 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Macrophomina phaseolina is a polyphagous phytopathogenic fungus that causes serious yield losses in economically important crop plants. The most appropriate control measure for this fungus is the utilization of resistance sources. To achieve this a fast and reliable screening method, in addition to a characterized pathogen population, is a prerequisite to accelerate breeding programmes. The aim of this study was to develop an efficient inoculation method for soybean and sunflower seedlings and to test the effect of inoculum maturity on disease development under controlled environmental conditions. Three inoculation methods, stem-tape, cut-stem and toothpick, and two inoculum types, hyphal and microsclerotial, were tested on sunflower and soybean seedlings. The fastest disease development on sunflower was obtained with the cut-stem and stem-tape inoculation methods using hyphal inoculum, and both methods had high repeatability. The cut-stem method, however, is not applicable for germplasm screening studies due to the predisposition of sunflower seedlings to pathogen attack. Application of the stem-tape inoculation method was useful for a variety of host reaction studies and for rapid germplasm screening. In soybean, the cut-stem inoculation method yielded consistent results in pathogenicity and variety reaction studies, but the stem-tape and toothpick inoculation methods had poor repeatability and long incubation periods, respectively. The cut-stem method in soybean and the stem-tape method in sunflower appear to be the most appropriate for pathogenicity and rapid germplasm screening studies with M. phaseolina under controlled conditions.
Résumé
Macrophomina phaseolina est un champignon phytopathogène polyphage qui cause de graves pertes de rendement dans des cultures de grand intérêt économique. Le mode de gestion le plus efficace pour lutter contre ce champignon est d’utiliser des sources de résistance. Afin d’y parvenir, une méthode rapide et fiable de criblage jointe à la caractérisation de la population d’agents pathogènes est préalable à l’accélération des programmes de sélection. Le but de cette étude était de développer une méthode efficace d’inoculation pour les semis de soya et de tournesol et de tester, dans des conditions contrôlées, l’effet de la maturité de l’inoculum sur le développement de la maladie. Trois méthodes d’inoculation, celles du ruban adhésif sur la tige, de la tige coupée, des cure-dents, et deux types d’inoculum, avec hyphes et avec microsclérotes, ont été testés sur des semis de tournesol et de soya. Le développement le plus rapide de la maladie sur le tournesol a été obtenu par les méthodes de la tige coupée et du ruban adhésif avec l’inoculum d’hyphes, et les deux méthodes ont affiché un haut taux de répétabilité. La méthode de la tige coupée, toutefois, ne s’appliquait pas aux études de criblage du germoplasme à cause de la prédisposition des semis de tournesol aux attaques de l’agent pathogène. L’application de la méthode d’inoculation par ruban adhésif a été utile pour diverses études sur la réaction de l’hôte ainsi que pour le criblage rapide du germoplasme. Chez le soya, la méthode d’inoculation de la tige coupée a fourni des résultats uniformes dans le cadre d’études sur la pathogénicité et la réaction des variétés, mais les méthodes d’inoculation du ruban adhésif et des cure-dents affichaient un faible taux de répétabilité et nécessitaient de longues périodes d’incubation, respectivement. La méthode de la tige coupée chez le soya et celle du ruban adhésif chez le tournesol, avec M. phaseolina, semblent, dans des conditions contrôlées, les plus appropriées pour les études sur la pathogénicité et le criblage rapide du germoplasme.
Introduction
Cultivated plant species are subjected to various biotic and abiotic stresses that limit yield, and oilseed crops are no exception. Oilseed plants are an economically important crop group cultivated for nutritional and industrial purposes, and 754 million tons of oilseed crops were produced worldwide in 2018 (FAOSTAT Citation2018). Oilseed crops are becoming increasingly important as industrial raw materials, supplementing livestock feed and being included in crop rotations. Accordingly, 34.7% and 4.7% of the total oilseed crop production worldwide are attributed to soybean and sunflower, respectively. The yield of this crop can be diminished severely, however, by charcoal rot (Macrophomina phaseolina Tassi. Goid.); losses can reach 80%–90% under conditions (high temperature and drought) favourable for disease development (Khan Citation2007; Gupta et al. Citation2012).
Macrophomina phaseolina is an anamorphic phytopathogenic fungus that can infect more than 500 species, including maize, soybean, sunflower, and clover. Generally, disease symptoms become visible during the flowering period of plants under abiotic stress conditions. Microsclerotia of M. phaseolina can remain viable in plant debris and soil for up to 15 years, and may constitute the main inoculum source for primary infection (Papavizas and Klag Citation1975; Ijaz et al. Citation2013). Owing to its polyphagous nature, crop rotations are not helpful for mitigating this disease and the soil-borne nature of M. phaseolina decreases the effectiveness of fungicide applications (Tyler Citation2008). While solarization and fumigation have been reported to suppress primary inoculum, their application in large fields is a tedious process (Lodha Citation1995).
