Abstract
Pythium spp. are the most common pathogens associated with soybean damping-off in the United States. The diversity of Pythium spp. recovered is extensive and the relative pathogenicity or aggressiveness of species common across states has varied among studies. We compared the aggressiveness and fungicide sensitivity of 118 isolates of four Pythium spp. (P. lutarium, P. oopapillum, P. sylvaticum, and P. torulosum) from 11 states (AR, IA, IL, IN, KS, MI, MN, ND, NE, SD, and WI). All isolates were screened using seed and seedling assays at two temperatures (13°C and 23°C). Seed rot and root rot severity varied by state of origin, with isolates from AR always the most aggressive. In general, isolates of P. sylvaticum and P. lutarium were more aggressive at 23°C compared with 13°C, while isolates of P. oopapillum and P. torulosum were more aggressive at 13°C than at 23°C. Fungicide sensitivity to five fungicides (metalaxyl, azoxystrobin, ethaboxam, captan, and thiram) was assessed using a plate assay at 13°C and 23°C. The EC50 values for each fungicide-isolate combination varied by state of origin, and were greatest for the isolates from AR. Temperature affected EC50 values for metalaxyl, azoxystrobin, and ethaboxam for P. oopapillum, P. sylvaticum, and P torulosum. The EC50 values for thiram were not affected by temperature for any species. Results from this study indicate that aggressiveness and fungicide sensitivity of Pythium spp. vary geographically, which suggests that management of damping-off of soybean should target local rather than regional Pythium populations.
Résumé
Pythium spp. sont les agents pathogènes les plus communément associés à la fonte des semis chez le soya aux États-Unis. La diversité de Pythium spp. récupérés est très grande et la pathogénicité relative ou l’agressivité des espèces communes aux États ont varié d’une étude à l’autre. Nous avons comparé l’agressivité et la sensibilité aux fongicides de 118 isolats de 4 Pythium spp. (P. lutarium, P. oopapillum, P. sylvaticum et P. torulosum) provenant de 11 États (AR, IA, IL, IN, KS, MI, MN, ND, NE, SD et WI). Tous les isolats ont été criblés au cours de tests effectués sur des semences et des semis à deux températures (13°C et 23°C). La gravité de la pourriture des semences et des racines variait selon l’État d’origine, les isolats de l’Arkansas étant toujours les plus agressifs. Généralement, les isolats de P. sylvaticum et P. lutarium étaient plus agressifs à 23°C qu’à 13°C, tandis que les isolats de P. oopapillum et P. torulosum étaient plus agressifs à 13°C qu’à 23°C. La sensibilité aux fongicides pour cinq fongicides (métalaxyl, azoxystrobine, éthaboxam, captane et thirame) a été évaluée au moyen d’un test sur plaque à 13°C et 23°C. Les valeurs de CE50 pour chaque combinaison de fongicide et d’isolat ont varié en fonction de l’État d’origine et étaient plus élevées pour les isolats provenant de l’Arkansas. La température a influencé les valeurs de CE50 du métalaxyl, de l’azoxystrobine et de l’éthaboxam pour P. oopapillum, P. sylvaticum et P torulosum. Les valeurs de CE50 du thirame n’ont pas été influencées par la température, et ce, pour aucune des espèces. Les résultats de cette étude indiquent que l’agressivité et la sensibilité aux fongicides de Pythium spp. varient géographiquement, ce qui suggère que la gestion de la fonte des semis chez le soya devrait davantage cibler les populations locales de Pythium plutôt que les populations régionales.
Introduction
In the United States, seedling disease was ranked second to third amongst all soybean (Glycine max (L.) Merr.) diseases from 2015 to 2017, with yield loss accounting for 163.6 million bushels during that period (Bradley et al. Citation2015, Citation2016, Citation2017; Allen et al. Citation2017). Pythium spp. are considered one of the most important pathogens causing seedling disease, as they are frequently isolated from symptomatic seedlings (Rizvi and Yang Citation1996; Yang and Feng Citation2001; Murillos-Williams and Pedersen Citation2008; Rojas et al. Citation2017a).
Pythium spp. favour saturated soils and cool temperatures for infection to occur (Rizvi and Yang Citation1996; Yang Citation1997; Mueller et al. Citation2016; Rojas et al. Citation2017a; Serrano and Robertson Citation2018). Under these conditions, Pythium spp. cause pre- and post-emergence damping-off of soybean, which can affect the seed, roots, and hypocotyl (Brown and Kennedy Citation1965; Griffin Citation1990; Rizvi and Yang Citation1996; Martin and Loper Citation1999; Hartman et al. Citation2015; Mueller et al. Citation2016). Temperature influenced the pathogenicity of Pythium spp. isolated from diseased seedlings collected from the north central region of the U.S. and Ontario, Canada (Rojas et al. Citation2017a). Temperature also influenced the aggressiveness of various Pythium spp. in Iowa, Minnesota, and North Dakota (Zitnick-Anderson and Nelson Citation2015; Matthiesen et al. Citation2016; Radmer et al. Citation2017); however, the aggressiveness and temperature preference of species recovered from different states has not been compared.
The diversity of Pythium spp. across states is extensive (Broders et al. Citation2007, Citation2009; Jiang et al. Citation2012; Marchand et al. Citation2014; Zitnick-Anderson and Nelson Citation2015; Matthiesen et al. Citation2016; Radmer et al. Citation2017; Rojas et al. Citation2017a). Rojas et al. (Citation2017a) reported 67 species of Pythium (32 of which were pathogenic) recovered from symptomatic soybean seedlings across 11 states and Ontario, Canada. In addition, several studies have identified and reported the pathogenicity of Pythium species from an individual state or province. In North Dakota, 26 Pythium species were isolated from symptomatic soybean seedlings and 20 species were pathogenic (Zitnick-Anderson and Nelson Citation2015), whereas 31 Pythium spp. were identified from soil or soybean seedling samples in Minnesota and nine species were pathogenic (Radmer et al. Citation2017). In Illinois, Jiang et al. (Citation2012) identified 27 Pythium spp. recovered from soil samples with 10 species being pathogenic. In these studies, some, but not all, species of Pythium were common across states. In addition, the relative pathogenicity or aggressiveness of species common across states varied among studies. Although experimental conditions varied among studies, the results of these studies suggest there might be inherent differences in local populations of these species. Therefore, it is important to compare the aggressiveness of Pythium spp. recovered from different states to understand better under what conditions individual species infect soybean and cause disease across states.
Currently, management of damping-off of soybean caused by Pythium spp. includes improving soil drainage, conventional tillage, host resistance, and fungicide seed treatments (Hartman et al. Citation1999; Grau et al. Citation2004; Kirkpatrick et al. Citation2006; Broders et al. Citation2007, Citation2009; Bates et al. Citation2008; Rosso et al. Citation2008; Ellis et al. Citation2013; Lamichhane et al. Citation2017; Klepadlo et al. Citation2019). However, fungicide seed treatments are typically the most convenient and effective management strategy to protect seeds and seedlings from infection. Metalaxyl, mefenoxam, and strobilurins are common active ingredients used to control Pythium spp. on soybean (Cohen and Coffey Citation1986; Ypema and Gold Citation1999; Broders et al. Citation2007; Ellis et al. Citation2013; Hartman et al. Citation2015). Reduced sensitivity to these fungicide active ingredients has been reported for Pythium spp., which increases the risk of damping-off of soybean (Dorrance et al. Citation2004; Broders et al. Citation2007; Mueller et al. Citation2013). Comparison of fungicide sensitivity of Pythium spp. common across many states is currently unknown.
