Abstract
The genus Exobasidium includes a number of species that are pathogenic to members of the Ericales, including blueberries (Vaccinium spp.), and Exobasidium leaf spot is an emerging concern for commercial blueberry production. Atypical Exobasidium leaf spot-like symptoms were observed in wild V. angustifolium (lowbush blueberry) in eastern Newfoundland and Labrador, Canada, beginning in 2016. A fungus with morphological characteristics typical of Exobasidium spp. was isolated from symptomatic tissue. The ITS regions from five isolates, and the LSU regions from two, were sequenced and compared with publicly available Exobasidium spp. sequences, including from E. maculosum, cause of Exobasidium leaf spot on V. angustifolium. The isolates collected in this study were genetically distinct from all isolates of E. maculosum, as well as from the other Exobasidium spp. sequences available, suggesting that the isolates described here represent a genetically distinct population belonging to the genus Exobasidium.
Résumé
Le genre Exobasidium inclut un nombre d’espèces pathogènes à l’égard des membres de la famille des Éricacées, y compris les bleuets (Vaccinium spp.), et le rouge du bleuet est une nouvelle préoccupation qui touche la production commerciale de cette baie. À partir de 2016, des symptômes atypiques du rouge ont été observés sur des plants de V. angustifolium sauvages dans l’est de Terre-Neuve-et-Labrador, au Canada. Un champignon affichant les caractéristiques morphologiques typiques d’Exobasidium spp. a été isolé à partir de tissu symptomatique. Les régions de l’ITS de cinq isolats et les régions LSU de deux autres ont été séquencées et comparées à des séquences d’Exobasidium spp. disponibles publiquement, y compris E. maculosum, cause du rouge chez V. angustifolium. Les isolats collectés dans le cadre de cette étude étaient génétiquement distincts de tous les isolats d’E. maculosum ainsi que des autres séquences disponibles d’Exobasidium spp., ce qui suggère que les isolats décrits ici représentent une population génétiquement dist, .incte appartenant au genre Exobasidium.
Introduction
Lowbush blueberry (Vaccinium angustifolium Ait.) is native to the northeastern United States and Canada, and its berries are harvested both commercially and recreationally. Blueberries of all kinds are an economically significant crop: high and lowbush blueberries were cultivated on 76 656 ha and were Canada’s most valuable fruit crop in 2018, with a total farm gate value of 270 USD M CAD (Statistics Canada Citation2019). Blueberries are culturally significant to Indigenous and non-Indigenous harvesters and are enjoyed as fresh fruit or in a variety of preparations, including in baked goods and as jams and jellies. Relative to highbush blueberries (V. corymbosum), the berries of lowbush blueberry plants are higher in phenolics, anthocyanins, and antioxidants (Kalt et al. Citation2001), contributing to their healthful reputation.
The productivity of blueberry plants can be limited by a number of diseases. Valdensinia leaf spot, Septoria leaf spot, and red leaf are among the most common and economically significant foliar diseases of V. angustifolium in Atlantic Canada (Hildebrand et al. Citation2016). While flower and fruit diseases reduce yield by directly damaging fruits and limiting fruit set, foliar diseases limit yield by reducing photosynthetic capacity, ultimately reducing the total number of flower buds (Percival and Dawson Citation2009). Among the diseases that affect blueberry production, Exobasidium fruit and leaf spots are an emerging challenge in blueberry production, affecting both highbush (Cline Citation2014) and lowbush (Hildebrand et al. Citation2016) plants with increasing frequency since the time they were first reported (Nickerson and Vander Kloet Citation1997; Cline Citation1998). The disease is characterized by small (5 mm), pale green spots on leaves (Nickerson and Vander Kloet Citation1997), and white-pink unripened spots on fruit. The genus Exobasidium (Ustilaginomycotina, Basidiomycota) contains a number of plant pathogens that cause a variety of growth abnormalities in infected plants, including many members of the order Ericales. For example, E. vexans causes blister blight, which can result in more than 40% yield loss in Camellia sinensis (tea) (Saravanakumar et al. Citation2007); E. maculosum causes fruit and leaf spot on a variety of blueberry species (Vaccinium spp.) in North America (Brewer et al. Citation2014); and E. vaccinii is reported as the causal agent of red leaf in lowbush blueberry (Mims and Nickerson Citation1986). Exobasidium species are also responsible for striking leaf diseases in V. vitis-idaea (known by amany regional common names, including but not limited to redberry, partridgeberry, and lingonberry) and V. oxycoccos (common names include cranberry and bog cranberry); on both plants, infection is characterized by significant deformity and swelling of the leaf, leading to a ‘cupped’ appearance with the adaxial leaf surface typically a bright, cherry-red colour and the abaxial surface developing a white, powdery appearance caused by the development of fungal hymenium (Brewer et al. Citation2014).
