Abstract
Colletotrichum higginsianum is an economically important anthracnose pathogen of crucifers that causes losses worldwide. For comprehensive control of anthracnose disease, it is essential to understand the mechanisms of pathogenicity in C. higginsianum. The Ser/Thr kinase Sch9 belonging to the AGC (protein kinase A, G, and C) kinase family, commonly found in eukaryotes, plays a vital role in various cell signalling pathways and development. However, it has not been studied in Colletotrichum species. Three domains, Protein kinase C conserved region 2 (C2), Serine/Threonine protein kinase catalytic (S_TKc) and Extension to Ser/Thr-type protein kinases (S_TK_X), were identified in the C. higginsianum Sch9 (ChSch9) protein. Phylogenetic analysis indicated that ChSch9 is closely related to the AGC kinase family, which is conserved in fungi. Deletion of ChSch9 caused defects in hyphal morphology, growth and conidiation. In addition, deletion of ChSch9 was associated with reduced tolerance to hyperosmotic, cell wall and oxidative stresses in C. higginsianum, and ChSch9 deletion mutants were more tolerant to heat. Furthermore, we found that ChSch9 might be involved in the pyruvate–acetaldehyde fermentation pathway. Importantly, deletion of ChSch9 led to a reduction in appressoria and infective hyphae formation, resulting in defects in virulence. Collectively, the results of this study indicate that ChSch9 is required for infection-related morphogenesis and virulence in C. higginsianum.
Résumé
Colletotrichum higginsianum est un agent pathogène causant l’anthracnose, une importante maladie des crucifères sur le plan économique qui cause des pertes partout dans le monde. Afin de maîtriser complètement l’anthracnose, il est essentiel de comprendre les mécanismes de la pathogénicité chez C. higginsianum. Une Ser/Thr kinase Sch9 appartenant à la famille AGC (protéine kinase A, G et C) de la kinase, couramment trouvée chez les eucaryotes, joue un rôle vital dans diverses voies de signalization de la cellule et son développement. Toutefois, elle n’a pas été étudiée chez les Colletotrichum. Trois domaines, la région 2 conservée (C2) de la protéine kinase C, le domaine catalytique de la sérine/thréonine kinase (S_TKc) et l’extension à des protéines kinases de type Ser/Thr (S_TK_X), ont été identifiés dans la protéine Sch9 de C. higginsianum (ChSch9). L’analyse phylogénétique a révélé que ChSch9 est étroitement lié à la famille AGC de la kinase, qui est conservée dans les champignons. La délétion de ChSch9 altère la morphologie des hyphes, la croissance et la conidiation. En outre, la délétion de ChSch9 a été associée aux stress hyperosmotique et oxydatif ainsi que de la paroi cellulaire chez C. higginsianum, et les mutants résultant de la délétion de ChSch9 étaient plus tolérants à la chaleur. De plus, nous avons trouvé que ChSch9 pourrait être impliqué dans la voie de la fermentation du pyruvate–acétaldéhyde. Notamment, la délétion de ChSch9 a engendré une réduction du nombre d’appresseurs et inhibé la formation des hyphes infectieux, ce qui a entraîné des anomalies sur le plan de la virulence. Collectivement, les résultats de cette étude indiquent que ChSch9 est nécessaire à la morphogénèse liée à l’infection et à la virulence chez C. higginsianum.
Introduction
The ascomycete genus Colletotrichum contains more than 200 recognized species, many of which can cause devastating diseases in dicot and monocot crops worldwide (Crouch et al. Citation2014; Jayawardena et al. Citation2016; Marin-Felix et al. Citation2017). Colletotrichum higginsianum Sacc. is an important plant pathogenic fungus causing economic losses to Brassicaceae in humid and subtropical regions, and the pathogen can also infect Arabidopsis thaliana (L.) Heynh. (O’Connell et al. Citation2004, Citation2012; Birker et al. Citation2009; Zampounis et al. Citation2016; Dallery et al. Citation2017). In host plants, conidia germinate on the leaf surface and form germ tubes, which later produce appressoria. Melanized appressoria penetrate the host cuticle into epidermal cells, and then produce primary biotrophic hyphae for further ingress into host cells. After that, biotrophic hyphae produce necrotrophic hyphae to accelerate disease and devastate host tissues. Acervuli form in necrotic tissues to produce numerous conidia, which are spread by wind and rain-splash to infect new foliage under suitable conditions (O’Connell et al. Citation2004, Citation2012; Plaumann et al. Citation2018).
Protein kinases play a pivotal role in various cell signalling pathways that regulate the development of eukaryotes (Cohen Citation2000). Among these, Ser/Thr kinase Sch9 belongs to the AGC (protein kinase A, G, and C) kinase family (Hunter and Plowman Citation1997), which is commonly found in eukaryotes, including animals, plants and fungi (Arencibia et al. Citation2013). Numerous functions dependent on TOR (target of rapamycin) are carried through the AGC kinase Sch9 that is phosphorylated by TOR (Jacinto and Hall Citation2003). After in vivo phosphorylation of seven serine/threonine residues on the C-terminus of Sch9 (Urban et al. Citation2007), it is involved in a signalling pathway that affects fungal filamentation (Wang et al. Citation2019). In Saccharomyces cerevisiae Meyen ex E.C. Hansen, deletion of sch9 causes a slower growth rate, because of defective transcriptional regulation of ribosomal proteins required for nitrogen and carbon metabolism (Crauwels et al. Citation1997; Huber et al. Citation2009). Otherwise, sch9 plays various roles in stress resistance, longevity, nutrient sensing, and protein kinase A (PKA) activity regulation in S. cerevisiae (Jorgensen et al. Citation2002; Pedruzzi et al. Citation2003; Pascual-Ahuir and Proft Citation2007; Huber et al. Citation2009). In Schizosaccharomyces pombe Lindner, overexpression of two homologues of Sch9, named Sck1 and Sck2, can overcome the loss of PKA activity (Jin et al. Citation1995; Fujita and Yamamoto Citation1998). In a human pathogen, Cryptococcus neoformans (San Felice) Vuill., the Sch9 deletion mutants showed increased thermal tolerance and also modulated capsule formation and virulence (Wang et al. Citation2004). For Candida albicans (C.P. Robin) Berkhout, CaSch9 is required for cell size, log-phase growth, stress response and virulence (Stichternoth et al. Citation2011). In Fusarium graminearum Schwabe, FgSch9 is important for stress responses, production of mycotoxins such as deoxynivalenol, conidiogenesis, and pathogenesis (Chen et al. Citation2014). In the rice blast fungus, Magnaporthe oryzae (T.T. Hebert) M.E. Barr, the Mosch9 deletion mutant is also defective in conidiogenesis and pathogenesis (Chen et al. Citation2014). The role of the Sch9 gene, however, is still not known in Colletotrichum species.
In this study, we deleted a Sch9 ortholog named ChSch9 in C. higginsianum using a homologous recombination strategy. Deletion of ChSch9 caused a reduction in vegetative growth, increased sensitivity to various stresses, and also a reduction in virulence. Then, we assessed the role of the ChSch9 gene in the utilization of carbon sources and found that ChSch9 might be involved in the pyruvate–acetaldehyde fermentation pathway. Overall, our data support that ChSch9 is essential for fungal infection-related morphogenesis and virulence in C. higginsianum.
