Abstract
Cannabis (Cannabis sativa L., marijuana) plants with symptoms of crown rot, root decay, wilting and plant death were sampled during 2018 and 2019 from seven licensed production greenhouses. Affected tissues from 140 diseased plants were surface-sterilized and plated onto potato dextrose agar. Ninety-five isolates morphologically resembling Pythium species were subcultured and subjected to PCR of the ITS1-5.8-ITS2 region of ribosomal DNA. The following species were identified based on >99% sequence identity to reference isolates in GenBank: P. myriotylum (43 isolates), P. dissotocum (35 isolates), P. aphanidermatum (3 isolates) and Globisporangium ultimum (syn. P. ultimum) (2 isolates). A fifth species – P. catenulatum (12 isolates), was distinguished from P. rhizo-oryzae using the cytochrome oxidase c subunit I (COI) sequence. Cannabis licensed production facilities in British Columbia had all five species present, while P. dissotocum was found in two facilities in Ontario, and P. myriotylum was present in one facility in northern California. Isolates selected to represent each Pythium species were grown on potato dextrose agar at 25°C and they all showed comparable colony growth after 6 days. The same isolates caused root browning, decay and stunting of cannabis plants grown in a coco: perlite potting medium. Plant mortality was similar after 21 days but rates of disease progression varied depending on the isolate tested. Wounding of roots and prolonged periods of saturation enhanced disease development. These results demonstrate for the first time that crown and root rot on greenhouse-grown cannabis plants can be caused by up to five Pythium species.
Résumé
Des plants de cannabis (Cannabis sativa L., marijuana) provenant de sept serres de production commerciale et affichant des symptômes de pourriture du collet, de pourriture des racines, de flétrissement, et qui en sont morts, ont été échantillonnés en 2018 et 2019. Les tissus atteints de 140 plants malades, dont la surface a été stérilisée, ont été déposés sur de la gélose dextrosée à la pomme de terre. Quatre-vingt-quinze isolats ressemblant à l’espèce Pythium ont été repiqués, puis la région de l’ITS1-5.8-ITS2 de l’ADN ribosomique a été soumise à une PCR. Les espèces suivantes ont pu être identifiées en se basant sur une identité de séquences de plus de 99% à l’égard des isolats de la GenBank: P. myriotylum (43 isolats), P. dissotocum (35 isolats), P. aphanidermatum (3 isolats) et Globisporangium ultimum (syn. P. ultimum) (2 isolats). Une cinquième espèce, P. catenulatum (12 isolats), a été différenciée de P. rhizo-oryzae en se référant à la séquence de la sous-unité I de la cytochrome c oxydase (COI). On trouvait les cinq espèces dans les installations de production autorisées de la Colombie-Britannique, tandis que P. dissotocum a été trouvé dans deux installations en Ontario et P. myriotylum, dans une installation du nord de la Californie. Les isolats sélectionnés pour représenter chaque espèce de Pythium ont été cultivés sur de la gélose dextrosée à la pomme de terre à 25°C et, au bout de six jours, toutes les colonies affichaient une croissance similaire. Les mêmes isolats causaient le brunissement des racines, la pourriture et le rabougrissement des plants de cannabis cultivés dans un terreau de fibre de coco et de perlite. Le taux de mortalité était analogue après 21 jours, mais le taux de progression de la maladie variait selon l’isolat testé. Des blessures infligées aux racines et des périodes prolongées de saturation ont accru le développement de la maladie. Ces résultats démontrent pour la première fois que jusqu’à cinq espèces de Pythium peuvent causer la pourriture du collet et des racines chez les plants de cannabis cultivés en serre.
Introduction
Pathogens infecting the root systems of plants, particularly species of Fusarium, Pythium and Rhizoctonia, cause significant reduction in yields of crops grown under greenhouse and field conditions. Many of these pathogens can also cause crown rot, leading to stunted growth, yellowing and wilting, and frequently death of plants. Root infections generally occur during the early stages of plant growth, and symptoms may manifest soon thereafter or later during growth. In some instances, a diverse group of pathogens may be present on root systems, making pathogen diagnosis and disease management more difficult (Garcia and Mitchell Citation1975; Pieczarka and Abawi Citation1978).
On cannabis (Cannabis sativa L., marijuana) grown in Canada, both Fusarium and Pythium species were previously reported to cause a range of disease symptoms. For example, in hydroponic systems, Fusarium oxysporum, F. solani, Pythium myriotylum, P. dissotocum and P. aphanidermatum were reported to cause root browning, yellowing of plants and stunting, and plant death (Punja and Rodriguez Citation2018; Punja et al. Citation2019). Similarly, on field-grown cannabis plants, a complex consisting of F. oxysporum, F. brachygibbosum and P. aphanidermatum caused plant mortality under extremely warm temperatures (Punja et al. Citation2018). On greenhouse-grown cannabis plants, symptoms of damping-off, crown rot, pith discoloration, and plant mortality were reported to be caused by F. oxysporum, F. proliferatum and F. solani (Punja et al. Citation2021; Punja Citation2021a, Citation2021b).
During 2018 and 2019, disease symptoms on greenhouse-grown cannabis plants in British Columbia that did not match these previous descriptions were observed in four commercial greenhouses. On vegetatively propagated plants, total destruction of the root system followed by plant collapse was observed. Affected plants had crown lesions extending up to 10 cm on the stem. On flowering plants, extensive root decay led to rapid wilting and plant death, while on stock (mother) plants, root browning with no apparent foliar symptoms was observed. Isolations made from symptomatic tissues yielded 95 isolates of Pythium originating from seven licensed production facilities located in British Columbia, Ontario and northern California during 2018–2019.
The objectives of this study, therefore, were to: (i) identify the Pythium species recovered from diseased cannabis plants using sequence analysis of the ITS1-5.8S-ITS2 region of rDNA; (ii) develop a protocol to assess the pathogenicity of isolates of the different Pythium species on cannabis plants; and (iii) compare the growth characteristics and pathogenicity of selected isolates.
