Abstract
Fusarium oxysporum causes root browning and crown infection on marijuana (Cannabis sativa L.) plants, resulting in stunted growth, yellowing of leaves, and plant death. Pathogen presence and diversity were assessed in samples of diseased crowns, stems, pith tissues and roots from five commercial production facilities in British Columbia and Ontario. PCR of the elongation factor (EF-1 α) region and sequence analysis confirmed the identity and phylogenetic relationships among 33 representative isolates from over 200 isolates collected. Stock (mother) plants of eight cannabis strains (genotypes) with symptoms of yellowing, crown rot and internal stem decay yielded F. oxysporum at a frequency of 70–100%. Cuttings obtained from asymptomatic plants showed pathogen recovery rates of 1–2% on potato dextrose agar at distances of 90–150 cm from the crowns. Symptoms of damping-off were observed on cuttings in a rooting and propagation facility. Pathogenicity tests confirmed the development of characteristic symptoms on cuttings and rooted plants inoculated with F. oxysporum. Phylogenetic analysis indicated that isolates of F. oxysporum from BC belonged to a separate clade from ON isolates and that clonal spread was occurring within licenced facilities. Air sampling conducted in a greenhouse facility revealed F. oxysporum was present in areas used for rooting, vegetative propagation, and where stock plants were grown. The pathogen was also recovered from inflorescences. These findings indicate that F. oxysporum is present in several commercial production facilities in Canada and reduces root development and establishment of rooted cuttings, and causes yellowing and stunting on flowering plants of many cannabis strains.
Résumé
Fusarium oxysporum cause le brunissement des racines et l’infection du collet chez les plants de marijuana (Cannabis sativa L.), ce qui entraîne le ralentissement de la croissance, le jaunissement des feuilles et la mort du plant. L’occurrence des agents pathogènes et leur diversité ont été évaluées à partir d’échantillons de collets, de tiges, de moelle et de racines infectés provenant de cinq installations commerciales de production de Colombie-Britannique et d’Ontario. La PCR de la région du facteur d’élongation (EF-1 α) et l’analyse des séquences a confirmé l’identité et les relations phylogénétiques chez 33 isolats représentatifs de plus de 200 isolats collectés. À une fréquence de 70 à 100%, les plants mères de huit souches (génotypes) de cannabis affichant les symptômes du jaunissement, de la pourriture du collet et de la pourriture interne de la tige ont produit F. oxysporum. Des boutures obtenues de plants asymptomatiques ont affiché, sur gélose dextrosée à la pomme de terre, des taux de récupération de 1 à 2% liés à l’agent pathogène, et ce, de 90 à 150 cm des collets. Des symptômes de la fonte des semis ont été observés sur des boutures dans une installation d’enracinement et de propagation végétale. Des tests de pathogénicité ont confirmé le développement caractéristique des symptômes chez les boutures et les plants enracinés inoculés avec F. oxysporum. L’analyse phylogénétique a montré que les isolats de F. oxysporum provenant de la Colombie-Britannique appartenaient à un clade différent de ceux de l’Ontario, et que la propagation clonale se produisait dans les installations autorisées. Un échantillonnage d’air effectué dans une des serres a révélé la présence de F. oxysporum dans les salles utilisées pour l’enracinement ainsi que la multiplication végétative et où les plants mères étaient cultivés. L’agent pathogène a également été collecté sur des inflorescences. Ces résultats indiquent que F. oxysporum est présent dans plusieurs installations commerciales de production au Canada, et que ce dernier interfère avec le développement racinaire ainsi qu’avec l’établissement des boutures racinées. De plus, il cause le jaunissement et le rabougrissement chez les plants en fleur de plusieurs souches de cannabis.
Introduction
Cannabis (Cannabis sativa L., marijuana) production in Canada is growing rapidly to meet the increasing demand for the recreational and medicinal markets. As of May 2020, there were 391 licenced holders in Canada who are approved by Health Canada to grow, harvest and/or sell cannabis inflorescences or products derived from them (Health Canada Citation2020). Some of the commercial production occurs in greenhouses previously used for fresh market vegetable production, including peppers, tomatoes and cucumbers. In other instances, new facilities were built in anticipation of continuing market demand by consumers following cannabis legalization in Canada on October 17, 2018. Among several challenges currently facing cannabis producers, management of diseases remains a high priority. Many of the recently reported diseases that affect cannabis production in Canada, such as root and crown rots, powdery mildew, and bud rots caused by Botrytis and Penicillium species, are appearing in both indoor controlled environment facilities and greenhouse production (Punja et al. Citation2019a).
Fusarium root and crown rot of C. sativa, caused by F. oxysporum Schlecht. emend. Snyder & Hansen, was first reported in 2018 in Canada and in California following isolations made from diseased plants during 2015–2017 (Punja and Rodriguez Citation2018; Punja et al. Citation2018). These studies showed the pathogen could be isolated from roots showing necrotic symptoms, from infected crown areas, and from vegetatively propagated rooted cuttings (Punja and Rodriguez Citation2018). Pathogenicity studies demonstrated the ability of two isolates originating from British Columbia (BC) and Ontario (ON) to cause stunting and yellowing symptoms on inoculated plants (Punja and Rodriguez Citation2018). However, the extent to which this pathogen is distributed in other production facilities, and its ability to infect other tissue types of cannabis plants, is unknown. Furthermore, the extent to which F. oxysporum is distributed within a production facility, and the mechanism(s) by which spread of inoculum of the pathogen is occurring have not been determined.
The objectives of this study were to: (i) determine the prevalence of F. oxysporum through analysis of samples originating from five production facilities in BC and ON; (ii) establish the types of tissues from which the pathogen could be recovered, inclusive of roots, crown, stems, and pith; (iii) determine the methods by which the pathogen could spread within a production facility; and (iv) establish the phylogenetic relationships among isolates of F. oxysporum originating from different sources.
Materials and methods
Isolations from plants
Cannabis plants at different stages of growth with symptoms of yellowing, stunted growth, wilting and necrosis of leaves, or browning of roots, were included in the study. Plants were grown indoors in controlled environments or in greenhouses in Health Canada approved licenced facilities (three were located in British Columbia and two in Ontario). Samples were selected from various stages during the production cycle, ranging from early propagation (1–2 weeks old) to vegetative growth (3–4 weeks of age) to onset of full flowering period (6–12 weeks of age) (). In addition, stock (mother) plants grown in large containers (10 L) over a period of 6–10 months were included. The isolations were conducted at various times during July 2018-September 2019 and a total of up to 350 symptomatic plants (as stock plants, rooted cuttings, vegetative plants or flowering plants) of 10 strains (genotypes) were included depending on what was in production (). Plants were propagated either in coco fibre (coco coir) substrate from various suppliers or in rockwool blocks (Grodan) and were provided with the appropriate nutrient regimes and lighting conditions as required for commercial hydroponic production (Small Citation2017). The environmental conditions during the periods over which isolations were conducted varied according to the production facility, with temperatures in the range of 24–32°C and relative humidity in the range of 50% to 85%.
