Abstract
This study aimed to evaluate the antifungal activity of oregano and clove essential oils and their main components (thymol, carvacrol and eugenol) against Penicillium digitatum, as well as against isolates of Penicillium italicum and Geotrichum candidum resistant to imazalil or guazatine, respectively. In vitro tests were conducted by a direct contact method on potato dextrose agar medium. The results indicated that the essential oils and their main components were effective in inhibiting fungal growth, with thymol displaying the highest efficacy. The antifungal activity of thymol was evaluated further on orange fruit infected experimentally with a suspension of spores of P. digitatum, G. candidum or a mixture of both fungi, using isolates resistant to chemical fungicides. Treatment of the oranges with the thymol solutions reduced or totally inhibited the development of fruit decay, depending on the thymol concentration used. These essential oils and their main components demonstrated a strong antifungal effect against Penicillium spp. and G. candidum resistant to chemical fungicides. They may have potential as active compounds for the formulation of natural preparations to use as alternatives to synthetic fungicides for the management of green, blue and sour rot in citrus fruit.
Résumé
Le sujet de cette étude est l’évaluation in vitro de l’activité antifongique des huiles essentielles d’origan, de clou de girofle et de leurs composés majoritaires (thymol, carvacrol et eugénol) contre des isolats de Penicillium digitatum et Penicillium italicum imazalil-résistants, et contre un isolat de Geotrichum candidum guazatine-résistant. Le test in vitro a été réalisé par la méthode de contact direct sur milieu PDA (Potato Dextrose Agar medium). Les résultats obtenus indiquent que les huiles essentielles et leurs composés majoritaires ont un effet inhibiteur de croissance mycélienne, avec une forte efficacité montrée par le thymol. L’activité antifongique préventive du thymol a été évaluée sur des oranges expérimentalement infectées avec une suspension de spores de P. digitatum et G. Candidum ou le mélange de spores des deux isolats résistants aux fongicides chimiques. Les résultats montrent que le trempage des oranges dans la solution de thymol réduit ou inhibe totalement la pourriture des fruits selon la concentration utilisée. Les huiles essentielles et leurs composés majoritaires montrent une forte activité antifongique contre Penicillium spp. et G. candidum résistants aux fongicides chimiques. Elles pourraient être utilisées comme composés actifs dans la formulation de préparations naturelles utilisées comme alternatives aux fongicides synthétiques pour lutter contre la pourriture verte, bleue et amère des agrumes.
Introduction
Citrus fruits are among the most cultivated in the world. According to the Food and Agriculture Organization (FAO Citation2017), the production of citrus fruit exceeded 124 million tons in 2017. In Morocco, the cultivation of citrus is an important component of the agricultural sector, covering a cultivated area of 118 000 hectares with an annual production of 2.4 millions of tons, with exports of approximately 600 000 tons (Ali Citation2017).
Some fungal species cause postharvest infections in citrus fruit, resulting in important economic losses (Moss Citation2008). The most important pathogenic fungi causing postharvest infections include Penicillium digitatum and P. italicum (Ascomycota; Eurotiomycetes; Eurotiales), responsible for green and blue rot, respectively, and Geotrichum candidum (Ascomycota; Saccharomycetes; Saccharomycetales), responsible for sour rot (Zheng et al. Citation2005; Valero & Serrano Citation2010).
The use of fungicides is the primary method of postharvest disease control in citrus fruit. The fungicides used for this purpose are primarily imazalil (IMZ), thiabendazole (TBZ), sodium orthophenyl phenol (SOPP), fludioxonil (FLU), guazatine (GUZ), pyrimethanil, or various mixtures of these compounds (Smilanick et al. Citation2005).
However, the use of artificial or synthetic products for disease control is becoming increasingly restricted due to their potential carcinogenicity, high residual toxicity, long degradation period and environmental pollution (Gupta & Dikshit Citation2010). Repeated use of chemical fungicides has also resulted in the development of fungal populations with multiple fungicide resistance, which further complicates disease management (Boubaker et al. Citation2009). In fact, resistance to IMZ, TBZ and SOPP has been reported in several citrus production areas (Kinay et al. Citation2007; Cabañas et al. Citation2009; Sánchez-Torres & Tuset Citation2011). The mode of action of these chemical fungicides is also limited (Smilanick et al. Citation2005). Hence, there is a need to develop efficient alternatives that are both environmentally friendly and meet consumer requirements.