Materials and methods
Sampling of plants
Cannabis plants at different stages of growth with symptoms of leaf yellowing, stunted growth, wilting, browning and decay of roots, or plant death, were included in the study. Plants were grown indoors in controlled environments or in greenhouses in Health Canada-approved licensed facilities (four were located in British Columbia and two in Ontario). One sample originated from a production facility in northern California. Samples of symptomatic plants were obtained at various stages during the production cycle, ranging from early propagation (1–2 weeks old) to vegetative growth (3–4 weeks of age) () to onset of full flowering period (6–12 weeks of age) (). In addition, stock (mother) plants that were grown in large containers (10 L) over a period of 6–10 months were sampled. The isolations were conducted during February 2018–December 2019 and a total of 140 symptomatic plants (stock plants, vegetative plants or flowering plants) of eight strains (genotypes) of cannabis were included, depending on what was in production. The symptoms displayed on these plants are shown in . Plants were propagated either in cocofibre (coco coir) substrate from various suppliers or in rockwool blocks (Grodan) and were grown with the appropriate nutrient regimes and lighting conditions required for commercial hydroponic production (Small Citation2017). The environmental conditions during the times at which sampling was conducted varied according to the production facility; temperatures were in the range of 24–38°C and relative humidity was in the range of 60% to 85%.
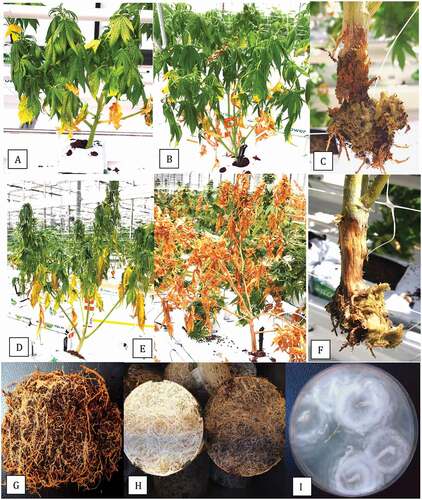
Fig. 1 (Colour online) The propagation system used for cannabis to produce vegetatively propagated plants and symptoms of Pythium infection. (a) Root development from a cutting that was first dipped in rooting powder (containing IAA) and inserted into a rockwool cube and incubated under high humidity for two weeks. (b) Placement of the cutting into a coco coir block. The rockwool cube is inserted into the block, potentially causing some root damage. (c) Transfer of coco blocks onto a greenhouse table where irrigation is provided by flooding the table, as shown in (d). (e, f) Early symptoms of wilting and yellowing of leaves on rooted cuttings. (g) Complete destruction of roots and crown discolouration on rooted cuttings. (h) Advanced root rot and crown and stem infection on vegetative plants. (i) Internal discolouration of tissues of plant shown in (h) with browning and rotting of the cortical and pith tissues. (j) Sunken brown lesions on upper crown and root rot symptoms on flowering plants. (k) Recovery of Pythium myriotylum from surface-sterilized tissue segments from the plant shown in (j).

Fig. 2 (Colour online) Symptoms of root and crown rot on flowering plants of cannabis caused by several species of Pythium. (a) Early symptoms of wilting with darkening and yellowing of leaves in early stages of flowering. (b) Advanced stages of wilting on affected plant. (c) Crown decay and root destruction on plant shown in (b). From this affected plant, P. myriotylum was recovered. (d) Extensive wilting and yellowing of leaves of plants at the flowering stage. (e) Total collapse and death of plants just prior to harvest. (F) Sunken crown lesion and destruction of the root system on plant shown in (e). From this affected plant, P. aphanidermatum was isolated. (g) Browning of roots on flowering plants grown in a coco coir medium from which P. dissotocum was recovered. (h) Browning of roots on stock plant (right) compared to healthy plant (left). The affected plant had been subjected to long periods of water saturation. (i) Colony of P. catenulatum recovered from the affected root system shown in (h).
Isolation from plants
Tissue sources for pathogen isolation included root segments, stem pieces and crown tissues from symptomatic plants. From each tissue type, small pieces, approximately 0.5 cm in length for roots or 0.2–0.4 cm2 for cuttings and stem pieces, were surface-disinfested by dipping them in a 10% bleach solution (containing 0.625% NaOCl) for 1 min, followed by 30 s in 70% EtOH. They were rinsed thrice in sterile water and blotted on sterile paper towels. Tissue pieces were plated onto potato dextrose agar (PDA, Sigma Chemicals, St. Louis, MO) amended with 130 mg L−1 of streptomycin sulphate (PDA+S). Dishes were incubated under ambient laboratory conditions (temperature range of 21–24°C with 10–12 hr day−1 fluorescent lighting) for 5–10 days. Emerging colonies that resembled Pythium spp. (rapidly growing colonies with white aerial mycelium) were transferred to fresh PDA+S for identification to species level. A total of 95 isolates of presumed Pythium spp. were obtained during 2018–2019. Cultures of all Pythium spp. were grown on PDA+S for 7 days under ambient laboratory conditions prior to molecular identification as described below.
Molecular identification
DNA was extracted from 10–50 mg of mycelium scraped from the surface of each colony using the QIAGEN DNeasy Plant Mini Kit (cat. no. 69 104). Species-level identification was done by conducting PCR of the ITS1-5.8S-ITS2 region of ribosomal DNA with 2–50 ng uL−1 DNA. The ITS region was amplified using the universal eukaryotic primers UN-UP18S42 (5′-CGTAACAAGGTTTCCGTAGGTGAAC-3′) and UN-LO28S576B (5′-GTTTCTTTTCCTCCGCTTATTAATATG-3′) (Schroeder et al. Citation2006) to produce a DNA template for sequencing. PCR conditions were as follows: initial denaturation at 94°C for 3 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, and extension at 72°C for 2 min, and a final extension at 72°C for 7 min, followed by 4°C hold. The PCR products (approximate size 950 bp) were cut from the gel and collected using the MinElute Gel Extraction Kit (cat. no. 28604) and 8 uL of DNA was sent to Eurofins Genomics (https://www.eurofinsgenomics.com/en/home/) for sequencing. The resulting sequences were compared to the ITS1-5.8S-ITS2 sequences from the National Center for Biotechnology Information (NCBI) GenBank database and those that had the highest sequence match (over 99%) were used to confirm species identity. Multiple sequence alignment of the respective isolates was done using the CLUSTAL W program (http://www.genome.jp/tools/clustalw). For the analysis, previously published reference isolates of different Pythium species, as described in Hyde et al. (Citation2014), that belonged to designated clades were included. The sequences of eight Pythium isolates were selected to represent each of the previously described species and they were subsequently included in a phylogenetic analysis using the neighbour-joining (NJ) method and a bootstrap consensus tree was inferred from 1000 replicates as described previously (Punja and Rodriguez Citation2018). The isolates were selected to represent the species based on characteristic colony and growth rates as described below, as well as showing 100% ITS sequence homology with reference isolate sequences.
