Abstract
Soybean plants showing symptoms of root rot were collected from fields in Manitoba, Canada, in 2017, to determine the causal agent of the disease. Of 240 isolated strains, including some known as root rot pathogens, five were identified as Fusarium sporotrichioides Sherb. based on morphological characteristics. All five isolates were pathogenic when inoculated on soybean under controlled conditions, and resulted in typical root rot symptoms. In addition, shoot length, root length, and fresh weight were significantly reduced. A PCR-based diagnostic test was developed, which specifically amplified the Trichothecene gene cluster in F. sporotrichioides. The primer SPO1 amplified a 541 bp fragment in fungal isolates originally isolated from commercial soybean crops, and from artificially infected roots of soybean. Trichothecene (TRI) gene expression was detected in infected soybean roots using RT-PCR analysis with TRI gene-specific primers. This is the first report of F. sporotrichioides as one of causal agents of root rot in soybean in Canada.
Résumé
En 2017, des plants de soya montrant des symptômes du pourridié ont été collectés dans des champs manitobains, au Canada, afin de déterminer l’agent causal de la maladie. Parmi 240 souches isolées, y compris certaines connues comme agents pathogènes provoquant le pourridié, 5 ont été identifiées en tant que Fusarium sporotrichioides Sherb. en se basant sur leurs caractéristiques moléculaires. Les cinq isolats étaient pathogènes suite à leur inoculation au soya dans des conditions contrôlées, et ont provoqué les symptômes typiques du pourridié. De plus, la longueur des pousses, la longueur des racines et le poids à l’état frais ont été significativement réduits. Un test de diagnostic, basé sur la PCR, qui amplifie spécifiquement le groupe de gènes codant la synthèse de trichothécènes chez F. sporotrichioides, a été mis au point. L’amorce SPO1 a amplifié un fragment de 541 bp d’isolats fongiques isolés originalement à partir de cultures commerciales de soya ainsi que de racines infectées artificiellement. L’expression des gènes trichothécène (TRI) a été détectée par RT-PCR avec des amorces spécifiques des gènes TRI dans des racines de soya infectées. Il s’agit de la première mention de F. sporotrichioides en tant qu’agent causal du pourridié chez le soya au Canada.
Introduction
Root rot disease is a major threat to agriculture worldwide causing reduced yield of several crop species each year (Bodah Citation2017). Root rots remain an ongoing threat to soybean (Glycine max L.) production in Canada in absence of efficient management strategies (Chang et al. Citation2015). In particular, Fusarium is one of the most common root rot pathogens of soybean in both Canada and the United States (Wrather et al. Citation2001). The infection starts below the ground, where the first symptoms are not discernible. When the symptoms become apparent on the above ground parts of the plant, yield is compromised and plant survival is jeopardized (Bodah Citation2017). Several Fusarium species have been reported to infect soybean roots (Nelson Citation1999), including F. oxysporum and F. solani (Diaz Arias et al. Citation2013). Fusarium oxysporum can cause a range of symptoms including wilt and root rot, but pathogenicity has not always been demonstrated due to significant variation among fungal isolates (Farias and Griffin Citation1989). More recently, other species of Fusarium such as F. graminearum, F. proliferatum, F. pseudograminearum, and F. redolnes have been associated with soybean root rot on seedlings and older plants (Pioli et al. Citation2004; Xue et al. Citation2007; Bienapfl et al. Citation2010; Diaz Arias et al. Citation2011; Ellis et al. Citation2012). Fusarium sporotrichioides is one of many Fusarium species associated with fusarium head blight in cereals (Nielsen et al. Citation2014; Nazari et al. Citation2019), but few studies have reported isolation of F. sporotrichioides from soybean roots exhibiting root rot symptoms (Diaz Arias Citation2012). The objectives of this study were to use Koch’s postulates to verify pathogenicity of F. sporotrichioides on soybean, examine the virulence of isolates and to develop specific PCR-based primers to detect the pathogen in infected soybean by targeting the trichothecene gene cluster.
Material and methods
Fungal isolates
Soybean plots of cultivar 24–10RY showed poor plant stand, stunting and leaf chlorosis at two locations in Carman and Melita, Manitoba, in 2017. The root system had few nitrogen fixing nodules and sparse lateral roots with reddish brown-to-black lesions on both tap and lateral roots (). The roots and lower stems showed cortical decay symptoms when cut lengthwise. Root pieces (1–2 cm) from 48 collected plants were surface sterilized in 0.5% NaOCl for 2 minutes, rinsed twice in sterilized water, air dried on sterilized filter papers, and placed on potato dextrose agar (PDA) (Becton, Dickinson, and Company, Sparks, Maryland, United States) amended with 100-mg streptomycin sulphate. The PDA dishes were incubated for 2 days at 25 ± 2°C with a 12 h light/12 h dark cycle. Growing hyphae were transferred using the hyphal tip method to new PDA dishes and incubated under the same conditions (Leyronas et al. Citation2012). The morphology of the growing cultures was observed after 10 days. The identity of all isolates was determined by sequencing the translational elongation factor 1-alpha (TEF1) gene. TEF1 was amplified by PCR using the universal primers EF1 and EF2 (O’Donnell et al. Citation1998). The amplification process involved an initial denaturation of 2 min at 95°C, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 40 s. The final extension was at 72°C for 10 min. Amplicons were resolved by horizontal gel electrophoresis in 1.5% agarose gels in 0.5× (Tris-borate EDTA, TBE) buffer at 100 V cm−1 for 45 min. Gels were pre-stained with ethidiumbromide (0.5 μg mL−1) digitally visualized and photographed on a Gel Doc-lt™ Imaging System (UVP-LLC, Upland, California, United States).
