentage were assessed at intervals of 5 d up to 30 d. The fruit weight loss percentage was calculated using the following equation:
Fruit weight loss % = [(Initial weight – Weight after storage)/Initial weight] x 100
Scanning electron microscopy of the pathogens’ mycelia following treatment with the Mint-T-G formulation
Petri dishes containing PDA medium (4% agar), mixed with Mint-T-G formulation (1:1, v/v) were seeded with mycelial discs (5-mm in diameter) from a 4 days old culture of the fungal pathogens. The plates were maintained for 3–5 d at 27 ± 2°C to obtain visible growth of the fungi. Agar discs with the fungal mycelia were cut with a scalpel into pieces (about 1 cm2), rinsed several times in distilled water and prepared for examination using the scanning electron microscopy (SEM) (JEOL JSM 5300 Lv SEM). Preparation and examination of mycelial samples were completed according to Bozzola and Russell (Citation1999).
Statistical analysis
All statistical analyses were performed with SPSS 22.0 software. The data were initially examined for their normal distribution of errors using Shapiro-Wilk’s W test and for homogeneity of variances using Levene’s test. Data collected from the pathogenicity test of the three fungal pathogens on peaches () were analysed for significance of variation using one-way analysis of variance (ANOVA). The least significant difference (LSD) test was used at P < 0.05 to identify the significant differences among the means of the treatments. The other data, which included the effect of essential oil formulations on the growth of the fungal pathogens in vitro (), the effect of essential oil formulations on disease incidence (%) and disease severity (%) at different temperatures () or in cold storage (), as well as the effect of the oil formulations on fruit weight loss under cold storage (), were analysed statistically using the two-way analysis of variance (ANOVA-2).
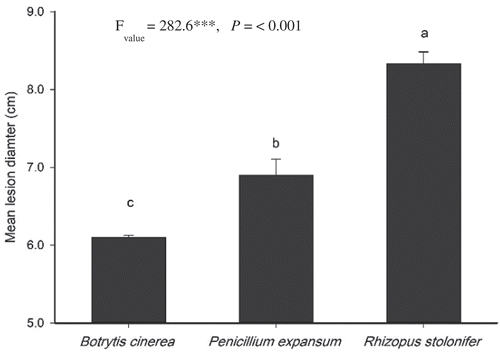
Fig. 1 Pathogenicity of three fungal pathogens on peaches estimated by measurement of lesion diameter (cm) at 27 ± 2°C after 7 days of incubation. The F value represents the result of a one-way ANOVA. Different letters indicate significant differences in the means at LSD (P < 0.05). Bars represent the standard error (n = 10).
Results
Pathogenicity test
The findings confirmed the infection of peaches by the three pathogens–B. cinerea, P. expansum and R. stolonifer––and their contribution to the appearance of the postharvest rot symptoms. Statistical analysis using one-way ANOVA showed a significant difference (P < 0.05) among the means of the three pathogens at 27ºC. Following a 7 d incubation period the mean lesion diameter for each pathogen was recorded as 8.3 ± 0.13 cm, 6.9 ± 0.2 cm and 6.1 ± 0.05 cm for R. stolonifer, P. expansum and B. cinerea, respectively ( and ). Thus, R. stolonifer was classified as the most destructive and B. cinerea as the least destructive based on the width of their lesions. In all cases, the infection started as soft brown areas on the infected fruit, with the growth of the fungus subsequently extending to occupy more healthy portions of the fruit that were covered by heavy fungal mycelium.
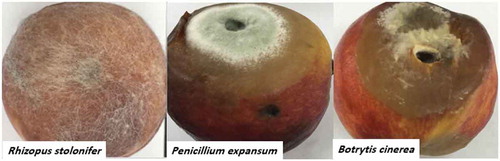
Fig. 2 (Colour online) Rot appearance on peach fruit infected with each of three fungal pathogens after 7 days of incubation at 27 ± 2°C. The infection started as soft soaked brown areas on the fruit, followed by fungal spread into healthy portions and the subsequent appearance of heavy mycelium on infected areas.