Recent genetic investigations have revealed a high level of genetic diversity in E. maculosum (Stewart et al. Citation2015), and have provided evidence that the Exobasidium sp. isolates originally collected from V. angustifolium by Mims and Nickerson (Citation1986) are genetically distinct from isolates collected from both highbush (V. corymbosum) and rabbiteye (V. virgatum) blueberries from the USA (Brewer et al. Citation2014; Stewart et al. Citation2015). Although Exobasidium leaf spot has been reported from Nova Scotia (NS), the presence or absence of this disease in Newfoundland and Labrador (NL) have not been reported in the literature.
Exobasidium-spot-like symptoms were observed on the leaves of wild lowbush blueberry plants near Flatrock, NL, in the summer of 2016. During the course of unrelated sample collection in wild blueberry fields, as well as during recreational berry picking, similar symptoms were observed across eastern NL in 2016 and in each year thereafter. While generally similar to the symptoms of Exobasidium leaf blight, which were first reported from the neighbouring province of NS (Nickerson and Vander Kloet Citation1997), plants displaying atypical symptoms were observed on several occasions: as opposed to numerous, small spots, leaves with atypical symptoms had just one or two large, highly conspicuous lesions. An experiment was undertaken to isolate and identify causal agents from leaves displaying these atypical symptoms of an Exobasidium leaf spot-like disease.
Materials and methods
Sample collection, isolation, and preservation
Leaf tissue displaying symptoms of an atypical Exobasidium leaf spot-like disease was collected from wild blueberry stands on the Avalon Peninsula on the island of Newfoundland during JJuly–August 2017 (; ). Symptomatic leaves were affixed to the lid of a Petri dish using Vaseline, and positioned over a plate of potato dextrose agar (PDA) amended with streptomycin (100 µg mL−1) and tetracycline (50 µg mL−1) (PDA+AB). Symptomatic leaves were also sliced with a sterile scalpel into sections approximately 5 mm × 5 mm and surface-sterilized by rinsing in sterile distiled H2O (dH2O) (20 s), 70% EtOH (15 s), 1% NaClO (30 s or 60 s), and finally in fresh sterile dH2O (20 s) before plating onto PDA+AB. After 48 h, single germinating spores, or fresh mycelial growth were subcultured onto fresh plates of PDA+AB. Colonies were maintained on the PDA at 4°C.
