Abstract
Black rot, caused by Xanthomonas campestris pv. campestris (Pammel) Dowson (Xcc), is a devastating disease of cruciferous crops worldwide and a threat to the economic production of Brassica oleracea L. in China. Strategies to control this disease are limited. In addition, knowledge of the molecular mechanisms of pathogenicity and host resistance are still only poorly understood. In this study, we performed transcriptomic analyses of the leaves of two contrasting B. oleracea lines (Xcc-resistant line QP07 and Xcc-susceptible line DBP71) to study the early defence response induced by infection with Xcc. We identified 3357 upregulated and 4091 downregulated genes between QP07 and DBP71 at 0, 12, 24, 48 and 96 h post-inoculation (hpi). A functional annotation pathways analysis of the differentially expressed genes indicated that the glucosinolate biosynthetic and catabolic pathways were enhanced, and ROS scavenging, hormonal, receptor-kinase-related genes and nucleotide binding site (NBS)-encoding resistance genes were expressed during the early infection period. In addition, we found that the host plants may respond to Xcc infection by actively regulating photosynthetic energy metabolism. The results provide new insights to help understand the different responses to Xcc in resistant and susceptible host genotypes, and to aid in the development of effective strategies for the prevention of black rot.
Résumé
La pourriture noire, causée par Xanthomonas campestris pv. campestris (Pammel) Dowson (Xcc), est une maladie dévastatrice qui s’attaque aux crucifères partout dans le monde et qui constitue une menace pour la production économique de Brassica oleracea L. en Chine. Les stratégies de lutte contre cette maladie sont limitées. De plus, les connaissances relatives aux mécanismes moléculaires de la pathogénicité et à la résistance de l’hôte sont à ce jour fragmentaires. Dans cette étude, nous avons procédé à des analyses trancriptomiques des feuilles de deux lignées opposées de B. oleracea (lignée résistante QP07 à Xcc et lignée réceptive DBP71 à l’égard de Xcc) afin d’étudier la réaction de défense première induite par l’infection à Xcc. Nous avons identifié 3 357 gènes régulés à la hausse et 4 091 gènes régulés à la baisse chez QP07 et DBP71, respectivement, à 0, 12, 24, 48 et 96 heures après inoculation. Une analyse fonctionnelle des voies d’annotation des gènes différentiellement exprimés a indiqué que les voies biosynthétiques et cataboliques du glucosinolate étaient améliorées et que le pillage des FRO, les gènes d’hormones et de récepteurs kinases ainsi que le site de fixation des nucléotides codant les gènes de résistance étaient exprimés au début de la phase infectieuse. En outre, nous avons noté que les plants hôtes peuvent réagir à l’infection à Xcc en régulant vivement le métabolisme de l’énergie photosynthétique. Les résultats fournissent un nouvel éclairage quant à la compréhension des diverses réactions à Xcc chez les différents génotypes hôtes, tant résistants que réceptifs, et contribuent à l’élaboration de stratégies efficaces pour prévenir la pourriture noire.
Introduction
Black rot disease affects cruciferous crops worldwide and is caused by Xanthomonas campestris pv. campestris (Xcc), a vascular pathogen spread via infected seeds (Cook et al. Citation1952; Vicente & Holub Citation2013). In addition, the pathogen can be disseminated by insects, diseased plants, farm implements, irrigation water and the seed trade (Sharma et al. Citation2017). Under natural conditions, the Xcc enters the margins of the leaves across the hydathodes, or through wounds on the plant, forming a triangular chlorotic yellow spot along the leaf edge spreading with the vein, gradually expanding into a V-shaped chlorosis around the leaf. The vein then becomes necrotic. When the pathogen spreads along the veins and petioles to the stems and roots of the plants, the vascular tissue in the roots and stems turns black, and the whole plant dies, sometimes accompanied by a secondary soft rot (Vicente & Holub Citation2013). Traditional measures used to manage black rot include the control of weeds and diseased plants that harbour the pathogen, the use of disease-free seeds and bactericidal seed treatments. However, these methods are all preventative, and there is no effective method to control black rot once the plant is infected (Vicente & Holub Citation2013; Singh et al. Citation2018). Therefore, the development and planting of disease-resistant varieties is a more effective solution for black rot prevention and reduction of yield losses than on other management measures.
Currently, Xcc is divided into 11 races, and races 1 and 4 are the most pathogenic and important, with over 90% of the black rot in the world caused by these two physiological races (Kamoun Citation1992; Vicente et al. Citation2001; Fargier & Manceau Citation2007; Vicente & Holub Citation2013; Cruz et al. Citation2017). A number of sources of resistance have been identified in the A and B genome of the brassicas, including Brassica rapa (AA), Brassica nigra (BB), Brassica juncea (AABB), Brassica napus (AACC), and Brassica carinata (BBCC) (Taylor et al. Citation2002; Tonguç & Griffiths Citation2004; Griffiths et al. Citation2009; Sharma et al. Citation2016, Citation2017). However, there are only a few reports of resistance genes in the C genome; moreover, Brassica oleracea (CC) is more sensitive to Xcc than other brassica species and reports of resistance in B. oleracea are mostly race-non-specific (Sharma et al. Citation1972; Jamwal & Sharma Citation1986; Griesbach et al. Citation2003; Tonu et al. Citation2013). Recently, Dey et al. (Citation2015) reported the creation of black rot-resistant lines in cauliflower through a combination of interspecific hybridization and embryo rescue. Saha et al. (Citation2016) identified new sources of resistance to race 1 by screening B. oleracea germplasm sources. Interestingly, the majority of Arabidopsis thaliana genotypes showed broad-spectrum resistance to the main pathogenic races of Xcc (Holub Citation2007). Although some resistant lines have been identified in brassica crops, and some resistance genes have been mapped, none have been cloned so far. Thus, an understanding of host resistance mechanisms and the interaction between B. oleracea and Xcc is necessary to improve black rot management.
Plants have evolved a range of effective defence systems over the course of long-term interactions and co-evolution with pathogens (Keshavarzi et al. Citation2004; Jones & Dangl Citation2006;). The first layer of the plant immune system, known as PTI (PAMP trigger immunity), is triggered by pattern recognition receptor (PRR) proteins that identify pathogen-related molecular patterns (PAMPs) and provide a type of underlying defence. However, pathogens can inhibit PTI by secreting effectors into host cells. Therefore, the second layer of the plant immune system can play a significant role in the cell. Plant disease resistance (R) genes identify cognate pathogen effectors (avirulence proteins) and trigger the activation of defence (Flor Citation1971; Dangl & Jones Citation2001). This type of resistance is commonly referred to as ETI (effector-triggered immunity). Resistance (R) proteins are considered to be important regulators of plant defence signalling. It has been reported that many R genes are involved in the resistance of brassica crops to different pathogens. Two clubroot resistance genes CRa and Crr1a of B. rapa have been cloned and found to be classic R genes (Ueno et al. Citation2012; Hatakeyama et al. Citation2013). Lv et al. (Citation2014) mapped Fusarium wilt-resistance gene FOC1 in B. oleracea, which encodes a TIR-NBS-LRR type R protein. Lee et al. (Citation2015) identified four quantitative trait loci (QTL) acting against Xcc by whole-genome resequencing and identified different NBS-LRR genes. Afrin et al. (Citation2018) identified R genes related to black rot resistance in B. oleracea, reporting that seven NBS-encoding genes were more highly expressed in Xcc-resistant vs. Xcc-susceptible lines. However, the response mechanisms related to these R genes have yet to be fully elucidated in B. oleracea when challenged with Xcc.
