Abstract
Under cool and wet conditions, high levels of botrytis grey mould (BGM) can occur on lentil in western Canada. Identification of local Botrytis isolates from lentil based on conidial morphology (N = 74) and sequence analysis (N = 5) indicated that B. cinerea was the predominant cause of BGM in this region. Eight field experiments at two sites (Saskatoon, SK and Outlook, SK) over two seasons were conducted to evaluate the optimal timing of the fungicide boscalid for the control of BGM in the lentil cultivar ‘CDC Grandora’. Weather conditions limited disease development in one year, but moderate levels were observed in four experiments in the other year. In all experiments, a single application at mid-flower in early-seeded experiments and at early flower in late-seeded experiments was as efficacious as a double or triple application of boscalid. There were no differences among double application treatments, and at Outlook they were all as efficacious in lowering BGM as the triple application. Despite some differences in BGM severity among fungicide applications, none of the treatments had an effect on yield or seed infection, and there was no correlation between petal colonization by B. cinerea and BGM disease severity under the study conditions.
Résumé
Dans l’Ouest canadien, lorsque le temps est frais et humide, la pourriture grise causée par le champignon botrytis (BGM) peut se développer abondamment sur les lentilles. L’identification des isolats locaux de Botrytis provenant de lentilles, basée sur la morphologie des conidies (N = 74) et l’analyse de la séquence (N = 5), a indiqué que B. cinerea était la principale cause de la BGM dans cette région. Huit expériences en champ ont été menées à deux sites (Saskatoon, Sask. et Outlook, Sask.) durant deux saisons pour évaluer la période optimale d’application du fongicide boscalide afin de lutter contre la BGM chez le cultivar de lentille ‘CDC Grandora’. Durant une année, les conditions météorologiques ont limité le développement de la maladie, mais, l’année suivante, des taux modérés ont été observés dans le cadre de quatre expériences. Au cours de toutes les expériences, une seule application durant celles menées à la mi-floraison des semis précoces et celles menées à la floraison précoce des semis tardifs a été aussi efficace que deux ou trois applications de boscalide. Il n’y a eu aucune différence entre les traitements basés sur deux applications et, à Outlook, ils ont été tout aussi efficaces quant à l’atténuation de la BGM que le traitement comportant trois applications. Malgré certaines différences relatives aux applications de fongicide quant à la gravité de la BGM, aucun des traitements n’a eu d’effet sur le rendement ni l’infection des semences, et, dans les conditions de l’étude, il n’y a eu aucune corrélation entre la colonization des pétales par B. cinerea et la gravité de la BGM.
Introduction
Botrytis grey mould (BGM) caused by Botrytis cinerea Pers. [Botryotinia fuckeliana (de Bary) Whetzel] is an important disease that has been reported on more than 200 crop species, reflecting the non-specific necrotrophic nature of the pathogen (MacFarlane Citation1968). Some of the major crops affected by this pathogen are faba bean, flax, grape, lettuce, onion, field pea, raspberry, strawberry, apple, tobacco and tomato (Jarvis Citation1980; Volpin and Elad Citation1991). Botrytis grey mould has also been reported on lentil in Australia (Lindbeck et al. Citation2008), Bangladesh (Bahl et al. Citation1993), Nepal (Karki Citation1993), and Pakistan (Iqbal et al. Citation1992). In Australia, B. cinerea and B. fabae Sardiña are both involved in BGM of lentil and are considered equally pathogenic (Davidson et al. Citation2007). The disease is seed and stubble-borne, with polycyclic disease development driven by wind dispersed conidia (Morrall Citation1997).
In western Canada, BGM typically occurs late in the growing season, under cool, moist environmental conditions (Morrall Citation1997; Gossen Citation1999) and as a result, BGM infection levels in harvested seeds often reflect the moisture conditions during the growing season. For example, in 2000, 2004, 2010 and 2016 between 72% and 85% of all seed samples tested in commercial labs were infected with Botrytis, and mean infection ranged from 1.7% to 5.3% (Morrall et al. Citation2001, Citation2005, Citation2011; Olson et al. Citation2019a). These were years with widespread and excessive late-season precipitation. In years with little late-season precipitation, such as 2001, 2017 and 2018, only 8% to 37% of seed samples tested were infected with Botrytis, and the maximum mean infection level was 1.1% (Morrall et al. Citation2002; Olson et al. Citation2019b, Citation2020). Generally, BGM in western Canadian lentil has been associated with B. cinerea, although detailed surveys of the prevailing species have not been conducted previously. Infection of pods often leads to the infection of the seeds which impacts seed viability, seedling emergence and stand establishment (Morrall Citation1997). As a highly seed-borne disease, B. cinerea can cause significant seedling blight due to secondary seedling infection from spores produced on seedlings emerging from infected seeds. Several seed treatments are registered for management of Botrytis seedling blight in Canada (Saskatchewan Ministry of Agriculture Citation2020a).
Partial resistance to BGM is available in lentil, but does not provide adequate protection to the crop under conditions conducive to disease development. Several BGM resistant lines were identified in a germplasm collection in Pakistan (Ilyas et al. Citation2007). The resistant cultivar ‘Nipper’ is recommended in regions of Australia prone to BGM (Hawthorne et al. Citation2012), and the Canadian lentil cultivar ‘Indianhead’ was identified as a source of resistance to BGM (Lindbeck et al. Citation2008). Although this cultivar is part of the parentage of many western Canadian lentil varieties as a source of partial resistance to Ascochyta lentis Vassiljevsky (cause of ascochyta blight) and Colletotrichum lentis Damm (race1, cause of anthracnose), none of them have high levels of resistance to BGM.