Pathogenicity test
Koch’s postulates were used to demonstrate the pathogenicity of five isolates tentatively identified as F. sporotrichiodies (M2, M9, M30, C21, and C38) on soybean. Inoculum was prepared by infecting wheat kernels with each isolate separately following the methodology described by Luckew et al. (Citation2012). In brief, wheat kernels were soaked overnight in water, drained, placed in plastic boxes (500 gm kernels per 1 L box), and autoclaved for 1 h at 121°C on two consecutive days. After cooling, the wheat kernels in each box were inoculated with six mycelial plugs (6 mm in diameter) from 5- to 7-day-old PDA cultures of each of the five F. sporotrichiodies isolates under sterile conditions and allowed to grow at 25°C with 12 h light/12 h dark cycle for 10 days. The boxes were shaken twice after 3 days and 6 days to obtain uniform distribution of inoculum. Autoclaved wheat kernels inoculated with six sterile PDA plugs served as a control. Twenty-five grams of infested wheat kernels were mixed with 1 kg of pasteurized ready mix (Sunshine® Mix#4, Sungro, Maryland, United States) in disinfected 6 metric pots for each isolate. In control pots 25 g. non-inoculated sterilized wheat kernels were used. There were five pots per treatment and three seeds of soybean cv. 24–10RY were seeded per pot and incubated in the greenhouse under a 24/16°C day/night temperature, 13/11 h light/dark cycle and 70–80% relative humidity. Plants were watered three times per week and maintained until they reached growth stage V5. Whole plants were uprooted, the roots carefully washed, and the severity of root rot was visually rated using the scoring method of Taheri et al. (Citation2011) (). Plants were blotted with a paper towel to remove excess moisture. Shoot length (from soil line to shoot tip), root length (from soil line to root tip) and root fresh weight were measured. The pathogen was re-isolated from infected roots and identified as above. The experiment was repeated twice. The data sets were tested for homogeneity of variance using a normal probability plot and no transformation of the data was required. Analysis of variance was performed using Proc Mixed Model, and means were compared using LSD at P < 0.05.
Table 1. Disease scoring scale
DNA extraction from fungal cultures and artificially infected soybean roots
Roots from three plants infected with each of the five isolates were cut into small pieces, bulked and frozen in liquid nitrogen. Each sample was ground into a powder with a mortar and a pestle. Genomic DNA was extracted using a Qiagen DNeasy plant DNA extraction kit (Qiagen, Valencia, California, United States). Similarly, DNA was extracted from the five isolates grown on PDA and from roots of healthy soybean roots.
Development of PCR primers
The Nucleotide Sequences Search program provided by the National Centre for Biotechnology Information (NCBI) (Bethesda, Maryland, United States) (http://www.ncbi.nlm.nih.gov/Entrez) was used to retrieve several Fusarium spp. trichothecene and translational elongation factor 1 alpha (TEF1) sequences from GenBank. The sequences were subsequently aligned using the program CLUSTAL-X 2.0.12 (http://www.clustal.org/) (Thompson et al. Citation1997) and examined for conserved regions at each locus. Percentage identity matrices and nucleotide sequence alignments for specify species were generated using GeneDoc (Nicholas and Nicholas Citation1997). Species-conserved nucleotide regions were selected to design PCR primers for amplification of DNA sequences specific to F. sporotrichiodes using Primer3 program (Rozen and Skaletsky Citation2000). The specificity of each primer was confirmed in silico by screening the primer sequences with BLASTn (Altschul et al. Citation1990). Four primer pairs were designed and tested with fungal and plant genomic DNA. The PCR primers designed are listed in . To determine the species specificity of the designed primers, each primer set was tested on genomic DNA of the five isolates of F. sporotrichioides; the closely related species F. cerealis, F. poae, F. equesiti, F. graminearum, F. acuminatum, F. oxysporum, F. sambucinum, F. avenaceum, and other soil-borne pathogens, Rhizoctonia solani, Colletotrichum coccodes, Verticillium albo-atrum, and Verticillium dahliae listed in (). Assays were performed as follows. Each 25 μL PCR reaction contained 1.4 μL MgCl2 25 mM (Promega, Madison, Wisconsin, United States), 5 μL of 5× Green GoTaq Flexi Buffer (Promega), 2 μL of dNTP 10 mM (Promega), 0.16 μL of 0.5 U of Taq polymerase (Promega), 2 μL of each forward and reverse primer (10 μM; ), 1μL of DNA template (25 ng/μL), and 13.44 μL of dH2O. The amplification process involved an initial denaturation of 2 min at 95°C, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 20 s and extension at 72°C for 40 s. The final extension was at 72°C for 3 min. The specificity of the designed primers was also tested on total DNA extracted from artificially infected soybean roots following the same PCR protocol as above as well as from uninoculated roots.