Owing to global warming and water scarcity, M. phaseolina may become a more economically important pathogen in the future (Jordaan et al. Citation2019). Under such circumstances, the utilization of resistance sources identified from cultivated and wild germplasm can provide resilient and sustainable control methods (Shehbaz et al. Citation2018).
A suitable inoculation method for creating artificial epiphytotics is a prerequisite for successful germplasm screening in breeding programmes. While various inoculation methods are applied to the phyllosphere of the plant, there is still a need to identify suitable inoculation techniques considering the structure of the plant (anatomy, morphology, developmental stage, etc.) for in vitro pathogenicity studies of M. phaseolina (Zazzerini and Tosi Citation1989; Pratt et al. Citation1998; Twizeyimana et al. Citation2012).
Inoculation methods used for in vitro pathogenicity and germplasm screening studies under semi-controlled conditions include the toothpick, stem-tape and cut-stem inoculation techniques, and these have been widely applied to a diverse array of crop plants (Zazzerini and Tosi Citation1989; Pratt et al. Citation1998; Twizeyimana et al. Citation2012). Among them, the cut-stem inoculation method is widely applied to members of the Fabaceae family, and the stem-tape method is applicable to crop plants with thick stems, such as sunflower (Zazzerini and Tosi Citation1989; Pratt et al. Citation1998; Avilés et al. Citation2008; Pickel et al. Citation2020). Although toothpick inoculation is not limited to any plant part or plant species, it is generally used on plants approaching the end of vegetative development (Pratt et al. Citation1998; Avilés et al. Citation2008; Pickel et al. Citation2020).
While several M. phaseolina inoculation methods have been reported in the literature and used on a variety of crop plants, there is a lack of information on standardized methods for undertaking phytopathological studies with this fungus in sunflower and soybean crops under controlled conditions. Hence, the present investigation was conducted to standardize the inoculation techniques for screening soybean and sunflower germplasm under controlled conditions.
Materials and methods
Plant materials
Plant materials used for the pathogenicity, inoculation and varietal reaction studies were obtained from the Trakya Agricultural Research Institute (Edirne, Turkey) and Eastern Mediterranean Agricultural Research Institute (Adana, Turkey). The susceptible varieties ‘Yesilsoy’ and ‘Deray’ were used in the pathogenicity and inoculation experiments for soybean and sunflower, respectively. A total of six soybean and nine sunflower genotypes were evaluated; in addition to the susceptible varieties, these included the soybean cultivars ‘Ataem 7ʹ, ‘Türksoy’, ‘Mono’, ‘Bravo’, ‘Lider’ and ‘Mersoy’, and the sunflower cultivars ‘11 TR 077ʹ, ‘Kayra’, ‘Palanci’, ‘Ahmetbey’, ‘Metinbey’, ‘Orfey’, ‘Cetinbey’, ‘Sems OR’ and ‘17 IMI TR 003ʹ.
Isolation and characterization of M. phaseolina
Soybean and sunflower plants exhibiting charcoal rot disease symptoms were collected from the experimental plots of the Trakya Agricultural Research Institute and Eastern Mediterranean Agricultural Research Institute during September–October 2018 and 2019. Plants were inspected for the presence of microsclerotia in stem sections under a binocular microscope. Surface-sterilized plant tissue was placed in Petri dishes containing potato dextrose agar (PDA) and incubated at 30°C for 3 days. The developing colonies were transferred to fresh PDA plates, and single hyphal isolates were obtained and stored at −80°C in cryotubes filled with 20% glycerol solution for further use (Altinok and Can Citation2010). Macrophomina phaseolina genomic DNA was extracted according to Peever et al. (Citation1999), and MpKFI-MpKRI primers developed by Babu et al. (Citation2007) were used for molecular identification of the M. phaseolina isolates.
Inoculation methods, variety screening and disease scoring
Susceptible soybean (‘Yesilsoy’) and sunflower (‘Deray’) cultivars were sown in pots (9 cm) containing a peat:perlite:sand (2:1:1) mixture at a density of four and three seeds per pot for soybean and sunflower, respectively, and placed into a controlled environment growth chamber adjusted to a 12 h light and 12 h dark photoperiod at 26°C and 60% relative humidity. Inoculation methods conducted during the growth chamber studies included the cut-stem, stem-tape and toothpick techniques. TAE 7 isolate (GenBank Acc. no.: MK285654) from sunflower was used throughout the study. All inoculation methods were performed on 3-week-old sunflower and soybean seedlings. After inoculation, the growth chamber conditions were adjusted to a 14 h light and 10 h dark photoperiod at 30°C and 60% relative humidity (Zazzerini and Tosi Citation1989; Pratt et al. Citation1998; Twizeyimana et al. Citation2012).
