Abstract
Stem and bulb nematode (Ditylenchus dipsaci) is a plant parasite that can cause severe damage to garlic crops in Ontario, Canada, and other garlic growing regions. Accurate soil sampling is important to determine the risk of nematode damage before planting garlic in a field. However, it is not clear where the nematode is most concentrated in the soil profile or how the nematode is best extracted. A field survey and laboratory experiments were conducted in the autumn of 2015 and 2018 to determine the distribution of stem and bulb nematode in the soil profile and to determine the most effective extraction method. The soil in 20 garlic fields throughout southern Ontario was sampled and the top 5 cm and the bottom 5–20 cm of soil were collected in a single core and then separated. Nematodes were extracted from all soil samples using both the Baermann pan and sugar centrifugal flotation methods. Significantly more stem and bulb nematodes were extracted from the top 5 cm of soil and using the sugar centrifugal flotation method. An additional extraction efficacy experiment was conducted using a known quantity of stem and bulb nematodes in soil to compare various extraction methods, and the sugar centrifugal flotation method continued to be the more effective method. These results demonstrate that only the top 5 cm of soil should be collected and assessed for populations of stem and bulb nematode in fields intended for garlic production, and the sugar centrifugal flotation method should be the extraction method of choice.
Résumé
Les anguillules des tiges et des bulbes (Ditylenchus dipsaci) sont des phytoparasites qui peuvent causer de graves dommages aux cultures d’ail en Ontario, au Canada, et dans d’autres régions productrices également. L’échantillonnage précis des sols est important pour déterminer le risque causé par les anguillules avant de planter l’ail dans un champ. Toutefois, on ne sait pas exactement où dans le profil du sol l’anguillule est la plus concentrée ou comment l’extraire le plus efficacement possible. Une étude au champ et des expériences en laboratoire ont été menées à l’automne 2015 et 2018 afin d’évaluer la distribution de l’anguillule des tiges et des bulbes dans le profil du sol et pour déterminer la méthode d’extraction la plus efficace. Le sol de 20 champs d’ail du sud de l’Ontario a été échantillonné, et les cinq premiers centimètres de la surface ainsi que les 5 à 20 cm du fond ont été prélevés dans une carotte unique, puis séparés. Les anguillules ont été extraites de tous les échantillons de sol par la méthode de Baermann ainsi que par centrifugation et flottaison dans une solution sucrée. Un nombre plus important d’anguillules ont été extraites des cinq premiers centimètres de la surface par centrifugation et flottaison dans une solution sucrée. Une expérience supplémentaire sur l’efficacité de l’extraction a été menée avec une quantité connue d’anguillules contenues dans le sol afin de comparer les différentes méthodes d’extraction, et la centrifugation-flottaison s’est toujours révélée la plus efficace. Ces résultats démontrent que seulement les cinq premiers centimètres de la surface des champs destinés à la culture de l’ail devraient être collectés pour évaluer les populations d’anguillules des tiges et des bulbes, et que la méthode de centrifugation et flottaison dans une solution sucrée devrait être la méthode d’extraction privilégiée.
Introduction
Stem and bulb nematode (SBN) (Ditylenchus dipsaci Kühn) is an important parasite of many plants and can cause extensive damage if left unmanaged. Stem and bulb nematode is an obligate migratory ecto- and endoparasite that feeds primarily on the parenchyma tissues of plants (Bridge and Starr Citation2007). It has a wide host range of over 500 plants and over 30 races of the species have been proposed based on crop-specific feeding preferences (Dropkin Citation1980; Shurtleff and Averre Citation2000; Bridge and Starr Citation2007; Qiao et al. Citation2013). However, variation among races has shown to be inconsistent and these races are under constant review as new information becomes available (Janssen Citation1994; Tenente Citation1996).
