Abstract
Smutted Bermudagrass (Cynodon dactylon L.) plants with diseased spikelets and completely deformed inflorescences covered with black teliospores were collected from five locations at the borders of plantations in Kafr El-Sheikh province, Egypt, in April 2018. The fungus was isolated from infected plants and identified as Ustilago cynodontis (Passerini) Henn. based on its cultural and morphological characteristics, molecular techniques, and pathogenicity tests. Slender colonies of U. cynodontis with irregular edges were observed on potato dextrose agar (PDA) after 6 days at 28°C. The mature teliospores were examined by scanning electron microscopy. Crystal-like structures with a pyramidal shape were observed to be produced by the fungus in culture. The crystals were further examined using an energy dispersive spectroscopy system connected to the scanning electron microscope and their dimensions were measured (18 ± 2 µm × 16 ± 2 µm × 19 ± 2 µm). PCR analysis with internal transcribed spacer (ITS) specific primers yielded an amplicon that shared a 99% identity with U. cynodontis sequences in GenBank. Phylogenetic analysis indicated that the Egyptian isolate of U. cynodontis shared the greatest similarity with isolates of U. cynodontis from Ethiopia, Iran, India, United States, Mexico, Spain, and China. Inoculation of Bermudagrass with the isolated fungus resulted in symptoms of smut similar to those observed in the field. To our knowledge, this is the first report concerning the occurrence and characterization of U. cynodontis, the causal agent of Bermudagrass smut, in Egypt.
Résumé
Des plants de chiendent pied-de-poule (Cynodon dactylon L.) infectés par le charbon, dont les épillets étaient malades et les inflorescences étaient entièrement déformées et couvertes de téleutospores noires, ont été collectés à cinq endroits à la limite de plantations dans la province de Kafr el-Cheik, en Égypte, en avril 2018. Le champignon a été isolé à partir de plants infectés et, en se basant sur les caractéristiques culturales et morphologiques, les techniques moléculaires et les tests de pathogénicité, il a été identifié en tant qu’Ustilago cynodontis (Passerini) Henn. Après six jours à 28°C, des colonies élancées d’U. cynodontis aux contours irréguliers ont été observées sur de la gélose dextrosée à la pomme de terre. Les téleutospores matures ont été examinées par microscopie à balayage. Des structures cristallines de forme pyramidale produites par le champignon sur le milieu de culture ont été observées. Les cristaux ont été examinés plus en détail par spectroscopie à dispersion d’énergie couplée au microscope à balayage, puis ils ont été mesurés (18 ±2 µm × 16 ±2 µm × 19 ±2 µm). Une analyse par PCR utilisant des amorces spécifiques de l’espaceur transcrit interne (ITS) a généré un amplicon qui partageait 99% de similitude avec les séquences d’U. cynodontis de la GenBank. L’analyse phylogénétique a indiqué que l’isolat égyptien d’U. cynodontis partageait le plus haut degré de similitude avec les isolats d’U. cynodontis provenant d’Éthiopie, d’Iran, d’Inde, des États-Unis, du Mexique, d’Espagne et de Chine. L’inoculation du chiendent pied-de-poule avec les isolats du champignon a provoqué des symptômes du charbon analogues à ceux observés en plein champ. À notre connaissance, il s’agit du premier rapport traitant de l’occurrence et de la caractérisation d’U. cynodontis, l’agent causal du charbon chez le chiendent pied-de-poule en Égypte.
