Epichloё glyceriae Schardl & Leuchtm., Mycologia 91(1): 103. 1999. MB: 450322
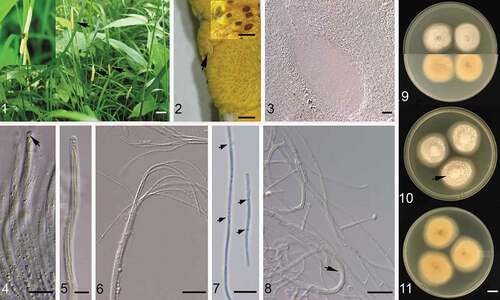
Figs. 1–11 (Colour online) (1) Yellowish-orange coloured stromata (arrows) encasing flowering tillers of Glyceria striata in the field (Mer Bleue Bog, Ottawa); inset showing close-up of a stroma with a symbiotic insect (Botanophila sp.), which could facilitate dispersal of ascospores and crossing of different fungal strains (see more details in Notes). (2) Close-up of a stroma dotted with greyish-yellow to brownish-orange (inset) ostioles, the arrow showing an egg of Botanophila sp. embedded on stroma. (3) A pear-shaped empty perithecium surrounded with stroma tissue textura oblita to porrecta. (4) Young asci, the arrow showing a thick cap with a central pore. (5–6) Mature asci with 8 filiform ascospores. (7) Fragmented ascospores, arrows showing multiple septa. (8) Conidiogenous cells producing obovoid conidia in culture, the arrow showing the aggregation of hyphae to fascicles. (9) Colonies on PDA obverse (top) and reverse (bottom). (10, 11) Colonies on CYA obverse and reverse, the arrow showing aerial hyphae loosely aggregated to numerous ‘clumps’ arranged more or less in rings. (1–7) Canadian National Mycological Herbarium DAOM 984743. (8–11) The Canadian Collection of Fungi Canadenses DAOMC 251848. Bar = 20 mm for 1; 10 mm for 9–11; 1 mm for 2; 200 µm for 2 inset, 20 µm for 3, 4, 6, 8; 10 µm for 5, 7.
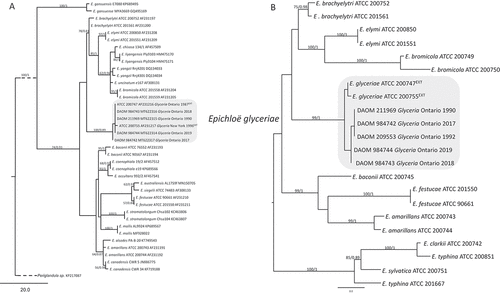
Fig. 12 Phylogenetic trees showing E. glyceriae in relation with other Epichloë species. (a) A rooted tree based on translational elongation factor 1-alpha (tef1) of 39 taxa (20 Epichloë spp.), using outgroup Periglandula sp. Informative characters = 98, Length = 257, CI = 0.891, RI = 0.937, RC = 0.835, HI = 0.109, G-fit = −91.300. Dashed branch is shorter than actual length. (b) A mid-point rooted tree based on concatenated sequences of actin (act1), β-tubulin (tub2), and tef1 of 22 taxa (10 Epichloë spp.). Informative characters = 140, Length = 216, CI = 0.889, RI = 0.924, RC = 0.822, HI = 0.111 G-fit = −134.100. Values on the branch represent bootstrap of parsimony analyses/posterior probability of Bayesian inference, EXT stands for ex-type. GenBank accession numbers for sequences generated in this study: act1, MT633792–MT633796, tef1, MT622314–MT622317, tub2, MT633797–MT633801
LECTOTYPE: Dried stromata developed on Glyceria striata plant # 2772, here designated MBT394123, separated from the enclosed dried culture of the male mating strain as detailed below (lectotype CUP 64922, image available at https://mycoportal.org/portal/collections/individual/index.php?occid=834803&clid=0) produced by experimental mating of original as follows: UNITED STATES New York, Madison Co. Canastota 1987, leg. K. Clay (infected plant with the isolate subsequently deposited as ATCC 200755 ♀) × CANADA Ontario, Osgoode, on Glyceria striata, 1987, leg. K. Clay and A. Leuchtmann (ATCC 200747 ♂), mating experiment and deposit by C.L. Schardl, Fayette Co., Kentucky, 1997. Epitype (CUP70726, designated here, MBT 394124) a dried culture of ATCC 200747, preserved on Jul 18, 1997.
