?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Downy mildew caused by Plasmopara viticola is a devastating disease of grapes. Four formae speciales have been identified in the pathogen, with P. viticola f. sp. aestivalis and P. viticola f. sp. riparia responsible for epidemics in eastern Canada. The objective of this study was to evaluate the effect of temperature on zoospore release from sporangia and the aggressiveness of these two formae speciales. The effect of temperature on zoospore release was determined for incubation time by estimating the per cent empty sporangia. The effect of temperature on aggressiveness was assessed on the grape variety ‘Vidal’ using a leaf disc assay; incidence, latency, severity, sporulation efficiency during infection, penetration and sporulation processes were evaluated. The optimum temperature for zoospore release was 20°C. A significant interaction between temperature and forma specialis for zoospore release was observed; however, zoospore release did not differ between formae speciales. During infection, penetration and sporulation, the optimum temperature for aggressiveness of the two formae speciales was between 17 and 21°C. Indices of aggressiveness for P. viticola f. sp. aestivalis were higher (P < 0.001) than for P. viticola f. sp. riparia. At the optimum temperature, P. viticola f. sp. aestivalis had a 14.3% higher incidence, 13.3% higher severity, produced 2.3-times more sporangia, and had an infection/sporulation cycle that was one day shorter than P. viticola f. sp. riparia. These results suggest that the two formae speciales have different epidemiological profiles, and that decision-support tools should be adjusted to take into account this differential response to temperature.
Résumé
Originaire de l’Amérique du nord, le mildiou causé par Plasmopara viticola, est l’une des plus dévastatrices maladies de la vigne. Des études récentes sur la génétique des populations de P. viticola ont démontré qu’il existe quatre formae speciales, dont deux (P. viticola f. sp. aestivalis et P. viticola f. sp. riparia) sont responsables des épidémies dans l’Est du Canada. Puisque l’épidémiologie de ces nouvelles formae speciales est très peu connue, l’objectif de ce travail était d’évaluer l’influence de la température sur l’éclosion des sporanges et l’agressivité des deux formae speciales de P. viticola. L’effet de la température sur l’éclosion des sporanges a préalablement été déterminé pour différentes fourchettes de température et de durée, en estimant le pourcentage de sporanges vides. Puis, l’effet de la température sur l’agressivité a été étudié sur le cépage Vidal par la technique des disques de feuilles. L’incidence, la sévérité, la latence, et l’efficacité de sporulation durant le processus d’infection, de pénétration et de sporulation sont les variables d’agressivité qui ont été évaluées. La température optimale d’éclosion des sporanges était de 20°C. Une interaction significative entre la température et les formae speciales pour l’éclosion des sporanges était observée. Cependant, il n’y a pas eu de différences significatives d’éclosion des sporanges entre les deux formae speciales. Pendant les processus d’infection, de pénétration et de sporulation, la température optimale pour l’agressivité des deux formae speciales était comprise entre 17 et 21°C. Globalement, P. viticola f. sp. aestivalis était significativement plus agressif que P. viticola f. sp. riparia. Les indices d’agressivité de P. viticola f. sp. aestivalis étaient plus grands (P < 0,001) que ceux de P. viticola f. sp. riparia. En effet, en moyenne, à la température optimale, P. viticola f. sp. aestivalis avait une incidence plus grande de 14.3%, une sévérité plus grande de 13.3%, produisait 2,3 fois plus de sporanges et réduisait de un jour le cycle d’infection/sporulation comparativement à P. viticola f. sp. riparia. Ces résultats suggèrent qu’en plus des différences génétiques et morphologiques, le profil épidémiologique des deux formae speciales est différent et que les outils d’aide à la décision devraient être adaptés pour tenir compte de cette réponse à la température qui est différente entre les deux formae speciales.
Introduction
Native to North America, downy mildew caused by Plasmopara viticola (Berk. & M.A. Curtis) Berl. & De Toni is one of the most devastating diseases of grapevines. The disease appears as oily patches (‘oil spots’) on the upper surface of leaves and as whitish down on the underside of leaves, berries, inflorescences and young stems (Gessler et al. Citation2011; Carisse Citation2016). When conditions are favourable, epidemics of downy mildew result in partial or total destruction of leaves and berries, abortion or drying of flowers, and weakening of vines, all of which have a serious impact on plant vigour, grape yield and grape quality (Gessler et al. Citation2011; Caffi et al. Citation2013; Carisse Citation2016).
Although a number of control methods have been used against this disease, the most effective method is still chemical fungicide applications coupled with decision-support tools (Park et al. Citation1997; Hill Citation2000; Rossi et al. Citation2000; Caffi et al. Citation2007). Such tools are based on an understanding of the influence of weather conditions on the disease cycle.
A number of studies have shown that downy mildew epidemics start with primary infection in the spring, caused by zoospores produced by the maturation and germination of oospores (Caffi et al. Citation2007; Kennelly et al. Citation2007; Gessler et al. Citation2011). A number of secondary infections then follow. During these secondary infections, sporangia are produced and disseminated, the sporangia open and release zoospores, the zoospores germinate and penetrate stomata, haustoria form, mycelia grow, and lastly, sporulation occurs (Lalancette et al. Citation1988; Gessler et al. Citation2011; Rossi et al. Citation2013). All these steps in the infection and sporulation processes are greatly influenced by environmental conditions such as light, temperature, wind, relative humidity and the presence of free water (Kast and Stark-Urnau Citation1999; Caffi et al. Citation2013). Caffi et al. (Citation2016) demonstrated that temperature and leaf wetness duration significantly influenced zoospore release and infection, with the optimum temperature being from 15–20°C.