Introduction
Bermudagrass (Cynodon dactylon L.) Pers. also known as couch grass, which is cultivated as a turf grass, is a perennial warm-season grass that reproduces by seeds, runners (branching stolons), and rhizomes. Propagation in nature mainly occurs by rhizomes (Dung et al. Citation2014). Bermudagrass is also known to be one of the most rapidly invasive and competitive weeds belonging to family Poaceae (Auddy et al. Citation2003). The weed is considered to be a potential host for many pests and pathogens (Marley Citation1995). Infection by Ustilago cynodontis (Pass.) Henn., the causal agent of smut on C. dactylon, causes serious damage to the development and growth of Bermudagrass. Smut disease of Bermudagrass causes complete damage to the spikelets that develop into sori consisting of black teliospores (Garcìa-Guzmán and Burdon Citation1997). The fungus destroys the whole inflorescence as a result of the infection, with no seed production, because the seeds are replaced with a fine powder of black teliospores 6–8 µm in diameter (Shivas and Vánky Citation2001). Ustilago cynodontis and other smut fungi belong to the family Ustilaginaceae and are parasites on Poaceae (Begerow et al. Citation2006). Ustilago cynodontis was reported by Kruse et al. (Citation2018) as a sister species to the U. striiformis complex, which is responsible for leaf-stripe smuts on grasses. Basidiomycete fungi are known to produce oxalic acid, which plays an essential role in virulence during cell wall degradation by using calcium from pectin and forming calcium oxalate (Do Rio et al. Citation2008). Oxalic acid secreted by Sclerotinia sclerotiorum plays an essential role in suppressing the production of reactive oxygen species during the oxidative burst of invaded host plant cells and helps in pathogenesis mechanisms (Cessna et al. Citation2000). Guimaraes and Stotz (Citation2004) reported that oxalate produced by S. sclerotiorum modified the guard cell function and let the stomata pores open during the infection process.
The aim of this work was to report the occurrence of Bermudagrass smut disease in Egypt, in addition to characterizing the causal pathogen using cultural, morphological and molecular techniques, and pathogenicity tests and to study the potential alternative hosts for the pathogen.
Materials and methods
Sample collection and fungal isolation
Smutted C. dactylon plants were observed and collected in April 2018 from five locations in the border of plantations located in Sakha Research Institute and Kafrelsheikh University in Kafr El-Sheikh province, Egypt (31.3°N 30.93°E). Sterile paper bags were used to collect smutted spikes without contamination, and these were transferred to the Plant Pathology Laboratory at the Agricultural Botany Department of the Faculty of Agriculture, Kafrelsheikh University, Egypt, for further investigation and photographic imaging. Climatic conditions that led to the development of smut disease were recorded during the months of March, April, and May 2018. Spores of the studied fungus were cultured on potato dextrose agar (PDA). Cultures were incubated at 28°C for six days. Spore germination was observed and the fungal colonies were photographed.
Pathogenicity test and potential alternative hosts
To study the potential alternative hosts for the fungus, a series of possible alternative grass hosts were inoculated. Seeds of C. dactylon 001602, barnyard grass (Echinochloa crus-galli 001604), cocograss or purple nutsedge (Cyperus rotundus 001607), common reed (Phragmites communis 001608), elephant or napier grass (Pennisetum purpureum 001609), Indian sandbur (Cenchrus biflorus 0016010), jungle rice (Echinochloa colona 001605), smallflower umbrella-sedge (Cyperus difformis 0016011) and viper grass (Dinebra retroflexa 0016016) were collected as landraces from Kafr El Sheikh Province, Egypt, in 2016 and were conserved by the Department of Agricultural Botany, Faculty of Agriculture, Kafrelsheikh University, Egypt. Grains of maize (Zea mays) cv. Giza 2 were obtained from the Sakha Research Institute, Kafr El Sheikh Province, Egypt.
Twenty-five seeds from each of the above-mentioned plant species were vacuum-inoculated for 15 min under room temperature in a suspension of 100 mg of U. cynodontis teliospores per litre of water for each of five isolates of the pathogen as described by Garcìa-Guzmán and Burdon (Citation1997), while another 25 seeds of each species were treated with sterilized water as a control treatment. The seeds were left in the suspension for a further one day at room temperature. Inoculated and non-inoculated seeds were sown separately in plastic trays filled with a mixture of sand and clay soil (3:1), which had been autoclaved at 121°C for 2 h. Maize grains were sown in pots filled with a mixture of sand and clay soil (3:1), which had been autoclaved at 121°C for 2 h. Each treatment was replicated three times. The plastic trays and pots were kept in a growth chamber at 24 ± 2°C under a 10 h dark and 14 h light cycle with 250 µmol m−2 s−1 photon flux density supplied by high-output white fluorescent tubes and watered daily to field capacity. The experiment was repeated twice. All plants were observed at flowering for disease development.