Materials and methods
Strains, plant and culture condition
The C. higginsianum wild-type strain IMI349061 (Ch-1) (supplemental Table S1), kindly provided by Prof. Yangdou Wei from the University of Saskatchewan, Canada, is virulent on A. thaliana (Col-0). The wild-type and derived strains were cultured on potato dextrose agar (PDA) at 25°C. TheEscherichia coli strain DH5α was cultured in Luria-Bertani (LB) medium at 37°C and used for plasmid preparation and multiplication. TheAgrobacterium tumefaciens strain EHA105 was cultured in LB medium at 28°C and used for the transformation of C. higginsianum. The Arabidopsis (Col-0) plants were grown in a chamber with a 16-h light period at 40–45 µmol m−2 s−1 (450–750 nm), 65%–75% relative humidity, 22°C/20°C day/night temperatures (Yuan et al. Citation2016) and used in virulence tests. Conidia washed from 7-d-old cultures were used in transformation via the Agrobacterium-mediated transformation (ATMT) method following Liu et al. (Citation2013). Potato dextrose agar amended with 50 µg mL−1 hygromycin B (Roche, USA) or 150 μg mL−1 neomycin (Ameresco, USA) was used for the selection of hygromycin-resistant and neomycin-resistant transformants, respectively.
Deletion and complementation of ChSch9
Approximately 1180 bp and 825 bp flanking sequences of the ChSch9 gene were amplified using primer pairs F1F/F1R and F2F/F2R (supplemental Table S2), respectively. The polymerase chain reaction (PCR) conditions were set as follows: 5 min at 94°C, 33 cycles consisting of 30 s at 94°C, 1 min at 55–60°C, 90 s at 72°C, and 10 min at 72°C. The amplification products of the upstream and downstream flanking sequences were then separately digested with HindIII/SalI and XbaI/KpnI and joined into the related restriction sites of vector pMD18T-HYG, resulting in the initial vector F1-HYG-F2 (Gu et al. Citation2017). The vector F1-HYG-F2 was then linearized with HindIII/KpnI and ligated with pNeo3300III to form the gene replacing vector pNeo3300IIIChSch9-Ko (Gu et al. Citation2017). The final vector was then inserted into the A. tumefaciens strain EHA105 and then transformed into Ch-1 by the ATMT procedure (Wei et al. Citation2016; Liu et al. Citation2017). The transformants were cultured on PDA supplemented with 50 µg mL−1 of HygB (Merck, Germany) and 500 µg mL−1 of cephalosporin (Amresco, USA), and then tested for resistance to neomycin (G418). Subsequently, the candidate transformants were verified by PCR with the four pairs of primers to amplify separately the ChSch9 gene (ChSch9FP/ChSch9RP), Hph (HygFP/HygRP), upstream flanking region/Hph overlapping sequence (ChSch9_5ʹFP/ChSch9_5ʹRP) and downstream flanking region/Hph overlapping sequence (ChSch9_3ʹFP/ChSch9_3ʹRP) (supplemental Table S2).
To verify that the ChSch9 deletion mutants were responsible for the phenotype, a ChSch9 deletion mutant was complemented with a 4.2-kb sequence containing a 1.5-kb upstream sequence and full length of ChSch9 gene coding region. PCR product amplified from gDNA of Ch-1 with primers CompSch9F and CompSch9R (supplemental Table S2) was ligated into pNEO3300III to form the complementation vector (Gu et al. Citation2019) and then transformed into ChSch9 deletion mutant via ATMT. The complemented transformants were selected on PDA supplemented with 150 μg mL−1 antibiotic G418 and confirmed by PCR and reverse transcription-PCR (RT-PCR).
Phenotype analysis
For the determination of growth rate, 5-mm-diameter mycelial plugs from colony margins were placed in the centre of PDA plates and incubated at 25°C. Colony diameters at 5–7 d post inoculation (dpi) were measured. Strains were also grown on cellophane membranes over PDA for 4 d and hyphal tips were observed under a microscope. To produce spores, the strains were grown on PDA in the dark at 25°C for 7 d, and conidia were harvested by washing each plate with 2 mL ddH2O and gently removing them from the surface mycelium using a sterilized spatula. The concentration of the conidial suspension was assessed with a haemocytometer. To assess appressorium formation, 10 μL conidial suspension (1 × 105 conidia mL−1) of each strain were placed on hydrophobic coverslips and incubated at 25°C for 24 h. The percentage of conidia that formed appressoria was determined by microscopic examination of at least 300 conidia (Zhu et al. Citation2017). Each experiment was replicated three times with three repeats each time.
Carbon source utilization analysis
To determine the involvement of ChSch9 in the fermentation process, all strains were cultured on Czapek medium supplied separately with 80 mM glucose, 40 mM potassium acetate, 40 mM ethanol and 5 mM acetaldehyde, and then incubated at 25°C for 7 d for growth measurements (Gu et al. Citation2019). Each treatment was replicated three times and the experiment was repeated three times.
Stress response assays
To test the sensitivity to stress, the strains were cultured on PDA supplemented with different stress agents (300 μg mL−1 Congo Red (CR), 300 μg mL−1 Calcofluor White (CFW), 0.005% sodium dodecyl sulphate (SDS), 0.7 M NaCl, 1.2 M KCl, 1 M Sorbitol, and 10 mM H2O2). After 7 d of incubation, colony diameters were measured and inhibition rates (%) were calculated with the formula: [(a – b) ÷ a] × 100% (where: a is the mean colony diameter on PDA; and b is the mean colony diameter on compound-amended PDA). The tolerance of these strains to high temperatures was assessed by inoculating 5-mm-diameter mycelial plugs on to the PDA and then incubating at 25°C or 35°C for 5 d. The growth inhibition rate (%) of the strains was then calculated. Each treatment was replicated three times and the experiment was repeated three times.
Virulence and plant infection assays
Virulence assays for the strains were carried out as reported previously (Zhu et al. Citation2017). Briefly, conidia harvested from 7-d-old PDA cultures at 25°C were washed twice with sterile ddH2O and the concentration of the conidial suspension was adjusted to 1 × 105 conidia mL−1. Virulence assays were conducted using two methods. First, a conidial suspension (10 μL) was spotted on to 4-week-old leaves of A. thaliana. Second, a conidial suspension was sprayed on to 4-week-old Arabidopsis plants, and 3 mL of conidial suspension was sprayed on each plant. After 4 d of incubation in a humid chamber at 27°C, water-soaked lesions on the inoculated A. thaliana leaves were then assessed. Fungal infections and hyphal colonization were observed by light microscopy after clearing with methanol:chloroform:glacial acetic acid (6:3:1) solution and staining with Trypan blue (Bhadauria et al. Citation2010).
