Abstract
Anthracnose was observed on postharvest avocados (Persea americana) in a commercial market in Jinju, South Korea, in 2019. Symptoms included soft rot and sunken lesions leaking necrotic fluid. Orange conidial masses were observed on the outer surfaces of these unappealing avocados. Colletotrichum fungi producing grey to olive colonies with orange conidial masses were consistently recovered from the lesions on the avocados. The aim of this study was to isolate and identify the causative pathogen. Koch’s postulates were completed by conducting pathogenicity tests on avocado fruit and wounded green Arabica coffee (Coffea arabica) berries. The symptoms induced artificially on avocado fruit were the same as those of the original infections, whereas negative control avocados were asymptomatic. The fungus associated with this anthracnose produced lesions on wounded coffee berries, but not on unwounded berries. Using morphological characterization, pathogenicity testing, and partial sequencing of the internal transcribed spacer (ITS) rDNA region and the glutamine synthetase (GS) gene, the fungus was identified as Colletotrichum kahawae subsp. ciggaro B. Weir & P.R. Johnst. The results of this study are expected to provide important information for avocado transportation and quarantine.
Résumé
En 2019, l’anthracnose a été observée dans un marché commercial de Jinju, en Corée du Sud, sur des avocats (Persea americana) qui avaient étépréalablement entreposés. Les symptômes incluaient de la pourriture molle et des lésions concaves d’où suintait un fluide nécrotique. Des masses conidiennes orangées étaient observables sur la surface de ces avocats peu attrayants. Des champignons du genre Colletotrichum, produisant des colonies variant de gris à olive comportant des masses conidiennes orangées, étaient invariablement prélevés sur les lésions des avocats. Le but de cette étude était d’isoler et d’identifier l’agent pathogène causal. Les postulats de Koch ont été vérifiés par des tests de pathogénicité menés sur des avocats et des baies vertes de café arabica (Coffea arabica). Les symptômes provoqués artificiellement sur les avocats étaient les mêmes que ceux des infections originales, alors que les avocats témoins négatifs étaient asymptomatiques. Le champignon associé à cette anthracnose a produit des lésions sur les baies de café meurtries, mais pas sur les baies saines. À l’aide de la caractérisation morphologique, de tests de pathogénicité et du séquençage partiel de la région de l’espaceur transcrit interne (ITS) de l’ADNr et du gène de la glutamine synthétase (GS), les champignons ont été identifiés en tant que Colletotrichum kahawae subsp. ciggaro B. Weir & P.R. Johnst. On s’attend des résultats de cette étude qu’ils fournissent de précieux renseignements sur le transport et la quarantaine des avocats.
Introduction
Avocado (Persea americana L.), which is native to South Central Mexico, is a nutritionally valuable and popular fruit worldwide. Avocado trees are cultivated in tropical and Mediterranean climates. The fruit contains large amounts of mono-unsaturated fatty acid, and is rich in vitamins, minerals, antioxidants and antimicrobials (Dreher & Davenport Citation2013). These factors make avocados popular as salad foods. In South Korea, avocado consumption is entirely dependent on imports because it cannot be cultivated in temperate climate zones.
Various postharvest diseases of avocado have been reported previously (Hartill Citation1991; Dann et al. Citation2013). Anthracnose is among the most serious, causing quality deterioration in avocado during storage and transportation (Peres et al. Citation2002; Everett Citation2003; Giblin et al. Citation2010; Hunupolagama et al. Citation2015; Farr and Rossman Citation2019). Management of avocado quality during storage and transportation is entirely dependent on fungicide treatments (Sivakumar and Bautista-Baños Citation2014).
Anthracnose was observed on imported avocados in a market in Jinju, South Korea in 2019. Symptoms included sunken necrotic lesions and soft rot which leaked fluid. Orange conidial masses and hyphal growth were observed on the outer surfaces of infected avocados, producing an unpleasant appearance that could cause consumer aversion. To identify the fungal agent causing anthracnose on these avocados, we performed fungal isolation, morphological characterization, pathogenicity testing and molecular identification.