‘Omics’ technologies have been important tools to explore and understand the molecular mechanisms of the B. oleracea-Xcc interaction. Izzah et al. (Citation2014) developed many EST-based markers by RNA sequencing of two cabbage parental lines (Xcc-resistant and Xcc-susceptible). Santos et al. (Citation2019b) focused on the changes in microRNA expression in B. oleracea and identified four miRNAs that were up-regulated in a resistant genotype and down-regulated in a susceptible genotype. Various groups have explored changes in protein abundance after inoculation of Xcc-resistant and Xcc-susceptible B. oleracea lines with Xcc, finding that most of the differentially abundant proteins were involved in photosynthesis, energetic metabolism, hormone metabolics and disease/defence responses (Villeth et al. Citation2016; Ribeiro et al. Citation2018; Santos et al. Citation2019a).
In previous studies, growth of the Xcc bacterial population in a resistant line was observed only in the first 24 h following inoculation, and then decreased significantly, thus suggesting that resistant hosts block bacterial growth at 24 h (Villeth et al. Citation2009; Andrade et al. Citation2010). To clarify the response mechanisms of B. oleracea in response to Xcc, we used transcriptome sequencing to analyse the changes in gene expression in B. oleracea 12, 24, 48 and 96 h following infection by the pathogen.
Materials and methods
Isolation and identification of bacterial strains
The bacterial strains were isolated from brassica crop leaves, with typical ‘V’-shaped chlorosis of black rot disease, collected from diverse regions of Shaanxi Province, China. Surface contamination of the leaves was removed by rinsing the surface with distilled water and disinfecting it with 75% ethanol for 1 min. Leaf tissue segments were excised from the lesion margins by the addition of sterile 0.85% saline; the saline suspension was streaked on nutrient agar (NA) medium with an inoculation loop and the petri dishes were cultured at 28°C for 2 days (Burlakoti et al. Citation2018). Pale yellow colonies were picked using an inoculation loop and streaked in the new NA medium for further purification. We isolated a total of 20 strains (Supplementary Table 1). Presumptive Xcc isolates were identified to species using a species-specific PCR assay suggested by the International Seed Testing Association (ISTA; Roberts and Koenraadt Citation2014), and the races were identified using race 1 and 4 specific molecular markers for Xcc (Rubel et al. Citation2017).
Pathogen and plant material preparation
Two contrasting B. oleracea DH lines were used in our study, including line QP07 (Xcc-resistant) and line DBP71 (Xcc-susceptible). These two lines were developed by microspore culture after three rounds of inbreeding using a variety moderately resistant to Xcc. The plants were grown in pots under greenhouse conditions at the Northwest Agriculture and Forestry University, Yangling, China. Inoculations were done on 45-day-old plants. This stage was well suited for diseases resistance screening as the plants were showing vigorous growth. The Xcc strain YL-1 that we had isolated and purified was grown in agar-free nutrient medium at 28°C and 200 rpm in a shaking incubator. The bacterial suspension was prepared at the rate of 1 × 108 cfu mL−1 for inoculation.
Pathogen inoculation and sample collection
Forty-five-day-old plants of QP07 and DBP71 were inoculated with Xcc by spraying the leaves with the bacterial suspension. The inoculated seedlings were cultured in a greenhouse with a 14 h light photoperiod at 28°C and kept humid during the treatment stage. Sample leaves of QP07 and DBP71 seedlings were collected at five time points (before inoculation, 12, 24, 48, and 96 h after inoculation) for RNA-Seq analysis. Three biological replicates were used with five plants per replicate at each infected stage. Following collection, the leaves were immediately frozen in liquid nitrogen and stored at −80°C for RNA isolation.
RNA isolation
Total RNA was extracted using a plant RNA Extraction kit (Takara, Dalian, China). The quality of the extracted RNA was evaluated with a Nanodrop spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA) and by running aliquots on a 1.0% agarose gel. The total RNA after treatment with gDNase was used as the template to synthesize first strand cDNA with a FastKing RT Kit (TIANGEN BIOTECH, Beijing, China).
RNA Sequencing
cDNA library construction and sequencing were performed by the Biomarker Technology Company (Beijing, China). The mRNA was enriched from the total RNA using beads with Oligo(dT), and mRNA was then disrupted to form short fragments under an elevated temperature. The first-strand cDNA was synthesized using the mRNA fragments as templates with random primers, and then the second-strand cDNA was synthesized by adding DNA polymerase I, dNTPs, RNase H and buffer. The cDNA was subjected to end reparation and single nucleotide adenine addition. The short fragments were then connected with adapters, and the suitable fragments were selected from an agarose gel and enriched by PCR amplification. Finally, RNA-Seq was performed using the Illumina Hi-Seq X Ten sequencing platform. The raw RNA-Seq data have been deposited at SRA (http://www.ncbi.nlm.nih.gov/sra/) under the accession number SUB6182237.
Digital gene expression analysis
After sequencing, the sub-sequences and low-quality sequence reads were removed from the original data. The clean reads were then mapped to the B. oleracea genome (http://plants.ensembl.org/Brassica_oleracea/Info/Index) and reference gene sequences using TopHat2 (Parkin et al. Citation2014). The gene expression levels were estimated by the amount of reads mapped to the reference sequences using the RPKM method (Mortazavi et al. Citation2008). The resulting P values were corrected using the Benjamini and Hochberg approach (Benjamini Citation1995), and the corrected P-values were used to determine the false discovery rate (FDR). Sequences were regarded to be significantly differentially expressed if the |log2 (fold change)| was ≥1 and the FDR was <0.01.
DEGs analysis and annotation
For functional annotation, gene functions were subjected to the following databases: NCBI nonredundant protein sequences (Nr, ftp://ftp.ncbi.nlm.nih.gov/blast/db), Swiss-Prot database (http://ftp.ebi.ac.uk/pub/databases/swissprot), the cluster of Orthologous Groups (KOG/COG) databases (http://www.ncbi.nlm.nih.gov/COG), and protein family (PFam, http://pfam.xfam.org/). Additionally, to perform GO and pathway annotation to the DEGs, the Blast2GO programme was used to acquire the GO annotation of the unigenes. The WEGO and Top GO software programmes were used to perform GO functional classifications and enrichment analyses to understand the distributions of the gene functions. The KEGG pathway annotations and enrichment analyses were performed using BLASTALL software against the KEGG databases (http://www.genome.jp/kegg/).