Effect of essential oil formulations on fungal growth in vitro
The efficiency of the essential oil formulations in suppression of the fungal pathogens at 27ºC was shown in vitro. Statistical analysis of the results using two-way ANOVA showed that treatments, incubation time and their interaction had significant effects on lesion size (P < 0.001). At this level of probability, the null hypothesis was rejected. After 6 d of incubation, the radial growth of B. cinerea treated with Mint-T-G and Thyme-T-G was 1.05 cm and 1.20 cm, respectively, compared with 6.30 cm in the control. This is a growth reduction of 83% and 80% relative to the control. However, when the oils were applied without preparation, the radial growth of the fungus was 2.20 cm and 2.35 cm for the mint oil and the thyme oil, respectively. Application of either Arabic gum (AG) or Tween 20 (TW) did not show any significant inhibitory effects on mycelial growth of the fungus compared with the control (). The same pattern of inhibition of mycelial growth of both P. expansum and R. stolonifer following application of both formulations was observed and was found to be more significant than for the essential oils without preparations. After 6 d of incubation, the growth of P. expansum was recorded as 1.53 cm and 1.93 cm when treated with Mint-T-G and Thyme-T-G, respectively, compared with the control (6.8 cm). The radial growth of R. stolonifer did not exceed 1.07 cm and 1.20 cm when treated with Mint-T-G and Thyme-T-G, respectively, compared with the control (9.0 cm). Tween 20 did not have any suppressive effect on fungal growth of P. expansum and R. stolonifer, however, Arabic gum showed a very low inhibitory effect on the growth of the pathogens ().
Effect of essential oil formulations on disease progress under different temperatures in vivo
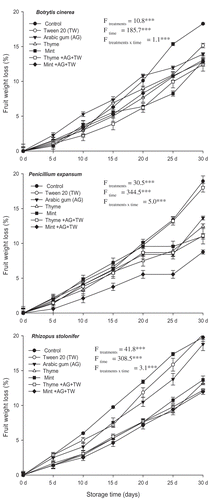
The essential oil formulations were tested for their efficacy in controlling disease progress under various temperatures (30°C, 20°C and room temperature 5–18°C). Results showed that the essential oil formulations contributed to the reduction of disease incidence and disease severity of the three pathogens significantly (P < 0.05) under the three temperatures (). It was observed that at 30ºC, disease incidence of R. stolonifer reached the maximum value (100%) and disease severity was 93.3%. The disease incidence of R. stolonifer on peaches was reduced to 38.3% when Thyme-T-G was applied; however, it was reduced to 28.3% when Mint-T-G was applied instead. It was noted that use of the essential oils on their own without formulation resulted in a significant reduction in disease incidence and severity compared with the control, but the reduction (56.7–51.7% for the incidence and 64.0% for the severity) was significantly lower than that of the essential oil formulations.
Fig. 4 Effect of essential oil formulations on disease incidence (%) and disease severity (%) caused by three fungal pathogens on peaches stored under different temperatures, after 7 days of incubation. Vertical bars indicate the standard error of the mean (N = 15). F-values represent two-way ANOVA results, ***: P < 0.001, **: P < 0.01, *: P < 0.05, ns: not significant.
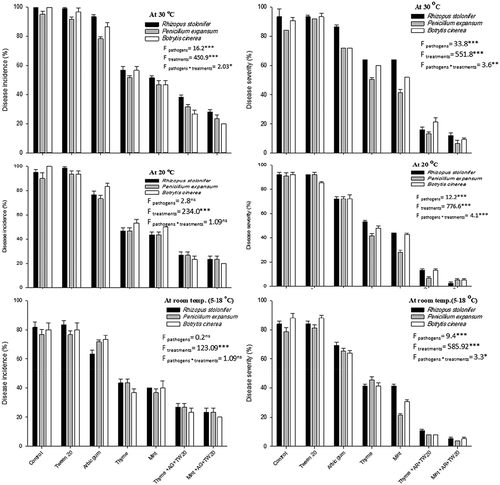
At 20°C, the disease incidence of B. cinerea was 100.0% and the disease severity was 92.0% on peach fruit after 7 d of incubation. The disease severity and incidence of this pathogen were reduced to 5.3% and 20.0%, respectively when the peach fruit were treated with Mint-T-G (). At the same temperature, R. stolonifer caused a severe infection of the peach fruit (92.0% of disease severity and 95.0% of disease incidence). Penicillium expansum showed 90.7% and 90.0%, of disease severity and incidence, respectively. Application of the essential oil formulations led to a significant decrease in both disease severity and incidence compared to the control or the essential oils alone without formulation. It was obvious that the oil formulations succeeded in reducing disease severity and incidence of the three pathogens compared with the carriers (Tween 20 or Arabic gum) or the oils without formulation.