Materials and methods
Pathogen isolation and morphological characterization
Isolations of the pathogen were made as previously described (Kwon et al. Citation2017) with some modifications. Five avocado fruit with anthracnose symptoms were collected. Tissue samples (3–5 mm) were collected from both symptomatic and healthy fruit tissues, surface-sterilized by immersion in 1% (w/v) sodium hypochlorite solution for 30 s, washed twice in sterile distilled water, and dried on sterile filter paper. The sterilized tissues were placed on water agar (WA) and incubated at 25°C for 2 days. Mycelial tips were then transferred to potato dextrose agar (PDA). The morphological characteristics of fungi grown on PDA for 7 days were examined by light microscopy (Axioplan; Carl Zeiss, Jena, Germany) under 400× magnification.
Pathogenicity test
For pathogenicity testing, three fungal isolates (MHGNU F131–3) were grown on PDA at 25°C for about 10 days prior to inoculation. To prepare conidial suspensions, PDA cultures were flooded with sterile distilled water, gently shaken, and filtered through sterile cheesecloth to remove the mycelia. The conidial concentration was measured using a hemocytometer, and adjusted to 3 × 105 conidia mL−1 in sterile distilled water. Three healthy avocados purchased from a commercial market were surface-sterilized with 70% ethanol, and 30 μL of the conidial suspension was placed on each avocado. The inoculated avocados were placed in a plastic box with a lid (20 × 22 × 15 cm) containing moistened sterile paper towels to maintain humidity and incubated at 25°C for 10 days. Three additional avocados were inoculated with sterile distilled water as a negative control.
Pathogenicity testing was also performed on green coffee berries. Green Arabic coffee (Coffea arabica L.) berries were collected from a coffee farm in Goseung, South Korea. Ten surface-sterilized green coffee berries were wounded using a sterile needle. A 30-μL aliquot of conidial suspension (3 × 105 conidia mL−1) was placed on the surface of wounded or unwounded berries. The inoculated coffee berries were placed in a plastic box with a lid and incubated as above. Sterile distilled water was used to inoculate negative controls. Symptoms on coffee berries were observed for 12 days after inoculation.
Molecular identification and phylogenetic analysis
To identify the fungal pathogen, the internal transcribed spacer (ITS) region and a region of the glutamine synthetase (GS) gene of isolate MHGNU F131 were amplified with the primer pairs ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. Citation1990), and GSF1 (5′-ATGGCCGAGTACATCTGG-3′) and GSR1 (5′-GAACCGTCGAAGTTCCAG-3′) (Stephenson et al. Citation1997), respectively. Total DNA was extracted using the Exgene Plant-Fungal SV Mini Kit (GeneAll Biotechnology Co., Seoul, South Korea). Polymerase chain reactions (PCR) were performed using Taq polymerase (TaKaRa, Tokyo, Japan) on a T100 Thermal Cycler (Bio–Rad, Hercules, CA) under the following conditions: 94°C for 2 min; 30 cycles of denaturation at 98°C for 30 s, annealing at 55°C for 30 s, and extension at 70°C for 1 min; followed by a final extension at 72°C for 4 min. Amplification products were examined by electrophoresis in 0.8% agarose gels and purified using Expin Gel SV (GeneAll Biotechnology, Seoul, Korea) following the manufacturer’s instructions. Sequencing was performed at Macrogen (Daejeon, South Korea) using the same primer pairs used for PCR amplification. DNA sequences were compared using the BLAST nucleotide programme of the National Center for Biotechnology Information (NCBI) reference database.
Sequence alignment of the ITS region and GS partial gene was performed using ClustalW. Phylogenetic trees were generated using MEGA software (ver. 7.0) with the Maximum likelihood method based on the Tamura–Nei model (Tamura and Nei Citation1993; Kumar et al. Citation2016). Sequences of related Colletotrichum strains and Fusarium redolens were downloaded from the GenBank database. Fusarium redolens M72 (GenBank accession no. KP295497) and C. theobromicola CBS124945 (JX010139) were used as outgroups.
