Abstract
Powdery mildew on cannabis (Cannabis sativa L., marijuana), caused by Golovinomyces cichoracearum, reduces plant growth and overall quality. To investigate disease management options, biological, chemical and physical approaches were assessed. A mildew-susceptible strain, ‘Copenhagen Kush’, was grown indoors with continual exposure to mildew inoculum. Treatments were applied weekly over a four-week period to groups of four plants once mildew infection had established itself. Trials were repeated thrice under varying initial disease pressures. Disease assessments were made weekly and the percentage of area infected on 30 leaflets per plant was used to calculate a disease rating score for treated and control plants. Disease progress curves were plotted and AUDPC values were determined for each treatment. To test the effect of UV-C light on mildew development, plants were exposed daily for 3–5 s over 28 days to UV-C light. The response of 12 cannabis strains to powdery mildew infection was assessed after exposing them to inoculum over a period of two weeks. The most effective treatments that significantly (P < 0.05) reduced disease in three trials were Luna Privilege SC (fluopyram), Regalia® Maxx, MilStop®, Rhapsody ASOTM, neem oil, and Stargus®. Treatments that were less effective included ZeroTol®, boric acid, and Actinovate® SP. Daily exposure of plants to UV-C light significantly reduced disease (by 45.2%, P < 0.05). Seven of 12 cannabis strains had significantly lower disease severity compared with the other five strains. The disease management strategies evaluated in this study have potential for reducing powdery mildew development on cannabis.
Résumé
Sur le cannabis (Cannabis sativa L., marijuana), l’oïdium, causé par Golovinomyces cichoracearum, réduit la croissance et altère la qualité des plants. Afin d’étudier la gestion de la maladie, des approches biologiques, chimiques et physiques ont été évaluées. Une souche sensible à l’oïdium, ‘Copenhagen Kush’, a été cultivée à l’intérieur et exposée continuellement à l’inoculum de l’oïdium. Des traitements ont été appliqués hebdomadairement pendant quatre semaines à des groupes de quatre plants à partir du moment où l’infection est survenue. Les essais ont été répétés trois fois sous différentes pressions initiales de la maladie. Cette dernière a été évaluée toutes les semaines et le pourcentage de surface foliaire infectée de 30 folioles par plant a été utilisé pour calculer une cote de classement de la maladie pour les plants traités et les plans témoins. Des courbes d’évolution de la maladie ont été tracées et les valeurs de l’AUDPC ont été déterminées pour chaque traitement. Pour tester l’effet des rayons UV-C sur l’évolution de l’oïdium, les plants y ont été exposés quotidiennement pendant 3 à 5 s pendant 28 jours. La réaction de 12 souches de cannabis à l’oïdium a été évaluée après les avoir exposées à l’inoculum pendant deux semaines. Les traitements qui ont le plus efficacement (P > 0,05) réduit l’incidence de la maladie au cours de trois essais étaient Luna Privilege SC (fluopyrame), Regalia Maxx, MilStop, Rhapsody ASO, l’huile de margousier et Stargus. Les traitements qui étaient moins efficaces incluaient ZeroTol, l’acide borique et Actinovate SP. L’exposition quotidienne des plants aux rayons UV-C a également réduit significativement l’incidence de la maladie (de 45,2%, P < 0,05). Sept des douze souches de cannabis affichaient une plus faible gravité de la maladie comparativement aux cinq autres souches. Les stratégies de gestion de la maladie évaluées au cours de cette étude peuvent réduire l’évolution de l’oïdium sur le cannabis.
Introduction
Powdery mildew diseases affect a wide range of host species and are caused by a large number of different obligate parasites belonging to several genera (Agrios Citation2005). On cannabis (Cannabis sativa L., marijuana), powdery mildew in Canada has been reported to be caused by Golovinomyces cichoracearum sensu lato (Pépin et al. Citation2018), and by closely related Golovinomyces species on hemp in other parts of North America (Schoener and Wang Citation2018; Gwinn et al. Citation2019; Ocamb and Pscheidt Citation2019; Szarka et al. Citation2019; Weldon et al. Citation2020). Powdery mildew is a prevalent disease that affects plant production in indoor controlled environments, greenhouses, and outdoor locations (Punja Citation2018; Punja et al. Citation2019). Following spore germination and infection, which can occur on leaf and stem surfaces, colonies that develop can limit photosynthesis and reduce nutrient availability to the plant, causing premature leaf drop as well as reducing overall vigour and potential yield of plants. Powdery mildew diseases, in general, have been managed by applications of fungicides and other chemical products, biological control agents, induction of disease resistance, and development of cultivars with genetic resistance (Agrios Citation2005). On cannabis, powdery mildew disease management relies on cultural control methods, such as removal of diseased leaves (deleafing), maintaining relative humidity at levels not conducive for pathogen development, applications of vaporized sulphur or reduced risk chemicals such as potassium bicarbonate (MilStop®) or hydrogen peroxide (ZeroTol®) (Health Canada Citation2019). There are currently no synthetic chemical fungicides registered for powdery mildew control on cannabis in Canada unlike on other crops. While reduced risk chemical products including potassium bicarbonate, hydrogen peroxide and lactic acid are registered for use on cannabis in Canada (Health Canada Citation2019), data on their comparative efficacy is lacking. In addition, the assessment of other potential methods for disease control, including biological control agents, has not been previously conducted. The rapid expansion of the cannabis industry in Canada requires that adequate disease control options for powdery mildew be identified for producers through an assessment of available options that do not impose additional risk to consumers and that meet Health Canada’s requirements for use (Health Canada Citation2019). Since strains (genotypes) of cannabis can also vary in their susceptibility to powdery mildew infection, an assessment of the levels of resistance to this disease requires further study.
In this study, the efficacy of nine reduced risk chemical or biological control products in managing powdery mildew development on cannabis plants was evaluated (). These products included three biological control agents, namely Actinovate® SP (containing Streptomyces lydicus strain WYEC 108), Rhapsody ASOTM (containing Bacillus subtilis strain QST 713), and Stargus® (containing Bacillus amyloliquefaciens strain F727). Chemical treatments evaluated included MilStop (potassium bicarbonate), ZeroTol (hydrogen peroxide), Silamol® (orthosilicic acid), neem oil, boric acid, and the plant defence inducer Regalia® Maxx (containing an extract of Reynoutria sachalinensis). The fungicide Luna® Privilege SC 500 (fluopyram) was included as an industry standard for comparative purposes. Luna Privilege Greenhouse, which makes use of the same active compound, but at a higher concentration, is registered for use in Canada for the control of powdery mildew and botrytis diseases on greenhouse grown cucumbers, peppers, tomatoes as well as other diseases (Bayer Citation2019). In addition, the efficacy of UV-C light to manage powdery mildew was also examined by daily exposure of leaves. Finally, the susceptibility of 12 strains (genotypes) of cannabis to disease development was assessed to determine their resistance to powdery mildew.
Table 1. Products evaluated for management of powdery mildew on cannabis plants and the average percent disease reduction values obtained over three trials compared to untreated control plants
Materials and methods
Plant materials and inoculation
Vegetative cuttings (approximately 12 cm in height) of the powdery mildew susceptible strain ‘Copenhagen Kush’ (CPH) were obtained from stock (mother) plants maintained in an indoor growing room with 24-hr lighting and rooted in a mixture (3:1) of coco (Canna Coco) and perlite (Dutch Treat) in individual 8.5 cm2 pots. The pots were placed under humidity domes (18-cm height dome with vents) for 10–14 days at room temperature (22–25 °C) in an indoor growing room with ambient lighting. To maintain high humidity during this time, the insides of the domes were misted with water every other day. After this time, the domes were removed, and plants were placed under two Sunblaster brand (Langley, British Columbia, Canada) 54 watt 6400 k T5HO lights with a 24-h photoperiod. Plants were watered as needed with a solution of 1 mL/L Sensi Grow Coco pH Perfect A + B (Advanced Nutrients, West Hollywood, California, USA) and 1 mL/L General Hydroponics Calimagic (General Hydroponics, Santa Rosa, California, USA) adjusted to a pH of 5.8–6.2 using Advanced Nutrients pH-Down (Advanced Nutrients, West Hollywood, California, USA). To initiate powdery mildew, initial inoculum originating from cannabis plants grown in a licenced facility in British Columbia was transferred to healthy plants by collecting spores and dusting plants. A single heavily infected plant was then placed in the centre of the room and allowed to release conidia. This ensured that background levels of inoculum in the indoor room where the experiments were to be conducted were sufficient for the disease to establish naturally. Rooted plants were removed from the high humidity environment, placed on a table, and received one spray of MilStop to minimize any variance in the initial levels of infection. The plants were then maintained for seven days in the growing room before treatments were applied ().