Stem and bulb nematode is present in Ontario, Canada, and has been found in garlic (Allium sativum L.) and other vegetable fields throughout the province (Mountain Citation1957; Sayre and Mountain Citation1962; Johnson and Kayler Citation1972; Fushtey and Kelly Citation1975; Hughes et al. Citation2013; Celetti and Paibomesai Citation2014). The race of the nematode found in Ontario prefers garlic, onion and leek as hosts (Celetti and Potter Citation2011; Poirier et al. Citation2019b). In a 2011 garlic survey in Ontario, stem and bulb nematode was found in 73% of garlic clove samples (Hughes et al. Citation2013). This serious pathogen of garlic can kill plants before harvest and render the crop unmarketable. The nematode feeds on the bulb, stem and basal plate of the garlic plant (Dropkin Citation1980; Poinar Citation1983). It excretes enzymes into the plant cells that break down cell walls, releasing nutrients and providing an avenue for secondary pathogens to invade, resulting in rotting and separation of the basal plate from the garlic bulb (Dropkin Citation1980; Maggenti Citation1981; Celetti and Paibomesai Citation2014; Celetti and Cranmer Citation2017). It is a motile nematode that travels between plants through soil water films, runoff and splashing, and can then move into the scales of garlic bulbs near the soil surface (Dropkin 1988; Celetti and Cranmer Citation2017). Stem and bulb nematode overwinters in soil and host plant debris, and the fourth stage, juvenile larvae, can survive desiccation for many years through anhydrobiosis (Dropkin 1988; Perry Citation1999).
It is not clear where this nematode is located in the soil profile. Since these nematodes are one of the main limiting factors to the garlic industry’s expansion in Ontario, it is important to understand not only where they live in the soil profile, but also the best method for extracting and quantifying them from the soil. Since these nematodes are known to infect the near-surface and above-ground portions of plants, it is believed that they mainly inhabit the top layers of soil. Thus, soil sampling procedures would need to take this into consideration.
Standard nematode soil sampling procedures involve using a soil probe to take a 20-cm deep soil core, collecting only the bottom 15 cm of soil (Celetti and Potter Citation2011). Usually, the top 5 cm of soil is discarded during this sampling procedure since most plant-parasitic nematode species live lower in the soil profile around plant roots (Celetti and Potter Citation2011). This standard soil sampling procedure may miss much of the population of stem and bulb nematodes in garlic fields, resulting in underestimating the population.
In addition to effective soil sampling, it is also important to use an extraction method that is the most effective at approximating population levels of the specific nematode of interest from soil. There are many methods available to extract nematodes from soil; two of the most common methods are the Baermann pan (BP) and the sugar centrifugal flotation (SCF) methods (Baermann Citation1917; Oostenbrink Citation1954; Jenkins Citation1964; EPPO Citation2013).
The BP method was modified from the Baermann funnel (Citation1917) method by Oostenbrink (Citation1954). In this method, a known quantity of soil is placed on a set of tissues (often three staggered 2-ply sheets) on top of a coarse mesh (~2 mm pore size) in a small plastic container partially filled with water up to the soil line. Motile nematodes move from the soil through the tissues and into the water basin, and are later collected in a #500 sieve (0.025 mm pore size) and transferred to a counting dish to be quantified. The BP method is better for extracting smaller, living and motile nematodes (EPPO Citation2013).
The SCF method is a more complex procedure, requiring a centrifuge and more labour than the BP method. In this method, the quantity of soil is mixed thoroughly in a bucket with water and left to settle for 30 seconds before being drained through a #500 sieve. The contents collected in the sieve are rinsed into a centrifuge tube and centrifuged at 1800 g for 6 minutes. After, the supernatant is decanted, a solution of 454 g sugar litre−1 water is added to the tube, and the contents are stirred thoroughly and centrifuged at 1800 g for 2 minutes. The supernatant is again drained through a #500 sieve, rinsed well with water, and transferred to a counting dish for nematode quantification. The SCF method extracts all types of nematodes, including living, dead, motile, sedentary and large nematodes (Jenkins Citation1964; Shurtleff and Averre Citation2000; EPPO Citation2013).