Nucleic acid manipulations, RT-PCR and qRT-PCR
Fungal gDNA was isolated from 5-d-old mycelia by the CTAB method (Damm et al. Citation2008). Total RNA was extracted using a TRIzol® Plus RNA Purification Kit (Invitrogen, Carlsbad, USA). For cDNA synthesis, a TransScript® synthesis kit (TransGen Biotech, Beijing, China) was used. To verify deletion and complementary mutants, RT-PCR was performed as described previously (Huang et al. Citation2017). Gene expression patterns of C. higginsianum from infected Arabidopsis plants were analyzed by qRT-PCR and amplification conditions were set as follows: 5 min at 95°C, 40 cycles consisting of 10 s at 95°C, 20 s at 55–60°C, and 20 s at 72°C (Zhu et al. Citation2019). The actin gene (CH063_05065) from C. higginsianum was used for normalization of expression. The qRT-PCR analyses were repeated at least twice and each repetition had three independent replicates.
Bioinformatics analysis
The full sequence and flanking sequences of the ChSch9 gene were downloaded from the C. higginsianum genome database (http://www.broadinstitute.org annotation/genome/colletotrichum group/MultiHome.html). Primer Premier 5.0 was used to design the primers (http://www.premierbiosoft.com/primerdesign/). FGENESH (Softberry Inc., Mount Kisco, NY) gene prediction software was then used for the analysis of open reading frames (ORF). The protein domain is predicated via SMART software (http://smart.embl-heidel-berg.de/). For phylogenetic analysis, the protein sequences of various organisms were obtained from NCBI, followed by their alignment with ClustalX (v. 2.0, http://www.clustal.org/clustal2/), and finally, a phylogenetic tree was generated with Mega software (v. 7.0, http://www.megasoftware.net/index.php) with the Neighbour-joining (NJ) method with 1000 bootstrap replicates and using S. cerevisiae as the outgroup.
Statistical analysis
All statistical analyses were performed using the R environment by constructing different libraries to assess significant treatment effects, and the least significant difference test (LSD) was performed with the Agricolae package (version 1.3–1) in R to separate means (P < 0.05) (De Mendiburu Citation2014).
Results
Identification and characterization of ChSch9
The SCH9 protein sequence (GenBank CAA40853.1) from S. cerevisiae was compared against the C. higginsianum genome database with BLASTP. The results showed that CH063_01372 (named ChSch9), which had 897 amino acids, shared 54% identity with S. cerevisiae SCH9.
In the domain analysis, three domains (Protein kinase C conserved region 2 (C2), Serine/Threonine protein kinase catalytic (S_TKc) and Extension to Ser/Thr-type protein kinases (S_TK_X)) were predicted in the ChSch9 protein (). A phylogenetic tree of SCH9 proteins from various fungal genomes indicated that most SCH9 homologues in filamentous ascomycetes were grouped with high bootstrap support, with yeasts as basal lineages (). This showed that ChSch9, closely related to the AGC kinase family, is conserved among fungi.
Fig. 1 (Colour online) Functional domains, phylogenetic tree and expression of ChSch9. (a) Domain structures of ChSch9 as annotated by SMART. (b) Neighbour-joining tree of ChSch9 in Colletotrichum higginsianum and other selected fungi, including some model and pathogenic fungi. All the sequences were downloaded from NCBI and their accession numbers are shown following the gene names. The numbers at branch nodes are bootstrap percentages out of 1000 replicates. (c) Expression profiles of ChSch9 relative to actin gene (CH063_05065) from C. higginsianum was used for normalization of expression in Mycelium from PDB culture and at different infection stages on Arabidopsis (24, 48, 72 and 90 hpi) by qRT-PCR. Relative abundance of ChSch9 transcripts during infectious growth was normalized by comparing with vegetative growth in PDB (relative expression level = 1, hyphae used as reference sample and normalized with actin gene). Three independent biological experiments with three replicates in each treatment were performed. Error bars represent the standard deviation
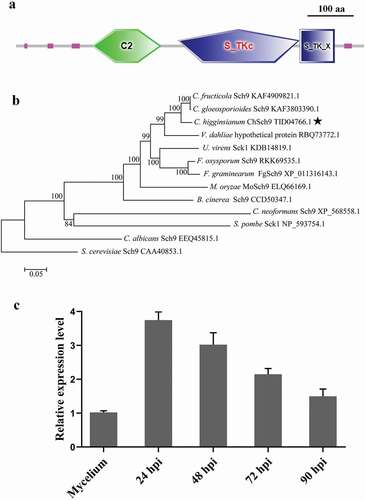
To gain an understanding of the functions of ChSch9, we initially quantified gene expression at different infection stages of C. higginsianum using qRT-PCR. Gene expression levels for ChSch9 were highest during appressorium formation and lower during later infection stages, suggesting that ChSch9 might be necessary for fungal penetration in C. higginsianum ().
ChSch9 is required for vegetative growth and sporulation
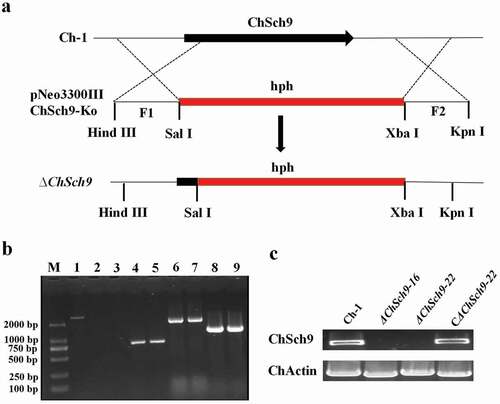
To explore the function of ChSch9 in C. higginsianum, a gene deletion vector, pNeo3300IIIChSch9-Ko, was constructed and introduced into the wild-type to generate deletion mutants by homologous recombination (). Fifty deletion mutant candidates could grow on PDA containing hygromycin, but only 13 did not grow on PDA supplemented with neomycin antibiotic (G418). Among these transformants, two deletion mutants, ΔChSch9-16 and ΔChSch9-22, were confirmed by a strict PCR strategy with four pairs of primers (supplemental Table S2), which were used to amplify separately the ChSch9 gene, Hph, the upstream flanking region/Hph overlapping sequence, and the downstream flanking region/Hph overlapping sequence. In ΔChSch9-16 and ΔChSch9-22, the ChSch9 gene was not detected whereas an Hph fragment was detected as an 800 bp band (). Moreover, 2100 and 1800 bp bands of two overlapping regions were amplified (), indicating that the ChSch9 gene was indeed deleted in both ΔChSch9-16 and ΔChSch9-22 mutants. In RT-PCR assays, expression of ChSch9 was not detected in these two deletion mutants (). The mutant ΔChSch9-22 was further selected for a complementation experiment. After transformation with a complementation vector pNEO3300III, expression of ChSch9 was detected in the complementation strain CΔChSch9-22 by RT-PCR ().