Treatment applications and disease assessments
The plants were arranged in groups of four per treatment, unless otherwise noted. In each experiment, two or three different treatments were compared to a control group. Products to be tested were prepared on the day of application and sprayed onto plants using a pressurized hand held spray bottle to runoff. At the start of the experiment (week 0), plants were assessed for powdery mildew infection, after which they received the appropriate treatment. Disease was assessed on the 30 most diseased leaflets (assessed visually) per group by placing them into one of five categories, based on the percent coverage of mildew (1–20%, 21–40%, 41–60%, 61–80%, or 81–100%). To calculate a disease rating score, the number of leaflets in each category was multiplied by a factor of 0.1, 0.3, 0.5, 0.7, or 0.9, respectively (with these numbers representing the midpoint of each category). Plants received four weekly sprays in total and five disease assessments were made over the span of five weeks, each at weekly intervals. Luna Privilege SC 500 (Bayer Cropscience, Research Triangle Park, North Carolina, USA) was only applied once, at the beginning of the experiment. Each experiment was repeated three times for each treatment (referred to as trials).
Data analysis
The average disease rating score for each treatment per week was plotted over the duration of the experiment to obtain a disease progress curve for each treatment in each of the trials. The results from each trial are presented separately as there were differences in the initial levels of infection on the plants as well as the overall disease pressure during the trial (see ). To statistically show these differences between trials, the disease ratings were compared by ANOVA followed post hoc by Tukey’s HSD test at P < 0.05 and are presented in . To test for significant differences between treatments within each trial, these data were also subjected to ANOVA followed post hoc by Tukey’s HSD test at P < 0.05 and are presented in . Finally, area under the disease progress curve (AUDPC) was calculated from the disease progress curves (Simko and Piepho Citation2012) and compared for differences among treatments within each trial by ANOVA followed post hoc by Tukey’s HSD test at P < 0.05. These results are presented in .
Fig. 1 Development of powdery mildew on cannabis leaves. (a) Initial infections are visible as white colonies on the upper surface of leaves. (b) Scanning electron microscopic image through a cross section of a diseased leaf showing powdery mildew mycelium growing over the surface of epidermal cells. The underlying cells of the epidermis and mesophyll layer, and the lower epidermis, can be seen in this section. (c) Abundant spore production from an older powdery mildew colony on the leaf surface

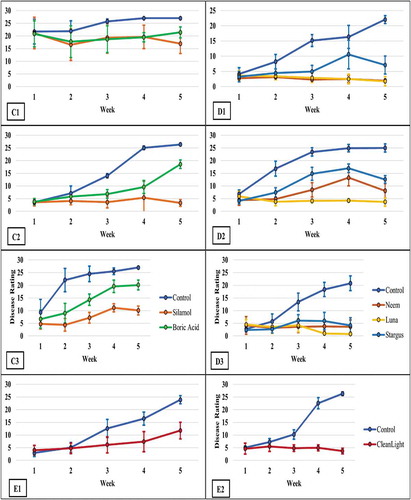
Fig. 2 The disease progress curves for powdery mildew development on cannabis plants when treated with Regalia Maxx, Rhapsody ASO, MilStop® (A1-3), ZeroTol®, Actinovate® (B1-3), boric acid, Silamol® (C1-3), Stargus®, neem oil, Luna Privilege SC 500 (D1-3), CleanLightTM UV-C (E1-2), and their respective untreated controls. Data represent the average disease score for each treatment in each week. There were four replicate plants per trial except in the case of the CleanLight trials (n = 8) and the second trial of ZeroTol, Actinovate and their respective control (n = 3). Error bars are 95% confidence intervals (continued on next page)
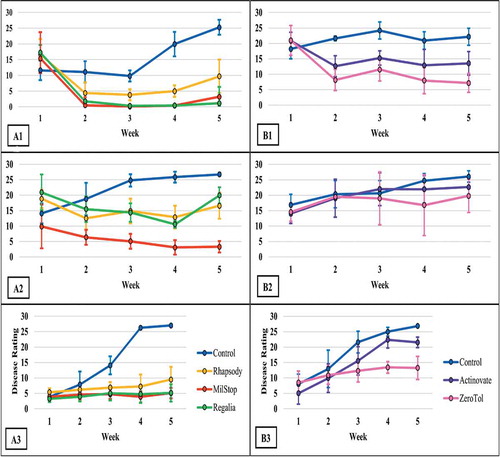
Fig. 3 Effect of four weekly applications of Regalia Maxx, Rhapsody ASO and MilStop® on development of powdery mildew on cannabis plants compared to a nontreated control. Data represent areas under the disease progress curves (AUDPC) from three repeated trials, each with four replicate plants. Error bars are 95% confidence intervals. Letters above the error bars represent significant differences in the AUDPC values of the treatments, as determined through ANOVA and Tukey’s post hoc test (P < 0.05)
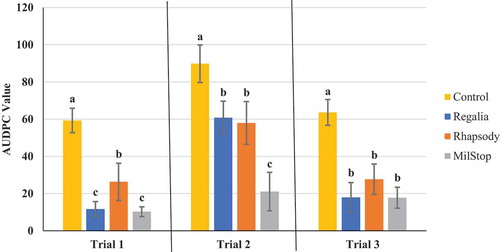
Fig. 4 Effect of four weekly applications of ZeroTol® and Actinovate® on development of powdery mildew on cannabis plants compared to a nontreated control. Data represent areas under the disease progress curves (AUDPC) from three repeated trials, each with four replicate plants (except in the case of trial 2 where an asterisk denotes three replicate plants were used instead of four). Error bars are 95% confidence intervals. Letters above the error bars represent significant differences in the AUDPC values of the treatments, as determined through ANOVA and Tukey’s post hoc test (P < 0.05)
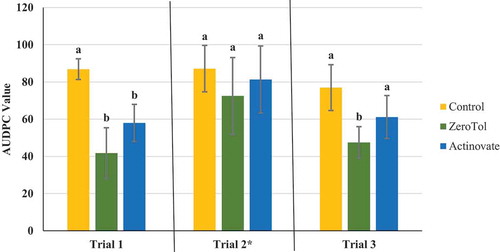
Fig. 5 Effect of four weekly applications of boric acid and Silamol® on development of powdery mildew on cannabis plants compared to a non-treated control. Data represent areas under the disease progress curves (AUDPC) from three repeated trials, each with four replicate plants. Error bars are 95% confidence intervals. Letters above the error bars represent significant differences in the AUDPC values of the treatments, as determined through ANOVA and Tukey’s post hoc test (P < 0.05)
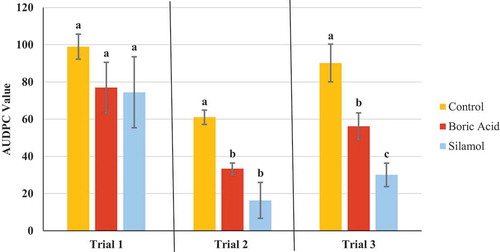
Fig. 6 Effect of four weekly applications of Stargus®, neem oil and Luna Privilege SC 500 on development of powdery mildew on cannabis plants. Data represent areas under the disease progress curves (AUDPC) from three repeated trials, each with four replicate plants. Error bars are 95% confidence intervals. Letters above the error bars represent significant differences in the AUDPC values of the treatments, as determined through ANOVA and Tukey’s post hoc test (P < 0.05)
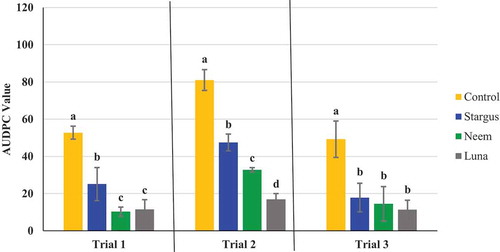
Table 2. Comparisons of final (week 5) disease rating scores in response to different treatments to manage powdery mildew on cannabis plants. Comparisons were made between trials and between treatments within a trial
UV treatments for powdery mildew control
Plants of strain CPH were grown as previously described and arranged in groups of 8, with 8 untreated (control) plants per trial. The treated plants received daily exposure to UV-C light from a hand-held CleanLightTM Pro (Honselersdijk, NL) unit over a period of four weeks. Plants were treated for 3–5 s per day (equal to 3–6 mJ/cm2 of radiation, as per the manufacturer) by moving the unit uniformly over and around the plant at a distance of 5 cm away. Treated and control plants were rated for disease as previously described, with the 30 most diseased leaflets being assessed at weekly intervals five times over the duration of the trial. Disease rating scores were calculated for treated and control plants each week and graphed, and AUDPC values were calculated as previously described. This experiment was conducted two times. Data are presented separately for each trial (). The disease rating scores and AUDPC values were compared for significant differences between treated and control plants using ANOVA followed post hoc by Tukey’s test at P < 0.05 ().