Table 2. Primer codes, targets and sequences
Table 3. List of the fungal species used in the specificity test
Trichothecene gene expression
Total RNA was extracted from soybean roots inoculated individually with five F. sporotrichioides isolates and from uninoculated control plants using Qiagen RNeasy Plant Mini Kit™ (Hilden, Germany) following the manufacturer’s instructions. Subsequently 200 µg of the total RNA was used for cDNA synthesis using a RevertAid First Strand cDNA Synthesis Kit™ (ThermoScientific, United States), following the manufacturer’s instructions. RT-PCR was conducted using trichothecene gene-specific primers; Tri-F 5ʹ CAATGACGCAGACTCCACATAT 3ʹand Tri-R 5ʹ AATCGCTTTGGTCATCTGATTT 3ʹ. Several Fusarium spp. Trichothecene sequences were retrieved from (GenBank: AF359360.3) to design this set of primers using the same method indicated above. RT-PCR was performed using Dream Taq DNA Polymerase (ThermoScientific, United States) in a 25 µL reaction volume containing 100 ng cDNA as a template. The RT-PCR reactions were run on Bio-Rad C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, California, United States) as follows; initial denaturation of 2 min at 95°C, followed by 35 cycles of denaturation at 95°C for 30 s, followed by annealing at 59°C for 30 s and then extension at 72°C for 20 s. The final extension was at 72°C for 5 min. The RT-PCR products were separated on 1% agarose gel contained 0.01% ethidium bromide and bands were visualized on AlphaImager Gel Documentation System (Alpha Tec, California, United States).
Results
Identification of F. sporotrichioides
Five isolates were identified as F. sporotrichioides based on cultural and morphological characteristics using microscopic examination. Colonies were fast growing with pink-white and dense mycelia that turned red after 7 days (). Microconidia were abundant, oval or pear-shaped or spindle-shaped, thin walled, hyaline, and ranged from 5.0 μm to 12.5 μm × 2.5 μm to 4 μm. Macroconidia were moderately curved, 3 to 5 septate, hyaline, and ranged from 21.0 μm to 42.9 μm × 3.5 μm to 5.0 μm (). Morphology of colonies and conidia matched the description of F. sporotrichioides Sherb (Leslie and Summerell Citation2006). In addition, alignment of TEF1 gene sequences showed a 100% homology with sequences of several isolates of F. sporotrichioides from GenBank. TEF1 gene sequence of isolates C21, C38, M2, M9, and M30 of F. sporotrichioides were deposited in GenBank with the following accession numbers: MH299928, MH299945, MH299948, MH299952, MN699963, respectively.
Pathogenicity test
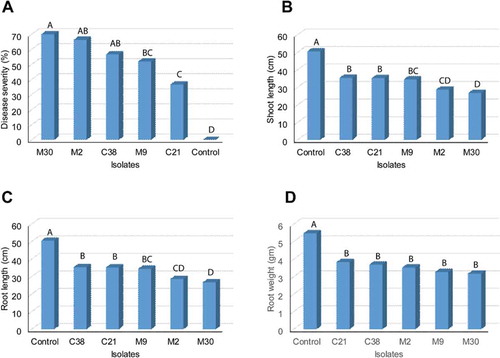
Inoculation of soybean with the five isolates of F. sporotrichiodes resulted in formation of dark brown lesions and typical root rot symptoms on the hypocotyl and main root ( and c) leading to significant reductions in the shoot and root length, and root weight as compared to the control (). Control plants were asymptomatic. Data presented in (a–d) showed there were significant differences among isolates on plant growth. Isolate M30 was more virulent than other isolates causing 70.0% root rot severity, while isolate C21 caused 36.6% root rot severity (). Similarly, isolate M30 caused the highest reduction in shoot length (26.8 cm), while isolates C38 and C21 caused the least reduction (35.4 and 35.2 cm, respectively) (). Isolates M30 and M2 caused the highest reduction in root length with values of (26.8 and 28.7 cm) (). However, there was no significant differences among isolates in root weight but all isolates reduced the root weight significantly compared to the control (). The pathogen was re-isolated from infected roots and identified as F. sporotrichiodes.