Fig. 2 (Colour online) Deletion and complementation of ChSch9. (a) Strategic map of gene deletion vector construction and sites for restriction enzymes. (b) Amplification of ChSch9 with four pairs of primers in deletion mutants. Line 1 shows the presence of ChSh9 gene in the wild-type, whereas lines 2–3, 4–5 ,6–7, 8–9 represent the HYG, upstream flanking region/Hph overlapping sequence, and downstream flanking region/Hph overlapping sequence in ΔChSch9-16 and ΔChSch9-22, respectively. (c) Confirmation of deletion and complemented strains with RT-PCR
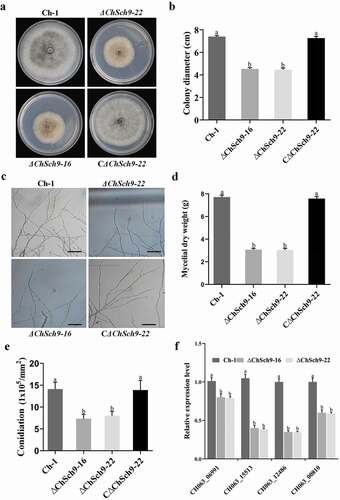
The wild-type, deletion mutants, ΔChSch9-16 and ΔChSch9-22, and complemented strain CΔChSch9-22 were cultured on PDA plates. The ΔChSch9 mutants grew significantly slower than the wild-type and complemented strains (P < 0.05) (, b). Microscopic examination of the hyphae of young colonies revealed that the hyphal tips of ChSch9 deletion mutants were less dense and branched (). The mycelial dry weights of the ΔChSch9 mutants were also significantly reduced (P < 0.05) (). Moreover, ΔChSch9 mutants produced a smaller number of spores compared with the wild-type and complemented strains (P < 0.05) (). In M. oryzae, several genes have been reported to regulate sporulation, such as MoAps2, MoCon7, MoAcr1 and MoCom1 (Kim and Lee Citation2012; Huang et al. Citation2017). To test whether the deletion of ChSch9 affects the expression level of conidiation-related genes in C. higginsianum, mRNA levels of homologues of these genes (COM1: CH063_15513; CON7: CH063_06991; APS2: CH063_12486; ACR1: CH063_00810) were quantified using qRT-PCR. The results showed that the expression levels of all these four conidiation-related genes were significantly decreased in the deletion mutants (P < 0.05) (). Thus, we conclude that ChSch9 was essential for controlling vegetative growth and sporulation in C. higginsianum.
Fig. 3 (Colour online) ChSch9 is critical for vegetative growth and conidiation in Colletotrichum higginsianum. (a) Morphology of colonies of mutant strains. Ch-1 and mutants were grown on PDA plates for 7 d. (b) Statistical analysis of growth rate of Ch-1 and mutants. Diameter of each strain was measured after culturing at 25°C for 7 d. (c) Hyphal tips of strains. Ch-1 and mutants were grown on cellophane overlying PDA for 4 d and hyphal tips were observed by microscopy. Scale bar, 50 μm. (d) Statistical analysis of mycelial dry weight. (e) Statistical analysis of conidial production. (f) Relative expression of different conidiation-related genes in the wild-type and deletion mutants of ChSch9 (relative expression level = 1, wild-type used as reference sample and normalized with actin gene). Error bars represent standard deviation. Three independent biological experiments with three replicates in each treatment were performed. Different letters represent significant differences between the mutant and wild-type at P < 0.05
ChSch9 is essential for tolerance towards various stresses
Since ∆ChSch9 mutants were defective in vegetative growth, ChSch9 mutant strains were grown on PDA to examine their responses to hyperosmotic, cell wall and oxidative stresses (). Compared with the wild-type and complementation strains, the growth of ChSch9 deletion mutants was significantly inhibited by the chemicals CR, KCl, NaCl, SDS and H2O2 (P < 0.05), indicating that the deletion of ChSch9 could reduce the tolerance to hyperosmotic, cell wall and oxidative stresses in C. higginsianum ().
Fig. 4 (Colour online) ChSch9 is essential for various stress tolerances. (a) Colony morphology of Ch-1 and ChSch9 deletion mutants in the presence of different stresses. Strains were cultured on PDA alone or PDA medium with 300 μg mL−1 CR, 300 μg mL−1 CFW, 0.005% SDS, 0.7 M NaCl, 1.2 M KCl, 1 M Sorbitol and 10 mM H2O2. Cultures were photographed after 7 d at 25°C. (b) Inhibition rate was calculated based on colony diameter of strains subjected to different stresses after 7 d. For each strain, the inhibition ratio in growth by different stresses was estimated in comparison with its growth on regular PDA. (c) Expression of seven chitin synthase genes in the wild-type and ChSch9 deletion mutants. (d) Expression levels of catalase and peroxidase in the wild-type and deletion mutants (relative expression level = 1, wild-type used as reference sample and normalized with actin gene). Error bars represent standard deviation. Three independent biological experiments with three replicates in each treatment were performed. Different letters represent significant differences between treatments at P < 0.05
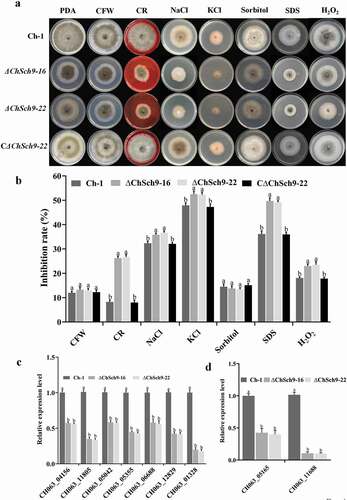
A significant proportion of fungal cell walls consists of chitin, and it plays a vital role in hyphal tip growth and hyphal morphology (Huang et al. Citation2017). Thus, we assessed the mRNA level of coding genes of seven chitin synthases (CH063_11805; CH063_04156; CH063_05042; CH063_12829; CH063_01328; CH063_05355; CH063_06688) by qRT-PCR. The results indicated that the expression levels of all seven chitin synthesis genes were significantly reduced in the ∆ChSch9 mutants (P < 0.05) (). Moreover, qRT-PCR assays demonstrated that the expression levels of catalase (CH063_11688) and peroxidase (CH063_05165) genes were somewhat reduced in ChSch9 deletion mutants (P < 0.05) ().
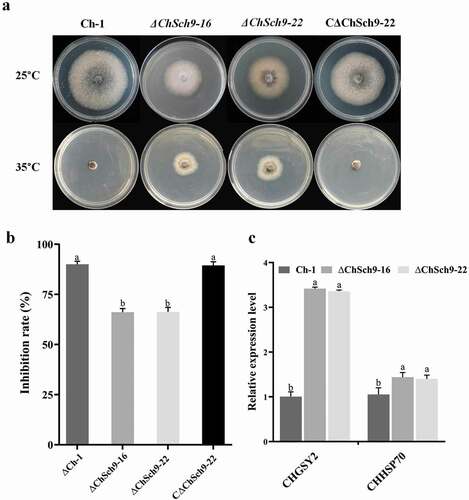
SCH9 is important for survival since Sch9 deletion mutants in S. cerevisiae have reduced survival, and in F. graminearum the PKA mutants are more tolerant of heat stress than the wild-type (Fabrizio et al. Citation2001; Zhu et al. Citation2017). To assess the role of ChSch9 in heat tolerance, the mutant strains were incubated at 25°C and 35°C () and the results demonstrated ChSch9 deletion mutants were more tolerant to heat compared with the wild-type and complemented strains (P < 0.05) (). To validate the results, we also assessed the expression levels of the heat tolerance-related genes Chgsy2 (CH063_00792) and Chhsp70 (CH063_01329) in ChSch9 deletion mutants and the wild-type. The results of qRT-PCR assays showed that the expression levels of genes related to heat tolerance were significantly up-regulated in the deletion mutants compared with the wild-type (P < 0.05) ().