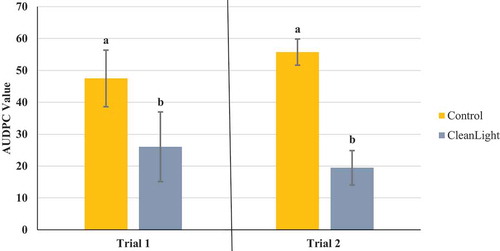
Fig. 7 Effect of daily exposure of cannabis leaves to UV-C light on powdery mildew development compared to a nontreated control. Data represent areas under the disease progress curves (AUDPC) from two repeated trials, each with eight replicate plants. Error bars are 95% confidence intervals. Letters above the error bars represent significant differences in the AUDPC values of the treatments, as determined through ANOVA and Tukey’s post hoc test (P < 0.05)
Scanning electron microscopy
To compare the extent of powdery mildew development (mycelial growth and sporulation) between control plants and those receiving specific treatments, leaf samples ca. 0.5 cm2 in size were prepared for scanning electron microscopy as described by Punja (Citation2018). The leaves were obtained from untreated (control) plants with young and old powdery mildew colonies, as well as from plants receiving four weekly treatments of Regalia, Rhapsody, or MilStop. In addition, leaves from plants exposed to CleanLight (daily exposure for 28 days) were included. The extent of mycelial growth of powdery mildew and the presence of sporulation were visually compared for control and treated leaves.
Effect of Regalia Maxx on chlorophyll ‘a’ and ‘b’ levels
On plants receiving four applications of Regalia Maxx, it was observed that leaves appeared darker green when compared to control plants. Therefore, methanol extraction and spectrophotometric analysis of chlorophyll a and b in leaf samples was performed according to Sumanta et al. (Citation2014). Two trials were conducted – one in an indoor growing room and the other under greenhouse conditions. Leaf samples were collected from approximately mid-height after the plants had received 4 applications, with 3–5 replicate samples. Mean values for chlorophyll a and b from the Regalia Maxx treated plants and the untreated control plants were compared for significant differences using ANOVA followed post hoc by Tukey’s test at P < 0.05. Data for the two trials are presented separately.
Screening of strains for susceptibility to powdery mildew
Stock plants of 12 cannabis strains grown in an indoor room were used as a source of cuttings. The plants received two weekly sprays of MilStop (BioWorks, Victory, New York, USA) to reduce powdery mildew infection, after which cuttings were taken and rooted as described previously. The cuttings were dusted with powdery mildew spores from fresh infected leaf tissue before being placed under the humidity dome. Two visibly infected plants of strain CPH were also placed under the domes to provide an additional source of inoculum. In each trial, there were two groups of four plants arranged around two diseased plants. Cuttings were left under the domes for a period of two weeks, during which the inside of the domes was misted on alternate days to maintain high humidity. At the end of two weeks, individual plants were rated for disease by placing them into one of five categories based on the percentage coverage of powdery mildew on the leaves, as described previously. The average disease score per strain was determined and graphed. Significant differences between the disease scores were determined using ANOVA followed post hoc by Tukey’s test at P < 0.05. The experiment was conducted twice.
Results
Development of the powdery mildew fungus
The symptoms of powdery mildew infection on cannabis leaves were quite apparent as white mycelial growth and sporulation of the pathogen on the upper surface of leaves on plants in the growing room (). Under the scanning electron microscope, a cross-section through a leaf showed mycelial growth on the epidermal surface (), and sporulation was abundant on the leaf surface (). The production of spores in chains is a characteristic of the genus Golovinomyces (Pépin et al. Citation2018).
Treatment applications and disease assessments
The foliar treatments applied to manage powdery mildew had different levels of efficacy when compared to the untreated controls. The results from each of the three trials conducted for each product showed some variation between trials, which was attributed to differing levels of initial infection and/or disease pressure during the trials. The disease progress curves for each trial for each product tested are shown in . They show the varying rates and levels of disease development over time. A comparison of final, week 5, disease rating scores in response to different treatment applications to manage powdery mildew on cannabis plants is presented in . Statistical comparisons were made between trials and between treatments within a trial. The statistical analysis showed that disease levels on control plants in each of the experiments, i.e., Control 1 to Control 5 values, were in the range of 20.8 to 27, and thus showed fairly consistent final disease levels in all trials. However, in a number of treatments, the data from different trials were significantly different. For example, in trial 2, the values for disease ratings were significantly different for Regalia, ZeroTol, Actinovate, Stargus, and neem when compared to the values for trials 1 and 3 (). The week five disease rating scores, however, showed that most treatments significantly reduced the development of powdery mildew in all three trials, except for ZeroTol, boric acid and Actinovate (), which were the poorest performing products.
Product efficacy based on area under the disease progress curve
The AUDPC values were calculated from the disease progress curves and are presented in . The most effective products, which provided significant (P < 0.05) reductions in AUDPC values consistently in all three trials were: Luna Privilege SC 500; MilStop; neem oil; Regalia Maxx; Rhapsody ASO; and Stargus. These products also provided average disease reductions of over 50% () for the majority of the trials. Products showing significantly reduced AUDPC values in two out of three trials were ZeroTol, boric acid, and Silamol, although these products often reduced disease by less than 50% in most of the trials (), with the exception of Silamol. Actinovate only provided significant disease control in one of the three trials. Plants receiving daily exposure to CleanLight had significantly (P < 0.05) lower AUDPC values in both trials, when compared to their appropriate controls (). The AUDPC values and the week five disease rating scores, therefore, provided similar conclusions on the efficacy of the products tested. The percentage reduction in disease levels for each of the products in each trial based on AUDPC values is summarized in . These values were derived by comparison of AUDPC values for the treatments with the untreated control used in each trial.
Visual representation of product efficacy
Representative leaves were sampled from plants in each of the treatments and photographed to show the differences in product efficacy (). While the leaves were from different experiments, they were selected to represent the disease rating values shown in . When compared to the untreated control leaves, treatments with CleanLight, Luna, Regalia Maxx and MilStop showed the lowest disease severity and incidence of powdery mildew, and leaves from treatments such as neem oil, Stargus, Rhapsody and ZeroTol also showed a visible reduction in disease development. By comparison, leaves treated with Actinovate and boric acid had the highest infection levels ().
Fig. 8 A representation of the comparative efficacy of 11 treatments at managing powdery mildew development on leaves of cannabis strain ‘Copenhagen Kush’. (a) Untreated control. (b) CleanLight Pro Unit. (c) Luna SC Privilege 500. (d) Regalia Maxx. (e) MilStop. (f) Neem oil. (g) Stargus. (h) Rhapsody ASO. (i) ZeroTol. (j) Silamol. (k) Boric acid. (l) Actinovate. Representative leaves were sampled from plants in each of the treatments and photographed to show the differences in product efficacy

Scanning electron microscopy
Scanning electron microscopic images showing growth of the powdery mildew pathogen on leaf surfaces of untreated cannabis plants were compared to those following applications of disease management products. Weekly applications of three products (Rhapsody, Regalia, MilStop) or daily exposure of plants to UV-C light were made. On control leaves, mycelium from a young developing colony was observed ramifying over the surface (), followed by more extensive mycelial proliferation and spore production in a mature colony on an untreated leaf (). Following four applications of Rhapsody, the extent of mycelial proliferation was noticeably reduced (), while some sporulation was evident. On leaves treated with Regalia Maxx, there was an absence of mycelial growth on the leaf surface, and trichomes could be seen (). Following applications of MilStop, mycelial growth was sparse and hyphae and conidia appeared to be plasmolysed (). Exposure of leaves to UV light also revealed a total absence of mycelial growth (). These observations show that there were obvious differences in pathogen development following various treatments when compared to the control and lend support to the disease measurement values reported above.