Table 1. Isolates of Fusarium oxysporum recovered from cannabis (Cannabis sativa L., marijuana) plants in this study
Fig. 1 Schematic representation of the production cycle of cannabis plants grown in commercial greenhouses. (Top left) Stock (mother) plants are used as a source of cuttings for vegetative propagation. The cuttings are rooted in an appropriate substrate, such as rockwool, peat or coco coir, and transferred after 2 weeks to a growth medium (rockwool, coco coir, peat, soil) for vegetative growth. After 2–3 weeks, the plants are transferred to a flowering room and placed under a photoperiod of 12 hr light:12 hr darkness to induce flowering. After 8–10 weeks, flowers are harvested and dried, usually over a period of 5–6 days
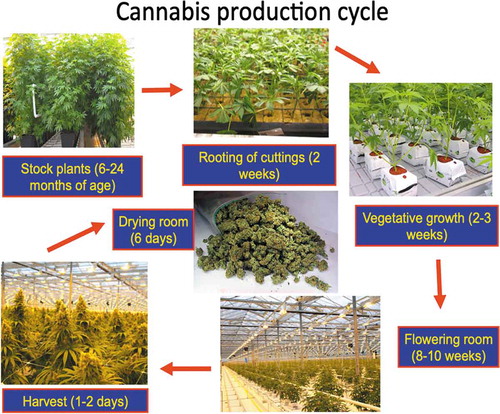
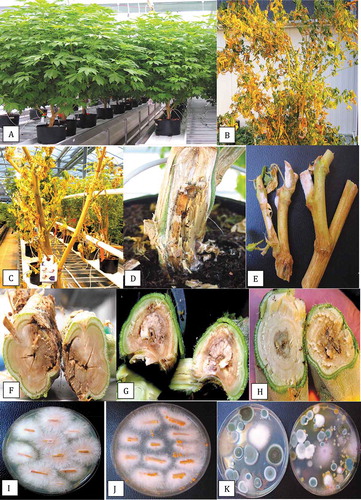
Fig. 2 Symptoms of infection caused by Fusarium oxysporum on stock (mother plants). (a) Healthy plants approximately 8 months old. (b) Advanced infection causing yellowing and necrosis of leaves, and wilting symptoms. (c) Total collapse and defoliation of diseased plant. (d) Symptoms of crown decay in the plant shown in (b) with white mycelial growth. (e) Growth of F. oxysporum on pruning sites of stock plants under conditions of high humidity. (f) Internal stem discoloration and decay at the crown of diseased plant shown in (d). (g) Internal stem discoloration and decay of the stem at a distance of 100 cm from the crown. (h) Internal stem discoloration and decay at a distance of 150 cm from the crown. (i) Colonies of F. oxysporum recovered from decayed tissue pieces from the crown of a diseased plant. (j) Formation of orange sporodochia after growth for one month on PDA. (k) Colonies of F. oxysporum (pinkish-white) recovered on Petri dishes left exposed in the growing environment of stock plants for one hour. Other colonies are those of Penicillium species
Tissues that included root segments, stem pieces, and crown tissues from plants showing symptoms at different stages of production (cuttings, vegetative growth, flowering plants, stock plants) (, ) were used for pathogen isolation. Two samples of diseased bud tissues were also included. From each tissue type, small pieces, ca. 0.5 cm in length for roots or 0.2–0.4 cm2 for cuttings, stem pieces and bud tissues, were surface-disinfested by dipping them in a 10% bleach solution (containing 0.625% NaOCl) for 1 min, followed by 30 s in 70% EtOH. They were rinsed thrice in sterile water and blotted on sterile paper towels. For larger diameter stem and pith tissues, the sterilization protocol was increased to 2 min in NaOCl and 30 s in EtOH. Tissue pieces were plated onto potato dextrose agar (PDA, Sigma Chemicals, St. Louis, MO) amended with 130 mg L−1 of streptomycin sulphate (PDA+S). Dishes containing the tissues were incubated under ambient laboratory conditions (temperature range of 21–24°C with 10–12 hr day−1 fluorescent lighting) for 5–10 days. Emerging colonies were transferred to fresh PDA+S dishes for subsequent identification to genus level using morphological criteria, including colony colour and size and microscopic examination of spores. Hyphal-tip transfers from colonies that resembled F. oxysporum were made as they formed the basis for this study. Colonies were grown on water agar for 72 hr and hyphal tip transfers made onto PDA+S. Other microbes observed growing on the isolation dishes were recorded and included Penicillium, Pythium and Trichoderma species, as well as a number of unidentified bacteria. Up to 200 isolates of F. oxysporum were obtained from the different tissue types, and 33 isolates were selected for molecular analysis () to represent the different tissue sources, licenced facilities and cannabis strains sampled.
Pathogen distribution in stock plants
The main stem of several diseased stock plants (6–10 months of age) was cut down and partitioned into 30-cm long increments, beginning at the crown and proceeding alongside branches to the top of the plant (> 200 cm). From each increment, a 5-cm long stem piece was dissected from the mid-point and surface-sterilized in a 10% bleach solution (Javex, containing 6.25% NaOCl) for 2 min followed by 70% EtOH for 30 s and rinsed with sterile distilled water for 1 min. The segments were transferred to a sterile Petri dish, where they were cut lengthwise with a scalpel and small tissue pieces, measuring approximately 0.5 cm2, were dissected and plated. A total of 20 tissue pieces from each segment were placed on five Petri dishes containing PDA+S and incubated under ambient laboratory conditions for one week after which the presence of F. oxysporum or other microbes was recorded. The experiment was conducted twice, with different groups of plants. Molecular confirmation was conducted as described below for select cultures.
To determine if cuttings from visibly healthy appearing stock plants (without symptoms of yellowing) could harbour Fusarium internally, cuttings (15 cm in length) were taken from several ‘Hash Plant’ stock plants, at incremental distances of 15 cm from the crown and proceeding up to 200 cm. The cuttings were obtained from the main and lateral branches from several stock plants and combined; a total of 20 cuttings were obtained from each of the distances sampled. The pruning shears used to obtain the cuts were dipped into 70% EtOH for 2 s at frequent intervals. The cuttings were inserted directly into PDA+S in 18 × 2.5 cm glass test-tubes. The percentage of cuttings that yielded F. oxysporum was rated after 7 days of incubation at 21–24°C. The experiment was conducted twice using different groups of stock plants each time. The data from both experiments was averaged and variation was expressed as standard errors.
Molecular identification
Species-level identification was conducted using PCR with primers for the elongation factor 1α (EF-1α) region: EF- 1 (5ʹATG GGT AAG GAG GAC AAG AC 3ʹ) and EF-2 (5ʹ GGA GGT ACC AGT GAT CAT GTT 3ʹ) (O’Donnell et al. Citation1998). Cultures of representative isolates from different tissue sources () were grown in potato dextrose broth at room temperature for 7 days and DNA was extracted from harvested mycelium using the QIAGEN DNeasy Plant Mini Kit. Aliquots of 1 µL containing 5–20 ng DNA was used for PCR in a 25 µL reaction volume consisting of 2.5 µL 10X buffer (containing 15 mM MgCl2), 0.5 µL 10 mM dNTP, 0.25 µL Taq DNA Polymerase (QIAGEN), 0.25 µL 10 mM forward and reverse primers, as well as 20.25 µL DNAse- and RNAse-free water (Invitrogen). All PCR amplifications were performed in a MyCycler thermocycler (BIORAD) with the following program: 3 min at 94°C; 30 s at 94°C, 30 s at 60°C, 3 min at 72°C (35 cycles); and 7 min at 72°C. PCR products were separated on 1% agarose gels and bands of the expected size (ca. 700 bp) were purified with QIAquick Gel Extraction Kit and sent to Eurofins Genomics (Eurofins MWG Operon LLC 2016, Louisville, KY) for sequencing. The resulting sequences were compared to the corresponding EF-1α sequences from the National Centre for Biotechnology Information (NCBI) GenBank database. Multiple sequence alignment of the respective isolates was done using the CLUSTAL W program (http://www.genome.jp/tools/clustalw). The sequences of F. oxysporum were subsequently included in a phylogenetic analysis using the neighbour-joining (NJ) method and a bootstrap consensus tree was inferred from 1000 replicates as described previously (Punja and Rodriguez Citation2018) using MEGA 7.0 (Kumar et al. Citation2016).