Three- (hyphae) and 10-day-old (microsclerotia) M. phaseolina colonies grown on PDA were used for the cut-stem and stem-tape inoculation methods. Toothpick inoculum was prepared according to Pratt et al. (Citation1998). For the stem-tape inoculation method, sunflower seedlings were wounded 2 cm below the lowest leaf, soybean seedlings were wounded 2 cm below the cotyledon using a sterile scalpel, and agar plugs with 3-day-old or 10-day-old colonies of M. phaseolina were placed on the wound and wrapped with parafilm (Zazzerini and Tosi Citation1989). During application of the toothpick inoculation method, toothpicks containing inoculum were pinned to the hypocotyl of sunflower and soybean seedlings with minimal damage (Pratt et al. Citation1998). The cut-stem method was applied according to Twizeyimana et al. (Citation2012), wherein the tops of 3-week-old sunflower and soybean seedlings were cut with a sterile scalpel 2 cm above the top leaf, and 100 µL pipette tips containing 3-day-old or 10-day-old colonies of M. phaseolina were placed on the wound.
Scoring of the cut-stem inoculation method was performed according to the 0–8 scale used by Viteri and Linares (Citation2017). All other methods were scored according to the 0–4 scale used by Pratt et al. (Citation1998). Scoring was performed every 3 days, and disease severity (%) was calculated (Townsend and Heuberger Citation1943) (S = score, P = number of plants, TP = total number of plants, HS = highest score).
Each application had three and four replicates (number of plants per pot) for sunflower and soybean, respectively, with 16 repeats (number of pots) for each inoculation method and inoculum combination.
Cut-stem inoculation and stem-tape inoculation methods were applied during pathogenicity and variety reaction studies using 3-day-old inoculum on soybean and sunflower, respectively. Pathogenicity tests were performed on the susceptible cultivars ‘Deray’ (sunflower) and ‘Yesilsoy’ (soybean). Nine sunflower and six soybean cultivars were assessed for their reaction to M. phaseolina, with three and four replicates (number of plants per pot) for sunflower and soybean, respectively, and five repeats (number of pots) for each variety.
All trials except for the pathogenicity assay were repeated twice, and area under the disease progress curve (AUDPC) values were computed based on disease severity (%) obtained on the scoring days (Simko and Piepho Citation2012) (yi = disease severity % at the ith observation, ti = time of observation, n = total number of observations).
One-way ANOVA, Tukey’s B test and independent sample t-test together with Levene’s test for equality of variance analyses were applied to the AUDPC data using SPSS 23.00 software (IBM) at a significance level of 0.05 (Korolev et al. Citation2000).
Results
Isolation and molecular characterization
Ten M. phaseolina isolates were obtained from soybean and sunflower plants exhibiting charcoal rot symptoms. After successful establishment of single hyphal isolates, DNA extraction was undertaken. All isolates were characterized as M. phaseolina based on PCR amplification using a species-specific primer pair set.
Inoculation methods applied to soybean and sunflower
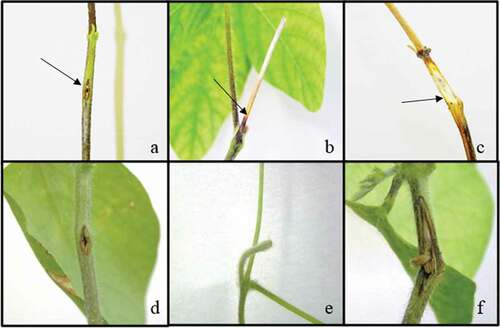
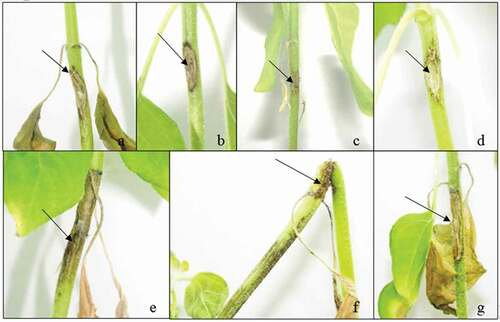
Disease symptoms progressing as thin lesions were observed on sunflower after removal of the inoculum applied through the stem-tape and toothpick inoculation methods. Symptoms that developed on cut-stem-applied plants progressed as rot starting at the inoculation point ().
Fig. 1 (Colour online) Control and diseased sunflower seedlings inoculated with Macrophomina phaseolina using different methods. (a) Toothpick method control and (d) inoculated seedlings; (b) stem-tape method control and (e) inoculated seedlings; and (c) cut-stem method inoculated and (f) control seedlings. Arrows indicate the symptoms

Application of the cut-stem inoculation method to soybean plants resulted in rapid disease progression until the first node and slowed down thereafter. The opposite result was observed for soybean plants inoculated using the stem-tape inoculation method; during the first 2 weeks after inoculation, disease development was relatively slow, then disease progression accelerated, and mortality was observed by the third week after inoculation (). No symptoms were observed in control plants. The pathogens from both hosts were isolated to meet Koch’s postulates and typical M. phaseolina colonies on PDA supplemented with antibiotics were observed.