Disease management strategies for BGM such as reducing plant density and applying fungicide are not straightforward, as conditions conducive to growth and sporulation of the pathogen also promote vegetative growth of lentil, thereby negating the effect of reduced seeding rates and impeding the penetration of fungicide sprays. In Australia, products used for the control of BGM in lentil include mancozeb, chlorothalonil, carbendazim and procymidone, each under multiple trade names (Lindbeck et al. Citation2002; Hawthorne et al. Citation2012). Fungicides currently registered for management of BGM in lentil in Canada include boscalid (Lance®, BASF Mississauga, ON), prothioconazole and trifloxystrobin (Delaro®, Bayer Crop Science Calgary, AB), fluxapyroxad and pyraclostrobin (Priaxor®, for suppression, BASF), penthiopyrad (Vertisan®, Belchim Crop Protection Canada Inc. Guelph, ON) and a Bacillus subtilis biofungicide (Serenade® OPTI, Bayer Crop Science). An additional 17 foliar fungicides are registered to manage ascochyta blight and/or anthracnose in lentil (Saskatchewan Ministry of Agriculture Citation2020a).
The timing of fungicide applications is one of the most important aspects of managing diseases in lentil. Under Australian conditions, where lentil is grown as a winter crop, fungicide application prior to canopy closure is recommended for BGM management, with additional applications considered based on weather conditions at mid-flower and again at end of flowering to the mid pod-fill stage (Lindbeck et al. Citation2002; Hawthorne et al. Citation2012). In Saskatchewan, the recommended application timing for management of ascochyta blight and anthracnose in lentil is the 10–12-node or early flower stage, prior to canopy closure (Saskatchewan Ministry of Agriculture Citation2020b). Previous research on fungicide application in lentil for BGM management was inconclusive due to low disease pressure in field experiments that resulted from dry weather conditions (Gossen Citation1999).
In fruit crops, it is well documented that colonization of flowers by B. cinerea correlates with disease incidence and severity of BGM in fruit and post-harvest symptoms (Elmer and Michailides Citation2007). In strawberries and grapes, fungicide application during flowering is required to mitigate the risk of latent infection of flowers (Powelson Citation1960; Nair et al. Citation1987; Legard et al. Citation2001; Mertley et al. Citation2002). In Saskatchewan disease surveys, high levels of lentil flower infection was associated with high precipitation during flowering, and early flower infection was deemed to have the highest impact on yield (Gossen Citation1999). Application of benomyl fungicide at mid-flowering in faba bean plants decreased the development of chocolate spot caused by B. fabae (Elliot and Whittington Citation1980), but increased yield only in seasons when epidemics were severe (Bainbridge et al. Citation1985; Creighton et al. Citation1985).
In Australia, delayed seeding has been recommended in areas with higher fall and winter rainfall that promote high vegetative growth (Hawthorne et al. Citation2012). Both B. cinerea and B. fabae were shown to survive in surface crop residues for 15 months, with some reduction in recovery of the pathogens by burying trash to a depth of 10 cm, under Australian growing conditions (Lindbeck et al. Citation2009). Canadian recommendations to follow a four-year crop rotation would avoid inoculum build-up of Botrytis and other pathogens in fields (Saskatchewan Ministry of Agriculture Citation2020b), but short-term economic pressures make these recommendations unattractive to many producers. Reduced tillage systems are recommended in this region, making stubble management strategies less accessible.
The objectives of this study were to confirm that BGM in Canadian lentil is caused by B. cinerea, to determine the optimum timing of boscalid application for control of BGM in lentil, and to evaluate if a correlation exists between flower petal infection and disease severity of BGM.
Materials and methods
Identification of Botrytis species causing BGM in Saskatchewan
Isolate collection
Infected lentil, chickpea, dry bean and field pea seed from commercial farms across the province of Saskatchewan were incubated on potato dextrose agar medium (PDA, DIFCO, Mississauga, ON) at a commercial seed testing laboratory (Discovery Seed Labs Ltd., Saskatoon, SK) and supplied to the University of Saskatchewan. Mycelial plugs of Botrytis sp. colonies in these Petri dishes were transferred to fresh PDA amended with 0.1 g L−1 of streptomycin sulphate (EM Science, Gibbstown, NJ). A total of 81 single-conidium-derived isolates were obtained, of which one was from dry bean, two from field pea, five from chickpea and 73 from lentil. An additional six isolates from alfalfa (Sept0301, Aug9811-Aug9814, Oct9902), one from chickpea (Feb9804) and one from lentil (Feb9807) were obtained from Agriculture and Agri-Food Canada, Saskatoon SK. Botrytis cinerea isolates from strawberry (DAOM 192631) and grape (DAOM 231368), and a B. fabae isolate from faba bean (DAOM 137563A) were obtained from the Canadian Collection of Fungal Cultures at AAFC in Ottawa, ON (Supplemental ).
Table 1. Growth stages of ‘CDC Grandora’ lentil, dates of inoculation with Botrytis cinerea and dates of boscalid applications at three timings (A, B, C) in field experiments at Outlook and Saskatoon, Saskatchewan, in 2002
Morphological characterization
Fungal cultures were incubated on PDA at 22°C with a 12 or 16 h photoperiod for 15 days. Conidia were harvested from PDA plates by adding 20 mL sterile distilled water along with one drop of Tween 20 and gently dislodging conidia with the edge of a sterile glass slide. The length and width of conidia were measured at 200× magnification (Zeiss Axioskop 40, Carl Zeiss, Germany) using an eyepiece micrometer. For B. cinerea isolate DAOM 192631 and B. fabae isolate DAOM 137563A, which were used for comparison, the length and width of 40 conidia were measured. Between three and 10 conidia were measured for each of the remaining isolates, with five or more conidia for more than 60% of the isolates. The experiment was designed as a completely randomized experiment.