Sampling for inoculum presence in the growing environment
Air sampling
To assess the potential for airborne dispersal of F. oxysporum spores within commercial production facilities, 9-cm diameter Petri dishes containing PDA+S were placed with the lids removed on benches in areas between rows of plants, at approximately 1-m intervals, in one greenhouse in BC. Sampling was conducted in areas used for maintenance of stock plants for vegetative propagation, in areas used for propagation of cuttings (propagation room) and for vegetative growth (vegetative room). A minimum of 15 replicate dishes was included at each sampling site and the sampling was repeated at various different time points. The dishes were left open for 60 min, following which the lids were replaced and the dishes brought back to the laboratory. Control dishes were placed in similar locations with the lids left on. Fungal colonies that developed after 5–7 days were identified to genus level where possible using morphological criteria and representative colonies were subcultured onto fresh medium. Species-level identification was done by conducting PCR of the ITS1-5.8S-ITS2 region of ribosomal DNA. The ITS regions (ITS1 and ITS2) as well as the 5.8S gene of the rDNA were amplified using the universal eukaryotic primers UN-UP18 S42 (5′-CGTAACAAGGTTTCCGTAGGTGAAC-3′) and UN-LO28 S576B (5′-GTTTCTTTTCCTCCGCTTATTAATATG-3′) (Schroeder et al. Citation2006) to produce a DNA template for sequencing. PCR conditions were as follows: initial denaturation at 94°C for 3 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, and extension at 72°C for 2 min, and a final extension at 72°C for 7 min, followed by 4°C hold. PCR products were purified from the gel and sent to Eurofins Genomics for sequencing. The resulting sequences were compared to the ITS1-5.8 S-ITS2 sequences from the National Centre for Biotechnology Information (NCBI) GenBank database that showed the highest sequence match (over 98%) to confirm species identity.
Sampling of growing substrate
Samples of approximately 5–10 g of coco fibre (coco coir) substrate used to grow plants were taken at multiple times during the production cycle () to assess for the presence of F. oxysporum. Samples originated from the root zone of symptomatic plants during vegetative propagation and continuing through to flowering at different sampling times in one production facility during 2019. Replicates of five samples were collected each time. A subsample of 0.5 g was suspended in 10 mL of sterile distilled water and mixed using a Vortex mixer for 20 s. A 1 mL suspension was transferred to 9 mL of water, shaken, and a further dilution was made in 9 mL of water. This was repeated up to a 10−3 dilution. Aliquots (0.5 mL) of each suspension were streaked onto two replicate PDA +S plates and repeated three times for each sample. The plates were incubated for 5–7 days under ambient laboratory conditions and then examined for colonies of Fusarium which were subcultured onto fresh medium and used for DNA extraction and molecular identification using the ITS primers as described above.
Pathogenicity studies
Test-tube inoculations
The pathogenicity of an isolate of F. oxysporum obtained from damped-off cuttings (isolate BC-4, ) was tested. A cotton plug was placed in the bottom of 18 × 2.5 cm glass test-tubes and moistened with sterile distilled water. A mycelial plug (8-mm diameter) taken from a 7-day old PDA culture of the isolate was placed on top of the cotton plug, mycelial side facing up. A stem cutting (approx. 15 cm in height) was then inserted fully into the test-tube to ensure contact was made between the base of the cutting and the mycelial plug. Control tubes received a PDA plug. The test-tubes were sealed with caps and placed upright in a test-tube rack for 7–10 days under ambient conditions. After this time, the extent of mycelial development on the stem cutting was measured with a ruler. A total of five cannabis strains were compared: ‘OG Kush’, ‘Hash Plant’, ‘Copenhagen Kush’, ‘White Rhino’ and ‘Island Honey’. There were four replicates for each strain and the experiment was conducted twice. The data from both experiments was averaged and variation was expressed as standard errors.
Stem cutting inoculations
For inoculation of stem cuttings, the base of stem segments (15-cm height) from stock plants of strains ‘Hash Plant’ and ‘White Rhino’ were dipped into a spore suspension of F. oxysporum for 5 min and inserted into pre-moistened 4 cm2 rockwool cubes (Grodan). The isolate was previously grown on PDA plates for 7–10 days under ambient laboratory conditions. The plates were flooded with 10 mL of sterile distilled water, the colony surface was rubbed with a glass rod, and the resulting spore suspension was poured through two layers of cheesecloth. Water was added as required to achieve a concentration of 1 × 10 6 spores mL−1 as quantified in a haemocytometer. Alternatively, the isolate was grown in potato dextrose broth shake cultures (each 100 mL) for 7–10 days at 150 rpm and the mycelial mat from two flasks was blended with 200 mL of water for 20 s. The cuttings were dipped into the mycelial+spore suspension for 3–5 min. To determine the inoculum level, dilutions of the mycelial+spore suspension were made in sterile distilled water (up to 10−5) and 50 µL was spread onto the surface of PDA+S plates and incubated at 21–24°C for 5 days, at which time colony-forming units (CFU mL−1) was quantified visually. All rockwool cubes containing inoculated cuttings were placed inside a plastic container, a 1 cm layer of water was added, and covered with a plastic dome to maintain high humidity and placed in a Conviron incubator set at 24°C with a 12-hr photoperiod for 2 weeks. Controls consisted of noninoculated cuttings. For each experimental trial, there were five replicate cuttings used. Symptoms of damping-off or stem infection of the cuttings was rated after 1 week and 2 weeks. Re-isolations were made from diseased tissues following the surface-sterilization procedure described previously and plating tissues onto PDA+S. The experiment was conducted three times.
Rooted plant inoculation
Cuttings were inserted into Jiffy-7® peat pellets (http://www.jiffypot.com/) or rockwool cubes that had been pre-soaked in Current Culture H2O® hydroponic nutrient solution (https://www.cch2o.com/) to root (Punja and Rodriguez Citation2018). After two weeks, the rooted cuttings were transferred to a coco coir: perlite (3:1) potting medium in 10 cm2 pots. Plants of strains ‘Hash Plant’, ‘Critical Kali Mist’ and ‘White Rhino’ were inoculated with 20 mL of the mycelial+spore suspension prepared as described above which was poured around the base of each plant. The pots were placed in a plastic tray containing a 1-cm layer of water, and covered with a plastic dome to maintain high humidity and placed in a Conviron incubator set at 24°C with a 12-hr photoperiod. Symptoms of yellowing or stunting of the plants were recorded after 2 and 4 weeks. There were four replicate plants for each strain. Re-isolations were made from diseased tissues following the surface-sterilization procedure described previously and plating tissues onto PDA+S. The experiment was conducted twice.
Results
Pathogen distribution in stock plants
Stock (mother) plants that were sampled in this study had attained a height of 2–3 m and a basal stem diameter of 5–6 cm after 6–10 months (). Symptoms of disease that affected some plants (5–10% of the total) included yellowing of leaves and defoliation (). At advanced stages of disease development, necrosis of leaves and stem death led to plant mortality (). At the crown region of many diseased plants, the stem was decayed and white mycelial growth was evident (). At pruning wounds, pinkish-white mycelial growth was observed under conditions of high relative humidity (). A brown mealy rot was visible internally when the plants were cut down (). The internal discoloration was present in the stem at distances of 100–150 cm from the crown (). From surface-sterilized crown and stem tissues of diseased plants, colonies of Fusarium oxysporum (identified as described below) were recovered at a frequency of 70–100% at the crown and 30–35% at distances of 150–200 cm from the crown (). Orange sporodochia formed on cultures grown on PDA+S after 4 weeks (). On air sampling plates placed around the vicinity of stock plants, colonies of F. oxysporum were observed to be present as well as a range of Penicillium species () that were identified as P. sphathulatum, P. olsonii, and P. simplicissimum by PCR of the ITS1-5.8 S-ITS2 region.