At room temperature, the three pathogens were able to cause similar levels of infection. The disease incidence ranged from 81.7% in the case of R. stolonifer and 76.7% in the case of P. expansum; however, the disease severity ranged from 88.0% in the case of B. cinerea and 78.7% in the case of P. expansum (). The two oil-formulations were effective under the room temperature and reduced disease severity and incidence of the three pathogens to 13.3–4.0% and 26.7–20.0%, respectively. Mint-T-G was more effective in suppression of fruit rot caused by the three fungi than Thyme-T-G under the three temperatures. In all cases, Tween 20 did not lead to a significant change in disease incidence or disease severity compared to the control treatment. However, the Arabic gum reduced the disease severity and the disease incidence by a very low percentage.
Effect of essential oil formulations on disease progress and fruit shelf-life under cold storage conditions (4ºC)
Under cold storage conditions, two-way ANOVA analysis showed that treatments, storage time and their interaction had a significant effect on disease incidence and severity at P < 0.001. At this level of probability, the null hypothesis was rejected, and the alternative hypothesis was supported. Peach rot following the fruit’s infection with any of the three pathogens progressed slowly from day one to 10 and worsened gradually up to day 30 at which point the whole fruit was rotten. The same pattern was observed on application of Tween 20 and Arabic gum alone. In contrast, application of essential oils as part of the formulation or on their own resulted in significant reduction of disease severity and incidence over the 30-days period, slowing the progress of the three pathogens (). In the case of B. cinerea, the signs of fruit rot appeared on day five but when treated with the essential oils alone or in formulation, signs of rot did not appear until day 15. After 30 days of storage, the disease incidence was 28.0–25.0% upon application of Thyme-T-G and Mint-T-G, in comparison with 100% in control and Tween 20 treatments.
Fig. 5 Effect of essential oil formulations on disease severity and incidence caused by three fungal pathogens on peaches kept under cold storage (4°C) for up to 30 days in vivo. Vertical bars indicate the standard error of the mean (N = 15). F-values represent two-way ANOVA results, ***: P < 0.001.
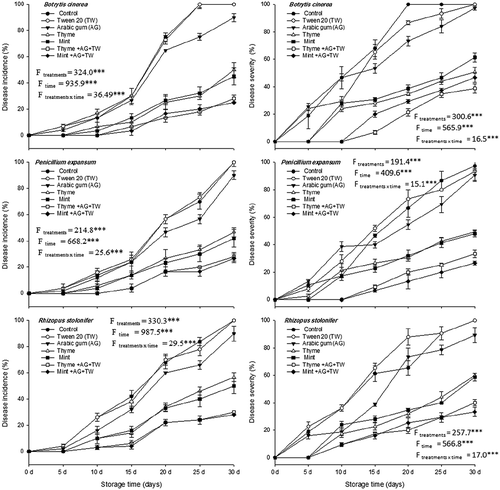
Application of the formulated essential oils significantly reduced disease incidence and severity compared with treatment with oils alone (without formulation) in the case of P. expansum. The results showed that Mint-T-G and Thyme-T-G delayed the appearance of rot symptoms on peaches until day 15 of incubation. At the end of the storage period, the disease incidence was 26.7% and 28.0%, respectively, as a result of treatment with these formulations (). The corresponding disease severity, at the end of the storage period, was 26.7% and 33.3%, in comparison with a disease severity of 97.3%, 93.3% and 90.7% that was detected in control, Tween 20 and Arabic gum. When the oils were applied without formulation, disease severity was reduced to 48.0–49.3%.
In a similar pattern, disease symptoms caused by R. stolonifer were observed after day 5 of storage, while fruit treated with the essential oils or the formulations did not exhibit any signs of rot until day 10. At the end of the storage period, disease incidence and severity was 100.0%. However, when the Mint-T-G and Thyme-T-G were applied, disease incidence was reduced to 33.3% and 40.0%, respectively. The corresponding disease severity for the two treatments were 28.0% and 30.0%, respectively. In all cases, Arabic gum alone showed a very low reduction of both disease severity and disease incidence ().