Fig. 9 Scanning electron microscopic images showing growth of the powdery mildew pathogen on leaf surfaces of untreated cannabis plants or following applications of disease management products. Weekly applications of three products or daily exposure of plants to UV-C light were made as indicated. Samples were collected after four applications of products or following a 28-day exposure to UV-C light. (a) Mycelium of a young developing colony on an untreated leaf. (b) Mycelium and spore production (arrow) of a mature colony on an untreated leaf. (c) Reduced mycelial growth on the leaf surface, with some sporulation (arrow) following applications of Rhapsody. (d) Total absence of mycelial growth on the leaf surface following applications of Regalia®. Hair-like projections that are visible are the trichomes. (e) Sparse mycelial growth and collapsed conidia (arrow) following applications of MilStop®. (f) Total absence of mycelial growth following daily exposure to UV-C light. Arrow shows conidia attempting to germinate

Effect of Regalia Maxx on chlorophyll a and b levels
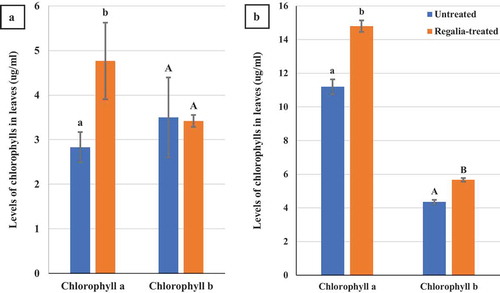
Samples of leaves from plants receiving four applications of Regalia Maxx and grown in an indoor environment had mean chlorophyll a levels that were significantly (P < 0.05) higher than leaves from untreated plants, while there was no significant difference in chlorophyll b levels between treated and untreated plants (). The chlorophyll a/b ratios for untreated and Regalia Maxx treated plants were 0.8 and 1.4, respectively. On plants grown in the greenhouse, there were significant (P < 0.05) differences between the mean levels of both chlorophyll a and chlorophyll b in Regalia treated leaves compared to untreated leaves (). The chlorophyll a/b ratios for untreated and Regalia Maxx treated plants were 2.67 and 2.61, respectively. The total chlorophyll content in leaves was about three-fold higher in plants grown in the greenhouse compared with the indoor environment, likely due to differences in light intensity levels and growing conditions.
Fig. 10 Effect of four weekly applications of Regalia Maxx on mean chlorophyll a and b levels in leaves of: (a) indoor grown plants (n = 3) and (b) greenhouse grown plants (n = 5) compared to their respective controls. Levels of chlorophyll a were significantly increased in both trials, while chlorophyll b was increased in the greenhouse trial. Error bars are 95% confidence intervals. Letters above the error bars represent significant differences in chlorophyll levels, as determined through ANOVA and Tukey’s post hoc test (P < 0.05)
Screening of strains for susceptibility to powdery mildew
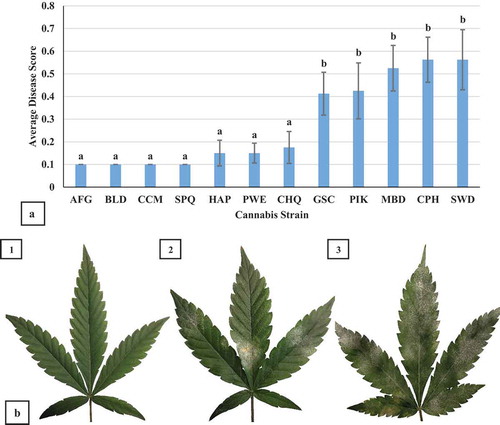
The disease scores for 12 cannabis strains are shown in . Seven of the strains had significantly lower (P < 0.05) disease scores compared to the five highly susceptible strains, suggesting they were resistant to infection. A comparison of disease development on leaves selected from strains ‘Space Queen’, ‘Pennywise’, and ‘Sweet Durga Mata’ is shown in .
Fig. 11 (a) Response of 12 different strains (genotypes) of cannabis plants to powdery mildew infection. Mean disease score values were calculated for infected plants of each strain to evaluate their susceptibility to powdery mildew two weeks after pathogen introduction. Error bars are 95% confidence intervals. Letters above the error bars represent significant differences in the AUDPC values of the treatments, as determined through ANOVA and Tukey’s post hoc test (P < 0.05). The strain abbreviations are: AFG = ‘Afghani’; BLD = ‘Blue Deity’; CCM = ‘Critical Cali Mist’; SPQ = ‘Space Queen’; HAP = ‘Hash Plant’; PWE = ‘Pennywise’; CHQ = ‘Cheese Quake’; GSC = ‘Girl Scout Cookies’; PIK = ‘Pink Kush’; MBD = ‘Moby Dick’; CPH = ‘Copenhagen Kush’; and SWD = ‘Sweet Durga Mata’. (11B) Comparison of powdery mildew development on leaves of three strains of cannabis. Representative leaves were sampled to illustrate a range of susceptibility to disease. (1) ‘Space Queen’. (2) ‘Pennywise’. (3) ‘Sweet Durga Mata’
Discussion
Powdery mildew occurs on cannabis (marijuana) and hemp plants grown indoors, in greenhouses and in outdoor environments and is a difficult disease to manage because of the rapid rate at which the pathogen can spread and a lack of available fungicides that are used to manage powdery mildew diseases on other crops (Agrios Citation2005). The spread of inoculum within a cannabis production facility from diseased plants is from the release of air-borne spores that are produced in large numbers (Punja Citation2018) as well as by infected plant materials that growers may move within and between different production facilities and from which diseased cuttings may be taken. In this study, introduction of a diseased plant within the growing room where experiments were conducted resulted in sufficient spread and consistent disease development on control plants in repeated trials over time.
The taxonomic placement of the cannabis powdery mildew pathogen was confirmed to be in the genus Golovinomyces (formerly Erysiphe) by Pépin et al. (Citation2018). In that study, and in subsequent work by other researchers from the United States, Golovinomyces has been shown to be the cause of powdery mildew on hemp (Schoener and Wang Citation2018; Gwinn et al. Citation2019; Ocamb and Pscheidt Citation2019; Szarka et al. Citation2019; Weldon et al. Citation2020). In these published studies, the ITS1-5.8S-ITS2 region of rDNA was used to identify the pathogen. Based on the homology to sequences previously deposited in GenBank®, three species with 100% sequence homology to each other, have been proposed to infect cannabis and hemp plants: G. ambrosiae, G. spadiceus, and G. cichoracearum. Further distinction among these species will require additional molecular markers other than ITS and phylogenetic analysis.
Currently, there are several products registered for use on cannabis in Canada to manage powdery mildew. These include wettable and vaporized sulphur, MilStop (potassium bicarbonate), ZeroTol (hydrogen peroxide), Regalia Maxx (an extract of giant knotweed), and Lacto-San (lactic acid) (Health Canada Citation2019.) Actinovate was also previously registered but has since been deregistered. Comparative efficacy studies utilizing these products for powdery mildew control on cannabis have not been previously conducted. Our results confirm that MilStop and Regalia Maxx were the most effective at reducing powdery mildew infection and spread when applied weekly. Biocontrol products such as Rhapsody ASO and Stargus (both containing Bacillus sp.) also suppressed powdery mildew to some degree. Lower efficacy was observed with other products tested, such as boric acid, Silamol, ZeroTol, and Actinovate. While the fungicide Luna Privilege SC 500 was the most effective product in reducing disease development, it is not registered for use on cannabis in Canada or in the United States and was included in this study for comparative purposes. In a recent study, Betz and Punja (Citation2020) demonstrated that Regalia Maxx effectively controlled powdery mildew development on wasabi plants (caused by Erysiphe cruciferarum), while Rhapsody also reduced disease progression to a lesser extent and Actinovate was not effective. On cucumber plants, Ni and Punja (Citation2020b) demonstrated that weekly applications of Rhapsody reduced powdery mildew development (caused by Podosphaeria xanthii) to the same extent as the fungicide Luna Privilege SC 500.