Table 1. List of isolates included in this study
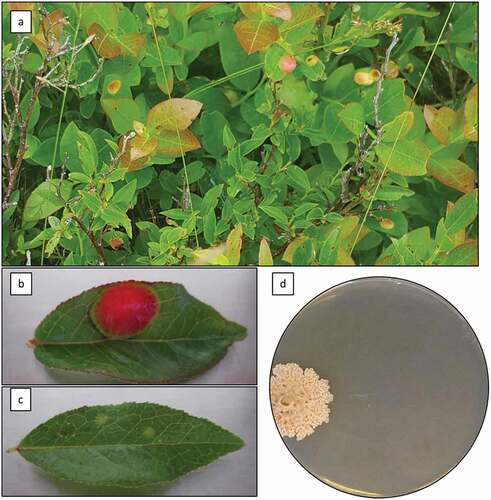
Fig. 1 (Colour online) Atypical Exobasidium-leaf-spot-like disease symptoms on Vaccinium angustifolium caused by Exobasidium sp. Symptoms on wild plants in Holyrood, NL, July 23, 2018 (a); close-up of atypical symptoms on detached leaf of V. angustifolium showing large, raised lesion (b); typical Exobasidium leaf spot symptoms on detached leaf of V. angustifolium (c); two-month old colony of Exobasidium sp., isolated from atypical leaf spot symptoms, on PDA+AB after 2 months of growth at 23 °C in the dark (d)
Pathology and Koch’s postulates
Twenty-five wild, non-symptomatic shoots of V. angustifolium were collected. Two leaves per shoot were wounded by a sterile pipet tip. The innoculum was prepared from three isolates (2017.07.23_TT_03, 2017.06.30_AV_01, and 2017.07.10_RL_02) by pipetting 2 mL of sterile dH2O onto the surface of an actively growing colony and agitating with a sterile hockey stick cell spreader. The suspension was filtered through sterile cheesecloth and the concentration of spores was adjusted to 106 spores mL−1. Two leaves in each of the five shoots per treatment were inoculated by pipetting 20 µL of inoculum prepared as described above directly onto the tissue. A mock inoculation, using sterile water in place of the spore suspension was also prepared; in addition, a check (non-wounded, non-inoculated) was also included in the experiment. Plants were covered with a transparent plastic bag to maintain high humidity and prevent cross-contamination between treatments, and were incubated in a growth chamber set to a cycle of 25°C, 16 h days and 18°C, 8 h nights at 80% relative humidity. After o1 month of incubation, the inoculated leaves from each of the plants were cultured using the VVaseline-based method as described above, and the incidence of symptoms recorded.
DNA sequencing and phylogenetic analysis
All primers were ordered from Integrated DNA Technologies (IDT; Coralville, IA), and PCR reagents were ordered from BioBasic, (Markham, ON). For DNA extraction, fungal tissue was collected by gently scraping the surface of an actively growing culture with a sterile scalpel. Genomic DNA was extracted from 100 mg of fungal tissue using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI) according to the manufacturer’s protocol. Following extraction, DNA was further purified by sodium acetate precipitation. For this precipitation, 0.1 volumes of 3 M NaOAc and 2 volumes of isopropanol were added to the DNA sample. The mixture was incubated at −20°C for at least 1 h, and the DNA was collected by centrifugation (10 000 g, 4°C, 10 min). The DNA was washed with 70% ethanol, dried and resuspended in dH2O. The quantity and quality of the resulting DNA was assessed on a JENWAY GenovaNano spectrophotometer (Bibby Scientific Ltd., Stone, Staffordshire, UK), and the concentration was adjusted to 20 ng µL−1 in sterile dH2O before proceeding. The primer pair ITS1-F (5ʹ-CTT GGT CAT TTA GAG GAA GT AA-3ʹ) and ITS4-B (5ʹ-CAG GAG ACT TGT ACA CGG TCC AG-3ʹ) (Gardes and Bruns Citation1993) were used to amplify the ITS region. The PCR mixture consisted of 3 µL of 10x PCR buffer, 3.3 µL of 20 mM MgSO4, 0.6 µL of a mixture of 10 mM dNTPs, 0.6 uL of a 10 µM solution of each primer, 0.08 µL of 5U Taq polymerase, 20.82 µL of sterile H2O, and 1 µL of 20 ng µL−1 DNA template. The PCR program consisted of an initial denaturation at 94°C for 3 min, followed by a total of 35 repetitions of denaturation at 94°C for 45 s; annealing at 54°C for 45 s; and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min. The primer pair NL-1 (5ʹ-GCA TAT CAA TAA GCG GAG GAA AAG-3ʹ) and NL-4 (5ʹ-GGT CCG TGT TTC AAG ACG G-3ʹ) (O’donnell Citation1993) was used to amplify the D1-D2 segment of LSU rDNA. The PCR reaction mixture was as described for the ITS segment. The PCR program was also identical to the ITS program, with the exception that the annealing was performed at 60°C for 30 s.