Fig. 2 (Colour online) Control and diseased soybean seedlings inoculated with Macrophomina phaseolina using different methods. (a) Toothpick method inoculated and (d) control seedlings; (b) cut-stem inoculated and (e) control seedlings; and (c) stem-tape inoculated and (f) control seedlings. Arrows indicate the symptoms
The highest mean AUDPC and fastest disease development were observed for sunflower under the cut-stem and stem-tape inoculation methods applied using 3-day-old inoculum (). On soybean, the highest AUDPC and disease severity (%) values were observed for the stem-tape inoculation method applied using 3-day-old inoculum (); however, a significant difference was observed between the two trials based on an independent sample t-test ().
Table 1. Results of Tukey’s B test applied to the area under the disease progress curve (AUDPC, %) values from different inoculation method–inoculum combinations of two separate trials to determine significant differences between methods. A significant difference between the methods was observed for sunflower (‘Deray’) and for soybean (‘Yesilsoy’) (p = 0.05)
Table 2. Results of independent sample t-tests applied to area under the disease progress curve (AUDPC, %) values to determine significant differences between two repeats (p = 0.05) (n = 16 per method) of an inoculation experiment
Fig. 3 Mean disease severity (%) on sunflower (‘Deray’) and soybean (‘Yesilsoy’) over time following inoculation in different Macrophomina phaseolina inoculation method–inoculum combinations. (a) Results of first trial of method–inoculum combinations on sunflower (n = 16); (b) results of second trial of method–inoculum combinations applied on sunflower (n = 16); (c) results of first trial of method–inoculum combinations on soybean (n = 16); (d) results of second trial of method–inoculum combinations on soybean (n = 16)
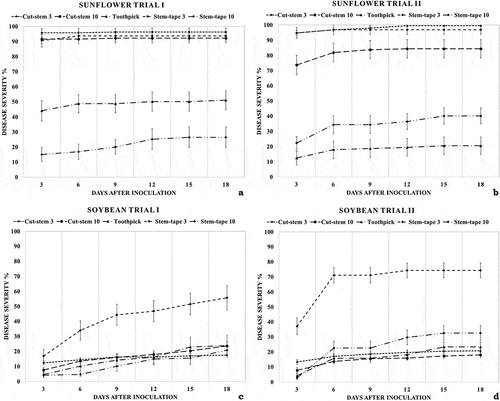
Fig. 4 Mean area under the disease progress curve (AUDPC, %) (n = 8) for different Macrophomina phaseolina inoculation method–inoculum combinations applied on sunflower and soybean. Vertical lines indicate the standard error of the AUDPC. Numbers next to each inoculation method indicate the number of days the inoculum was grown on potato dextrose agar (PDA). The AUDPC is shown as a percentage of the maximum possible area, starting from the day of inoculation until the final disease assessment
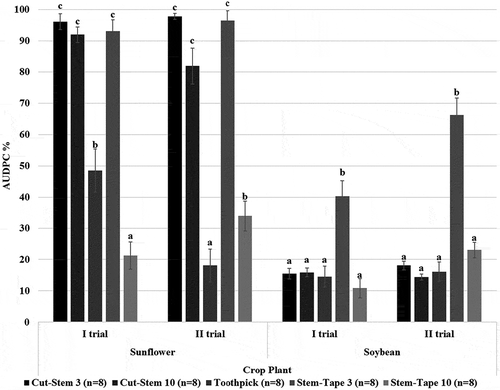
The results of the one-way ANOVA and Tukey’s B test applied to the AUDPC data of the different methods did not indicate a significant difference between repeats; however, there was a significant difference between inoculum maturity types for the stem-tape inoculation method applied to sunflower and soybean but not for the cut-stem inoculation method applied to either plant ().
As a result of the independent sample t-test applied to the AUDPC data, statistically significant differences between repeats were observed for the toothpick inoculation method applied to sunflower and the stem-tape inoculation method applied to soybean for both inoculum maturity types, and no significant differences between repeats were observed for the other inoculation methods ().
Variety reactions and pathogenicity assays
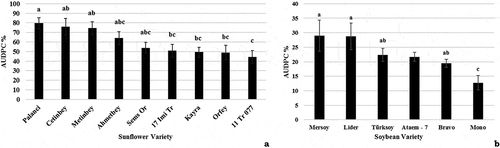
A stem-tape inoculation method with a 3-day-old inoculum was applied for varietal reaction assays of sunflower as this method exhibited a minimal difference between repeats and a short incubation period. Charcoal rot symptoms appeared on all the varieties; however, control plants (uninoculated) did not develop any symptoms. None of the tested varieties was resistant to M. phaseolina. The results of Tukey’s B applied to the AUDPC data suggest the presence of three different groups ().
