Abstract
In two glasshouse experiments, colonization of potato (Solanum tuberosum L.) plants by the bacterial pathogens Dickeya solani and Pectobacterium parmentieri was studied after leaf infection. Leaves, whether or not artificially wounded, were spray-inoculated with various densities of green-fluorescent protein tagged strains of the pathogens, avoiding contamination of soil during inoculation. Microscopy analysis indicated that both pathogens were able to penetrate and colonize hydathodes, stomata and wounds of inoculated leaves. Dickeya solani was detected at 42 days after inoculation in leaves, stems, stolons and occasionally in tubers, whereas P. parmentieri was restricted to leaves, stems and stolons, and could not be detected in tubers. The infection percentage was higher for plants with wounded leaves than for plants with untouched leaves, and higher at higher inoculum densities. Nevertheless, infection of leaves could also occur at low densities of D. solani (102 cfu mL−1). We further investigated the risks for translocation of the pathogens from infected haulms through soil into progeny tubers after haulm destruction. In a glasshouse experiment, populations of the pathogens increased in haulms in the first week after chemical or mechanical destruction, but decreased in the second week. For P. parmentieri, transmission occurred from destroyed haulms via soil into progeny tubers in soil, but not for D. solani.
Résumé: Lors de deux expériences menées en serre, la colonization de plants de pommes de terre (Solanum tuberosum L.) par les agents pathogènes bactériens Dickeya solani et Pectobacterium parmentieri a été étudiée après que les feuilles ont été infectées. Les feuilles, blessées naturellement ou artificiellement, ont été inoculées par vaporization avec diverses densités de souches des agents pathogènes marquées avec une protéine fluorescente verte, évitant ainsi la contamination du sol durant l’opération. L’analyse par microscopie a indiqué que les deux agents pathogènes pouvaient pénétrer et colonizer les hydathodes, les stomates et les blessures de feuilles inoculées. D. solani a été détecté 42 jours après inoculation dans les feuilles, les tiges, les stolons et, occasionnellement, dans les tubercules, tandis que P. parmentieri s’est limité aux feuilles, aux tiges et aux stolons; il n’a pas pu être détecté dans les tubercules. Le taux d’infection était plus élevé chez les plants dont les feuilles affichaient des blessures que chez ceux dont les feuilles étaient intactes, et plus élevé à des densités plus fortes d’inoculum. Néanmoins, l’infection des feuilles pouvait également se produire à de faibles densités de D. solani (102 cfu mL−1). Nous avons étudié plus en profondeur les risques de transfert, par le sol, des agents pathogènes des fanes infectées aux tubercules issus des tubercules mères après la destruction des fanes. Au cours d’une expérience menée en serre, les populations d’agents pathogènes dans les fanes ont augmenté durant la première semaine après leur destruction chimique ou mécanique, mais ont décliné au cours de la deuxième semaine. Quant à P. parmentieri, la transmission s’est effectuée des fanes détruites aux tubercules issus des tubercules mères par le sol, mais pas pour D. solani.
Introduction
Blackleg and slow wilt, caused by Pectobacterium or Dickeya (sub)species, are diseases with a major impact on seed potato (Solanum tuberosum L.) production (Tsror et al. Citation2012). Blackleg refers to the basal stem rot of potato haulms and slow wilt is characterized by a yellowing and wilting of the leaves. Infections by these genera of soft rot Pectobacteriaceae (SRP) may be a cause for downgrading to a lower certification class or even rejection of the seed lot. Management of diseases caused by SRP is complicated. Most commercial cultivars are susceptible to the pathogens and no effective control agents are currently available (Czajkowski et al. Citation2011). In addition, the pathogens can spread undetected in a seed lot over several generations before symptom expression occurs. Finally, if rotten infected tubers are present during harvest and post-harvest handling, the pathogens are readily disseminated within and between seed lots (Perombelon & Kelman Citation1980).
Seed potato production starts with the use of mini-tubers or seed lots from clonal selections which are basically pathogen free (Boomsma et al. Citation2013). However, infections can occur in the first year of multiplication in the field (Saddler, SASA, Edinburgh, UK, personal communication, Burgess et al. Citation1994; Boomsma et al. Citation2013). In a survey of 11 propagators of mini-tubers in the Netherlands, one seed lot was already found infected with blackleg-causing pathogens after the first field generation, and 6 of the 11 seed growers encountered infections after the third generation (Boomsma et al. Citation2013).
Seed potatoes can become infected during planting, plant growth, at harvest or postharvest, during sorting and grading. The role of sorting-belts during grading in the spread of the pathogens in infected seed lots has been recognized, in particular if rotten tubers are present (Elphinstone & Pérombelon Citation1986a). Contaminated machines may also contribute to initial infections of pathogen-free seed lots. In studies in the Netherlands, contamination was frequently found on machines used during haulm destruction (45% of the cases). Nevertheless, the source responsible for progeny tuber infection could not be reliably determined (Boomsma et al. Citation2013). Several other infection sources potentially responsible for primary infections have been identified, such as contaminated rainwater, irrigation water, insects, aerosols, animal fur, or human clothes (Perombelon & Kelman Citation1980; Czajkowski et al. Citation2011). The relative importance of these inoculum sources in the epidemiology of soft rot Pectobacteriaceae is still unclear.
In seed potato crops, haulm destruction is carried out to avoid dissemination of aphid-borne viruses, to manipulate tuber-size distribution and to advance development of the periderm (Struik Citation1992). The haulms can be destroyed chemically, by burning or mechanically by pulling or chopping, or by a combination of methods. There is relatively little information on the risks that haulm infections by soft rot Pectobacteriaceae pose on progeny tubers. If haulms are infected by these pathogens, populations may increase in leaves and stems upon haulm destruction as the defence mechanisms of destroyed potato tissue will be impaired. Field studies showed that populations of Pectobacterium carotovorum and P. atrosepticum on leaves were generally low (< 102 cfu g−1), but occasionally higher densities exceeding 104 cfu g−1 were found later in the growing season (Burgess et al. Citation1994). However, after haulm destruction when weather conditions were wet, densities could be high (Elphinstone & Pérombelon Citation1986b; Burgess et al. Citation1994). In the studies of Burgess et al. (Citation1994) it was suggested that the high densities in haulm debris were mainly from epiphytic populations.
In theory, seed potatoes may become infected after leaf infection via systemic translocation of the pathogen through stems and stolons into tubers. In parallel experiments conducted previously, in which stems or severely wounded leaves were inoculated with high pathogen densities, translocation of Dickeya solani from leaves to the top of stems and from stems to tubers, respectively, has been shown to occur (Czajkowski et al. Citation2010). Alternatively, inoculum that has leaked from infected haulms may disperse by water into the soil and infect progeny tubers. This might particularly be a risk after haulm destruction when high densities of the pathogen build up in degrading tissues. Rainfall on the haulm debris may cause inoculum present on this debris to leak, via the soil, to progeny tubers, and contaminate their skin. After haulm destruction, tubers remain in the soil, often for several weeks (Struik Citation1992). This results in maturation of the periderm and a reduction in tuber damage during harvest. It also results in further degradation of the mother tubers, thus reducing contamination by smearing at harvest. A last potential infection pathway is via direct contact of tubers with haulm debris still present on the soil ridges at harvest.