The active compound in Luna Privilege SC 500 (fluopyram) is a systemic fungicide that has been shown to control both powdery mildew and leaf spot on tart cherry (Proffer et al. Citation2012), with preventative and moderately curative effects on grey mould caused by Botrytis cinerea on strawberry (Veloukas and Karaoglanidis Citation2012) and on table grapes (Vitale et al. Citation2016). One application of Luna Privilege SC 500 significantly reduced powdery mildew development on treated cannabis plants in all three trials in this study. It could be an effective treatment to manage powdery mildew on stock (mother) plants to prevent subsequent spread of the pathogen during vegetative propagation. Product registration for use on cannabis is still required in Canada.
Regalia Maxx is a formulated extract of giant knotweed (Reynoutria sachaliensis) that has been shown to induce systemic resistance in plants, resulting in the production and accumulation of phytoalexins, phenolic compounds (Daayf et al. Citation1997, Citation2000) and flavonoids (Fofana et al. Citation2002; McNally et al. Citation2003). Increased activity of pathogenesis-related proteins such as glucanase, chitinase, and peroxidase has also been reported (Schneider and Ullrich Citation1994). Regalia was previously shown to be effective in reducing powdery mildew development on a number of crops, including cucumber (Fofana et al. Citation2002; Rur et al. Citation2018), tomato (Konstantinidou-Doltsinis et al. Citation2006), squash (Zhang et al. Citation2016), and wasabi (Betz and Punja Citation2020). When tested on cannabis, Regalia Maxx reduced powdery mildew development in all three trials, showing efficacy under a range of disease pressures. The mechanism of action may include production of the previously mentioned biochemical compounds, but this requires further study. Scanning electron microscopic observations showed that Regalia-treated leaves had no visible development of mycelium on the leaf surface. This may be the result of a combination of fungitoxic and induced resistance mechanisms.
It was observed that applications of Regalia Maxx caused a dark green appearance on leaves and significantly increased chlorophyll a levels were observed although untreated and Regalia Maxx treated plants had similar chlorophyll a/b ratios. The enhanced chlorophyll levels may provide a benefit to the plants, as powdery mildew infection is known to decrease photosynthesis levels in infected leaf tissues (Gordon and Duniway Citation1982; Balkema-Boomstra and Mastebroek Citation1995).
Formulated potassium bicarbonate products, including MilStop and Armicarb®, act as fungitoxic compounds through a range of mechanisms, including altering the pH of leaf and fruit surfaces and osmotic pressure, which in turn inhibit the development of fungal mycelium and spores (Mitre et al. Citation2018). Potassium bicarbonate products have been reported to be effective at controlling apple scab and powdery mildew on apples (Jamar et al. Citation2008; Mitre et al. Citation2018) and significantly reduced the incidence and severity of powdery mildew on gooseberry (Wenneker Citation2016). Moyer and Peres (Citation2008) demonstrated that when applied to gerbera daisies to control powdery mildew, MilStop was effective at reducing disease, and depending on the cultivar of daisy, it was comparable to treatments of systemic fungicides. Scanning electron microscopic observations of MilStop treated cannabis leaves showed significantly reduced mycelial development compared to the control, with some evidence of plasmolysed cells that may be the result of osmotic or pH changes.
Two Bacillus spp. products were tested to evaluate their efficacy at managing powdery mildew on cannabis plants. These were Rhapsody ASO, which contains Bacillus subtilis strain QST 713, and Stargus, which contains Bacillus amyloliquefaciens strain 727. Bacillus spp. are known to produce many antifungal compounds, including the lipopeptides iturins and fengycins (Romero et al. Citation2007; Siahmoshteh et al. Citation2018; Ni and Punja Citation2019), proteases (Takami et al. Citation1989; Degering et al. Citation2010), and siderophores (Shoda Citation2000), which act collectively to reduce pathogen development. Additionally, application of these bacteria induces host resistance that can suppress subsequent infection by pathogens (Ongena et al. Citation2005; Lahlali et al. Citation2013) as well as enhance plant growth (Kloepper and Ryu C-M Citation2004; Pérez-García et al. Citation2011). Bacillus based products have also been shown to be effective in managing powdery mildew, particularly when plants are treated preventatively, or if it is used in combination with systemic fungicides (Keinath and DuBose Citation2004; Romero et al. Citation2007; Gilardi et al. Citation2008; Elmhirst et al. Citation2011; Ni and Punja Citation2019). Scanning electron microscopic observations of treated cannabis leaves showed that Rhapsody applications reduced mycelial growth to some extent, but visible development of the pathogen still occurred and spores were produced.
Neem oil from the evergreen tree Azadirachta indica is known to contain an array of antifungal compounds, with the triterpenoids present acting in a synergistic or additive manner in order to achieve their antifungal effects (Govindachari et al. Citation1998). When neem oil fractions were purified and single triterpenoids were tested against a range of common plant pathogens, such as Fusarium oxysporum and Alternaria tenuis, there was a loss of effect (Govindachari et al. Citation1998). When applied to whole plants, neem products had a significant effect on reducing powdery mildew disease development on rose (Pasini et al. Citation1997), pea (Patil et al. Citation2017; Vivek et al. Citation2017; Deshmukh et al. Citation2018), okra (Moharam and Obiadalla Ali Citation2012), and other crops. Our results are consistent with previously published reports that show that neem oil or neem extracts are effective at reducing powdery mildew development. There are several azadirachtin or neem oil extract-based products registered for use on cannabis and hemp in the USA, but no products with these active ingredients are registered for use on cannabis in Canada.
The use of ultraviolet light (UV-C and UV-B) to reduce pathogen and mould development in agricultural production has been previously described, including for management of post-harvest decay of strawberries, potatoes, and tomatoes (Liu et al. Citation1993; Stevens et al. Citation1999; Nigro et al. Citation2000). As well, UV light has been used for management of powdery mildew on crops such as roses, strawberries, cucumbers, and tomatoes (Willocquet et al. Citation1996; Suthaparan et al. Citation2012, Citation2014, Citation2016) and as a seed treatment to reduce fungal infection (Siddiqui et al. Citation2011). The CleanLight Pro unit, which uses UV-C light, was effective at reducing powdery mildew development in both trials conducted, with few powdery mildew colonies developing once treatment was started. Previous studies suggest that the efficacy of UV light in managing diseases is due to both its direct germicidal activity as well as its indirect ability to induce defence responses in plants, resulting in increased levels of phenolic compounds and pathogenesis-related proteins (Douillet-Breuil et al. Citation1999; Bonomelli et al. Citation2004; Hijnen et al. Citation2006). Scanning electron microscopic observations of treated cannabis leaves showed that mildew spores had not germinated, and mycelial growth was inhibited, suggesting a direct toxic effect. The application of UV-C light could be done on a larger scale in current cannabis production facilities through the use of mounted booms or in-row units which are available commercially and utilized for other crops (Gadoury Citation2019).
The use of boron to manage diseases as a soil amendment, as well as a foliar application, has been previously investigated. Boron applied to soil was reported to significantly reduce the development of clubroot on canola in field trials (Deora et al. Citation2011). However, when applied as a foliar treatment in the form of boric acid to field grown potatoes, it did not have a significant effect on late blight and was ineffective against Phytophthora infestans growth based on ED50 data (Frenkel et al. Citation2010). Boric acid as a foliar treatment decreased the severity of late blight in potted greenhouse tomatoes, both on leaves that were treated and in distal leaves, possibly through the induction of phenolic compounds and peroxidase enzymes (Frenkel et al. Citation2010). Boric acid as a foliar spray also significantly reduced the number of lesions per flag leaf when used to treat tan spot on wheat (Simoglou and Dordas Citation2006). Canola plants treated with boric acid had twice the levels of boron in leaves compared to control plants and development of Sclerotinia sclerotiorum was significantly reduced (Ni and Punja Citation2020a). In our study, although applications of boric acid provided a significant reduction in disease in two out of three trials, the low average disease reduction in all three trials (less than 50%) indicates it is not an effective option for powdery mildew management.