Effect of essential oil formulations on fruit weight loss under cold storage (4ºC)
The results showed that application of essential oils either alone or in preparation with Arabic gum and Tween 20 reduced the weight loss percentage of peaches significantly (P < 0.05) during cold storage. Two-way ANOVA results did not support the null hypothesis. Thus, the evidence generated indicates the existence of significant differences between the various treatments and the various incubation periods and between treatments and storage times. After day 30, the decrease in the weight of fruit infected with B. cinerea and treated with Mint-T-G and Thyme-T-G was 12.82% and 12.39%, respectively compared to 12.9% and 13.0% when the essential oils were applied alone. However, the decrease in fruit weight in the control, Tween 20 and Arabic gum treatments was 18.27%, 15.14% and 13.9%, respectively (). Penicillium expansum caused 18.9% loss in the fruit weight after 30 days in cold storage, but the decrease in weight of fruit treated with essential oils either alone or in formulation did not exceed 12.5% (). Mint-T-G had the highest protective effect and contributed to the lowest weight loss (8.8%). Rhizopus stolonifer caused the the greatest weight loss compared with the other two pathogens; contributing to a 19.8% weight loss after day 30 of storage. Application of Mint-T-G and Thyme-T-G reduced the weight loss to 11.97% and 12.18%, respectively (). When the essential oils were applied alone, the weight loss was 13.2–13.7%. Neither Arabic gum nor Tween 20 alone resulted in a significant reduction in the fruit weight loss percentage compared to the control.
Effect of Mint-T-G on the mycelial structure of the pathogens
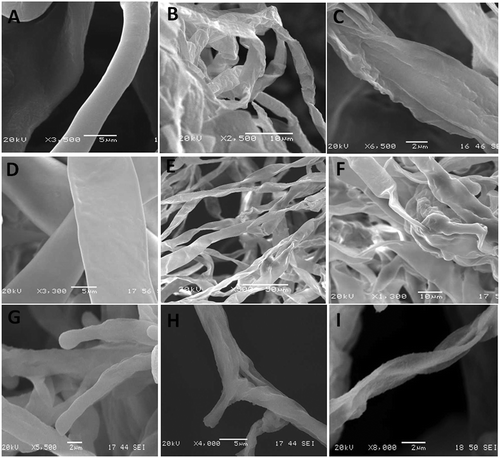
The effect of Mint-T-G, the most effective formulation against the three pathogens, was examined by scanning electron microscopy (SEM) to evaluate its efficacy in suppressing or killing the fungal pathogens. The SEM micrographs generated illustrated the fungitoxicity of the essential oil formulations following fungal treatment. The treatment, for all three fungi, resulted in a visible degradation of the hyphae. Nearly all cases exhibited compressed, indistinct and plasmolysed hyphae compared to the untreated hyphae which exhibited smooth normal cells full of cytoplasm ().
Fig. 7 Scanning electron micrographs of hyphae of three fungal pathogens treated with a peppermint essential oil formulation with Arabic gum and Tween 20 (Mint-T-G) at a concentration of 1 mL L−1. Botrytis cinerea (a–c), Penicillium expansum (d–f), and Rhizopus stolonifera (g–i). The untreated hyphae (control) show well-developed inflated cells having normal smooth walls (a, d, and g). The treated hyphae showing plasmolysis, distortion, squashed appearance and complete collapse (b, c, e, f, h, and i).
Discussion
Botrytis cinerea, P. expansum and R. stolonifer are major pathogens contributing to postharvest fruit rot globally (Fan et al. Citation2000; Parveen et al. Citation2016; Yin et al. Citation2017). The findings of this study confirmed the contribution of these pathogens to peach decomposition at a wide range of temperatures. At 27ºC, the most destructive pathogen was R. stolonifer. This fungus has been confirmed as the primary pathogen responsible for postharvest rot of many fruits and vegetables and in this study led to infection of peaches under different conditions of temperature and humidity including cold storage conditions (Al-Hindi et al. Citation2011; Parveen et al. Citation2016). Botrytis cinerea was ranked as the second most common fungal plant pathogen responsible for fruit decay (Dean et al. Citation2012). Penicillium expansum is also a chief causative agent of postharvest decay in a variety of fruits, causing significant loss of productivity and impacting human health through production of the mycotoxin patulin (Marek et al. Citation2003). The broad range of temperatures in which these pathogens can spread signifies the potential for their infection of fruits leading to decay at various stages of production including growth, transportation, commercialization and storage including cold storage. From this perspective, the application of an effective strategy to manage these pathogens using one formula is a big challenge facing the production sector.