There is very limited information available on effective methods for extracting SBN from soil. Most SBN studies have extracted the nematode from plant tissue using the BP method in nematicide efficacy trials, which is suitable for tissue and root samples. However, there have been a few studies that used the SCF method to extract SBN from soil (Andres and Lopez-Fando Citation1996; Castillo et al. Citation2007). To our knowledge, a recent study conducted by Poirier et al. (Citation2019a) is the only study to date that has compared the BP and SCF methods to extract SBN from soil. The researchers found that the BP method was the most effective method for extracting SBN. The methods for the BP and SCF used by this group were similar, but not the same, as the standard methods used in Ontario labs.
Specific nematode extraction methods are important for relating populations found in a soil sample to a historical threshold, whether a damage or economic threshold. This is most certainly the case for garlic and for SBN. For Ontario’s agriculture industry, nematode thresholds are important when determining management practices, both with respect to choice of practice and timing. In Ontario, the current economic threshold for stem and bulb nematode in garlic is 100 nematodes per kilogram of soil (Celetti and Potter Citation2011), based on the Baermann pan extraction method. If nematode counts are above this threshold, it is recommended to avoid planting garlic in the field or to fumigate prior to planting. Both options can be quite extreme, so ensuring that population levels are accurately determined is essential.
The two objectives of this study were to a) determine the distribution of stem and bulb nematode in the soil profile to optimize soil sampling, and b) determine the best method for extracting and quantifying this nematode from field soil. Other plant-parasitic nematode genera extracted from the soil samples were also quantified to determine their location in the soil profile and extraction efficacy using the SCF and BP methods.
Materials and methods
Garlic field sampling
Twenty garlic fields in southern Ontario, Canada, were sampled in the autumn of 2015 after garlic was harvested (15 fields) and 2018 (5 fields) before garlic was planted. Garlic fields ranged in size from small, 0.15 ha fresh market grower fields, to 8 ha commercial producer fields. Soil samples were taken from each field in an ‘X’ pattern, and approximately forty 20 cm soil cores were taken from each field using a 30 cm deep by 2 cm wide soil probe. Each 20 cm soil core was separated into two sections, the top 5 cm and the bottom 5–20 cm of soil, and placed in respective designated buckets. Soil samples were transferred to labelled bags and kept in a cooler with ice packs during transportation. Samples were stored in a 4°C refrigerator the same day and were kept refrigerated for no longer than one day before extraction.
Nematode extraction
Nematodes from the top 5 cm and bottom 5–20 cm soil samples from each field were extracted separately. Each soil sample was homogenized by mixing the soil in a bucket and a 50 g aliquot of soil was used in the Baermann pan and sugar centrifugal flotation extraction methods (Baermann Citation1917; Oostenbrink Citation1954; Jenkins Citation1964; EPPO Citation2013) as described above. Soil samples remained in the Baermann pans for 7 days before quantification.
Extractions and quantifications were conducted by both the University of Guelph Lab Services (2015 samples) and the Ontario Crops Research Centre – Bradford (2018 samples). All plant-parasitic nematodes extracted were identified to genus and counts were recorded per kilogram of soil.
Additional extraction methods comparison
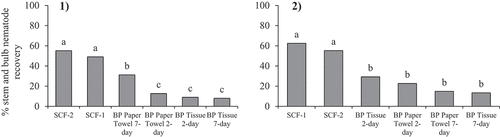
Extraction methods were compared further to assess variations in methods that have been used in other experiments. In addition to the SCF and BP methods described above, an additional sugar centrifugal flotation method and three other Baermann pan methods were compared to assess the ability to extract SBN from soil in September and December 2020. The additional SCF method (SCF-2) was similar to the aforementioned method (SCF-1), but used a #70 sieve (0.212 mm pore size) on top of the #500 sieve during the first sieving step, centrifuge tubes were centrifuged for 4 and 1 minutes, all supernatant was collected and a 484 g litre−1 sugar solution was used. The four Baermann pan methods included either three staggered 2-ply tissue paper (Kleenex®) sheets or two staggered paper towel (Bounty©) sheets with an extraction time of 2 or 7 days. One hundred cubic centimetres of moist pasteurized mineral soil (organic matter 3.1%, pH 7.4) were measured for each extraction method. Each quantity of soil was inoculated with 200 SBN in 1 mL of water by distributing the nematode solution into a 1 cm wide by 2 cm deep hole in the soil sample. The hole was immediately covered and set aside at room temperature for 2 hours before extraction. Active stem and bulb nematode collected from infested garlic cloves were used for inoculation. After extraction, the number of SBN recovered were counted under a compound microscope. The trial consisted of five replicates and was repeated twice.