Fig. 1 (a) Symptoms of root rot caused by Fusarium sporotrichioides observed on soybean roots in commercial field in Melita, MB. (b) Non-infected soybean plants (cv. 24–10 RY) (right) and artificially infected plants showing significant reduction in shoot length (left). (c) Difference between control plants cv.24–10RY (left) and artificially infected plants (middle and right)
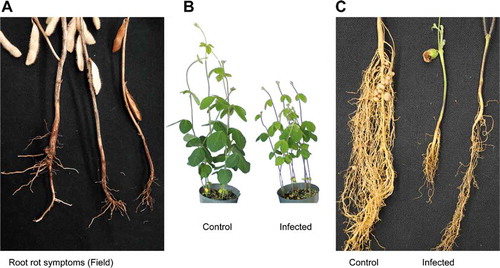
Fig. 2 (a) Three weeks old, F. sporotrichioides culture on PDA medium. (b) Conidia spores of F. sporotrichioides grown on PDA medium at 25°C for 3 weeks
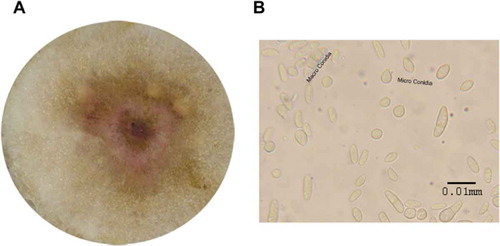
Fig. 3 (a) Percentage of root rot severity on soybean caused by five isolates of F. sporotrichioides, (b) Means of shoot length of 15 plants for each isolate of F. sporotrichioides, (c) Means of root length of 15 plants for each isolate of F. sporotrichioides, (d) Means of root weight of 15 plants for each isolate of F. sporotrichioides. Means with the same letter are not significantly different at P < 0.05 using LSD. The experiment was repeated twice
Primer specificity test
Primer SPO1 () amplified the target sequence of the trichothecene gene when tested on genomic DNA from all five F. sporotrichioides isolates in pure culture and produced amplicons size as expected 541bp (). No amplification products were obtained from 28 isolates representing closely related Fusarium spp. or other fungal species listed in . The SPO1 primer set also detected F. sporotrichioides in artificially infected soybean roots, but not in uninoculated roots ().
Fig. 4 (a) Specificity of the primer set SPO1 with gDNA isolated from pure fungal cultures listed in . (b) Specificity of SPO1 with gDNA isolated from artificially inoculated soybean roots using the five isolates of F. sporotrichioides. (c) Detection of trichothecene gene expression in infected roots using RT-PCR
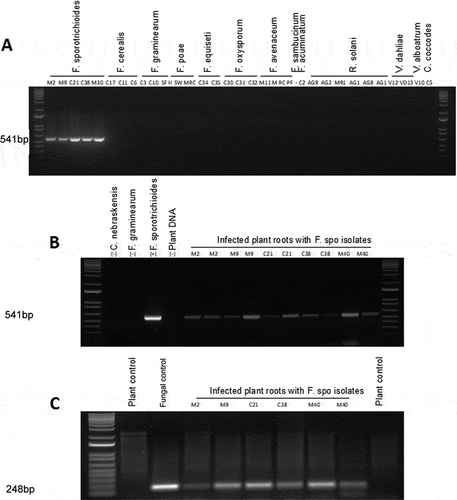
Additional verification of F. sporotrichioides was obtained with TRI gene expression in the artificially infected soybean roots using RT-PCR gene primers. The agarose gel image showed the expected band size of 248 bp for all F. sporotrichioides isolates in artificially infected roots and in a pure culture of the fungus ( and c). No amplification products were found in the uninoculated plant DNA.
Discussion
Soilborne pathogens occur worldwide and are adapted to a wide range of environmental conditions, causing serious root rots in many economical important crops (Macedo et al. Citation2017). Fusarium root rot causes substantial yield losses in soybean worldwide (Chang et al. Citation2013). In 2002, a survey was conducted to determine the casual pathogens of soybean root rot in Eastern Canada, which showed that Fusarium species represented 68% of fungal strains isolated from the infected root samples (Zhang et al. Citation2010). In 2006, soybean yield in Canada was one of the highest on record due to above average precipitation and optimal temperatures. At the same time, Fusarium root rot was one of the yield limiting diseases under these weather conditions (Wrather et al. Citation2010). In 2017, our research group conducted isolation of pathogens from soybean roots collected from two Manitoba locations: Carman and Melita and we found a predominance of Fusarium species in the infected roots (unpublished data). Five isolates of F. sporotrichioides were recovered from soybean samples collected from two locations in Manitoba showing root rot symptoms. Following Koch’s postulates, all five isolates caused obvious root rot symptoms on soybean and were successfully re-isolated. In addition, we showed differences in virulence among isolates that also caused significant reductions in shoot and root length and root weight. We designed PCR primers to specifically detect F. sporotrichioides in pure culture and in planta. Methods for use in diagnostics and detection of plant pathogens need to be quick, simple, reliable, and cost-effective (Abd-Elmagid et al. Citation2013). Several molecular methods have also been developed to detect several important plant pathogenic Fusarium spp. (Nicholson et al. Citation2003; Chandra et al. Citation2011). Many DNA regions have been used to define differences between the important pathogens within the genus Fusarium (Edel et al. Citation1997; Mach et al. Citation2004; O’Donnell et al. Citation2008, Citation2013; Ortiz et al. Citation2017), among these ITS has been for a long time considered as the universal barcode in fungi and has been intensively used to detect and identify F. sporotrichioides (Kulik et al. Citation2004; Wilson et al. Citation2004; Yli-Mattila et al. Citation2004; Jurado et al. Citation2005). The closely related species (i.e., cryptic species like F. sporotrichioides, F. poae, F. graminearum, and other members in the Fusarium sambucinum species clomps ‘FSSC’) are usually different in a few nucleotide positions at the Internal Transcribed Spacers (ITS) level, which makes it extremely difficult to differentiate between these species (O’Donnell et al. Citation2004; Starkey et al. Citation2007; Sarver et al. Citation2011; Cai et al. Citation2011). For this reason, using ITS is usually not recommended to differentiate between closely related species such as within the genus Fusarium (Kiss Citation2012). Therefore, we endeavoured to develop a primer set to specifically detect F. sporotrichioides and accurately differentiate between this important pathogen and other closely related Fusarium species (mainly members of the FSSC) involved in soybean root rots.