Molecular species determination
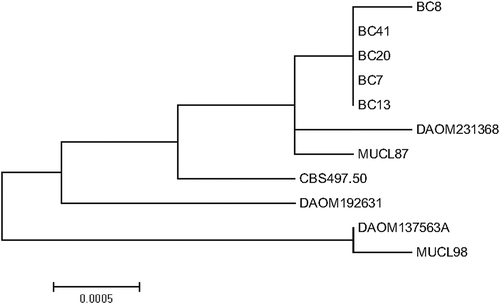
Mycelium of five lentil isolates (BC7, BC8, BC13, BC20, BC41), B. cinerea DAOM 192631 and DAOM 231368, and B. fabae DAOM 137563A (Supplemental ) were grown in glucose yeast medium on a rotary shaker for two to three days, filtered, freeze dried and pulverized. Extraction of DNA was conducted using a DNeasy plant mini kit (Qiagen Canada, Toronto ON). Partial sequences of heat-shock protein 60 (HSP60), glyceraldehyde-3-phosphate dehydrogenase (G3PDH) and DNA-dependent RNA polymerase subunit II (RPB2) genes were amplified using primer sets G3PDHfor/rev, HSP60for/rev, RPB2for/rev (Staats et al. Citation2005). Amplicons were sequenced at the National Research Council Saskatoon Research Centre. Consensus contigs were constructed using DNAbaser v4 (Heracle BioSoft) and compared to published sequences in NCBI Blast (Altschul et al. Citation1990). Sequence data of the three gene regions were concatenated and used to construct a maximum likelihood phylogenetic tree in MEGA 6 (Tamura et al. Citation2013). Published sequence data of ex-type isolates B. cinerea MUCL87, B. fabae MUCL98 and B. pelargonii CBS 497.50 were included in the tree construction (Staats et al. Citation2005).
Effect of boscalid application frequency and timing on BGM severity
Establishment of field experiments
Field experiments were conducted in 2002 and 2003 at the Canada-Saskatchewan Irrigation Diversification Centre (CSIDC) at Outlook, and at the University of Saskatchewan in Saskatoon, SK. Each experiment was a randomized complete block design of seven fungicide frequency treatments plus an untreated control, with four replications (). In order to increase the potential for development of adequate disease severity, two experiments were conducted each season at each site, with an early and late seeding date.
Cultivar ‘CDC Grandora’, a late maturing, large-seeded green lentil (Vandenberg et al. Citation2002) was chosen for its high biomass production and tendency for lodging (Ball et al. Citation2006), which are traits that promote a canopy conducive to BGM development. Plots of 1.4 × 3.6 m were seeded at the recommended rate of 132 seeds m−2. Row spacing was reduced from the recommended 20 cm to 16 cm with 27 cm inter-plot spacing in order to reduce air flow and thus promote BGM development. Guard rows of the BGM susceptible cultivar ‘Northfield’ (Ali and Gammie Citation1995; Davidson et al. Citation2004) were seeded on either side of each experiment. Early seeding in 2002 took place on May 10 at Saskatoon and May 11 at Outlook, and in 2003 on May 16 at Saskatoon and May 20 at Outlook. Late seeding at both sites occurred on June 3, 2002 and June 5, 2003.
Seeds were planted into a heavy clay soil at Saskatoon, a silty loam soil at Outlook in 2002, and a fine, sandy loam soil at Outlook in 2003. Granular lentil inoculant (Nodulator®, BASF) was added to seed at a rate of 5.6 kg ha−1. Adequate amounts of nutrients were present at both locations in both years, so no fertilizer was applied. Weed control consisted of a spring application of trifluralin (Treflan®, Gowan Canada, Winnipeg MB) or ethalfluralin (Edge®, Gowan Canada), a pre-emergence application of imazethapyr (Pursuit®, BASF) and sethoxydim (Poast® Ultra, BASF) application as needed for annual grassy weed control. Extensive hand weeding was also done at both locations. Dessicants were applied prior to harvest, using diquat (Reglone®, Syngenta Guelph ON) in 2002 and glyphosate (Roundup Transorb®, Bayer Crop Science) in 2003.
At Outlook, the experiments were conducted under a linear irrigation system and a misting irrigation system was utilized at Saskatoon. In 2002, combined precipitation and irrigation from the beginning of May until the end of September amounted to 188 mm at Outlook and 203 mm at Saskatoon, whereas in 2003, experiments received precipitation and irrigation totalling 133 mm at Outlook and 149 mm at Saskatoon.
Early-seeded lentil experiments in 2002 were harvested on September 23 or 24, whereas late-seeded experiments were harvested after an early snowfall on October 9 at Outlook and on October 18 at Saskatoon. In 2003, early-seeded experiments were harvested on August 22 and late-seeded experiments were harvested on August 27 at both locations. Seed yield was measured for each plot, and seed samples were retained for testing seed-borne B. cinerea infection.
Despite irrigation, no BGM developed in the early or late-seeded lentil experiments at Outlook in 2003 nor in the late-seeded experiment at Saskatoon in 2003. In the early-seeded experiment at Saskatoon in 2003, disease pressure was low and final disease severity only reached 19% in non-sprayed control plots. Therefore, only data from the four 2002 experiments are presented.