Several population genetic studies (Kast et al. Citation2000; Gobbin et al. Citation2005; Schröder et al. Citation2011; Fontaine et al. Citation2013) revealed the existence of a number of genetically different P. viticola populations at the vineyard, regional and continental scales. Gobbin et al. (Citation2005) showed that not all the genetic populations of P. viticola present in a vineyard during primary infection are capable of initiating a secondary infection. Some genotypes dominate over others. Indeed, Roatti et al. (Citation2013) found that three genotypes of P. viticola (BO, VOL1 and VOL2) isolated in climatically different regions of Italy had similar aggressiveness but differed in competitiveness when the genotypes were co-inoculated. Competition studies using those genotypes demonstrated that VOL1 had low competitiveness against BO and VOL2. Observations for potato late blight were reported by Fall et al. (Citation2015), who showed that the various clonal lineages of Phytophthora infestans present in Canada differed in aggressiveness.
In addition, a study by Rouxel et al. (Citation2013) on the genetics of P. viticola populations showed that there are four formae speciales of P. viticola, three of which infect cultivated grapes: P. viticola f. sp. aestivalis (P.v. aestivalis), P. viticola f. sp. riparia (P.v. riparia), and P. viticola f. sp. vinifera (P.v. vinifera). The presence of a forma specialis of P. viticola in a region depends on the grape variety grown. In Canada, there are essentially two formae speciales of P. viticola: P.v. aestivalis and P.v. riparia (Rouxel et al. Citation2014; Dugan and Everhart Citation2016; Camargo et al. Citation2019). That raises the question of whether these two formae speciales have epidemiological differences in addition to genetic differences. It would thus be valuable to evaluate the influence of temperature on the aggressiveness of these two formae speciales. The objective of this study was to look at the effect of temperature on zoospore release and aggressiveness in the two formae speciales during the infection, penetration and sporulation processes. This study is the first in an investigation of the comparative epidemiology of the two formae speciales of P. viticola encountered in eastern Canada.
Materials and methods
Collection and storage of P.v. riparia and P.v. aestivalis isolates
Samples of leaves or grapevine tissues showing symptoms of downy mildew were collected from eight vineyards in the Montérégie region of Quebec (latitude: 45°23′14.03″ N; longitude: −73°06′2.76″W). The samples were collected from seven grape cultivars (‘Vidal’, ‘Pinot Noir’, ‘Chardonnay’, ‘Chancellor’, ‘Saint Croix’, ‘Saint Pepin’ and ‘Vandal Cliche’). Portions of tissues containing P. viticola sporangia were collected and placed in plastic bags, the bags were sealed with air inside and stored at room temperature until processed. In the laboratory, 1 cm2 of tissue containing fresh sporangia was collected for identification of the formae speciales using a PCR assay targeting the ITS1 and ITS2 regions as described in Camargo et al. (Citation2019) and in Hong et al. (Citation2019). The rest of the material collected was placed in plastic boxes containing wet filter paper and the plastic boxes were stored overnight in the dark at room temperature to promote the production of fresh spores. Then, for preservation of the P. viticola strains, portions of tissue containing fresh sporangia were placed in empty Petri dishes and stored at −20°C.
Preparation of P.v. riparia and P.v. aestivalis inocula
As P. viticola is a biotrophic parasite, the detached leaf method was used to produce the inocula (Caffi et al. Citation2016). For the first assay, a mixture of five P.v. riparia and three P.v. aestivalis isolates were used to prepare inoculum. For the second and third assays, mixtures of three isolates were used to prepare inoculum of both P.v. riparia and P.v. aestivalis. The inocula were prepared by collecting 1 cm2 of tissue containing fresh P. viticola sporangia in an Eppendorf tube. Then, 1 mL of sterile distilled water was added, and the suspension was shaken by hand for a few seconds to promote the release of sporangia. Next, the sporangial solution thus prepared was counted using a hemocytometer and diluted to 104 sporangia mL−1. The spore suspension was used to inoculate leaf discs from (‘Vidal’) grapevine leaves (fourth to sixth leaves of the apical part of 10- to 12-week-old plants that had been grown in greenhouses) placed in Petri dishes on wet absorbent paper. Inoculation was performed by placing 50 µL of spore suspension per 15-mm diameter leaf disc. The dishes were stored for 24 h at 20°C, 95–100% relative humidity and a photoperiod of 12 h/12 h, after which the excess inoculum was removed, and the dishes were stored under the same conditions for 7 days. The leaf discs on which P. viticola had sporulated were recovered and placed in 50 mL Falcon tubes containing 25 mL of sterile distilled water. The tubes were shaken by hand for a few seconds to release the sporangia. The solutions thus obtained were used as inocula in the following experiments. In this study, the grape cultivar ‘Vidal’ (a hybrid grape produced from Vitis vinifera 75% and Vitis aestivalis 6.4%) was used as host because, based on our preliminary experiments, it is equally susceptible to both forma speciales.
Effect of temperature on zoospore release (empty sporangia)
The effect of temperature (5, 10, 15, 20, 25 and 30°C) and incubation time (0.5, 1, 3, 6 and 24 h) on zoospore release (empty sporangia) for the two formae speciales was evaluated under controlled conditions. Zoospore release was estimated based on the number of empty sporangia (sporangia that had released their zoospores). For each forma specialis, 0.5 mL of inoculum (104 sporangia mL−1) obtained as described above was transferred into 90 1.5 mL Eppendorf tubes and placed in the dark in incubators set to the temperatures under study, with 15 tubes per incubator. For each temperature and incubation time pair, three tubes were randomly removed from the incubator, and then 20 µL of solution was collected from the tubes and sporangia were counted using a hemocytometer under 250× magnification (Caffi et al. Citation2016). For each sample, the number of empty sporangia in 100 randomly selected sporangia was counted. This experiment was conducted as a completely randomized design with three replicates. The experiment was conducted four times, and the incubators and the arrangement of samples in the incubators were selected at random.