Silicon is known to be effective at managing powdery mildew when applied as an amendment to soil or in nutrient solution as well as a foliar spray (Bowen et al. Citation1992; Schuerger and Hammer Citation2003; M-H et al. Citation2007; Shetty et al. Citation2011). When applied to the roots, soluble silicon products resulted in increased silica deposition in leaves, especially in the apoplast of epidermal cells as well as induction of host defences. This host response included silica deposition around areas of appressoria penetration, resulting in a reduction in haustoria formation, and an increase in the production of phenolic compounds (Menzies et al. Citation1991; Samuels et al. Citation1991; Shetty et al. Citation2011). When applied as a foliar treatment, plants accumulated considerably lower levels of silicon, suggesting that management of disease from foliar silica products is due to a direct interaction with the pathogen rather than through a plant mediated mode of action (M-H et al. Citation2007). It is possible that the efficacy of Silamol is partially due to the low pH of the solution, with the rate used in our trials having a pH of 3.2. In two of three trials, Silamol significantly reduced powdery mildew development on cannabis plants when applied as a foliar spray. Silamol was not effective in the first trial due to the higher initial disease levels, suggesting that it is most effective when used as a preventative treatment under low disease pressure. The efficacy of silicon applied as a fertilizer to the roots of cannabis plants on powdery mildew suppression has not been investigated.
There is a limited amount of research on the efficacy of hydrogen peroxide, present in products such as ZeroTol, for disease management. Hydrogen peroxide has been shown to have some efficacy at reducing powdery mildew development on cucumber plants (Hafez et al. Citation2008) and for post-harvest reduction of Botrytis cinerea infection on white pepper fruits (Hafez Citation2010) as well as inducing resistance to downy mildew in pearl millet (Geetha and Shetty Citation2002). Hydrogen peroxide may work through two modes of action – it is known to have biocidal effects and may act as an inducer to trigger the hypersensitive response and signal transduction, inducing genes involved in systemic acquired resistance (Allan and Fluhr Citation1997; Alvarez et al. Citation1998; Kuźniak and Urbanek Citation2000; Hückelhoven and Kogel Citation2003). Our results showed that ZeroTol was partially effective in reducing powdery mildew development on cannabis plants, but was not as effective as some of the other treatments described above.
Previous research has shown Actinovate AG to be effective at reducing powdery mildew disease severity compared to unsprayed controls but it was ineffective compared to a water control on grape (Janousek et al. Citation2009), and compared to both water and unsprayed treatments, it was ineffective at lowering disease incidence. On squash and cantaloupe, Actinovate alone was not found to reduce powdery mildew severity compared to a water control (Zhang et al. Citation2011). The efficacy of Actinovate is most likely due to antibiotic and chitinase production by Streptomyces spp. as well as induction of plant defences following application (Conn et al. Citation2008; Vurukonda et al. Citation2018). However, in this study, Actinovate did not provide significant disease reduction and it is not currently registered for disease control on cannabis in Canada.
Genetic resistance has been one of the most effective approaches for powdery mildew management on a wide range of crops. The genetic basis of powdery mildew resistance is in part based on R-genes, known to be present in crops such as tomato, cucumber, wheat, hops, melon, and numerous others (Huang et al. Citation2000; Bai et al. Citation2003; Sakata et al. Citation2006; Cao et al. Citation2011; Ning et al. Citation2014; Wolfenbarger et al. Citation2014). The mechanistic basis for resistance of plant tissues to powdery mildew infection is complex, and numerous changes in gene expression and biochemical compounds are described (Qiu et al. Citation2015). Among the 12 cannabis strains that were tested for their susceptibility to powdery mildew in this study, it was observed that seven showed partial or complete resistance to infection. This qualitative rating may suggest that one major gene for resistance is present. Differing levels of susceptibility to powdery mildew infection have also been observed among hemp cultivars (Cala et al. Citation2019). The basis for this resistance in cannabis and hemp is not yet known. The isolation and cloning of R-genes in the C. sativa genome to powdery mildew resistance is needed to enhance breeding of cannabis strains with disease resistance.
This research represents an initial investigation into the management of powdery mildew on cannabis. Further research is needed to determine how potential adjustments to the rates of these products may affect their efficacy, the possible benefits to using products in tandem (alternating Regalia Maxx and MilStop rather than just using MilStop, for example), as well as the effects of spraying these products at different intervals, not just weekly. In addition, further research is needed to establish modes of action of some of the most effective products described in this study.
Acknowledgements
We thank S. Lung, L. Ni, and D. Sutton for providing assistance.
Additional information
Funding
References
- Agrios GN. 2005. Plant pathology. 5th ed. Amsterdam: Elsevier Academic Press.
- Allan AC, Fluhr R. 1997. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 9:1559–1572.
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. 1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 92:773–784.
- Bai Y, Huang -C-C, Hulst RVD, Meijer-Dekens F, Bonnema G, Lindhout P. 2003. QTLs for tomato powdery mildew resistance (Oidium lycopersici) in Lycopersicon parviflorum G1.1601 co-localize with two qualitative powdery mildew resistance genes. Mol Plant-Microbe Inter. 16(2):169–176. doi:10.1094/MPMI.2003.16.2.169.
- Balkema-Boomstra AG, Mastebroek HD. 1995. Effect of powdery mildew (Erysiphe graminis f.sp. hordei) on photosynthesis and grain yield of partially resistant genotypes of spring barley (Hordeum vulgare L.). Plant Breed. 114:126–130.
- Bayer. 2019. Luna privilege Greenhouse. [accessed 2019 Dec 9]. https://www.environmentalscience.bayer.ca/greenhouse-and-nursery/products/luna-privilege.
- Betz EC, Punja ZK. 2020. Management of powdery mildew, caused by Erysiphe cruciferarum, on wasabi (Wasabia japonica) plants in British Columbia. Can J Plant Pathol. 42. doi:10.1080/07060661.2020.1764109
- Bonomelli A, Mercier L, Franchel J, Baillieul F, Benizri E, Mauro MC. 2004. Response of grapevine defenses to UV-C exposure. Am J Enol Vitic. 55:51–59.
- Bowen P, Menzies J, Ehret D, Samuels L, Glass A. 1992. Soluble silicon sprays inhibit powdery mildew development on grape leaves. J Am Soc Hort Sci. 117:906–912.
- Cala A, Portilla N, Weldon B, Gura W, King N, Stack G, Toth J, Carlson C, Gadoury D, Sapkota S, et al. 2019. Evaluating disease resistance in CBD hemp cultivars. Cornell Hemp Field Day 2019. Handout. [accessed 2019 Oct 20]. https://hemp.cals.cornell.edu/hemp-resources/our-research/.
- Cao A, Xing L, Wang X, Yang X, Wang W, Sun Y, Qian C, Ni J, Chen Y, Liu D, et al. 2011. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc Nat Acad Sci USA. 108:7727–7732.
- Conn VM, Walker AR, Franco CM. 2008. Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol Plant-Microbe Inter. 21:208–218.
- Daayf F, Ongena M, Boulanger R, Hadrami IE, Bélanger RR. 2000. Induction of phenolic compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extracts of Reynoutria sachalinensis. J Chem Ecol. 26:1579–1593.
- Daayf F, Schmitt A, Belanger RR. 1997. Evidence of phytoalexins in cucumber leaves infected with powdery mildew following treatment with leaf extracts of Reynoutria sachalinensis. Plant Physiol. 113:719–727.
- Degering C, Eggert T, Puls M, Bongaerts J, Evers S, Maurer K-H, Jaeger K-E. 2010. Optimization of protease secretion in Bacillus subtilis and Bacillus licheniformis by screening of homologous and heterologous signal peptides. App Environ Microbiol. 76:6370–6376.
- Deora A, Gossen BD, Walley F, McDonald MR. 2011. Boron reduces development of clubroot in canola. Can J Plant Pathol. 33:475–484.
- Deshmukh NJ, Deokar CD, Deshmukh S. 2018. Effect of bio-control agents against powdery mildew of pea caused by Erysiphe polygoni DC. Agrica. 7:114–120.
- Douillet-Breuil AC, Jeandet P, Adrian M, Bessis R. 1999. Changes in the phytoalexin content of various Vitis spp. in response to ultraviolet C elicitation. J Agric Food Chem. 47:4456–4461.
- Elmhirst JF, Haselhan C, Punja ZK. 2011. Evaluation of biological control agents for control of botrytis blight of geranium and powdery mildew of rose. Can J Plant Pathol. 33:499–505.