The results of each PCR were assessed by electrophoresis of 10 µL of the reaction mixture on a 1% agarose gel (OmniPur Agarose, Calbiochem, USA) containing GelRed (Biotium, Hayward, CA) using TAE buffer (2 M Tris base, 1 M acetic acid, 50 mM EDTA; pH 8.5). The sizes and concentrations of each sample were compared to reference bands in a LowRanger DNA ladder (Norgen Biotek, Thorold, ON). The remaining volume of the PCR products were purified using either gel extraction or the EZ-10 Spin Column PCR Products Purification Kit (BioBasic, Amherst, NY) according to the manufacturer’s protocol, with the exception that the final DNA elution was performed using 15 μL of sterile water after incubating the column at 45°C for 2 min, and the elution was repeated twice. The purified DNA was quantitated on an agarose gel by comparison to the LowRanger ladder as described above. Ten ng of purified DNA was utilized for each unidirectional sequencing reaction performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Austin, TX) and an AB3500 instrument. The sequencing reaction consisted of 2 μL BigDye Terminator 3.1 Ready Reaction Mix; 2 μL BigDye Terminator v1.1 & v3.1 5X Sequencing Buffer; 2 μL of a suitable primer (either ITS1-F, NL-1, or NL-4) (3.2 μM); 10 ng DNA; and sterile dH2O to bring the total reaction volume to 20 μL. The cycle-sequencing program consisted of an initial denaturation of 1 min at 96°C followed by 25 cycles of 10 s at 96°C, 5 s at 50°C, and 4 min at 60°C. Sample purification consisted of an ethanol/EDTA precipitation as per the manufacturer’s protocol. The resulting trace files were visualized, corrected, and trimmed using MEGA X software v. 10.2.2 (Kumar et al. Citation2018). The sequences were queried against the National Centre for Biotechnology Information (NCBI) database using the nucleotide Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using both the default parameters and also limiting the results to sequences from type specimens only. Neighbour-joining trees for each gene segment were constructed using MEGA X and the sequences listed in ; the ITS (NR_073343.1) and LSU sequences (NG_042394.1) from Meira geulakonigii were included as the outgroup in each tree.
Results and discussion
Leaves displaying typical symptoms () had a single raised lesion of approximately 10 mm in diameter, initially chlorotic to yellow, but later becoming bright red with a light green margin (). The underside of the lesion was pale green to white. This is in contrast to the typical symptoms of Exobasidium leaf spot, which on lowbush blueberry consist of multiple small lesions, typically up to 5 mm in diameter () (Nickerson and Vander Kloet Citation1997). Plants with symptomatic leaves were observed during the months of June, July, and August each year from 2016 onward, and were generally found in shaded areas (e.g. at the base of trees). Symptomatic plants are frequently clustered together, growing directly beside unaffected plants, suggesting that perhaps some clonal populations are more susceptible to infection (data not shown). Occasionally, spots that resembled exobasidium fruit spots were observed on mature berries, but isolations were not performed from these tissues.
When symptomatic leaves were cultured by surface-sterilizing and culturing plant tissues directly, a variety of fungi, including Cladosporium sp., Aspergillus sp., Fusarium sp., and Aureobasidium pullulans were obtained (data not shown). Using the Vaseline-based method, a variety of fungi were again obtained, but, in addition, a slow-growing, cream-coloured fungus with a yeast-like growth form was obtained from all the symptomatic leaves assessed. Isolates were beige with a wrinkled, convolute appearance after 14 days of incubation at 18°C in the dark (). The fungus was slow-growing, with an average growth rate of approximately 3 mm per week.