This study aimed to further investigate the risks of potato leaf infections with D. solani and P. parmentieri. Both pathogens are responsible for a substantial number of blackleg outbreaks in Europe (Sławiak et al. Citation2009; Toth et al. Citation2011; Pasanen et al. Citation2013; Zoledowska et al. Citation2018). In two greenhouse experiments, the ability of these pathogens to colonize plants systemically after leaf infection was studied. Complementary to former studies (Czajkowski et al. Citation2010) we also used low-pathogen densities and inoculated plants with leaves that were not artificially damaged (untouched). In addition, the dispersion of the pathogens from leaves via soil into tubers was studied after mechanical and chemical haulm destruction. Damaged and untouched leaves were spray-inoculated with a low or high pathogen density. The initial stage of infection was visualized microscopically using bacterial strains tagged with a green fluorescent protein.
Materials and methods
Bacterial strains and media used for cultivation
In this study, the GFP tagged D. solani strain IPO2254 (parental strain IPO2222; Czajkowski et al. Citation2010) and the GFP-tagged P. parmentieri strain IPO3399 (parental strain IPO1955) were used. Both wild-type strains were found to be virulent in field experiments using vacuum-infiltrated tubers (Van der Wolf et al. Citation2017). Strains were tagged with the pPROBE-AT-gfp plasmid and expressed GFP in a stable way. The construction and testing of strain IPO2254 is described by Czajkowski et al. (Citation2010). Strains IPO2254 and IPO3399 were constructed and tested following the procedure of Czajkowski et al. (Citation2010), and produced similar results (unpublished data).
Prior to use, strains were grown at 25°C for 24–48 h on tryptone soya agar (TSA, Oxoid; Basingstoke, UK) supplemented with 150 µg mL−1 ampicillin. Suspensions were prepared by washing the cells from the agar with a quarter strength Ringer’s solution (Sigma-Aldrich; St. Louis, USA). Stock suspensions were made with an optical density of 0.1 at 600 nm containing approximately 108 cfu mL−1.
Plant and soil extracts were pour-plated in PT medium (per litre: 7.1 g polygalacturonic acid (Sigma-Aldrich), 0.71 g Bacto Tryptone (Becton Dickinson & Co; Franklin Lakes, USA), 1.4 g NaNO3, 5.7 g K2HPO4, 0.28 g MgSO4.7H2O, 0.14 mL Tween20, 11.4 g Agar, pH 7.0; Van Vuurde et al. Citation2002) supplemented with 150 µg mL−1 of ampicillin and with 200 µg mL−1 of cycloheximide (PTAC). Plates were incubated at 25°C for 24 h. Re-isolation of the GFP tagged strains from symptomatic tissues was done on the double-layer crystal violet pectate (DL-CVPAG366) medium described by Hélias et al. (Citation2012).
For in-situ enrichment of D. solani and P. parmentieri in plant tissues for microscopic studies, whole leaflets or leaf pieces were incubated at 25 °C for 1 or 2 days on 1.5% water agar supplemented with 150 µg mL−1 of ampicillin and with 200 µg mL−1 of cycloheximide (WAAC).
Greenhouse experiments
In 2013 and 2014 greenhouse experiments were conducted to study the systemic colonization of plants after leaf inoculation with SRP (Experiments on systemic colonization). In addition, in 2014 an experiment was set up to study the dispersion of SRP from contaminated leaves via soil into tubers after haulm destruction (Experiment on dispersion via soil).
In all experiments, plants raised from pre-basic seed tubers of the cultivar ‘Kondor’ (Agrico; Emmeloord, NL) were used. For the experiments on systemic colonization, 100 pre-sprouted mini-tubers were planted at approximately 5 cm depth in potting soil (black peat amended with 50 kg m−3 clay and 4.7 kg m−3 carbonic magnesia lime; mixture WUR no 4) in 5 L plastic pots on 26 March 2013 and on 28 April 2014. The 50 mini-tubers for the experiment on the dispersion via soil were planted on 13 May 2014 in 5 L pots filled with river clay soil collected at Grebbedijk experimental farm, Wageningen.
Before use in experiments, plants were grown at 23°C, 16 h photoperiod and ca. 70% relative humidity. A fluid nutrient solution (Yara Nederland; Vlaardingen, NL) for potted tomato plants was applied once a week following the manufacturer’s guidelines.
Experiments on systemic colonization: inoculation of potato plants. Spray-inoculation of potato plants was performed 4–5 weeks after planting, when plants were 27–30 cm high and stolons had already formed. Eighty plants were inoculated after deliberately wounding the leaves and 16 plants were inoculated without artificially wounding the leaves.
To minimize the risk of soil contamination with the pathogens during spray-inoculation, the soil surface of the pots was covered with a plastic film. Stems were selected with two fully expanded and freely accessible leaves just below the apical bud. These leaves were successively labelled with a coloured twist tie, (if applicable) wounded and inoculated. For wounding, a soft toothbrush was gently moved once over the adaxial surface of each leaflet of the compound leaves resulting in striped superficial injuries. Tagged leaves were spray-inoculated using a 1.25 L domestic air pressure plant sprayer (Gardena; Ulm, DE). Both sides of the tagged leaves were sprayed until run off (3–4 mL per plant) with a tap water suspension of the pathogens at a concentration of 0, 102, 104 or 106 cfu mL−1, on the basis of an optical density (OD600) of 0.1 corresponding to a cell density of 108 cfu mL−1. Four sets of 8 plants with wounded leaves and 4 plants with untouched leaves were inoculated with a pathogen suspension of 102 or 104 cfu mL−1. Two sets of 10 plants with wounded leaves and 6 plants with untouched leaves were inoculated with a pathogen suspension of 106 cfu mL−1. Half of the sets were inoculated with D. solani and the other half with P. parmentieri. A set of 10 plants with wounded leaves and 6 plants with untouched leaves were sprayed with water. For complementary microscopic observations, suitable leaves of the remaining set of 4 plants were spray-inoculated with water or high densities of D. solani (108–109 cfu mL−1).
Directly after inoculation, plants were placed in humid chambers for 24 h, with each pathogen and inoculum concentration combination in a separate chamber. After removal from the humid chambers the plants were randomized within four groups consisting of two plants with wounded leaves and one plant with untouched leaves per pathogen concentration. The remaining plants inoculated with 0, 106 or 108–109 cfu mL−1 pathogen density were randomly distributed among the four groups. During the infection process plants were kept at a relative humidity of 75–80% and water was supplied via a watering mat to avoid splash dispersal of pathogen cells present on above-ground plant parts.
Experiments on systemic colonization: monitoring of the infection process
The infection process was monitored by assessing bacterial densities in extracts of inoculated leaves, stems, stolons and tubers by microscopic examination of the inoculated leaves and by weekly inspections for symptoms. Symptoms were recorded and symptomatic plants withdrawn from the experiment for isolation of SRP.
Experiments on systemic colonization: assessment of bacterial densities by pour-plating
To assess initial contamination levels, two tagged leaves of three plants with wounded leaves and two tagged leaves of one plant with untouched leaves per treatment (pathogen and inoculum density combination) were sampled 4–6 h after inoculation.
To monitor the infection process, one stem with two tagged leaves of four asymptomatic plants per wounded leaf treatment was sampled at 1 and 21 days post-inoculation (dpi). Of the plants with untouched leaf treatments, one asymptomatic stem with two tagged leaves was sampled at these time-points. Depending on the availability of asymptomatic plants at the end of the experiments (42 dpi in 2013 and 35 dpi in 2014), stems of 2–5 plants per treatment were sampled. In addition to stems with tagged leaves, the stolons and tubers of these plants were collected.