Fungal identification
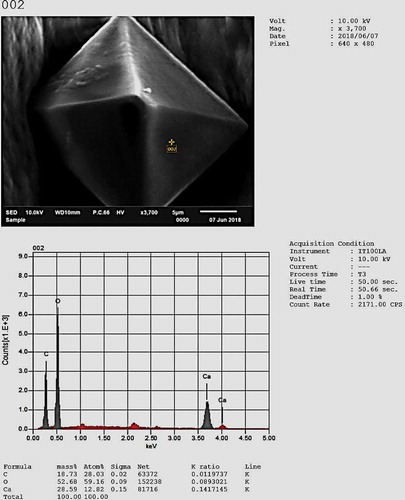
Black teliospores from sori were gently collected in sterilized Eppendorf tubes from the infected and completely distorted inflorescence of C. dactylon plants for microscopic investigation. Light microscopy (Lieca DM 1000) was used to observe the smut spores before and after germination on sterilized glass slides. Under sterile conditions, sterilized filter papers were cut into small pieces and put in sterilized Petri dishes and wetted with sterilized water, then sterilized glass slides were placed in the centre of the plate and dusted with the teliospores and left for 24 h at 28°C to allow for germination. Cultured spores on PDA were also examined microscopically after an incubation period of 6 days at 28°C and the agar was observed for secretions of the fungus. Teliospores were also sent to the Electron Microscopy Unit at Mansoura University for scanning electron microscope SEM (Joel JSM-6510LV) examination and media with germinated spores were sent to the scanning electron microscope (Joel JSM-T 330A) unit, Faculty of Science, Kafrelsheikh University. Microanalysis of the crystals produced by the fungus was performed by SEM with energy dispersive X-ray spectrometry (EDS) with an accelerated voltage 10 kV for measuring the energy used for X-ray distribution during focusing of the electron beam.
About 100 mg teliospores from each one of two isolates of the fungus were ground in liquid nitrogen using a mortar and pestle. DNA was extracted from the powdered tissue using an i-genomic plant DNA extraction Mini Kit (iNtRON Biotechnology, Inc., Cat. No. 17371) according to the manufacturer’s instructions. The eluted DNA was stored at −20ºC.
Amplification of the internal transcribed spacer (ITS) region was conducted in an automated thermal cycler (C1000TM Thermal Cycler, Bio-RAD) using ITS4 (5`-TCCTCCGCTTATTGATATGC-3`) and ITS5 (5`-GGAAGTAAAAGTCGTAACAAGG-3`) primers (White et al. Citation1990). The following parameters were used: 35 cycles of 94ºC for 30 s, 51ºC for 1 min, 72ºC for 1.5 min, and a final extension at 72ºC for 3 min. Each PCR mixture (25 µL) contained 1 µL of 25 ng nucleic acid, 1 µL of each primer (10 pmol), 12.5 µL of GoTaq Colourless Master Mix [containing Taq DNA polymerase, dNTPs, MgCl2 and reaction buffers at optimal concentrations for efficient amplification of DNA templates by PCR (Promega Corporation, Madison, Wisconsin, USA)] and 9.5 µL of Nuclease-free water (Promega). Fifteen µl of all PCR products were analysed by electrophoresis through a 1% agarose gel, stained with ethidium bromide, and DNA bands were visualized using a UV transilluminator.
The PCR product was purified using a QIAquick© Gel Extraction Kit (Qiagen Inc., Chatsworth, California, USA) and both strands were sequenced with an ABI 377 automated DNA sequencer (Applied Biosystems Inc., Foster City, California, USA). The ITS regions of the two samples representing the U. cynodontis isolates, were sequenced using ITS4 and ITS5 primers (White et al. 1990). Raw sequence chromatograms were assembled and edited using GAP4 (Bonfield et al. Citation1995) to correct the ambiguous bases or remove low-quality stretches from the termini of the sequences. The BLASTN algorithm (Altschul et al. Citation1997) was used to search the non-redundant GenBank database (http://www.ncbi.nlm.nih.gov/blast) for homologies to known sequences. Multiple alignments were performed using ClustalW (Thompson et al. Citation1994) and the phylogenetic analyses were conducted with MEGA 4 software (Kumar et al. Citation2018) and a neighbour-joining tree was generated with the same program using the Maximum parsimony method (Tamura et al. Citation2004). The ITS4/ITS5 sequences of isolates of U. cynodontis, U. hordei, U. maydis and U. tritici used for comparisons were retrieved from the GenBank database (www.ncbi.nlm.nih.gov).