Results and discussion
Anthracnose on avocado fruit was observed in a commercial market in Jinju, South Korea. The symptoms included sunken lesions and soft rot with leaking fluid. Orange conidial masses formed on the outer surfaces of the avocados (). Longitudinal sections of infected avocado fruits from the commercial market exhibited severe brown soft rot symptoms (). Transverse sections of infected avocado fruits showed a white mycelial growth ().
Fig. 1 (Colour online) Symptoms caused by Colletotrichum kahawae subsp. ciggaro on (a) avocado fruit from a commercial market in South Korea, (b) avocado fruit inoculated with a fungal isolate (MHGNU F131) from the fruit above causing identical symptoms, and (c) coffee berries inoculated with the same isolate causing symptoms similar to coffee berry disease
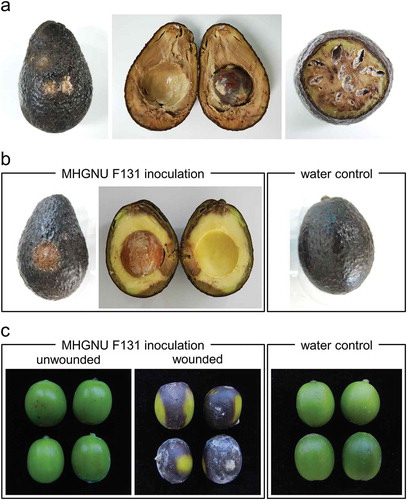
In pathogenicity tests, avocados inoculated with three fungal isolates including the isolate used for molecular identification, MHGNU F131, showed a sunken appearance after 4 days, and numerous orange conidial masses were observed on the lesions after 10 days. Cross sections of inoculated avocados exhibited dark brown discolouration and soft rot similar to the fruit originally obtained from the commercial market (). In contrast, negative control avocados were asymptomatic.
To fulfil Koch’s postulates, the pathogen was re-isolated from lesions of infected avocado fruit. The morphological characteristics and the ITS rDNA sequences of the fungus isolated from the inoculated fruit were identical to those of the original isolate.
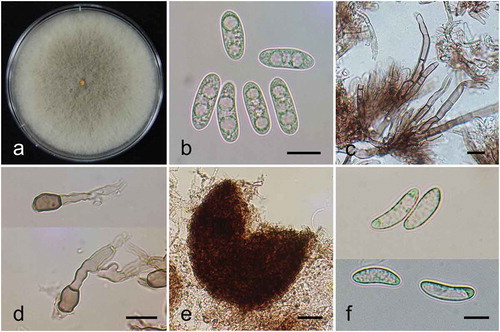
Grey to olive fungal colonies were consistently observed on PDA (). On PDA, the aerial mycelium was initially white to grey, gradually turned greenish-grey, and became dark greenish on the surface and pale orange on the underside. The conidia of isolate MHGNU F131 were straight, cylindrical and aseptate, with a rounded apex and measuring 14–18 × 4.5 μm (). Orange conidial ooze was scattered throughout the culture with numerous dark brown setae (). Appressoria were clavate or slightly irregular, variable in size, dark-walled, and light brown in colour (). Abundant perithecia formed tightly packed clumps, and were dark brown in colour and about 240 μm in size (). Ascospores were slightly curved with rounded ends, measuring 13–16 × 4 μm (). The morphological characteristics of the fungus were similar to those previously described for the C. kahawae group (Weir et al. Citation2012).