The two tagged leaves, the top of the stem and the stem base were processed from each stem. One of the leaves was analyzed without prior disinfection. The other leaf was analyzed after surface-sterilization to detect deeper seated infections below the epidermis. For this, each individual leaf was subsequently treated with 70% (v/v) ethanol for 1 min, 1% (v/v) chlorine bleach for 4 min and four times with tap water. For sampling the top of stems, first the flower (if present) and leaf buds were cut-off just above the youngest expanded almost horizontal leaf. The 10 cm stem section directly below this point was considered the top of the stem, while a 10 cm section directly above the soil line was considered as the stem base. Long stolons were cut into pieces of at most 7–8.5 cm and the stolon material from each plant was pooled. The stolon end (ca. 5 mm diameter) was cut off from the tubers and the stolon ends per plant were pooled. Stem tops, stem bases, stolons and tubers were analyzed without prior disinfection.
Tissue samples were transferred to Universal Extraction bags (BIOREBA; Reinach, CH) and weighed. Each sample was crushed in its extraction bag using a hammer and, directly after crushing, a volume equivalent to twice the sample weight was added of 0.02% diethyldithiocarbamic acid (Acros Organics; Fair Lawn, USA) amended quarter strength Ringer’s buffer. One hundred µL of undiluted, 10, 100 and 1000 times diluted samples were added to the wells of a 24-well plate (Greiner BioOne; Kremsmünster, AT) and mixed with 300 µL of liquefied PTAC medium cooled down to 45–50°C. After the medium had solidified, plates were incubated for 24–48 h at 25°C. Pour-plates were examined for the presence of GFP tagged D. solani or P. parmentieri colonies under 495 nm blue light using a Leica MZ FL III epifluorescence stereo microscope (Leica Microsystems; Wetzlar, DE) equipped with a mercury high pressure photo-optic lamp (Leica Hg 50 W/AC) and GFP 2 filter cube at a low magnification of 10 and 20 times. Green fluorescent colonies were counted to enable calculation of numbers of colony forming units per gram of tissue (cfu g−1).
Experiments on systemic colonization: microscopic examination of leaves
A short time (2–3 h) after inoculation and at 1 and 7 dpi, 10 leaflets were sampled from plants with wounded leaves inoculated with 0, 106 or 108–109 cfu mL−1 of D. solani, or 106 cfu mL−1 of P. parmentieri, and 8 leaflets from plants with untouched leaves inoculated with the same inoculum densities for microscopic examination. Samples were analyzed with or without prior incubation. Leaflets were incubated in the dark at 25°C for 1, 2 or 7 days on WAAC plates with the abaxial side of the leaf touching the agar surface.
The surface of the leaflets was inspected for the presence of green fluorescent signal using a Leica MZ FL III epifluorescence stereo microscope (ESM) with a zoom magnification objective of 0.8 up to 10×. Photographs of fluorescent parts were taken with an AxioCam MR05 camera (Carl Zeiss; Oberkochen, DE) and AxioVision software programme Release 4.8 (Carl Zeiss). Square pieces of leaf tissue (±1 × 1 cm) with fluorescent signal were then cut out with a scalpel blade. In some samples, the square pieces of leaf were divided into thin strips using a razor blade. Whole squares and millimetre-thin cross sections were transferred onto microscope slides and examined by fluorescence microscopy (FM) or confocal laser scanning microscopy (CLSM). For FM, the specimens were mounted in water, covered with a cover glass and inspected using a Zeiss Axioplan optical microscope equipped with an Osram HBO 103 W/2 mercury high pressure photo-optic lamp (Osram; Munich, DE) and a 09 filter (blue 450–490) and 10, 20 and 40× objectives. Photographs were taken with an AxioCam IC.c 3 camera (Carl Zeiss) and AxioVision Release 4.8 software. For CLSM, the specimens were subsequently embedded in molten 0.3% multipurpose agarose (Agarose MP, Roche; Basel, CH) at 50°C, covered with a cover glass and cooled to room temperature before further inspection using a Leica DM5500Q confocal laser-scanning microscope. For excitation of GFP, a 488 nm blue laser was used and the emitted light was detected through a 505 nm filter. Photographs were taken with the Leica Digital System connected to the CLSM using 10, 40 and 63× objectives. For some samples photographs were composed by stacking multiple images.
Experiments on systemic colonization: processing symptomatic plants
For isolation of bacteria from blackleg affected stems, a piece of stem tissue of approximately 5 cm was dissected from just above the margin of the rot, and transferred to an Universal Extraction bag and processed as described above in the section ‘Assessment of bacterial densities by pour-plating’. In addition to pour-plating in PTAC medium, diluted stem extracts were also spread-plated onto DL-CVPAG366. After incubation for 48–72 h at 25°C, DL-CVPAG366 plates were examined by ESM for the presence of green-fluorescent colonies with a cavity typical of soft rot Pectobacteriaceae.
Experiment on dispersion via soil: inoculation of potato plants and haulm destruction
On day 83 after planting, one day before inoculation, the soil surface of the pots was covered with plastic film to avoid soil contamination with the pathogens during spray-inoculation, and the plants were placed in humid chambers. The next day the haulms were sprayed with water (16 plants) or a bacterial suspension of 108 cfu mL−1 D. solani (16 plants) or P. parmentieri (16 plants) in water until run off. Plants were kept in the humid chambers for another three days.
Four days after inoculation, the plants were taken out of the humid chambers. The pots were removed from 12 plants per treatment (water or SRP). Sets of three plants per treatment were placed in a plastic crate (79 × 58 × 32 cm) the perforated bottom of which was covered with a 10-cm-thick layer of hydroponic granules. The spaces between the root balls of the plants were filled with river clay to construct ridges (20 cm high, 15 cm wide at the top). Crates with ridged plants were arranged using an RCB design. The remaining potted plants were placed in front of the crates, one plant of each treatment in front of a crate with plants of the corresponding treatment.
On the fifth day after inoculation,the haulms of the ridged and the potted plants were destroyed. For vine killing, 10 mL L−1 Reglone (active compound diquat, Syngenta, Basel, CH) was atomized onto the leaves with a 1.25 L domestic air pressure plant sprayer (Gardena). Mechanical haulm destruction was performed per crate and pot. The foliage was subsequently cut off 5–10 cm above soil level, fragmented in a chopper (Stephan UM44-S tiltable cutter, Pacific Food Machinery; Thomastown, AU) and spread over the ridge. After chopping the haulms, the stalks sticking out of the ridges were sprayed with Reglone to prevent regrowth.
After haulm destruction, rain was simulated by irrigating plants with an overhead spray line using Micro sprinklers (Dan type 8966, NaanDan Jain Irrigation Ltd; Na’an, IL). For the first five days after haulm destruction a total of 4 L per m2 per day was applied in 4 intervals of 6 h. The next five days a total of 1 L per m2 per day was applied in 4 intervals of 6 h and at day 8 an extra application of 4 L per m2 in order to enhance the translocation of bacteria with free water from haulms into the soil.
Experiment on dispersion via soil: monitoring pathogen populations after haulm destruction
To assess initial contamination levels, the foliage, soil and tubers of the potted plants standing in front of the crates with artificial potato ridges were sampled 3–4 h after haulm destruction. The haulms of the ridged plants were sampled 3, 6 and 14 days after haulm destruction to monitor population dynamics of the pathogens in the decaying plant tissues. Soil and tubers were sampled 14 days after haulm destruction to assess pathogen translocation from haulms via the soil into tubers.
Experiment on dispersion via soil
Sampling to estimate bacterial densities. At each sampling time, material from or around each plant was collected and the material from the three plants of a crate was pooled. After chemical vine killing, four leaflets or parts of leaves per plant were collected, whereas after mechanical haulm destruction about 15 mL debris around the stalks of each plant was collected. To sample the soil, the plant debris in a diameter of about 20 cm around the plants was removed from the soil surface and about 1 cm topsoil of the exposed area stripped off. About 15 mL soil was then scraped from the exposed soil layer. If present, tubers partly exposed to the air were collected and the exposed peel area of these tubers was marked. Furthermore, for each plant two tubers positioned just below the soil surface were collected.