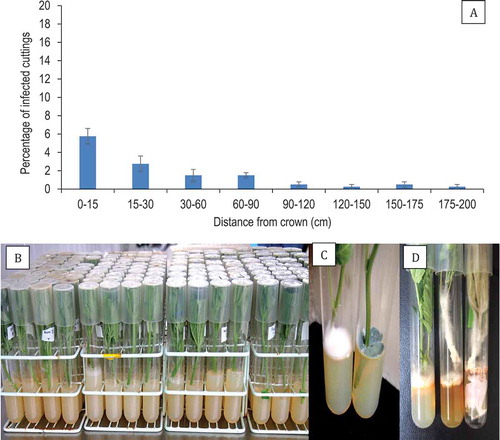
From healthy appearing (asymptomatic) stock plants of ‘Hash Plant’ from which cuttings were taken at approximately 15-cm incremental distances, beginning at the crown and proceeding alongside branches to the top of the plant (> 200 cm), the percentage of cuttings harbouring F. oxysporum was 2–6% at distances of 0–90 cm from the crown and 0.5–1% at 90–200 cm distances (). These data represent the averages from two experiments with 20 replicates at each distance. The cuttings are shown in . Fusarium oxysporum could be seen emerging from the base of the cutting and growing onto PDA (. The most frequently encountered fungal genus emerging from > 50% of the cuttings was Penicillium ().
Fig. 3 Frequency of recovery of Fusarium oxysporum from stem cuttings that originated from asymptomatic mother plants. (a) Percentage recovery of F. oxysporum from stem cuttings obtained at various distances from the crown of stock plants. (b) Method used to assay stem cuttings by inserting the cut end into a test-tube containing PDA. (c) Colony of Fusarium (left) and Penicillium (right) developing after 7 days of incubation at 21–24° C. (d) Growth of F. oxysporum from ends of stem cuttings onto PDA and onto the foliage after 18 days of incubation. Cuttings were taken at distances of 30–120 cm from the crown. The method described in (b) was used to assess frequency of internal infection. Data are from 20 cuttings at each of the distances and the experiment was conducted twice
Pathogen presence in the propagation room
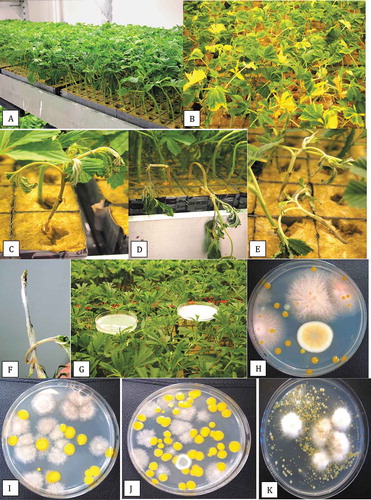
Stem cuttings originating from stock plants were transferred into rockwool cubes and grown in the commercial propagation room under conditions of 25–27°C and 85% relative humidity for two weeks. Healthy cuttings remained green (). However, some of the cuttings displayed yellowing of the lower leaves (). These affected cuttings wilted and collapsed, displaying symptoms of damping-off and soft rot (. White mycelial growth was visible at the base of some stems () which was identified as F. oxysporum. Petri dishes placed within the propagation room at canopy height for one hour () or on surrounding benches showed development of colonies of F. oxysporum after 5 days of incubation (). Other colonies that developed on the Petri dishes were light-brown and feathery (; when subcultured, they were identified as Peziza ostracoderma (99% sequence homology to GenBank no. MF567527.1 from soil and JQ764766.1 from air). In addition, a large number of bright yellow pigmented bacterial colonies were also present; these were identified as Sphingobium sp. ( using the 16S/18S ITS region of rDNA (conducted by the University of Guelph Agriculture and Food Laboratory). Samples of the drainage water from the propagation room showed that colonies of both F. oxysporum and Sphingobium were present ().
Fig. 4 Development of Fusarium oxysporum on cannabis cuttings in a propagation room. (a) Healthy cuttings one day after insertion into rockwool blocks. (b) Symptoms of yellowing associated with F. oxysporum infection after 2 weeks. (c-e) Symptoms of damping-off on cuttings, showing wilting of leaves and total collapse. (f) White mycelial growth and necrosis due to infection by F. oxysporum on a diseased cutting. (g) Sampling for air-borne fungi by placing Petri dishes on the canopy for a 1 hour exposure. (h) Pink colonies of F. oxysporum recovered on exposed Petri dishes placed in the propagation room. (i, j) Recovery of light brown colonies of Peziza ostracoderma and bright yellow colonies of Sphingobium sp. on exposed Petri dishes in the propagation room. (k) Colonies of F. oxysporum recovered from plating of drainage water from the rooting room
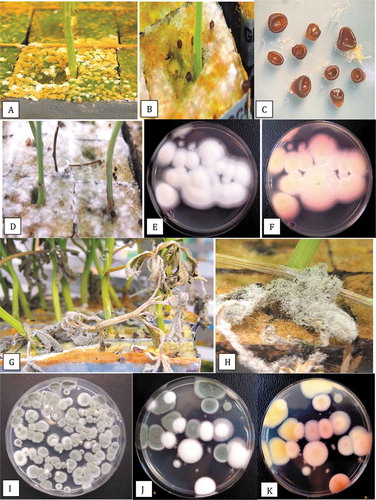
The rockwool blocks into which cuttings were inserted were frequently colonized by various fungi within the 2-week period required for rooting. A brown surface growth with abundant sporulation of the ‘peat mold’ fungus Peziza ostracoderma was common (). The fungus formed brown fruiting structures (apothecia) on the rockwool block () which were removed from the rockwool and placed on water agar to obtain close-up photographs (). In addition, there was also extensive white mycelial growth over the surface of rockwool blocks (); when subcultured, this was identified as of Lecanicillium aphanocladii (submitted to GenBank as accession no. MT311365). On PDA plates, colonies of L. aphanocladii developed white cottony growth with a light-dark pink pigmentation on the underside (. On dead leaves, sporulation of Penicillium olsonii that superficially resembled Botrytis grey mould was common ( and air-borne spores were captured on PDA plates over a 1-hr exposure (). Colonies of Penicillium citrinum (green) and Lecanicillium (white) also developed on exposed Petri dishes in the propagation room; the underside of these colonies were yellow and pink, respectively (.
Fig. 5 The surface colonizing fungi present on rockwool blocks used to root cannabis cuttings. All photographs were taken 2 weeks after cuttings were initiated. (a) Surface growth and sporulation of the peat mould fungus Peziza ostraderma. (b) Formation of brown fruiting structures (apothecia) on the rockwool block. (c) Apothecia of Peziza removed from the rockwool and placed on water agar to obtain close-up photographs. (d) Extensive white mycelial growth of Lecanicillium aphanocladii over the surface of rockwool blocks. (e, f) Growth of L. aphanocladii on PDA plates showing white cottony growth and light-dark pink pigmentation on the underside. (g, h) Sporulation of Penicillium olsonii on dead leaves that superficially resembles Botrytis grey mould. (i) Growth of P. olsonii colonies on PDA. (j, k) Colonies of Penicillium citrinum (green) and Lecanicillium (white) on exposed Petri dishes in the propagation room; the underside of these colonies are yellow and pink, respectively
Pathogen presence in the vegetative phase
Rooted cuttings were transferred from the propagation room into blocks of coco coir substrate for growth to the vegetative stage. Some plants continued to display symptoms of yellowing, leaf necrosis, stunted growth and plant death (). A comparison of a healthy plant of strain ‘Hash Plant’ to diseased plants is shown in . Affected plants had necrotic and yellowing leaves (). At the base of the cuttings that was inserted into the rockwool cube for rooting, visible decay of the stem and roots was evident () and the crown area was discoloured (). Isolations made from diseased roots and crown regions showed a high percentage (up to 85%) of recovery of F. oxysporum (), with some Pythium spp. also present. The reduction in the volume of the root system of vegetative plants infected by F. oxysporum is shown in . Roots that emerged from the bottom of the coco coir block were brown and decayed, symptomatic of Fusarium infection ().