Fig. 5 (Colour online) ChSch9 is involved in the negative regulation of heat tolerance. (a) Ch-1, ChSch9 deletion mutants and complemented strains were cultured on PDA at 25°C and 35°C. Images were taken after 5 d of incubation at 25°C and 35°C. (b) Inhibition rate of the radiated growth of each strain on the PDA at 35°C compared with that of growth at 25°C. (c) Relative expression of Chgsy2 and Chhsp70 in the hyphae of mutants incubated at 35°C for 3 h (relative expression level = 1, wild-type used as reference sample and normalized with actin gene). Error bars represent the standard deviation. Three independent biological experiments with three replicates in each treatment were performed. Different letters represent significant differences between treatments at P < 0.05
ChSch9 might be involved in the acetaldehyde fermentation pathway
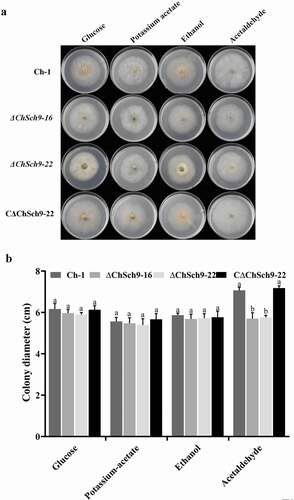
We further studied the role of ChSch9 in the fermentation pathways by culturing on Czapek medium supplemented separately with different carbon sources (). The results indicated that when exposed to acetaldehyde, the ChSch9 deletion mutants showed less growth compared with the wild-type and complemented strains (P < 0.05), suggesting ChSch9 might function in the pyruvate–acetaldehyde fermentation pathway in C. higginsianum ().
Fig. 6 (Colour online) Carbon source utilization of the ChSch9 deletion mutants. (a) Colony morphology of the wild-type, ChSch9 deletion mutants and complemented strain grown on Czapek medium supplemented with 80 mM glucose, 40 mM potassium acetate, 40 mM ethanol and 5 mM acetaldehyde for 7 d at 25°C. (b) Mycelial growth rates were determined 7 d after incubation at 25°C under different supplied carbon sources. Error bars represent the standard deviation. Three independent biological experiments with three replicates in each treatment were performed. Different letters represent significant differences within the same carbon source (P < 0.05)
ChSch9 plays an important role in virulence in C. higginsianum
Differentiation and maturation of appressoria are key steps for successful infection by C. higginsianum. Appressorium formation was investigated on artificial hydrophobic surfaces. At 24 hpi, appressorium formation by the ΔChSch9 mutants was reduced and lower than that of the wild-type and complemented strain (P < 0.05) (). For pathogenicity of mutants, all strains were first spot-inoculated on Arabidopsis leaves and, at 4 dpi, the wild-type and complemented mutants produced water-soaked lesions whereas tiny lesions were produced by the ChSch9 deletion mutants (). We also sprayed conidial suspensions on to attached Arabidopsis leaves with these strains. At 4 dpi, the ChSch9 deletion mutants produced fewer lesions on infected leaves whereas the wild-type and complemented strains could cause severe disease ().
Fig. 7 (Colour online) ChSch9 is essential for plant infection by Colletotrichum higginsianum. (a) Appressorial formation of Ch-1 and mutants on hydrophobic surface. Total 10 µL of conidial suspension of each strain was dropped on to the hydrophobic surface and these were incubated at 25°C for 24 h. (b) Pathogenicity assays with attached Arabidopsis leaves. Ten microlitres of conidial suspensions of each strain were spotted on to the Arabidopsis leaves and incubated at 25°C for 4 d. (c) Pathogenicity assays with Arabidopsis plants. Conidial suspensions of each strain were sprayed on to the Arabidopsis plants and incubated at 25°C for 4 d, 3 mL of conidial suspension was sprayed on each plant. (d) At 4 dpi, inoculated leaves were fixed, stained with Trypan blue and photographed under a microscope. AP, appressoria; PH, primary hyphae; SH, secondary hyphae. (e) Percentage penetration rates of the strains on Arabidopsis leaves. Approximately 300 conidia or appressoria were observed per incubated site. Error bars represent the standard deviation. All treatments had three independent biological repeats. Different letters represent significant differences between treatments at P < 0.05
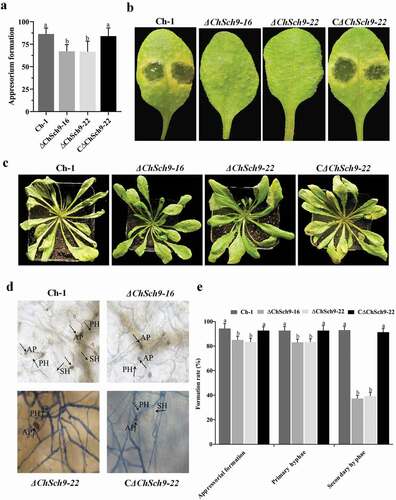
To measure the formation rate of various infection structures, the Trypan blue-stained leaf tissues were examined by microscopy at 4 dpi. Most of the appressoria of the wild-type and complemented strain had penetrated to form both biotrophic hyphae and necrotrophic hyphae that had extensively colonized the leaf by 4 dpi (, e). In the ΔChSch9 mutants, formation rates of appressoria and biotrophic hyphae were slightly reduced, and less necrotrophic hyphae were produced (, e). Overall, these results indicate that deletion of ChSch9 affected the formation of appressoria and infective hyphae and caused reduced virulence in C. higginsianum.
Discussion
In eukaryotic organisms, protein kinases are required for the alteration of several developmental processes and responses to environmental inducements by reversible protein phosphorylation (Serrano Citation1996). Sch9 kinase performs functions associated with TORC1 band cAMP-signalling pathways in S. cerevisiae (Urban et al. Citation2007; Zhang et al. Citation2011), which has been reported to play a role in several developmental and infection processes in C. higginsianum and other fungi (Bormann et al. Citation2014; Hu et al. Citation2014; Gu et al. Citation2017; Zhu et al. Citation2017). As a major downstream effector of TORC1, the protein kinase Sch9 plays multiple roles in stress resistance, survival rate and nutrient sensing in S. cerevisiae (Pedruzzi et al. Citation2003; Pascual-Ahuir and Proft Citation2007; Huber et al. Citation2009). In this study, we discovered that the ChSch9 deletion mutants grow more slowly than the wild-type, which is comparable to the SCH9 deletion mutants of S. cerevisiae (Geyskens et al. Citation2000; Jorgensen et al. Citation2002, Citation2004). In F. graminearum, deletion mutants of FgSch9 showed a reduction in growth rate and conidiation. Moreover, in C. albicans, CaSch9 deletion mutants showed less cell growth in comparison with a wild-type strain (Liu et al. Citation2010; Chen et al. Citation2014). We observed that the conidia of ChSch9 were less numerous compared with the wild-type conidia. CaSch9 mutants showed a striking hyperfilamentous phenotype under hypoxia (Stichternoth et al. Citation2011). Instead, the hyphae of ΔChSch9 showed less dense and narrower branches, which is similar to what has been reported with FgSch9 mutants (Gu et al. Citation2015). These results suggest that ChSch9 regulates growth rate and hyphal morphogenesis in yeast and C. higginsianum. Thus, Sch9 orthologs may have a conserved role in the regulation of fungal growth.