Leaves of healthy cuttings inoculated with Exobasidium sp. turned red; some distal, non-inoculated leaves turned brown and dehisced. In contrast, the non-inoculated shoots remained green and appeared healthy. Cultures of Exobasidium sp. identical to that used for inoculation were recovered from all the symptomatic leaves, but from none of the mock-inoculated or checked leaves. Failure to produce disease symptoms that exactly match the symptoms in the field is confounding. Nickerson and Vander Kloet (Citation1997) successfully completed Koch’s postulates for exobasidium leaf spots on V. angustifolium, and Pehkonen et al. (Citation2008) also reported successful inoculation of V. vitis-idaea by E. splendidum and E. vaccinii in the field. Koch’s postulates are not addressed in several recent reports of new host–pathogen relationships involving Exobasidium spp. (Cline Citation1998; Nagao et al. Citation2001, Citation2004, Citation2006; Kennedy et al. Citation2012; Piątek et al. Citation2012; Brewer et al. Citation2014; Pusz et al. Citation2019). The use of detached shoots, rather than whole plants, may account for the inability to reproduce disease symptoms in this study.
A portion of the ITS region was successfully amplified and sequenced for the purpose of comparison with sequences available for related Exobasidium spp. The ITS sequences obtained from the NL isolates were highly similar to one another, with 95.6% identity across all isolates for the 483 bp-long segment used for alignment with other Exobasidium spp. sequences. When these sequences were queried against the BLAST database, the top match for isolates 2017.03.23_TT_03, 2017.06.30_AV_01, 2017.07.14_SC_02, and 2017.07.10_RL_02 was E. rostrupii (FJ896132.1) with 94.88%, 95.32%, 92.45%, or 95.91% identity across 98% (2017.03.23_TT_03), 100% (2017.06.30_AV_01, 2017.07.14_SC_02), or 88% (2017.07.10_RL_02) of the sequence length; and for isolate 2017.07.14_SC_05b, the top match was E. uvae-ursi (KY424482.1) with 94.94% identity across 99% of the sequence (Supplemental ). When the BLAST search was limited to matches originating only from type specimens, the top match for all the samples from this study was E. ferrugineae (NR_120076.1), with 88.02%, 88.39%, 88.65%, 86.57%, and 87.67% identity across 87%, 88%, 79%, 96%, or 87% of the query length for isolates 2017.03.23_TT_03, 2017.06.30_AV_01, 2017.07.14_SC_02, 2017.07.14_SC_05b, and 2017.07.10_RL_02, respectively (Supplemental Table 2). The sequence from E. ferrugineae is the only ITS sequence available in GenBank for any member of the genus Exobasidium. The second-best match in the type-strain-specific BLAST search was Meira geulakonigii (NR_073343.1), a member of the Exobasidiales that was originally isolated from citrus rust mite Phyllocoptruta oleivira (Ashmead) (Eriophyidae) that was observed on Citrus grandis (Boekhout et al. Citation2003).
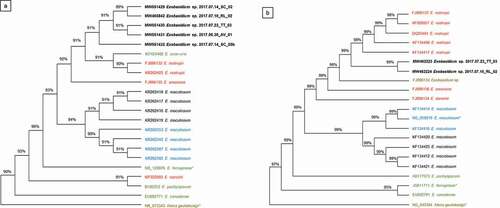
A neighbour-joining tree was constructed from the ITS sequences to examine the relationship between the Exobasidium sp. isolates from this study and related species of Exobasidium from V. angustifolium and from other host plants, including the sequences that produced the highest matches in the BLAST search (). The ITS tree demonstrates that the isolates from this study are genetically distinct from Exobasidium spp. previously studied from related host plants, with a bootstrap value of 91%, the isolates from this study clustered together. The nearest neighbours to the NL isolates include sequences from E. rostrupii (FJ896132.1 and KR262425.1), E. uvae-ursi (KY424482.1), and E. arescens (FJ896135.1). The ITS sequences from isolates collected in this study are distinct from those of the E. maculosum isolates collected from any of the other host plants, including V. angustifolium from the neighbouring province of NS. The ITS sequences also did not cluster with Exobasidium spp. that cause similar symptoms in other host plants, such as E. pachysporum (pathogenic on V. uliginosum) or E. arescens (pathogenic on V. myrtillus).