Fig. 6 Symptoms of infection due to Fusarium oxysporum in the vegetative stage of growth of cannabis plants. (a) Severely diseased cuttings one week after transfer from the propagation room into coco coir blocks. (b) Comparison of growth of a healthy plant of ‘Hash Plant’ (left) with two diseased plants (right) one week after transfer from the propagation room. (c) Stunted growth and foliar necrosis of diseased plants of strain ‘Afghani Kush’ two weeks after transfer from the propagation room. (d) Yellowing of leaves of infected ‘White Rhino’ plants. (e) Total collapse of plants of ‘Hash Plant’ due to infection by F. oxysporum. (f) Infection of exposed ends of cutting and decay of the stem tissues of ‘Hash Plant’ within the rockwool blocks. (g) Crown lesion and browning and decay of roots. (h) Recovery of colonies of F. oxysporum from crown and root tissues of diseased plants. (i) Reduction of root volume on diseased plant (right) compared to a healthy plant (left). (j) Browning of emerging roots on the underside of coco block of a diseased plant

Population levels of F. oxysporum in coco coir substrate when plated following serial dilution onto PDA+S showed considerable variability in numbers in CFU between different plants and replicate samples (data not shown); however, the general trend observed was an increase in population levels from vegetative growth of plants to the flowering stage for samples taken from symptomatic plants. Colonies of F. oxysporum in coco coir at the onset of vegetative growth were < 101 CFU cm−3. There were no colonies present in unused coco coir. At the end of the vegetative growth stage (2 weeks), population levels were > 102; at the flowering stage, populations had increased to > 103 CFU cm−3. Numerous other fungi were present in the coco samples, including species of Penicillium, Aspergillus, Rhizopus, and unidentified bacteria and yeasts.
Pathogen presence in the flowering stage
Vegetative plants that survived and continued to the flowering stage of production frequently displayed yellowing of leaves (. The roots of these plants were brown and the root mass was reduced, with few feeder roots present (). The yellowing symptom was seen in strains ‘Purple God’ and ‘Hash Plant’ at the time of flower development (, as well as in other strains (). As the plants progressed to maturity, the diseased plants were visibly stunted and the leaves were necrotic (). Wilting symptoms were evident during hot sunny conditions. Cultures of F. oxysporum were recovered from all symptomatic plants ().
Fig. 7 Symptoms of infection due to Fusarium oxysporum in the flowering stage of growth of cannabis plants. (a) Yellowing of leaves of strain ‘White Rhino’. (b) Yellowing of lower leaves of strain ‘Purple God’. (c) Reduced root volume and browning of roots of plant shown in (b). (d) Pronounced yellowing and stunting of plant of strain ‘Purple God’ at the onset of flowering. (e-f). Advanced stages of disease development and plant collapse of strain ‘Hash Plant’ four weeks into the flowering stage. Healthy plants can be seen in the background

Pathogen identification by PCR
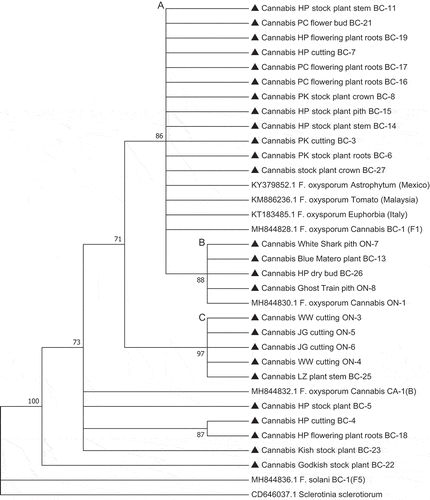
PCR using the EF-1α primers produced a band of approximately 700 bp in size in all 33 isolates () and BLAST analysis identified them as F. oxysporum. Phylogenetic analysis showed the isolates were placed in three clades (A-C) that were comprised of isolates of similar origins (licenced producers, LP) or geographic origin (BC and ON) (). In the largest sampling facility located in BC (LP-1), isolates originating from stock plants, cuttings, crown, stem, and root tissues, and flower buds of three cannabis strains all belonged to clade A (), suggesting pathogen spread was occurring clonally within the facility between various stages of plant production. Isolates from one LP in ON (LP-4) clustered in clade C, while isolates from a different LP (LP-5) grouped with isolates from BC in clade B. Previously identified isolates from diseased cannabis plants in BC (BC-1) and ON (ON-1) (Punja and Rodriguez Citation2018) were clustered within clades A and B, respectively ().
Fig. 8 PCR band of size ca. 700 bp obtained for 8 isolates of Fusarium oxysporum from cannabis plants (BC-3, BC-6, BC-7, BC-9, BC-12, BC-16, BC-21, BC-26) using the EF-1 primer set. Lane 9 is the water control
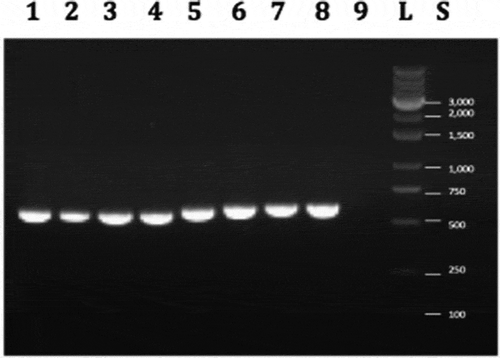
Fig. 9 Phylogenetic analysis of Fusarium oxysporum isolates originating from cannabis plants (see ) using EF-1α sequences compared to isolates from other hosts (GenBank numbers are shown). Isolates were obtained from a range of tissue sources and from different licenced facilities in BC and ON. The evolutionary history was inferred using the Neighbour-Joining method. The optimal tree with the sum of branch length = 0.35416035 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. Branches corresponding to partitions reproduced in less than 70% bootstrap replicates were collapsed. The evolutionary distances were computed using the p-distance method and are in the units of the number of base differences per site. The analysis involved 33 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 412 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al. Citation2016). The distinguishable clades are represented by letters from A to C
Pathogenicity studies
Test-tube inoculations
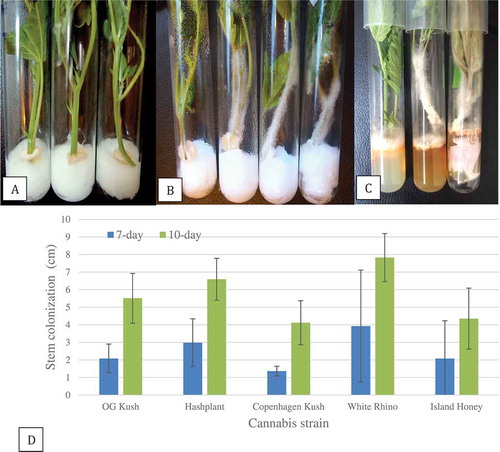
The placement of stem cuttings in contact with mycelial plugs of F. oxysporum in test-tubes () resulted in mycelium growing upwards on the cuttings (). Measurements made after 7 days and 10 days for five cannabis strains showed that all were colonized to the same extent by the mycelium after 10 days (). Mycelial growth on ‘White Rhino’, for example, was 8 cm after 10 days.