REFERENCE SEQUENCES: E277 (=ATCC 200747) genome sequence PRJNA67247 (NCBI, Submitted by University of Kentucky, Department of Plant Pathology); ATCC 200747 act1 = AF240101, tub2 = L78275, tef1 = AF231216; ATCC 200755 act1 = AF240102, tub2 = L78276, tef1 = AF231217.
STROMATA: 15–35 mm long (n = 10) surrounding undeveloped inflorescence and leaf sheath, white velvet mycelium mass and conidiogenous cells at the bottom covered with a continuous layer of closely arranged yellow orange perithecia (); PERITHECIA: pyriform 250–380 × 106–133 µm (n ≥ 20, ); OSTIOLE: greyish-yellow to brownish-yellow (), 57–126 µm diam (n ≥ 30), slightly elevated when stromata are fresh, severely elevated when stromata are dried after detachment from living plant, making stroma surface severely tuberculate; STROMA TISSUE: around perithecium textura oblita to porrecta in longitudinal view (); ASCI: cylindrical, 110–350 × 6–8 µm, caps 4–5.5 µm thick (n ≥ 30), with a clear central pore (); ASCOSPORES: filiform, 8, hyaline, 110–320 × 1.2–1.8 µm (n ≥ 30, ), multiple septa developed upon maturity and break to fragments of varied length ().
COLONIES: grown on potato dextrose agar (PDA) and CYA 30–40 mm for 21 days at room temperature (19–22 ⁰C) and natural daylight, creamy white with abundant aerial mycelia, velvety, during sporulation aerial hyphae loosely aggregate to numerous ‘clumps’ arranged more or less in rings (, arrow), hyphae arranged in fascicles (, arrow); colony reverse light yellow to orange (); CONIDIOGENOUS CELLS: Neotyphodium-type, arising singly, slender cylindrical, tapering towards apex (6–)15–28(–31) × 2–3 µm; CONIDIA: hyaline, aseptate, obovoid, 6–8 × 3–4 µm (n ≥ 30, ).
HOST: Glyceria striata
DISTRIBUTION: CANADA Ontario, USA New York.
COLLECTIONS: CANADA Ontario, Dundas County, Winchester Township, 45°04ʹ15”N, 75°16ʹ45”W, 1 July 1990 by S. Darbyshire DAOM 211969; Ottawa, Mer Bleue Bog, Anderson Road, 45°24ʹ59.7”N, 75°33ʹ06.5”W, 24 June 2017 by S. Darbyshire (DAOM 984742); Ottawa (Nepean), Stony Swamp, Moodie Drive, 25 June 2018 by M. Liu (DAOM 984743); 10 July 2019 by M. Liu, P. Shoukouhi and K. R. Bisson (DAOM 984744); Ottawa (Waterson Corners), 10 June 1992 by S. Darbyshire (DAOM 209533).
NOTES: Schardl and Leuchtmann (Citation1999) described this species based on the fertile stromata obtained through a mating experiment using a strain from New York as female (ATCC 200755) and a strain from Ontario as male (ATCC 200747). During the experiment, the plant was first inoculated with ATCC 200755 (♀), and vernalized over winter. When the young stromata developed in the following spring, they were fertilized by rubbing the fresh culture of ATCC 200747 (♂) onto the stromata as spermatia. The holotype of Epichloё glyceriae (CUP 64922) included the resulting stromata and also the dried cultures of the male parent (ATCC 200747) in Petri dishes. Although the male parent and resulting stromata share a haplotype, the two parts belong to separate gatherings wherein the male parent was collected from the field, and the resulting stromata were from the laboratory experimental cross. These two separate gatherings should, therefore, not be considered as a single specimen to be designated as holotype (Art. 8.1, 8.2) (Turland et al. Citation2018). Here we designated only the stromata on plant 2772 as lectotype and one dried culture of ATCC 200747 (preserved on Jul 18, 1997) as epitype (CUP 70726). The second culture plate was given a different specimen number CUP 70727.