Validation of the RNA-Seq data by qRT-PCR
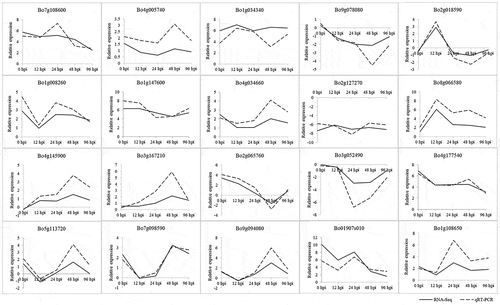
To confirm the RNA-Seq results obtained, we selected 20 DEGs for qRT-PCR analysis. The gene-specific primers were designed using Primer3 web (http://primer3.ut.ee/) and are listed in Supplementary Table 2. The qRT-PCR reactions were conducted on a CFX 96 real-time PCR system (Bio-Rad, Hercules, CA). The thermal cycling conditions were as follows: 95°C for 3 min, followed by 40 amplification cycles of 95°C for 5 s, and 60°C for 30 s, and melting curves were generated by increasing the temperature from 55°C to 95°C in increments of 0.5°C per 10 s to verify the specificity of the PCR amplification. An analysis of the gene expression was performed for all the samples at 0, 12, 24, 48, and 96 h post-inoculation of QP07 and DBP71 with Xcc. B. oleracea Actin2 (BoActin2) was used as the housekeeping gene and three independent biological and technical replicates were performed per primer. The relative expression levels of 20 DEGs were calculated using the 2−ΔΔCt method and results were analysed in MS Excel (Livak and Schmittgen Citation2001).
Results
Identification of bacterial strains and symptoms of the inoculated B. oleracea leaves
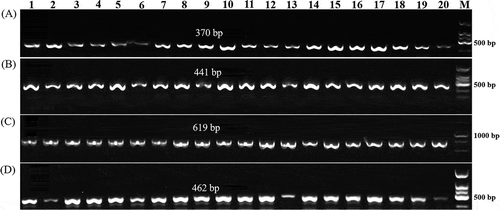
The bacterial isolates were identified to the species level using the species-specific PCR assay suggested by the International Seed Testing Association (ISTA; Roberts and Koenraadt Citation2014). Among the 20 strains that we isolated, we found three amplicons (370, 441 and 619 bp) that identified them all as Xcc (). The Xcc strains that we isolated were identified using race 1 and 4 specific molecular markers (Rubel et al. Citation2017). None of the 20 Xcc strains were amplified by the Xcc race 1 primer Xcc_47R1, and the 462 bp amplicon specific for the Xcc race 4 was observed in 19 of the Xcc isolates (). Therefore, Xcc strain YL-1 (race 4) was selected for inoculation.
Fig. 1 PCR amplification products from 20 Xanthomonas campestris pv. campestris (Xcc) strains in a species-specific PCR assay and race 4 specific molecular markers. (a), (b) and (c): Three amplicons (370, 441 and 619 bp) from all 20 strains in the PCR assay; (d): Amplicons (462 bp) obtained with Xcc-Race 4 specific molecular markers from all 20 strains. Lanes 1–20: 20 Xcc strains (the corresponding strains, host and sampling location for each lanes are included in Supplementary Table 1)
At approximately 72 h post-inoculation, DBP71 began to show slight chlorosis along the leaf edge. At approximately 1 week post-inoculation, a typical V-shaped chlorosis appeared around the leaf. Up through 2 weeks after the inoculation, we found severe V-shaped chlorosis around the leaf of line DBP71, but no black rot symptoms were detected in line QP07 (). This result indicated that QP07 showed more resistance to Xcc than DBP71.
RNA-Seq analysis
To evaluate the molecular mechanisms of B. oleracea against Xcc, RNA-Seq analysis was performed on three biological replicates of QP07 and DBP71 samples collected at each time point (0, 12, 24, 48 and 96 hpi) after Xcc inoculation. Raw reads were generated for RNA-Seq using 150-bp paired end Illumina sequencing. After sequencing quality control, a total of 237.32 Gb of data were obtained, and the filtered data of each of the 30 cDNA libraries reached 6.06 Gb. The GC percentage of the sequence data from the 30 libraries was approximately 48%, and 91% of bases were Q30 or better, indicating that the accuracy and quality of the sequencing data were adequate for further analysis. Almost 89% of the sequenced reads were mapped to the reference B. oleracea genome (Supplementary Table 3). The mapped reads are aligned with different regions of the reference genome, and approximately 85% of the reads mapped to exons (Supplementary Table 3).
Analysis of differentially expressed genes (DEGs)
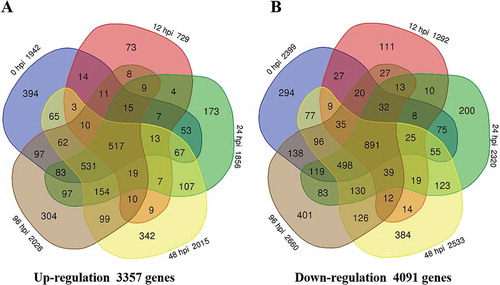
A total of 7430 DEGs between the two lines (QP07-vs-DBP71) were included, including 3357 up-regulated and 4091 down-regulated genes (, Supplementary Table 6). Compared with DBP71, 1942 genes were up-regulated, and 2399 genes were down-regulated in the 0 hpi QP07 sample. Following inoculation with Xcc, the numbers of DEGs in QP07 (as shown in ) included 729 up-regulated and 1292 down-regulated at 12 hpi; 1856 genes were up-regulated and 2320 genes were-down regulated at 24 hpi; 2015 genes were up-regulated and 2533 genes were down-regulated at 48 hpi; 2026 genes were up-regulated and 2660 genes were down-regulated 96 hpi. Moreover, only 1388 DEGs were detected as common DEGs at all five points, while 517 genes were up-regulated, and 891 genes were down-regulated ().