Comparison of growth rates and pathogenicity
One isolate each of P. aphanidermatum, P. myriotylum, P. dissotocum, P. ultimum and P. catenulatum was selected from the eight isolates that were included in the phylogenetic analysis () and for which GenBank accessions numbers are provided in . The isolates were selected to represent the morphological features of colonies that most closely matched those of the four species as shown in . The cultures were grown on PDA+S for 1 week and then 5 mm diameter plugs were transferred to fresh medium, in five replicates, and placed at 22°C in a controlled environment chamber with 12 hr light/dark. Colony growth measurements were made daily in two perpendicular directions for 6 days and averaged. The experiment was conducted twice and the data averaged. The growth comparisons of the representative isolates was made by examining radial growth at each daily interval.
Table 1. Pythium species recovered from root and crown tissues of symptomatic plants of Cannabis sativa L. during 2018 and 2019a
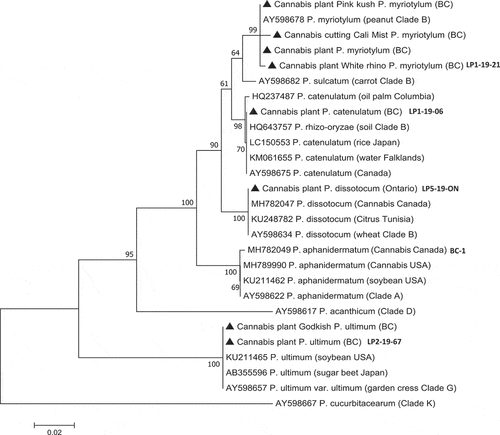
Fig. 3 Phylogenetic relationships of eight isolates of Pythium spp. recovered from cannabis plants from British Columbia (BC) and Ontario (ON) (marked with ⏏) using ITS1-ITS2 sequences compared to isolates from other hosts and geographic regions (GenBank numbers are shown). For the analysis, reference isolates of different Pythium species described in Hyde et al. (Citation2014) that belonged to designated Clades A, B, D, G, K were included in the tree. Isolates of P. dissotocum and P. aphanidermatum recovered from cannabis plants in previous studies (Punja and Rodriguez Citation2018; Punja et al. Citation2019) were included (GenBank accession nos. MH782047 and MH782049, respectively). The relationship was inferred using the Neighbour-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The tree is drawn to scale, with branch lengths in the same units as those used for the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. The analysis involved 27 nucleotide sequences. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA7 (Kumar et al. Citation2016). The outgroup used was Phytophythium cucurbitacearum.
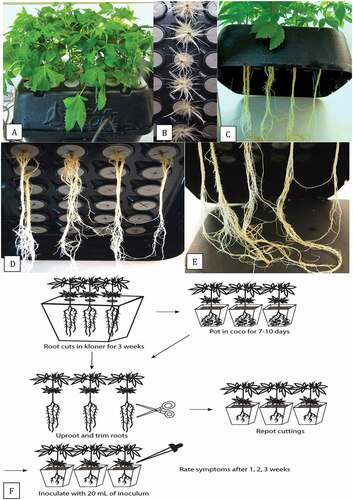
To obtain rooted plants for pathogenicity testing, stem cuttings (10–15 cm in length) taken from stock plants of cannabis strains ‘Hash Plant’ and ‘White Rhino’ were placed upright in the polyethylene collar plugs designed to hold them in a TurboKlone T24 Turbo mini aeroponic cloning system (www.turboklone.com) with a humidity dome (). The ends of the stem cuttings were dipped in Remo Roots rooting hormone (with IBA/NAA and nutrients, https://indoorgrowingcanada.com/products/remo-nutrients-remos-roots) to promote rooting (). The aeroponics unit was driven by a pump that provided continuous misting with water containing General Hydroponics Rapid Start (with plant extracts and nutrients N-P-K 1–0.5-1, https://www.greencorner.ca/gh-rapid-start-1-oz) in a bottom reservoir of the unit. At a later stage, 1 ml L−1 Sensi Grow Coco pH Perfect A + B and 1 ml L−1 General Hydroponics Calimagic (adjusted to a pH of 5.8–6.2 using Advanced Nutrients pH-Down) were added to the TurboKlone to enhance root growth. Throughout the experiment, the solution in the aeroponic cloners was adjusted daily to a pH of 5.8–6.2 using the previously mentioned pH-Down solution. After 3 weeks, the rooted cuttings (c–e) were removed from the TurboKlone. For inoculation, the plants were either used directly from the Turboklone, or were potted in a coco coir: perlite (3:1) potting medium in 10 cm2 pots. The pots were placed in a plastic tray containing a 1 cm layer of water, and covered with a plastic dome to maintain high humidity and placed in a Conviron incubator set at 24°C with a 24-hr photoperiod for 7–10 days. At the time of inoculation, the plants were uprooted and the coco growing medium was shaken off. The bottom 10 cm of the root systems of these plants, and those taken directly from the cloner, were cut with scissors (). The wounded plants were re-potted and inoculated with 20 mL of a mycelial suspension of each Pythium isolate which was poured around the base of each plant. To prepare inoculum, the isolates were grown in potato dextrose broth (100 mL) for 7 days at 150 rpm on a shaker and the mycelial mat from two flasks was blended with 200 mL of water for 20 s. The blended slurry (which contained mostly mycelium, and occasionally a few sporangia) was then used to inoculate plants at 20 mL per pot. Aliquots of the slurry were serially diluted in sterile distilled water and plated onto PDA to estimate colony-forming units per mL after 5 days of incubation.
Fig. 4 (Colour online) Propagation of vegetative cuttings by rooting in a TurboKlone T24 Turbo mini aeroponic cloning system. (a) Cuttings with foliage growth 2 weeks after they were introduced into the cloner. (b) Roots emerging from the exposed end of the cutting following treatment with rooting hormone. (c) Elongated roots developing from the cuttings 3 weeks after they were introduced into the cloner. (d, e) Total root length from the cut end of the stem to the bottom of the reservoir is about 30 cm. (f) Schematic representation of the inoculation method for testing the pathogenicity of isolates of Pythium spp. recovered from cannabis plants. Details are presented in the materials and methods.