Statistical analyses
Nematode counts for all assessments were analyzed using SAS® Version 9.4 (SAS Institute Inc., Cary, NC). The soil profile and initial extraction method experiment was designed as a factorial, with soil depth and extraction method as the factors. Data for both years were pooled for each nematode genus presented as there was no significant effect of year. An analysis of variance (ANOVA) was performed using PROC GLIMMIX to analyze the interaction between soil depth and extraction method for the different nematode genera. Multiple means comparisons were done to separate the nematode number for soil depth, extraction method and interactions using Tukey’s multiple comparison test. The alpha value for all statistical analyses was set to 0.05.
For the additional extraction methods trials, an analysis of variance (ANOVA) was performed using PROC GLIMMIX to assess the number of SBN recovered. Multiple means comparisons were done to separate the number of SBN recovered from each extraction method using Tukey’s multiple comparison test with an alpha value set to 0.05.
Results
Stem and bulb nematodes were found in 14 of the 20 garlic fields surveyed (Table S1). Populations of stem and bulb nematode ranged from 0 to 100 kg−1 soil and averaged 7 kg−1 soil when nematodes were extracted using the Baermann pan method. When the sugar centrifugal flotation method was used, stem and bulb nematode counts ranged from 0 to 2400 kg−1 soil and averaged 222 kg−1 soil. There was an interaction between soil depth and the extraction method among stem and bulb, spiral (Helicotylenchus spp.) and total root nematodes. The SCF method led to the extraction of significantly more stem and bulb nematodes from the top 5 cm of soil (). In the deeper portion of the soil core, however, there was no difference between the two extraction methods. In addition, significantly more spiral and total root pathogenic nematodes were extracted from the 5–20 cm deep section of soil using the SCF method compared to the BP method from the top 5 cm of soil. There was no interaction between the extraction methods and soil profile distribution for all other nematode genera, including root-knot (Meloidogyne spp.), root lesion (Pratylenchus spp.) and ring nematode (Criconemoides spp.). Significantly more spiral nematodes were extracted from the bottom 5–20 cm of soil (474 kg−1 soil as compared to 243 kg−1 soil in the top 5 cm), regardless of the extraction method. In addition, the SCF method extracted more spiral and ring nematodes than the BP method (516 vs 201 and 76 vs 0 nematodes kg−1 soil, respectively).
Table 1. Populations of plant-parasitic nematode genera in the top 5 cm and bottom 5–20 cm of the soil profile determined using the sugar centrifugal flotation and Baermann pan extraction method
Both sugar centrifugal flotation methods extracted significantly more SBN than the Baermann pan methods in both trials (). The maximum percent SBN recovered were 54% and 64.5% in the first trial and 77% and 58% in the second trial for SCF-1 and SCF-2, respectively. In the first trial, the BP method using paper towel for 7 days extracted significantly more SBN than the other BP methods, but less than the SCF methods.
Discussion
The results from this study show that both sampling method and extraction method can lead to significantly different results when attempting to determine population levels of stem and bulb nematode from the soil. The highest population levels of stem and bulb nematode were found in the top 5 cm of soil when nematodes were extracted using the sugar centrifugal flotation method. Thus, the best approach to accurately approximating the populations of SBN in soil is to sample only the top 5 cm and use the sugar flotation method for extraction.