We hypothesized that Pythium spp. from 11 states () would vary in aggressiveness and fungicide sensitivity at two temperatures (13°C and 23°C), and that individual species would differ in aggressiveness across states. Therefore, the objectives of this research were to (i) determine the aggressiveness of four Pythium spp. that were recovered from symptomatic soybean seedlings in 11 states, (ii) for each Pythium spp. compare aggressiveness across all states, (iii) quantify fungicide sensitivity of all Pythium isolates, and (iv) evaluate the effect of temperature (13°C and 23°C) on aggressiveness and fungicide sensitivity. Results from this research will provide an improved understanding of the aggressiveness and fungicide sensitivity of Pythium spp. from 11 states and how geographic location might influence the occurrence of damping-off of soybean caused by Pythium spp.
Table 1. Number of Pythium isolates recovered from symptomatic soybean seedlings collected in 11 states of the United States of America and evaluated for aggressiveness and fungicide sensitivity at 13°C and 23°Ca.
Materials and methods
Pythium isolates
One hundred and eighteen isolates, representing four species of Pythium (P. lutarium, P. oopapillum, P. sylvaticum, and P. torulosum) present in 11 states, were compared for their aggressiveness on soybean and fungicide sensitivity (Supplementary ; ). All isolates were recovered from diseased soybean seedlings as part of a survey conducted in 2011 and 2012 across 11 states and Ontario Canada and identified as described in Rojas et al. (Citation2017a). Isolates were stored at 23°C in the dark on 4% V8 juice media [40 mL V8 juice, 0.6 g calcium carbonate, 0.2 g bacto yeast agar, 1.0 g sucrose, 0.01 g cholesterol, 20.0 g bacto agar, 960 mL deionized water] (DV8), and amended with neomycin sulphate (100 mg L−1) and chloramphenicol (10 mg L−1) (DV8++) (Matthiesen et al. Citation2016).
Seed and seedling assays
Seed and seedling assays at 13°C and 23°C were used to assess the aggressiveness of each Pythium isolate on soybean cv. Sloan. Temperatures (13°C and 23°C) were chosen to represent the range of soil temperatures during soybean planting in the 11 states. A Petri dish seed assay, similar to that reported by Matthiesen et al. (Citation2016), was used. Briefly, isolates on DV8++ were incubated at 23°C for 3 to 4 days in the dark. From the edge of the colony, a 3 mm plug was removed and placed at the centre of a new 100 mm x 15 mm Petri dish containing 20 mL of DV8++. Sloan seeds were surface sterilized in 0.525% sodium hypochlorite solution for 3 min, rinsed continuously with sterile water for 1 min, and air dried for 15 min. When cultures were 3 to 4 days old, 10 seeds were placed 5 mm from the edge of the Petri dish. Petri dishes were incubated in the dark in growth chambers at two temperatures: (i) 13°C or (ii) 23°C. For each species, a non-inoculated control of 10 surface sterilized seeds was placed on Petri dishes of DV8++ only. Petri dishes for all treatments were arranged in a randomized complete block design at each temperature with three replicates per isolate (N = 118). Each growth chamber experiment was completed three times per temperature with each run separated by at least 24 hr. After 7 days, seeds and/or roots were visually assessed for disease severity. For the seed assay, disease severity was estimated as the percentage of seed and radicle tissue that was rotted.
A seedling-cup assay similar to that reported by Matthiesen et al. (Citation2016) was used to assess seedling disease with the following revision: inoculum of each isolate was prepared on white grain millet. Millet was soaked in deionized water for 24 hr and then excess water was drained. Spawn bags (0.2-micron pore patch) (MycoSupply Company, Pittsburgh, PA) containing 500 mL of soaked millet were autoclaved for 30 min and cooled for 24 hr twice with each cycle separated by 24 hr. Isolates were placed on DV8++ and incubated at 23°C for 5 to 7 days in the dark. Each colonized Petri dish was cut into 10 mm2 pieces that were mixed with one spawn bag of sterile millet, which was incubated at 23°C in the dark for 7 to 10 days. Every 2 to 3 days, the inoculated millet was gently mixed by rotating the spawn bag to incorporate mycelia evenly throughout the spawn bag. After 7 to 10 days, the millet inoculum was dried for 2 days in fume hood. For the seedling assay, polystyrene cups (237 mL), with four 3 mm holes on the bottom of the cup for drainage were used. Each cup was separated into layers, beginning from the bottom of the cup: coarse vermiculite (150 mL), inoculum (10 mL), coarse vermiculite (50 mL), seeds (10), and coarse vermiculite (25 mL). Filled cups were incubated in growth chambers at either 13°C or 23°C on a light cycle of 16 h light and 8 h dark. Control cups contained sterile millet in place of inoculated millet. For each temperature, cups were arranged in a randomized complete block design with three replicates per isolate (N = 118). Each isolate was tested at each temperature three times, with each run separated by at least 24 hr. After 14 days, seedlings were carefully removed from the cups, roots were washed for 5 min under running tap water to remove any vermiculite, and disease severity was assessed as described above.
Koch’s postulates were performed for each isolate using the above-mentioned seedling assay and a modified isolation method from Broders et al. (Citation2007). After 14 days, one symptomatic seedling was selected arbitrarily and washed as described above. Symptomatic tissue (3 to 4 mm from the lesion edge) was cut from the roots, pressed between two sterile paper towels and placed under corn meal agar (17 g corn meal agar, 1000 mL deionized water) (CMA) amended with pimaricin (5 mg L−1), ampicillin (250 mg L−1), rifampicin (10 mg L−1), pentachloronitrobenzene (50 mg L−1), and benomyl (10 mg L−1) (CMA+PARP+B). Isolation plates were incubated at 23°C for 3 to 5 days in the dark. Isolates recovered from symptomatic soybean seedling tissues were morphologically identical to that of the isolate used to inoculate seedlings and thus fulfilled Koch’s postulates (Middleton Citation1943; Waterhouse Citation1967; Van der Plaats-niterink Citation1981; Ali-Shtayeh and Dick Citation1985; Bala et al. Citation2010).
Fungicide sensitivity assay
Isolates were evaluated for fungicide sensitivity to metalaxyl (Allegiance®; Bayer CropScience), azoxystrobin (Dynasty®; Syngenta Crop Protection Inc.), ethaboxam (Intego®; Valent U.S.A. Corporation), captan (Captan 400; Bayer CropScience), and thiram (Bayer CropScience) by determining the EC50 of each isolate. To provide the correct concentration of active ingredient, each fungicide was dissolved in acetone. Each fungicide solution was added to clarified DV8 (cDV8), after autoclaving when media temperature had cooled to 50°C (Broders et al. Citation2007), to final concentrations of 0, 0.1, 1.0, 10, and 100 μg a.i. mL−1 of commercial-grade product. For azoxystrobin, 50 µg mL−1 of salicylhydroxamic acid (SHAM) was added to the media to inhibit the alternative oxidase respiratory pathway (Olaya et al. Citation1998; Broders et al. Citation2007).