Many studies have reported the efficiency of essential oils (EOs) and their main components (MCs) to manage postharvest fruit diseases (Burt Citation2004; Tripathi & Dubey Citation2004). The antifungal activity of essential oils makes them a promising alternative to replace chemical fungicides, given their non-phytotoxicity and their biodegradability (Gatto et al. Citation2011).
Many studies have shown the efficiency of EOs and MCs in the control of Penicillium and Geotrichum species that are sensitive to chemical fungicides (Plaza et al. Citation2004; Du Plooy et al. Citation2009). In the present work, we determine their efficiency against Penicillium and Geotrichum isolates that are resistant to chemical fungicides. Specifically, the objectives of this study were to: (i) evaluate the in vitro antifungal activity of EOs and their MCs (thymol, carvacrol and eugenol) from oregano and clove on fungal isolates that cause postharvest citrus rot and are resistant to chemical fungicides; and (ii) evaluate the antifungal activity of thymol on experimentally infected citrus fruit.
Material and methods
Fungal species and cultures
The pathogenic fungi used in this study included an IMZ resistant isolate of P. digitatum, an IMZ resistant isolate of P. italicum and a GUZ resistant isolate of G. candidum. The minimal inhibitory concentration (MIC) of spores of P. digitatum and P. italicum grown on IMZ-amended potato dextrose agar (PDA) medium was 0.4 and 0.8 mg mL−1, respectively. The MIC of spores of G. candidum grown on GUZ-amended PDA was 0.5 mg mL−1. Spore suspensions were prepared using 7-day-old cultures on PDA medium. Spores were detached with a sterile handle and transferred to a tube containing sterile saline solution (0.9% NaCl). The concentration of the spore suspensions was determined using a Malassez cell and were diluted to obtain an inoculum concentration of approximately 106 spores mL−1 (Neimann & Baayen Citation1989).
EOs and their MCs
EOs used in this study were from oregano (Origanum compactum) and clove (Eugenia caryophylata). Oregano EO is composed of 30.5% carvacrol and 27.5% thymol, whereas clove EO is 90.0% eugenol. EOs were provided by Menthol India (Delhi, India). The thymol, carvacrol and eugenol used in this study were provided by Fluka (Steinhein, Germany).
Antifungal activity of EOs and their MCs in vitro
Antifungal activity was evaluated in vitro according to the methodology reported by Remmal et al. (Citation1993a), which consists of dispersing EOs/MCs in an agar solution at 0.2 %. EOs/MCs were added to sterile molten PDA to obtain the following final concentrations: 0 (control); 0.125, 0.25, 0.5 and 1.0 mg mL−1.
Three Petri dishes were used for each concentration of EOs/MCs. The Petri dishes filled with PDA were inoculated with 10 µL of spore suspension (106 spores mL−1) of each isolate (P. italicum, P. digitatum or G. candidum) and incubated at 27°C for 7 days. The experiment was performed three times. After 7 days of incubation, the fungal colony diameter of the three isolates was measured. The antifungal activity of EOs and MCs was expressed as the percentage of mycelium growth inhibition (MGI), which was calculated according to the following formula (Boubaker et al. Citation2016):
%MGI = [(control diameter-treated diameter) / control diameter] ×100
The antifungal activity of thymol in vivo
‘Maroc-late’ orange fruit (Citrus sinensis)were provided by Dellassus Company (Casablanca, Morocco). The fruits used in this experiment were harvested from 10-year-old trees with a volkameriana rootstock. Harvests occurred at 10 AM in March when the temperature was 25°C with 30.0% humidity. Only healthy, commercially mature and freshly harvested fruits (48 h after harvest) were used for the study.
Orange fruits were injected under the mesocarp with 250 µL of a spore suspension (106 spores mL−1) using an insulin syringe equipped with a fine needle (30 gauges) inclined tangentially on mesocarp surface. This volume of inoculum was chosen after preliminary trials to determine the adequate amount needed to cause visual symptoms of fruit rot.