- Fofana B, McNally D, Labbé C, Boulanger R, Benhamou N, Séguin A, Bélanger R. 2002. Milsana-induced resistance in powdery mildew-infected cucumber plants correlates with the induction of chalcone synthase and chalcone isomerase. Physiol Mol Plant Pathol. 61:121–132.
- Frenkel O, Yermiyahu U, Forbes GA, Fry WE, Shtienberg D. 2010. Restriction of potato and tomato late blight development by sub-phytotoxic concentrations of boron. Plant Pathol. 59:626–633.
- Gadoury DM. 2019. The potential of light treatments to suppress certain plant pathogens and pests. Research Focus Cornell Univ. Viticulture Enol. 2:1–7.
- Geetha HM, Shetty HS. 2002. Induction of resistance in pearl millet against downy mildew disease caused by Sclerospora graminicola using benzothiadiazole, calcium chloride and hydrogen peroxide—a comparative evaluation. Crop Prot. 21:601–610.
- Gilardi G, Manker DC, Garibaldi A, Gullino ML. 2008. Efficacy of the biocontrol agents Bacillus subtilis and Ampelomyces quisqualis applied in combination with fungicides against powdery mildew of zucchini. J Plant Dis Prot. 115:208–213.
- Gordon TR, Duniway JM. 1982. Effects of powdery mildew infection on the efficiency of CO2 fixation and light utilization by sugar beet leaves. Plant Physiol. 69:139–142.
- Govindachari TR, Suresh G, Gopalakrishnan G, Banumathy B, Masilamani S. 1998. Identification of antifungal compounds from the seed oil of Azadirachta indica. Phytoparasitica. 26:109–116.
- Gwinn KD, Bernard EC, Hansen ZR, Boggess S, Chaffin A, Trigiano RN 2019. Powdery mildew in field-grown hemp. In: Proceedings of the 1st Annual Scientific Conference of the Science of Hemp: Production and Pest Management, October 10 11 Lexington, Kentucky: Poster.
- Hafez YM. 2010. Control of Botrytis cinerea by the resistance inducers benzothiadiazole (BTH) and hydrogen peroxide on white pepper fruits under postharvest storage. Acta Phytopathol Et Entomol Hung. 45:13–29.
- Hafez YM, Bayoumi YA, Pap Z, Kappel N. 2008. Role of hydrogen peroxide and Pharmaplant-turbo against cucumber powdery mildew fungus under organic and inorganic production. Int J Hortic Sci. 14:39–44.
- Health Canada. 2019. Pest control products for use on cannabis. [accessed 2019 Sept 27]. https://www.canada.ca/en/health-canada/services/cannabis-regulations-licensed-producers/pest-control-products.html.
- Hijnen W, Beerendonk EF, Medema GJ. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 40:3–22.
- Huang CC, Cui -Y-Y, Weng C, Zabel P, Lindhout P. 2000. Development of diagnostic PCR markers closely linked to the tomato powdery mildew resistance gene Ol-1 on chromose 6 of tomato. Theor Appl Genet. 101:918–924.
- Hückelhoven R, Kogel K-H. 2003. Reactive oxygen intermediates in plant-microbe interactions: who is who in powdery mildew resistance? Planta. 216:891–902.
- Jamar L, Lefrancq B, Fassotte C, Lateur M. 2008. A during-infection spray strategy using sulphur compounds, copper, silicon and a new formulation of potassium bicarbonate for primary scab control in organic apple production. Eur J Plant Pathol. 122:481–493.
- Janousek CN, Bay IS, Gubler WD. 2009. Control of grape powdery mildew with synthetic, biological, and organic fungicides: 2009 field trials. UC Davis: Department of Plant Pathology. [accessed 2019 Sept 30]. https://escholarship.org/uc/item/8fz3p4vc.
- Keinath AP, DuBose VB. 2004. Evaluation of fungicides for prevention and management of powdery mildew on watermelon. Crop Prot. 23:35–42.
- Kloepper JW, Ryu C-M ZS. 2004. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 94:1259–1266.
- Konstantinidou-Doltsinis S, Markellou E, Kasselaki A-M, Fanouraki MN, Koumaki CM, Schmitt A, Liopa-Tsakalidis A, Malathrakis NE. 2006. Efficacy of Milsana®, a formulated plant extract from Reynoutria sachalinensis, against powdery mildew of tomato (Leveillula taurica). Biocontrol. 51:375–392.
- Kuźniak E, Urbanek H. 2000. The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol Plant. 22:195–203.
- Lahlali R, Peng G, Gossen BD, McGregor L, Yu FQ, Hynes RK, Hwang SF, McDonald MR, Boyetchko SM. 2013. Evidence that the biofungicide Serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology. 103:245–254.
- Liu J, Stevens C, Khan VA, Lu JY, Wilson CL, Adeyeye O, Kabwe MK, Pusey PL, Chalutz E, Sultana T, et al. 1993. Application of ultraviolet-C light on storage rots and ripening of tomatoes. J Food Prot. 56:868–873.
- McNally DJ, Wurms KV, Labbé C, Bélanger RR. 2003. Synthesis of C-glycosyl flavonoid phytoalexins as a site-specific response to fungal penetration in cucumber. Physiol Mol Plant Pathol. 63:293–303.
- Menzies JG, Ehret DL, Glass ADM, Samuels AL. 1991. The influence of silicon on cytological interactions between Sphaerotheca fuliginea and Cucumis sativus. Physiol Mol Plant Pathol. 39:403–414.
- M-H G, Menzies JG, Bélanger RR. 2007. Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants. Eur J Plant Pathol. 119:429–436.
- Mitre V, Buta E, Lukács L, Mitre I, Teodorescu R, Hoza D, Sestraş AF, Stănică F. 2018. Management of apple scab and powdery mildew using bicarbonate salts and other alternative organic products with fungicide effect in apple cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 46:115–121.
- Moharam MH, Obiadalla Ali HA. 2012. Preventative and curative effects of several plant derived agents against powdery mildew disease of okra. Notulae Scientia Biologicae. 4:76–82.
- Moyer C, Peres NA 2008. Evaluation of biofungicides for control of powdery mildew of gerbera daisy. In: Proceedings Florida State Horticultural Society, 2008 Jun 10–12 Fort Lauderdale, Florida, vol. 121: 389–394.
- Ni L, Punja ZK. 2019. Management of fungal diseases on cucumber (Cucumis sativus L.) and tomato (Solanum lycopersicum L.) crops in greenhouses using Bacillus subtilis. In: Islam M, Rahman M, Pandey P, Boehme M, Haesaert G, editors. Bacilli and agrobiotechnology: phytostimulation and biocontrol. Bacilli in climate resilient agriculture and bioprospecting (Vol. 2). Cham: Springer; p. 1–28.
- Ni L, Punja ZK. 2020a. Effects of a foliar fertilizer containing boron on development of Sclerotinia stem rot (Sclerotinia sclerotiorum) on canola (Brassica napus L.) leaves. J Phytopathol. 168:47–55.
- Ni L, Punja ZK. 2020b. Management of powdery mildew on greenhouse cucumber (Cucumis sativus L.) plants using biological and chemical approaches. Can J Plant Pathol. 42. doi:10.1080/07060661.2020.1746694
- Nigro F, Ippolito A, Lattanzio V, Di Venere D, Salerno M. 2000. Effect of ultraviolet-C light on postharvest decay of strawberry. J Plant Pathol. 82:29–37.
- Ning X, Wang X, Gao X, Zhang Z, Zhang L, Yan W, Li G. 2014. Inheritances and localization of powdery mildew resistance gene in melon Edisto47. Euphytica. 195:345–353.
- Ocamb CM, Pscheidt JW. 2019. Hemp (Cannabis sativa)-Powdery Mildew. Pacific Northwest plant disease management handbook. A Pacific Northwest Extension Publication. [accessed 2020 Sept 15]. https://pnwhandbooks.org/plantdisease/host-disease/hemp-cannabis-sativa-powdery-mildew.
- Ongena M, Duby F, Jourdan E, Beaudry T, Jadin V, Dommes J, Thonart P. 2005. Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl Microbiol Biotech. 67:692–698.