Fig. 10 Pathogenicity testing and response of five cannabis strains to inoculation with Fusarium oxysporum in a test-tube assay. (a) Initial set-up showing a mycelial plug placed on the surface of a moistened cotton plug and a stem cutting placed over the plug. (b) Mycelial growth over the stem cutting after 10 days of incubation. Strains shown from left to right in each test-tube are: ‘Copenhagen Kush’, ‘Island Honey’ ‘White Rhino’ and ‘Hash Plant’. (c) Mycelial growth over the stem cuttings after 18 days. Necrosis of the foliage can be seen
Stem cutting inoculations
Stem cuttings that were dipped into a spore suspension of F. oxysporum for 5 min and inserted into pre-moistened 4 cm2 rockwool cubes showed symptoms of wilting and collapse on ‘Hash Plant’ () and stunted growth and yellowing of leaves on ‘White Rhino’ () after two weeks. Cuttings of ‘Hash Plant’ dipped into a mycelial+spore suspension for 3 min showed pronounced yellowing after two weeks (). Cuttings dipped for five min had extensive mycelial growth that progressed upwards along the cutting and there was visible wilting (). The pathogen also caused necrosis and soft rot of the cutting (. The inoculum level used was determined to be in the range of 1 × 106 −1×107 CFU mL−1 in repeated experiments. Most non-inoculated controls did not show symptoms of infection; occasionally, control cuttings did develop symptoms of Fusarium infection which was attributed either to aerial spread of inoculum from the inoculated plants or to natural infection present within the stock plants used to obtain the cuttings.
Fig. 11 Pathogenicity testing of Fusarium oxysporum using stem cuttings in rockwool blocks and coco coir substrate. (a) Response of ‘Hash Plant’ cuttings to inoculation for five min using a spore suspension (right) compared to non-inoculated controls (left) after 7 days. (b) Response of ‘White Rhino’ cuttings to inoculation for five min using a spore suspension (right) compared to non- controls (left) after 7 days. (c) Response of strain ‘Hash Plant’ to inoculation for three min using a mycelium+spore suspension. Non-inoculated controls are shown on the left. (d) Response of ‘Hash Plant cuttings to inoculation for five min using a mycelium+spore suspension. Extensive growth of mycelium up the stem and wilting can be seen. (e, f) Close-up of inoculated ‘Hash Plant’ showing mycelial growth up the stem and development of necrosis. Photos shown in (c-f) were taken 2 weeks after inoculation. (g) Symptoms on strain ‘Hash Plant’ (far right) and ‘Critical Kali Mist’ (middle) compared to non-inoculated plant of ‘Hash Plant’ (far left) four weeks after inoculation of rooted cuttings with a mycelium+spore suspension in coco coir growing substrate. (h) Symptoms on strain ‘White Rhino’ after inoculation of a rooted cutting. Crown lesion and root decay are evident

Rooted plant inoculation
Four weeks after inoculation of rooted plants with a mycelial+spore suspension, strains ‘Critical Kali Mist’ and ‘Hash Plant’ showed yellowing of the lower leaves and some necrosis (). The development of roots on strain ‘White Rhino’ was significantly reduced (). The pathogen was recovered from all symptomatic plants and not from control plants.
Discussion
The results from this study expand on the first reported occurrence of F. oxysporum on hydroponically grown marijuana (cannabis) plants (Punja and Rodriguez Citation2018) by demonstrating the presence of the pathogen in five licenced production facilities in British Columbia and Ontario. This study also showed that roots, crowns, stems and vegetative cuttings were susceptible to colonization by F. oxysporum, and that inoculum was present in the growing environment in the air, in coco substrate used to grow plants, and in drainage water. Cuttings obtained from symptomatic and asymptomatic infected stock plants gave rise to disease symptoms at the propagation stage; subsequently, symptoms were observed in the vegetative and flowering stages. Infection that resulted in damping-off on cuttings in the propagation phase could frequently be traced back to diseased stock plants as the source. The spread of F. oxysporum within stock plants likely occurs in the xylem and pith tissues, which were visibly decayed. The pathogen may also reside in these tissues as an endophyte, as reported for F. oxysporum in the stems of asymptomatic tomato plants (Demers et al. Citation2015). Stock plants that have been maintained for an extended period of time (8–12 months) may therefore harbour internal infections that can subsequently be transmitted to newly established cuttings. The pathogen was recovered from tissues of stock plants at distances of up to 200 cm from the crown region; the frequency of cuttings taken at this distance that showed recovery of F. oxysporum was about 0.5%. The pith tissues in cannabis plants could allow spread of potential pathogens such as F. oxysporum to occur, as demonstrated previously for endophytic fungi (Punja et al. Citation2019a). The ability of F. oxysporum to colonize exposed pruning wounds from airborne inoculum and spread into the pith tissues could also provide opportunities for internal colonization of cannabis plants. Inoculum produced on diseased crown tissues can potentially also spread to the inflorescences of mature plants (Punja et al. Citation2019a). Aerial dispersal of inoculum of F. oxysporum has been reported in several greenhouse crops (Rowe et al. Citation1977; Gamliel et al. Citation1996; Katan et al. Citation1997; Rekah et al. Citation2000; Scarlett et al. Citation2015) and colonies were detected in this study on Petri dishes left exposed in the growing environment. Isolates of F. oxysporum from diseased pith and stem tissues were identical in EF-1α sequence to isolates from stem cuttings with damping-off symptoms and from flower buds, suggesting a similar source of origin. They also clustered with F. oxysporum isolates from other hosts and geographic origins. Some sequence diversity was present among the 33 isolates assessed in this study, based on the EF-1α gene, possibly reflecting the diverse origins of isolates, depending on the source of the cannabis strain, province of origin, and year of isolation. The clustering of some BC isolates with a group from ON could suggest a similar origin of the plant material used to propagate specific strains.
During the rooting stage of cannabis propagation, the warm and humid environment required to promote rooting of cuttings encouraged the proliferation of several fungi on the rockwool substrate. These included Peziza, Lecanicillium and Penicillium species, all of which are considered to be saprophytic contaminants and not associated with symptoms of yellowing on the plants attributed to Fusarium. However, their presence could influence the quality of the rooted plants and some could become established as endophytes at this early stage of plant growth. Colonization of the surfaces of peat-based growing media and wet rockwool by Peziza ostracoderma has been previously reported (Meinken et al. Citation2016). Lecanicillium (formerly Verticillium lecanii) has been recovered from a wide range of sources and is known for its mycoparasitic and entomopathogenic activity (Peciulyte and Kacergius Citation2012; El-Debaiky Citation2017). It was reported to parasitize the fruiting bodies of the white jelly-leaf mushroom Tremella fuciformis (Liu et al. Citation2018). Penicillium olsonii was the main species found on decaying leaf litter in the rooting room and can similarly be found on a range of substrates (Chatterton et al. Citation2012). In addition to the fungal contaminants, a Gram – ve saprophytic bacterium Sphingobium was present in the propagation room. Members of the genus Sphingobium (Sphingomonas) are characterized as non-sporulating, rod-shaped, motile or non-motile, strictly aerobic, chemo-organotrophic and catalase-positive bacteria (Sheu et al. Citation2013). They are widely distributed in nature and are known for their abilities to degrade a wide variety of xenobiotic pollutants, such as aromatic compounds (Cai et al. Citation2015) and are present in soil, water, and in the plant rhizosphere (Sheu et al. Citation2013). The source of Sphingobium in this study is likely from water that was disseminated through the humidifier as aerosolized propagules. Sphingobium spp. have been previously detected from biofilms in public water municipal systems (Koskinen et al. Citation2000; Gulati and Ghosh Citation2017), in tap water (Yun et al. Citation2016) and in natural spring water (Guðmundsdóttir et al. Citation2019). Interestingly, some samples of dried harvested flowers (buds) of cannabis were found to be contaminated with a Sphingobium sp. (unpublished observations).