A fungicide sensitivity assay, similar to that described by Matthiesen et al. (Citation2016) was used. After isolates were grown on DV8++ in the dark for 3 to 4 days at 23°C, a 3 mm plug from the edge of the colony was removed and placed at the edge of a new 100 mm × 15 mm Petri dish containing 20 mL of clarified DV8 (cDV8) amended with each fungicide. Petri dishes containing either cDV8 media only or cDV8 media with SHAM were used as controls. Two temperature conditions were used: (i) 13°C or (ii) 23°C. For each fungicide, a randomized complete block design with a factorial arrangement (4 species × 5 fungicide concentrations) blocked by temperature (13°C and 23°C) was used. There were three replicate plates of each isolate (N = 118) for each fungicide concentration. Each experiment was repeated two times for a total of three independent runs with each run separated by at least 24 hr. At 48, 72, and 96 h after inoculation, mycelial growth was measured from the edge of the plug to the edge of the colony growth. A change in mycelial growth of each isolate at each concentration was calculated as: [(colony length (non-amended) – colony length (amended))/colony length (non-amended)] × 100. For azoxystrobin, a change in mycelial growth of each isolate at each concentration amended with SHAM was calculated as: [(colony length (SHAM amended) – colony length (fungicide-SHAM amended))/colony length (SHAM amended)] × 100.
Statistical analysis
Analysis of variance (ANOVA) was performed to determine the main effects and interactions on disease severity (seed rot and root rot) at each assessment time using PROC MIXED of SAS (version 9.4; SAS Institute Inc. Cary, NC). Temperature, species, and state of origin were treated as fixed effects and replication and run as random effects. Results were not compared among species, as assays for each species were conducted separately. Tukey’s Honestly Significant Difference (HSD) test was used to compare differences in treatment means for disease severity at P = 0.05 (Sokal and Rohlf Citation1989). Percentage of mycelia inhibition was transformed into probits and plotted against a log base 10 value of fungicide concentrations to calculate EC50 values (Zadoks and Schein Citation1979). Temperature, species, state of origin, fungicide, and fungicide concentration were treated as fixed effects and replication and run as random effects. PROC GLM of SAS (version 9.4) was used to analyse EC50 values. Tukey’s Honestly Significant Difference (HSD) test was used to separate treatment means of fungicide sensitivity at P = 0.05.
Results
Seed and seedling assays
All isolates of Pythium lutarium, P. oopapillum, P. sylvaticum, and P. torulosum were pathogenic on soybean cultivar ‘Sloan’ at 13°C and 23°C in both assays compared to the noninoculated control (P < 0.0001). For all four species tested, isolates from AR were more aggressive than isolates from all other states at both temperatures in both assays. Seed plus radicle rot severity (seed assay) and root rot severity (seedling assay) scores of individual Pythium isolates within each species, state of origin, and temperature did not differ in either assay. Therefore, severity ratings for all isolates of each species were combined per state of origin, temperature, and assay. As experimental runs were not significantly different, all runs of each species per state of origin and temperature were combined for each assay.
Seed assay
Pythium lutarium
Seed rot severity differed by state of origin at 13°C (P = 0.0142) and 23°C (P = 0.0117). Severity ranged from 37.5% (ND) to 100% (AR) at 13°C and 41.6% (MI) to 100% (AR) at 23°C. Temperature had no effect on seed rot severity for isolates from AR, IL, IN and MI, while more seed rot occurred at 23°C compared to 13°C for isolates from the remaining four states (IA, KS, ND, and NE) (P < 0.0109; ).
Fig. 1 Aggressiveness, as a percentage of seed/root rot severity, of four Pythium spp. collected from 11 states of the United States of America and tested on soybean cultivar, ‘Sloan’, using a seed assay. Isolates of Pythium lutarium (A), P. oopapillum (B), P. sylvaticum (C), and P. torulosum (D) incubated at 13°C and 23°C. Columns showing capitalized letters or lower-case letters indicate the level of significance across states for isolates incubated at 13°C or 23°C, respectively. Values followed by the same letter are not significantly different according to Tukey’s Honestly Significant Difference (HSD) test (α = 0.05). Columns denoting an asterisk (*) indicate significant difference between 13°C and 23°C per state
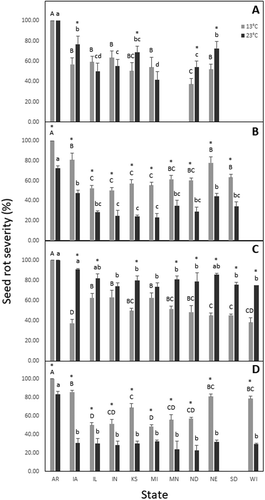
Pythium oopapillum
At 13°C and 23°C, seed rot severity differed by state of origin (P = 0.0135 and P = 0.0098, respectively) with the isolate from AR at 13°C being the most aggressive and those isolates from IL and IN the least aggressive. Severity ranged from 50.4% (IN) to 100% (AR) at 13°C and 23.3% (MI) to 72.6% (AR) at 23°C. More seed rot was observed at 13°C compared to 23°C for all states (P < 0.0129); ).
Pythium sylvaticum
Severity differed by state of origin at 13°C (P = 0.0176), while less differentiation in severity was detected among states at 23°C (P = 0.0299). Severity ranged from 37.5% (IA) to 100% (AR) at 13°C and 73.6% (MI) to 100% (AR) at 23°C. Seed rot severity was greater at 23°C compared to 13°C for isolates from most states (P < 0.0189), except AR, IN, and MI ().
Pythium torulosum
At 13°C, seed rot severity differed by state of origin (P = 0.0166) with isolates from AR being the most aggressive and those from IL and MI the least aggressive. At 23°C, severity was greatest for AR compared to all other states. Severity ranged from 48.3% (MI) to 100% (AR) at 13°C and 22.5% (ND) to 83.3% (AR) at 23°C. Isolates from all states were more aggressive at 13°C compared to 23°C (P < 0.0109; ).
Seedling assay
Pythium lutarium
Root rot severity differed by state of origin at 13°C (P = 0.0102) and 23°C (P = 0.0159). Severity ranged from 38.9% (ND) to 100% (AR) at 13°C and 43.3% (MI) to 100% (AR) at 23°C. Similar to seed rot severity, an effect of temperature was detected on isolates from four states (IA, KS, ND, and NE) with severity greater at 23°C compared to 13°C (P < 0.0273; ).
Fig. 2 Aggressiveness, as a percentage of root rot severity, of four Pythium spp. collected from 11 states of the United States of America and tested on soybean cultivar, ‘Sloan’, using a seedling assay. Isolates of Pythium lutarium (A), P. oopapillum (B), P. sylvaticum (C), and P. torulosum (D) incubated at 13°C and 23°C. Columns showing capitalized letters or lower-case letters indicate the level of significance across states for isolates incubated at 13°C or 23°C, respectively. Values followed by the same letter are not significantly different according to Tukey’s Honestly Significant Difference (HSD) test (α = 0.05). Columns with an asterisk (*) indicate greater aggressiveness (P < 0.05) for either 13°C and 23°C for each state
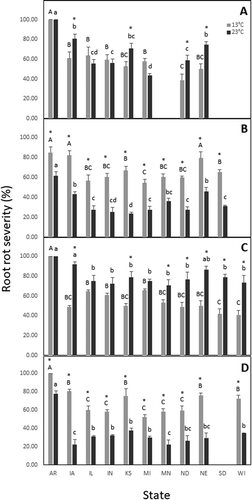
Pythium oopapillum
At 13°C and 23°C, root rot severity differed by state of origin (P = 0.0108 and P = 0.0095, respectively) ranging from 54.5% (MI) to 85.2% (AR) at 13°C and 23.6% (KS) to 61.9% (AR) at 23°C. Greater root rot severity was observed at 13°C compared to 23°C for all states (P < 0.0207; ).