Symptoms of yellowing or stunting of the plants and extent of mycelial growth were rated on a scale of 0 to 5 after 1, 2 and 3 weeks. The scale was as follows: 0 = healthy plant, no visible symptoms; 1 = plants wilting, some stunting; 2 = as in (1) with leaves yellowing, stem discoloured; 3 = as in (2) with mycelial growth visible on stem, leaves necrotic; 4 = as in (3) but with more mycelial growth and necrotic leaves; 5 = plants dead, mycelial growth covering plants. There were five replicate plants for each Pythium isolate. The experiment was conducted twice on each of strains ‘Hash Plant’ and ‘White Rhino”. Standard errors of the mean were determined from the replicates and repetitions of the experiments. The data are presented as average disease rating at 7, 14 and 21 days and as mean final disease rating at 21 days. Re-isolations were made from diseased tissues following the surface-sterilization procedure described previously and tissues were plated onto PDA+S.
Results
Disease symptoms and pathogen recovery
The procedure used for commercial propagation of cannabis plants is shown in . Cuttings are rooted in rockwool cubes under high humidity conditions for 2 weeks (), after which they are transferred into cocofibre blocks () and placed on benches to undergo vegetative growth for an additional 2 weeks (). The benches are flooded to provide irrigation at least twice daily (). Initial symptoms of disease were observed on propagated rooted cuttings as sudden wilting and yellowing of the lower leaves (, f). The crown tissues were soft and necrotic. On older vegetative plants, wilting and darkening (greening) of the upper foliage, accompanied by yellowing of the lower leaves, was commonly observed (). When these plants were pulled up, the root system had been destroyed and the crowns were rotted. In many instances, the crown infection progressed upward on the stems for distances of up to 10 cm (). Longitudinal sections made through these stems showed an internal darkening and rotting of the cortical and pith tissues (). Similar symptoms were observed on young flowering plants, which showed dark brown sunken lesions around the crown and a decayed root system (). Isolations made from these plants yielded primarily colonies identified as P. myriotylum () and occasionally P. dissotocum.
The progression of disease symptoms on flowering plants caused by Pythium is shown in . Wilting and darkening (greening) of the leaves, with yellowing and necrosis of the lower leaves, accompanied by destruction of the crown and root tissues, was associated with P. myriotylum (–c). Rapid and extensive wilting and yellowing of the lower leaves accompanied by a completely destroyed root system was associated with P. aphanidermatum (–f). A total collapse and rapid death of the plants with a completely destroyed root system was observed (). The root systems of larger flowering plants with no apparent foliar systems showed surface browning () and when these were surface-sterilized and plated onto PDA, they yielded primarily P. dissotocum. On large stock plants grown for 6–8 months in 10 L pots, many roots developed a surface browning compared to healthy white roots on unaffected plants (). These roots yielded P. catenulatum (). These species identifications were based on the PCR analysis described below.
Pythium species identification
Following PCR of the ITS1-5.8-ITS2 region of ribosomal DNA using the primer set UN-UP18S42 and UN-LO28S576B, a band size of approximately 950 bp was obtained for all Pythium isolates (data not shown). Sequence comparison against reference isolates described in Hyde et al. (Citation2014) that belonged to distinct clades were used to infer the presence of four Pythium species (one of which, P. ultimum, is also synonymous with Globisporangium ultimum) within the collection of 95 isolates obtained during 2018 and 2019. A fifth species – P. catenulatum – could not be resolved from P. rhizo-oryzae using the ITS region. Therefore, the cytochrome oxidase c subunit I (COI) region was compared following PCR of mitochondrial DNA using primers OomCoxILevup (5ʹ-TCAWCWMGATGGCTTTTTTCAAC-3ʹ) and Fm85mod (5ʹ-RRHWACKTGACTDATRATACCAAA-3ʹ) as described by (Robideau, et al., Citation2011). A 730 bp size fragment was obtained for two isolates (data not shown) and sequence analysis demonstrated a 97.14% sequence identity to P. catenulatum (accession no. MT981120.1 from tomato roots in Australia) and a 96.55% identity to P. rhizo-oryzae (accession no. HQ708798.1 from rice roots in India). The identity of the isolates was ascribed to P. catenulatum.
The number of isolates of each of the Pythium species recovered in this study are presented in . The most frequently recovered isolates were of P. myriotylum (43 isolates), followed by P. dissotocum (35 isolates) and P. catenulatum (12 isolates). Isolates of both P. aphanidermatum and P. ultimum were recovered at low frequencies. Isolates belonging to all five species were present in British Columbia, with four licensed production facilities having different combinations of isolates. Samples from Ontario contained P. dissotocum and one sample from northern California had P. myriotylum present (). Phylogenetic analysis was conducted with eight isolates selected to represent each of the Pythium species identified in the present study based on ITS analysis () and based on Hyde et al. (Citation2014). Isolates of each Pythium species were found to group with the selected reference isolate chosen to represent that species from different hosts and geographic regions worldwide (). The reference isolates were selected from clades that contained these species as described in Hyde et al. (Citation2014). Isolates of Pythium species recovered from cannabis plants in previous studies (Punja and Rodriguez Citation2018; Punja et al. Citation2019) were also found to group with isolates corresponding to the same species in the present study (GenBank accessions nos. MH782047 and MH782049).
Pathogenicity tests
To ensure development of a uniform root system for inoculation with Pythium isolates, cannabis cuttings were rooted in a TurboKlone aeroponic rooting system () supplemented with hydroponic rooting and nutrient solution. After 10 days, roots were initiated from the cut stem ends (), and by 3 weeks, extensive roots had developed, measuring >30 cm in length (). The roots were white with many lateral branches and root hairs (, e). The inoculation methods whereby plants were removed from the cloner, roots were wounded and plants potted in a coco: perlite medium or immediately inoculated with 20 mL of a mycelial suspension (), resulted in disease symptoms. The symptoms caused by an isolate of P. myriotylum inoculated on strain ‘White Rhino’ are shown in . After 2 weeks, diseased plants were wilted and stunted, with an initial darkening of the leaves which then turned yellow, and the root system was brown (, b). After 3 weeks, leaves were necrotic and some plants were dead with extensive mycelial growth (, d). The effect of inoculation with isolates selected to represent each of the four Pythium species (P. aphanidermatum was excluded) on disease development on strains ‘Hash Plant’ and ‘White Rhino’ is shown in . After 3 weeks, all inoculated plants showed severe symptoms of disease, ranging to total collapse with P. myriotylum (, b) and severely stunted surviving plants for P. catenulatum (, h).
Fig. 5 (Colour online) Symptoms on cannabis plants resulting from inoculation with an isolate of Pythium myriotylum. Cuttings of strain ‘White Rhino’ were rooted in the cloner and inoculated with 20 mL of a mycelial suspension after roots were trimmed and plants were potted. (a) Initial wilting symptoms 7–10 days after inoculation on plant on the right compared to the noninoculated control plant on the left. (b) Wilted plants showing yellowing of the lower leaves and browning of the root system at 14 days. (c) Advanced stages of disease development after 3 weeks on two inoculated plants. (d) Growth of mycelium of P. myriotylum under conditions of high humidity.