Based on the results obtained in vitro, thymol was selected for further testing on the orange fruit. Forty-eight hours after harvesting, the fruits were washed with sterilized water and then air dried for 1 h. afterwards; the fruits were split into three lots of 40. One lot was dipped in a 0.5 mg mL−1 thymol solution, a second was dipped in a 1.0 mg mL −1 thymol solution, and a third lot (control) was dipped in water. After 5 min of dipping, the fruits from each lot were air-dried and divided into four groups, which were then inoculated as described above with either P. digitatum, G. candidum, a mixture of both fungi (125 μL of spore suspension of each of the two isolates). Controls were inoculated with 250 µL of distilled water. The inoculation was performed at two equidistant sites marked on the equator line of the fruits. After that, the fruits were placed in a storage room, inside transparent plastic boxes incubated at 22°C with a high humidity level (90.0%). The experiment was performed three times. The percentage of fruits infected, and the diameter of the lesions were determined after 3, 5 and 7 days of incubation.
Statistical analysis
All data was analysed using IBM SPSS v. 21. Results are expressed as average values. Mycelium growth inhibition (MGI) values from the in vitro experiments were compared by analysis of variance (ANOVA) using the Least Significant Difference (LSD) post-hoc test. The results of the fruit infection study were compared using the Student’s t-test. Results are considered significant at P < 0.05.
Results
The inhibitory effect of EOs and their MCs on fungus growth in vitro
The effects of EOs and their MCs on P. digitatum, P. italicum and G. candidum mycelial growth were evaluated. The mycelial growth of the isolates was partially inhibited by 0.125 mg mL−1 of all of the EOs/MCs used (). However, thymol caused the greatest inhibition of mycelial growth (73.0–83.0%), followed by carvacrol (52.4–75.0%). Eugenol was the least active compound, inhibiting mycelial growth by 10.6–34.2%. A comparison between oregano and clove oil showed that the oregano oil was more active against P. digitatum, P. italicum and G. candidum, with percentages of inhibition of 61.4%, 29.0% and 74.0%, respectively.
Table 1. Effect of essential oils (EOs) and their main components (MCs) on mycelial growth of Penicillium digitatum.
Table 2. Effects of essential oils (EOs) and their main components (MCs) on mycelial growth of Penicillium italicum.
Table 3. Effects of essential oils (EOs) and their main components (MCs) on mycelial growth of Geotrichum candidum.
At a concentration of 0.25 mg mL−1, thymol showed a total inhibition of mycelial growth of all three isolates (MIC = 0.25 mg mL−1). Carvacrol totally inhibited the mycelial growth of the two Penicillium species (MIC = 0.25 mg mL−1) () and partially inhibited mycelial growth of G. candidum (). At this concentration, eugenol, oregano oil and clove oil partially inhibited all three isolates. At 0.5 mg mL−1, both EOs and eugenol completely inhibited mycelial growth of the fungal isolates (MIC = 0.25 mg mL−1).
The results indicated that thymol was the most active antifungal agent amongst the EOs and MCs tested. Consequently, this compound was assessed for activity against P. digitatum and G. candidum on experimentally infected fruits.
The antifungal activity of thymol in vivo test
Dipping of the oranges for 5 min in solutions of thymol at 0.5 and 1.0 mg mL−1 significantly delayed the appearance of rot symptoms and reduced the diameter of lesions on fruit under laboratory conditions (). A 0.5 mg mL−1 solution of thymol decreased the surface rot by 80.0% and 60.0% in oranges inoculated with P. digitatum () and G. candidum (), respectively. However, this concentration of thymol did not result in any significant reduction in the percentage of the infected fruits, nor did it reduce the diameter of rot lesions in oranges inoculated with a mixture of P. digitatum and G. candidum (). The 1.0 mg mL−1 thymol solution totally inhibited rot development during the first 7 days of incubation. In contrast, 7 days after inoculation with P. digitatum, G. candidum or a mixture of both fungal pathogens, the surfaces of all control oranges were rotten. No rot symptoms were detected on fruits inoculated only with sterilized water (data not shown). No phytotoxicity was observed on the fruits treated with various thymol concentrations.