Pythium sylvaticum
Root rot severity differed by state of origin at 13°C (P = 0.0319) and 23°C (P = 0.0452). Severity ranged from 40.8% (WI) to 100% (AR) at 13°C and 70.4% (MN) to 100% (AR) at 23°C. More root rot occurred at 23°C compared to 13°C for isolates from IA, KS, MN, ND, NE, SD and WI (P < 0.0227; ).
Pythium torulosum
At both 13°C and 23°C, root rot severity differed by state of origin (P = 0.0233 and P = 0.0122, respectively). Severity ranged from 52.5% (MI) to 100% (AR) at 13°C and 22.5% (MN) to 77.5% (AR) at 23°C. An effect of temperature on root rot severity was detected for all states with more root rot observed at 13°C compared to 23°C (P < 0.0196; ).
Fungicide sensitivity assay
The growth of all 118 isolates evaluated was less in the presence of all fungicides tested at 13°C and 23°C compared with the non-amended media control (P < 0.0222). The EC50 values of all isolates were greater for azoxystrobin compared to metalaxyl, ethaboxam, captan, or thiram at both temperatures ( and ). EC50 values of Pythium isolates within each species, state, and temperature did not differ; therefore, EC50 values for all Pythium isolates of each species were combined for each state and temperature. All runs of each species, state, and temperature were combined, as experimental runs were not significantly different.
Table 2. Fungicide sensitivity, reported as EC50 values (µg mL−1) ± SE (Range), of four Pythium spp. to metalaxyl and azoxystrobin across 11 states of the United States of America at 13°C and 23°Ca.
Table 3. Fungicide sensitivity, reported as EC50 values (µg mL−1) ± SE (Range), of four Pythium spp. to ethaboxam, captan, and thiram across 11 states of the United States of America at 13°C and 23°Ca
Pythium lutarium
EC50 values differed by state of origin at 13°C (P = 0.0398) and 23°C (P = 0.0417) for all fungicides. At 23°C, the EC50 value of azoxystrobin for the AR isolate was seven times greater than the value observed for the isolates from MI. Temperature had no effect on EC50 values of all fungicides among states, except azoxystrobin with isolates from AR, IA, KS, ND, and NE having greater EC50 values at 23°C compared to 13°C (P < 0.0242; and ).
Pythium oopapillum
At 13°C (P = 0.0440) and 23°C (P = 0.0467), EC50 values differed by state of origin for all fungicides, except thiram at 13°C. At 23°C, the EC50 values of metalaxyl and thiram for the AR isolate were 63 times and 65 times greater, respectively, than the EC50 values of metalaxyl and thiram for the isolates from KS and MI. An effect of temperature on EC50 values of azoxystrobin and ethaboxam was detected with EC50 values of all states greater at 13°C compared to 23°C (P < 0.0277). Isolates from all states, except AR and IA, had greater EC50 values for metalaxyl at 13°C compared to 23°C (P < 0.0241) and isolates from all states, except AR, IL, and MI, had greater EC50 values for captan at 13°C compared to 23°C (P < 0.0331; and ).
Pythium sylvaticum
At both 13°C and 23°C, EC50 values differed by state of origin for all fungicides (P = 0.0397 and P = 0.0409, respectively). The EC50 value of metalaxyl at 13°C for the AR isolates was 11 times greater than the EC50 value of metalaxyl for the WI isolate; whereas at 23°C, the EC50 value of metalaxyl for the AR isolates was only two times greater than the EC50 value of metalaxyl for the WI isolate. Temperature had an effect on EC50 values of azoxystrobin for all states, with isolates having greater EC50 values at 23°C compared to 13°C (P < 0.0246). For metalaxyl and captan, no effect of temperature was detected for isolates from IL, IN, and MI, whereas isolates from all other states had greater EC50 values at 23°C compared to 13°C (P < 0.0115; and ).
Pythium torulosum
At 13°C (P = 0.0310) and 23°C (P = 0.0436), EC50 values differed by state of origin for all fungicides. At 13°C, the EC50 value of metalaxyl for the AR isolates was five times and four times greater than the EC50 values of metalaxyl for the MI and MN isolates, respectively. Whereas at 13°C, the EC50 value of azoxystrobin for the AR isolates was three times and four times greater than the EC50 values of azoxystrobin for the MI and MN isolates, respectively. An effect of temperature on EC50 values of metalaxyl, azoxystrobin, and ethaboxam was detected with isolates from all states having greater EC50 values at 13°C compared to 23°C (P < 0.0344; and ).
Discussion
This study builds on previous research that examined the prevalence of Pythium spp. associated with soybean damping-off in Iowa (Matthiesen et al. Citation2016) by comparing the aggressiveness and fungicide sensitivity of four Pythium species recovered from symptomatic seedlings in 11 states. Over the past two decades, numerous studies have described the diversity and pathogenicity of Pythium spp. recovered from soybean seedlings collected across several states (Rizvi and Yang Citation1996; Zhang and Yang Citation2000; Broders et al. Citation2007, Citation2009; Jiang et al. Citation2012; Marchand et al. Citation2014; Zitnick-Anderson and Nelson Citation2015; Matthiesen et al. Citation2016; Radmer et al. Citation2017; Rojas et al. Citation2017a). This study further contributes to our understanding of each of the four most prevalent species of Pythium (P. lutarium, P. oopapillum, P. sylvaticum, P. lutarium) that infect soybean because it compares the aggressiveness and fungicide sensitivity of isolates representing each species collected from 11 states in a single experiment (). We found aggressiveness and fungicide sensitivity of Pythium spp. varied geographically, which suggests that management of damping-off of soybean should target state rather than regional (multi-state) Pythium populations.
In our experiments, we found that all P. oopapillum and P. torulosum isolates were aggressive at 13°C and all P. lutarium and P. sylvaticum isolates were aggressive at 23°C. Similarly, we previously reported that P. torulosum isolates were more aggressive at 13°C compared to 23°C, whereas P. sylvaticum isolates were more aggressive at 23°C compared to 13°C (Matthiesen et al. Citation2016). Other studies also have demonstrated an effect of temperature on Pythium spp. recovered from soybean seedlings. Radmer et al. (Citation2017) reported that P. sylvaticum caused a larger decrease in root length at 25°C than at 15°C or 20°C, and Rojas et al. (Citation2017a) showed that P. oopapillum and P. torulosum caused greater seed rot at 13°C than at 20°C. The influence of temperature on the aggressiveness of Pythium spp. illustrates the importance of soil temperature associated with planting date of soybean in the United States. Typically, soybean is planted when soil temperatures are >10°C (Hoeft et al. Citation2000); however, soil temperatures at planting often fluctuate and fall below 10°C, depending on the movement of weather systems (De Bruin and Pedersen Citation2008; Serrano and Robertson Citation2018; Serrano et al. Citation2018). Low soil temperatures and wet soils can increase the risk of damping-off due to the seed being susceptible to disease for an extended period of time (Martin and Loper Citation1999). Serrano et al. (Citation2018) reported that when pots containing soybean seed and P. sylvaticum were exposed to a period of cold stress (4°C or 10°C for 96 h) 2 to 4 days after planting, stand emergence was significantly reduced as a result of damping-off. For many cropping systems, adjusting the planting date can be an effective management strategy for combating disease (Nelson Citation1999; Diaz et al. Citation2005; Bekkerman et al. Citation2008; Naseri Citation2013). Our data suggest that adjusting the planting date may have little effect in minimizing disease severity for fields with a diverse population of Pythium spp.