Sampling the top 5 cm of soil is different from what is typically recommended for sampling to extract plant-parasitic nematodes from soil. Most government, industry and academic institutions recommend sampling for plant-parasitic nematodes by taking a 20–25 cm soil core and discarding the top 2.5–5 cm of soil (Hafez Citation2003; Abawi and Gugino Citation2007; Celetti and Potter Citation2011). Although this sampling protocol is effective for estimating most root-feeding nematode populations, discarding the top 5 cm of soil does not appear to be as effective when sampling and estimating stem and bulb nematode populations as it would miss the majority of these stem feeders in a soil sample. In addition, full 20–25 cm soil cores, including the top 5 cm of soil, would dilute the numbers of stem and bulb nematode. For the root-feeding genera found in the samples, however, using the standard sampling protocol that discards the top 5 cm of soil would still be the best option.
Since garlic is planted in the autumn in Ontario and not in the spring following winter, it is likely that the SBN recovered from the top 5 cm of soil were generally healthy and pathogenic at the time of the fallautumn sampling period. Active SBN during enumeration helped confirm this. If immotile SBN were recovered in general, or after the winter months, there may be speculation as to the pathogenicity of SBN found in the upper layer of soil, but this was not the case in this study.
Specific soil sampling guidelines for the stem and bulb nematode will create an accurate standard and provide more consistency among surveys, which is important when interpreting and comparing results. Many academic institutions already have sampling guidelines for specific nematodes and crops and this makes developing recommendations for growers more reliable (Jagdale and Arnold-Smith Citation2011; Camp Citation2012; Celetti and Cranmer Citation2017). Also, the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) recently published an article on stem and bulb nematode management in garlic that recommends collecting the top 5 to 7.5 cm of soil when sampling for this nematode, based on the first year of data from the current study (Celetti and Cranmer Citation2017).
In addition to the sampling protocol, the method used to extract stem and bulb nematodes from soil can lead to large differences in population estimates. The Baermann pan and sugar centrifugal flotation methods are two of the most common methods used in laboratories (EPPO Citation2013). The present study found that more stem and bulb nematodes were extracted from the soil using the SCF method compared to the BP method in the section of the soil profile where the highest populations of these nematodes were found. Although the SCF method is a slightly more complex process and requires more equipment and technical experience, it has been shown to be more effective for the extraction of stem and bulb nematodes, as well as a wide variety of other nematode genera (Harrison and Green Citation1976; Wallace Citation2016; Blauel Citation2018). It also provides results within a day compared to 5–7 days using the BP method. Most research conducted on stem and bulb nematodes makes use of the BP method for nematode extraction from soil, which was found to be effective for multiple soil types in a study conducted by Poirier at el. (Citation2019a). However, stem and bulb nematode studies conducted by Andres and Lopez-Fando (Citation1996) and Castillo et al. (Citation2007) used the SCF method for nematode extraction, indicating that there are researchers who may have concluded that this is a more accurate extraction method for SBN.
Diagnostic clinics and research laboratories regularly use the Baermann pan method, but this method is limited by its ability to only extract smaller and motile nematodes. However, the present study also found that the BP method significantly underestimates actual stem and bulb nematode populations in the soil as compared to the SCF method. Diagnostic clinics may be starting to change their protocols, as the University of Guelph Agriculture and Food Laboratory now recommends using the SCF method to assess for SBN in soil.