Fig. 2 (Colour online) Neighbour-joining trees depicting the relationship between sequences from Exobasidium spp. isolates originally collected from Vaccinium spp. and related host species (). Bootstrap values are expressed as percentages on the nodes of each branch. Colours indicate the host from which the isolate was originally obtained: black text represents samples collected from V. angustifolium; blue text represents isolates from V. virgatum or V. corymbosum; red text indicates isolates from other Vaccinium spp.; green text indicates isolates from other Ericaceae; and brown text indicates a host origin outside the Ericaceae. Isolates in bold were collected in this study, and an asterisk indicates type strains. Sequences from either (a) ITS or (b) LSU were used to construct each tree
For LSU, sequenced amplicons were obtained from isolates 2017.07.23_TT_03 and 2017.07.10_RL_02. The sequences were 99.08% identical across the 544 bp segment used for alignment with other sequences. When these sequences were queried against the BLAST database, the top match for both was E. rostrupii (KF134408.1) with 99.08% and 98.90% identity across 100% of the length of each sequence for 2017.07.23_TT_03 and 2017.07.10_RL_02, respectively (Supplemental Table 3). When the BLAST search was limited to matches originating only from type specimens, the top match for both LSU sequences from this study was E. maculosum (NG_059219.1), with 96.15% identity across 100% of the query length for both isolates (Supplemental Table 4).
The neighbour-joining tree constructed for LSU sequences from this study and from sequences from related Exobasidium spp. () was consistent with the ITS tree in demonstrating that the isolates from this study are genetically distinct from other Exobasidium spp. With a bootstrap value of 99%, the isolates from this study clustered together, and were most similar to sequences from E. rostrupii (FJ896137, AF009857, DQ25494, KF134408, and KF134417). Notably, as for the ITS sequences, the LSU tree indicated that the sequences from this study are genetically distinct from E. maculosum, the cause of exobasidium leaf spots on V. angustifolium.
While Exobasidium spp. are known to cause berry and foliar symptoms in V. angustifolium, and to the best of our knowledge, the unusual symptoms observed in this study have not been previously reported, and the putative causal pathogen appears to be genetically distinct from other Exobasidium spp. that cause similar symptoms in related host plants. Recent work by Stewart et al. (Citation2015) has demonstrated that the pathogen causing typical Exobasidium leaf spot symptoms in V. angustifolium is E. maculosum. Despite notable differences between isolates collected on V. angustifolium as opposed to V. corymbosum and V. virgatum (Brewer et al. Citation2014; Stewart et al. Citation2015), the Exobasidium sp. reported from NS on V. angustifolium is likely E. maculosum (Stewart et al. Citation2015). In contrast, using both the ITS and LSU regions, the isolates in this study were clearly distinct from the isolates studied by Stewart et al., suggesting that the pathogen observed in eastern NL belongs to a genetically distinct population that could not be identified to the level of species with the available data. Given that the samples were collected from the island portion of the province of NL, and considering also the comparatively small commercial blueberry industry in this province relative to other parts of mainland Atlantic Canada and tthe USA it is feasible that the population in NL has evolved separately from other populations that have been previously detected and studied.