Effect of temperature on the aggressiveness of the two formae speciales
The effect of temperature on the aggressiveness of the two formae speciales was evaluated at the same temperatures as in the previous tests on leaf discs in accordance with the method of Caffi et al. (Citation2016). Each test used three Petri dishes containing 15 discs from ‘Vidal’ grapevine leaves, for a total of 36 dishes (6 temperatures × 2 formae speciales × 3 replicates). The leaves used in this study were the fourth to sixth leaves of the apical part of 10- to 12-week-old plants grown in a greenhouse. The 15-mm diameter leaf discs, which were taken from leaves that had been washed with tap water, were placed in Petri dishes (abaxial leaf side up) lined with wet absorbent paper. Then, the underside of each disc was inoculated with one drop (50 µL; 104 sporangia mL−1) of spore solution obtained as described above. Following inoculation, the dishes were stored in incubators set to the experimental temperatures. After 24 h, the leaf discs were washed with tap water to remove excess inoculum, and the dishes were put back into the incubators until day 10 so that the effect of temperature during the infection, penetration and sporulation processes could be evaluated. The experiment was conducted as a completely randomized design with three replicates, repeated twice. In this study, the only source of variation was the temperature. All incubators were calibrated at 95–100% relative humidity and a 12 h/12 h photoperiod.
Effect of temperature during the infection, penetration and sporulation processes on aggressiveness
The inoculated leaf discs were placed in the incubators to evaluate the effect of temperature on aggressiveness of the two formae speciales of P. viticola during three key processes (infection, penetration and sporulation). The following processes were defined in Caffi et al. (Citation2016), Williams et al. (Citation2007) and Lalancette et al. (Citation1988). In the infection process, the dishes were subject to each of the incubation temperatures for 10 days (except those at 20 and 25°C for which the incubation time was 7 days). In the penetration process, the dishes were subject to each temperature for 24 h and then incubated for 6 days at the optimum growth temperature of 20°C (Caffi et al. Citation2016). In the sporulation process, the dishes were incubated at 20°C for 3 days and then at each of the incubation temperatures for sporulation.
Estimation of aggressiveness variables
The Petri dishes were observed daily to estimate latency (LAT), which is defined as the time elapsed from inoculation to the beginning of sporulation (Roatti et al. Citation2013). Incidence (INC) was estimated as the proportion of discs with sporulation in relation to the total number of inoculated discs (15 discs). Severity (SEV) was estimated only in assays two and three as the percentage of leaf disc surface with sporulation. Sporulation efficiency (SPO) was evaluated as the number of sporangia produced per unit of inoculated sporangia. Sporulation efficiency was evaluated based on three leaf discs per Petri dish for assay one, and 15 leaf discs per Petri dish for assays two and three. An index of aggressiveness (IA) for each forma specialis was calculated according to the formula proposed by Roatti et al. (Citation2013) and Corio-Costet et al. (Citation2011): IA = LN [SPO × INC × (1/LAT)].
Statistical analysis
Analyses of variance (ANOVAs) (alpha = 0.05) were used to determine whether there was a significant effect of temperature, of forma specialis, and of the interaction between temperature and forma specialis on each of the aggressiveness variables. To model the effect of temperature, the Gaussian model was chosen based on the shape of the temperature response curves and because the parameters have biological significance. The Gaussian model was fitted as:
where Y is the different aggressiveness variables, X is the temperature, X0 is the optimum temperature, a is the asymptote (maximum value of Y), and b is the dispersion around X0. This model was fitted separately to the data for each forma specialis of P. viticola (P.v. riparia and P.v. aestivalis). To estimate the value of the parameters a, X0 and b, partial derivative equations were obtained and the Marquardt iterative method of the non-linear regression (NLIN) procedure in SAS was used. However, for the aggressiveness variables for the penetration process, it was not possible to fit the Gaussian model (convergence criterion not met), hence, a linear regression model in the form of a second-degree polynomial was used. The model was fitted as: Y = β0 + β1X + β2X2, where Y is aggressiveness variables, and X is the temperature; these were fitted separately to the data for each forma specialis. The resulting models were used to derive the cardinal temperatures (optimum, minimum and maximum temperatures) for the aggressiveness variables. The minimum and the maximum temperature were estimated as the temperature when incidence was 0.05, severity was 1% and sporulation efficiency was 1.0. The different analyses were performed with SAS v. 9.4.
Results
Effect of temperature on zoospore release (empty sporangia)
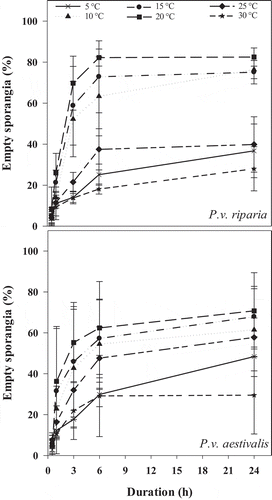
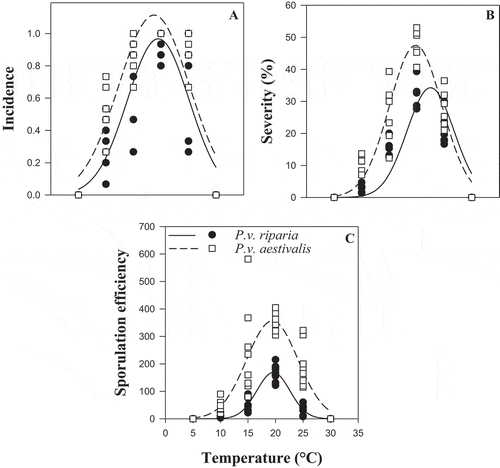
An effect of temperature (P < 0.001) and incubation time (P < 0.001) on per cent empty sporangia was observed, but there was no effect of forma specialis on zoospore release (P = 0.182) (). However, there were interactions between temperature and forma specialis (P < 0.001) and between temperature and incubation time (P < 0.001) (). Observation of the per cent empty sporangia curves for each forma specialis as a function of incubation time showed that the patterns of zoospore release were similar for both formae speciales (). The optimum temperature for zoospore release was 20°C for both formae speciales (), however, a maximum of 83% empty sporangia was observed for P.v. riparia and 75% for P.v. aestivalis (). For a given temperature, the maximum number of zoospores released for the formae speciales was reached after 6 h of incubation at all temperatures except at 5°C, for which optimum zoospore release was not achieved after 24 h ().