In plant-pathogenic fungi, invasive structure development and polarized hyphal growth and morphology depend on the regular synthesis and distribution of chitin in the cell wall (Samalova et al. Citation2017). In the present study, we have shown that the deletion of ChSch9 altered the resistance to cell wall inhibitors. The results of qRT-PCR analysis confirm the conclusion that the expression levels of chitin synthase genes were extensively inhibited in the ChSch9 deletion mutants. The protein kinase Sch9 performs numerous roles in stress resistance, longevity in life span, and nutrient sensing in S. cerevisiae (Jorgensen et al. Citation2002; Pedruzzi et al. Citation2003; Pascual-Ahuir and Proft Citation2007; Huber et al. Citation2009). Sch9 hinders PKA activity, and deletion of Sch9 increases PKA activities (Zhang et al. Citation2011). In S. pombe, overexpression of two homologues of Sch9, named Sck1 and Sck2, could overcome the loss of PKA activity (Jin et al. Citation1995; Fujita and Yamamoto Citation1998). Furthermore, Sch9 and PKA manage similar pathways that congregate with Rim15 kinase in S. cerevisiae (Pedruzzi et al. Citation2003; Roosen et al. Citation2005). The deletion mutants of Rim15 in M. oryzae have a slow growth rate and are sensitive to external environmental stresses such as osmotic stresses (Motoyama et al. Citation2008). In F. graminearum, the Fgrim15 mutant was reduced in conidiation and virulence (Wang et al. Citation2011), results which are comparable to our study. Moreover, in S. cerevisiae the SCH9 mutant showed an increased survival rate in response to H2O2 stress resistance (Fabrizio et al. Citation2001). In C. neoformans, the Sch9 deletion mutant showed increased thermal tolerance, and the deletion appeared to modulate capsule formation and affect virulence (Wang et al. Citation2004). In F. graminearum, the ∆Fgsch9 mutant had increased tolerance to elevated temperatures (Chen et al. Citation2014). In this study, we found that ChSch9 is important for various stresses and increased tolerance to elevated temperatures. The Chsch9 deletion mutants were sensitive to 0.7 M NaCl, CR and SDS, in findings comparable to earlier studies (Chen et al. Citation2014). In S. cerevisiae, Sch9 is involved in hyperosmotic stress, and in this study the ChSch9 deletion mutants were sensitive to H2O2 stress. Hence, it is expected that ChSch9 plays an important role in responding to exterior environmental stress in C. higginsianum.
Unlike the cpk1 cpk2 double mutant reported earlier (Hu et al. Citation2014), the ChSch9 mutant was still pathogenic. However, it showed significantly reduced virulence on Arabidopsis. Although Sch9 orthologs are well conserved in plant pathogenic fungi and filamentous ascomycetes, few have been studied. In the human pathogens C. albicans and C. neoformans, however, the orthologs are recognized to be vital for virulence (Wang et al. Citation2004; Liu et al. Citation2010). The CaSch9 deletion mutant was attenuated in virulence in a mouse model of systemic candidiasis due to its defects in yeast growth and filamentation (Liu et al. Citation2010). For C. neoformans, the Sch9 ortholog functions both independently of and in conjunction with the cAMP-PKA pathway in pathogenesis (Wang et al. Citation2004). Moreover, its involvement in pathogenicity was reported in F. graminearum (Chen et al. Citation2014). In this study, we observed that ΔChSch9 showed dramatically reduced virulence in Arabidopsis plants, which may result from multiple defects in appressorium formation and development of secondary hyphae. In plants, the quick production and accumulation of ROS are considered to be the first response against invading pathogens (Shetty et al. Citation2007; Imam et al. Citation2016). Hydrogen peroxide (H2O2), an important ROS that can be produced by host plants in reaction to fungal infection, can initiate lipid peroxidation, DNA damage, formation of hydroxyl radicals and protein oxidation (Rolke et al. Citation2004). In this study, the ChSch9 deletion mutants showed increased sensitivity to hydrogen peroxide. Second, the deletion of ChSch9 in C. higginsianum led to increased sensitivity to cell wall-damaging agents. Earlier studies on numerous fungi have shown that proper cell wall integrity is needed for virulence (Kim et al. Citation2009; Xu et al. Citation2010). Based on the collective results of this study, we conclude that ChSch9 is necessary for virulence in C. higginsianum due to its role in fungal development and stress resistance.
ChSch9_supplementry_table_R1.docx
Download MS Word (28.9 KB)Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2021.1921850.
Additional information
Funding
References
- Arencibia JM, Pastor-Flores D, Bauer AF, Schulze JO, Biondi RM. 2013. AGC protein kinases: from structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim Biophys Acta Proteins Proteom. 1834(7):1302–1321. doi:https://doi.org/10.1016/j.bbapap.2013.03.010
- Bhadauria V, Miraz P, Kennedy R, Banniza S, Wei Y. 2010. Dual trypan-aniline blue fluorescence staining methods for studying fungus-plant interactions. Biotech Histoch. 85(2):99–105. doi:https://doi.org/10.3109/10520290903132196
- Birker D, Heidrich K, Takahara H, Narusaka M, Deslandes L, Narusaka Y, Reymond M, Parker JE, Connell RO. 2009. A locus conferring resistance to Colletotrichum higginsianum is shared by four geographically distinct Arabidopsis accessions. Plant J. 60(4):602–613. doi:https://doi.org/10.1111/j.1365-313X.2009.03984.x
- Bormann J, Boenisch MJ, Brückner E, Firat D, Schäfer W. 2014. The adenylyl cyclase plays a regulatory role in the morphogenetic switch from vegetative to pathogenic lifestyle of Fusarium graminearum on wheat. PLoS One. 9(3):e91135. doi:https://doi.org/10.1371/journal.pone.0091135
- Chen D, Wang Y, Zhou X, Wang Y, Xu J-R. 2014. The Sch9 kinase regulates conidium size, stress responses, and pathogenesis in Fusarium graminearum. PLoS One. 9(8):e105811.
- Cohen P. 2000. The regulation of protein function by multisite phosphorylation–a 25 year update. Trends Biochem Sci. 25(12):596–601. doi:https://doi.org/10.1016/S0968-0004(00)01712-6
- Crauwels M, Donaton MCV, Pernambuco MB, Winderickx J, De Winde JH, Thevelein JM. 1997. The Sch9 protein kinase in the yeast Saccharomyces cerevisiae controls cAPK activity and is required for nitrogen activation of the fermentable-growth-medium-induced (FGM) pathway. Microbiol. 143(8):2627–2637. doi:https://doi.org/10.1099/00221287-143-8-2627
- Crouch J, O’Connell R, Gan P, Buiate E, Torres MF, Beirn L, Shirasu K, Vaillancourt L. 2014. The genomics of Colletotrichum. In: Dean RA, Lichens-Park A, Kole C, editors. Genomics of plant-associated fungi: monocot pathogens. Berlin (Heidelberg): Springer; p. 69–102.