The SPO1 primer set developed and reported herein is accurate and can be used alone in singleplex PCR or can be incorporated in future work in a multiplex PCR with other primers that are specific for other species of the genus Fusarium, which are known to cause root rots of soybean. Fusarium sporotrichioides is widespread on both plants and in soil throughout cool temperate regions of the world (Visconti et al. Citation1985; Mateo et al. Citation2002). The pathogen is often associated with fusarium head blight (FHB) in wheat, barley and oats (Xu et al. Citation2008; Nielsen et al. Citation2011). FHB is an economically important fungal disease in many cereal crops including wheat, barley, and oats (Parry et al. Citation1995; Amarasinghe et al. Citation2015). Fusarium sporotrichioides Sherb. was one of several other Fusarium spp. including F. acuminatum Ellis and Everhart, F. avenaceum (Corda: Fr.) Sacc., F. culmorum (W.G. Smith) Sacc., F. equiseti (Corda) Sacc., F. graminearum Schwabe (teleomorph Gibberella zeae (Schwein.) Petch.) and F. poae (Peck) Wollenw that have been frequently isolated from cereals infected by Fusarium spp. (Clear et al. Citation1996; Tekauz et al. Citation2011; Xue et al. Citation2015). Many Fusarium species produce mycotoxins that can contaminate the harvested grains (Amarasinghe et al. Citation2015). Fusarium sporotrichioides produces type A trichothecenes such as T-2 toxin, HT-2 toxin, neosolaniol, and diacetoxyscirpenol (Beardall and Miller Citation1994; Mateo et al. Citation2002). At least two serious events of mycotoxicoses have been associated with the consumption of food infected with Fusarium spp. The first was the Alimentary Toxic Aleukia that occurred in Orenberg Oblast USSR during World War II (Joffe and Yagen Citation1977; Mateo et al. Citation2002) and the other one was the bean-hull poisoning of horses in Japan (Ueno et al. Citation1972; Mateo et al. Citation2002). Another toxin producer is F. cerealis, which has been associated with FHB in winter wheat in Manitoba (Amarasinghe et al. Citation2015), Japan (Sugiura et al. Citation1994), and in wheat fields in the eastern United States (Schmale et al. Citation2011). In 2018, Abdelmagid et al. (Citation2018) reported for the first time that F. cerealis causes root rot on soybean. With both F. sporotrichioides (this study) and F. cerealis (Abdelmagid et al. Citation2018) being able to cause root rots of soybean, cultivation of soybean following wheat in fields with a history of FHB may increase the risk of soybean root rots. Analyses for Fusarium detection in soils and/or plants grown in these fields can also complement proactive measures to protect the crops. However, methods to selectively and accurately detect more of the closely related pathogens in the Fusarium group are needed to identify species with higher risks for both soybeans and cereal crops. In a recent study, we developed a very specific tool to discriminate F. graminearum sensu stricto from other Fusarium species that cause FHB (Hafez et al. Citation2020), which will help achieve such a goal.
Our findings will have an impact on future research investigating the ability of these Fusarium species to produce mycotoxins in infected soybeans, the prevalence of F. sporotrichioides and F. cerealis in soybean fields, and further interaction with other species to form root rot pathogen complexes. To our knowledge, this is the first time in Canada where F. sporotrichioides has been proven to cause root rot of soybean.
Acknowledgements
We would like to thank: Manitoba Pulse and Soybean Growers (MPSG); Western Grains Research Foundation; The Canadian Agricultural Partnership; Ag Action Manitoba Research and Innovation: Grain Innovation Hub; the Manitoba Wheat and Barley Growers Association; and MITACS for their financial support.
Additional information
Funding
References
- Abd-Elmagid A, Garrido PA, Hunger R, Lyles JL, Mansfield MA, Gugino BK, Smith DL, Melouk HA, Garzón CD. 2013. Discriminatory simplex and multiplex PCR for four species of the genus Sclerotinia. J Microbiol Methods. 92:293–300. doi:10.1016/j.mimet.2012.12.020.