Fig. 3 Number of differentially expressed genes in the Brassica oleracea line QP07 (a) up-regulated or (b) down-regulated (|log2 (fold change)| ≥1 and the FDR < 0.01) compared with DBP71. Numerals inside the parentheses indicate the number of genes expressed at each time-point. The total number of DEGs is noted at the bottom of each Venn diagram. hpi, h post-inoculation
Functional annotation analysis of the DEGs
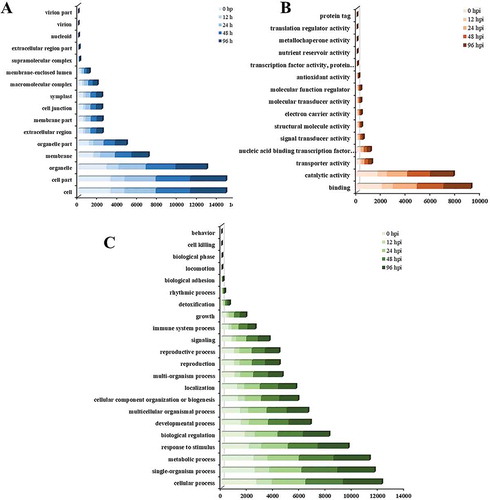
Based on the GO terms, DEGs between the two lines (QP07-vs-DBP71) were assigned to three GO ontologies: cellular component, molecular function and biological process. For the five time-points, there were 6014 DEGs enriched in the cellular component ontology, and more DEGs were involved in the ‘cell,’ ‘cell part’ and ‘organelle’ categories (). In the molecular function ontology, 4873 DEGs were assigned to 15 GO categories (). The main enriched categories were ‘binding’ and ‘catalytic activity.’ There were 5477 DEGs enriched in the biological process ontology, with most of the genes involved in ‘cellular process,’ ‘single-organism process,’ ‘metabolic process,’ ‘response to stimulus’ and ‘biological regulation’ (). The GO enrichment analysis of the DEGs in the biological process ontology shows that the top GO term at each time-point is represented as a directed acyclic graph (Supplementary Figs. 1–5). We extracted the top 10 GO terms at each time-point, and a total of 25 GO terms appeared in five stages (). Among these, the GO terms ‘glucosinolate catabolic process’ (GO:0019762) and ‘defense response signaling pathway, resistance gene-independent’ (GO:0010204) were present at all five time-points. Furthermore, the GO term ‘immune response-regulating signaling pathway’ (GO:0002764) was present at 0, 12, 24, and 96 hpi. In addition, we also found that ‘defense response to fungus, incompatible interaction’ (GO:0009817) and ‘regulation of plant-type hypersensitive response’ (GO:0010363) were only present at 48 hpi ().
Table 1. Top 10 Gene Ontology (GO) terms (biological process) that were significantly enriched at five time-points after inoculation of Brassica oleracea with Xanthomonas campestris pv. campestris.
Fig. 4 GO assignment of the differentially expressed genes (DEGs) in Brassica oleracea lines QP07 and DBP71. The unigenes were assigned to three main categories: cellular component (a), molecular function (b), and biological process (c). The x-axis indicates the number of annotated DEGs. hpi, h post-inoculation
The differentially expressed gene sequences were mapped to the reference canonical pathways in KEGG. A total of 1468 DEGs were annotated in the KEGG database and assigned to 126 KEGG pathways, of which 17 significantly enriched pathways (P ≤ 0.01) were detected (Supplementary Table 4). The most common term was ‘Cysteine and methionine metabolism,’ followed by ‘Photosynthesis – antenna proteins.’ The ‘Glutathione metabolism’ and ‘Sulfur metabolism’ pathways were also enriched. Based on the KEGG annotation analyses, we also extracted the top 10 metabolic pathways with the highest number of DEGs, and the top three metabolic pathways were ‘Biosynthesis of amino acids,’ ‘Carbon metabolism’ and ‘Plant hormone signal transduction’. The ‘Plant-pathogen interaction’ pathway was also enriched (Supplementary Table 5).
DEGs involved in the resistance to XCC
To survive infection by pathogens, plants require an effective response to restrict the further propagation of the pathogen. We utilized our transcriptome data analysis and the reported data in the literature to better understand the defence mechanism that B. oleracea employs against infection by Xcc. We analysed the response of photosynthesis (45 DEGs), ROS scavenging (40 DEGs), plant hormones (JA 17 DEGs, ET 16 DEGs, SA 9 DEGs), glucosinolates metabolites (32 DEGs), receptor kinase (30 DEGs) and resistance (R) genes (18 DEGs) after inoculation with Xcc (Supplementary Table 7).
The existence of ROS is a double-edged sword for plants, as they can inhibit the damage caused by pathogens, but also directly damage plant cell components. Therefore, the balance of ROS in cells is very important. Forty DEGs were identified as related to ROS scavenging in QP07 and showed an irregular regulation.
Identification of the DEGs involved in photosynthesis
According to the results of KEGG pathway analyses, photosynthesis was differentially regulated in QP07 compared with DBP71 after Xcc infection. Forty-five DEGs were identified in the KEGG pathway as related to photosynthesis in QP07, including ‘Photosynthesis,’ ‘Photosynthesis – antenna proteins,’ and ‘Carbon fixation in photosynthetic organisms’ (Supplementary Table 7). Compared with DBP71, the most DEGs associated with these pathways were down-regulated in QP07 after Xcc infection. However, interestingly, the number of up-regulated DEGs was significantly increased at 48 hpi, which included chlorophyll a-b binding protein, malate dehydrogenase, fructose-bisphosphate aldolase and PsbQ-like protein.
Identification of the DEGs involved in the SA, JA and ET signalling pathways
SA, JA and ET play a significant role in the plant’s defence system as the most important plant defence hormones. This study indicates that the biosynthesis of a number of these hormones was affected by Xcc infection. The results of the plant hormone pathway analysis in GO and KEGG indicated that there were 17 genes (10 up-regulated and seven down-regulated) related to the JA pathway. Sixteen genes (11 up-regulated and five down-regulated) were involved in the ET pathway, and nine genes (six up-regulated and three down-regulated) were associated with the ET pathway (Supplementary Table 7).
Quantitative RT-PCR validation
To confirm the results of the RNA-Seq analysis, 20 DEGs were selected for qRT-PCR analysis, including some involved in photosynthesis, ROS scavenging, plant hormone metabolism, glucosinolate metabolism, as well as resistance (R) genes and receptor like proteins (Supplementary Table 2). The results showed that all 20 DEGs were expressed in a manner similar to that observed via RNA-Seq analysis (), confirming the high degree of reliability of the RNA-Seq method used in this study.
Discussion
Black rot leads to a significant decline in the yield and quality of B. oleracea, and is one of the most destructive and widespread seed-borne bacterial diseases of this crop (Williams Citation1980; Singh et al. Citation2011, Citation2018). Unfortunately, few highly resistant lines have been identified in B. oleracea. Therefore, to develop an effective strategy for Xcc-resistance breeding, it is important to understand the mechanisms of resistance that different host lines utilize in response to black rot. In this study, we conducted a transcriptomic analysis of the defence responses to Xcc in resistant and susceptible varieties of B. oleracea.