Fig. 6 (Colour online) Disease symptoms 3 weeks after inoculation of rooted cuttings of two cannabis strains with isolates selected to represent four Pythium species. In the left panel (a, c, e, g) is strain ‘Hash Plant; in the right panel (b, d, f, h) is strain ‘White Rhino’. The four species inoculated were P. myriotylum (a, b), P. ultimum (c, d), P. dissotocum (e, f) and P. catenulatum (g, h). In each photo, noninoculated plants are shown on the left. The isolates used are shown in .

Growth rate and disease ratings
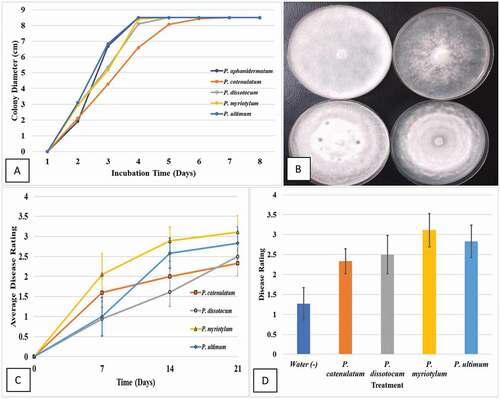
The comparative radial growth of isolates representing five Pythium species on PDA at 22°C over a 7-day period is shown in . All isolates grew rapidly and reached the edge of the 9 cm diameter Petri dish by 6 days () and they displayed distinct colony morphologies (). The disease ratings obtained 1, 2 and 3 weeks after inoculation with isolates representing four Pythium species (P. aphanidermatum was not included) is shown in . Both P. myriotylum and P. ultimum caused foliar necrosis and wilting by 14 days (disease severity rating of 3), while P. dissotocum and P. catenulatum had a disease severity rating of 1.5–2 at 14 days (). At 21 days, however, the disease ratings for isolates representing all four species were comparable and in the range of 2.5 to 3.5 ().
Fig. 7 (Colour online) Growth and pathogenicity of isolates of Pythium spp. on cannabis plants. (a) Comparison of radial growth of isolates representing five Pythium species on potato dextrose agar (PDA) after 7 days. (b) Appearance of representative colonies of isolates of P. ultimum (top left), P. myriotylum (upper right), P. dissotocum (lower left) and P. catenulatum (lower right) after 7 days of growth on PDA. (c) Disease development over a 3-week period following inoculation of cannabis plants with an isolate each of four Pythium species. The disease rating scale used is described in the materials and methods. Vertical bars represent standard errors of the mean. (d) Final disease ratings taken at day 21. Vertical bars are standards errors of the mean for each species. There were no significant differences among the four Pythium species in final disease ratings. The water (negative control) showed symptoms of yellowing which was attributed to poor rooting and not due to Pythium.
Discussion
Root discolouration (browning) is a common early symptom of infection due to Pythium species, which is generally followed by decay of lateral roots and crown tissues (Hendrix and Campbell Citation1973; Martin and Loper Citation1999; Sutton et al. Citation2006; Troth and Thiessen Citation2019; Osterbauer and Ocamb Citation2020). This study demonstrates that isolates of as many as five different Pythium species, identified based on comparison of ITS1-5.8S-ITS2 sequences with reference isolates deposited in GenBank, and from the cytochrome oxidase c subunit I (COI) sequence for P. catenulatum, can cause root browning, root rot and crown rot of cannabis plants grown under greenhouse conditions, resulting in stunting, wilting and ultimately plant death. In the phylogenetic analysis used for species confirmation, the ITS sequences of reference isolates of Pythium species that belonged to distinct clades in the study of Hyde et al. (Citation2014) were used to validate the species identification of representative isolates from cannabis in this study, with one exception. Pythium catenulatum was shown to be identical to P. rhizo-oryzae HQ643757 in ITS sequences and both are members of Pythium Clade B (Hyde et al. Citation2014). Differences in the formation of intercalary and terminal hyphal bodies and absence of sporangia and zoospores were proposed to define P. rhizo-oryzae (Bala et al. Citation2006). Since these two species are reported to be distinguished by their mitochondrial cytochrome c oxidase subunit I (COI) sequences (Robideau et al. Citation2011), we utilized this approach to confirm the presence of P. catenulatum. Isolates of this species have previously been recovered from soybeans in Ohio, causing seed rot and damping-off (Dorrance et al. Citation2004). Pythium catenulatum was considered to be moderately pathogenic on cool season turfgrasses, causing seed rot, root rot and damping-off symptoms and formed sporangia and oospores in culture (Rudsari et al. Citation2015). It has also been reported from rosemary plants and from sugarcane cuttings, where it caused root rot and damping-off, respectively, in Taiwan (Ho Citation2009). In other studies, isolates of P. catenulatum were weakly pathogenic or non-pathogenic on hosts such as sugarcane in Louisiana (Lee and Hoy Citation1992) and bentgrass in North Carolina (Abad et al. Citation1994). On pepper plants in Florida, P. catenulatum caused limited root necrosis without causing above-ground symptoms and was categorized as a ‘subclinical pathogen’ (Chellemi et al. Citation2000). The host range of P. catenulatum is therefore wide, and it is described as a root rot pathogen on cannabis plants for the first time.
We developed a method utilizing an aeroponic cloning system to induce rapid root formation on cannabis cuttings to establish uniform populations of plants for pathogenicity studies (). After 3 weeks, rooted plants were ready to be inoculated. Wounding was performed prior to inoculation by trimming the bottom 10 cm of the root system to provide wound sites for Pythium infection. Wounding of roots is known to enhance infection by Pythium spp. on a range of host plants (Hendrix and Campbell Citation1973; Martin and Loper Citation1999; Sutton et al. Citation2006). Under commercial growing conditions, wounding of roots likely first occurs when rooted cuttings at the propagation stage () are transferred into coco coir blocks to initiate vegetative growth (). The blocks are then placed on tables and subjected to intermittent periods of daily flooding for two weeks () before being transferred to the flowering room. Under these conditions, water-saturated and damaged roots emerging from the bottom of the coco block () are likely to be predisposed to infection by Pythium spp. In saturated soils and temperatures of 26–30°C, P. myriotylum destroyed ginger rhizomes within 7 days (Stirling et al. Citation2009).