Inoculation of field plots
In 2002, all experiments were inoculated with a conidia suspension containing a mixture of six isolates of B. cinerea (BC1, BC6, BC9, BC18, BC26, and BC31) originating from lentil seed samples in Saskatchewan (Supplemental ). Media for conidia production was prepared by pouring sterilized PDA media (Difco, Sparks MD, USA) into large tinfoil trays containing slices of autoclaved bread (Wonderbread, Weston Bakeries, Toronto ON). A conidia suspension containing all six isolates was spread over the medium in trays and incubated for 14 days. Conidia were harvested by adding sterile distilled water to the medium surface and scraping it with a glass slide. The resulting suspension was filtered through miracloth (EMD Millipore, Burlington MA USA). At each location, all plots including the guard rows were inoculated with 5.5 L of 5 × 105 B. cinerea conidia mL−1 applied with a hand-held, CO2-powered sprayer with three standard flat-fan nozzles (XR8004) at 140 kPa. Two inoculations were performed in early-seeded experiments, at pre-flower (Outlook) or early flower (Saskatoon) and again at mid-flower (Outlook) or post-flower (Saskatoon). Late-seeded experiments were inoculated once, at early flower (Outlook) and at late flower (Saskatoon) ().
Fungicide treatments
All boscalid (Lance®, BASF) treatments were applied at the recommended rate of 300 g active ingredient ha−1, equal to 420 g of formulated product ha−1. Fungicide treatments consisted of one, two or three applications at three different times during the growing season. Growth stages of lentil plants at the three application timings varied with location, seeding date and year, ranging from first flower to late flower (). In addition to unsprayed control plots, the seven fungicide application treatments included: one application at timing A (first to early flower), one application at timing B (early to mid flower), one application at timing C (late flower), two applications at timings A and B, two applications at timings A and C, two applications at timings B and C, and three applications at timings A, B and C ().
Disease assessment
Disease severity ratings were initiated in all experiments in July when the crop was at the early flowering stage, one week after the first inoculation. Disease assessments were conducted on a weekly basis following the second inoculation in 2002, and continued until the middle of September. Five randomly selected plants per plot were rated using the Horsfall-Barratt scale to measure BGM severity (Horsfall and Barratt Citation1945). Anthracnose, caused by Colletotrichum lentis Damm, and white mould, caused by Sclerotinia sclerotiorum (Lib.) de Bary, were also rated when present in field plots. Final disease severity ratings were converted into percentage data for analysis.
Flower petal evaluation
Lentil flower petal evaluations were completed in the early and late-seeded experiments weekly for four weeks beginning in early August 2002. Flowers were sampled at random from each replicate of the unsprayed ‘CDC Grandora’ control plots in each experiment. Sixteen petals per replication were incubated on PDA medium in sealed Petri dishes at 23°C with a 16 h photoperiod for 2 weeks. Following incubation, flower petals were assessed for the presence of B. cinerea colonies. Flower petals were not evaluated in 2003, as flower abortion occurred in August at both locations resulting in insufficient tissue available for assessment.
Evaluation of Botrytis seed infection
Seed samples from early and late-seeded lentil experiments in 2002 and early-seeded experiments at both locations in 2003 were tested for the presence of seed-borne B. cinerea. Seed from late-seeded experiments in 2003 was not tested due to low levels of BGM in the plots. One hundred lentil seeds from each plot for a total of 400 seeds per treatment were tested. Seed was surface sterilized for 3 minutes in 10% bleach (0.6% NaOCl), dried on sterile paper towel and placed on PDA media at 10 seeds per Petri dish. Dishes were incubated at room temperature under incandescent lighting for 10 days. The presence of Botrytis and other seed-borne pathogens was recorded after 7 and 10 days.
Statistical analysis
All statistical analyses were conducted with SAS v. 9.4 (SAS Institute Inc., Cary NC). Length and width of conidia, and disease severity in field experiments were analysed using the mixed model procedure. Variances were assessed and heterogeneity was modelled with the repeated statement in SAS (Littell et al. Citation2006).
Final disease severity and yield data from each experiment were analysed separately because plant development at the time of fungicide application varied (). Fungicide timing was considered a fixed effect and blocks were considered random effects. Mean separation was conducted with Tukey’s Honestly Significant Difference test. Linear contrasts were used to compare fungicide treatments with the control, one versus two or three boscalid applications, and two versus three boscalid applications. Flower petal data from the 2002 experiments at two locations were combined and a Pearson’s correlation analysis was completed to determine whether a relationship existed between flower petal infection and BGM severity in the field.
Results
Identification of Botrytis species causing BGM in Saskatchewan
Morphological characterization
Conidia of B. cinerea isolate DAOM 192631 were on average 13.1 ± 1.5 μm long and 8.7 ± 1.1 μm wide. Conidia of B. fabae isolate DAOM 137563 were significantly larger with a mean length of 22.2 ± 2.4 μm and a mean width of 12.9 ± 1.9 μm. Measurements for both isolates fall into the ranges given for each species (Ellis and Waller Citation1974a, Citation1974b). All field isolates had conidia significantly shorter than B. fabae DAOM 137563 (P < 0.0001), and with the exception of BC37, BC48, BC55, BC66, BC68, BC69 and BC78, were of similar length as those of B. cinerea DAOM 192631 (Supplemental ). Similarly, conidia of all isolates were significantly narrower than those of B. fabae DAOM 137563 (P < 0.0001), although conidia BC37, BC48, BC55, BC68, BC69 and BC78 were also slightly wider than B. cinerea DAOM 192631.
Molecular species determination
Identification of five isolates from lentil (BC7, BC8, BC13, BC20, BC41) as B. cinerea was further supported by sequence data of a 881 bp region of G3PDH, a 978 bp region of HSP60 and a 1093 bp region of RPB2, all of which had high identity (≥99.7%) with B. cinerea sequences in NCBI Blast (Altschul et al. Citation1990). A maximum likelihood phylogenetic tree of concatenated sequences showed that all five lentil isolates grouped closely with B. cinerea DAOM 231368 and ex-type isolate MUCL87 (AJ745676.1, AJ716065.1, AJ705004.1, Staats et al. Citation2005). Data from DAOM 137563A did not group tightly with the other B. cinerea sequences due to the presence of four SNPs in common with B. fabae MUCL98 and/or B. pelargonii CBS 497.50 reference sequences. Sequence data of B. fabae DAOM 137563A grouped tightly with ex-type isolate MUCL98 (AJ745686.1, AJ716075.1, AJ705014.1, Staats et al. Citation2005) ().