Table 1. Analysis of variance of the effect of temperature on the zoospore release (empty sporangia) of two formae speciales of Plasmopara viticola
Effect of temperature during the infection process on the aggressiveness of P.v. riparia and P.v. aestivalis
For both formae speciales, all aggressiveness variables were null at 5, 10 and 30°C (). For all the aggressiveness variables (incidence, severity, latency and sporulation efficiency), the ANOVA showed an effect of temperature (P < 0.001) and of forma specialis (P ≤ 0.010) (). There was an interaction between temperature and forma specialis for severity (P < 0.001) and for sporulation efficiency (P < 0.001) (). The coefficients of determination (R2) of the Gaussian models were between 0.79 and 0.95 depending on the variable (). The curves obtained by fitting the Gaussian model showed that the patterns of temperature response for incidence, severity and sporulation efficiency of both formae speciales were similar but differed in amplitude (). There was a significant difference between the asymptote and the dispersion parameters estimated by the Gaussian model for the two formae speciales (), while the optimum temperature (X0) for the two formae speciales did not differ (). Based on the Gaussian model, during the infection process, the optimum temperature was between 17 and 21°C for both formae speciales (). The minimum temperature for P.v. riparia was between 6.9 and 9.8°C and the maximum temperature was >30°C (estimated between 31.0 and 31.9°C). For P.v. aestivalis, the minimum estimated temperature was between 4.8 and 8.3°C and the maximum temperature was >30°C (estimated between 33.0 and 34.9°C). The forma specialis P.v. aestivalis was more aggressive during the infection process than P.v. riparia as illustrated in . When conditions were favourable for the two formae speciales, P.v. aestivalis was capable of infecting 14% more leaf discs with 15% higher severity, produced two times more sporangia, and shortened the length of the cycle by one day () compared with P.v. riparia.
Table 2. Analysis of variance of the effect of temperature on the aggressiveness variables of two formae speciales of Plasmopara viticola during the infection, penetration and sporulation processes
Table 3. Parameter estimates of the Gaussian models fitted to the aggressiveness variables (incidence, severity and sporulation efficiency) as a function of temperature for the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), during the infection and sporulation processes
Fig. 2 Effect of temperature on the latency of the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), (a) during the infection process, (b) during the penetration process, (c) during the sporulation process, and (d) during all three processes (the average of all three processes). Error bars represent the standard error of mean (STD) of the latency. For each temperature, values of bars with the same letter are not significantly different according to Tukey’s test (P ≤ 0.05)
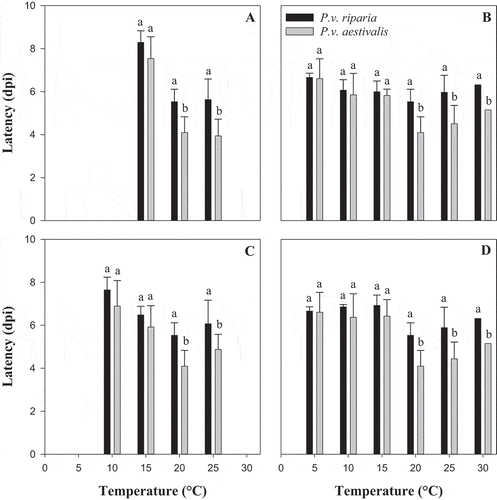
Fig. 3 Representation of the Gaussian models of the effect of temperature on the aggressiveness of the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), during the infection process. (a) Incidence, (b) Severity and (c) Sporulation efficiency
Effect of temperature during the penetration process on the aggressiveness of P.v. riparia and P.v. aestivalis
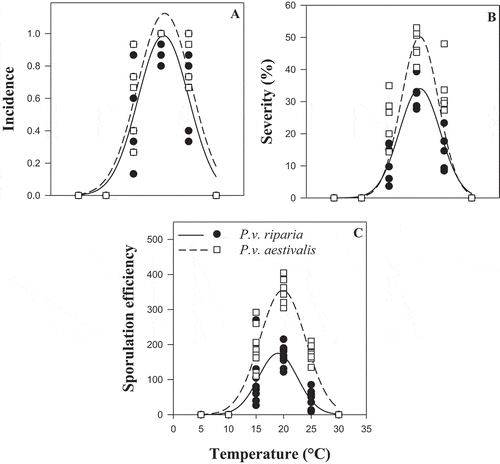
Exploration of the distribution of incidence for P.v. riparia and P.v. aestivalis as a function of temperature ( and 4) showed that, during the penetration process, the aggressiveness variables were not-null for either formae speciales at all the temperatures under study (). The ANOVA for the aggressiveness variables (incidence, severity, latency and sporulation efficiency) revealed an effect of temperature (P < 0.001) and forma specialis (P ≤ 0.001) for all aggressiveness variables (). Additionally, there were interactions between temperature and forma specialis for latency (P = 0.015) and sporulation efficiency (P = 0.001), but not for incidence (P = 0.904) and severity (P = 0.387) (). The estimate of the linear model parameters is presented in . The coefficients of determination (R2) of the linear models were between 0.46 and 0.85 (). The temperature response patterns for incidence, severity and sporulation efficiency of both formae speciales were similar but differed in amplitude (). The optimum temperature during the penetration process was between 17 and 20°C for P.v. riparia and P.v. aestivalis (). The minimum estimated temperature of P.v. riparia was 3.8 and 5.2°C and the maximum temperature was >30°C (estimated between 30.4 and 31.2°C). For P.v. aestivalis, the minimum temperature was <5°C (estimated between 1.9 and 3.9°C) and the maximum temperature was >30°C (between 32.8 and 34.2°C). When all aggressiveness variables at all temperatures during the penetration process are considered, P.v. aestivalis was significantly more aggressive than P.v. riparia. The models obtained showed that when penetration conditions were favourable, P.v. aestivalis had a 15% higher incidence, 12% higher severity, produced 2.9 times more sporangia (), and the length of the infection/sporulation cycle was shortened by one day (), compared with P.v. riparia.