Fig. 1 Effect of thymol on rot development in orange fruit. (a) percentage of rotten fruit after seven days of incubation. (b) lesion diameter after seven days of incubation. Fruits were treated with thymol at different concentrations, inoculated with 250 µL of an aqueous suspension of spores of Penicillium digitatum (106 spores per mL) and incubated for seven days at 22°C. Results are expressed as means of three replicates. Significance in comparison with control (fruits dipped in water only) according to Student test at P < 0.05
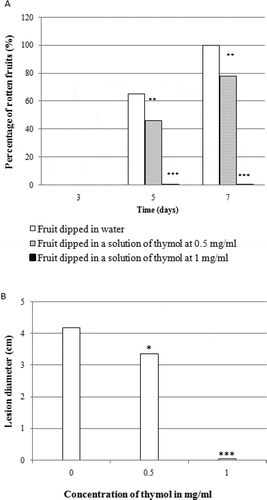
Fig. 2 Effect of thymol on rot development in orange fruit. (a) percentage of rotten fruits after seven days of incubation. (b) lesion diameter after seven days of incubation. Fruits were treated with thymol at different concentrations, inoculated with 250 µL of an aqueous suspension of spores of Geotrichum candidum (106 spores per mL) and incubated for seven days at 22°C. Results are expressed as means of three replicates. Significance in comparison with control (fruit dipped in water only) according to Student test at P < 0.05
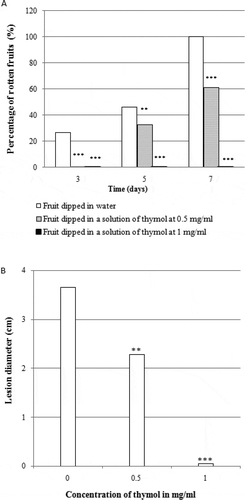
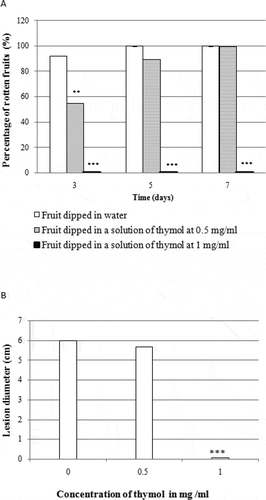
Fig. 3 Effect of thymol on rot development in orange fruit. (a) percentage of rotten fruits after seven days of incubation. (b) lesion diameter after seven days of incubation. Fruits were treated with thymol at different concentrations, inoculated with 250 µL of spores suspension of a mixture of Penicillium digitatum and Geotrichum candidum isolates and incubated for seven days at 22°C. Results are expressed as means of three replicates. Significance in comparison with control (fruit dipped in water only) according to Student test at P < 0.05
Discussion
An evaluation of the fungicide sensitivity of the three isolates included in the current study indicated that the MIC for G. candidum spores grown on GUZ-amended PDA was 0.5 mg mL−1 (results not published). According to Brown (Citation1979), the MIC for GUZ against sensitive isolates of G. candidum was 0.025 mg mL−1. Kuramoto and Yamada (Citation1975) also reported G. candidum isolates resistant to GUZ with a MIC of 1.0 mg mL−1. The MIC for spores of P. digitatum and P. italicum grown on IMZ-amended PDA medium was 0.4 and 0.8 mg mL−1, respectively (results not published). These values are also much higher than those that have been reported for IMZ sensitive Penicillium spp. isolates, which are around 0.1 mg L−1 (Brown & Miller Citation1999; Pérez et al. Citation2011).