Experiment on dispersion via soil: processing samples
Leaf samples from chemically killed haulms were transferred to Universal Extraction bags (BIOREBA) and processed as described in the section ‘Experiments on systemic colonization: extraction of plant material and pour-plating’. Samples of the debris of mechanically destroyed haulms were mixed before transferring 10–20 g material to an extraction bag and processing as described for leaf samples.
Before processing tubers, clay adhering to their peel was carefully wiped off with tissue paper. When tubers had been exposed to the air, the peel of the exposed part was removed and processed for each individual tuber as described for leaf samples. The other tubers were completely peeled, after which the peel of the six tubers of the same crate were mixed and a 15–40 g subsample was transferred to an extraction bag and processed as described for leaf samples.
Each soil sample was mixed before transferring 6 g of soil to a 180 mL container (Gibco BioOne) containing 57 mL 0.1% tetrasodium pyrophosphate (Sigma-Aldrich) and 6 g gravel (size 5–8 mm). The container was mounted on a Stuart SF1 Flask Shaker (Bibby Scientific Ltd; Staffordshire, UK) and agitated for 10 min at 800 oscillations min−1. After agitation, serial dilutions of the soil-pyrophosphate suspensions were pour-plated in PTAC medium as described in the section ‘Experiments on systemic colonization: extraction of plant material and pour-plating’.
Statistics
Data on plants sprayed with water were not included in the statistical analysis. For data on leaf infections, restricted maximum likelihood (REML) analysis was performed on log (cfu + 1) transformed pathogen densities with treatment factors ‘inoculum’ (D. solani or P. parmentieri), ‘inoculum density’ (102, 104 or 106 cfu mL−1), ‘wounding’ (wounded or not-wounded), ‘disinfection’ (disinfected or not-disinfected) and ‘time point’ (0, 1, 21, 42 or 35 dpi). The Linear Mixed models directive of Genstat 19 (VSN International; Hemel Hempstead, UK) was used to perform the analysis. The plate counts to estimate the bacterial density (in cfu g−1) formed the random term. Main effects were estimated. F-probabilities for these estimates are presented. Next to this, pairwise least significant difference (LSD) tests were performed with probability 0.05.
Results
Experiments on systemic colonization
Microscopic observations of the infection process in leaves. On fresh leaves spray-inoculated with a density of 106 cfu mL−1 SRP suspensions, D. solani and P. parmentieri could not be detected by epifluorescence stereo microscopy (ESM, results not shown). Therefore, plants spray-inoculated with high densities of D. solani (108–109 cfu mL−1) were used in subsequent ESM studies on early stages of leaf infections. For workable confocal laser scanning microscopy (CLSM) studies, samples had to be incubated first on WAAC plates.
At 1 dpi, on the surface of untouched leaves, fluorescent bacterial cells were relatively frequently observed along the veins and at the tip of the leaves. Using CLSM the presence of single fluorescent cells was observed at the entrance of stomata on the adaxial side of untouched leaves of which tissue pieces had been incubated overnight after sampling at 1 dpi (, Fig. S1A and B), but were not present on mock-inoculated leaves (). Using FM, in tissues of leaves that had been incubated for 48 h after sampling, a strong green fluorescent signal with FM was observed in association with stomata at 7 dpi (), but was not present on mock-inoculated leaves (). Multiple fluorescent cells were observed in and around hydathodes at leaf margins of untouched leaves that had been incubated overnight after sampling at 0 dpi (), but not observed on mock-inoculated leaves ().
Fig. 1 Microscopic images of leaf tissues after spray-inoculation with a GFP-tagged strain of Dickeya solani, or mock-inoculated. Green fluorescent bacterial cells or signals in the images are indicated with an arrow. (a) Confocal laser scanning microscopy (CLSM) image of a stoma on the adaxial side of an inoculated potato leaf. (b) Mock-inoculated. Leaflets sampled at 1 day after inoculation, were incubated overnight on water agar at 25°C prior to observation. Photomicrographs were taken with a 40x (a) or 10× (b) objective lens. (c) Epifluorescence stereomicroscopy (ESM) image of a stoma on the adaxial side of an inoculated potato leaf. (d) Mock-inoculated. Leaflets were sampled 7 days after inoculation and incubated on water agar for 2 days at 25°C to support bacterial growth. Photomicrographs were taken with a 20× (c) or 40× (d) objective lens. (e) CLSM image of a hydathode of an inoculated potato leaf. (f) Mock-inoculated. Leaflets sampled at 0 dpi were placed on water agar and incubated overnight at 25°C prior to observation. Photomicrographs were taken with a 40× objective lens. (g) CLSM image of vascular tissue of the abaxial side of an inoculated potato leaf. (h) Mock-inoculated. Photomicrographs were taken with a 10× objective lens 7 days after inoculation. (i and j) ESM images of the adaxial side of wounded potato leaf tissue. Leaflets were collected shortly after leaf inoculation, placed on water agar and incubated overnight at 25°C prior to observation. Notice the striped pattern of the infected tissues consistent with the striped pattern of the superficial wounds at the time of inoculation. Photomicrographs were taken with a zoom magnification objective at 10×
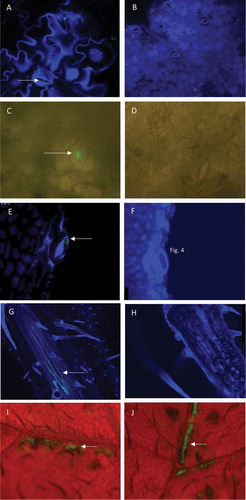
Occasionally, in leaves wounded prior to inoculation, colonization of xylem vessels was observed 7 dpi, and also in tissues without prior incubation (), but not in mock-inoculated leaves (). Directly after spray-inoculation and overnight incubation of wounded leaves, harmed tissues were found frequently infected by D. solani as observed by ESM ( and J). A strong fluorescent signal was found in approximately 40–50% of the wounded tissue (data not shown).
Disease development
During the 2013 experiment, one plant with untouched leaves inoculated with a high density (106 cfu mL−1) of P. parmentieri developed blackleg symptoms. After inoculation of wounded leaves with 106, 104, or 102 cfu mL−1 of D. solani, blackleg symptoms developed in 1, 2 and 1 plants, respectively. After inoculation of untouched leaves with D. solani, blackleg developed in 1 plant inoculated with 106 cfu mL−1 and 1 plant with 104 cfu mL−1. GFP-producing, cavity-forming bacteria were isolated from affected tissues (data not presented). None of the plants from the 2014 experiment showed blackleg symptoms. Plants with leaves that had been sprayed with water remained symptomless throughout both experiments.
Infection of leaves
In the 2013 experiment, directly after spray-inoculation of the plants with wounded leaves, both GFP-tagged pathogens could be detected in disinfected leaf tissues of only 67% of the plants by dilution-plating, and only if inoculated with a high density of 106 cfu mL−1 ().