- Abdelmagid A, Hafez M, Lawley Y, Adam LR, Daayf F. 2018. First report of Fusarium cerealis causing root rot on soybean. Plant Dis. 102:1225. doi:10.1094/PDIS-04-18-0556-PDN.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215:403–410. doi:10.1016/S0022-2836(05)80360-2.
- Amarasinghe CC, Tittlemierb SA, Fernandoa WGD. 2015. Nivalenol-producing Fusarium cerealis associated with Fusarium head blight in winter wheat in Manitoba, Canada. Plant Pathol. 64:988–995.
- Beardall JM, Miller JD. 1994. Diseases in humans with mycotoxins as possible causes. In: Miller JD, Trenholm HL, editors. Mycotoxins in grain. Compounds other than aflatoxin. St. Paul: Eagan Press; p. 487–539.
- Bienapfl JC, Malvick DK, Percich JA 2010. First report of Fusarium redolens causing root rot of soybean in Minnesota. Plant Dis. 94:1069.
- Bodah ET. 2017. Root rot diseases in plants: A review of common causal agents and management strategies. Agr Res & Tech. 5:55661. doi:10.19080/ARTOAJ.2017.04.555661.
- Cai L, Giraud T, Zhang N, Begerow D, Cai G, Shivas RG. 2011. The evolution of species concepts and species recognition criteria in plant pathogenic fungi. Fungal Divers. 50:121–133. doi:10.1007/s13225-011-0127-8.
- Chandra NS, Wulff EG, Udayashankar AC, Nandini BP, Niranjana SR, Mortensen CN, Prakash HS. 2011. Prospects of molecular markers in Fusarium species diversity. Appl Microbiol Biot. 90:1625–1639. doi:10.1007/s00253-011-3209-3.
- Chang KF, Hwang SF, Ahmed H, Gossen BD, Turnbull GD, Strelkov SE. 2013. Management strategies to reduce losses caused by fusarium seedling blight of field pea. Can J Plant Sci. 93:619–625. doi:10.4141/cjps2012-293.
- Chang KF, Hwang SF, Conner RL, Ahmed HU, Zhou Q, Turnbull GD, Strelkov SE, McLaren DL, Gossen BD. 2015. First report of Fusarium proliferatum causing root rot in soybean (Glycine max L.) in Canada. Crop Prot. 67:52–58. doi:10.1016/j.cropro.2014.09.020.
- Clear RM, Patrick SK, Platford RG, Desjardins M. 1996. Occurrence and distribution of Fusarium species in barley and oat seed from Manitoba in 1993 and 1994. Can J Plant Pathol. 18:409–414. doi:10.1080/07060669609500596.
- Diaz Arias MM 2012. Fusarium species infecting soybean roots: frequency, aggressiveness, yield impact and interaction with the soybean cyst nematode [Doctoral thesis]. Iowa State University Digital Repository. https://lib.dr.iastate.edu/etd/12314/.
- Diaz Arias MM, Leandro LF, Munkvold GP. 2013. Aggressiveness of Fusarium species and impact of root infection on growth and yield of soybeans. Phytopathology. 103:822–832. doi:10.1094/PHYTO-08-12-0207-R.
- Diaz Arias MM, Munkvold GP, Leandro LF. 2011. First report of Fusarium proliferatum causing root rot on soybean (Glycine max) in the United States. Plant Dis. 95:1316. doi:10.1094/PDIS-04-11-0346.
- Edel V, Steinberg C, Gautheron N, Alabouvette C. 1997. Evaluation of restriction analysis of polymerase chain reaction (PCR)-amplified ribosomal DNA for the identification of Fusarium species. Mycol Res. 101:179–187. doi:10.1017/S0953756296002201.
- Ellis ML, Díaz Arias MM, Leandro LF, Munkvold GP 2012. First report of Fusarium armeniacum causing seed root on soybean (Glycine max) in the United States. Plant Dis. 96:1693.
- Farias GM, Griffin GJ. 1989. Roles of Fusarium oxysporum and Fusarium solani in Essex disease of soybean in Virginia. Plant Dis. 73:38–42. doi:10.1094/PD-73-0038.
- Hafez M, Abdelmagid A, Adam LR, Daayf F. 2020. Specific detection and identification of Fusarium graminearum sensu stricto using a PCR-RFLP tool and specific primers targeting the translational elongation factor 1 alpha gene. Plant Dis. 104:1076–1086. doi:10.1094/PDIS-03-19-0572-RE.
- Joffe AZ, Yagen B. 1977. Comparative study of the yield of T-2 toxic produced by Fusarium poae, F. sporotrichioides and F. sporotrichioides var. tricinctum strains from different sources. Mycopathologia. 60:93–97. doi:10.1007/BF00490378.
- Jurado M, Vázquez C, Patiño B, González-Jaén MT. 2005. PCR detection assays for the trichothecene-producing species Fusarium graminearum, Fusarium culmorum, Fusarium poae, Fusarium equiseti and Fusarium sporotrichioides. Syst Appl Microbiol. 28:562–568. doi:10.1016/j.syapm.2005.02.003.