Previous results showed that the direct contact methodology is the most effective to evaluate the antifungal activity of EOs and their MCs in vitro (Bouddine et al. Citation2012). The use of this methodology in this study showed that oregano oil, clove oil and their MCs are effective against three fungal isolates resistant to the fungicides IMZ or GUZ. Thymol, with a MIC of 0.25 mg mL−1, was the most effective antifungal component. Carvacrol was the second most-effective compound, with an MIC in the range of 0.25–0.5 mg mL−1. Eugenol, oregano oil and clove oil were significantly less effective against the three fungal isolates evaluated, with a MIC around 0.5 mg mL−1. A comparative study of the antifungal activity of carvacrol, thymol and eugenol showed that carvacrol has the highest activity (Abbaszadeh et al. Citation2014). A study conducted by Markovic et al. (Citation2011) also showed that carvacrol has a higher antifungal activity than thymol. These variations in results may reflect several factors, including the botanical source (some commercial oils are derived from different species), the origin of the plant material, the time of harvest and stage of development, the freshness of the plant material (fresh or dried), and the isolation technique (steam distillation, hydro distillation, extraction). Ultee et al. (Citation2002) also hypothesized that the hydroxyl group and the presence of a system of delocalized electrons are important for the antimicrobial activity of phenolic compounds such as carvacrol and thymol.
Although EOs and their MCs displayed different MIC values, all the low concentrations used in this study, at least partly inhibited the growth of the fungal isolates tested. This suggests that even low concentrations of EOs or their MCs could be more efficient than chemical fungicides in protecting citrus fruit against postharvest fungal attacks.
The antifungal effect of thymol, carvacrol and eugenol on pathogenic fungi of citrus fruit has already been reported (Regnier et al. Citation2014; Tao et al. Citation2014). Other researchers have demonstrated an antifungal effect of thyme and lemon oils against P. digitatum, P. italicum and G. candidum (Zhou et al. Citation2014; Boubaker et al. Citation2016).
The oregano EO used in this study contains 30.5% carvacrol and 27.5% thymol, whereas the clove EO contains 90.0% eugenol. Other studies of the composition of these two EOs reported similar data (Velluti et al. Citation2003; Bouhdid et al. Citation2008; Yahyazadeh et al. Citation2008).
The antifungal activity of the oregano and clove EOs is mainly due to the presence of phenolic compounds such as thymol, carvacrol and eugenol. In fact, Faid et al. (Citation1996) reported that the antifungal activity of EOs increases with an increase in the content of phenolic compounds, which are characterized by lipophilic properties and functional groups such as the free OH group. The presence of an aromatic ring with a phenolic hydroxyl group forms hydrogen bonds with the active sites of target enzymes (Farag et al. Citation1989). Nevertheless, while in this study the oregano EO had a lower concentration of phenolic components than the EO of clove it still showed higher antifungal activity. According to Farag et al. (Citation1989), this may reflect the relative position of the hydroxyl group, which could enhance the activity of phenolic components. Yahyazadeh et al. (Citation2008) have reported similar results.
Our data also shows that EOs were less effective than their MCs as antifungal agents. Similar results have been shown previously (Nostro et al. Citation2004; Bouddine et al. Citation2012). This may be related to the fact that MCs represent only a fraction of the whole essence and they interact in an additive rather than a synergistic way (Lambert et al. Citation2001).
The antimicrobial action of EOs may be owed to the degradation of various enzymatic systems, including those involved in energy production and synthesis of structural components (Conner et al. Citation1984). Other studies have shown that oregano EO, thymol and carvacrol, act at the level of the cell membrane, causing a significant permeabilization that leads to a leakage of various substances, such as ions, ATP and nucleic acids (Helander et al. Citation1998; Lambert et al. Citation2001). Clove oil acts on P. digitatum by modifying its hyphal morphology. This may be related to the interference of EO components with enzymatic reactions in wall synthesis, which affects fungal morphogenesis and growth (Romagnoli et al. Citation2005; Yahyazadeh et al. Citation2008).
MICs observed in our study are low in comparison with some studies (Pérez-Alfonso et al. Citation2012; Regnier et al. Citation2014). This could be explained by the fact that dispersion of thymol and clove using triton-X 100 could reduce their antifungal activity. Indeed, we have previously demonstrated that detergents such as triton-X100, Tween 80 or solvents like ethanol lower the efficiency of EOs/MCs (Remmal et al. Citation1993a, Citation1993b). The use of agar at 0.2% as a dispersion agent could also explain the lower MICs obtained in this study.