Results and discussion
Disease symptoms and development
Disease symptoms observed in the field appeared on C. dactylon as dark black powdered teliospore masses surrounding a central columella of host tissue on almost all branches of the same plant (). Smutted plants were smaller and weaker than healthy C. dactylon plants (). Disease symptoms developed gradually from closed to open inflorescences, fully occupied with black powdery sori, which could reach 4 cm in length (). Sometimes infection was localized in the basal parts of the inflorescence, with abortive spikelets in its distal parts; immature sori were often hidden by enveloping leaf sheaths. These symptoms are similar to those observed by Garcìa-Guzmán and Burdon (Citation1997) who reported that smut disease of Bermudagrass causes a destruction of the spikelets and deformation into sori containing a black powder of teliospores. Some Ustilaginaceae species like the covered smut of oats and barley caused by Ustilago hordei are economically significant, causing huge losses in production because the disease is highly specific on the florescence, replacing the kernels with black sori (Laurie et al. Citation2012). Observed symptoms were similar to Bermudagrass smut disease, caused by U. cynodontis, described by Shivas and Vánky (Citation2001). Climatic conditions for disease development such as temperature and relative humidity were recorded during March, April and May 2018 as follows: 23°C and 64%, 30°C and 46%, 36°C and 40%, respectively.
Fig. 1 (a) Smutted Cynodon dactylon plants (right) showing development of sori filled with black teliospores (smutted inflorescence with no seeds produced) compared with healthy inflorescence with seeds (left); (b) smutted C. dactylon plant (right) in comparison with a healthy plant (left); (c) the development stages of a smutted inflorescence of C. dactylon in comparison with a healthy one (left)

Morphological identification of the pathogen
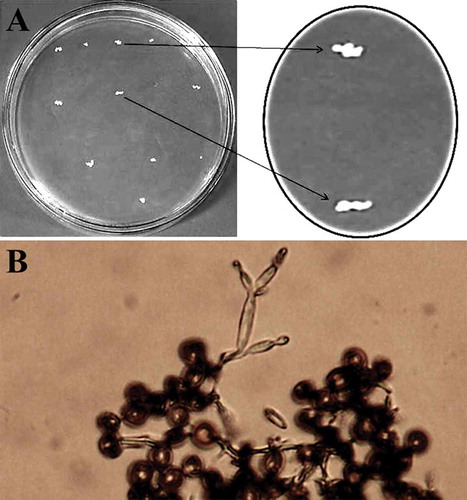
Fungal colonies were slender with irregular edges (). Germination of teliospores was also observed. Teliospores germinated within 24 h when plated at 28°C and produced basidia that gave rise to lateral and terminal, ovoid to long ellipsoidal basidiospores (). Potato dextrose agar may promote the skinny form (yeast-like colonies) from teliospores. These findings are similar to those of Durieu-Trautmann and Tavlitzki (Citation1975).
Fig. 2 (a) Colonies of Ustilago cynodontis grown on PDA medium for 6 days at 28°C. Note the irregular edge (slender form); (b) germinated teliospores of U. cynodontis produced basidia with ovoid to long ellipsoidal basidiospores, as seen under a light microscope (40 X)
Immature teliospores were often in readily separable chains, connected by minute hyaline hyphal remnants. Mature teliospores were homogeneous, single, irregularly globose or subglobose (from 5.85 to 6.055 µm diameter) (), yellowish brown to light olivaceous brown; smooth with dense low warts, 1-μm thick and were consistent with previous descriptions of U. cynodontis teliospores by Brook (Citation1957); Shivas and Vánky (Citation2001); Vánky (Citation1994, Citation2003)); Dung et al. (Citation2014). Crystal-like structures were produced by U. cynodontis colonies that were at least four days old. The crystal-like structures were bright with a pyramidal shape under the light microscope. These crystals were reported to be produced by Basidiomycete fungi such as Moniliophthora perniciosa grown on malt medium, and to play an essential role in virulence during cell wall degradation by using calcium from pectin and forming calcium oxalate crystals (Do Rio et al. Citation2008). Crystals were clearly observed and measured using ESM with dimensions of 18.275 ± 2 × 15.804 ± 2 × 19.104 ± 2 µm. Microanalysis of U. cynodontis crystal structures was conducted using an energy dispersive spectroscopy (EDS) system in SEM, revealing that the crystals were calcium oxalate ().