Fig. 5 Screening soybean and sunflower varieties with Macrophomina phaseolina under controlled environmental conditions using the optimum inoculation methods for the crop species. The area under the disease progress curve (AUDPC) is shown as a percentage of the maximum possible area, starting from the day of inoculation until the final disease assessment. (a) Mean AUDPC (n = 10 per germplasm) for soybean varieties inoculated with M. phaseolina using the cut-stem inoculation method; values denoted by the same letter are not significantly different. (b) Mean AUDPC (n = 10 per germplasm) for sunflower varieties inoculated with M. phaseolina using the stem-tape inoculation method; values denoted by the same letter are not significantly different (p = 0.05)
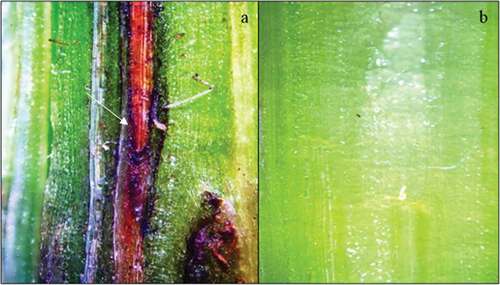
Differences in symptoms between varieties were observed. The ‘Sems OR’, ‘11 TR 077ʹ and ‘17 TR Imi 003ʹ varieties exhibited subepidermal lesions that were not visible without careful examination ().
Fig. 6 (Colour online) Subepidermal lesions on sunflower seedlings inoculated with Macrophomina phaseolina (a) using the stem-tape inoculation method vs. (b) control plants under 12× magnification. Longitude tangential section of the stem
Lesions that reached the node induced yellowing of the leaf and petiole. The cultivar ‘11 TR 077ʹ showed slow development of the disease in the first week, but later, disease progression was faster. Lesions caused by M. phaseolina surrounded stems completely at the inoculation point on the cultivars ‘Palanci’, ‘Ahmetbey’ and ‘Metinbey’, and after that disease progressed in both the vertical and horizontal directions. Lesions were also observed on petioles of the ‘Kayra’ variety, and lesions advanced as two–three thin lines on the ‘17 IMI TR 003ʹ variety. Lesions on the genotypes ‘Orfey’ and ‘Sems OR’ remained restricted to the inoculation points ().
Fig. 7 (Colour online) Disease reactions observed on sunflower inoculated with Macrophomina phaseolina. The cultivars (a) ‘Kayra’, (b) ‘17 TR IMI 003ʹ, (c) ‘Sems OR’, (d) ‘11 TR 077ʹ, (e) ‘Palanci’, (f) ‘Metinbey’ and (g) ‘Deray’ are shown. The arrows indicate lesions
Disease screening of the soybean cultivars was performed following the cut-stem inoculation method with 3-day-old inoculum. Tukey’s B analysis was applied to the AUDPC values of the two tested germplasm groups. Furthermore, resistant hosts were identified, although no complete resistance was observed (). Variations in the expression of symptoms of different varieties were as follows: local lesions on the cultivar ‘Ataem’, yellowing of the oldest leaves on ‘Bravo’, slight yellowing of all plants of the cultivar ‘Lider’ independent of lesion progression and yellow lesions on ‘Mersoy’.
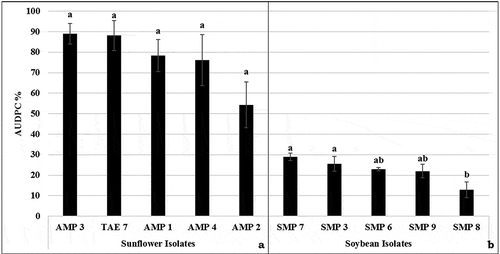
All isolates collected from soybean and sunflower plants caused disease development on their original hosts. The results of Tukey’s B test grouped soybean isolates into three different groups, although no difference between sunflower isolates was observed ().
Fig. 8 Results of pathogenicity tests with Macrophomina phaseolina isolates recovered from sunflower and soybean and inoculated on sunflower (‘Deray’) and soybean (‘Yesilsoy’) using the stem-tape and cut-stem methods, respectively. The area under the disease progress curve (AUDPC), starting from the day of inoculation until the final disease assessment. Bars topped by the same letter are not significantly different (n = 5 per isolate) at p < 0.05
Discussion
Macrophomina phaseolina causes charcoal rot disease in many economically important crops (Ijaz et al. Citation2013). It has previously been reported on maize, sunflower and soybean from Turkey (Karcılıoğlu et al. Citation1985; Tatlı and Sağır Citation1992; Mahmoud and Budak Citation2011). There are a number of diverse factors affecting disease development, such as environmental conditions, inoculum load, exposure period and plant age (Jordaan et al. Citation2019; Khamari et al. Citation2019; Chaturvedi Citation2020). In the present study, the pathogen was isolated successfully from plant tissues with embedded microsclerotia, and all isolates were identified as M. phaseolina through PCR using species-specific primers.