Table 1. Progress of soft rot Pectobacteriaceae colonization process1 in wounded leaves during the 2013 and 2014 systemic colonization experiments
Population densities of D. solani increased rapidly and at 1 dpi the pathogen could be detected in tissues of all wounded leaves sampled from plants inoculated with inoculum densities 106 or 104 cfu mL−1 and half of the leaves inoculated with the low inoculum density of 102 cfu mL−1. Infection rates of wounded leaves inoculated with 102 cfu mL−1 increased to 75 and 100% at 21 and 42 dpi, respectively. At 42 dpi, the density of D. solani in wounded leaf tissue was 101–105 cfu g−1 for plants inoculated with 102 cfu mL−1, 104–105 cfu g−1 for plants inoculated with 104 cfu mL−1 and ca. 106 cfu g−1 for plants inoculated with 106 cfu mL−1 (). In leaves which had not been disinfected prior to plating, higher densities were found than in disinfected leaves, indicating that epiphytical populations of the pathogen were also present. In inoculated plants with untouched leaves, a similar course of population growth was observed, except that D. solani was not detected in leaves of plants inoculated with 102 cfu mL−1.
Fig. 2 Population densities of Dickeya solani and Pectobacterium parmentieri (respectively, the upper and lower row of graphs) in leaves at 0, 1, 21 and 42 days after spraying potato leaves with suspensions of 102, 104 or 106 cfu mL−1. Results are summarized of the glasshouse experiment in 2013. W = leaves wounded prior to inoculation, NW = leaves not wounded prior to inoculation, D = leaves disinfected prior to analysis, ND = leaves not disinfected prior to analysis. Error bars show standard deviations
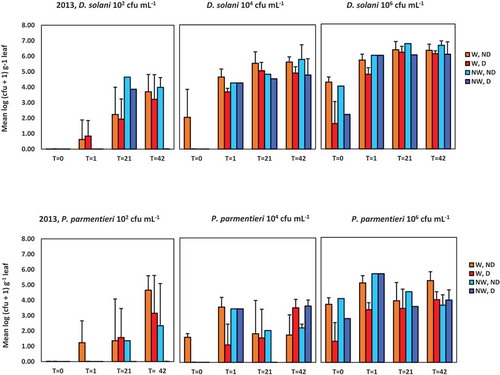
Infection rates in plants with wounded leaves inoculated with P. parmentieri also increased with time, but the rate increase was slower in leaves inoculated with densities of 102 or 104 cfu mL−1, as was found for D. solani. After disinfection of wounded leaves inoculated with 102 cfu mL−1, infection rates of 0, 50 and 100% were found at 1 dpi to 21 and 42 dpi, respectively. For leaves inoculated with 104 cfu mL−1, infection rates of 50, 50 and 100% were found at 1 dpi to 21 and 42 dpi, respectively. Pathogen densities also increased in plants inoculated with wounded leaves, but at 42 dpi the maximum densities were 100–1000 fold lower than for D. solani. At 42 dpi, 100% of the sampled leaves were infected for all treatments, except for those on plants inoculated with 102 cfu mL−1 which had not been artificially wounded and which also were disinfected. This indicated that an inoculum density of 102 cfu mL−1, similarly for D. solani, was not sufficient to establish infection in leaves that were not wounded.
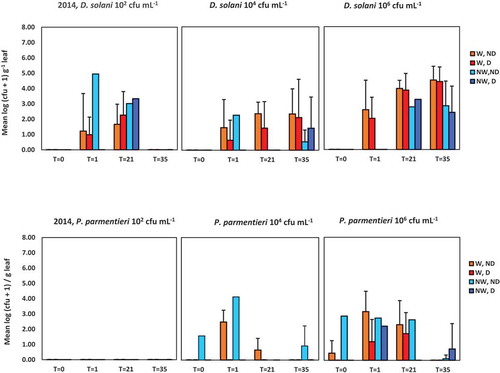
In 2014, during the entire course of the experiment, the infection rates and levels were generally lower than in 2013 for both pathogens at all densities (, ). At 0 dpi, in plants with untouched leaves inoculated with P. parmentieri, the pathogen could only be detected in an extract of a non-disinfected leaf of a single plant at the highest inoculum density of 106 cfu mL−1. For the higher inoculum densities of 104 cfu mL−1 and 106 cfu mL−1, the populations of D. solani increased rapidly in leaf material. At 35 dpi, infection rates of 50 and 100% were found after disinfection of wounded leaves that were inoculated with 104 and 106 cfu mL−1, respectively. Population densities were still approximately a 100-fold lower than in 2013. In particular, in tissues of untouched leaves inoculated with D. solani, population densities remained small compared with the 2013 experiment, even at a density of 106 cfu mL−1. In plants treated with P. parmentieri, positive samples were found only occasionally. In leaves sprayed with water, no GFP-tagged pathogens were detected throughout either experiment.
Fig. 3 Population densities of Dickeya solani and Pectobacterium parmentieri (respectively, the upper and lower row of graphs) in leaves at 0, 1, 21 and 35 days after spraying potato leaves with suspensions of 102, 104 and 106 cfu mL−1. Results are summarized of the glasshouse experiment in 2014. W = leaves wounded prior to inoculation, NW = leaves not wounded prior to inoculation, D = leaves disinfected prior to analysis, ND = leaves not disinfected prior to analysis. Error bars show standard deviations
Supplementary Tables S1 – S4 show the results of the REML analysis per time-point and per year for the variance components inoculum, inoculum density, wounding and disinfection, respectively. For both years (2013 and 2014) and at most time-points (except time points 0 and 1 dpi in 2014), the analysis indicated statistically significant higher densities in leaves inoculated with D. solani than for leaves inoculated with P. parmentieri (Table S1). Similarly, in both years and at most time-points, statistically higher densities were found in leaves inoculated at a higher inoculum density (106 cfu mL−1) than in leaves inoculated with a low (102 cfu mL−1) inoculum density (Table S2). In both years, at most time-points (except for 42 dpi in 2013), no effect was found for wounding (Table S3). The REML analysis indicated no effect of leaf disinfection before further processing the samples on overall pathogen densities for the time-points 21 dpi in 2013 and for 21 and 35 dpi in 2014 (Table S4). It seemed that the contribution of the pathogens to the total counts, externally present on leaves and exposed to the disinfectants, was variable. Table S5 shows the results of the REML analysis for both years for the time-point variance component, and indicates a statistically significant effect of the time after inoculation on overall pathogen densities for both years. In 2013, at all time-points, the overall pathogen densities were higher compared with the preceding time-point, whereas in 2014 overall pathogen densities increased between day 0 and day 1 and remained at about that level after day 1.
Infection of stems, stolons and tubers
In 2013, the distribution of both D. solani and P. parmentieri was assessed in 23 plants per pathogen, 42 days after inoculation. Translocation of D. solani and P. parmentieri from leaves to lower plant parts was found in 10 and 3 plants, respectively. In most of these plants the distribution of the pathogens was not homogeneous. In 7 plants inoculated with D. solani, the pathogen was recovered from either the stem tops, the stem bases, or the stolons. In the other 3 plants, D. solani was present in both stem bases and stolons (2 plants) or in the stem top, stolon and stolon ends of tubers (1 plant). After inoculation with P. parmentieri, the pathogen was recovered from both stem tops and stem bases (2 plants) or from stem bases and stolons (1 plant).
D. solani was recovered from stem tops of plants of which wounded leaves were inoculated with inoculum densities 102 and 106 cfu mL−1, respectively (). Densities were relatively low (between 101 and 104 cfu g−1). The pathogen was also recovered from stem bases of plants of which the untouched leaves had been inoculated with a density of 104 cfu mL−1 and from stem bases of plants of which leaves of both wounding treatments (wounded or untouched) were inoculated with a density of 106 cfu mL−1. Infection levels between 102 and 106 cfu g−1 were found. Dickeya solani was not recovered from stem samples from plants of which untouched leaves were inoculated with an inoculum density of 102 cfu mL−1. The pathogen was also not recovered from stolons of plants (irrespective whether their leaves were wounded or not) inoculated with an inoculum density of 102 cfu mL−1. However, D. solani was found in stolons of plants of which wounded leaves were inoculated with a density of 104 cfu mL−1 and of plants of which leaves (irrespective of being wounded or untouched before inoculation) were inoculated with a density of 106 cfu mL−1. Densities between 101 and 107 cfu g−1 were found. Dickeya solani was found only in stolon ends of progeny tubers collected from one of five plants of which wounded leaves were inoculated with a density of 106 cfu mL−1. The population density was relatively low (1.3 102 cfu g−1).