The findings of this study confirmed that both of the essential oil formulations tested effectively suppressed the three pathogens in vitro. Preparation of the essential oils in Arabic gum and Tween 20 (thyme-T-G and Mint-T-G) caused a reduction in fungal growth of up to 88.0%. The efficiency of mint and thyme oils in controlling the postharvest pathogens was previously reported (Lopez-Reyes et al. Citation2013; Sivakumar and Bautista-Baños Citation2014; Elshafie et al. Citation2015). This study is the first examining these essential oils as part of an Arabic gum and Tween 20 formulation for their ability to suppress the primary fungal pathogen of peach postharvest rot. These findings are supported by those of Maqbool et al. (Citation2011), who applied the mixture of lemongrass and cinnamon essential oils and Arabic gum to control anthracnose disease of banana and papaya fruit. Tween 20 did not show any antifungal effect and Arabic gum showed a very weak inhibitory effect. We presume that Tween 20 acts solely as an emulsifier in these preparations enhancing the dispersion of oils in the solution, but that it does not have any antifungal activity. Arabic gum is not known to contain any antifungal constituents, but its low antifungal ability can be attributed to its adhesive-like texture that could cause growth retardation of the fungal germ tubes.
An important outcome of this study was the success of the essential oil formulations in protecting peaches against postharvest rot caused by any of the three pathogens and at a broad range of temperatures (5–30ºC). The disease incidence and severity of the three pathogens were reduced significantly under the three temperatures, and the effectiveness of mint oil was higher than that of thyme oil. Similarly, preparation of Mint-T-G showed a higher inhibitory effect than Thyme-T-G. The Tween 20 and Arabic gum alone treatments did not exhibit a significant change compared with the control. The antifungal activity of mint and thyme was previously demonstrated in many studies (Lopez-Reyes et al. Citation2013; Jhalegar et al. Citation2015; Santoro et al. Citation2018). In accordance with these findings, Matan et al. (Citation2009) demonstrated the antifungal effect of mint oil and its major constituents. In the same context, Bouchra et al. (Citation2003) showed the efficiency of the volatile oil of thyme in the inhibition of mycelial growth of B. cinerea.
The findings of this study demonstrate the efficacy of the essential oil formulations – as safe and edible coating materials – in protecting peaches against common fungal pathogens active over a broad range of temperatures and throughout temperature fluctuations. For example, variations in room temperature under our conditions were found to range from a maximum of 18°C and a minimum of 5°C. Under cold storage at a constant temperature (4°C), the essential oil formulations protected peaches against the three fungal pathogens tested during the first 10 days of incubation and retarded disease progress for up to 30 days. Disease incidence remained low at 25.0–30.0% after 30 days and disease severity reached 26.6–46.7%. Furthermore, the oil formulations reduced the percentage of peach weight loss during the cold storage period. These outcomes show the highly protective impact the essential oil formulations impart on the peaches compared to use of essential oils on their own. Based on the final results and consistency of the data for both in vitro and in vivo experiments, it can be assumed that the use of formulated essential oils in Arabic gum and Tween 20 as coating materials for the protection of peaches from fungal infections can be achieved under a wide range of temperatures and can also prolong the shelf-life of the fruit in cold storage. To the best of the authors’ knowledge, this is the first report on the impact of the application of formulations of mint or thyme essential oils in an Arabic gum and Tween 20 coating material and its ability to protect peaches against postharvest decay whilst also prolonging their shelf-life. A review of the literature revealed that only one study has dealt with the application of formulations of Arabic gum and essential oils in the management of anthracnose and the quality of banana and papaya (Maqbool et al. Citation2011).
As this study’s target is an edible fruit, it is crucial that the selected essential oils and the coating materials are safe for human consumption. The essential oils of both mint and thyme were deemed safe materials that have been used in various public and pharmaceutical applications globally (Ali et al. Citation2015). Thyme is consumed orally for the treatment of many infectious diseases (Conrad and Kemmerich Citation2006). Mint is added to food or as a tea and also has many other uses. It has a long history of use for digestive disorders. Both clinical and in vitro research demonstrated its safety on human health (Keifer et al. Citation2008). In this study, these two oils were used at very low concentrations of 2 mL L−1 to avoid the taste associated with them.
Arabic gum is an arabinogalactan polysaccharide that is obtained naturally from the Acacia tree and used in industry for many purposes due to its emulsifying properties, low solution viscosity and encapsulation properties (Motlagh et al. Citation2006). Mixing essential oils with Arabic gum could act as a barrier retarding the growth of fungi and protecting the fruit tissues from penetration by the pathogens’ germ tubes (Palhano et al. Citation2004; Maqbool et al. Citation2011). Various studies have supported the use of numerous essential oil based edible films, and coatings have been developed to control postharvest pathogenic microorganisms. Bosquez-Molina et al. (Citation2010) reported the successful application of mesquite gum-based coating incorporated with thyme and Mexican lime essential oils to control Colletotrichum gloeosporioides and R. stolonifer growth in vitro and for the protection of papaya fruit from rot during storage.