The prevalence of Pythium spp. diversity associated with diseased soybean seedlings collected within and among individual states often differs (Broders et al. Citation2007, Citation2009; Zitnick-Anderson and Nelson Citation2015; Radmer et al. Citation2017). In 2011 and 2012, Rojas et al. (Citation2017a) found that P. sylvaticum was the most prevalent species recovered in 11 states. In the present study, P. sylvaticum isolates from all states were generally more aggressive (caused more severe seed and root rot) than the other Pythium spp. tested. In our previous study, P. sylvaticum isolates from Iowa were more aggressive than the other Pythium spp. evaluated (Matthiesen et al. Citation2016). Specifically, this species caused more severe seed and root rot when temperatures are optimal (23°C) compared to seed and root rot caused by P. lutarium, P. oopapillum, and P. torulosum at their optimum temperatures for pathogenicity (23°C, 13°C, and 13°C, respectively). Therefore, as P. sylvaticum was both the most prevalent and aggressive species of Pythium across the 11 states we tested, seedling disease management practices should perhaps target P. sylvaticum before considering another Pythium spp., such as P. lutarium, P. oopapillum, or P. torulosum, that also may be present in the region.
All Pythium isolates from AR, regardless of species, caused the most seed and root rot compared to all other Pythium isolates evaluated. These isolates were collected from a research field with a history of a rice-soybean rotation (Urrea et al. Citation2013). Seedling disease associated with both soybean and rice can be caused by several Pythium spp., including P. sylvaticum and P. torulosum (Binggan and Bingxin Citation2003; Eberle Citation2008; Matsumoto and Aye Citation2011; Stetina Citation2013). One possible explanation for the greater aggressiveness observed with these isolates is excess water availability provided when rice paddy fields are flooded or when planted to soybean. Rice paddy fields have poor drainage, which can lead to frequent flooding when fields are planted to soybean (Kato et al. Citation2013). Excess water availability combined with a crop rotation, which includes two susceptible hosts, could have allowed for the continued reproduction and growth of Pythium throughout the soybean-rice rotation. Pythium spp. are oomycetes and prefer saturated soil conditions in which they produce zoospores that are attracted to roots by chemotaxis (Hendrix and Campbell Citation1973; Stanghellini Citation1974; Rupe et al. Citation2011). Kirkpatrick et al. (Citation2006) showed that the isolation frequency of Pythium spp. increased when flooding occurred, indicating that greater reproduction occurs under flooded conditions. Other studies have reported an increase in disease severity caused by Pythium spp. when soils are flooded. Kato et al. (Citation2013) demonstrated that there was an increase in pre-emergence damping-off of soybean seedlings caused by Pythium spp. as the length of time the soil was flooded increased; roots were collected from a soybean field converted from a paddy field in Japan. In addition, Li et al. (Citation2015) found that increasing the time soil was waterlogged from 1 hr to 24 hr, increased the severity of hypocotyl and root rot of common bean caused by P. irregulare. Saturated soils increase the diffusion of soybean seed or root exudates throughout the soil, resulting in increased sporangia and zoospore production of Pythium spp. (Stanghellini and Hancock Citation1971; Nelson Citation2004; Rupe et al. Citation2011). Flooding can exacerbate Pythium infection causing repeated growth of sporangia and zoospores, which could potentially increase the likelihood of species evolution. This could change the dynamic of Pythium populations present in the field and provide selection pressure for the emergence of more aggressive isolates.
The effect of temperature on the aggressiveness of isolates varied by state of origin. Isolates of P. sylvaticum from all states, except AR, IN, and MI, showed a significant difference in aggressiveness between 13°C and 23°C. Isolates of P. lutarium, P. oopapillum, and P. torulosum from IA and NE were similar to each other in their aggressiveness at 13°C and similar to each other in their aggressiveness at 23°C. The role of environmental factors on the expression of disease in plant pathogens has been studied extensively worldwide (Pariaud et al. Citation2009; Mariette et al. Citation2016; Velasquez et al. Citation2018; Delgado-Baquerizo et al. Citation2020). However, only one study that we are aware of has compared Pythium spp. across several states. Rojas et al. (Citation2017b) found that states adjacent to one another had similar communities and grouped together, for example: IL, IN, and MI, and IA, NE, and SD. In their study, they showed a correlation between latitude and oomycete community composition suggesting edaphic and environmental factors were responsible. Other studies have also found that community structure differs by geographic region. Tedersoo et al. (Citation2014) showed taxonomic fungal groups varied depending on their distance from the equator. Likewise, Mariette et al. (Citation2016) found that Phytophthora infestans isolates from three geographical locations (Northern and Western Europe, and the Mediterranean Basin) differed in their aggressiveness components (latent period, lesion growth rate, sporangia production, and fitness index) depending on temperature (10°C, 14°C, 18°C, 24°C). Our data suggests that the Pythium spp. we studied could be exhibiting geographic adaptation to temperature. Plant breeders should focus on creating soybean cultivars that show resistance against Pythium spp. that encompass the local community structure in specific states.
Implementation of fungicide management practices should first be based on an assessment of fungicide sensitivity levels. Numerous studies have shown variation in fungicide sensitivity of Pythium spp. recovered from various crops (Kato et al. Citation1990; Dorrance et al. Citation2004; Broders et al. Citation2007; Lu et al. Citation2012; Weiland et al. Citation2014; Matthiesen et al. Citation2016; Radmer et al. Citation2017). Similar to previous findings (Matthiesen et al. Citation2016), the Pythium spp. tested in this study showed variability in fungicide sensitivity with some Pythium spp. less sensitive (having greater EC50 values) to some fungicides. One reason for this variation of fungicide sensitivity amongst Pythium spp. could be an association between sporangia morphology and fungicide active ingredient. Broders et al. (Citation2007) evaluated the fungicide sensitivity of 58 Pythium isolates representing 11 species and 2 groups. They reported that Pythium species with globose sporangia (i.e. P. sylvaticum) were less sensitive to azoxystrobin and trifloxystrobin (quinone outside inhibitors) than species with filamentous sporangia (i.e. P. torulosum). In this study, P. sylvaticum (globose sporangia) was less sensitive to azoxystrobin at its optimum temperature of 23°C than P. lutarium, P. oopapillum, or P. torulosum (filamentous sporangia) at their optimum temperatures of 23°C, 13°C, and 13°C, respectively. This suggests that species morphology could be related to fungicide sensitivity, especially at temperatures where the species is most aggressive.