Research has indicated that a range of resistance proteins have evolved in plants, which activate immune responses and prevent pathogen infection (Jones & Dangl Citation2006). Among these, plants use cell surface specific receptor proteins to perceive pathogen-secreted effectors to regulate immunity, resulting in PTI (Jones & Dangl Citation2006; He et al. Citation2018). In this study, we found a multitude of PRR genes differentially expressed between QP07 and DBP71. However, we found no differentially expressed key PRR genes that trigger PTI, such as flagellin sensing 2 (FLS2), chitin elicitor-binding protein (CEBiP), brassinosteroid insensitive 1-associated kinase 1 (BAK1) and chitin elicitor receptor kinase (CERK). These phenomena were also observed in the studies of Chen et al. (Citation2016) and Wang et al. (Citation2019). In addition, there are many examples of the differential expression of cysteine-rich receptor-like kinase genes (CRKs), some of which are related to plant defence and are up-regulated after bacterial infection in Arabidopsis (Czernic et al. Citation1999; Chen et al. Citation2003). We also noticed that the differentially expressed genes were more apparent on 0, 24, and 48 hpi. This suggests that the recognition between B. oleracea and Xcc occurs at an early stage of infection and within a relatively short period.
Disease resistance in plants can result from the interaction between pathogen avirulence (avr) genes and the corresponding host resistance (R) genes (Dangl & Jones Citation2001; Bent & Mackey Citation2007). Eighteen genes for R proteins were found to be differentially expressed between the two lines, including five transcripts homologous to Arabidopsis ‘ADR1-like 1,’ ‘Resistance to P. syringae pv maculicola 1,’ ‘Resistant to P. syringae 5,’ ‘Recognition of P. parasitica 8’ and ‘RPP8L4’, which were up-regulated at different time-points in QP07. Previous studies indicated that ADR1 contributed to resistance to the biotrophic pathogens Peronospora parasitica and Erysiphe cichoracearum (Grant et al. Citation2003). RPM1 can perceive the Pseudomonas syringae TTSS effectors AvrRpm1 and AvrB, leading to the hypersensitive reaction (HR) and restriction of Pseudomonas growth; overexpression of RPM1 conferred broad-spectrum resistance to the fungal pathogen Magnaporthe oryzae and the bacterial pathogen Xanthomonas oryzae pv. oryzae (Xoo) in rice (Gao et al. Citation2011; El Kasmi et al. Citation2017; Li et al. Citation2019). Ade et al. (Citation2007) showed that RPS5 confers resistance to the bacterium P. syringae. It was also reported that RPP8 confers resistance to downy mildew in Arabidopsis (McDowell et al. Citation1998; Mohr et al. Citation2010). Therefore, these R genes may be vital for mediating resistance to Xcc in B. oleracea, and the gene functions may be conserved across species.
According to the lifestyles and infection strategies of pathogens, plant hormones participate in plant-pathogen interactions by regulating defence response signal networks, enabling plants to produce the most suitable defence response to pathogens (O’Donnell et al. Citation2001; Anderson et al. Citation2004; Prerostova et al. Citation2018). Salicylic acid (SA) and jasmonic acid (JA) are involved in the reaction to biotrophic pathogens and necrotrophic pathogens, respectively (Glazebrook Citation2005; Pieterse et al. Citation2009). To study whether SA- and JA/ET-mediated pathways were also involved in B. oleracea resistance to Xcc, we examined the pattern of expression of hormone-related DEGs. We found that the JA biosynthetic pathway was significantly induced and that LOX1, LOX2, LOX3, OPR1 and OPR2 were up-regulated. Lipoxygenase (LOX) is a master regulator of the JA biosynthetic pathways (Wasternack Citation2007). In addition, some studies have reported that LOX1 was involved in the hypersensitive response to X. campestris in plants (Jalloul et al. Citation2002; Patil et al. Citation2005). Some key genes involved in ET biosynthesis (e.g., ACO1, ACO4, SAM2, and SAM3) and the signalling pathway (e.g., EIN3, ERF4, and ERF15) were up-regulated in QP07. However, we found that NIMIN1 and NIMIN2 were only up-regulated at 0 hpi for the SA-mediated pathway. Combined with previous data, our results suggest that the JA signalling pathway and not the SA signalling pathway may play a critical role in host resistance to Xcc.
Photosynthetic metabolism plays a significant role in the interactions between pathogens and host plants, and the host can act against pathogens by reducing photosynthesis (Berger et al. Citation2007; Garavaglia et al. Citation2010). Previous research showed that most photosynthesis-related proteins were increased after Xcc infection in susceptible plants (Santos et al. Citation2019a). Compared with DBP71, most of the differentially expressed genes were down-regulated in QPO7. However, the differentially expressed genes increased significantly at 48 hpi. This may indicate that the susceptible B. oleracea requires more energy to cope with infection by Xcc. Among these DEGs, two fructose-bisphosphate aldolase (FBA) genes were up-regulated at 48 hpi in QP07 compared with DBP71. Cai et al. (Citation2016) reported that overexpression of the FBA gene in tomato significantly increased the expression level and activity of some enzyme genes in the Calvin cycle, and the rate of regeneration of RuBP increased significantly. In addition, the accumulation of malondialdehyde (MDA) and ROS decreased significantly. A population dynamics study of the interaction between Xcc and host plants has shown that population growth was observed only in the first 24 h after inoculation, followed by a significant reduction in the bacterial population up to 48 h (Villeth et al. Citation2009). Taken together, our analysis suggests that resistant plants may reduce the energy supply of Xcc by reducing photosynthetic metabolism, thereby inhibiting its growth. In addition, the resilience of the resistant plants seems to be greater than that of the susceptible plants, which can quickly restore their normal photosynthetic energy metabolism. This result was consistent with previous observations in proteomic analyses of the interaction between Xcc and B. oleracea (Ribeiro et al. Citation2018; Santos et al. Citation2019a).
ROS are vital signalling molecules that regulate the onset of the hypersensitive response (Levine et al. Citation1994). Moreover, the accumulation of ROS can be toxic to the pathogen by inhibiting and/or reducing its survival (Jones & Dangl Citation2006; Zhang & Zhou Citation2010). However, ROS accumulation can lead to biofilm system damage and the oxidation of important cell components (Lushchak Citation2011). To adapt to ROS toxicity, plants usually protect themselves against ROS by enzymatic and non-enzymatic antioxidant systems, such as catalase activity, glutathione metabolism and ascorbate peroxidase. The B. oleracea antioxidants include catalase (Bo3g167210, Bo7g117190), superoxide dismutase (Bo8g112320), glutathione peroxidase 5 (Bo4g098230) and several glutathione S-transferases, which were up-regulated at different time-points in QP07 compared with DBP71 (Supplementary Table 7). Similar results were observed in a proteome analysis of the interaction between B. oleracea and Xcc (Santos et al. Citation2019a). These results may indicate a balance of oxidative stress response in B. oleracea in response to Xcc infection, maintaining some genes by up-regulation and others by down-regulation, which may be important to efficiently control the pathogen without causing extensive damage to the plant.