Different inoculum maturity types and inoculation methods were tested on sunflower and soybean seedlings under controlled environmental conditions using equal inoculum amounts and consistent inoculum exposure periods. This facilitated the selection of a suitable inoculation method for pathogenicity and varietal reaction assays. The cut-stem and toothpick inoculation methods are among the most popular inoculation techniques used in pathology studies under field and greenhouse conditions on various crop plant species (Zazzerini and Tosi Citation1989; Pratt et al. Citation1998; Twizeyimana et al. Citation2012; Viteri and Linares Citation2017). There is a lack of information on the applicability of inoculation methods other than the cut-stem inoculation under controlled environmental conditions. In addition, the stem-tape inoculation method has not been applied to germplasm screening studies. Toothpick inoculation techniques have limitations, such as low repeatability, owing to the vegetative developmental stage of the seedlings. Hence, the toothpick inoculation method was not suitable for rapid screening studies on soybean and sunflower seedlings under growth chamber conditions. According to Twizeyimana et al. (Citation2012), an equal distribution of the amount of inoculum among plants is an important factor during phytopathological studies. The results of studies performed by Khamari et al. (Citation2019) and Chaturvedi (Citation2020) suggest that older plants become more susceptible to charcoal rot disease. The toothpick inoculation method was used mainly for pathogenicity studies on a diverse array of hosts under greenhouse or field conditions and on mature plants between 6 and 8 weeks old, depending on the crop species (Pratt et al. Citation1998; Avilés et al. Citation2008). At the same time, the stem-tape and cut-stem inoculation methods applied to sunflower and soybean, respectively, showed few differences between trials, and faster disease development occurred on sunflower, making them suitable for work such as rapid resistance screening of varieties and evaluation of fungal pathogenicity under controlled conditions.
The effect of inoculum maturity on disease development was evaluated by Meyer et al. (Citation1974). However, their study was performed under field conditions, and the effect of inoculum maturity on disease development in vitro had not previously been reported. Hyphal and microsclerotial inocula were applied to soybean and sunflower plants to study the effect of inoculum type on the incubation period of the disease under controlled conditions. Hemmati et al. (Citation2018) performed histopathological studies and reported that susceptible and resistant cultivars did not show differences in the amount of microsclerotia attached to the roots or in the formation of appressoria, and that variation in disease progression in host tissues was observed after penetration of hyphae into inner plant tissues. As fresh inoculum consists mainly of hyphal structures, rapid disease progression can occur by bypassing the microsclerotial germination stage. The AUDPC and disease severity values obtained with the stem-tape inoculation method on sunflower and soybean using 3-day-old inoculum showed significant differences, suggesting that inoculum maturity is also important for controlled studies and that inoculation with fresh hyphae significantly accelerates disease development.
Almost all young leaves were removed from 3-week-old sunflower seedlings at the V4 development stage in plants subjected to the cut-stem inoculation method; new branches did not develop, and sunflower seedlings became highly susceptible to necrotrophic pathogen attack. The opposite situation was observed in soybean seedlings. The formation of new branches on lower nodes and the development of new leaves were observed for soybean subjected to the cut-stem inoculation method. Tuite (Citation1969) mentioned that predisposed and susceptible hosts are among the important factors affecting the success of pathogenicity studies. Therefore, we propose a cut-stem inoculation method for sunflower as a fast pathogenicity screening method under fully controlled conditions. However, this method is not suitable for other studies, such as germplasm evaluation, since the plants become more susceptible to pathogen attack, resulting in rapid disease development. The cut-stem method can be used for fast soybean germplasm and fungal population screening assays under fully controlled conditions, as suggested by Twizeyimana et al. (Citation2012).
The results of the present study indicate that the AUDPC is very informative when applied to disease severity (%) obtained during growth chamber studies when appropriate inoculation and scaling methods are used. Mengistu et al. (Citation2007) noted that the AUDPC value is greatly affected by environmental conditions when pathological studies are performed under field conditions, and the disease developmental stages reported by Hemmati et al. (Citation2018) suggest that the time necessary for successful infection is different between susceptible and resistant cultivars. Since AUDPC represents the speed of disease progression over time, we suggest that the AUDPC values, together with disease severity and disease incidence, should be considered for the identification of plant sources resistant to charcoal rot disease under controlled conditions.
The results of the sunflower variety reactions did not identify any resistance among the tested genotypes, and only ‘11 TR 077ʹ was moderately resistant to charcoal rot. However, differences in the severity and type of symptoms were observed among varieties, suggesting the presence of diverse resistance mechanisms. Among cultivated sunflower genotypes, only moderately resistant and susceptible germplasms have been reported to date, but potential resistance sources have been found in wild relatives (Shehbaz et al. Citation2018). Similarly, our results suggest the presence of resistance sources in screened soybean germplasm, but no complete resistance was observed. Soybean cultivars with moderate resistance to charcoal rot were reported by Mengistu et al. (Citation2011).