Molecular identification of the pathogen
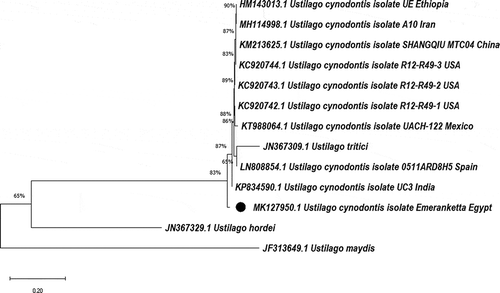
The ITS sequence of our isolate ‘Emeranketta’ was deposited in the NCBI and the GenBank with accession number MK127950.1, while the other isolate was excluded because of its low-quality sequence data. Phylogenetic analysis involving 13 nucleotide sequences of several U. cynodontis, U. hordei, U. maydis, and U. tritici isolates from different places around the world was conducted to show the position of our isolate ‘Emeranketta’ between related isolates (). Phylogenetic analysis showed that our isolate shared the greatest similarity with U. cynodontis isolates deposited from Ethiopia, Iran, India, United States, Mexico, Spain, and China. Therefore, the fungal isolate ‘Emeranketta’ was identified as U. cynodontis based on morphological and molecular identification.
Fig. 5 Neighbour-joining tree sequences showing the position of our isolate ‘Emeranketta’ of Ustilago cynodontis from Egypt versus related isolates. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method. The numbers at the nodes indicate the levels of bootstrap support (%). The analysis included 13 nucleotide sequences of U. cynodontis, U. hordei, U. maydis, and U. tritici isolates from different origins around the world. The scale bar at the bottom indicates the number of substitutions per site
Koch’s postulates and potential alternative hosts
No symptoms were observed on C. dactylon plants of the control seed treatment. Also, no symptoms were observed on E. crus-galli, C. rotundus, P. communis, P. purpureum, C. biflorus, E. colona, Z. mays, C. difformis and D. retroflexa plants grown from inoculated seeds with all five isolates of U. cynodontis (). Symptoms appeared on the inflorescence only on inoculated C. dactylon plants, and teliospores of the five isolates were collected and used to repeat the pathogenicity test, to fulfil Koch’s postulates. All inoculated Bermudagrass plants, with all five fungal isolates, displayed typical symptoms of dark black powdered teliospore masses surrounding a central columella of host tissue appearing on almost all inflorescences of the same plant. Smutted plants were smaller and weaker than healthy C. dactylon plants. Infections led to complete devastation of the inflorescences, while seeds were replaced with black teliospores arranged in sori that reached to 4-cm long. Sometimes infection was localized to the basal parts of the inflorescence, with abortive spikelets in its distal parts; immature sori were often hidden by enveloping leaf sheaths. These symptoms are similar to those described by Garcìa-Guzmán and Burdon (Citation1997) who reported that smut disease of Bermudagrass causes a destruction of the spikelets, turning them into black sori full of teliospores. Thus, the fungal isolate ‘Emeranketta’ was identified as U. cynodontis based on morphological and molecular identification, as well as pathogenicity tests.
Table 1. Potential alternative grass hosts of Ustilago cynodontis, belonging to two different botanical families, Poaceae and Cyperaceae.
It could be concluded from our results that the smut fungus, U. cynodontis, exists in Egypt and infects C. dactylon plants causing complete distortion of inflorescences turning them into sori. The pathogen was found throughout all sample locations in Kafr El-Sheikh Province, Egypt. Pathogenicity tests on C. dactylon and potential alternative grass hosts confirmed that the fungus infects only Bermudagrass and not the other species tested. Crystals were clearly observed by SEM. The fungus was identified using both a PCR technique and sequence analysis. PCR amplification confirmed that the amplicon from the fungus shared a 99% identity with U. cynodontis sequences. Phylogenetic analysis was done and the U. cynodontis isolate ‘Emeranketta’ shared the greatest similarity with isolates of U. cynodontis from Ethiopia, Iran, India, United States, Mexico, Spain, and China.