Plants defend themselves against pathogens by synthesizing and accumulating metabolites with antibiotic properties (Pastorczyk & Bednarek Citation2016). Glucosinolates, secondary metabolites of cruciferous plants, are implicated in the defence against plant pathogens (Grubb & Abel Citation2006). Previous research showed that glucosinolates could enhance resistance against Xcc in B. oleracea. Aires et al. (Citation2009) reported the effect of glucosinolate hydrolytic products (GHP) on Xcc and showed that GHP could inhibit the growth of this bacterium. Similar results were obtained in a study by Velasco et al. (Citation2013). Recently, Madloo et al. (Citation2019) evaluated the role of three glucosinolates (the aliphatics sinigrin, glucoiberin and the indolic glucobrassicin) in the defence of kale against Xcc, showing that only the indolic glucobrassicin can inhibit infection by Xcc. In this study, the expression of MYB28, FMO GS-OX2, BASS5, MAM2, GSL-OH and BCAT4, which encode proteins involved in the glucosinolate biosynthetic pathway, was up-regulated after Xcc infection. The double mutant MYB28/29, which does not accumulate aliphatic GSLs, has been shown to negatively affect the plant’s ability to defend against Botrytis cinerea and Alternaria brassicicola (Buxdorf et al. Citation2013). In addition, many key genes involved in the glucosinolate catabolic process (e.g., TGG2, UGT74 C1, ESP, NSP4, and NSP5) were also up-regulated in QP07 compared with DBP71. It is interesting that the up-regulation of the genes involved in glucosinolate biosynthesis and catabolic processes is concentrated at 48 h. This is similar to previous findings reported for the interaction between glucosinolates and Xcc (Velasco et al. Citation2013). Collectively, our results suggest that glucosinolates are synthesized and catabolized to enhance resistance to Xcc at the early stages of infection.
In this study, we comparatively analysed gene expression profiles in the leaves of Xcc-resistant and Xcc-susceptible lines of B. oleracea during the early stages of Xcc infection. A total of 7430 DEGs were identified. During this early phase, the glucosinolate biosynthetic and catabolic pathways were enhanced, and ROS scavenging, hormonal, nucleotide binding site (NBS)-encoding resistance (R) and receptor-kinase related genes were differentially expressed in response to Xcc. Taken together, the results suggest that upon infection by Xcc, B. oleracea activates its defences to restrict pathogen development and spread by (i) synthesizing antibacterial substances and reducing the energy supply of Xcc to inhibit its growth (e.g., enhancing GSL biosynthesis and catabolism, reducing photosynthetic metabolism), and (ii) maintaining a balance of in the host oxidative stress response to efficiently control Xcc.
Supplementary_Material_revised.rar
Download (6.2 MB)Acknowledgements
We thank Professor Baohua Li and Professor Xiaofeng Wang from our University for experimental advice and critical reading of this manuscript. We also wish to thank the reviewers and editor for their valuable comments.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ade J, DeYoung BJ, Golstein C, Innes RW. 2007. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc Natl Acad Sci USA. 104:2531–2536. doi:10.1073/pnas.0608779104.
- Afrin KS, Rahim MA, Park J, Natarajan S, Kim H, Nou I. 2018. Identification of NBS-encoding genes linked to black rot resistance in cabbage (Brassica oleracea var. capitata). Mol Biol Rep. 45:773–785. doi:10.1007/s11033-018-4217-5.
- Aires A, Mota VR, Saavedra MJ, Monteiro AA, Simões M, Rosa EAS, Bennett RN. 2009. Initial in vitro evaluations of the antibacterial activities of glucosinolate enzymatic hydrolysis products against plant pathogenic bacteria. J Appl Microbiol. 106:2096–2105. doi:10.1111/j.1365-2672.2009.04181.x.
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. 2004. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. The Plant Cell. 16:3460–3479. doi:10.1105/tpc.104.025833.
- Andrade AE, Silva LP, Pereira JL, Noronha EF, Reis FB, Carlos B, Santos MFD, Domont GB, Franco OL, Angela M. 2010. In vivo proteome analysis of Xanthomonas campestris pv. campestris in the interaction with the host plant Brassica oleracea. FEMS Microbiol Lett. 281:167–174. doi:10.1111/j.1574-6968.2008.01090.x.
- Benjamini YHY. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 57:289–300.
- Bent AF, Mackey D. 2007. Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol. 45:399–436. doi:10.1146/annurev.phyto.45.062806.094427.
- Berger S, Sinha AK, Roitsch T. 2007. Plant physiology meets phytopathology: plant primary metabolism and plant pathogen interactions. J Exp Bot. 58:4019–4026. doi:10.1093/jxb/erm298.
- Burlakoti RR, Chen JR, Hsu CF, Burlakoti P, Kenyon L. 2018. Molecular characterization, comparison of screening methods, and evaluation of cross‐pathogenicity of black rot (Xanthomonas campestris pv. Campestris) Strains from Cabbage, Choy Sum, Leafy Mustard and Pak Choi from Taiwan. Plant Pathol. 67:1589–1600.
- Buxdorf K, Yaffe H, Barda O, Levy M. 2013. The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS One. 8:e70771. doi:10.1371/journal.pone.0070771.
- Cai B, Li Q, Xu Y, Yang L, Bi H, Ai X. 2016. Genome-wide analysis of the fructose 1,6-bisphosphate aldolase (FBA) gene family and functional characterization of FBA7 in tomato. Plant Physiol Bioch. 108:251–265. doi:10.1016/j.plaphy.2016.07.019.
- Chen J, Pang W, Chen B, Zhang C, Piao Z. 2016. Transcriptome analysis of Brassica rapa near-isogenic lines carrying clubroot-resistant and -susceptible alleles in response to Plasmodiophora brassicae during early infection. Front Plant Sci. 6:1183. doi:10.3389/fpls.2015.01183.
- Chen K, Du L, Chen Z. 2003. Sensitization of defense responses and activation of programmed cell death by a pathogen-induced receptor-like protein kinase in Arabidopsis. Plant Mol Biol. 53:61–74. doi:10.1023/B:PLAN.0000009265.72567.58.
- Cook A, Walker J, Larson R. 1952. Studies on the disease cycle of crucifer black rot. Phytopathology. 42:162–167.
- Cruz J, Tenreiro R, Cruz L. 2017. Assessment of diversity of Xanthomonas campestris pathovars affecting cruciferous plants in portugal and disclosure of two novel X. campestris pv. campestris races. J Plant Pathol. 99:403–414.
- Czernic P, Visser B, Sun W, Savouré A, Deslandes L, Marco Y, Van Montagu M, Verbruggen N. 1999. Characterization of an Arabidopsis thaliana receptor-like protein kinase gene activated by oxidative stress and pathogen attack. Plant J. 18:321–327. doi:10.1046/j.1365-313X.1999.00447.x.
- Dangl JL, Jones JDG. 2001. Plant pathogens and integrated defence responses to infection. Nature. 411:826–833. doi:10.1038/35081161.
- Dey SS, Sharma K, Bhatia Dey R, Sandeep Kumar GM, Singh D, Kumar R, Parkash C. 2015. Inter specific hybridization (Brassica carinata × Brassica oleracea) for introgression of black rot resistance genes into indian cauliflower (B. oleracea var. botrytis L.). Euphytica. 204:149–162. doi:10.1007/s10681-015-1352-0.