Fig. 1 Maximum likelihood phylogenetic tree based on the concatenated partial sequences of heat-shock protein 60 (HSP60), glyceraldehyde-3-phosphate dehydrogenase (G3PDH) and DNA-dependent RNA polymerase subunit II (RPB2) for field isolates BC7, BC8, BC13, BC20 and BC41 from lentil, isolates DAOM 137563A identified as Botrytis fabae, DAOM 192631 and DAOM 231368 identified as B. cinerea from the Canadian Collection of Fungal Cultures, and ex-type isolates B. cinerea MUCL87, B. fabae MUCL98 and B. pelargonii CBS 497.50 (published by Staats et al. Citation2005)
Effect of boscalid application frequency and timing on BGM severity
BGM developed slowly at both locations because of extreme heat during the flowering period in 2002, in particular at Saskatoon where the average maximum temperature for ten days prior to the first inoculation was 31.2°C. Nevertheless, the lentil canopy was almost completely closed at the second application date (B) and plants were at the late flowering stage at the last application timing in the middle of August (C, ). At Saskatoon, BGM symptoms appeared at low levels (5%) in control plots when Treatment C was applied (late flowering stage in both experiments), and then increased to 55% in early-seeded and 33% in late-seeded control plots in 2002. At Outlook BGM was evident at 2% in early-seeded control plots when Treatment A was applied, had reached 40% at the time of the last fungicide application, and 55% in mid September. BGM development was slower in late-seeded plots at Outlook where a severity of 5% in control plots was observed at the second fungicide application time, 20% at the time of Treatment C and 39% at the final rating in mid September.
Anthracnose was found in trace amounts at Saskatoon in 2002, but reached 30% and 24% severity in early and late-seeded control plots at Outlook, respectively. No significant differences in anthracnose severity were observed among plots treated with one, two or three applications of boscalid (data not shown). White mould severity was very low, with means ranging from 1% to 5.8% in untreated plots, and was considered negligible.
A significant impact of fungicide treatments on BGM severity was observed in all four experiments in 2002 (P < 0.007). Levels of BGM in control plots were significantly higher than in fungicide-treated plots in the early-seeded experiment at Saskatoon and the late-seeded experiment at Outlook. Spraying boscalid at the last application timing (Treatment C) in the early-seeded experiment at Outlook resulted in BGM levels similar to the control (). Application treatments A or B in late-seeded plots at Saskatoon produced highly variable results, so could not be differentiated from effects in the control plot either. In three experiments, a single application at early or mid flower (Treatment A in early-seeded lentil at Saskatoon, Treatment B at Outlook) was as efficacious as a double or triple application of boscalid. There were no differences among double application treatments and at Outlook and in early-seeded lentil at Saskatoon they were all as efficacious in lowering BGM as the triple application ().
Table 2. Effect of one, two or three boscalid application timings (A, B, C) at three different crop stages on the severity of Botrytis cinerea on, and seed yield of early and late-seeded CDC Grandora lentil in experiments at Saskatoon and Outlook, Saskatchewan, in 2002. Disease severity means with the same letter in each column are not different at P = 0.05. Values in brackets behind mean seed yields are standard errors of the mean
Lentil yields were below average in most experiments due to very dry growing conditions and late maturing nature of ‘CDC Grandora’. Losses due to grasshoppers occurred at both locations in 2002. Average yield was 738 kg ha−1 in the early-seeded and 457 kg ha−1 in the late-seeded experiment at Saskatoon, 1401 kg ha−1 in the early-seeded and 1154 kg ha−1 in the late-seeded experiment at Outlook. Boscalid applications had no effect on lentil yields except for the early-seeded experiment at Saskatoon (P = 0.01134) where an early- (Treatment A) and late-flower application (Treatment AC) revealed reduced yield compared to plots with Treatments C, BC and ABC ().
Flower infection was low in unsprayed control plots, with up to 9% infection at Outlook and 8% infection at Saskatoon in 2002. There was no correlation between flower infection and the resulting disease severity or seed yields in control plots (data not presented).
Botrytis seed infection was only detected in seed samples from the late-seeded experiment at Outlook in 2002, where it reached 1.5% in the control treatment. There was no effect of fungicide frequency and timing on seed infection (P = 0.19).
Discussion
Conidial morphology and sequencing data indicate that Botrytis cinerea was the predominant species causing BGM of lentil in western Canada during the time of isolate collection (1997–2002), which represents a valuable bench mark for the causal organism of BGM in lentil prior to the resurgence of faba bean production in Saskatchewan in recent years. The prevalence of B. cinerea contrasts with the situation in Australia, where B. fabae and B. cinerea are equal contributors to BGM on lentil (Davidson et al. Citation2007). Botrytis fabae is a major pathogen of faba bean, which almost equals lentil in acreage in Australia, and could explain the frequent recovery of that species from Australian lentil. Faba bean has been adopted as a speciality crop in western Canada in recent years, with an average of 20 000 ha seeded in Saskatchewan from 2016 to 2019 (Saskatchewan Ministry of Agriculture Citation2019), hence it will be interesting to see whether B. fabae will become more prominent on lentil grown in Saskatchewan as well. As demonstrated in Australia, neighbouring faba bean crops can serve as a reservoir of inoculum for lentil (Davidson et al. Citation2007), and on faba bean it is well established that B. fabae is the more aggressive pathogen compared to B. cinerea (Mansfield and Deverall Citation1974).