Although the methods tested in the current study have been evaluated previously by other researchers, to our knowledge no such study has been conducted under controlled growth chamber conditions. The stem-tape inoculation method is suggested as a reliable and repeatable inoculation method that could potentially be applied to sunflower seedlings at every developmental stage, although its success depends mostly on inoculum maturity and type (microsclerotia or hyphae). We propose that the cut-stem inoculation method applied to sunflower is easy to use and works well independently of inoculum quality; however, this method is not suitable for analyses other than pathogenicity. For soybean, the most suitable method was cut-stem inoculation since inoculum maturity did not have an effect on disease severity and progression. Rapid disease development resulting in plant death after 14 days after inoculation in soybean can lead to misinterpretation in germplasm screening studies, and the low repeatability makes the stem-tape method inappropriate for application to soybean seedlings. Although the toothpick inoculation method is widely used in various crop species under semi-controlled conditions, it was not suitable for studies on soybean or sunflower in growth chamber studies due to slow disease development.
Additional information
Funding
References
- Altinok HH, Can C. 2010. Characterization of Fusarium oxysporum f. sp. melongenae isolates from eggplant in Turkey by pathogenicity, VCG and RAPD analysis. Phytoparasitica. 38(2):149–157. doi:https://doi.org/10.1007/s12600-010-0081-0.
- Avilés M, Castillo S, Bascon J, Zea-Bonilla T, Martín-Sánchez PM, Pérez-Jiménez RM. 2008. First report of Macrophomina phaseolina causing crown and root rot of strawberry in Spain. Plant Pathol. 57(2):382. doi:https://doi.org/10.1111/j.1365-3059.2007.01717.x.
- Babu BK, Saxena AK, Srivastava AK, Arora DK. 2007. Identification and detection of Macrophomina phaseolina by using species-specific oligonucleotide primers and probe. Mycologia. 99(6):797–803. doi:https://doi.org/10.1080/15572536.2007.11832511.
- Chaturvedi D. 2020. Identification of moth bean plant susceptibility and variability factors to Macrophomina phaseolina and pathogenicity assay for the root rot in India. Indian Phytopathol. 73(2):253–256. doi:https://doi.org/10.1007/s42360-019-00192-z.
- FAOSTAT. 2018. The Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). [accessed 2020 Apr 11]. fao.org.
- Gupta GK, Sharma SK, Ramteke R. 2012. Biology, epidemiology and management of the pathogenic fungus Macrophomina phaseolina (Tassi) Goid with special reference to charcoal rot of soybean (Glycine max (L.) Merrill). J Phytopathol. 160(4):167–180. doi:https://doi.org/10.1111/j.1439-0434.2012.01884.x.
- Hemmati P, Zafari D, Mahmoodi SB, Hashemi M, Gholamhoseini M, Dolatabadian A, Ataei R. 2018. Histopathology of charcoal rot disease (Macrophomina phaseolina) in resistant and susceptible cultivars of Soybean. Rhizosphere. 7:27–34. doi:https://doi.org/10.1016/j.rhisph.2018.06.009.
- Ijaz S, Sadaqat HA, Khan MN. 2013. A review of the impact of charcoal rot (Macrophomina phaseolina) on sunflower. J Agric Sci. 151(2):222–227. doi:https://doi.org/10.1017/S0021859612000512.
- Jordaan E, van der Waals JE, McLaren NW. 2019. Effect of irrigation on charcoal rot severity, yield loss and colonization of soybean and sunflower. Crop Prot. 122:63–69. doi:https://doi.org/10.1016/j.cropro.2019.04.026.
- Karcılıoğlu A, Onan E, Esentepe M, Sezgin E. 1985. Ege Bölgesinde İkinci Ürün Soya ve Susam Ekim Alanlarında Görülen Fungal Hastalıklar Üzerinde Araştırmalar [Research on fungal diseases seen in aftercrop soy and sesame cultivation fields in Aegean region]. Zirai Mücadele Araştırma Yıllığı. 1:25. [ Turkish].
- Khamari B, Satapathy SN, Patra C. 2019. Effect of inoculum load and duration of exposure to Macrophomina phaseolina on disease incidence of sesame. Int J Chem Stud. 7(1):1728–1730.
- Khan SN. 2007. Macrophomina phaseolina as causal agent for charcoal rot of sunflower. Mycopath. 5(2):111–118.
- Korolev N, Katan J, Katan T. 2000. Vegetative compatibility groups of Verticillium dahliae in Israel: their distribution and association with pathogenicity. Phytopathology. 90(5):529–536. doi:https://doi.org/10.1094/PHYTO.2000.90.5.529.