- El Kasmi F, Chung E, Anderson RG, Li J, Wan L, Eitas TK, Gao Z, Dangl JL. 2017. Signaling from the plasma-membrane localized plant immune receptor RPM1 requires self-association of the full-length protein. Proc Natl Acad Sci USA. 114:E7385–E7394. doi:10.1073/pnas.1708288114.
- Fargier E, Manceau C. 2007. Pathogenicity assays restrict the species Xanthomonas campestris into three pathovars and reveal nine races within X. campestris pv. campestris. Plant Pathol. 56:805–818. doi:10.1111/j.1365-3059.2007.01648.x.
- Flor HH. 1971. Current status of the gene-for-gene concept. Annu Rev Phytopathol. 9:275–296. doi:10.1146/annurev.py.09.090171.001423.
- Gao Z, Chung EH, Eitas TK, Dangl JL. 2011. Plant intracellular innate immune receptor resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc Natl Acad Sci USA. 108:7619–7624. doi:10.1073/pnas.1104410108.
- Garavaglia BS, Thomas L, Gottig N, Zimaro T, Garofalo CG, Gehring C, Ottado J. 2010. Shedding light on the role of photosynthesis in pathogen colonization and host defense. Commun Integr Biol. 3:382–384. doi:10.4161/cib.3.4.12029.
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 43:205–227. doi:10.1146/annurev.phyto.43.040204.135923.
- Grant JJ, Chini A, Basu D, Loake GJ. 2003. Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol Plant Microbe In. 16:669–680. doi:10.1094/MPMI.2003.16.8.669.
- Griesbach E, Loptien H, Miersch U. 2003. Resistance to Xanthomonas campestris pv. campestris (Pammel) Dowson in cabbage Brassica oleracea L. J Plant Dis Protect. 110:461–475. doi:10.1007/BF03356123.
- Griffiths PD, Marek LF, Robertson LD. 2009. Identification of crucifer accessions from the NC-7 and NE-9 plant introduction collections that are resistant to black rot (Xanthomonas campestris pv. campestris) races 1 and 4. Hortscience. 44:284–288. doi:10.21273/HORTSCI.44.2.284.
- Grubb CD, Abel S. 2006. Glucosinolate metabolism and its control. Trends Plant Sci. 11:89–100. doi:10.1016/j.tplants.2005.12.006.
- Hatakeyama K, Suwabe K, Tomita RN, Kato T, Nunome T, Fukuoka H, Matsumoto S. 2013. Identification and characterization of Crr1a, a gene for resistance to clubroot disease (Plasmodiophora brassicae Woronin) in Brassica rapa L. PLoS One. 8:e54745. doi:10.1371/journal.pone.0054745.
- He Y, Zhou J, Shan L, Meng X. 2018. Plant cell surface receptor-mediated signaling – a common theme amid diversity. J Cell Sci. 131:jcs209353. doi:10.1242/jcs.209353.
- Holub EB. 2007. Natural variation in innate immunity of a pioneer species. Curr Opin Plant Biol. 10:415–424. doi:10.1016/j.pbi.2007.05.003.
- Izzah N, Lee J, Jayakodi M, Perumal S, Jin M, Park B, Ahn K, Yang T. 2014. Transcriptome sequencing of two parental lines of cabbage (Brassica oleracea L. var. capitata L.) and construction of an EST-based genetic map. BMC Genomics. 15:149. doi:10.1186/1471-2164-15-149.
- Jalloul A, Montillet JL, Assigbetsé K, Agnel JP, Delannoy E, Triantaphylidès C, Daniel JF, Marmey P, Geiger JP, Nicole M. 2002. Lipid peroxidation in cotton: xanthomonas interactions and the role of lipoxygenases during the hypersensitive reaction. Plant J. 32:1–12. doi:10.1046/j.1365-313X.2002.01393.x.
- Jamwal RS, Sharma PP. 1986. Inheritance of resistance to black rot (Xanthomonas campestris pv. campestris) in cauliflower (Brassica oleracea var. Botrytis). Euphytica. 35:941–943. doi:10.1007/BF00028603.
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature. 444:323–329. doi:10.1038/nature05286.
- Kamoun S. 1992. Incompatible interactions between crucifers and Xanthomonas campestris involve a vascular hypersensitive response: role of the hrpX locus. Mol Plant Microbe In. 5:22–33. doi:10.1094/MPMI-5-022.
- Keshavarzi M, Soylu S, Brown I, Bonas U, Nicole M, Rossiter J, Mansfield J. 2004. Basal defenses induced in pepper by lipopolysaccharides are suppressed by Xanthomonas campestris pv. vesicatoria. Mol Plant Microbe In. 17:805–815. doi:10.1094/MPMI.2004.17.7.805.
- Lee J, Izzah N, Jayakodi M, Perumal S, Joh H, Lee H, Lee S, Park J, Yang K, Nou I, et al. 2015. Genome-wide SNP identification and QTL mapping for black rot resistance in cabbage. BMC Plant Biol. 15:32. doi:10.1186/s12870-015-0424-6.
- Levine A, Tenhaken R, Dixon R, Lamb C. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 79:583–593. doi:10.1016/0092-8674(94)90544-4.
- Li Z, Huang J, Wang Z, Meng F, Zhang S, Wu X, Zhang Z, Gao Z. 2019. Overexpression of Arabidopsis nucleotide-binding and leucine-rich repeat genes RPS2 and RPM1(D505V) confers broad-spectrum disease resistance in rice. Front Plant Sci. 10:417. doi:10.3389/fpls.2019.00417.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. doi:10.1006/meth.2001.1262.
- Lushchak VI. 2011. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Phys C. 153:175–190.
- Lv H, Fang Z, Yang L, Zhang Y, Wang Q, Liu Y, Zhuang M, Yang Y, Xie B, Liu B, et al. 2014. Mapping and analysis of a novel candidate Fusarium wilt resistance gene FOC1 in Brassica oleracea. BMC Genomics. 15:1094. doi:10.1186/1471-2164-15-1094.
- Madloo P, Lema M, Francisco M, Soengas P. 2019. Role of major glucosinolates in the defense of kale against sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris. Phytopathology. 109:1246–1256. doi:10.1094/PHYTO-09-18-0340-R.
- McDowell JM, Dhandaydham M, Long TA, Aarts MGM, Goff S, Holub EB, Dangl JL. 1998. Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. The Plant Cell. 10:1861–1874.
- Mohr TJ, Mammarella ND, Hoff T, Woffenden BJ, Jelesko JG, McDowell JM. 2010. The Arabidopsis downy mildew resistance gene RPP8 is induced by pathogens and salicylic acid and is regulated by W box cis elements. Mol Plant Microbe In. 23:1303–1315. doi:10.1094/MPMI-01-10-0022.