- Dallery JF, Lapalu N, Zampounis A, Pigné S, Luyten I, Amselem J, Wittenberg AH, Zhou S, De Queiroz MV, Robin GP. 2017. Gapless genome assembly of Colletotrichum higginsianum reveals chromosome structure and association of transposable elements with secondary metabolite gene clusters. BMC Genomics. 18(1):667. doi:https://doi.org/10.1186/s12864-017-4083-x
- Damm U, Mostert L, Crous P, Fourie P. 2008. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia. 20:87. doi:https://doi.org/10.3767/003158508X324227
- De Mendiburu F. 2014. Agricolae: statistical procedures for agricultural research. R Package Version 1.1.
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. 2001. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 292(5515):288–290. doi:https://doi.org/10.1126/science.1059497
- Fujita M, Yamamoto M. 1998. S. pombe sck2+, a second homologue of S. cerevisiae Sch9 in fission yeast, encodes a putative protein kinase closely related to PKA in function. Curr Genet. 33(4):248–254. doi:https://doi.org/10.1007/s002940050333
- Geyskens I, Kumara SH, Donaton MC, Bergsma JCT, Thevelein J, Stefaan W. 2000. Expression of mammalian PKB partially complements deletion of the yeast protein kinase Sch9. In: Bos JL, editor. Proceedings of NATO conference. Vol. 316. Amsterdam (Netherlands): IOS Press; p. 117–126.
- Gu Q, Chen M, Huang J, Wei Y, Hsiang T, Zheng L. 2017. Multifaceted roles of the rasguanine nucleotide exchange factor ChRgf in development, pathogenesis, and stress responses of Colletotrichum higginsianum. Phytopathol. 107(4):433–443. doi:https://doi.org/10.1094/PHYTO-03-16-0137-R
- Gu Q, Yuan Q, Zhao D, Huang J, Hsiang T, Wei Y, Zheng L. 2019. Acetyl‐coenzyme A synthetase gene ChAcs1 is essential for lipid metabolism, carbon utilization and virulence of the hemibiotrophic fungus Colletotrichum higginsianum. Mol Plant Pathol. 20(1):107–123. doi:https://doi.org/10.1111/mpp.12743
- Gu Q, Zhang C, Yu F, Yin Y, Shim WB, Ma Z. 2015. Protein kinase FgSch9 serves as a mediator of the target of rapamycin and high osmolarity glycerol pathways and regulates multiple stress responses and secondary metabolism in Fusarium graminearum. Environ Microbiol. 17(8):2661–2676. doi:https://doi.org/10.1111/1462-2920.12522
- Hu S, Zhou X, Gu X, Cao S, Wang C, Xu JR. 2014. The cAMP-PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum. Mol Plant-Microbe Interact. 27(6):557–566. doi:https://doi.org/10.1094/MPMI-10-13-0306-R
- Huang L, Zhang S, Yin Z, Liu M, Li B, Zhang H, Zheng X, Wang P, Zhang Z. 2017. MoVrp1, a putative verprolin protein, is required for asexual development and infection in the rice blast fungus Magnaporthe oryzae. Sci Rep. 7:1–16. doi:https://doi.org/10.1038/s41598-016-0028-x
- Huber A, Bodenmiller B, Uotila A, Stahl M, Wanka S, Gerrits B, Aebersold R, Loewith R. 2009. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev. 23(16):1929–1943. doi:https://doi.org/10.1101/gad.532109
- Hunter T, Plowman GD. 1997. The protein kinases of budding yeast: six score and more. Trends Biochem Sci. 22(1):18. doi:https://doi.org/10.1016/S0968-0004(96)10068-2
- Imam J, Mandal NP, Variar M, Shukla P. 2016. Advances in molecular mechanism toward understanding plant-microbe interaction: a study of M. oryzae versus rice. In: Shukla P, editor. Frontier discoveries and innovations in interdisciplinary microbiology. New Delhi: Springer; p. 79–96.
- Jacinto E, Hall MN. 2003. Erratum: Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 4(3):117–126. doi:https://doi.org/10.1038/nrm1018
- Jayawardena RS, Hyde KD, Damm U, Cai L, Liu M, Li XH, Zhang W, Zhao WS, Yan JY, Rs J, et al. 2016. Notes on currently accepted species of Colletotrichum. Mycosphere. 7(8):1192–1260. doi:https://doi.org/10.5943/mycosphere/si/2c/9
- Jin M, Fujita M, Culley BM, Apolinario E, Yamamoto M, Maundrell K, Hoffman CS. 1995. sck1, a high copy number suppressor of defects in the cAMP-dependent protein kinase pathway in fission yeast, encodes a protein homologous to the Saccharomyces cerevisiae SCH9 kinase. Genetics. 140(2):457–467. doi:https://doi.org/10.1093/genetics/140.2.457
- Jorgensen P, Nishikawa JL, Breitkreutz B-J, Tyers M. 2002. Systematic identification of pathways that couple cell growth and division in yeast. Science. 297(5580):395–400. doi:https://doi.org/10.1126/science.1070850
- Jorgensen P, Rupeš I, Sharom JR, Schneper L, Broach JR, Tyers M. 2004. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 18(20):2491–2505. doi:https://doi.org/10.1101/gad.1228804
- Kim JE, Lee HJ, Lee J, Kim KW, Yun S-H, Shim WB, Lee YW. 2009. Gibberella zeae chitin synthase genes, GzChs5 and GzChs7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr Genet. 55(4):449. doi:https://doi.org/10.1007/s00294-009-0258-6
- Kim KS, Lee YH. 2012. Gene expression profiling during conidiation in the rice blast pathogen Magnaporthe oryzae. PLoS One. 7(8):1–10.
- Liu L, Yan Y, Huang J, Hsiang T, Wei Y, Li Y, Gao J, Zheng L. 2017. A novel MFS transporter gene ChMfs1 is important for hyphal morphology, conidiation, and pathogenicity in Colletotrichum higginsianum. Front Microbiol. 8(OCT):1–11. doi:https://doi.org/10.3389/fmicb.2017.01953
- Liu L, Zhao D, Zheng L, Hsiang T, Wei Y, Fu Y, Huang J. 2013. Microbial pathogenesis identification of virulence genes in the crucifer anthracnose fungus Colletotrichum higginsianum by insertional mutagenesis. Microb Pathog. 64:6–17. doi:https://doi.org/10.1016/j.micpath.2013.06.001
- Liu W, Zhao J, Li X, Li Y, Jiang L. 2010. The protein kinase CaSch9p is required for the cell growth, filamentation and virulence in the human fungal pathogen Candida albicans. FEMS Yeast Res. 10(4):462–470. doi:https://doi.org/10.1111/j.1567-1364.2010.00617.x
- Marin-Felix Y, Groenewald J, Cai L, Chen Q, Marincowitz S, Barnes I, Bensch K, Braun U, Camporesi E, Damm U. 2017. Genera of phytopathogenic fungi: GOPHY 1. Study Mycol. 86:99–216.