Table 4. Parameters of the linear models (LM) of the effect of temperature on the aggressiveness of the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), during the penetration process
Fig. 4 Representation of linear regression analysis of the effect of temperature on the aggressiveness of the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), during the penetration process. (a) Incidence, (b) Severity and (c) Sporulation efficiency
Effect of temperature during the sporulation process on the aggressiveness of P.v. riparia and P.v. aestivalis
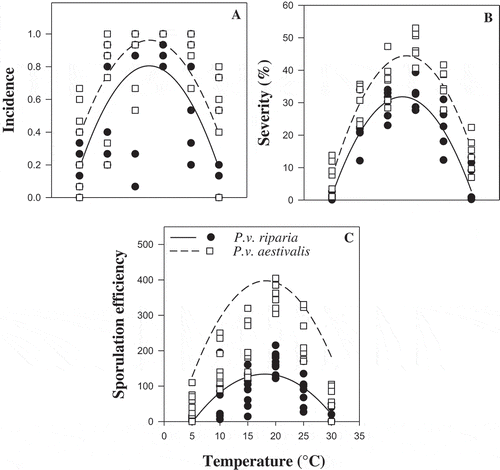
During the sporulation process, the aggressiveness variables did not differ for either forma specialis at 5 and 30°C ( and 5). There was an effect of temperature (P < 0.001) and forma specialis (P < 0.001) for all aggressiveness variables (). The coefficients of determination (R2) of the Gaussian models were between 0.76 and 0.94. The Gaussian models for incidence, severity and sporulation efficiency showed that the distributions of these aggressiveness variables as a function of temperature were similar for both P.v. riparia and P.v. aestivalis (). Similar to the data for the infection processes, there was a difference between the asymptote and the dispersion parameters estimated by the Gaussian model but no significant difference for the optimum temperature parameter (X0) (). The optimum temperature during the sporulation process was between 17 and 21°C for both formae speciales. For P.v. riparia the estimated minimum temperature was between 5.6 and 9.4°C and the maximum temperature was ≥30°C (estimated between 29.8 and 33.4°C) and for P.v. aestivalis the minimum estimated temperature was between 2.5 and 5.5°C and the maximum temperature was >30°C (estimated between 34.1 and 36.3°C). At the optimum temperature during the sporulation process the incidence of P.v. aestivalis was 14% higher than the incidence of P.v. riparia (). Moreover, in comparison with P.v. riparia, P.v. aestivalis had a 13% higher severity (), produced about 2 times as much sporangia (), and the length of the sporulation cycle was shorter (). Although sporulation did not occur on the leaf discs incubated at 5 and 30°C, sporulation was observed after one day when they were reincubated at 20°C (results not shown).
Fig. 5 Representation of the Gaussian models (GM) of the effect of temperature on the aggressiveness of the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), during the sporulation process. (a) Incidence, (b) severity and (c) sporulation efficiency
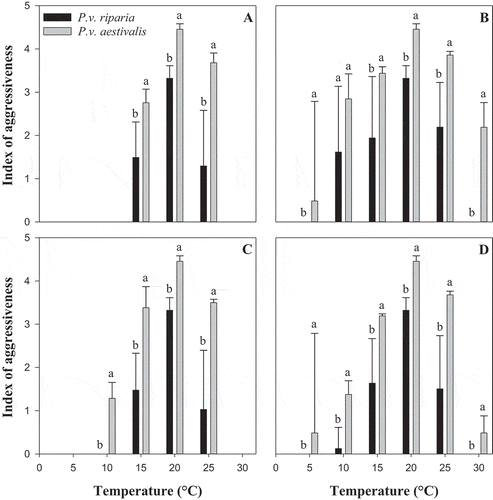
Index of aggressiveness
The indices of aggressiveness showed an effect of temperature and forma specialis on aggressiveness (P < 0.001) (). The index of aggressiveness for both formae speciales were similar (and null) at 5, 10 and 30°C during the infection process (), and at 5 and 30°C during the sporulation process (), however, they differed (and not null) at all temperatures during the penetration process (). At all other temperatures under study, the index of aggressiveness for P.v. aestivalis was higher than that for P.v. riparia (). The averages of the aggressiveness indices of all three key processes for P.v. aestivalis at the temperatures under study were significantly higher than those for P.v. riparia ().