Due to the scant morphological variations, many Epichloë specimens were originally identified as one species, Epichloë typhina (Craven et al. Citation2001). Molecular phylogenetic studies, combined with host specificity, mating experiments, and morphology have brought better resolution at the species level (Craven et al. Citation2001; Leuchtmann and Oberhofer Citation2013). To date, 35 Epichloë species, 3 subspecies, and 6 varieties are accepted (Leuchtmann et al. Citation2014). Interspecific hybrids of two or more biological Epichloë spp. are common, especially for species predominantly in asexual stages (Schardl Citation1996; Moon et al. Citation2004; Charlton et al. Citation2012). Based on tub2 gene phylogenies and morphological characteristics, Schardl and Leuchtmann (Citation1999) described Epichloë glyceriae while circumscribing a species specifically occurring in the Glyceria striata. Morphologically, E. glyceriae is similar to E. elymi; however, they can be distinguished by minute morphological differences. For example, E. glyceriae produces a more continuous layer of perithecia and longer perithecium necks, in addition to having differing host ranges from that of E. elymi (Schardl and Leuchtmann Citation1999). The phylogenetic trees based on tef1 () and the concatenated DNA sequences of three loci, actin (act1), β-tubulin (tub2) and tef1 (), supported the separation of E. glyceriae from E. elymi and other species although the statistical supports for deeper relationships were low. This could be a result of sampling bias or incomplete lineage sorting because multiple copies of these housekeeping genes were identified in some Epichloë species (Craven et al. Citation2001).
The grass endophytes Epichloë spp. (Clavicipitaceae, Ascomycota) are symbionts that systemically inhabit in cool season grasses (Poaceae subfam. Pooideae) (Schardl and Phillips Citation1997). Epichloë species can be transmitted vertically and/or horizontally (Schardl Citation1996). Obligate asexual species producing sterile hyphae inside plants propagate through plant seeds (Siegel et al. Citation1984; Philipson and Christey Citation1986; Gagic et al. Citation2018) or root tissues (Azevedo and Welty Citation1995), which often results in restricted host ranges. Alternatively, sexual species developing reproductive structures on the flowering tillers, thereby preventing the plant from seeding, are transmitted horizontally through the dispersal of sexual spores in the air (Chung and Schardl Citation1997) and/or vectored by insects, e.g., Botanophila spp. ( inset, Bultman et al. Citation1995; Leuchtmann Citation2003). The adult flies lay eggs on the unfertilized fungal stromata ( arrow). Upon hatching, the larvae feed on fungal tissues until they mature and then, during the following year after pupation in the ground, the adult flies can transfer the fungal spermatia to another stroma, causing cross-fertilization promoted by the flies’ attraction to the aromatic emissions of stromata (Leuchtmann Citation2003).
The roles and functions of Epichloë endophytes in agricultural and natural ecosystems have attracted a significant amount of research interest since the 1980s. Nonetheless, there are still large knowledge gaps and consequently unanswered questions. Examples of these questions include what factors trigger sexual versus asexual lifestyles and consequently affect their mode of transmission? With frequent inter-specific hybridization, what are the mechanisms that underlie genetic subdivision (speciation)? Answers to these questions will facilitate the application of Epichloë endophytes for biological control of pests in grassland ecosystems and agricultural systems, and also help to enhance sustainable agriculture by increasing crop fitness.
Acknowledgements
We thank Parivash Shoukouhi and the Molecular Technologies Laboratory (MTL) at the Ottawa Research & Development Centre for technical assistance, Jennifer Wilkinson (DAOM), Teresa Iturriaga (CUP), Kathie T. Hodge, Canadian Collection of Fungal Cultures (CCFC) for handling specimens and cultures, Stephen Darbyshire for providing samples and one photograph ( inset), Scott A. Redhead for pre-submission review, and two anonymous reviewers for constructive comments to improve the manuscript.