Fungicide seed treatments are commonly recommended to soybean farmers, especially when a field has a history of seedling disease and soil conditions at planting are expected to favour seedling disease development (Bradley Citation2008; Munkvold Citation2009; Popp et al. Citation2010; Coker et al. Citation2010; Esker and Conley Citation2012; Urrea et al. Citation2013; Gaspar et al. Citation2017; Serrano et al. Citation2018; Scott et al. Citation2020). Typically, seed treatments include a mixture of active ingredients that combine fungicides with oomycete activity (phenylamides, e.g. metalaxyl or mefenoxam; thiazole caroxamide, e.g. ethaboxam) and fungal activity (demethylation inhibitors, e.g. propiconazole; quinone outside inhibitors, e.g. azoxystrobin; succinate dehydrogenase, e.g. sedaxane) (Giesler and Ziems Citation2008; Coker et al. Citation2010; Urrea et al. Citation2013; Scott et al. Citation2020). Scott et al. (Citation2020) evaluated the efficacy of fungicide seed treatments mixtures with and without ethaboxam against Pythium spp. and Phytophthora sojae, and reported greater soybean stands and yield when ethaboxam was included in the mixture compared to mixtures containing metalaxyl or mefenoxam alone. Similarly, Urrea et al. (Citation2013) evaluated fungicide seed treatments in soils containing soybean seedling pathogens; Pythium spp., Fusarium spp., and Rhizoctonia solani and reported the greatest plant stands across three soybean cultivars occurred when seed was treated with mixtures (trifloxystrobin + metalaxyl and mefenoxam + fludioxonil + azoxystrobin). Reduced fungicide sensitivity has been reported for metalaxyl and mefenoxam, strobilurins, and ethaboxam for Pythium spp. recovered from soybean (Gisi et al. Citation2002; Taylor et al. Citation2002; Dorrance et al. Citation2004; Broders et al. Citation2007; Radmer et al. Citation2017; Noel et al. Citation2019). However, in this study, we found that EC50 values of azoxystrobin and metalaxyl for P. sylvaticum were similar across most states, whereas EC50 values of azoxystrobin and metalaxyl for both P. lutarium and P. torulosum differed across many states when species were at their optimum temperatures (23°C and 13°C, respectively).
In this study, we demonstrated that temperature influenced the aggressiveness of Pythium spp. recovered from 11 states, particularly when species were at their optimum growing temperature. Across all states, P. sylvaticum was most aggressive at 23°C compared to 13°C; however, as aggressiveness of P. sylvaticum at 23°C varied amongst states, this could suggest edaphic or geographic factors play a role in species aggressiveness. Similarly, fungicide sensitivity of Pythium spp. was also dependent on temperature and fungicide active ingredient. EC50 values of azoxystrobin were greater for P. sylvaticum at 23°C; while at 13°C, EC50 values of metalaxyl, azoxystrobin, and ethaboxam were greater for P. torulosum. Our data suggest that the influence of temperature on aggressiveness and fungicide sensitivity could be due to Pythium spp. exhibiting a geographic adaptation to temperature, thereby increasing the prevalence of damping-off of soybean in specific states.
Supplementary Table 1
Download MS Word (38.6 KB)Supplemental material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2021.1881162.
Additional information
Funding
References
- Ali-Shtayeh MS, Dick MW. 1985. Five new species of Pythium (Peronosporomycetidae). Bot J Linn Soc. 91:297–317. doi:https://doi.org/10.1111/j.1095-8339.1985.tb01152.x.
- Allen T, Bradley C, Sisson A, Byamukama E, Chilvers M, Coker C, Collins A, Damicone J, Dorrance A, Dufault N, et al. 2017. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 18:19–27. doi:https://doi.org/10.1094/PHP-RS-16-0066.
- Bala K, Robideau GP, Desaulniers N, de Cock A, Levesque CA. 2010. Taxonomy, DNA barcoding and phylogeny of three new species of Pythium from Canada. Persoonia Mol Phylogeny Evol Fungi. 25:22–31. doi:https://doi.org/10.3767/003158510X524754.
- Bates GD, Rothrock CS, Rupe JC. 2008. Resistance of the soybean cultivar Archer to Pythium damping-off and root rot caused by several Pythium spp. Plant Dis. 92:763–766. doi:https://doi.org/10.1094/PDIS-92-5-0763.
- Bekkerman A, Goodwin BK, Piggott NE. 2008. Spatio-temporal risk and severity analysis of soybean rust in the United States. J Agric Resour Econ. 33:311–331.
- Binggan L, Bingxin Z. 2003. Identification of Pythium sylvaticum and species-specific primers. Mycosystema. 23:356–365.
- Bradley C, Allen T, Chilvers M, Giesler L, Mehl K, Tenuta A, Sisson A, Wise K. 2015. Soybean disease loss estimates from the United States and Ontario, Canada – 2015. Crop Protection Network. CPN-1018-15-W. doi:https://doi.org/10.31274/cpn-20190620-041.
- Bradley C, Allen T, Tenuta A, Mehl K, Sisson A. 2016. Soybean disease loss estimates from the United States and Ontario, Canada – 2016. Crop Protection Network. CPN-1018-16-W. doi:https://doi.org/10.31274/cpn-20190729-000.
- Bradley C, Allen T, Tenuta A, Mehl K, Sisson A. 2017. Soybean disease loss estimates from the United States and Ontario, Canada – 2017. Crop Protection Network. CPN-1018-17-W. doi:https://doi.org/10.31274/cpn-20190729-001.
- Bradley CA. 2008. Effect of fungicide seed treatments on stand establishment, seedling disease, and yield of soybean in North Dakota. Plant Dis. 92:120–125. doi:https://doi.org/10.1094/PDIS-92-1-0120.
- Broders KD, Lipps PE, Paul PA, Dorrance AE. 2007. Characterization of Pythium spp. associated with corn and soybean seed and seedling disease in Ohio. Plant Dis. 91:727–735. doi:https://doi.org/10.1094/PDIS-91-6-0727.
- Broders KD, Wallhead MW, Austin GD, Lipps PE, Paul PA, Mullen RW, Dorrance AE. 2009. Association of soil chemical and physical properties with Pythium species diversity, community composition, and disease incidence. Phytopathology. 99:957–967. doi:https://doi.org/10.1094/PHYTO-99-8-0957.
- Brown GE, Kennedy BW. 1965. Pythium pre-emergence damping-off of soybean in Minnesota. Plant Dis Rep. 49:646.
- Cohen M, Coffey MD. 1986. Systemic fungicides and the control of oomycetes. Annu Rev Phytopathol. 24:311–338. doi:https://doi.org/10.1146/annurev.py.24.090186.001523.
- Coker C, Rupe JC, Monfort S, Kirkpatrick TL. 2010. Arkansas plant disease control products guide. MP 154. Little Rock (AR): University of Arkansas Division of Agriculture Cooperative Extension Service.
- De Bruin JL, Pedersen P. 2008. Soybean seed yield response to planting date and seeding rate in the upper Midwest. Agron J. 100:696–703. doi:https://doi.org/10.2134/agronj2007.0115.
- Delgado-Baquerizo M, Guerra CA, Cano-Diaz C, Egidi E, Wang J, Eisenhauser N, Singh BK, Maestre FT. 2020. The proportion of soil-borne pathogens increases with warming at the global scale. Nat Clim Change. 11:1–5.
- Diaz CG, Ploper LD, Galvez MR, Gonzalez V, Zamorano MA, Jaldo HE, Lopez C, Ramallo JC. 2005. Effect of late season diseases on the growth of different soybean genotypes in relation to planting date. AgriScientia. 22:1–7.
- Dorrance AE, Berry SA, Bowen P, Lipps PE. 2004. Characterization of Pythium spp. from three Ohio fields for pathogenicity on corn and soybean and metalaxyl sensitivity. Plant Health Prog. 2:1–7.
- Eberle ME 2008. Importance and characterization of seedling pathogens on stand establishment in rice [master’s thesis]. Fayetteville (AR): University of Arkansas.
- Ellis ML, McHale LK, Paul PA, St. Martin SK, Dorrance AE. 2013. Soybean germplasm resistant to Pythium irregulare and molecular mapping of resistance quantitative trait loci derived from the soybean accession PI424354. Crop Sci. 53:1008–1021. doi:https://doi.org/10.2135/cropsci2012.08.0461.