Table 5. Analysis of variance of the effect of temperature on the index of aggressiveness of the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), during infection, penetration, and sporulation and the global process
Fig. 6 Effect of temperature on the index of aggressiveness of the two formae speciales, Plasmopara viticola f. sp. aestivalis (P.v. aestivalis) and P. viticola f. sp. riparia (P.v. riparia), (a) during the infection process, (b) during the penetration process, (c) during the sporulation process and (d) during all three processes (the average of all three processes). Error bars represent the standard error of mean (STD) of the aggressiveness index. For each temperature, values of bars with the same letter are not significantly different according to Tukey’s test (P ≤ 0.05)
Discussion
Rouxel et al. (Citation2013) demonstrated that there are four formae speciales of P. viticola worldwide, which are genetically differentiated and specific to certain grape species and hybrids, as is evident from their names (P. viticola f. sp. vinifera, P. viticola f. sp. riparia, P. viticola f. sp. aestivalis and P. viticola f. sp. quinquefolia) (Dugan and Everhart Citation2016). Rouxel et al. (Citation2014) also showed that the four formae speciales of P. viticola have different geographic distributions. In eastern Canada, there are essentially two formae speciales (P.v. aestivalis and P.v. riparia). Despite these interesting advances in the characterization of these formae speciales, no studies have examined their epidemiological significance. A better understanding of the impact of these formae speciales on the development and management of downy mildew requires a full understanding of their respective epidemiology. With this in mind, we studied the influence of temperature on the aggressiveness of the two main formae speciales of P. viticola responsible for downy mildew of grape in eastern Canada.
Incidence, severity, latency and sporangium production (sporulation efficiency) are examples of the variables that are used to evaluate the aggressiveness of strains (Pariaud et al. Citation2009; Corio-Costet et al. Citation2011; Roatti et al. Citation2013). Given the variation in temperature that occurs in nature, it was important that our study evaluate the effect of temperature on aggressiveness during the three key processes (infection, penetration and sporulation) in the life cycle of P. viticola. The effect of temperature during these processes in P. viticola was reported by Caffi et al. (Citation2016), Williams et al. (Citation2007) and Lalancette et al. (Citation1988). To our knowledge, this is the first study to separately evaluate the influence of temperature during the infection, penetration and sporulation processes on the aggressiveness of P.v. aestivalis and P.v. riparia. The results of our study suggest that temperature has a significant influence on all processes of both formae speciales. Several authors demonstrated that temperature had an effect on P. viticola infection (Keil et al. Citation2006; Williams et al. Citation2007; Caffi et al. Citation2016). Similar work conducted on strains of Pythium spp. isolated in Minnesota found that temperature had a significant influence on aggressiveness (Radmer et al. Citation2017). Caffi et al. (Citation2016) showed that the optimum infection temperature for P. viticola was between 20 and 25°C, values similar to those obtained in our study. Indeed, the optimum temperature for incidence, severity, latency and sporulation efficiency for both formae speciales is between 17 and 21°C. The patterns of the change in incidence as a function of temperature for P.v. riparia and P.v. aestivalis are similar but differ in amplitude. The same observation applies to severity and sporulation efficiency. No matter which process is considered, the incidence, severity and sporulation efficiency of P.v. aestivalis are higher than those of P.v. riparia. Similarly, the latency values for P.v. aestivalis are lower than those for P.v. riparia. Those results differ from the ones obtained by Roatti et al. (Citation2013), who showed that P. viticola strains isolated in various regions of Italy and Israel did not differ in terms of latency in response to temperature. That difference in response between the P. viticola strains used by those authors and the strains used in our study can be explained by the fact that those authors used genetically different strains, but are members of the same forma specialis (P.v. vinifera), whereas the strains in our study belong to two different formae speciales (P.v. riparia and P.v. aestivalis). Rouxel et al. (Citation2014) demonstrated that P.v. vinifera is the main forma specialis of P. viticola encountered in Europe, whereas P.v. riparia and P.v. aestivalis are the two main formae speciales encountered in eastern Canada. Similar work conducted in Canada on clonal lineages of Phytophthora infestans found that the P. infestans clonal lineages encountered in Canada had different levels of aggressiveness (Peters et al. Citation1999; Fall et al. Citation2015).
Based on the Gaussian model, the predicted minimum temperature for infection by P.v. riparia was between 6.9 and 9.8°C and the maximum temperature was between 31.0 and 31.9°C. For P.v. aestivalis, the minimum temperature for infection was between 4.8 and 8.3°C and the maximum temperature was between 33.0 and 34.9°C. However, the evaluation of the aggressiveness variables during the infection cycle shows that P.v. riparia and P.v. aestivalis are unable to cause infection at the extreme temperatures of 5–10°C and 30°C, but are able to penetrate tissues at those temperatures. According to linear models, the minimum temperature for penetration by P.v. riparia was between 3.8 and 5.2°C and the maximum temperature was between 30.4 and 31.2°C. For P.v. aestivalis, the minimum temperature for penetration was between 1.9 and 3.9°C and the maximum temperature was between 32.8 and 34.2°C. That result is partially in keeping with the report by Williams et al. (Citation2007) that the zoospores of P. viticola were unable to penetrate tissues at 30°C but were able to weakly penetrate them at 5°C. Our results showed that both formae speciales were able to sporulate at temperatures between 10 and 25°C. However, during sporulation, the Gaussian models showed that for P.v. riparia the minimum temperature was between 5.6 and 9.4°C and the maximum temperature was between 29.8 and 33.4°C and for P.v. aestivalis the minimum temperature was between 2.5 and 5.5°C and the maximum temperature was between 34.1 and 36.3°C. In contrast, Lalancette et al. (Citation1988) found that sporulation was possible only between 15 and 25°C. Even though it is now clear that P. viticola strains are unable to sporulate at the extreme temperatures (5 and 30°C), it should be stressed that those temperatures inhibit sporulation but do not kill P. viticola, given that after incubation at 5 or 30°C without sporulation, rapid sporulation is observed when the strains are returned to the optimum growth temperature. These results are critically important from an epidemiological perspective, because failure to take into account the effect of temperature variations during the penetration and sporulation processes could have a major impact on grape downy mildew forecasts.