Fig. 2 (Colour online) Morphological characteristics of Colletotrichum kahawae subsp. ciggaro isolated from avocado in South Korea. (a), Mycelium grown on PDA. (b), Conidia. (c), Setae. (d), Appresoria. (e), Perithecium. (f), Ascospores. Bar = 10 μm in B, C, D and F and bar = 50 μm in E
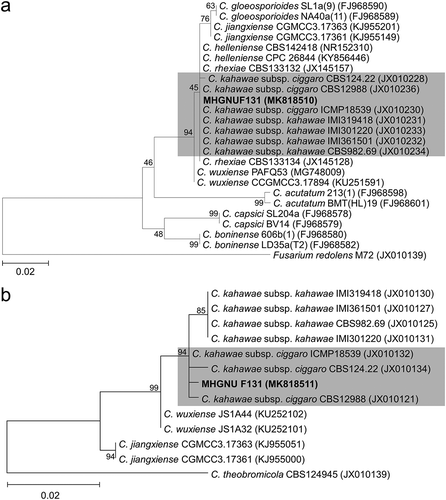
The ITS rDNA (566 bp; GenBank accession no. MK818510) sequence of isolate MHGNU F131 showed 100% identity with that of a C. kahawae subsp. ciggaro isolate VC097 (JN715837) that infects Andean blackberry in Colombia. A phylogenetic tree showed that isolate MHGNU F131 from avocado is located within a clade comprising reference isolates of the C. kahawae group (). Two genetically close subspecies, C. kahawae subsp. ciggaro and C. kahawae subsp. kahawae, can be distinguished using GS sequence analysis (Weir et al. Citation2012). The GS intron (1022 bp; MK818511) sequence of MHGNU F131 exhibited 98% sequence of maximum identity with that of C. kahawae subsp. ciggaro isolate 53 (KF170407), which causes olive anthracnose in Italy. The phylogenetic tree based on the GS sequence showed that isolate MHGNU F131 from avocado is located within a clade comprising reference isolates of C. kahawae subsp. ciggaro (). On the basis of the symptoms, morphological features, and ITS and GS sequence analyses, isolate MHGNU F131 from avocado was identified as Colletotrichum kahawae subsp. ciggaro B. Weir & P.R. Johnst.
Fig. 3 Phylogenetic trees based on ITS rDNA sequence (a) and partial sequence of the glutamine synthetase gene (b) showing the relationship among selected Colletotrichum species including an isolate recently obtained from imported avocado fruits in South Korea
Colletotrichum kahawae subsp. kahawae causes coffee berry disease, causing huge economic losses in Arabica green coffee production (Várzea et al. Citation2002; Chen et al. Citation2005; Silva et al. Citation2006, Citation2012). Therefore, this fungus is ranked as a quarantine pathogen. It is also registered as a biological weapon in Australia and South Korea, and is strictly regulated on imports in coffee-growing regions of Latin America and Asia. Recently, South Korea began to cultivate coffee in a small area. We determined that C. kahawae subsp. ciggaro isolate MHGNU F131 from imported avocado caused coffee berry disease on wounded green Arabica coffee berries (), but not on unwounded berries. These results are consistent with those of another study, which demonstrated that Colletotrichum spp. including C. gloeosporioides, C. acutatum, C. boninese and C. capsici caused moderate sunken lesions on wounded berries (Nguyen et al. Citation2010). In addition, Batista et al. (Citation2017) indicated that because of the close taxonomic relationship between C. kahawae subsp. kahawae and C. kahawae subsp. ciggaro, there has been an overestimation of C. kahawae subsp. ciggaro causing coffee epidemics. Thus, C. kahawae subsp. ciggaro isolated from imported avocados does not appear to pose a risk to coffee production in South Korea.
To our knowledge, this is the first report of avocado postharvest disease caused by C. kahawae subsp. ciggaro. The results of this study are expected to provide critical information for avocado transportation and quarantine.
Additional information
Funding
References
- Batista D, Silva DN, Vieira A, Cabral A, Pires AS, Loureiro A, Guerra-Guimarães L, Pereira AP, Azinheira H, Talhinhas P, et al. 2017. Legitimacy and implications of reducing Colletotrichum kahawae to subspecies in plant pathology. Front Plant Sci. 7:2051.