- Esker PD, Conley SP. 2012. Probability of yield response and breaking even for soybean seed treatments. Crop Sci. 52:351–359. doi:https://doi.org/10.2135/cropsci2011.06.0311.
- Gaspar AP, Mueller DS, Wish KA, Chilvers MI, Tenuta AU, Conley SP. 2017. Response of broad-spectrum and target-specific see treatments and seeding rate on soybean seed yield, profitability, and economic risk. Crop Sci. 57:2251–2262. doi:https://doi.org/10.2135/cropsci2016.11.0967.
- Giesler L, Ziems AD. 2008. Plant disease–field crops. Lincoln (NE): UNL Extension publications, University of Nebraska-Lincoln. Nebguide. Issue May. http://extension.unl.edu/publications.
- Gisi U, Sierotzki H, Cook A, McCaffery A. 2002. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manag Sci. 58:859–867. doi:https://doi.org/10.1002/ps.565.
- Grau CR, Dorrance AE, Bond J, Russin JS. 2004. Fungal diseases. In: Shibles RM, Harper JE, Wilson RF, Shoemaker RC, editors. Soybeans: improvement, production, and uses. 3rd ed. Vol. 16. Madison (WI): American Society of Agronomy; p. 679–763.
- Griffin GJ. 1990. Importance of Pythium ultimum in disease syndrome of cv. Essex soybean. Can J Plant Pathol. 12:135–140. doi:https://doi.org/10.1080/07060669009501016.
- Hartman G, Rupe J, Sikora E, Domier L, Davis J, Steffey K, eds. 2015. Compendium of soybean diseases and pests. St. Paul (MN): American Phytopathological Society.
- Hartman GL, Sinclair JB, Rupe JC, eds. 1999. Compendium of soybean diseases. St. Paul (MN): American Phytopathological Society.
- Hendrix FF Jr, Campbell WA. 1973. Pythiums as plant pathogens. Annu Rev Phytopathol. 11:77–98. doi:https://doi.org/10.1146/annurev.py.11.090173.000453.
- Hoeft RG, Nafziger ED, Johnson RR, Aldrich SR. 2000. Soybean as a crop. Modern corn and soybean production. Champaign (IL): MCSP Publications; p. 31–59.
- Jiang YN, Haudenshield JS, Hartman GL. 2012. Characterization of Pythium spp. from soil samples in Illinois. Can J Plant Pathol. 34:448–454. doi:https://doi.org/10.1080/07060661.2012.705326.
- Kato M, Minamida K, Tojo M, Kokuryu T, Hamagucki H, Shimada S. 2013. Association of Pythium and Phytophthora with pre-emergence seedling damping-off of soybean grown in a field converted from a paddy field in Japan. Plant Prod Sci. 16:95–104. doi:https://doi.org/10.1626/pps.16.95.
- Kato S, Coe R, New L, Dick MW. 1990. Sensitivities of various Oomycetes to hymexazol and metalaxyl. J Gen Microbiol. 136:2127–2134. doi:https://doi.org/10.1099/00221287-136-10-2127.
- Kirkpatrick MT, Rupe JC, Rothrock CS. 2006. Soybean response to flooded soil conditions and the association with soilborne pathogenic genera. Plant Dis. 90:592–596. doi:https://doi.org/10.1094/PD-90-0592.
- Klepadlo M, Balk CS, Vuong TD, Dorrance AE, Nguyen HT. 2019. Molecular characterization of genomic regions for resistance to Pythium ultimum var. ultimum in the soybean cultivar Magellan. Theor Appl Genet. 132:405–417. doi:https://doi.org/10.1007/s00122-018-3228-x.
- Lamichhane JR, Durr C, Schwanck AA, Robin MH, Sarthou JP, Cellier V, Messean A, Aubertot JN. 2017. Integrated management of damping-off disease: a review. Agron Sustain Dev. 37:10–20.
- Li YP, You MP, Colmer TD, Barbetti MJ. 2015. Effect of timing and duration of soil saturation on soilborne Pythium diseases of common bean (Phaseolus vulgaris). Plant Dis. 99:112–118. doi:https://doi.org/10.1094/PDIS-09-13-0964-RE.
- Lu XH, Davis RM, Livingston S, Nunez J, Hao JJ. 2012. Fungicide sensitivity of Pythium spp. associated with cavity spot of carrot in California and Michigan. Plant Dis. 96:384–388. doi:https://doi.org/10.1094/PDIS-07-11-0562.
- Marchand G, Chen Y, Berhane NA, Wei L, Lévesque CA, Xue AG. 2014. Identification of Pythium spp. from the rhizosphere of soybeans in Ontario, Canada. Can J Plant Pathol. 36:246–251. doi:https://doi.org/10.1080/07060661.2014.920921.
- Mariette N, Androdias A, Mabon R, Corbière R, Marquer B, Montarry J, Andrivon D. 2016. Local adaptation to temperature in populations and clonal lineages of the Irish potato famine pathogen Phytophthora infestans. Ecol Evol. 6:6320–6331. doi:https://doi.org/10.1002/ece3.2282.
- Martin FN, Loper JE. 1999. Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospects for biological control. Crt Rev Plant Sci. 18:111–181. doi:https://doi.org/10.1080/07352689991309216.
- Matsumoto M, Aye SS. 2011. Chemotaxonomic characterization of fungal genera, causal pathogens of rice seedling diseases, using fatty acid analysis. Bull Inst Trop Agric Kyushu Univ. 34:1–14.
- Matthiesen RL, Ahmad AA, Robertson AE. 2016. Temperature affects aggressiveness and fungicide sensitivity of four Pythium spp. that cause soybean and corn damping off in Iowa. Plant Dis. 100:583–591. doi:https://doi.org/10.1094/PDIS-04-15-0487-RE.
- Middleton JT. 1943. The taxonomy, host range and geographical distribution of the genus Pythium. Lancaster (PA): Published for the Club by Lancaster Press.
- Mueller D, Wise K, Dufault N, Bradley C, editors. 2013. Fungicides for field crops. St. Paul (MN): APS Press.
- Mueller D, Wise K, Sisson A, Smith D, Sikora E, Bradley C, Robertson AE, eds. 2016. A farmer’s guide to soybean diseases. St. Paul (MN): American Phytopathological Society.
- Munkvold GP. 2009. Seed pathology progress in academia and industry. Annu Rev Phytopathol. 47:285–311. doi:https://doi.org/10.1146/annurev-phyto-080508-081916.
- Murillos-Williams A, Pedersen P. 2008. Early incidence of soybean seedling pathogens in Iowa. Agron J. 100:1481–1487. doi:https://doi.org/10.2134/agronj2007.0346.
- Naseri B. 2013. Interpretation of variety x sowing date x sowing depth interaction for bean-Fusarium-Rhizoctonia pathosystem. Arch Phytopathol Pflanzenschutz. 46:2244–2252. doi:https://doi.org/10.1080/03235408.2013.792535.
- Nelson BD. 1999. Fusarium blight or wilt, root rot, and pod and collar rot. Compendium of soybean diseases. St. Paul (MN): American Phytopathological Society.
- Nelson EB. 2004. Microbial dynamics and interactions in the spermosphere. Annu Rev Phytopathol. 42:271–309. doi:https://doi.org/10.1146/annurev.phyto.42.121603.131041.