Fig. 4 Average densities of Dickeya solani and Pectobacterium parmentieri (respectively, the upper and lower row of graphs) in leaves, in top or base parts of potato stems, in stolons or in tubers at 42 days post-inoculation in the experiment conducted in 2013. Bars indicate standard deviations. Plants were inoculated with 102, 104 or 106 cfu mL−1 of Dickeya solani or Pectobacterium parmentieri on leaves that were wounded or not wounded prior to inoculation
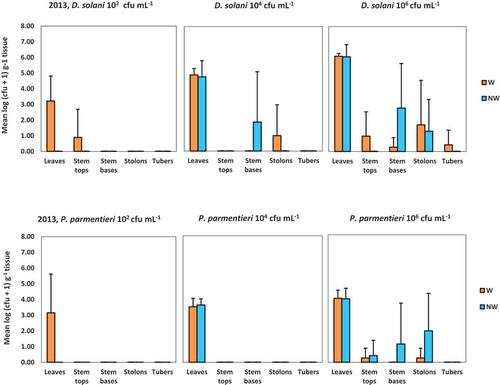
Translocation of P. parmentieri was found only in plants of which leaves were inoculated with a density of 106 cfu mL−1, irrespective of being wounded or not before inoculation (). Densities of P. parmentieri recovered from stem tops were relatively low (between 101 and 103 cfu g−1). Densities found in stem bases and stolons were between 105 and 106 cfu g−1 and between 101 and 106 cfu g−1, respectively.
Throughout the experiment, no GFP-tagged pathogens were detected in stem tops or bases, or stolons or stolon ends of progeny tubers of plants sprayed with water.
On day 35 of the 2014 experiment, only one plant was found with infected stem tissue following inoculation of wounded leaves with a 106 cfu mL−1 suspension of D. solani. Dickeya solani was found in the stolons of this plant, but not in stolon ends of the tubers. Due to the absence of infections in the stems, no stolons and no progeny tubers were analyzed for the other plants.
Experiment on dispersion via soil
Five days after inoculation of the plants, population densities in leaf and haulm tissues were between 106 and 108 cfu g−1 of plant material for P. parmentieri and ca. 104 cfu g−1 of plant material for D. solani (). At that time, haulms were destroyed, after which the population dynamics of P. parmentieri followed the same trend for haulms which were chemically or mechanically destroyed. Six days after destruction, populations increased by a factor of 100 to 1 000 000, after which populations declined rapidly (). Fourteen days after destruction, haulms were largely decayed and populations were at a level of 101–104 cfu g−1. After chemical haulm destruction, the populations of D. solani declined rapidly, but for the mechanically destructed haulms, population densities remained more or less stable for 3 days after which they declined. The bacteria were not detectable at 14 days after haulm destruction.
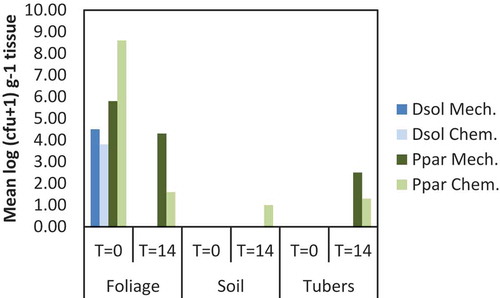
Fig. 5 Population densities of Dickeya solani (Dsol) and Pectobacterium parmentieri (Ppar) in foliage tissue at 0, 3, 5 and 14 days after chopping the haulms followed by herbicide treatment of the stalks (Mech.) or after chemical vine killing (Chem.). Haulms were spray-inoculated with 108 cfu mL−1 5 days before haulm destruction
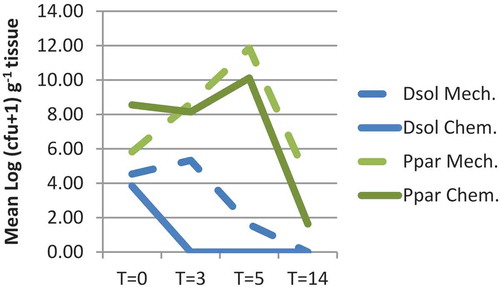
Water controls were negative, except for a positive result with chemically treated plants at 6 days after haulm destruction, possibly due to cross-contamination via splash dispersion during simulated irrigation.
The soil and the tubers were negative for D. solani, but for P. parmentieri low densities were found after chemical haulm destruction, both in the soil and in the peel of tubers, and after mechanical haulm destruction in the peel of tubers (). Water controls were negative (n = 2).
Discussion
Our experiments on the colonization of potato plants showed that contamination of potato haulms with D. solani or P. parmentieri poses a considerable risk of leaf infection and that translocation of bacteria from infected leaves to stems and underground plant parts, i.e., stolons and tubers, can occur. Blackleg-diseased plants were found even after spray-inoculation of the leaves. Downward migration of D. solani after injection of the upper part of the stems with high densities of the bacterium has previously been shown in greenhouse studies (Czajkowski et al. Citation2010). They also demonstrated that inoculation of severely wounded leaves with a high density (108 cfu mL−1) suspension of D. solani resulted in colonization of leaves and translocation to the petioles and adjacent parts of the stem, although no infections of the stem base were observed. In our study, leaf infections were also found after spray-inoculation of wounded potato leaves, even at low densities of both D. solani and P. parmentieri. Infections also occurred when untouched leaves were used, which mimics the situation that can occur during potato cropping.
The population densities in leaves were higher for D. solani than for P. parmentieri, which may be related to specific virulence factors found in D. solani, but not in P. parmentieri. Compared with P. parmentieri, D. solani exhibited higher pectinase, cellulase and protease activities, a lower siderophore production rate, a lower capacity to macerate potato tissue and a lower rate of swimming and swarming motility (Zoledowska et al. Citation2018). Dickeya solani also has a functional type III secretion system (T3SS), important for bacteria to suppress basal defence reactions of the host (Garlant et al. Citation2013; Zoledowska et al. Citation2018), which is not present in P. parmentieri (Nykyri et al. Citation2012). However, no clear correlation has been found between the presence of a T3SS and the virulence for soft rot of the Pectobacteriaceae (Kim et al. Citation2009).
Directly after inoculation, the plants were kept in a water-saturated atmosphere which resulted in the presence of free water on the surface of the leaves. After inoculation of undamaged leaves, Dickeya and Pectobacterium cells may have moved via this free water to natural openings, such as hydathodes and stomata, and entered the mesophyll. In several CLSM images, the pathogens were observed in or near hydathode and stomatal openings. Stomata are found both on the adaxial and abaxial side of potato leaves, although in higher numbers on the abaxial side. The entrance of the pathogen through stomata may be facilitated by coronatine produced by soft rot Pectobacteriaceae, which prevents closure of the stomata (Slawiak & Lojkowska Citation2009). Infected hydathodes and stomata may result in systemic infections when the pathogen migrates into vascular tissues via the intercellular spaces between the parenchyma cells of the mesophyll.