The symptoms of infection caused by Pythium spp. observed on cannabis plants in commercial facilities are consistent with those on other hydroponically grown greenhouse crops infected by Pythium spp., which may include an initial darkening (greening) of leaves on affected cucumber and pepper plants (Punja and Yip Citation2003; Sutton et al. Citation2006) followed by wilting and plant collapse. Total plant collapse due to Pythium infection was not uncommon during the hot summer months under greenhouse conditions. Occurrences of multiple Pythium species, in some cases up to 10 species, have been reported on a range of host plants (Abad et al. Citation1994; Benard and Punja Citation1995; Chellemi et al. 2000; Zhang and Yang Citation2000; Dorrance et al. Citation2004; Sutton et al. Citation2006; Wei et al. Citation2010; Rudsari et al. Citation2015; Rojas et al. Citation2017). Damping-off, a common symptom caused by Pythium spp. on several crops (Hendrix and Campbell Citation1973), was not observed on cannabis cuttings and was reported instead to be caused by several species of Fusarium (Punja Citation2021a, Citation2021b). On rooted vegetative cuttings, Pythium infection resulted in a dark sunken stem lesion that extended up to 10 cm from the crown region (, i). From some of these affected plants, F. oxysporum was also recovered at a low frequency. The presence of both pathogens infecting the same plants may enhance disease symptoms although double inoculations were not conducted.
For growth and pathogenicity studies, an isolate each of the four Pythium species was selected for testing, recognizing that there can be intra-specific variability within a given species that is not reflected by the inclusion of just one isolate in these studies. Therefore, direct comparisons of growth and pathogenicity between isolates selected to represent the species are difficult to make. However, among the Pythium species reported in the present study, P. myriotylum is considered to be the most important based on its high frequency of isolation and its ability to grow rapidly and cause plant mortality within 14 days. While isolates of P. aphanidermatum and P. ultimum similarly grew rapidly in culture and caused high plant mortality, their prevalence in the greenhouses sampled in BC during 2018–2019 was much lower, at three and two isolates, respectively, of the total isolates recovered. Pythium myriotylum has been previously reported to cause root rot and wilt symptoms on cannabis plants in Canada (Punja and Rodriguez Citation2018) and in California (Pitman et al. Citation2021a) and on hemp plants in Connecticut (McGehee et al. Citation2019), North Carolina (Thiessen et al. Citation2020) and Arizona (Hu Citation2021) and has a wide host range (Le et al. Citation2017; Punja and Rodriguez Citation2018). Isolates of both P. myriotylum and P. dissotocum were recovered from hemp plants in Oregon (Osterbauer and Ocamb Citation2020). A sample from northern California with symptoms of root rot yielded P. myriotylum in the present study. Pythium aphanidermatum and P. ultimum have been reported to cause crown and root rot on hemp in Indiana (Beckerman et al. Citation2017, Citation2018) and on cannabis plants in California (Punja et al. Citation2018; Pitman et al. Citation2021b). Finally, isolates of both P. aphanidermatum and P. ultimum were reported to cause crown and root rot of hemp in North Carolina (Troth and Thiessen Citation2019). Previously, P. aphanidermatum and P. ultimum were reported to cause damping-off on hemp seedlings (McPartland Citation1996). Recently, McGehee and Raudales (Citation2021) isolated G. irregulare and demonstrated its pathogenicity on hemp seedlings. These results confirm the ability of isolates representing several different species of Pythium to cause root rot on cannabis and hemp plants in different geographic regions.
Isolates of the highly pathogenic species P. aphanidermatum, P. myriotylum and P. ultimum are able to infect a wide range of host plant species (Martin and Loper Citation1999; Chellemi et al. 2000; Sutton et al. Citation2006; Ho Citation2009; Wei et al. Citation2010). Both P. aphanidermatum and P. myriotylum produce zoospores from sporangia and also form oospores (Sutton et al. Citation2006). The rapid growth rate and production of a range of cell wall degrading enzymes by isolates of these species likely contribute to their pathogenicity (Martin and Loper Citation1999; Wang et al. Citation2003; Boudjeko et al. Citation2006; Geethu et al. Citation2013). The destruction of roots and crown tissues on cannabis plants is the likely outcome of these enzymes. The optimal temperature range at which disease is reported to occur for P. aphanidermatum and P. myriotylum is 25–36°C (Chellemi et al. 2000; Ho Citation2009; Stirling et al. Citation2009) and 15–20°C for P. ultimum (Sutton et al. Citation2006; Owen-Going et al. Citation2008; Wei et al. Citation2010). Under greenhouse conditions used to cultivate cannabis, ambient air temperatures are generally in the range of 24–38°C during the summer season. This may, in part, account for the low incidence of recovery of P. ultimum in the present study as it has a lower temperature optimum.
Pythium dissotocum was the second most frequently isolated species from diseased cannabis plants in this study. This pathogen is considered to be a species complex taxonomically and infects a broad range of plant species, including spinach, lettuce, opium poppy, tobacco and cilantro (Bates and Stanghellini Citation1984; Stanghellini and Kronland Citation1986; Alam et al. Citation1996; Fortnum et al. Citation2000; Corrêa et al. Citation2011; Romero et al. Citation2012; McGehee et al. Citation2018) and is capable of producing zoospores and oospores. Isolates of this species complex were recovered at a frequency of almost 70% from diseased roots in a previous study and were equally pathogenic on cannabis cuttings as P. myriotylum (Punja and Rodriguez Citation2018). It was the only species recovered from a limited sample of greenhouse-grown plants from Ontario. In the present study, plants inoculated with isolates of P. dissotocum developed rapid symptoms similar to those caused by P. myriotylum and it is considered to be an important component of the diverse Pythium species affecting cannabis plants.
Inoculum of the various Pythium species present in cannabis production greenhouses may be introduced through infected planting material; from carry-over of inoculum from previous crops containing diseased plants; infested recirculating water and infected planting medium; tools and equipment, hydroponic pipes and tubing; and potentially by workers (Sutton et al. Citation2006). Movement of fungus gnats and shore flies may also spread Pythium inoculum (Sutton et al. Citation2006). Outbreaks of cannabis root aphids (Rhopalosiphum abdominalis) reported recently in greenhouses in British Columbia could further predispose roots to infection by Pythium due to insect feeding damage sites that provide entry points (unpublished observations). All Pythium species identified in the present study, with the exception of P. ultimum, can produce sporangia that release zoospores which are readily disseminated in the recirculating hydroponic nutrient solution (Sutton et al. Citation2006). Additional studies are required to understand the epidemiology of the various species of Pythium reported to infect cannabis plants.