Examining the fungal hyphae treated with Mint-T-G using the SEM revealed the strong fungicidal effect of the formulation on the tested fungi. The treated hyphae were plasmolysed and completely collapsed. This finding indicates that the formulations maintained the chemical constituents of essential oils that caused the destruction of the fungal hyphae. This is the first study to examine the effect of a formulated essential oil in Arabic gum and Tween 20 on fungal structure. Given the previous findings that examined the effect of pure or native essential oils on fungal mycelia, the mechanism of antifungal activity of these oils can be postulated. In this respect, Salem et al. (Citation2016) showed that lemongrass oil altered the mycelia of R. stolonifer and shrivelled their surfaces. Other studies showed that the mycelia of different pathogens, when subjected to volatile oils, suffered from collapse, squashing or deformation (Yahyazadeh et al. Citation2008; Salehan et al. Citation2013). Zhou et al. (Citation2018) proposed that the volatile oil’s main constituents were responsible for its mode of action. Examples of such volatile oils include carvacrol and eugenol that result in the shrinking and rupture of the fungal cell plasma membrane. These constituents may carry out their functions by preventing cell membrane synthesis of ergosterol, resulting in cell membrane impairment and breakage, consequently causing the seepage of the fungal cell’s internal material leading to its death (Ghannoum and Rice Citation1999; Ma et al. Citation2016). An alternative mode of action of the volatile oils could be due to the inhibition of extracellular enzyme synthesis, disruption of the cell wall structure, prevention of cytoplasm formation and integration of the mycelial structure, all of which will lead to mycelial death (Rasooli et al. Citation2006; Sharma and Tripathi Citation2008).
The findings of this study support the efficacy of mint and thyme essential oil formulations in Arabic gum and Tween 20 (Mint-T-G and Thyme-T-G) as a new coating material to protect peaches from postharvest fungal infection. The preparations successfully inhibited growth of the fungi in vitro and suppressed disease development in vivo under a wide range of temperatures. In cold storage, the application of the formulated materials protected the fruit up to one month with very low levels of disease incidence and severity. The results suggest the utility of these formulations in the protection of the fruit during harvesting, transportation, commercialization and storage. Additional studies are needed to determine the efficacy of these formulations against several other postharvest fruit diseases for their potential application on a larger scale.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under grant number R.G.P. 1/43/40.
Additional information
Funding
References
- Abata LK, Izquierdo AR, Viera W, Flores FJ. 2016. First report of Botrytis rot caused by Botrytis cinerea on peach in Ecuador. J Plant Pathol. 98:690.
- Abdolahi A, Hassani A, Ghosta Y, Bernousi I, Meshkatalsadat M. 2010. Study on the potential use of essential oils for decay control and quality preservation of Tabarzeh table grape. J Plant Prot Res. 50:45–52.
- Al-Hindi RR, Al-Najada AR, Mohamed SA. 2011. Isolation and identification of some fruit spoilage fungi: screening of plant cell wall degrading enzymes. Afr J Microbiol Res. 5:443–448.
- Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F. 2015. Essential oils used in aromatherapy: a systemic review. Asian Pac J Trop Biomed. 5:601–611.
- Baraldi E, Mari M, Chierici E, Pondrelli M, Bertolini P, Pratella GC. 2003. Studies on thiabendazole resistance of Penicillium expansum of pears: pathogenic fitness and genetic characterization. Plant Pathol. 52:362–370.
- Beever RE, Weeds PL. 2007. Taxonomy and genetic variation of Botrytis and Botryotinia. In: Elad Y, Williamson B, Tudzynski P, Delen N, editors. Botrytis: biology, pathology and control. Dordrecht: Springer; p. 29–52..
- Bosquez-Molina E, Ronquillo-de Jesús E, Bautista-Baños S, Verde-Calvo J, Morales-López J. 2010. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol Technol. 57:132–137.
- Boubaker H, Karim H, El Hamdaoui A, Msanda F, Leach D, Bombarda I, Vanloot P, Abbad A, Boudyach EH, Ait Ben Aoumar A. 2016. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind Crop Prod. 86:95–101.
- Bouchra C, Achouri M, Hassani LI, Hmamouchi M. 2003. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J Ethnopharmacol. 89:165–169.
- Bozzola JJ, Russell LD. 1999. Electron microscopy: principles and techniques for biologists. Boston (MA): Jones & Bartlett Learning.
- Brzozowski L, Mazourek M. 2018. A sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability. 10:2023.
- Chan Z, Tian S. 2005. Interaction of antagonistic yeasts against postharvest pathogens of apple fruit and possible mode of action. Postharvest Biol Technol. 36:215–223.