In 2013, inoculation of wounded leaves resulted in an overall higher infection rate than inoculation of untouched leaves, whereas in 2014, due to the low infection rate, no clear differences were found (data not presented). The risk of stem wounding for infections with aerosols has been previously reported (Graham Citation1985), but no information has been published on leaf wounding. Wounding is considered to favour the entrance of bacteria into leaves and the release of nutrients. Wounding also results in the production of jasmonate, a key signalling compound in plant defence, but also a strong chemo-attractant for Dickeya dadantii (Antunez-Lamas et al. Citation2009a). In addition, jasmonate may potentially induce genes involved in virulence and survival of the pathogen in the plant apoplast (Antunez-Lamas et al. Citation2009b).
Contamination of haulms of pathogen-free first-generation crops during the growing season can occur from various sources, including rainwater, aerosols, surface water used for irrigation, insects, machines, clothes, shoes, fur and feathers (Czajkowski et al. Citation2011). Flies can carry as much as 107 cfu on their bodies and may locally deposit high numbers on leaves (Harrison et al. Citation1977). Rainwater may contain only low densities (< 20 cfu L−1) of the pathogens (Franc et al. Citation1984; McCarter-Zorner et al. Citation1984). However, frequent deposition of SRP during rainy periods in the growing season may result in high pathogen numbers on the haulms. During haulm pulverization, high numbers of SRP cells at an estimated 108 cfu per ha can be generated as an aerosol (Perombelon et al. Citation1979). Inoculum aerosols will also be generated by raindrops splashing on an infected ware crop. The aerosols can be dispersed by wind several hundred metres before deposition on a crop, mainly by the action of rain (Perombelon et al. Citation1979). In our experiments on the colonization of potato plants, inoculum densities as low as 102 cfu mL−1 were sufficient to establish an infection. Because of these findings, it is plausible that in practice foliar contamination with SRP deposited via the air can result in infected potato haulms.
In our 2013 experiment, the two pathogens migrated from inside the leaves downwards and could be detected at 42 dpi in the stems. Dickeya solani was also found in the stolons and tubers when inoculated at high densities. Downward translocation of D. solani in stem- and leaf-inoculated plants has been demonstrated previously (Czajkowski et al. Citation2010). Czajkowski et al. (Citation2010) recovered the pathogen from stolons and progeny tubers after stem-inoculation, but not after leaf-inoculation. In the case of P. parmentieri, downward translocation has not been previously studied, but a rapid downward translocation of Pectobacterium brasiliense in stem-inoculated potato plants has been shown to occur (Moleleki et al. Citation2017).
Results of the experiments on the colonization of potato plants showed that haulm infections can result in tuber infection due to systemic colonization of plants, although the incidence was low. Even at high inoculum levels, only a single tuber infection was found at 42 dpi, despite the use of a highly susceptible cultivar and a temperature favourable for disease development. In practice, however, the time over which infections can be established after emergence of the plants is longer and can last for up to 80 days. Therefore, we do not exclude the possibility that, in practice, progeny tubers in seed production crops grown from mini-tubers can become infected during plant growth via the route of airborne haulm infections and systemic colonization of plants.
The mechanism behind the downward translocation observed in the 2013 experiment is not well understood. Our microscopy studies indicated the presence of D. solani in xylem vessels of contaminated leaves (). No degradation of vascular tissues was observed, as was the case with Czajkowski et al. (Citation2010). A reverse water transport due to negative hydraulic pressure as a result of a reduced water uptake by the roots and a reduced leaf evaporation as described by Tyree (Citation1997) is not expected either, as plants in our glasshouse experiments did not encounter water stress. The slowing down of water flow in xylem vessels at night (Průšová Citation2016) and an active movement of the pathogen may be involved in the downward translocation, but it is unclear by which type of motility. Pectobacterium brasiliense loses its flagella at 24 h days post-inoculation, leaving out the possibility of swimming or swarming motility (Moleleki et al. Citation2017). Twitching motility might possibly be the driving mechanism (Hosseinzadeh et al. Citation2013; Moleleki et al. Citation2017).
Progeny tubers may also become infected via rainwashing of bacteria from infected aboveground tissues, in particular after haulm destruction (Elphinstone & Pérombelon Citation1986b; Burgess et al. Citation1994). In the case of P. parmentieri, the experiment on pathogen dispersion from leaves through soil showed that tuber infections can occur from inoculum washed from infected haulm debris into the soil. In the first week after haulm destruction, the inoculum increased in particular after mechanical haulm killing, to densities of up to 1011 cfu g−1. These levels were higher than those found in field experiments in Scotland, in which densities in pulverized haulms were limited to 106–107 cfu g−1 (Burgess et al. Citation1994). Environmental conditions, such as temperature, wind and humidity, which determine haulm debris drying, may influence population development. The increase in P. parmentieri density was followed by a strong decrease in the second week. At 14 days after haulm killing, P. parmentieri was found both in soil and on tubers, although the infection incidence was low. Consequently, if the haulms or haulm fragments are located on the ridges above the tubers, P. parmentieri may be washed from haulms into the soil and tubers with rain or irrigation water. In the case of D. solani, the populations in the haulms died out much faster than for P. parmentieri, which may explain the absence of the pathogen in soil and on tubers.
In summary, if contamination of leaves occurs, both D. solani and P. parmentieri can cause haulm infections even at low inoculum density. Pectobacterium parmentieri, however, is better able to multiply and persist in destroyed haulms, posing a risk of soil contamination and tuber infection. The relative importance of the two pathways in initial infections, i.e., translocation from haulms to tubers via the vascular route or washing off the inoculum with water after haulm destruction, needs to be determined empirically under field conditions.
TableS5.docx
Download MS Word (15.2 KB)TableS4.docx
Download MS Word (16.4 KB)TableS3.docx
Download MS Word (15.8 KB)TableS2.docx
Download MS Word (14.8 KB)TableS1.docx
Download MS Word (15.8 KB)SupplementFigure1.pptx
Download MS Power Point (540.9 KB)Acknowledgements
This project was financially supported by the Dutch Ministry of Economic Affairs (knowledge base (KB) research). We thank Mrs. L. Hyman (Dundee, Scotland, UK) for her editorial work.
Supplemental Material
Supplemental data for this article can be accessed here.
References
- Antunez-Lamas M, Cabrera E, Lopez-Solanilla E, Solano R, González-Melendi P, Chico JM, Toth I, Birch P, Pritchard L, Liu H, et al. 2009b. Bacterial chemoattraction towards jasmonate plays a role in the entry of Dickeya dadantii through wounded tissues. Mol Microbiol. 74:1543. doi:10.1111/j.1365-2958.2009.06960.x.
- Antunez-Lamas M, Cabrera-Ordonez E, Lopez-Solanilla E, Raposo R, Trelles-Salazar O, Rodríguez-Moreno A, Rodríguez-Palenzuela. 2009a. Role of motility and chemotaxis in the pathogenesis of Dickeya dadantii 3937 (ex Erwinia chrysanthemi 3937). Microbiol. 155:434–442. doi:10.1099/mic.0.022244-0.
- Boomsma D, Velvis H, Kristelijn K, van Tent Becking T, Kastelein P, van der Zouwen PS, Krijger MC, Forch MG, van der Wolf JM, Czajkowski RL, et al. 2013. Het project “Deltaplan Erwinia Deel C - Pootaardappelen. Eindrapport van het onderzoek 2009-2012. The Hague (NL (in Dutch)): Dutch Potato Association. Accessed 2019 Feb 21. http://library.wur.nl/WebQuery/wurpubs/fulltext/252391
- Burgess PJ, Blakeman JP, Perombelon MCM. 1994. Contamination and subsequent multiplication of soft rot erwinias on healthy potato leaves and debris after haulm destruction. Plant Pathol. 43:286–299. doi:10.1111/j.1365-3059.1994.tb02687.x.