Cultural and biological control methods are the two main approaches for management of Pythium infection on cannabis plants in Canada since there are no fungicides registered for use and genetic resistance has not been identified among the genotypes (strains) currently in cultivation. In the present study, Pythium spp. were recovered from eight cannabis strains used in commercial production in two provinces. The avoidance of over-saturated growing conditions and minimizing wounding of roots can reduce disease development due to Pythium spp. (Kirkpatrick et al. Citation2006; Sutton et al. Citation2006; Stirling et al. Citation2009). However, under the current widely used hydroponic cultivation systems used to grow cannabis, over-saturation and wounding of roots occur frequently during production, as described above. Disinfectants such as Chemprocide (didecyldimethyl ammonium chloride), Zerotol (hydrogen peroxide) and sodium hypochlorite (bleach) may be partially effective in reducing Pythium inoculum build-up and survival in hydroponic nutrient solutions (Sutton et al. Citation2006); however, oospores, where present, would be more resilient to the effects of these chemicals. The registration of fungicides such as metalaxyl for application at the early stages of vegetative propagation to minimize Pythium infection and carry-over of infection from vegetative to older flowering plants should be considered to manage this destructive pathogen on cannabis plants in Canada.
Application of biological control agents offers an alternative approach to management of diseases caused by a range of Pythium spp. (Martin and Loper Citation1999; Punja and Yip Citation2003; Sutton et al. Citation2006; Nzungize et al. Citation2012; Halo et al. Citation2019). In Canada, four biological control agents are registered for use on cannabis to control root pathogens: Rootshield and Trianum (Trichoderma harzinanum strain T22), Asperello (Trichoderma asperellum strain T34) and Prestop (Gliocladium catenulatum strain J1446) (Health Canada Citation2019). Previous studies have demonstrated the efficacy of these and other biocontrol agents in reducing Pythium infection on a range of crop species (Martin and Loper Citation1999; Punja and Yip Citation2003; Rose et al. Citation2004; Kipngeno et al. Citation2015; Elshahawy and El-Mohamedy Citation2019; Halo et al. Citation2019). Efficacy data comparing the registered biological control products are required for cannabis, as well as an investigation into other potentially useful biocontrol agents such as Bacillus subtilis (Rhapsody) and Bacillus amyloliquefaciens (Stargus) (Utkhede et al. Citation2000; Ni and Punja Citation2019). These evaluations of additional biological control agents and other low-risk chemicals for use on cannabis in Canada for Pythium management is of utmost importance to support the continued production of high-quality cannabis by licensed growers. Together with Fusarium species, Pythium species represent the greatest challenge facing commercial producers of cannabis in Canada (Punja Citation2021c).
Acknowledgements
Technical assistance provided by Alastair Roberts, Li Ni and Sarah Chen during various aspects of this project is gratefully acknowledged.
Additional information
Funding
References
- Abad ZG, Shew HD, Lucas LT. 1994. Characterization and pathogenicity of Pythium species isolated from turfgrass with symptoms of root and crown rot in North Carolina. Phytopathology. 84:913–921. doi:https://doi.org/10.1094/Phyto-84-913.
- Alam M, Sattar A, Chourasia HK, Janardhanan KK. 1996. Damping-off, a new disease of opium poppy caused by Pythium dissotocum. Indian Phytopathol. 49:94–97.
- Bala K, Gautam N, Paul B. 2006. Pythium rhizo-oryzae sp. nov. isolated from paddy fields: taxonomy, ITS region of rDNA, and comparison with related species. Curr Microbiol. 52:102–107. doi:https://doi.org/10.1007/s00284-005-0116-9.
- Bates M, Stanghellini M. 1984. Root rot of hydroponically-grown spinach caused by Pythium aphanidermatum and P. dissotocum. Plant Dis. 68:989–991. doi:https://doi.org/10.1094/PD-68-989.
- Beckerman J, Nisonson H, Albright N, Creswell T. 2017. First report of Pythium aphanidermatum causing crown and root rot of industrial hemp in the United States. Plant Dis. 101:1038. doi:https://doi.org/10.1094/PDIS-09-16-1249-PDN.
- Beckerman J, Stone J, Ruhl G, Creswell T. 2018. First report of Pythium ultimum causing crown and root rot of industrial hemp in the United States. Plant Dis. 102:2045. doi:https://doi.org/10.1094/PDIS-12-17-1999-PDN.
- Benard D, Punja ZK. 1995. Role of Pythium species in cavity spot development on carrots in British Columbia. Can J Plant Pathol. 17:31–45. doi:https://doi.org/10.1080/07060669509500717.
- Boudjeko T, Andème-Onzighi C, Vicré M, Balangé AP, Ndoumou DO, Driouich A. 2006. Loss of pectin is an early event during infection of cocoyam roots by Pythium myriotylum. Planta. 223:271‐282. doi:https://doi.org/10.1007/s00425-005-0090-2.
- Chellemi DO, Mitchell DJ, Kannwischer-Mitchell ME, Rayside PA, Rosskopf EN. 2000. Pythium spp. associated with bell pepper production in Florida. Plant Dis. 84:1271–1274.
- Corrêa AS, Rocha AB, Willani SA, Dariva JM, Souza MV, Moraes MG. 2011. Yellow stunt, a tobacco disease caused by Pythium dissotocum in southern parts of Brazil. Plant Dis. 95:354. doi:https://doi.org/10.1094/PDIS-10-10-0759.
- Dorrance AE, Berry SA, Bowen P, Lipps PE. 2004. Characterization of Pythium spp. from Three Ohio fields for pathogenicity on corn and soybean and metalaxyl sensitivity. Plant Health Prog Plant Manage Network (On-line). 5(1):10. doi:https://doi.org/10.1094/PHP-2004-0202-01-RS
- Elshahawy IE, El-Mohamedy RS. 2019. Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J Plant Pathol. 101:597–608. doi:https://doi.org/10.1007/s42161-019-00248-z.
- Fortnum BA, Rideout J, Martin SB, Gooden D. 2000. Nutrient solution temperature affects Pythium root rot of tobacco in greenhouse float systems. Plant Dis. 84:289–294. doi:https://doi.org/10.1094/PDIS.2000.84.3.289.
- Garcia R, Mitchell DJ. 1975. Interactions of Pythium myriotylum with Fusarium solani, Rhizoctonia solani, and Meloidogyne arenaria in pre-emergence damping-off on peanut. Plant Dis Rept. 59:665–669.