A closed canopy in lentil provides an excellent microclimate for the development of BGM, where B. cinerea can sporulate abundantly on decaying lentil tissues in moist conditions (Kaiser Citation1992). Upon canopy closure, BGM can develop explosively, suggesting that infection may occur earlier but may remain latent for some time, which has been reported in other crops such as strawberry and grape (Keller et al. Citation2003). It is also known that B. cinerea can sporulate profusely under conducive conditions, contributing to the rapid increase in reinfection and disease progression (Morrall Citation1997).
Conducive conditions for the development of BGM consist of moderate temperatures and above average precipitation that results in increased vegetative growth of lentil plants and a dense, lush canopy (Morrall Citation1997). Under these conditions, an early application of boscalid prior to row closure is anticipated to result in better disease control due to better coverage of lentil foliage, with additional benefits from a second application to protect new growth. Early fungicide application can thus address early and potential latent infection, and ensure adequate canopy penetration. In field experiments conducted at Saskatoon and Outlook in 2002, however, weather conditions characterized by above average temperatures and below average precipitation supplemented with irrigation were not conducive to the development of the crop nor epidemic BGM development. Despite canopy closure at the mid-flower stage, irrigation of plots, and artificial inoculations, BGM development was slow and only reached moderate levels in experiments that year, leaving limited data from which to draw conclusions. Under these conditions, a single application of boscalid at mid-flower in early-seeded experiments, and at early flower in late-seeded experiments (Treatment B) was as efficacious as a double or triple application in suppressing BGM. In Australia, more than one application of a fungicide is typically considered in lentil for BGM control, with the first application planned prior to canopy closure, followed by applications during the flowering or pod fill stage (Davidson et al. Citation2007; Hawthorne et al. Citation2012). However, growing conditions in Australia differ markedly from those in Canada as lentil are typically seeded in the fall and tend to be exposed to precipitation mainly in the first half of the growing cycle whereas in Canada lentil are more likely to be subjected to rain in the second half of the growing cycle, before harvest in the fall.
The significant impact of an early to mid-flower fungicide application indicates that even under moderate disease pressure good canopy penetration and possibly the protection of flowers benefitted the plants. However, the low levels of flower infection of less than 10% observed in this study did not correlate with BGM severity. Previous research suggested that flower petals may be important sites of B. cinerea infection in lentil, as incidence of 45% to 50% in lentil flowers correlated with BGM development in commercial lentil fields (Gossen Citation1999).
Under moderate disease pressure observed in this study, fungicide treatments overall had no effect on yield or on seed infection. Low yields due to dry and hot growing conditions and additional stress due to grasshoppers probably confounded any potential yield effect in response to BGM reductions. However, a lack of yield response upon fungicide applications that reduced BGM severity has been reported in other crops. Fungicide applications (benomyl, prochloraz or iprodione) to faba beans with low levels of chocolate spot did not increase yield (Bainbridge et al. Citation1985) and benomyl sprays provided a yield benefit only in wet years when disease severity was high (Fitt et al. Citation1986). The most consistent and profitable yield response in winter faba bean was obtained from two fungicide applications (chlorothalonil + benomyl or vinclozolin) sprayed at early and mid-flower for control of chocolate spot (B. cinerea and B. fabae) (Dobson and Giltrap Citation1991). In cucumber, a single fungicide application of tebuconazole plus dichlofluanid reduced B. cinerea stem infections by 90%, resulted in only a 6% yield increase (Yunis et al. Citation1991).
BGM can negatively affect seed quality in two ways, by reducing the visual appearance of seeds in terms of colour and shape, thus lowering its commercial value as a food product, and by increasing the risk of seedling blight if infected seeds are used for seeding in subsequent years. As a result of the dry growing conditions, B. cinerea seed infection was detected in only one of four experiments and at low levels of 1.5%. In cool and wet years, Botrytis seed infection of lentil tends to be widespread, as was evident in 2000, 2004, 2010 and 2016, when between 72% and 85% of seed samples tested in commercial labs were infected with this pathogen with a mean infection level of samples of 1.7% to 5.3% (Morrall et al. Citation2001, Citation2005, Citation2011; Olson et al. Citation2019a). Up to 10% of seed infection is considered tolerable under Saskatchewan growing conditions (Saskatchewan Ministry of Agriculture Citation2020c). Seed treatment with fungicides such as carbathiin and thiabendazole can significantly reduce the impact of Botrytis seedling blight in seedling establishment (Morrall Citation1997).
To date, BGM has been considered a minor disease in lentil production in western Canada, although epidemic outbreaks are not unknown in years with below average temperatures and above average precipitation, particularly in the second half of the growing season. If faba bean production increases, it is feasible that BGM on lentil will also be caused by B. fabae, which may affect the prevalence and dynamics of this disease on lentil.
Supplemental Table 1
Download MS Word (31.6 KB)Acknowledgements
The researchers acknowledge that this study was conducted on Treaty 6 territory and the homeland of the Metis. The University of Saskatchewan is committed to reconciliation and recognizes the importance of our relationship to the Indigenous Peoples of this land as an integral component of all our research. Technical assistance was provided by Brent Barlow, Scott Ife, Stephanie Boechler, Patricia Gakis and the pulse crop field crew of the Crop Development Centre.
Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2021.1873865
Additional information
Funding
References
- Ali SM, Gammie RL. 1995. Register of Australian grain legume cultivars. Lens culinaris (lentil) cv. Northfield. Aust J Exp Agr. 35(8):1181–1182. doi:https://doi.org/10.1071/EA9951181.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215(3):403–410. doi:https://doi.org/10.1016/S0022-2836(05)80360-2.