- Czajkowski R, De Boer WJ, Van Veen JA, Van der Wolf JM. 2010. Downward vascular translocation of a green fluorescent protein-tagged strain of Dickeya sp. (Biovar 3) from STEM AND LEAF INOCULATION SITES ON POTATO. Phytopathology. 100(11):1128–1137. doi:10.1094/PHYTO-03-10-0093.
- Czajkowski R, Perombelon MCM, Van Veen JA, Van der Wolf JM. 2011. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol. 60:999–1013. doi:10.1111/j.1365-3059.2011.02470.x.
- Elphinstone JG, Pérombelon MCM. 1986a. Contamination of potatoes by Erwinia carotovora during grading. Plant Pathol. 35:25–33. doi:10.1111/j.1365-3059.1986.tb01977.x.
- Elphinstone JG, Pérombelon MCM. 1986b. Contamination of progeny tubers of potato plants by seed- and leaf-borne Erwinia carotovora. Potato Res. 29:77–93. doi:10.1007/BF02361983.
- Franc GD, Harrison MD, Powelson ML 1984. The presence of Erwinia carotovora in ocean water, rain water and aerosols. In: Graham DC, Harrison MD, eds. International conference on potato blackleg disease. Edinburgh, UK, pp 48–49.
- Garlant L, Koskinen P, Rouhiainen L, Laine P, Paulin L, Auvinen P. 2013. Genome sequence of Dickeya solani, a new soft rot pathogen of potato, suggests its emergence may be related to a novel combination of non-ribosomal peptide/polyketide synthetase clusters. Diversity. 5:824–842. doi:10.3390/d5040824.
- Graham DC 1985. Spread of Erwinia bacteria in atmospheric aerosols. In: Graham DC, Harrison MD, eds. International Conference on Potato Blackleg Disease. Edinburgh, 43–45.
- Harrison MD, Quinn CE, Sells IA, Graham DC. 1977. Waste potato dumps as sources of insects contaminated with soft rot coliform bacteria in relation to recontamination of pathogen-free potato stocks. Potato Res. 20(1):37–52. doi:10.1007/BF02362299.
- Hélias V, Hamon P, Huchet E, Van der Wolf J, Andrivon D. 2012. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathol. 61(2):339–345. doi:10.1111/j.1365-3059.2011.02508.x.
- Hosseinzadeh S, Shams-Bakhsh M, Yamchi A. 2013. A comparative study on effect of two different aiiA genes on pathogenicity factors of Dickeya chrysanthemi pv. chrysanthemi. Arch Phytopathol Plant Prot. 46(12):1468–1479. doi:10.1080/03235408.2013.769723.
- Kim H-S, Ma B, Perna NT, Charkowski AO. 2009. Phylogeny and virulence of naturally occurring Type III secretion system-deficient pectobacterium strains. Appl Environm Microbiol. 75(13):4539–4549. doi:10.1128/AEM.01336-08.
- McCarter-Zorner NJ, Franc GD, Harrison MD, Michaud JE, Quinn CE, Ann Sells I, Graham DC. 1984. Soft rot Erwinia bacteria in surface and underground waters in Southern Scotland UK and in Colorado USA. J Appl Bacteriol. 57:95–106. doi:10.1111/j.1365-2672.1984.tb02361.x.
- Moleleki LN, Pretorius RG, Tanui CK, Mosina G, Theron J. 2017. A quorum sensing-defective mutant of Pectobacterium carotovorum ssp. brasiliense 1692 is attenuated in virulence and unable to occlude xylem tissue of susceptible potato plant stems. Molec Plant Pathol. 18:32–44. doi:10.1111/mpp.12372.
- Nykyri J, Niemi O, Koskinen P, Nokso-Koivisto J, Pasanen M, Broberg M, Plyusnin I, Törönen P, Holm L, Pirhonen M, et al. 2012. Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathog. 8:e1003013. doi:10.1371/journal.ppat.1003013.
- Pasanen M, Laurila J, Brader G, Palva ET, Ahola V, van der Wolf J, Hannukkala A, Pirhonen M. 2013. Characterisation of Pectobacterium wasabiae and Pectobacterium carotovorum subsp. carotovorum isolates from diseased potato plants in Finland. Ann Appl Biol. 163(3):403–419. doi:10.1111/aab.12076.
- Perombelon MCM, Fox RA, Lowe R. 1979. Dispersion of Erwinia carotovora in aerosols produced by the pulverization of potato haulm prior to harvest. Phytopathol Zeitsch. 94(3):249–260. doi:10.1111/j.1439-0434.1979.tb01557.x.
- Perombelon MCM, Kelman A. 1980. Ecology of the soft rot erwinias. Annu Rev Phytopathol. 18(1):361–387. doi:10.1146/annurev.py.18.090180.002045.
- Průšová A 2016. Light on phloem transport (an MRI approach). PhD thesis. Wageningen, the Netherlands: wageningen University, 130.
- Sławiak M, Beckhoven JRCM, Speksnijder AGCL, Czajkowski R, Grabe G, Van der Wolf JM. 2009. Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. Eur J Plant Pathol. 125(2):245–261. doi:10.1007/s10658-009-9479-2.
- Slawiak M, Lojkowska E. 2009. Genes responsible for coronatine synthesis in Pseudomonas syringae present in the genome of soft rot bacteria. Eur J Plant Pathol. 124(2):353–361. doi:10.1007/s10658-008-9418-7.
- Struik PJ 1992. Potato cultivation and haulm destruction in the Netherlands. In: Bouman A, ed. Proceedings of the Meeting of the Section Engineering of the European association for Potato Research, 1992. Wieringerwerf (NL): IMAG-DLO Report 92-16, 8–13.
- Toth IK, Van der Wolf JM, Saddler G, Lojkowska E, Hélias V, Pirhonen M, Tsror L, Elphinstone JG. 2011. Dickeya species: an emerging problem for potato production in Europe. Plant Pathol. 60(3):385–399. doi:10.1111/j.1365-3059.2011.02427.x.
- Tsror L, Erlich O, Hazanovsky M, Ben Daniel B, Zig U, Lebiush S. 2012. Detection of Dickeya spp. latent infection in potato seed tubers using PCR or ELISA and correlation with disease incidence in commercial field crops under hot-climate conditions. Plant Pathol. 61:161–168. doi:10.1111/j.1365-3059.2011.02492.x.
- Tyree MT. 1997. The cohesion-tension theory of sap ascent: current controversies. J Experim Botany. 48:1753–1765.
- Van der Wolf JM, De Haan EG, Kastelein P, Krijger M, De Haas BH, Velvis H, Mendes O, Kooman-Gersmann M, Van der Zouwen PS. 2017. Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathol. 66:571–583. doi:10.1111/ppa.12600.
- Van Vuurde JWL, Van der Wolf JM, Pérombelon MCM. 2002. Immunofluorescence colony-staining (IFC). In: Pérombelon MCM, Van der Wolf JM, editors. Methods for the detection and quantification of Erwinia carotovora subsp. atroseptica (Pectobacterium carotovorum subsp. atrosepticum) on potatoes: a laboratory manual. Dundee (UK): Scottish Crop Research Institute; p. 25–33.
- Zoledowska S, Motyka A, Zukowska D, Sledz W, Lojkowska E. 2018. Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Dis. 102:154–164. doi:10.1094/PDIS-05-17-0761-RE.