Another factor that must be considered is the high genetic diversity among E. maculosum isolates that was first reported by Stewart et al. (Citation2015), and later confirmed in a multi-genome study (Abrahams and Brewer Citation2018). Interestingly, Abrahams and Brewer found a mutation in a gene related to DNA repair, which they suggest may account for the unexpectedly high mutation rate in E. maculosum. The Nova Scotian isolates of E. maculosum from V. angustifolium, for which genetic information is available, were originally collected more than 20 years ago. It would be very interesting to compare contemporary isolates from NS and NL in order to further elucidate the relationship between pathogenic Exobasidium spp. causing leaf spot diseases in Atlantic Canada. A concerted sample collection effort to describe the prevalence of the Exobasidium sp. from this study within the province of NL, as well as from the neighbouring province of NS, where exobasidium leaf spots were first described, is the next step in understanding the distribution of the population described here. Detailed morphological data collection and comparison with known Exobasidium spp. is also planned. In addition, further examination of the pathology of these isolates on multiple genotypes of V. angustifolium and related Vaccinium spp. is necessary to determine the potential threat that this population may pose to wild and cultivated berries.
Supplemental Tables 1-4
Download MS Word (33.7 KB)Acknowledgements
The island of Newfoundland is the ancestral homelands of the Mi’kmaq and Beothuk. The Inuit of Nunatsiavut and NunatuKavut and the Innu of Nitassinan, and their ancestors, are the original people of Labrador. Technical assistance from Diana Perrin and the assistance of research affiliate students David Martin, Mika MacKinnon, and Arielle Przybysz is gratefully acknowledged. The East Coast Trail Association is also acknowledged for allowing research samples from the trail to be collected for this project.
Supplemental data
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2021.1919926
Additional information
Funding
References
- Abrahams A, Brewer MT. 2018. Population genomics reveals high mutation rate and divergence among populations of blueberry pathogen Exobasidium maculosum. In International Congress of Plant Pathology. Boston (MA).
- Begerow D, Bauer R, Oberwinkler F. 1997. Phylogenetic studies on nuclear large subunit ribosomal DNA sequences of smut fungi and related taxa. Can J Bot. 75:2045–2056. doi:https://doi.org/10.1139/b97-916.
- Boekhout T, Theelen B, Houbraken J, Robert V, Scorzetti G, Gafni A, Gerson U, Sztejnberg A. 2003. Novel anamorphic mite-associated fungi belonging to the Ustilaginomycetes: Meira geulakonigii gen. nov., sp. nov., Meira argovae sp. nov. and Acaromyces ingoldii gen. nov., sp. nov. Int J Syst Evol. 53:1655–1664. doi:https://doi.org/10.1099/ijs.0.02434-0.
- Brewer MT, Turner AN, Brannen PM, Cline WO, Richardson EA. 2014. Exobasidium maculosum, a new species causing leaf and fruit spots on blueberry in the southeastern USA and its relationship with other Exobasidium spp. parasitic to blueberry and cranberry. Mycologia. 106:415–423. doi:https://doi.org/10.3852/13-202.
- Cline WO. 1998. An Exobasidium disease of fruit and leaves of highbush blueberry. Plant Dis. 82:1064. doi:https://doi.org/10.1094/PDIS.1998.82.9.1064B.
- Cline WO. 2014. New and emerging diseaes of blueberry. Acta Hortic. 1017:45–49. doi:https://doi.org/10.17660/ActaHortic.2014.1017.2.
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes ‐ application to the identification of mycorrhizae and rusts. Mol Ecol. 2:113–118. doi:https://doi.org/10.1111/j.1365-294X.1993.tb00005.x.
- Hildebrand PD, Renderos WE, Delbridge RW. 2016. Diseases of lowbush blueberry and their identification. Ottawa, Canada: Agriculture and Agri-Food Canada; p. 44.
- Kalt W, Ryan D, Duy JC, Prior RL, Ehlenfeldt MK, Kloet SPV. 2001. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (Vaccinium Section cyanococcus spp.). J Agric Food Chem. 49:4761–4767. doi:https://doi.org/10.1021/jf010653e.