Acknowledgements
We would like to thank Associate Professor Dr. A.M. Abu Shama, Faculty of Science, Kafrelsheikh University, Egypt, for the definition process of the crystals.
Additional information
Funding
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. doi:10.1093/nar/25.17.3389
- Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T, Mukherjee B. 2003. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol. 84:131–138. doi:10.1016/S0378-8741(02)00322-7
- Begerow D, Stoll M, Bauer R. 2006. A phylogenetic hypothesis of Ustilaginomycotina based on multiple gene analyses and morphological data. Mycologia. 98:906–916. doi:10.1080/15572536.2006.11832620
- Bonfield JK, Smith KF, Staden R. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23:4992–4999. doi:10.1093/nar/23.24.4992
- Brook SD. 1957. Additions to the smut fungi of New Zealand, 11. Trans Roy Soc New Zealand. 84:643–648.
- Cessna SG, Sears VE, Dickman MB, Low BS. 2000. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell. 12:2191–2199. doi:10.1105/tpc.12.11.2191
- Do Rio MCS, De Oliveira BV, Tomazella DPT, Da Silva JAF, Pereira GAG. 2008. Production of calcium oxalate crystals by the basidiomycete Moniliophthora perniciosa, the causal agent of Witches’ Broom Disease of cacao. Curr Microbiol. 56:363–370. doi:10.1007/s00284-007-9091-7
- Dung JKS, Carris LM, Hamm PB. 2014. First report of Ustilago cynodontis causing smut of Cynodon dactylon in Washington State, United States. Plant Dis. 98:280. doi:10.1094/PDIS-05-13-0560-PDN
- Durieu-Trautmann O, Tavlitzki J. 1975. Reversible and permanent effects of the carbon sources and various antibiotics on the morphology and metabolic properties of Ustilago cynodontis cells. J Cell Biol. 66:102–113. doi:10.1083/jcb.66.1.102
- Garcìa-Guzmán G, Burdon JJ. 1997. Impact of the flower smut Ustilago cynodontis (Ustilaginaceae) on the performance of the clonal grass Cynodon dactylon (gramineae). Am J Bot. 84:1565–1571. doi:10.2307/2446618
- Guimaraes RL, Stotz HU. 2004. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 136:3703–3711. doi:10.1104/pp.104.049650
- Kruse J, Dietrich W, Zimmermann H, Klenke F, Richter U, Richter H, Thines M. 2018. Ustilago species causing leaf-stripe smut revisited. IMA Fungus. 9:49–73. doi:10.5598/imafungus.2018.09.01.05
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 35:1547–1549. doi:10.1093/molbev/msy096
- Laurie JD, Ali S, Linning R, Mannhaupt G, Wong P, Güldener U, Münsterkotter M, Moore R, Kahmann R, Bakkeren G, et al. 2012. Genome comparison of barley and maize smut fungi reveals targeted loss of RNA silencing components and species specific presence of transposable elements. Plant Cell. 24:1733–1745. doi:10.1105/tpc.112.097261
- Marley PS. 1995. Cynodon dactylon: an alternative host for Sporisorium sorghi, the causal organism of sorghum covered smut. J Crop Prot. 14:491–493. doi:10.1016/0261-2194(95)00023-F
- Shivas RG, Vánky K. 2001. The smut fungi on Cynodon, including Sporisorium normanensis, a new species from Australia. Fungal Divers. 8:149–154.
- Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci (USA). 101:11030–11035. doi:10.1073/pnas.0404206101
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22(22):4673–4680. doi:10.1093/nar/22.22.4673
- Vánky K. 1994. European smut fungi. Jena, New York: Gustav Fischer Verlag Stuttgart; p. 570.
- Vánky K. 2003. The smut fungi (Ustilaginomycetes) of Hyparrhenia (Poaceae). Fungal Divers. 12:179–205.
- White TJ, Bruns TD, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Bruns T, Taylor J, Sninsky JJ, White TJ, editors. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. San Diego: Academic Press; p. 315–322. doi:10.1016/B978-0-12-372180-8.50042-1