Apart from incidence, severity, latency and sporulation efficiency, the duration of zoospore release can also be used to explain aggressiveness. Caffi et al. (Citation2016) evaluated the relationship between temperature and wetness duration on the zoospore release of P. viticola. That study showed that sporangia were able to open at temperatures from 5 to 30°C. Those results are similar to those obtained in our study, which found that the sporangia of both formae speciales were able to open at the same temperatures and that the optimum temperature for zoospore release was 20°C. In addition, the ANOVA revealed a significant effect of temperature and incubation time on zoospore release but no effect of forma specialis. Williams et al. (Citation2007) also showed that zoospore release of P. viticola occurred between 5 and 35°C. Similar studies carried out in New York on three clonal lineages of P. infestans (US-1, US-7 and US-8) revealed that the effect of temperature on sporangial germination differed depending on the lineage (Mizubuti and Fry Citation1998).
Williams et al. (Citation2007) demonstrated that the temperatures for zoospore release and zoospore germination were the same as the temperatures for zoospore penetration of tissues. Those authors showed that there is a difference between the number of zoospores that are released and the number of zoospores that penetrate tissues. Thus, although zoospore germination in that study occurred at temperatures between 5 and 30°C, penetration was impossible below 10°C and above 25°C. Their results are not fully in agreement with ours. Although zoospore release occurred between 5 and 30°C in our study as well, we showed that penetration of tissue by zoospores was also possible between 5 and 30°C for both formae speciales. Zoospore release is an important parameter for P. viticola aggressiveness. The faster zoospore release occurs, the faster zoospores penetrate and encyst in the stomata and, therefore, the faster the infection/sporulation cycle (Keil et al. Citation2006; Williams et al. Citation2007). Zoospore release also has an influence on the intensity of infection. Under environmental constraints, rapid zoospore release can make all the difference from an epidemiological perspective.
Although our study made it possible to evaluate the different aggressiveness variables, it would be useful to have an accurate estimate of the aggressiveness of the two formae speciales. The index of aggressiveness for the formae speciales was evaluated from variables such as incidence, sporulation efficiency and latency (Pariaud et al. Citation2009). Several authors have used these variables to calculate the index of aggressiveness (Corio-Costet et al. Citation2011; Roatti et al. Citation2013). Our results differ from those obtained with P. viticola strains originating from other geographic regions or with different fungicide resistance profiles (Corio-Costet et al. Citation2011; Roatti et al. Citation2013). In these studies, however, the formae speciales was unknown. Aggressiveness is an important parameter in terms of the adaptability of pathogens. Aggressiveness contributes to the selection of pathogen populations in a given region (Pariaud et al. Citation2009).
The results of our study clearly show that P.v. aestivalis is more aggressive than P.v. riparia, and regardless of the step in the infection/sporulation cycle, P.v. riparia and P.v. aestivalis have different epidemiological profiles. When conditions are favourable, P.v. aestivalis produces more sporangia, exhibits a higher level of incidence, and has a short infection/sporulation cycle compared with P.v. riparia. These results should be given serious consideration in grape downy mildew management programmes in eastern Canada. To improve the state of epidemiological knowledge of these two formae speciales, it would be advisable to determine competitiveness between them, measure their aggressiveness on the grape varieties grown in eastern Canada, and study the dynamics of primary inoculum production.
Acknowledgements
This work was funded by Agriculture and Agri-Food Canada. We would like to thank Audrey Levasseur, Geneviève Clément and Annie Lefebvre for their technical assistance. RM also gratefully acknowledges the Centre SÈVE and its AgroPhytoSciences scholarship programme for the scholarship that helped make this work possible.
References
- Caffi T, Gilardi G, Monchiero M, Rossi V. 2013. Production and Release of Asexual Sporangia in Plasmopara viticola. Phytopathology. 103:64–73. doi:10.1094/PHYTO-04-12-0082-R.
- Caffi T, Legler SE, González-Domínguez E, Rossi V. 2016. Effect of temperature and wetness duration on infection by Plasmopara viticola and on post-inoculation efficacy of copper. Eur J Plant Pathol. 144:737–750. doi:10.1007/s10658-015-0802-9.
- Caffi T, Rossi V, Cossu A, Fronteddu F. 2007. Empirical vs. mechanistic models for primary infections of Plasmopara viticola. Bull OEPP/EPPO Bull. 37:261–271. doi:10.1111/j.1365-2338.2007.01120.x.
- Camargo MP, Hong CF, Amorim L, Scherm H. 2019. Cryptic species and population genetic structure of Plasmopara viticola in São Paulo State, Brazil. Plant Pathol. 68:719–726. doi:10.1111/ppa.12993.
- Carisse O. 2016. Development of grape downy mildew (Plasmopara viticola) under northern viticulture conditions: Influence of fall disease incidence. Eur J Plant Pathol. 144:773–783. doi:10.1007/s10658-015-0748-y.
- Corio-Costet M-F, Dufour M-C, Cigna J, Abadie P, Chen W-J. 2011. Diversity and fitness of Plasmopara viticola isolates resistant to QoI fungicides. Eur J Plant Pathol. 129:315–329. doi:10.1007/s10658-010-9711-0.
- Dugan FM, Everhart S. 2016. Cryptic species: a leitmotif of contemporary mycology has challenges and benefits for plant pathologists. Plant Health Progr. 17:250–253. doi:10.1094/PHP-RV-16-0046.
- Fall ML, Tremblay DM, Gobeil-Richard M, Couillard J, Rocheleau H, Van der Heyden H, Lévesque AC, Beaulieu C, Carisse O. 2015. Infection efficiency of four Phytophthora infestans clonal lineages and DNA-based quantification of sporangia. PLoS ONE. 10:e0136312. doi:10.1371/journal.pone.0136312.