- Kennedy AH, Goldberg NA, Minnis AM. 2012. Exobasidium ferrugineae sp. nov., associated with hypertrophied flowers of Lyonia ferruginea in the southeastern USA. Mycotaxon. 120:451–460. doi:https://doi.org/10.5248/120.451.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549. doi:https://doi.org/10.1093/molbev/msy096.PMID:29722887
- Li Z, Guo L. 2009. Two new species and a new Chinese record of Exobasidium (Exobasidiales). Mycotaxon. 108:479–484. doi:https://doi.org/10.5248/108.479.
- Mims CW, Nickerson NL. 1986. Ultrastructure of the host-pathogen relationship in red leaf disease of lowbush blueberry caused by the fungus Exobasidium vaccinii. Can J Bot. 64:1339–1343. doi:https://doi.org/10.1139/b86-184.
- Nagao H, Ezuka A, Harada U, Sato T, Kakishima M. 2006. Two new species of Exobasidium causing Exobasidium diseases on Vaccinium spp. in Japan. Mycoscience. 47:277–283. doi:https://doi.org/10.1007/S10267-006-0307-7.
- Nagao H, Ezuka A, Ohkubo H, Kakishima M. 2001. A new species of Exobasidium causing witches’ broom on Rhododendron wadanum. Mycoscience. 42:549–554. doi:https://doi.org/10.1007/BF02460953.
- Nagao H, Kakishima M, Sato T. 2004. Three species of Exobasidium causing Exobasidium leaf blight on subgenus Hymenanthes, Rhododendron spp., in Japan. Mycoscience. 45:85–95. doi:https://doi.org/10.1007/S10267-003-0162-8.
- Nickerson NL, Vander Kloet SP. 1997. Exobasidium leaf spot of lowbush blueberry. Can J Plant Pathol. 19:66–68. doi:https://doi.org/10.1080/07060669709500575.
- O’donnell K. 1993. Fusarium and its relatives. In: Reynolds DR, Taylor JW, editors. The fungal holomorph: mitotic, meiotic, and pleomorphic speciation in fungal systematics. Wallingford: CAB International; p. 225–233.
- Ort BS, Bantay RM, Pantoja NA, O’Grady PM. 2012. Fungal diversity associated with Hawaiian Drosophila host plants. PLoS One. 7:e40550. doi:https://doi.org/10.1371/journal.pone.0040550.
- Pehkonen T, Koskimäki J, Riihinen K, Pirttilä AM, Hohtola A, Jaakola L, Tolvanen A. 2008. Artificial infection of Vaccinium vitis-idaea L. and defence responses to Exobasidium species. Physiol Mol Plant Path. 72:146–150. doi:https://doi.org/10.1016/j.pmpp.2008.08.002.
- Percival DC, Dawson JK. 2009. Foliar disease impact and possible control strategies in wild blueberry production. Acta Hortic. 810:345–354. doi:https://doi.org/10.17660/ActaHortic.2009.810.45.
- Piątek M, Lutz M, Welton P. 2012. Exobasidium darwinii, a new Hawaiian species infecting endemic Vaccinium reticulatum in Haleakala National Park. Mycol Prog. 11:361–371. doi:https://doi.org/10.1007/s11557-011-0751-4.
- Pusz W, Malicki M, Patejuk K, Ronikier M, Suchan T. 2019. First record of Exobasidium rhododendri (Fuckel) C. E. Cramer in Poland. Acta Soc Bot Pol. 88:3632. doi:https://doi.org/10.5586/asbp.3632.
- Saravanakumar D, Vijayakumar C, Kumar N, Samiyappan R. 2007. PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot. 26:556–565. doi:https://doi.org/10.1016/j.cropro.2006.05.007.
- Statistics Canada. 2019. Table 32-10-0364-01. Estimates, production and farm gate value of fresh and processed fruits.
- Stewart JE, Brooks K, Brannen PM, Cline WO, Brewer MT. 2015. Elevated genetic diversity in the emerging blueberry pathogen Exobasidium maculosum. PLoS One. 10:e0132545. doi:https://doi.org/10.1371/journal.pone.0132545.