- Chen ZJ, Liang J, Rodrigues CJJ. 2005. Colletotrichum gloeosporioides can overgrow Colletotrichum kahawae on green coffee berries first inoculated with C. kahawae. Biotechnol Lett. 27:679–682.
- Dann EK, Ploetz RC, Coates LM, Pegg KG. 2013. Foliar, fruit and soilborne diseases. In: Schaffer B, Wolstenholme BN, Whiley AW, editors. The Avocado: botany, production and uses. Wallingford: CABI Publishing; p. 380–422.
- Dreher ML, Davenport AJ. 2013. Hass avocado composition and potential health effects. Crit Rev Food Sci Nutr. 53:738–750.
- Everett KR. 2003. The effect of low temperatures on Colletotrichum acutatum and Colletotrichum gloeosporioides causing body rots of avocado in New Zealand. Plant Pathol. 32:441–448.
- Farr DF, Rossman AY 2019. Fungal databases, U.S. national fungus collections, ARS, USDA. [Internet]. [ accessed 2019 Apr 28]. https://nt.ars-grin.gov/fungaldatabases/.
- Giblin FR, Coates LM, Irwin JAG. 2010. Pathogenic diversity of avocado and mango isolates of Colletotrichum gloeosporioides causing anthracnose and pepper spot in Australia. Plant Pathol. 39:50–62.
- Hartill WFT. 1991. Post-harvest diseases of avocado fruits in New Zealand. N Z J Crop Hortic Sci. 19:297–304.
- Hunupolagama DM, Wijesundera RLC, Chandrasekharan NV, Wijesundera WSS, Kathriarachchi HS, Fernando THPS. 2015. Characterization of Colletotrichum isolates causing avocado anthracnose and first report of C. gigasporum infecting avocado in Sri Lanka. Plant Pathol Quar. 5:132–143.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Kwon J-H, Choi O, Kang B, Lee Y, Park J, Kang D-W, Han I, Park E-J, Kim J. 2017. Identification of Neocosmospora ipomoeae causing tomato stem rot in Korea. Australasian Plant Dis Notes. 12:34.
- Nguyen PTH, Pettersson OV, Olsson P, Liljeroth E. 2010. Identification of Colletotrichum species associated with anthracnose disease of coffee in Vietnam. Eur J Plant Pathol. 127:73–87.
- Peres NAR, Kuramae EE, Dias MSC, Ee Souza NL. 2002. Identification and characterization of Colletotrichum spp. affecting fruits after harvesting in Brazil. Phytopathology. 150:128–134.
- Silva DN, Talhinhas P, Cai L, Manuel L, Gichuru EK, Loureiro A, Várzea V, Paulo OS, Batista D. 2012. Host-jump drives rapid and recent ecological speciation of the emergent fungal pathogen Colletotrichum kahawae. Mol Ecol. 21:2655–2670.
- Silva MC, Várzea V, Guerra-Guimarães L, Azinheira HG, Fernandez D, Petitot A-S, Bertrand B, Lashermes P, Nicole M. 2006. Coffee resistance to the main diseases: leaf rust and coffee berry disease. Braz J Plant Physiol. 18:119–147.
- Sivakumar D, Bautista-Baños S. 2014. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 64:27–37.
- Stephenson SA, Green JR, Manner JM, Maclean DJ. 1997. Cloning and characterization of glutamine synthetase from Colletotrichum gloeosporioides and demonstration of elevated expression during pathogenesis on Stylosanthes guianensis. Curr Genet. 31:447–454.
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 10:512–526.
- Várzea VMP, Rodrigues CJ Jr, Lewis BG. 2002. Distinguishing characteristics and vegetative compatibility of Colletotrichum kahawae in comparison with other related species from coffee. Plant Pathol. 51:202–207.
- Weir BS, Johnston PR, Damm U. 2012. The Colletotrichum gloeosporioides species complex. Stud Mycol. 73:115–180.
- White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York (NY): Academic Press; p. 315–322.