A recent study on extraction methods to quantify stem and bulb nematode in soil (Poirier et al. Citation2019a) came to the opposite conclusion. They found that the Baermann funnel and Baermann pan methods often extracted more stem and bulb nematodes as compared to the SCF method. The SCF method used in their study was similar to the method used in the initial extraction method comparison in the present study, which used the same sieve sizes and centrifugation rates. However, they used a higher sugar solution concentration (484 g L−1 compared to 454 g L−1), less centrifugation time (4 and 1 min compared to 6 and 2 min) and also collected all supernatant. In addition, for the BP methods, paper towel was used instead of tissues, which have different pore sizes. These same methods, including using 100 cm3 of soil spiked with 200 SBN, were compared to the SCF and BP methods used throughout the present study, and our results continued to show that SCF methods extract significantly more SBN from soil compared to BP methods. Reasons for these contradictory results are unclear. Unfortunately, there are no other results in previous literature to compare these two studies. In our studies, the SCF method has continuously been the preferred method for extracting SBN in soil and these results support decisions by laboratories, such as the University of Guelph Agriculture and Food Laboratory, to switch to using this method when assessing for this nematode in soil.
Garlic is a growing industry in Ontario and the stem and bulb nematode is one of the most important pests affecting garlic production in the province. In Ontario, the current method of sampling for stem and bulb nematode in soil has already been changed to sample only the top 5 cm, based on previous research. Following the results from this study, it is also recommended to use the sugar centrifugal flotation method for extraction of these nematodes from soil. These two changes will help to accurately identify fields with stem and bulb nematode, allowing growers to avoid infested fields or use other management practices to protect garlic from this devastating pest.
Supplementary_Table_-_stem_and_bulb_nematode_soil_profile_and_extraction_methods.docx
Download MS Word (27.9 KB)Acknowledgements
The authors would like to thank Dennis Van Dyk and Kevin Vander Kooi for their technical assistance in sampling and nematode extraction in 2018; and Melody Melzer and Dr Xueschan Shan, University of Guelph Laboratory Services for nematode extraction, identification and enumeration in 2015.
Supplemental material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2021.1928756
References
- Abawi GS, Gugino BK. 2007. Soil sampling for plant-parasitic nematode assessment. Department of Plant Agriculture, Cornell University. https://hdl.handle.net/1813/43284
- Andres MF, Lopez-Fando S. 1996. Effect of granular nematicide applications on the population density of Ditylenchus dipsaci in garlic. Nematropica. 26:167–170.
- Baermann G. 1917. Eine einfache methode zur auffindung von ankylostomum (Nematoden) larven in erdproben. [A simple way to find ankylostomum (nematode) larvae in soil samples]. Geneeskd Tijdschr Ned-Indie. 57:131–137.
- Blauel T. 2018. Plant-parasitic nematode population dynamics and alternative management options for tomatoes in southwestern Ontario [M.Sc. thesis]. University of Guelph.
- Bridge J, Starr JL. 2007. Plant nematodes of agricultural importance. London (UK): Manson Publishing Ltd.
- Camp ML. 2012. How to take soil samples for nematode analysis from field crops. University of Tennessee Extension. https://utcrops.com/wp-content/uploads/2021/01/Nematode-Sampling-How-to-20121.pdf.
- Castillo P, Vovlas N, Azpilicueta A, Landa BB, Jiménez-Díaz RM. 2007. Host-parasite relationships in fall-sown sugar beets infected by the stem and bulb nematode, Ditylenchus dipsaci. Plant Dis. 91:71–79.
- Celetti M, Cranmer T. 2017. Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) – stem and bulb nematode management in garlic. http://www.omafra.gov.on.ca/english/crops/hort/news/hortmatt/2017/14hrt17a2.htm.
- Celetti M, Paibomesai M. 2014. Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) – managing stem and bulb nematode in garlic starts in the fall. http://www.omafra.gov.on.ca/english/crops/hort/news/hortmatt/2014/22hrt14a1.htm.
- Celetti M, Potter J. 2011. Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) – sampling soil and roots for plant parasitic nematodes. http://www.omafra.gov.on.ca/english/crops/facts/06-099.htm.
- Dropkin VH. 1980. Introduction to plant nematology. New York (NY): John Wiley and Sons Inc.