- Chastagner G, Ogawa J. 1979. A fungicide-wax treatment to suppress Botrytis cinerea and protect fresh-market tomatoes [Postharvest decay]. Phytopathology (USA).
- Conrad F, Kemmerich B. 2006. Clinical trial in acute bronchitis with a fixed combination of fluid extracts of thyme herb and ivy leaves. Focus Altern Complement Ther. 11:14–15.
- Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J. 2012. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 13:414–430.
- du Plooy W, Regnier T, Combrinck S. 2009. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol Technol. 53:117–122.
- El Ouadi Y, Manssouri M, Bouyanzer A, Majidi L, Bendaif H, Elmsellem H, Shariati M, Melhaoui A, Hammouti B. 2017. Essential oil composition and antifungal activity of Melissa officinalis originating from north-east Morocco, against postharvest phytopathogenic fungi in apples. Microb Pathogol. 107:321–326.
- El-Kheir MKS, Abu El Gasim AY, Baker AAA. 2008. Emulsion-stabilizing effect of gum from acacia senegal (L) willd. The role of quality and grade of gum, oil type, temperature, stirring time and concentration. Pakistan J Nutrit. 7:395–399.
- Elshafie HS, Mancini E, Camele I, Martino LD, De Feo V. 2015. In vivo antifungal activity of two essential oils from Mediterranean plants against postharvest brown rot disease of peach fruit. Ind Crop Prod. 66:11–15.
- Fan Q, Tian S, Xu Y, Wang Y, Jiang A. 2000. Biological control of Rhizopus rot of peach fruits by Candida guilliermondii. Acta Bot Sin. 42:1033–1038.
- Faust M, Timon B. 1995. Origin and dissemination of peach. Hort Rev. 17:331–379.
- Frisvad JC, Samson RA. 2004. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol. 49:1–174.
- Gamagae S, Sivakumar D, Wijeratnam RSW, Wijesundera R. 2003. Use of sodium bicarbonate and Candida oleophila to control anthracnose in papaya during storage. Crop Prot. 22:775–779.
- Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 12:501–517.
- Jhalegar MJ, Sharma RR, Singh D. 2015. In vitro and in vivo activity of essential oils against major postharvest pathogens of Kinnow (Citrus nobilis × C. deliciosa) mandarin. J Food Sci Technol. 52:2229–2237.
- Kamas J, Stein L, Nesbitt M. 2013. Peaches. AgriLife extension. Texas A&M. https://aggie-horticulture.tamu.edu/fruit-nut/files/2015/04/peaches_2015.pdf.
- Keifer D, Ulbricht C, Abrams TR, Basch E, Giese N, Giles M, Kirkwood CD, Miranda M, Woods J. 2008. Peppermint (Mentha xpiperita) an evidence-based systematic review by the natural standard research collaboration. J Herb Pharmacother. 7:91–143.
- Khan M, Rahim T, Naeem M, Shah M, Bakhtiar Y, Tahir M. 2008. Post harvest economic losses in peach produce in district Swat. Sarhad J Agric. 24:705–711.
- Liu H, Jiang W, Bi Y, Luo Y. 2005. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol Technol. 35:263–269.
- Lopez-Reyes JG, Spadaro D, Prelle A, Garibaldi A, Gullino ML. 2013. Efficacy of plant essential oils on postharvest control of rots caused by fungi on different stone fruits in vivo. J Food Prot. 76:631–639.
- Ma BX, Ban XQ, He JS, Huang B, Zeng H, Tian J, Chen YX, Wang YW. 2016. Antifungal activity of Ziziphora clinopodioides Lam. essential oil against Sclerotinia sclerotiorum on rapeseed plants (Brassica campestris L.). Crop Prot. 89:289–295.
- Maqbool M, Ali A, Alderson PG, Mohamed MTM, Siddiqui Y, Zahid N. 2011. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol Technol. 62:71–76.
- Marek P, Annamalai T, Venkitanarayanan K. 2003. Detection of Penicillium expansum by polymerase chain reaction. Int J Food Microbiol. 89:139–144.
- Mari M, Gregori R, Donati I. 2004. Postharvest control of Monilinia laxa and Rhizopus stolonifer in stone fruit by peracetic acid. Postharvest Biol Technol. 33:319–325.
- Matan N, Woraprayote W, Saengkrajang W, Sirisombat N, Matan N. 2009. Durability of rubberwood (Hevea brasiliensis) treated with peppermint oil, eucalyptus oil, and their main components. Int Biodeterior Biodegrad. 63:621–625.