- Bahl PN, Lal S, Sharma BM. 1993. An overview of the production and problems of lentil in South Asia. In: Erskine W, Saxena MC, editors. Lentil in south Asia. Aleppo (Syria): ICARDA; p. 1–10.
- Bainbridge A, Fitt BDL, Creighton NF, Cayley GR. 1985. Use of fungicides to control chocolate spot (Botrytis fabae) on winter field beans (Vicia faba). Plant Pathol. 34(1):5–10. doi:https://doi.org/10.1111/j.1365-3059.1985.tb02754.x.
- Ball RA, Hanlan TG, Vandenberg A. 2006. Stem and canopy attributes that affect lodging resistance in lentil. Can J Plant Sci. 86(1):78–81. doi:https://doi.org/10.4141/P05-037.
- Creighton NF, Bainbridge A, Fitt BDL. 1985. Epidemiology and control of chocolate spot (Botrytis fabae) on winter field beans (Vicia fabae). Crop Prot. 4(2):235–243. doi:https://doi.org/10.1016/0261-2194(85)90021-3.
- Davidson J, Materne M, Lindbeck K, Krysinska-Kaczmarek M, Hartley D 2004. Understanding botrytis gray mold of lentils in Australia and identifying useful resistance. Proceedings of the 5th European Conference on Grain Legumes; Jun 7-11, 2004; Dijon, France.
- Davidson J, Pande S, Bretag TW, Lindbeck KD, Krishana-Kishore G. 2007. Biology and management of Botrytis spp. in legume crops. In: Elad Y, Williamson B, Tudzynski P, Delen N, editors. Biology and management of Botrytis spp. in field crops. Dordrecht (Netherlands): Kluwer Academic Publishers; p. 295–318.
- Dobson SC, Giltrap NJ. 1991. Timing of sprays for control of rust and chocolate spot in spring and winter field beans. Asp Appl Biol. 27:111–116.
- Elliot JEM, Whittington WJ. 1980. The control of chocolate spot (Botrytis fabae) infection of field beans (Vicia faba L.) by the fungicides benlate, bavistin, cercobin and BAS 352F. J Agr Sci-Cambridge. 94:461–464.
- Ellis MB, Waller JM. 1974a. Sclerotinia fuckeliana (conidial state: botrytis cinerea). CMI Descriptions of Pathogenic Fungi and Bacteria; Kew (UK): Commonwealth Mycological Institute; p. 431.
- Ellis MB, Waller JM. 1974b. Botrytis fabae. CMI Descriptions of Pathogenic Fungi and Bacteria; Kew (UK): Commonwealth Mycological Institute; p. 432.
- Elmer PAG, Michailides TJ. 2007. Epidemiology of Botrytis cinerea in orchard and vine crops. In: Elad Y, Williamson B, Tudzynski P, Delen N, editors. Biology and management of Botrytis spp. in field crops. Dordrecht (Netherlands): Kluwer Academic Publishers; p. 243–272.
- Fitt BDL, Finney ME, Creighton NF. 1986. Effects of irrigation and benomyl treatment on chocolate spot (Botrytis fabae) and yield of winter-sown field beans (Vicia faba). J Agr Sci. 106(2):307–312. doi:https://doi.org/10.1017/S0021859600063899.
- Gossen BD. 1999. Biology and control of Botrytis blight of lentil and alfalfa. ADF project 94000184 final report. Regina (Saskatchewan): Saskatchewan Ministry of Agriculture; [ accessed 2020 May]. http://www.agriculture.gov.sk.ca/apps/adf/ADFAdminReport/19940184_242200095037.pdf.
- Hawthorne W, Materne M, Davidson J, Lindbeck K, McMurray L, Brand J. 2012. Lentil disease management strategy. Canberra (Australia): Australian Government Grains Research and Development Corporation.
- Horsfall JG, Barratt RW. 1945. An improved grading system for measuring plant diseases. Phytopathology. 35:655.
- Ilyas MB, Chaudhry MB, Muhammad F, Ul-Hassan I. 2007. Sources of resistance in lentil germplasm against botrytis gray mold. Pak J Phytopathol. 2:173–176.
- Iqbal SM, Hussain S, Malik BA. 1992. vitro evaluation of fungicides against Botrytis cinerea of lentil. Lens Newsletter. 19:49–51.
- Jarvis WR. 1980. Epidemiology. In: Coley-Smith JR, Verhoeff K, Jarvis WR, editors. Biology of Botrytis. London (UK): Academic Press Ltd; p. 219–245.
- Kaiser W. 1992. Fungi associated with the seeds of commercial lentils from the US pacific northwest. Plant Dis. 76(6):605–610. doi:https://doi.org/10.1094/PD-76-0605.
- Karki PB. 1993. Plant protection of lentil in Nepal. In: Erskine W, Saxena MC, editors. Lentil in south Asia. Aleppo (Syria): ICARDA; p. 187–193.
- Keller M, Viret O, Cole FM. 2003. Botrytis cinerea infection in grape flowers: defense reaction, latency, and disease expression. Phytopathology. 93(3):316–322. doi:https://doi.org/10.1094/PHYTO.2003.93.3.316.
- Legard DE, Xiao JC, Mertley CK, Chandler CK. 2001. Management of Botrytis fruit rot in annual winter strawberry using Captan, Thiram, and Iprodione. Plant Dis. 85(1):31–39. doi:https://doi.org/10.1094/PDIS.2001.85.1.31.
- Lindbeck K, Materne M, Davidson J, McMurray L, Panagiotopoulos K. 2002. Lentil disease management strategy for Southern Region. Canberra (Australia): Australian Government Grains Research and Development Corporation.
- Lindbeck KD, Bretag TW, Ford R. 2009. Survival of Botrytis spp. on infected lentil and chickpea trash in Australia. Australas Plant Path. 38(4):399–407. doi:https://doi.org/10.1071/AP09015.