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat Methods. 5:621–628. doi:10.1038/nmeth.1226.
- O’Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ, Chen J, Pang W, Chen B, Zhang C, Piao Z. 2001. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 25:315–323. doi:10.1046/j.1365-313x.2001.00968.x.
- Parkin IA, Koh C, Tang H, Robinson SJ, Kagale S, Clarke WE, Town CD, Nixon J, Krishnakumar V, Bidwell SL, et al. 2014. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 15:R77.
- Pastorczyk M, Bednarek P. 2016. The function of glucosinolates and related metabolites in plant innate immunity. Adv Bot Res. 7:171–198.
- Patil MA, Pierce ML, Phillips AL, Venters BJ, Essenberg M. 2005. Identification of genes up-regulated in bacterial-blight-resistant upland cotton in response to inoculation with Xanthomonas campestris pv. malvacearum. Physiol Mol Plant P. 67:319–335.
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. 2009. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 5:308–316.
- Prerostova S, Dobrev P, Konradyova V, Knirsch V, Gaudinova A, Kramna B, Kazda J, Ludwig-Müller J, Vankova R. 2018. Hormonal responses to Plasmodiophora brassicae infection in Brassica napus cultivars differing in their pathogen resistance. Int J Mol Sci. 19:4024.
- Ribeiro DG, Cunha GCRD, Santos CD, Silva LP, Oliveira Neto OBD, Labuto LBD, Mehta A. 2018. Brassica oleracea resistance-related proteins identified at an early stage of black rot disease. Physiol Mol Plant P. 104:9–14.
- Roberts SJ, Koenraadt H. 2014. 7-019a: detection of Xanthomonas campestris pv. campestris on Brassica spp. The International Seed Testing Association (ISTA). http://seedtest.org/upload/cms/user/SH-07-019a-2014.pdf.
- Rubel MH, Robin AHK, Natarajan S, Vicente JG, Kim H, Park J, Nou I. 2017. Whole-genome re-alignment facilitates development of specific molecular markers for races 1 and 4 of Xanthomonas campestris pv. campestris, the cause of black rot disease in Brassica oleracea. Int J Mol Sci. 18:2523.
- Saha P, Kalia P, Sharma M, Singh D. 2016. New source of black rot disease resistance in Brassica oleracea and genetic analysis of resistance. Euphytica. 207:35–48.
- Santos C, Nogueira FCS, Domont GB, Fontes W, Prado GS, Habibi P, Santos VO, Oliveira-Neto OB, Grossi-de-Sá MF, Jorrín-Novo JV, et al. 2019a. Proteomic analysis and functional validation of a Brassica oleracea endochitinase involved in resistance to Xanthomonas campestris. Front Plant Sci. 10:414.
- Santos LS, Maximiano MR, Megias E, Pappas M, Ribeiro SG, Mehta A. 2019b. Quantitative expression of microRNAs in Brassica oleracea infected with Xanthomonas campestris pv. campestris. Mol Biol Rep. 46:3523–3529.
- Sharma BB, Kalia P, Singh D, Sharma TR. 2017. Introgression of black rot resistance from Brassica carinata to cauliflower (Brassica oleracea botrytis Group) through embryo rescue. Front Plant Sci. 8:1255.
- Sharma BB, Kalia P, Yadava DK, Singh D, Sharma TR. 2016. Genetics and molecular mapping of black rot resistance locus Xca1bc on chromosome B-7 in ethiopian mustard (Brassica carinata A. Braun). PLoS One. 11:1–13.
- Sharma BR, Swarup V, Chatterjee SS. 1972. Inheritance of resistance to black rot in cauliflower. Genome. 14:363–370.
- Singh D, S D, Yadava DK. 2011. Genetic and pathogenic variability of indian strains of Xanthomonas campestris pv. campestris causing black rot disease in crucifers. Curr Microbiol. 63:551–560.
- Singh S, Dey SS, Bhatia R, Batley J, Kumar R. 2018. Molecular breeding for resistance to black rot [Xanthomonas campestris pv. campestris (Pammel) Dowson] in Brassicas: recent advances. Euphytica. 214:196.
- Taylor JD, Conway J, Roberts SJ, Astley D, Vicente JG. 2002. Sources and origin of resistance to Xanthomonas campestris pv. campestris in brassica genomes. Phytopathology. 92:105–111.
- Tonguç M, Griffiths PD. 2004. Development of black rot resistant interspecific hybrids between Brassica oleracea L. cultivars and Brassica accession A 19182, using embryo rescue. Euphytica. 136:313–318.
- Tonu NN, Doullah MA, Shimizu M, Karim MM, Kawanabe T, Fujimoto R, Okazaki K. 2013. Comparison of positions of QTLs conferring resistance to Xanthomonas campestris pv. campestris in Brassica oleracea. Am J Plant Sci. 04:11–20.
- Ueno H, Matsumoto E, Aruga D, Kitagawa S, Matsumura H, Hayashida N. 2012. Molecular characterization of the CRa gene conferring clubroot resistance in Brassica rapa. Plant Mol Biol. 80:621–629.
- Velasco P, Lema M, Francisco M, Soengas P, Cartea M. 2013. vivo and in vitro effects of secondary metabolites against Xanthomonas campestris pv. campestris. Molecules. 18:11131–11143.
- Vicente JG, Conway J, Roberts SJ, Taylor JD. 2001. Identification and origin of Xanthomonas campestris pv. campestris races and related pathovars. Phytopathology. 91:492–499.
- Vicente JG, Holub EB. 2013. Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol Plant Pathol. 14:2–18.
- Villeth GR, Reis FB, Tonietto A, Huergo L, de Souza EM, Pedrosa FO, Franco OVL, Mehta A. 2009. Comparative proteome analysis of Xanthomonas campestris pv. campestris in the interaction with the susceptible and the resistant cultivars of Brassica oleracea. FEMS Microbiol Lett. 298:260–266.
- Villeth GRC, Carmo LST, Silva LP, Santos MF, de Oliveira Neto OB, Grossi-de-Sá MF, Ribeiro IS, Dessaune SN, Fragoso RR, Franco OL, et al. 2016. Identification of proteins in susceptible and resistant Brassica oleracea responsive to Xanthomonas campestris pv. campestris infection. J Proteomics. 143:278–285.
- Wang S, Yu F, Zhang W, Tang J, Li J, Yu L, Wang H, Jiang J. 2019. Comparative transcriptomic analysis reveals gene expression changes during early stages of Plasmodiophora brassicae infection in cabbage (Brassica oleracea var. capitata L.). Can J Plant Pathol. 41:188–199.
- Wasternack C. 2007. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot London. 100:681–697.
- Williams PH. 1980. Black rot: a continuing threat to world crucifers. Plant Dis. 64:736–742.
- Zhang J, Zhou J. 2010. Plant immunity triggered by microbial molecular signatures. Mol Plant. 3:783–793.