Thymol was tested for its antifungal activity on fruits artificially inoculated with the fungi, given its higher efficiency relative to the EOs and other MCs (carvacrol and eugenol) in the in vitro results. After 7 days of incubation, fruits dipped in the different thymol concentrations for 5 min and inoculated with P. digitatum and/or G. candidum developed much reduced symptoms of disease. This included a significant delay in the appearance of rot symptoms, a reduced percentage of rotten fruits, a reduction in lesion diameter at 0.5 mg mL−1, and an almost complete absence of rot at 1.0 mg mL−1. Similar results were reported by Pérez-Alfonso et al. (Citation2012), who suggested that dipping artificially inoculated lemons with 20 µL of aqueous suspension of P. digitatum (107 spores mL−1) in a wax solution containing 0.25 mg mL−1 of thymol for 10 min could inhibit the development of rot on fruits.
No rot was observed in fruits treated with 1.0 mg mL−1 of thymol 7 days after inoculation with a mixture P. digitatum and G. candidum. However, no significant effect was observed following treatment with thymol at 0.5 mg mL−1, which was sufficient to control each fungus individually. These results suggest a synergism between the two species in causing rot of citrus fruit, a result also reported by Morris (Citation1982).
The present results indicate that thymol is effective against fungal pathogens that cause postharvest decay in citrus fruit. These findings are promising and may contribute to development of a stable and efficient natural product as an alternative to traditional fungicide treatments in packinghouses. Unlike previous studies on the use of EOs, our results suggest that thymol alone is sufficient for the treatment of fruits. Indeed, the cost for the low doses of thymol required to control the fruit rot fungi are comparable to those associated with chemical fungicides. As such, thymol, especially at low doses, may represent an attractive alternative to traditional fungicides, since it does not represent a hazard for consumers, the environment or the staff handling the fruit in packaging warehouses.
Acknowledgements
The authors thank M Pierre-paul Bringuier, Ms Laurence Giroldi, M Abdelilah Aboussekhra Miss Soukayna Remmal and Miss Imane Remmal for their assistance in checking the English of the manuscript. The authors would like to thank Dr. Anis Sfendla for his help in the revision process and his insights on statistical analysis. This work was supported by the Industrial Laboratory of Veterinary Alternatives (LIAV).
References
- Abbaszadeh S, Sharifzadeh A, Shokri H, Khosravi AR, Abbaszadeh A. 2014. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J Mycol. 24:51–56.
- Ali K. (2017, 18 avril) Agrumes L’export, maillon faible de la filière. L’Economiste. Edition N°:5005. [Internet]. [accessed Aug 2018]. Available from: http://leconomiste.com/article/1011196-agrumes-l-export-maillon-faible-de-la-filiere. French.
- Boubaker H, Karim H, El Hamdaoui A, Msanda F, Leach D, Bombarda I, Aoumar AAB. 2016. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind Crops Prod. 86:95–101. doi:10.1016/j.indcrop.2016.03.036.
- Boubaker H, Saadi B, Boudyach EH, Ait Benaoumar A. 2009. Sensitivity of Penicillium digitatum and Penicillium to imazalil and thiabendazole in Morocco. J Plant Pathol. 4:152–158. doi:10.3923/ppj.2009.152.158.
- Bouddine L, Louaste B, Achahbar S, Chami N, Chami F, Remmal A. 2012. Comparative study of the antifungal activity of some essential oils and their main phenolic components against Aspergillus niger using three different methods. Afr J Biotechnol. 11:14083–14087. doi:10.5897/AJB11.3293.
- Bouhdid S, Skali SN, Idaomar M, Zhiri A, Baudoux D, Amensour M, Abrini J. 2008. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr J Biotechnol. 7:10.
- Brown GE 1979. Biology and control of Geotrichum candidum, the cause of citrus sour rot. Proceedings of the Florida State Horticultural Society, Germany. 92:186–189.
- Brown GE, Miller WR. 1999. Maintaining fruit health after harvest. In: Timmer LW, Duncan LW, editors. Citrus Health Manag. USA: APS Press; p. 175–188.
- Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods -a review. Int J Food Microbiol. 94:223–253. doi:10.1016/j.ijfoodmicro.2004.03.022.
- Cabañas R, Abarca ML, Bragulat MR, Cabañas FJ. 2009. Comparison of methods to detect resistance of Penicillium expansum to thiabendazole. J Appl Microbiol. 48:241–246. doi:10.1111/j.1472-765X.2008.02521.x.
- Conner DE, Beuchat LR, Worthington RE, Hitchcock HL. 1984. Effects of essential oils and oleoresins of plants on ethanol production, respiration and sporulation of yeasts. Int J Food Microbiol. 1:63–74. doi:10.1016/0168-1605(84)90009-6.
- Du Plooy W, Regnier T, Combrinck S. 2009. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol Technol. 53:117–122. doi:10.1016/j.postharvbio.2009.04.005.
- Faid M, Charai M, Mosaddak M. 1996. Chemical composition and antimicrobial activities of two aromatic plants: origanum majorana L. and O. compactum Benth. J Essent Oil Res. 8:657–664. doi:10.1080/10412905.1996.9701036.
- Farag RS, Daw ZY, Abo-Raya SH. 1989. Influence of some spice essential oils on Aspergillus parasiticus growth and production of aflatoxins in a synthetic medium. J Food Sci. 54:74–76. doi:10.1111/j.1365-2621.1989.tb08571.x.
- Food And Agricultural Organization of United Nations. 2017. Citrus fruit - fresh and processed statistical Bulletin 2016. Rome. [Internet]. [accessed Aug 2018]. Available from: http://www.fao.org/economic/est/est-commodities/citrus-fruit/en/.
- Gatto MA, Ippolito A, Linsalata V, Cascarano NA, Nigro F, Vanadia S, Di Venere D. 2011. Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol Technol. 61:72–82. doi:10.1016/j.postharvbio.2011.02.005.
- Gupta S, Dikshit AK. 2010. Biopesticides: an eco-friendly approach for pest control. J Biopesticides. 3:186–188.
- Helander IK, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, von Wright A. 1998. Characterization of the action of selected essential oil components on Gram negative bacteria. J Agric Food Chem. 46:3590–3595. doi:10.1021/jf980154m.
- Kinay P, Mansour MF, Gabler FM, Margosan DA, Smilanick JL. 2007. Characterization of fungicide-resistant isolate of Penicillium digitatum collected in California. J Crop Prot. 26:647–656. doi:10.1016/j.cropro.2006.06.002.
- Kuramoto T, Yamada S. 1975. Occurence of sour rot in Satsuma mandarin. Bulletin of the Fruit Tree Research Station, Japan. Series B. 2:87–95.
- Lambert RJW, Skandamis PN, Coote PJ, Nychas GJE. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 91:453–462. doi:10.1046/j.1365-2672.2001.01428.x.
- Markovic T, Chatzopoulou P, Siljegovic J, Nikoli N, Glamoclija J, Ciric A. 2011. Chemical analysis and antimicrobial activities of the essential oils of Satureja thymbra L. and Thymbra spicata L and their main components. Arch Biol Sci. 63:457–464. doi:10.2298/ABS1102457M.
- Morris SC. 1982. Synergism of Geotriochum candidum and Penicillium digitatum in infected citrus fruit. Phytopathology. 72:1336–1339. doi:10.1094/Phyto-72-1336.
- Moss MO. 2008. Fungi, quality and safety issues in fresh fruits and vegetables. J Appl Microbiol. 104:1239–1243. doi:10.1111/j.1365-2672.2007.03705.x.
- Neimann CJ, Baayen RP. 1989. Inhibitory effects of pnenylserine and salicylic acid on phytoalexin accumulation in carnation infected by Fusarium oxysporum f. sp. dianthi. Rijksuniversiteit Faculteit Landbouwwetenschappen. Gent. 54/2a:435–438.
- Nostro A, Blanco AR, Cannatelli MA, Enea V, Flamini G, Morelli I, Alonzo V. 2004. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol Lett. 230:91–195. doi:10.1016/S0378-1097(03)00890-5.