- EPPO (European and Mediterranean Plant Protection Organization). 2013. PM 7/119 (1) Nematode extraction. EPPO Bull. 43(3):471–495. doi:https://doi.org/10.1111/epp.12077
- Fushtey SG, Kelly CB. 1975. A new record of stem and bulb nematode in Ontario. Can Plant Dis Surv. 55:27–28.
- Hafez SL. 2003. Sampling procedure to diagnose nematode infestations. College of Agricultural and Life Sciences, University of Idaho. https://www.extension.uidaho.edu/publishing/pdf/CIS/CIS1056.pdf
- Harrison JM, Green CD. 1976. Comparison of centrifugal and other methods for standardization of extraction of nematodes from soil. Ann Appl Biol. 82:299–308. doi:https://doi.org/10.1111/j.1744-7348.1976.tb00565.x
- Hughes B, Celetti MJ, Paibomesai M, Yu Q. 2013. Stem and bulb nematode (Ditylenchus dipsaci) in Ontario-grown garlic. Can J Plant Pathol. 35(1):114.
- Jagdale GB, Arnold-Smith LC. 2011. Sampling for plant-parasitic nematodes identification and diagnosis. Department of Plant Pathology, University of Georgia. https://extension.uga.edu/content/dam/extension-county-offices/cobb-county/anr/NemaGuide4-2017%20(5).pdf.
- Janssen GJW. 1994. The relevance of races in Ditylenchus dipsaci (Kiihn) Filipjev, the stem nematode. Fundam Appl Nematol. 17(5):469–473.
- Jenkins W. 1964. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis Rep. 48(9):692.
- Johnson PW, Kayler WE. 1972. Stem and bub nematode found in Erieau Marsh, Kent County, Ontario. Can Plant Dis Surv. 52(3):107.
- Maggenti A. 1981. General nematology. New York: Springer-Verlag.
- Mountain WB. 1957. Outbreak of the bulb and stem nematode in Ontario. Ann Rep Can Plant Dis Surv. 1957:62–63.
- Oostenbrink M. 1954. Een doelmatige methode voor het toetsen van aaltjesbestrijdingsmiddelen in grond met Hoplolaimus uniformis als proefdier. Mededelingen Landbouwhogeschool Gent. 19:377–408.
- Perry RN. 1999. Desiccation survival of parasitic nematodes. Parasitology. 119:S19–S30. doi:https://doi.org/10.1017/S0031182000084626
- Poinar GO. 1983. The naural history of nematodes. New Jersey: Prentice-Hall Inc.
- Poirier S, Dauphinais N, Bélair G, Gravel V, Mimee B. 2019a. Validation of extraction methods for diagnosis of the stem and bulb nematode Ditylenchus dipsaci. Can J Plant Sci. 41(4):597–602.
- Poirier S, Dauphinais N, Heyden HVD, Véronneau PY, Bélair G, Gravel V, Mimee B. 2019b. Host range and genetic characterization of Ditylenchus dipsaci populations from Eastern Canada. Plant Dis. 103:456–460. doi:https://doi.org/10.1094/PDIS-07-18-1201-RE
- Qiao Y, Zaidi M, Badiss A, Hughes B, Celetti MJ, Yu Q. 2013. Intra-racial variation of Ditylenchus dipsaci from garlic in Ontario as revealed by random amplified polymorphic DNA analysis. Can J Plant Pathol. 35:346–353. doi:https://doi.org/10.1080/07060661.2013.804883
- Sayre RM, Mountain WB. 1962. The bulb and stem nematode (Ditylenchus dipsaci) on onion in southwestern Ontario. Phytopathology. 52:510–516.
- Shurtleff MC, Averre CW. 2000. Diagnosing plant diseases caused by nematodes. The American Pathological Society. St. Paul (MN): APS Press.
- Tenente RCV. 1996. Nematode problems of bulbs, with special reference to Ditylenchus dipsaci. Nematropica. 26:91–99.
- Wallace T. 2016. Plant-parasitic nematodes in managed golf course greens throughout Canada [M.Sc. thesis]. University of Guelph.