- Motlagh S, Ravines P, Karamallah K, Ma Q. 2006. The analysis of Acacia gums using electrophoresis. Food Hydrocol. 20:848–854.
- Neri F, Mari M, Brigati S. 2006. Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 55:100–105.
- Nishijima WT, Ebersole S, Fernandez JA. 1990. Factors influencing development of postharvest incidence of Rhizopus soft rot of papaya. Acta Hortic. 269:495–502.
- Palhano FL, Vilches TT, Santos RB, Orlando MT, Ventura JA, Fernandes PM. 2004. Inactivation of Colletotrichum gloeosporioides spores by high hydrostatic pressure combined with citral or lemongrass essential oil. Int J Food Microbiol. 95:61–66.
- Pansera MR, Conte RI, E Silva SM, Sartori VC, Silva Ribeiro RT. 2016. Strategic control of postharvest decay in peach caused by Monilinia fructicola and Colletotrichum gloeosporioides. Appl Res Agrotechnol. 8:7–14.
- Parveen S, Wani A, Bhat M, Koka J, Wani F. 2016. Management of postharvest fungal rot of peach (Prunus persica) caused by Rhizopus stolonifer in Kashmir Valley, India. Plant Pathol Quarant. 6:19–29.
- Rasooli I, Rezaei MB, Allameh A. 2006. Growth inhibition and morphological alterations of Aspergillus niger by essential oils from Thymus eriocalyx and Thymus x-porlock. Food Control. 17:359–364.
- Roodbari NH, Badiei A, Soleimani E, Khaniani Y. 2016. Tweens demulsification effects on heavy crude oil/water emulsion. Arabian J Chem. 9:S806–S811.
- Salehan NM, Meon S, Ismail IS. 2013. Antifungal activity of Cosmos caudatus extracts against seven economically important plant pathogens. Int J Agric Biol. 15:864–870.
- Salem EA, Youssef K, Sanzani SM. 2016. Evaluation of alternative means to control postharvest Rhizopus rot of peaches. Scient Hortic. 198:86–90.
- Santoro K, Maghenzani M, Chiabrando V, Bosio P, Gullino ML, Spadaro D, Giacalone G. 2018. Thyme and savory essential oil vapor treatments control brown rot and improve the storage quality of peaches and nectarines, but could favor gray mold. Foods. 7:7.
- Sharma N, Tripathi A. 2008. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol Res. 163:337–344.
- Sivakumar D, Bautista-Baños S. 2014. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 64:27–37.
- Sivakumar D, Hewarathgamagae N, Wijeratnam RW, Wijesundera R. 2002. Effect of ammonium carbonate and sodium bicarbonate on anthracnose of papaya. Phytoparasitica. 30:486–492.
- Soliman EA, El-Moghazy AY, El-Din MSM, Massoud MA. 2013. Microencapsulation of essential oils within alginate: formulation and in vitro evaluation of antifungal activity. J Encapsul Adsorp Sci. 3:48.
- Tatsadjieu N, Dongmo PJ, Ngassoum M, Etoa F, Mbofung C. 2009. Investigations on the essential oil of Lippia rugosa from Cameroon for its potential use as antifungal agent against Aspergillus flavus Link ex. Fries. Food Control. 20:161–166.
- Turek C, Stintzing FC. 2013. Stability of essential oils: a review. Comprehen Rev Food Sci Food Saf. 12:40–53.
- Waller JM, Lenné JM, Waller SJ, editors. 2002. Plant pathologist’s pocketbook. Oxon (UK): Cabi.
- Xu B, Zhang H, Chen K, Xu Q, Yao Y, Gao H. 2013. Biocontrol of postharvest Rhizopus decay of peaches with Pichia caribbica. Curr Microbiol. 67:255–261.
- Yahyazadeh M, Omidbaigi R, Zare R, Taheri H. 2008. Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J Microbiol Biotechnol. 24:1445–1450.
- Yin G, Zhang Y, Pennerman KK, Wu G, Hua SST, Yu J, Jurick WM, Guo A, Bennett JW. 2017. Characterization of blue mold Penicillium species isolated from stored fruits using multiple highly conserved loci. J Fungi. 3:12.
- Zhou D, Wang Z, Li M, Xing M, Xian T, Tu K. 2018. Carvacrol and eugenol effectively inhibit Rhizopus stolonifer and control postharvest soft rot decay in peaches. J Appl Microbiol. 124:166–178.