- Kiss L. 2012. Limits of nuclear ribosomal DNA internal transcribed spacer (ITS) sequences as species barcodes for Fungi. P Natl A Sci. 109:E1811. doi:10.1073/pnas.1207143109.
- Kulik T, Fordoński G, Pszczółkowska A, Płodzień K, apiński M. 2004. Development of PCR assay based on ITS2 rDNA polymorphism for the detection and differentiation of Fusarium sporotrichioides. FEMS Microbiol Lett. 239:181–186. doi:10.1016/j.femsle.2004.08.037.
- Leslie JF, Summerell BA, Editors. 2006. The Fusarium laboratory manual. Hoboken: Blackwell Publishing Professional; p. 256–257.
- Leyronas C, Duffaud M, Nicot PC. 2012. Compared efficiency of the isolation methods for Botrytis cinerea. Exp Mycol. 3:221–225.
- Luckew AS, Cianzio SR, Leandro LF. 2012. Screening method for distinguishing soybean resistance to Fusarium virguliforme in resistant x resistant crosses. Crop Sci. 52:2215–2223. doi:10.2135/cropsci2011.09.0500.
- Macedo R, Sales LP, Yoshida F, Silva-Abud LL, Lobo MJ. 2017. Potential worldwide distribution of Fusarium dry root rot incommon beans based on the optimal environment for disease occurrence. PLoS ONE. 12(11):e0187770. doi:10.1371/journal.pone.0187770.
- Mach RL, Kullnig-Gradinger CM, Farnleitner AH, Reischer G, Adler A, Kubicek CP. 2004. Specific detection of Fusarium langsethiae and related species by DGGE and ARMS-PCR of a β-tubulin (tub1) gene fragment. Int J Food Microbiol. 95:333–339. doi:10.1016/j.ijfoodmicro.2003.12.011.
- Mateo JJ, Mateo R, Jime´ne M. 2002. Accumulation of type A trichothecenes in maize, wheat and rice by Fusarium sporotrichioides isolates under diverse culture conditions. Int J Food Microbiol. 72:115–123. doi:10.1016/S0168-1605(01)00625-0.
- Nazari L, Pattori E, Somma S, Manstretta V, Waalwijk C, Moretti A, Meca G, Rossi V. 2019. Infection incidence, kernel colonisation, and mycotoxin accumulation in durum wheat inoculated with Fusarium sporotrichioides, F. langsethiae or F. poae at different growth stages. Eur J Plant Pathol. 153:715–729. doi:10.1007/s10658-018-1558-9.
- Nelson BD. 1999. Fusarium blight or wilt, root rot, and pod and collar rot. In: Sinclair JB, Backman PA, Agricultural Experiment Station, editors. Compendium of Soybean Diseases. 3rd ed. St. Paul: American Phytopathological Society; p. 35–36.
- Nicholas KB, Nicholas HB Jr. 1997. GeneDoc: a tool for editing and annotating multiple sequence alignments. www.psc.edu/biomed/genedoc.
- Nicholson P, Chandler E, Draeger RC, Gosman NE, Simpson DR, Thomsett M, Wilson AH. 2003. Molecular tools to study epidemiology and toxicology of Fusarium head blight of cereals. Eur J Plant Pathol. 109:691–703. doi:10.1023/A:1026026307430.
- Nielsen LK, Cook DJ, Edwards SG, Ray RV. 2014. The prevalence and impact of Fusarium head blight pathogens and mycotoxins on malting barley quality in UK. Int J Food Microbiol. 179:38–49. doi:10.1016/j.ijfoodmicro.2014.03.023.
- Nielsen LK, Jensen JD, Nielsen GC, Jensen JE, Spliid NH, Thomsen IK, Justesen AF, Collinge DB, Jørgensen LN. 2011. Fusarium head blight of cereals in Denmark: species complex and related mycotoxins. Phytopathology. 101:960–969. doi:10.1094/PHYTO-07-10-0188.
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. PNAS USA. 95:2044–2049. doi:10.1073/pnas.95.5.2044.
- O’Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, Ward TJ, Frandsen RJN, Lysøe E, Rehner SA, Aoki T, et al. 2013. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet Biol. 52:20–31. doi:10.1016/j.fgb.2012.12.004.
- O’Donnell K, Ward TJ, Aberra D, Kistler HC, Aoki T, Orwig N, Klemsdal SS. 2008. Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genet Biol. 45:1514–1522. doi:10.1016/j.fgb.2008.09.002.
- O’Donnell K, Ward TJ, Geiser DM, Kistler HC, Aoki T. 2004. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet Biol. 41:600–623. doi:10.1016/j.fgb.2004.03.003.
- Ortiz CS, Bell AA, Magill CW, Liu J. 2017. Specific PCR detection of Fusarium oxysporum f. sp. vasinfectum California race 4 based on a unique Tfo1 insertion event in the PHO gene. Plant Dis. 101:34–44. doi:10.1094/PDIS-03-16-0332-RE.
- Parry DW, Jenkinson P, McLeod L. 1995. Fusarium ear blight scab in small grain cereals — a review. Plant Pathol. 44:207–238.