- Pérez E, Blanco O, Berreta C, Dol I, Lado J. 2011. Imazalil concentration for in vitro monitoring of imazalil resistant isolates of Penicillium digitatum in citrus packinghouses. Postharvest Biol Technol. 60:258–262. doi:10.1016/j.postharvbio.2011.01.002.
- Pérez-Alfonso CO, Martínez-Romero D, Zapata PJ, Serrano M, Valero D, Castillo S. 2012. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and Penicillium italicum involved in lemon decay. Int J Food Microbiol. 158:101–106. doi:10.1016/j.ijfoodmicro.2012.07.002.
- Plaza P, Torres R, Ussal J, Lamarca N, Vinas I. 2004. Evaluation of the potential of commercial postharvest application of essential oils to control citrus decay. J Hortic Sci Biotech. 79:935–940. doi:10.1080/14620316.2004.11511869.
- Regnier T, Combrinck S, Veldman W, Du Plooy W. 2014. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind Crops Prod. 61:151–159. doi:10.1016/j.indcrop.2014.05.052.
- Remmal A, Bouchikhi T, Tantaoui-Elaraki A, Ettayebi M. 1993b. Inhibition of antibacterial activity of essential oils by tween 80 and ethanol in liquid medium. J Pharm Belg. 48:352–356.
- Remmal A, Tantaoui-Elaraki A, Bouchikhi T, Ettayebi M. 1993a. Improved method for the determination of antimicrobial activity of essential oils in agar Medium. J Essent Oil Res. 5:1179–1184. doi:10.1080/10412905.1993.9698197.
- Romagnoli C, Bruni R, Andreotti E, Rai MK, Vicentini CB, Mares D. 2005. Chemical characterization and antifungal activity of essential oil of capitula from wild Indian Tagetes patula L. Protoplasma. 225:57–65. doi:10.1007/s00709-005-0084-8.
- Sánchez-Torres P, Tuset JJ. 2011. Molecular insights into fungicide resistance in sensitive and resistant Penicillium digitatum strains infecting citrus. Postharvest Biol Technol. 59:159–165. doi:10.1016/j.postharvbio.2010.08.017.
- Smilanick J, Mansour M, Margosan D, Gabler FM, Goodwine W. 2005. Influence of pH and sodium bicarbonate on effectiveness of imazalil to inhibit germination of Penicillium digitatum and to control postharvest green mold on citrus fruit. Plant Dis. 89:640–648. doi:10.1094/PD-89-0640.
- Tao N, Jia L, Zhou H. 2014. Anti-fungal activity of citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 153:265–271. doi:10.1016/j.foodchem.2013.12.070.
- Tripathi P, Dubey NK. 2004. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting on fruit and vegetables. Postharvest Biol Technol. 32:235–245. doi:10.1016/j.postharvbio.2003.11.005.
- Ultee A, Bennik MHJ, Moezelaar R. 2002. The phenolic hydroxyl group of carvacrol is essential for action against the food borne pathogen Bacillus cereus. Appl Environ Microbiol. 8:1561–1568. doi:10.1128/AEM.68.4.1561-1568.2002.
- Valero D, Serrano M. 2010. Postharvest biology and technology for preserving fruit quality. London (New York): CRC press.
- Velluti A, Sanchis V, Ramos AJ, Egido J, Marın S. 2003. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int J Food Microbiol. 89:145–154. doi:10.1016/S0168-1605(03)00116-8.
- Yahyazadeh M, Omidbaigi R, Zare R, Taheri H. 2008. Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J Microbiol Biotechnol. 24:1445–1450. doi:10.1007/s11274-007-9636-8.
- Zheng XD, Zhang HY, Sun P. 2005. Biological control of postharvest green mold decay of oranges by Rhodotorula glutinis. Eur Food Res Technol. 220:353–357. doi:10.1007/s00217-004-1056-5.
- Zhou H, Tao N, Jia L. 2014. Antifungal activity of citral, octanal and terpineol against Geotrichum citri-aurantii. Food Control. 37:277–283. doi:10.1016/j.foodcont.2013.09.057.