- Motoyama T, Ochiai N, Morita M, Iida Y, Usami R, Kudo T. 2008. Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr Genet. 54(4):185. doi:https://doi.org/10.1007/s00294-008-0211-0
- O’Connell R, Herbert C, Sreenivasaprasad S, Khatib M, Esquerré-Tugayé M-T, Dumas B. 2004. A novel pathosystem for the molecular dissection of plant-fungal interactions. Mol Plant-Microbe Interact. 17(3):272–282. doi:https://doi.org/10.1094/MPMI.2004.17.3.272
- O’Connell RJ, Thon MR, Hacquard S, Amyotte SG, Kleemann J, Torres MF, Damm U, Buiate EA, Epstein L, Alkan N. 2012. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat Genet. 44(9):1060. doi:https://doi.org/10.1038/ng.2372
- Pascual-Ahuir A, Proft M. 2007. The Sch9 kinase is a chromatin-associated transcriptional activator of osmostress-responsive genes. Embo J. 26(13):3098–3108. doi:https://doi.org/10.1038/sj.emboj.7601756
- Pedruzzi I, Dubouloz F, Cameroni E, Wanke V, Roosen J, Winderickx J, De Virgilio C. 2003. TOR and PKA signaling pathways converge on the protein kinase Rim15 to control entry into G0. Mol Cell. 12(6):1607–1613. doi:https://doi.org/10.1016/S1097-2765(03)00485-4
- Plaumann PL, Schmidpeter J, Dahl M, Taher L, Koch C. 2018. A dispensable chromosome is required for virulence in the hemibiotrophic plant pathogen Colletotrichum higginsianum. Front Microbiol. 9:1005. doi:https://doi.org/10.3389/fmicb.2018.01005
- Rolke Y, Liu S, Quidde T, Williamson B, Schouten A, Weltring KM, Siewers V, Tenberge KB, Tudzynski B, Tudzynski P. 2004. Functional analysis of H2O2‐generating systems in Botrytis cinerea: the major Cu‐Zn‐superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol Plant Pathol. 5(1):17–27. doi:https://doi.org/10.1111/j.1364-3703.2004.00201.x
- Roosen J, Engelen K, Marchal K, Mathys J, Griffioen G, Cameroni E, Thevelein JM, De Virgilio C, De Moor B, Winderickx J. 2005. PKA and Sch9 control a molecular switch important for the proper adaptation to nutrient availability. Mol Microbiol. 55(3):862–880. doi:https://doi.org/10.1111/j.1365-2958.2004.04429.x
- Samalova M, Mélida H, Vilaplana F, Bulone V, Soanes DM, Talbot NJ, Gurr SJ. 2017. The β‐1, 3‐glucanosyltransferases (Gels) affect the structure of the rice blast fungal cell wall during appressorium‐mediated plant infection. Cell Microbiol. 19(3):e12659. doi:https://doi.org/10.1111/cmi.12659
- Serrano R. 1996. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. In: Jeon KW, editor. International review of cytology. Academic Press; p. 1–52.
- Shetty NP, Mehrabi R, Lütken H, Haldrup A, Kema GH, Collinge DB, Jørgensen HJL. 2007. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 174(3):637–647. doi:https://doi.org/10.1111/j.1469-8137.2007.02026.x
- Stichternoth C, Fraund A, Setiadi E, Giasson L, Vecchiarelli A, Ernst JF, Du D. 2011. Sch9 kinase integrates hypoxia and CO2 sensing to suppress hyphal morphogenesis in Candida albicans. Eukaryot Cell. 10(4):502–511. doi:https://doi.org/10.1128/EC.00289-10
- Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H. 2007. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 26(5):663–674. doi:https://doi.org/10.1016/j.molcel.2007.04.020
- Wang C, Zhang S, Hou R, Zhao Z, Zheng Q, Xu Q, Zheng D, Wang G, Liu H, Gao X, et al. 2011. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog. 7(12):e1002460. doi:https://doi.org/10.1371/journal.ppat.1002460
- Wang P, Cox GM, Heitman J. 2004. A Sch9 protein kinase homologue controlling virulence independently of the cAMP pathway in Cryptococcus neoformans. Curr Genet. 46(5):247–255. doi:https://doi.org/10.1007/s00294-004-0529-1
- Wang Y, Deng YZ, Cui G, Huang C, Zhang B, Chang C, Jiang Z, Zhang L-H. 2019. The AGC kinase SsAgc1 regulates Sporisorium scitamineum mating/filamentation and pathogenicity. mSphere. 4(3):e00259–00219. doi:https://doi.org/10.1128/mSphere.00259-19
- Wei W, Xiong Y, Zhu W, Wang N, Yang G, Peng F. 2016. Colletotrichum higginsianum mitogen-activated protein kinase ChMK1: role in growth, cell wall integrity, colony melanization, and pathogenicity. Front Microbiol. 7:1–11. doi:https://doi.org/10.3389/fmicb.2016.01212
- Xu YB, Li HP, Zhang JB, Song B, Chen FF, Duan XJ, Xu HQ, Liao YC. 2010. Disruption of the chitin synthase gene CHS1 from Fusarium asiaticum results in an altered structure of cell walls and reduced virulence. Fungal Genet Biol. 47(3):205–215. doi:https://doi.org/10.1016/j.fgb.2009.11.003
- Yuan Q, Chen M, Yan Y, Gu Q, Huang J, Zheng L. 2016. ChSte7 is required for vegetative growth and various plant infection processes in Colletotrichum higginsianum. Biomed Res Int. 2016:1–11.
- Zampounis A, Pigné S, Dallery JF, Wittenberg AH, Zhou S, Schwartz DC, Thon MR, O’Connell RJ. 2016. Genome sequence and annotation of Colletotrichum higginsianum, a causal agent of crucifer anthracnose disease. Genome Announc. 4(4):816–821. doi:https://doi.org/10.1128/genomeA.00821-16
- Zhang A, Shen Y, Gao W, Dong J. 2011. Role of Sch9 in regulating Ras-cAMP signal pathway in Saccharomyces cerevisiae. FEBS Lett. 585(19):3026–3032. doi:https://doi.org/10.1016/j.febslet.2011.08.023
- Zhu W, Xu X, Peng F, Yan DZ, Zhang S, Xu R, Wu J, Li X, Wei W, Chen W. 2019. The cyclase-associated protein ChCAP is important for regulation of hyphal growth, appressorial development, penetration, pathogenicity, conidiation, intracellular cAMP level, and stress tolerance in Colletotrichum higginsianum. Plant Sci. 283:1–10. doi:https://doi.org/10.1016/j.plantsci.2019.02.012
- Zhu W, Zhou M, Xiong Z, Peng F, Wei W. 2017. The cAMP-PKA signaling pathway regulates pathogenicity, hyphal growth, appressorial formation, conidiation, and stress tolerance in Colletotrichum higginsianum. Front Microbiol. 8:1416. doi:https://doi.org/10.3389/fmicb.2017.01416