- Lindbeck KD, Bretag TW, Materne MA. 2008. Field screening in Australia of lentil germplasm for resistance to botrytis grey mould. Australas Plant Path. 37(4):373–378. doi:https://doi.org/10.1071/AP08012.
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. 2006. SAS for mixed models. 2nd ed. Cary (NC): SAS Institute Inc.
- MacFarlane HH. 1968. Review of applied mycology plant host-pathogen index to volumes 1-40 (1922-1961). Kew (UK):Commonwealth Mycological Institute.
- Mansfield JW, Deverall BJ. 1974. The rates of fungal development and lesion formation in leaves of Vicia faba during infection by Botrytis cinerea and Botrytis fabae. Ann Appl Biol. 76(1):77–89. doi:https://doi.org/10.1111/j.1744-7348.1974.tb01358.x.
- Mertley JC, MacKenzie SJ, Legard DE. 2002. Timing of fungicide applications for Botrytis cinerea based on development stage of strawberry flowers and fruit. Plant Dis. 86(9):1019–1024. doi:https://doi.org/10.1094/PDIS.2002.86.9.1019.
- Morrall RAA. 1997. Evolution of lentil diseases over 25 years in western Canada. Can J Plant Sci. 19:197–207.
- Morrall RAA, Carriere B, Cronje S, Schmeling D, Thompson L. 2002. Seed-borne pathogens of chickpeas, lentil and pea in Saskatchewan in 2001. Can Plant Dis Surv. 82:95–103.
- Morrall RAA, Carriere B, Cronje S, Schmeling D, Thomson L. 2001. Seed-borne pathogens of lentil in Saskatchewan in 2000. Can Plant Dis Surv. 81:126–129.
- Morrall RAA, Carriere B, Ernst B, Pearse C, Schmeling D, Thomson L. 2005. Seed-borne pathogens of lentil in Saskatchewan in 2004. Can Plant Dis Surv. 85:84–86.
- Morrall RAA, Carriere B, Ernst B, Schmeling D. 2011. Seed-borne pathogens of lentil and chickpea in Saskatchewan in 2010. Can Plant Dis Surv. 91:130–132.
- Nair NG, Emmett RW, Parker FE. 1987. Programming applications of dicarboximides to control bunch rot of grapes caused by Botrytis cinerea. Plant Pathol. 36(2):175–179. doi:https://doi.org/10.1111/j.1365-3059.1987.tb02218.x.
- Olson BD, Banniza S, Blois T, Ernst B, Junek S, Phelps S, Ziesman B. 2019a. Seed-borne pathogens of pulse crops in Saskatchewan in 2016. The Canadian phytopathological society Canadian plant disease survey: disease highlights 2019. Can J Plant Pathol. 41(Suppl):145–149.
- Olson BD, Banniza S, Blois T, Ernst B, Junek S, Phelps S, Ziesman B. 2019b. Seed-borne pathogens of pulse crops in Saskatchewan in 2017. The Canadian phytopathological society Canadian plant disease survey: disease highlights 2019. Can J Plant Pathol. 41(Suppl):149–154.
- Olson BD, Banniza S, Ernst B, Fatima S, Junek S, Phelps S, Prasad T, Risula D, Wenaus J. 2020. Seed-borne pathogens of pulse crops in Saskatchewan in 2019. The Canadian phytopathological society Canadian plant disease survey: disease highlights 2020. Can J Plant Pathol. in Press. doi:https://doi.org/10.1007/s42161-020-00644-w.
- Powelson RL. 1960. The initiation of strawberry fruit rot caused by Botrytis cinerea. Phytopathology. 50:491–494.
- Saskatchewan Ministry of Agriculture. 2019. Specialty crop report 2019; [ accessed 2020 May]. https://pubsaskdev.blob.core.windows.net/pubsask-prod/115207/Specialty%252BCrop%252BReport%252B2019_web.pdf.
- Saskatchewan Ministry of Agriculture. 2020a. Crop protection guide 2020. Regina (SK): Saskatchewan Ministry of Agriculture. accessed 2020 May. https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/crop-guides-and-publications/guide-to-crop-protection.
- Saskatchewan Ministry of Agriculture. 2020b. Lentil diseases – identification and management; [ accessed 2020 Mar]. https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/disease/lentil-diseases-identification-and-management#.
- Saskatchewan Ministry of Agriculture. 2020c. Seed quality and guidelines for seed borne diseases of pulse crops; [ accessed 2020 May]. https://pubsaskdev.blob.core.windows.net/pubsask-prod/98478/98478-Guidelines_for_seed_borne_diseases_of_pulse_crops.pdf.
- Staats M, van Baarlen P, van Kan JAL. 2005. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Mol Biol Evol. 22(2):333–346. doi:https://doi.org/10.1093/molbev/msi020.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729. doi:https://doi.org/10.1093/molbev/mst197.
- Vandenberg A, Kiehn FA, Vera C, Gaudiel R, Buchwaldt L, Dueck S, Morrall RAA, Wahab J, Slinkard AE. 2002. CDC Grandora lentil – cultivar description. Can J Plant Sci. 82(1):105–106. doi:https://doi.org/10.4141/P01-002.
- Volpin H, Elad Y. 1991. Influence of calcium nutrition on susceptibility of rose flowers to dicarboximide-resistant strains of Botrytis cinerea in plant debris during summer in Israel. Phytoparasitica. 17:13–21.
- Yunis H, Elad Y, Mahrer Y. 1991. Influence of fungicidal control of cucumber and tomato grey mould (Botrytis cinerea) on fruit yield. Pestic Sci. 31(3):325–335. doi:https://doi.org/10.1002/ps.2780310307.