- Pasini C, D’Aquila F, Curir P, Gullino ML. 1997. Effectiveness of antifungal compounds against rose powdery mildew (Sphaerotheca pannosa var. rosae) in glasshouses. Crop Prot. 16:251–256.
- Patil NB, Zacharia S, Kumari M. 2017. Eco-friendly management of powdery mildew of garden pea (Pisum sativum L.). Inter J Current Microbiol Appl Sci. 6:684–689.
- Pépin N, Punja ZK, Joly DL. 2018. Occurrence of powdery mildew caused by Golovinomyces cichoracearum sensu lato on Cannabis sativa in Canada. Plant Dis. 102:2644.
- Pérez-García A, Romero D, de Vicente A. 2011. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotech. 22:187–193.
- Proffer TJ, Lizotte E, Rothwell NL, Sundin GW. 2012. Evaluation of dodine, fluopyram and penthiopyrad for the management of leaf spot and powdery mildew of tart cherry, and fungicide sensitivity screening of Michigan populations of Blumeriella jaapii. Pest Manag Sci. 69:747–754.
- Punja ZK. 2018. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40:514–527.
- Punja ZK, Collyer D, Scott C, Lung S, Holmes J, Sutton D. 2019. Pathogens and molds affecting production and quality of Cannabis sativa L. Front Plant Sci. doi:10.3389/fpls.2019.01120.
- Qiu W, Feechan A, Dry I. 2015. Current understanding of grapevine defense mechanisms against the biotrophic fungus (Erysiphe necator), the causal agent of powdery mildew disease. Hortic Res. 2: 1–9. Article number: 15020.
- Romero D, De Vicente A, Zeriouh H, Cazorla FM, Fernandez-Ortuno, Torés JA, Pérez-Garcia. 2007. Evaluation of biological control agents for managing cucurbit powdery mildew on greenhouse-grown melon. Plant Pathol. 56:976–986.
- Rur M, Rämert B, Hökeberg M, Vetukuri RR, Grenville-Briggs L, Liljeroth E. 2018. Screening of alternative products for integrated pest management of cucurbit powdery mildew in Sweden. Eur J Plant Pathol. 150:127–138.
- Sakata Y, Kubo N, Morishita M, Kitadani E, Sugiyama M, Hirai M. 2006. QTL analysis of powdery mildew resistance in cucumber (Cucumis sativus L.). Theor Appl Genet. 112:243–250.
- Samuels AL, Glass ADM, Ehret DL, Menzies JG. 1991. Distribution of silicon in cucumber leaves during infection by the powdery mildew fungus (Sphaerotheca fuliginea). Can J Bot. 69:140–146.
- Schneider S, Ullrich WR. 1994. Differential induction of resistance and enhanced enzyme activities in cucumber and tobacco caused by treatment with various abiotic and biotic inducers. Physiol Mol Plant Pathol. 45:291–304.
- Schoener J, Wang S 2018. First detection of Golovinomyces ambrosiae causing powdery mildew on medical marijuana plants in Nevada. In: International Congress of Plant Pathology, 2018 August 1. Boston, USA. Oral Presentation.
- Schuerger AC, Hammer W. 2003. Suppression of powdery mildew on greenhouse-grown cucumber by addition of silicon to hydroponic nutrient solution is inhibited at high temperature. Plant Dis. 87:177–185.
- Shetty R, Jensen B, Shetty NP, Hansen M, Hansen CW, Starkey KR, Jørgensen HJ. 2011. Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa. Plant Pathol. 61:120–131.
- Shoda M. 2000. Bacterial control of plant diseases. J Biosci Bioeng. 89:515–521.
- Siahmoshteh F, Hamidi-Esfahani Z, Spadaro D, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. 2018. Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Contr. 89:300–307.
- Siddiqui A, Dawar S, Zaki MJ, Hamid N. 2011. Role of ultraviolet (UV-C) radiation in the control of root infecting fungi on groundnut and mung bean. Pak J Bot. 43:2221–2224.
- Simko I, Piepho HP. 2012. The area under the disease progress stairs: calculation, advantage, and application. Phytopathology. 102:381–389.
- Simoglou KB, Dordas C. 2006. Effect of foliar applied boron, manganese and zinc on tan spot in winter durum wheat. Crop Prot. 25:657–663.
- Stevens C, Khan VA, Lu JY, Wilson CL, Chalutz E, Droby S, Kabwe MK, Haung Z, Adeyeye O, Pusey LP, et al. 1999. Induced resistance of sweet potato to Fusarium root rot by UV-C hormesis. Crop Prot. 18:463–470.
- Sumanta N, Haque CI, Nishika J, Suprakash R. 2014. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species using various extracting solvents. Res J Chem Sci. 4:63–69.
- Suthaparan A, Solhaug KA, Stensvand A, Gislerød HR. 2016. Determination of UV action spectra affecting the infection process of Oidium neolycopersici, the cause of tomato powdery mildew. J Photochem Photobiol B. 156:41–49.
- Suthaparan A, Stensvand A, Solhaug KA, Torre S, Mortensen LM, Gadoury D, Gislerød HR. 2012. Suppression of powdery mildew (Podosphaera pannosa) in greenhouse roses by brief exposure to supplemental UV-B radiation. Plant Dis. 96:1653–1660. doi:10.1094/PDIS-01-12-0094-RE.
- Suthaparan A, Stensvand A, Solhaug KA, Torre S, Telfer KH, Ruud AK, Mortensen LM, Gadoury DM, Seem RC, Gislerød HR. 2014. Suppression of cucumber powdery mildew by supplemental UV-B radiation in greenhouses can be augmented or reduced by background radiation quality. Plant Dis. 98:1349–1357.
- Szarka D, Tymon L, Amsden B, Dixon E, Judy J, Gauthier N. 2019. First report of powdery mildew caused by Golovinomyces spadiceus on industrial hemp (Cannabis sativa) in Kentucky. Plant Dis. 103:7. doi:10.1094/PDIS-01-19-0049-PDN.
- Takami H, Akiba T, Horikoshi K. 1989. Production of extremely thermostable alkaline protease from Bacillus sp. no. AH-101. Appl Microbiol Biotech. 30:120–124.
- Veloukas T, Karaoglanidis GS. 2012. Biological activity of the succinate dehydrogenase inhibitor fluopyram against Botrytis cinerea and fungal baseline sensitivity. Pest Manag Sci. 68:858–864.
- Vitale A, Panebianco A, Polizzi G. 2016. Baseline sensitivity and efficacy of fluopyram against Botrytis cinerea from table grape in Italy. Ann Appl Biol. 169:36–45.
- Vivek M, Abhilasha AL, Sobita S. 2017. Efficacy of botanicals and bio-agents against powdery mildew disease of garden pea (Pisum sativum L.). J Pharmacog Phytochem. 6:1125–1126.
- Vurukonda SSKP, Giovanardi D, Stefani E. 2018. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Inter J Mol Sci. 19:952–978.
- Weldon WA, Ullrich MR, Smart LB, Smart CD, Gadoury DM. 2020. Cross-infectivity of powdery mildew isolates originating from hemp (Cannabis sativa) and Japanese hop (Humulus japonicus) in New York. Plant Health Prog. 21:47–53.
- Wenneker M. 2016. Controlling powdery mildew (Sphaerotheca mors-uvae) of gooseberry (Ribes uva-crispa) with potassium bicarbonate and risk of phytotoxicity. Acta Horticul. 1133:515–520.
- Willocquet L, Colombet D, Rougier M, Fargues J, Clerjeau M. 1996. Effects of radiation, especially ultraviolet B, on conidial germination and mycelial growth of grape powdery mildew. Eur J Plant Pathol. 102:441–449.
- Wolfenbarger SN, Eck EB, Ocamb CM, Probst C, Nelson ME, Grove GG, Gent DH. 2014. Powdery mildew outbreaks caused by Podosphaera macularis on hop cultivars possessing the resistance gene R6 in the Pacific northwestern United States. Plant Dis. 98:852.
- Zhang S, Mersha Z, Vallad GE, Huang CH. 2016. Management of powdery mildew in squash by plant and alga extract biopesticides. Plant Pathol J. 32:528–536.
- Zhang S, Vallad GE, White TL, Huang CH. 2011. Evaluation of microbial products for management of powdery mildew on summer squash and cantaloupe in Florida. Plant Dis. 95:461–468.