The development of disease symptoms in the early propagative and vegetative growth phases, as well as at the flowering stage, indicate that F. oxysporum is capable of infecting cannabis plants at all stages of growth. However, the onset of early infection i.e. symptoms developing at the propagative or vegetative stages, are likely to be the most important for disease development, as infections occurring later during plant growth (e.g. during flowering), from a build-up of inoculum in the growing substrate may not manifest themselves as pronounced symptoms in the same order of magnitude as those observed during the early stages of production. Spread of the pathogen from plant to plant is likely to occur during the propagation stage and the vegetative growth stage, as these plants were grown on benches in close proximity to one another and water was provided by flooding the benches. Drainage water was shown to contain inoculum of F. oxysporum. Symptoms due to Fusarium root and crown rot, as well as Pythium spp., were aggravated when plants encountered a prolonged period of saturation (unpublished observations). A proportion of the diseased samples (15%) yielded Pythium spp. which have been reported as pathogens on cannabis plants (Punja and Rodriguez Citation2018). At the flowering stage, plants were grown in large coco slabs and were irrigated through individual drip lines, with two plants per bag (); spread of Fusarium from plant to plant was therefore minimal and disease foci consisting of clusters of more than two plants were not observed. A build-up of inoculum of F. oxysporum was noted within the coco growing substrate as the plants progressed to flowering.
Up to 14 cannabis strains were sampled in commercial production and observed to be susceptible to F. oxysporum in the present study, particularly ‘Hash Plant’ and ‘Pink Kush’. The in vitro test-tube inoculation method did not reveal significant differences in the extent of stem colonization of five cannabis strains tested. Therefore, the potential for identifying genetic resistance to F. oxysporum in cannabis strains remains unknown. In contrast, differences in susceptibility to powdery mildew are known to exist among different cannabis strains. The inoculation methods developed to evaluate pathogenicity to F. oxysporum in this study could be used to screen for genetic resistance, as well as to assess virulence of different isolates. These include the test-tube method and the rockwool block inoculation procedure, both of which resulted in rapid development of symptoms on stem cuttings. Whole plant inoculations require a longer period of time for symptom development (Punja and Rodriguez Citation2018). The use of a mycelial+spore suspension as inoculum provided more consistent results compared to a spore suspension, and an inoculation time of 3 min gave consistent but not excessive levels of disease compared to 5 min on both strains evaluated (unpublished observations). The use of an inoculum concentration in the range of 1 × 106 – 1 × 107CFU mL−1 and exposure times of 3–5 min is comparable to inoculation methods used by other researchers to study host–pathogen interactions in a range of other crop species infected by F. oxysporum (Purwatti et al. Citation2008; Ortiz and Hoyos-Carvajal Citation2016; Paynter and Herrington Citation2016; Jarek et al. Citation2018). The symptoms resulting from the inoculation of cuttings was identical to those seen in the commercial propagation room. Symptoms on inoculated rooted plants also were similar to those observed on plants in the vegetative stage.
In other plant species in which genetic resistance to F. oxysporum has been described (mostly causing vascular wilt diseases), spread of the pathogen within the root cortex and xylem tissues was prevented through several mechanisms: the formation of physical barriers, secretion of phenolic compounds, alteration of cell wall structure, and production of fungitoxic compounds and pathogenesis-related proteins (Beckman and Roberts Citation1995; Di Pietro et al. Citation2003; Michielse and Rep Citation2009; Bani et al. Citation2018; Husaini et al. Citation2018; Chen et al. Citation2019; van der Does et al. Citation2019). Whether or not these responses may be induced in the xylem and pith tissues of cannabis plants in response to Fusarium infection requires further study. The potential reduction in yield and quality of cannabis plants due to infection by F. oxysporum in which root volume is reduced but foliar symptoms are absent has not been quantified.
One approach available currently for management of Fusarium root and crown rot of cannabis is the application of biological control agents such as Rootshield (containing Trichoderma harzianum strain T-22) and Prestop (containing Gliocladium catenulatum strain J1446) (Lung et al. Citation2020). Both products are registered for use on cannabis in Canada for management of root infecting pathogens and botrytis grey mould. Applications should be made early during the propagation stage and at transplantation of rooted cuttings. The ability of these biocontrol agents to colonize plants as endophytes (Bailey et al. Citation2008; Chatterton and Punja Citation2010) and persist in the rhizosphere of cannabis plants would enhance their biological activity, although the potential for limiting Fusarium spread in the xylem and pith tissues remains unexplored. Additional research on other approaches for management of F. oxysporum on cannabis plants is needed, as Fusarium root and crown rot is becoming more widespread and can be a limiting factor in cannabis production. Assessment of stock plants for internal infection and reducing the duration of maintenance of stock plants to less than 6 months should minimize pathogen build-up. Plant tissue culture methods to propagate pathogen-free plants from meristems or axillary buds of cannabis (Punja et al. Citation2019b) is worthy of investigation to reduce the potential for Fusarium transmission. In addition, screening of a broad cannabis strain selection to identify potential sources of genetic resistance should be conducted to determine whether the response of stem tissues or vegetative cuttings can be correlated with whole plant responses to infection by F. oxysporum.
Acknowledgements
Technical assistance provided by Cameron Scott, Samantha Lung, and Sarah Chen is gratefully acknowledged.
Additional information
Funding
References
- Bailey BA, H B, Strem MD, Crozier J, Thomas SE, Samuels GJ, Vinyard BT, Holmes KA. 2008. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol Contr. 46:24–35. doi:10.1016/j.biocontrol.2008.01.003.
- Bani M, Pérez-De-Luque A, Rubiales D, Rispail N. 2018. Physical and chemical barriers in root tissues contribute to quantitative resistance to Fusarium oxysporum f. sp. pisi in pea. Front Plant Sci. 9:199. doi:10.3389/fpls.2018.00199.
- Beckman CH, Roberts EM. 1995. On the nature and genetic basis for resistance and tolerance to fungal wilt diseases of plants. Adv Bot Res. 21:35–77.
- Cai S, Shi C, Zhao J-D, Cao Q, He J, Chen L-W. 2015. Spingobium phenoxybenzoativorans sp. nov., a 2-phenoxybenzoic-acid-degrading bacterium. Inter J Sys Evol Microbiol. 65:1986–1991. doi:10.1099/ijs.0.000209.
- Chatterton S, Punja ZK. 2010. Factors influencing colonization of cucumber roots by Clonostachys rosea f. catenulata, a biological disease control agent. Biocontr Sci Tech. 20:37–55. doi:10.1080/09583150903350253.
- Chatterton S, Wylie AC, Punja ZK. 2012. Fruit infection and postharvest decay of greenhouse tomatoes caused by Penicillium species in British Columbia. Can J Plant Pathol. 34:524–535. doi:10.1080/07060661.2012.710069.
- Chen A, Sun J, Matthews A, Armas-Egas L, Chen N, Hamill S, Mintoff S, LTT T-N, Batley J, Aitken EAB. 2019. Assessing variations in host resistance to Fusarium oxysporum f. sp. cubense race 4 in Musa species, with a focus on the subtropical race 4. Front Microbiol. 10:1062. doi:10.3389/fmicb.2019.01062.