- Fontaine MC, Austerlitz F, Giraud T, Labbé F, Papura D, Richard-Cervera S, Delmotte F. 2013. Genetic signature of a range expansion and leap-frog event after the recent invasion of Europe by the grapevine downy mildew pathogen Plasmopara viticola. Mol Ecol. 22:2771–2786. doi:10.1111/mec.12293.
- Gessler C, Pertot I, Perazzolli M. 2011. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol Mediterr. 50:3–44.
- Gobbin D, Jermini M, Loskill B, Pertot I, Raynal M, Gessler C. 2005. Importance of secondary inoculum of Plasmopara viticola to epidemics of grapevine downy mildew. Plant Pathol. 54:522–534. doi:10.1111/j.1365-3059.2005.01208.x.
- Hill GK. 2000. Simulation of P. viticola oospore-maturation with the model SIMPO. Bull OILB/SROP. 23:7–8.
- Hong C-F, Brewer MT, Brannen PM, Scherm H. 2019. Prevalence, geographic distribution and phylogenetic relationships among cryptic species of Plasmopara viticola in grape-producing regions of Georgia and Florida, USA. J Phytopathol. 167:1–8.
- Kast WK, Stark-Urnau M. 1999. Survival of sporangia from Plasmopara viticola, the downy mildew of grapevine. Vitis. 38:185–186.
- Kast WK, Stark-Urnau M, Seidel M, Gremmrich AR. 2000. Inter-isolate variation of virulence of Plasmopara viticola on resistant varieties. Mitt Klosterneubg. 50:38–42.
- Keil S, Immink H, Kassemeyer -H-H. 2006. Effect of temperature and leaf-wetness-duration on the infection severity of the grapevine downy mildew Plasmopara viticola (Berk. et Curtis ex. De Bary) Berl. et de Toni. In: Pertot I, Gessler C, Gadoury D, Gubler W, Kassemeyer -H-H, Magarey P, editors. Proceedings of the 5th International Workshop on Grapevine Downy and Powdery Mildew. San Michele all’Adige, Italy: SafeCrop Centre and Instituto Agrario di San Michele all’Adige; p. 122, June 18–23.
- Kennelly MM, Gadoury DM, Wilcox WF, Magarey PA, Seem RC. 2007. Primary infection, lesion productivity, and survival of sporangia in the grapevine downy mildew pathogen Plasmopara viticola. Phytopathology. 97:512–522. doi:10.1094/PHYTO-97-4-0512.
- Lalancette N, Madden LV, Ellis MA. 1988. A quantitative model for describing the sporulation of Plasmopara viticola on grape leaves. Phytopathology. 78:1316–1321. doi:10.1094/Phyto-78-1316.
- Mizubuti ESG, Fry WE. 1998. Temperature effects on developmental stages of isolates from three clonal lineages of Phytophthora infestans. Phytopathology. 88:837–843. doi:10.1094/PHYTO.1998.88..837.
- Pariaud B, Ravigné V, Halkett F, Goyeau H, Carlier J, Lannou C. 2009. Aggressiveness and its role in the adaptation of plant pathogens. Plant Pathol. 58:409–424. doi:10.1111/j.1365-3059.2009.02039.x.
- Park EW, Seem RC, Gadoury DM, Pearson RC. 1997. DMCAST: A prediction model for grape downy mildew development. Vitic Enol Sci. 52:182–189.
- Peters RD, Platt HW, Hall R. 1999. Use of allozyme markers to determine genotypes of Phytophthora infestans in Canada. Can J Plant Pathol. 37:144–153. doi:10.1080/07060669909501205.
- Radmer L, Anderson G, Malvick DM, Kurle JE, Rendahl A, Mallik A. 2017. Pythium, Phytophthora, and Phytopythium spp. Associated with Soybean in Minnesota, Their Relative Aggressiveness on Soybean and Corn, and Their Sensitivity to Seed Treatment Fungicides. Plant Dis. 68:62–72. doi:10.1094/PDIS-02-16-0196-RE.
- Roatti B, Gessler C, Perazzolli M, Pertot I. 2013. Co-inoculated Plasmopara viticola genotypes compete for the infection of the host independently from the aggressiveness components. Eur J Plant Pathol. 136:363–371. doi:10.1007/s10658-013-0171-1.
- Rossi V, Caffi T, Gobbin D. 2013. Contribution of molecular studies to botanical epidemiology and disease modelling: Grapevine downy mildew as a case-study. Eur J Plant Pathol. 135:641–654. doi:10.1007/s10658-012-0114-2.
- Rossi V, Ponti I, Cravedi P. 2000. The status of warning services for plant pests in Italy. Bull OEPP/EPPO Bull. 30:19–29. doi:10.1111/j.1365-2338.2000.tb00845.x.
- Rouxel M, Mestre P, Baudoin A, Carisse O, Delière L, Ellis MA, Gadoury D, Lu J, Nita M, Richard-Cervera S, et al. 2014. Geographic distribution of cryptic species of Plasmopara viticola causing downy mildew on wild and cultivated grapes in eastern North America. Phytopathology. 104:692–701. doi:10.1094/PHYTO-08-13-0225-R.
- Rouxel M, Mestre P, Comont G, Lehman BL, Schilder A, Delmotte F. 2013. Phylogenetic and experimental evidence for host-specialized cryptic species in a biotrophic oomycete. New Phytol. 197:251–263. doi:10.1111/nph.12016.
- Schröder S, Telle S, Nick P, Thines M. 2011. Cryptic diversity of Plasmopara viticola (Oomycota, Peronosporaceae) in North America. Org Divers Evol. 11:3–7. doi:10.1007/s13127-010-0035-x.
- Williams MG, Magarey PA, Sivasithamparam K. 2007. Effect of temperature and light intensity on early infection behaviour of a Western Australian isolate of Plasmopara viticola, the downy mildew pathogen of grapevine. Australas Plant Pathol. 36:325–331. doi:10.1071/AP07029.