- Noel ZA, Sang H, Roth MG, Chilvers MI. 2019. Convergent evolution of C239S mutation in Pythium spp. B-tubulin coincides with inherent insensitivity to ethaboxam and implications for other Peronosporalean Oomycetes. Phytopathology. 109:2087–2095. doi:https://doi.org/10.1094/PHYTO-01-19-0022-R.
- Olaya G, Zhen D, Koller W. 1998. Differential responses of germinating Ventura inaequalis conidia to Kresoxim-methyl. Pestic Sci. 54:230–236. doi:https://doi.org/10.1002/(SICI)1096-9063(1998110)54:3<230::AID-PS815>3.0.CO;2-O.
- Pariaud B, Ravigne V, Halkett F, Goyeau H, Carlier J, Lannou C. 2009. Aggressiveness and its role in the adaptation of plant pathogens. Plant Pathol. 58:409–424. doi:https://doi.org/10.1111/j.1365-3059.2009.02039.x.
- Popp M, Rupe JC, Rothrock CS. 2010. Economic evaluation of soybean fungicide seed treatments. J Am Soc Farm Managers Rural Appraisers. 73:50–62.
- Radmer L, Anderson G, Malvick DM, Kurle JE, Rendahl A, Mallik A. 2017. Pythium, Phytophthora, and Phytopythium spp. associated with soybean in Minnesota, their relative aggressiveness on soybean and corn, and their sensitivity to seed treatment fungicides. Plant Dis. 101:62–72. doi:https://doi.org/10.1094/PDIS-02-16-0196-RE.
- Rizvi SSA, Yang XB. 1996. Fungi associated with soybean seedling disease in Iowa. Plant Dis. 80:57–60. doi:https://doi.org/10.1094/PD-80-0057.
- Rojas A, Jacobs J, Napieralski S, Karaj B, Bradley C, Chase T, Esker P, Giesler L, Jardine D, Malvick D, et al. 2017a. Oomycete species associated with soybean seedlings in North America – part I: identification and pathogenicity characterization. Phytopathology. 107:208–292. doi:https://doi.org/10.1094/PHYTO-04-16-0177-R.
- Rojas A, Jacobs J, Napieralski S, Karaj B, Bradley C, Chase T, Esker P, Giesler L, Jardine D, Malvick D, et al. 2017b. Oomycete species associated with soybean seedlings in North America – part II: diversity and ecology in relation to environmental and edaphic factors. Phytopathology. 107:293–304. doi:https://doi.org/10.1094/PHYTO-04-16-0176-R.
- Rosso ML, Rupe JC, Chen P, Mozzoni LA. 2008. Inheritance and genetic mapping of resistance to Pythium damping-off caused by Pythium aphanidermatum in “Archer” soybean. Crop Sci. 48:2215–2222. doi:https://doi.org/10.2135/cropsci2008.01.0035.
- Rupe JC, Rothrock CS, Bates G, Rosso ML, Avanzato MV, Chen P. 2011. Resistance to Pythium seedling disease in soybean. Pages 261–276 in Soybean-molecular aspects of breeding. A. Sudaric, ed. Rijeka, Croatia: In Tech. doi:https://doi.org/10.5772/15301.
- Scott K, Eyre M, McDuffee D, Dorrance AE. 2020. The efficacy of ethaboxam as a soybean seed treatment toward Phytophthora, Phytopythium, and Pythium in Ohio. Plant Dis. 104:1421–1432. doi:https://doi.org/10.1094/PDIS-09-19-1818-RE.
- Serrano M, McDuffee D, Robertson AE. 2018. Damping-off caused by Pythium sylvaticum on soybeans subjected to periods of cold stress is reduced by seed treatments. Can J Plant Pathol. 40:571–579. doi:https://doi.org/10.1080/07060661.2018.1522516.
- Serrano M, Robertson AE. 2018. The effect of cold stress on damping-off of soybean caused by Pythium sylvaticum. Plant Dis. 102:2194–2200. doi:https://doi.org/10.1094/PDIS-12-17-1963-RE.
- Sokal RR, Rohlf FJ. 1989. Introduction to biostatistics. 2nd ed. New York (NY): W. H. Freeman and Co.
- Stanghellini M, Hancock J. 1971. Radial extent of the bean spermosphere and its relation to the behavior of Pythium ultimum. Phytopathology. 61:165–168. doi:https://doi.org/10.1094/Phyto-61-165.
- Stanghellini ME. 1974. Spore germination, growth and survival of Pythium in soil. Proc Am Phytopathol Soc. 1:211–214.
- Stetina T 2013. The effects of salinity on Pythium disease of rice and soybean [master’s thesis]. Fayetteville (AR): University of Arkansas.
- Taylor RJ, Salas B, Secor GA, Rivera V, Gudmestad NC. 2002. Sensitivity of North American isolates of Phytophthora erythroseptica and Pythium ultimum to mefenoxam (metalaxyl). Plant Dis. 86:797–802. doi:https://doi.org/10.1094/PDIS.2002.86.7.797.
- Tedersoo L, Bahram M, Polme S, Koljalg U, Yorou N, Wijesundersa R, Ruiz L, Vasco-Palacios AM, Thu PQ, Suija A, et al. 2014. Global diversity and geography of soil fungi. Science. 346:1256688. doi:https://doi.org/10.1126/science.1256688.
- Urrea K, Rupe JC, Rothrock CS. 2013. Effect of fungicide seed treatments, cultivars, and soils on soybean stand establishment. Plant Dis. 97:807–812. doi:https://doi.org/10.1094/PDIS-08-12-0772-RE.
- Van der Plaats-niterink AJ. 1981. Monograph of the genus Pythium. Stud Mycol. 21:1–242.
- Velasquez AC, Castroverde CM, He SY. 2018. Plant and pathogen warfare under changing climate conditions. Curr Biol. 28:619–634. doi:https://doi.org/10.1016/j.cub.2018.03.054.
- Waterhouse GM. 1967. Key to Pythium Pringsheim. Mycol Pap. 109:1–15.
- Weiland JE, Santamaria L, Grunwald NJ. 2014. Sensitivity of Pythium irregulare, P. sylvaticum, and P. ultimum from forest nurseries to mefenoxam and fosetyl-al, and control of Pythium damping-off. Plant Dis. 98:937–942. doi:https://doi.org/10.1094/PDIS-09-13-0998-RE.
- Yang XB. 1997. Soybean damping off. Iowa State University publication PM939. Ames (IA): Iowa State University.
- Yang XB, Feng F. 2001. Ranges and diversity of soybean fungal diseases in North America. Phytopathology. 91:769–775. doi:https://doi.org/10.1094/PHYTO.2001.91.8.769.
- Ypema HL, Gold RE. 1999. Kresoxim-methyl: modification of a naturally occurring compound to produce a new fungicide. Plant Dis. 83:4–19. doi:https://doi.org/10.1094/PDIS.1999.83.1.4.
- Zadoks JC, Schein RD. 1979. Epidemiology and plant disease management. New York (NY): Oxford University Press Inc.
- Zhang BQ, Yang XB. 2000. Pathogenicity of Pythium populations from corn-soybean rotation fields. Plant Dis. 84:94–99. doi:https://doi.org/10.1094/PDIS.2000.84.1.94.
- Zitnick-Anderson KK, Nelson BD Jr. 2015. Identification and pathogenicity of Pythium on soybean in North Dakota. Plant Dis. 99:31–38. doi:https://doi.org/10.1094/PDIS-02-14-0161-RE.