- Pioli RN, Mozzoni L, Morandi EN 2004. First report of pathogenic association between Fusarium graminearum and soybean. Plant Dis. 88:220.
- Rozen S, Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: methods in molecular biology. Totowa: Humana Press; p. 365–386.
- Sarver BA, Ward TJ, Gale LR, Broz K, Kistler HC, Aoki T, Nicholson P, Carter J, O’Donnell K. 2011. Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance. Fungal Genet Biol. 48:1096–1107. doi:10.1016/j.fgb.2011.09.002.
- Schmale DG, Wood-Jones AK, Cowger C, Bergstrom GC, Arellano C. 2011. Trichothecene genotypes of Gibberella zeae from winter wheat fields in the eastern USA. Plant Pathol. 60:909–917. doi:10.1111/j.1365-3059.2011.02443.x.
- Starkey DE, Ward TJ, Aoki T, Gale LR, Kistler HC, Geiser DM, Suga H, Tóth B, Varga J, O’Donnell K, et al. 2007. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet Biol. 44:1191–1204. doi:10.1016/j.fgb.2007.03.001.
- Sugiura Y, Saito H, Tanaka T, Tanaka T, Ichinoe M, Ueno Y. 1994. Fusarium crookwellense, a newly isolated fungus from wheat in Japan: its mycotoxin production and pathogenicity to wheat and barley. Mycoscience. 35:77–82.
- Taheri AE, Hamel C, Gan Y, Vujanovic V 2011. First report of Fusarium redolens from Saskatchewan and its comparative pathogenicity. Can J Plant Pathol. 33:559–564.
- Tekauz A, Gilbert J, Stulzer M, Beyene M, Kleiber F, Ghazvini H, Kaethler R, Hajipour Z. 2011. Monitoring Fusarium head blight of oat in Manitoba in 2010. Can Plant Dis Surv. 91:84–85.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-–4882. doi:10.1093/nar/25.24.4876.
- Ueno Y, Ishii K, Sakai K, Kanaeda S, Tsunoda H, Tanaka T, Enomoto M. 1972. Toxicological approaches to the metabolites of Fusaria. IV. Microbial examination on “Bean-hulls poisoning of horses” with the isolation of toxic trichothecenes, neosolaniol and T-2 toxin, of Fusarium solani M-1-1. Jap J Exp Med. 42:187–203.
- Visconti A, Mirocha CJ, Bottalico A, Chelkowsky J. 1985. Trichothecene mycotoxins produced by Fusarium sporotrichioides strain P-11. Mycotoxin Res. 1:3–10. doi:10.1007/BF03191948.
- Wilson A, Simpson D, Chandler E, Jennings P, Nicholson P. 2004. Development of PCR assays for the detection and differentiation of Fusarium sporotrichioides and Fusarium langsethiae. FEMS Microbiol Lett. 233:69–76. doi:10.1016/j.femsle.2004.01.040.
- Wrather A, Shannon G, Balardin R, Carregal L, Escobar R, Gupta GK, Ma Z, Morel W, Ploper D, Tenuta A, et al. 2010. Effect of diseases on soybean yield in the top eight producing countries in 2006. 11. Online. Plant Health Prog. 11:29. doi:10.1094/PHP-2010-0125-01-RS.
- Wrather JA, Stienstra WC, Koenning SR. 2001. Soybean disease loss estimates for the United States from 1996 to 1998. Can J Plant Pathol. 23:122–131. doi:10.1080/07060660109506919.
- Xu XM, Nicholson P, Thomsett MA, Simpson D, Cooke BM, Doohan FM, Brennan J, Monaghan S, Moretti A, Mule G, et al. 2008. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology. 98:69–78. doi:10.1094/PHYTO-98-1-0069.
- Xue AG, Chen Y, Marchand G, Guo W, Ren C, Savard M, McElroy ARB. 2015. Timing of inoculation and Fusarium species affect the severity of Fusarium head blight on oat. Can J Plant Sci. 95:517–524. doi:10.4141/cjps-2014-300.
- Xue AG, Cober E, Voldeng HD, Babcock C, Clear RM. 2007. Evaluation of pathogenicity of Fusarium graminearum and Fusarium pseudograminearum on soybean seedlings under controlled conditions. Can J Plant Pathol. 29:35–40. doi:10.1080/07060660709507435.
- Yli-Mattila T, Mach RL, Alekhina IA, Bulat SA, Koskinen S, Kullnig-Gradinger CM, Kubicek CP, Klemsdal SS. 2004. Phylogenetic relationship of Fusarium langsethiae to Fusarium poae and Fusarium sporotrichioides as inferred by IGS, ITS, beta-tubulin sequences and UP-PCR hybridization analysis. Int J Food Microbiol. 95:267–285. doi:10.1016/j.ijfoodmicro.2003.12.006.
- Zhang JZ, Xue AG, Zhang HJ, Nagasawa AE, Tambong JT. 2010. Response of soybean cultivars to root rot caused by Fusarium species. Can J Plant Sci. 90:767–776. doi:10.4141/CJPS09133.