- Lodha S. 1995. Soil solarization, summer irrigation and amendments for the control of Fusarium oxysporum f. sp. cumini and Macrophomina phaseolina in arid soils. Crop Prot. 14(3):215–219. doi:https://doi.org/10.1016/0261-2194(95)00014-D.
- Mahmoud A, Budak H. 2011. First report of charcoal rot caused by Macrophomina phaseolina in sunflower in Turkey. Plant Dis. 95(2):223. doi:https://doi.org/10.1094/PDIS-09-10-0631.
- Mengistu A, Arelli PA, Bond JP, Shannon GJ, Wrather AJ, Rupe JB, Chen P, Little CR, Canaday CH, Newman MA, et al. 2011. Evaluation of soybean genotypes for resistance to charcoal rot. Plant Health Prog. 12(1):6. doi:https://doi.org/10.1094/PHP-2010-0926-01-RS.
- Mengistu A, Ray JD, Smith JR, Paris RL. 2007. Charcoal rot disease assessment of soybean genotypes using a colony-forming unit index. Crop Sci. 47(6):2453–2461. doi:https://doi.org/10.2135/cropsci2007.04.0186.
- Meyer WA, Sinclair JB, Khare MN. 1974. Factors affecting charcoal rot of soybean seedlings. Phytopathology. 64(6):845–849. doi:https://doi.org/10.1094/Phyto-64-845.
- Papavizas GC, Klag NG. 1975. Isolation and quantitative determination of Macrophomina phaseolina from soil. Phytopathology. 65:182–187. doi:https://doi.org/10.1094/Phyto-65-182.
- Peever TL, Canihos Y, Olsen L, Ibañez A, Liu YC, Timmer LW. 1999. Population genetic structure and host specificity of Alternaria spp. causing brown spot of Minneola tangelo and rough lemon in Florida. Phytopathology. 89(10):851–860. doi:https://doi.org/10.1094/PHYTO.1999.89.10.851.
- Pickel B, Dai N, Maymon M, Elazar M, Tanami Z, Frenkel O, Toamy MA, Mor N, Freeman S. 2020. Development of a reliable screening technique for determining tolerance to Macrophomina phaseolina in strawberry. Eur J Plant Pathol. 157(7):707–718. doi:https://doi.org/10.1007/s10658-020-02051-4.
- Pratt RG, Mclaughlin MR, Pederson GA, Rowe DE. 1998. Pathogenicity of Macrophomina phaseolina to mature plant tissues of alfalfa and white clover. Plant Dis. 82(9):1033–1038. doi:https://doi.org/10.1094/PDIS.1998.82.9.1033.
- Shehbaz M, Rauf S, Al-Sadi AM, Nazir S, Bano S, Shahzad M, Hussain MM. 2018. Introgression and inheritance of charcoal rot (Macrophomina phaseolina) resistance from silver sunflower (Helianthus argophyllus Torr. & A. Gray) into cultivated sunflower (Helianthus annuus L.). Australas Plant Pathol. 47(4):413–420. doi:https://doi.org/10.1007/s13313-018-0573-9.
- Simko I, Piepho HP. 2012. The area under the disease progress stairs: calculation, advantage, and application. Phytopathology. 102(4):381–389. doi:https://doi.org/10.1094/PHYTO-07-11-0216.
- Tatlı F, Sağır A. 1992. Güneydoğu Anadolu Bölgesinde Ikinci Ürün Mısır, Susam ve Soya ‘da Görülen Bazı Fitopatolojik Sorunlar [Some phytopathological problems seen in the aftercrop corn, sesame and soy in the southeastern Anatolia region]. Güneydoğu Anadolu Bölgesi’nde İkinci Ürün Tarımı ve Sorunları Sempozyumu. [ Turkish].
- Townsend GR, Heuberger JW. 1943. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. 24:340–343.
- Tuite J. 1969. Plant pathological methods. Fungi and bacteria. Minneapolis (MN): Burgess Publishing Company.
- Twizeyimana M, Hill CB, Pawlowski M, Paul C, Hartman GL. 2012. A cut-Stem inoculation technique to evaluate soybean for resistance to Macrophomina phaseolina. Plant Dis. 96(8):1210–1215. doi:https://doi.org/10.1094/PDIS-02-12-0126-RE.
- Tyler BM. 2008. Genomics of fungal-and oomycete-soybean interactions. In: Stacey G, editor. Genetics and genomics of soybean. New York: Springer; p. 243–267.
- Viteri DM, Linares AM. 2017. Reaction of Phaseolus spp. genotypes to ashy stem blight caused by Macrophomina phaseolina. Euphytica. 213(8):1–8. doi:https://doi.org/10.1007/s10681-017-1989-y.
- Zazzerini A, Tosi L. 1989. Chlorate sensitivity of Sclerotium bataticola isolates from different hosts. J Phytopathol. 126(3):219–224. doi:https://doi.org/10.1111/j.1439-0434.1989.tb01107.x.