References
- Azevedo MD, Welty RE. 1995. A study of the fungal endophyte Acremonium coenophialum in the roots of tall fescue seedlings. Mycologia. 87(3):289–297. doi:https://doi.org/10.1080/00275514.1995.12026533.
- Bultman TL, White JF, Bowdish TI, Welch AM, Johnston J. 1995. Mutualistic transfer of Epichloë spermatia by Phorbia flies. Mycologia. 87(2):182–189.
- Charlton ND, Craven KD, Mittal S, Hopkins AA, Young CA. 2012. Epichloë canadensis, a new interspecific epichloid hybrid symbiotic with Canada wild rye (Elymus canadensis). Mycologia. 104(5):1187–1199. doi:https://doi.org/10.3852/11-403.
- Chung KR, Schardl CL. 1997. Sexual cycle and horizontal transmission of the grass symbiont, Epichloë typhina. Mycol Res. 101(3):295–301. doi:https://doi.org/10.1017/S0953756296002602.
- Craven K, Hsiau P, Leuchtmann A, Hollin W, Schardl CL. 2001. Multigene phylogeny of Epichloë species, fungal symbionts of grasses. Ann Mo Bot Gard. 88(1):14–34. doi:https://doi.org/10.2307/2666129.
- Gagic M, Faville MJ, Zhang W, Forester NT, Rolston MP, Johnson RD, Ganesh S, Koolaard JP, Easton HS, Hudson D, et al. 2018. Seed transmission of Epichloë endophytes in Lolium perenne is heavily influenced by host genetics. Front Plant Sci. 9(1580):1–16. doi:https://doi.org/10.3389/fpls.2018.01580.
- Leuchtmann A. 2003. Taxonomy and diversity of Epichloë endophytes. In: White JF, Bacon CW, Hywel-Jones NL, Spatafora JW, editors. Clavicipitalean fungi: evolutionary biology, chemistry, biocontrol, and cultural impacts. New York (NY); Basel: Marcel Dekker, Inc; p. 169–192.
- Leuchtmann A, Bacon CW, Schardl CL, White JF, Tadych M. 2014. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia. 106(2):202–215. doi:https://doi.org/10.3852/13-251.
- Leuchtmann A, Oberhofer M. 2013. The Epichloë endophytes associated with the woodland grass Hordelymus europaeus including four new taxa. Mycologia. 105(5):1315–1324. doi:https://doi.org/10.3852/12-400.
- Moon CD, Craven KD, Leuchtmann A, Clement SL, Schardl CL. 2004. Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol Ecol. 13(6):1455–1467. doi:https://doi.org/10.1111/j.1365-294X.2004.02138.x.
- Philipson MN, Christey MC. 1986. The relationship of host and endophyte during flowering, seed formation, and germination of Lolium perenne. N Z J Botan. 24(1):125–134. doi:https://doi.org/10.1080/0028825X.1986.10409724.
- Schardl CL. 1996. Epichloë species: fungal symbionts of grasses. Annu Rev Phytopathol. 34(1):109–130. doi:https://doi.org/10.1146/annurev.phyto.34.1.109.
- Schardl CL, Leuchtmann A. 1999. Three new species of Epichloë symbiotic with North American grasses. Mycologia. 91:95–107.
- Schardl CL, Phillips TD. 1997. Protective grass endophytes: where are they from and where are they going? Plant Dis. 8(5):430–438. doi:https://doi.org/10.1094/PDIS.1997.81.5.430.
- Siegel MR, Johnson MC, Varney DR, Nesmith WC, Buckner RC, Bush LP, Burrus PB II, Jones TA, Boling JA. 1984. A fungal endophyte in tall fescue: incidence and dissemination. Phytopathology. 74(8):932–937. doi:https://doi.org/10.1094/Phyto-74-932.
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, et al. editors. 2018. International code of nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the nineteenth international botanical congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten: Koeltz Botanical Books.