- Demers JE, Gugino BK, Jiminez-Gasco MM. 2015. Highly diverse endophytic and soil Fusarium oxysporum populations associated with field-grown tomato plants. Appl Environ Microbiol. 81:81–90. doi:10.1128/AEM.02590-14.
- Di Pietro A, Madrid MP, Caracuel Z, Delgado-Jarana J, Roncero MIG. 2003. Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Mol Plant Microbe Inter. 4(5):315–525.
- El-Debaiky SA. 2017. New record of Lecanicillium aphanocladii Family: cordycipitaceae from Egypt. J Bacteriol Mycol. 5(7):00161.
- Gamliel A, Katan T, Yunis H, Katan J. 1996. Fusarium wilt and crown rot of sweet basil: involvement of soilborne and airborne inoculum. Phytopathology. 86:56–62. doi:10.1094/Phyto-86-56.
- Guðmundsdóttir R, Kreiling AK, Kristjánsson BK, VÞ M, Pálsson S. 2019. Bacterial diversity in Icelandic cold spring sources and in relation to the groundwater amphipod Crangonyx islandicus. PLoS One. 14(10):e0222527. doi:10.1371/journal.pone.0222527.
- Gulati P, Ghosh M. 2017. Biofilm forming ability of Sphingomonas paucimobilis isolated from community drinking water systems on plumbing materials used in water distribution. J Water Health. 15(6):942–954. doi:10.2166/wh.2017.294.
- Health Canada. 2020. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/industry-licensees-applicants/licensed-cultivators-processors-sellers.html.
- Husaini AM, Sakina A, Cambay SR. 2018. Host-pathogen interactions in Fusarium oxysporum infections: where do we stand? Mol Plant Microbe Inter. 31(9):889–898. doi:10.1094/MPMI-12-17-0302-CR.
- Jarek TM, Dos Santos AF, Tessmann DJ, Vieira ESN. 2018. Inoculation methods and aggressiveness of five Fusarium species against peach palm. Ciência Rural. 48(4):e20170462. doi:10.1590/0103-8478cr20170462.
- Katan T, Shlevin E, Katan J. 1997. Sporulation of Fusarium oxysporum f. sp. lycopersici on stem surfaces of tomato plants and aerial dissemination of inoculum. Phytopathology. 87:712–719. doi:10.1094/PHYTO.1997.87.7.712.
- Koskinen R, Ali‐Vehmas T, Kämpfer P, Laurikkala M, Tsitko I, Kostyal E, Atroshi F, Salkinoja‐Salonen M. 2000. Characterization of Sphingomonas isolates from Finnish and Swedish drinking water distribution systems. J Appl Microbiol. 89:687–696. doi:10.1046/j.1365-2672.2000.01167.x.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874. doi:10.1093/molbev/msw054.
- Liu J, Chen B, Qiu X, Lian L, Huang Z, Xie B, Jiang Y. 2018. First report of Lecanicillium aphanocladii causing rot of Tremella fuciformis in China. Plant Dis. 102(3):675. doi:10.1094/PDIS-08-17-1214-PDN.
- Lung S, Betz EC, Roberts AJ, Punja ZK. 2020. Infection of Cannabis sativa cuttings by Fusarium oxysporum and Fusarium proliferatum and investigation into potential biofungicide control. Can J Plant Pathol. 42: (in press) (abstr.).
- Meinken E, Lohr D, Wock C. 2016. Colonization of growing media by saprophytic fungi – importance of carbon and nitrogen fractions. Acta Hortic. 1168:325–332.
- Michielse CB, Rep M. 2009. Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol. 10:311–324. doi:10.1111/j.1364-3703.2009.00538.x.
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concurrent evidence from nuclear and mitochondrial genealogies. Proc Natl Acad Sci USA. 95:2044–2049. doi:10.1073/pnas.95.5.2044.
- Ortiz E, Hoyos-Carvajal L. 2016. Standard methods for inoculations of F. oxysporum and F. solani in Passiflora. Afr J Agric Res. 11(17):1569–1575. doi:10.5897/AJAR2015.10448.
- Paynter ML, Herrington ME. 2016. Development of a glasshouse bioassay suitable for evaluating Fusarium wilt resistance in strawberry. Acta Hortic. 1127:125–132. doi:10.17660/ActaHortic.2016.1127.21.
- Peciulyte D, Kacergius A. 2012. Lecanicillium aphanocladii – a new species to the mycoflora of Lithuania and a new pathogen of tree leaves mining insects. Botan Lithuanica. 18(2):133–146. doi:10.2478/v10279-012-0015-5.
- Punja ZK, Collyer D, Scott C, Lung S, Holmes J, Sutton D. 2019a. Pathogens and molds affecting production and quality of Cannabis sativa L. Front Plant Sci. 10:1120. doi:10.3389/fpls.2019.01120.
- Punja ZK, Holmes J, Collyer D, Lung S. 2019b. Development of tissue culture methods for marijuana (Cannabis sativa L.) strains to achieve Agrobacterium-mediated transformation to enhance disease resistance. Vitro Cell Dev Biol Plant. 55:523. (abstr.).
- Punja ZK, Rodriguez G. 2018. Fusarium and Pythium species infecting roots of hydroponically grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40(4):498–513. doi:10.1080/07060661.2018.1535466.
- Punja ZK, Scott C, Chen S. 2018. Root and crown rot pathogens causing wilt symptoms on field-grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40(4):528–541. doi:10.1080/07060661.2018.1535470.
- Purwatti RD, Hidayah N, Sudjindro S. 2008. Inoculation methods and conidial densities of Fusarium oxysporum f.sp. cubense in Abaca. Hayati J Biosci. 15(1):1–7. doi:10.4308/hjb.15.1.1.
- Rekah Y, Shtienberg D, Katan J. 2000. Disease development following infection of tomato and basil foliage by airborne conidia of the soilborne pathogens Fusarium oxysporum f. sp. radicis-lycopersici and F. oxysporum f. sp. basilici. Phytopathology. 90:1322–1329. doi:10.1094/PHYTO.2000.90.12.1322.
- Rowe RC, Farley JD, Coplin DL. 1977. Airborne spore dispersal and recolonization of steamed soil by Fusarium oxysporum in tomato greenhouses. Phytopathology. 77:1513–1517. doi:10.1094/Phyto-67-1513.
- Scarlett K, Tesoriero L, Daniel R, Maffi D, Faora F, Guest DI. 2015. Airborne inoculum of Fusarium oxysporum f. sp. cucumerinum. Eur J Plant Pathol. 141:779–787. doi:10.1007/s10658-014-0578-3.
- Schroeder KL, Okubara PA, Tambong JT, Lévesque CA, Paulitz TC. 2006. Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology. 96:637–647. doi:10.1094/PHYTO-96-0637.
- Sheu S-Y, Shiau Y-W, Wei Y-T, Chen W-M. 2013. Sphingobium fontiphilum sp. nov., isolated from a freshwater spring. Inter J Syst Evol Microbiol. 63:1906–1911. doi:10.1099/ijs.0.046417-0.
- Small E. 2017. Cannabis. In: A complete guide. Boca Raton FL: CRC Press; 567 pp.
- van der Does HC, Constantin ME, Houterman PM, Takken FLW, Cornelissen BJC, Haring MA, van den Burg HA, Rep M. 2019. Fusarium oxysporum colonizes the stem of resistant tomato plants, the extent varying with the R-gene present. Eur J Plant Pathol. 154:55–65. doi:10.1007/s10658-018-1596-3.
- Yun SS, Siddiqi MZ, Lee SY, Kim MS, Choi K, Im WT. 2016. Sphingomonas hankyongensis sp. nov. isolated from tap water. Arch Microbiol. 198(8):767–771. doi:10.1007/s00203-016-1237-1.