- Geethu C, Resna AK, Nair AR. 2013. Characterization of major hydrolytic enzymes secreted by Pythium myriotylum, causative agent for soft rot disease. Antonie Van Leeuwenhoek. 104:749–757. doi:https://doi.org/10.1007/s10482-013-9983-4.
- Halo BA, Al-Yahyai RA, Maharachchikumbura SN, Al-Sadi AM. 2019. Talaromyces variabilis interferes with Pythium aphanidermatum growth and suppresses Pythium-induced damping- off of cucumbers and tomatoes. Scientific Rept. 9:11255. doi:https://doi.org/10.1038/s41598-019-47736-x.
- Health Canada. 2019. Pest control products for use on cannabis; [accessed 2020 Aug 28]. https://www.canada.ca/en/health-canada/services/cannabis-regulations-licensed-producers/pest-control-products.html.
- Hendrix FF, Campbell WA. 1973. Pythium as plant pathogens. Annu Rev Phytopathol. 11:78–98. doi:https://doi.org/10.1146/annurev.py.11.090173.000453.
- Ho -H-H. 2009. The genus Pythium in Taiwan, China (1) – a synoptic review. Front Biol China. 4:15–728. doi:https://doi.org/10.1007/s11515-009-0009-6.
- Hu J. 2021. First report of crown and root rot caused by Pythium myriotylum on hemp (Cannabis sativa) in Arizona. Plant Dis. (in press). doi:https://doi.org/10.1094/PDIS-12-20-2712-PDN
- Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SA, Goonasekara ID, et al. 2014. One stop shop: backbones trees for important phytopathogenic genera: I. Fungal Diver. doi:https://doi.org/10.1007/s13225-014-0298-1
- Kipngeno P, Losenge T, Maina N, Kahangi E, Juma P. 2015. Efficacy of Bacillus subtilis and Trichoderma asperellum against Pythium aphanidermatum in tomatoes. Biolog Contr. 90:92–95. doi:https://doi.org/10.1016/j.biocontrol.2015.05.017.
- Kirkpatrick MT, Rothrock CS, Rupe JC, Gbur EE. 2006. The effect of Pythium ultimum and soil flooding on two soybean cultivars. Plant Dis. 90:597–602. doi:https://doi.org/10.1094/PD-90-0597.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874. doi:https://doi.org/10.1093/molbev/msw054.
- Le DP, Smith MK, Aitken EAB. 2017. Species delimitation in Pythium species complexes: the case of Pythium myriotylum Drechsler and Pythium zingiberis Takahashi. Mycol Prog. 16:257–267. doi:https://doi.org/10.1007/s11557-017-1272-6.
- Lee YS, Hoy JW. 1992. Interactions among Pythium species affecting root rot of sugarcane. Plant Dis. 76:735–739.
- Martin FN, Loper JE. 1999. Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospects for biological control. Crit Rev Plant Sci. 8(2):111–181. doi:https://doi.org/10.1080/07352689991309216
- McGehee C, Raudales RE. 2021. Pathogenicity and mefenoxam sensitivity of Pythium, Globisporangium and Fusarium isolates from coconut coir and rockwool in marijuana production. Front Agron. (in press).
- McGehee C, Raudales RE, Elmer WH. 2018. First report of Pythium dissotocum causing Pythium root rot on hydroponically grown lettuce in Connecticut. Plant Dis. 102:2043. doi:https://doi.org/10.1094/PDIS-02-18-0365-PDN.
- McGehee CS, Apicella P, Raudales R, Berkowitz Y, Ma S, Durocher S, Lubell J. 2019. First report of root rot and wilt caused by Pythium myriotylum on hemp (Cannabis sativa) in the United States. Plant Dis. 103(12):3288. doi:https://doi.org/10.1094/PDIS-11-18-2028-PDN
- McPartland JM. 1996. A review of Cannabis diseases. J Intern Hemp Assoc. 3:19–23.
- Ni L, Punja ZK. 2019. Management of fungal diseases on cucumber (Cucumis sativus L.) and tomato (Solanum lycopersicum L.) crops in greenhouses using Bacillus subtilis. In: Islam MT, Rahman MM, Pandey P, Boehme MH, Haesaert G, editors. Bacilli and agrobiotechnology: phytostimulation and biocontrol. Cham (Switzerland): Springer; p. 1–28.
- Nzungize JR, Lyumugabe F, Busogoro J-P, Baudoin J-P. 2012. Pythium root rot of common bean: biology and control methods. A review. Biotecnol Agron Soc Environ. 16:405–413.
- Osterbauer NK, Ocamb CM. 2020. Hemp (Cannabis sativa) – Root and crown rot. In Pscheidt JW, Ocamb CM, Eds. Pacific Northwest Plant Disease Management Handbook. Oregon State University, Corvallis. Available at: https://pnwhandbooks.org/plantdisease/host-disease/hemp-cannabis-sativa-root-crown-rot
- Owen-Going TN, Beninger CW, Sutton JC, HallJC. 2008. Accumulation of phenolic compounds in plants and nutrient solution of hydroponically-grown peppers inoculated with Pythium aphanidermatum. Can J Plant Pathol. 30:214–225. doi:https://doi.org/10.1080/07060661.2008.10540537.
- Pieczarka DJ, Abawi GS. 1978. Effect of interaction between Fusarium, Pythium and Rhizoctonia on severity of bean root rot. Phytopathology 68:403–408.
- Pitman TL, Philbrook RN, Warren JG. 2021a. First report of Pythium myriotylum causing root rot in Cannabis sativa (L.) in California. Plant Dis. (in press). https://doi.org/http://doi.org/10.1094/PDIS-02-21-0336-PDN
- Pitman TL, Philbrook RN, Vetterli MR, Warren JG. 2021b. First report of pythium ultimum causing Crown rot in Greenhouse-Grown Cannabis sativa in California. Plant Dis. 105(4):1230. (in press). doi:https://doi.org/10.1094/PDIS-10-20-2228-PDN
- Punja ZK. 2021a. Epidemiology of Fusarium oxysporum causing root and crown rot of cannabis (Cannabis sativa L., marijuana) plants in commercial greenhouse production. Can J Plant Pathol. 43:216–235. doi:https://doi.org/10.1080/07060661.2020.1788165.
- Punja ZK. 2021b. First report of Fusarium proliferatum causing crown and stem rot, and pith necrosis, in cannabis (Cannabis sativa L., marijuana) plants. Can J Plant Pathol. 43:236–255. doi:https://doi.org/10.1080/07060661.2020.1793222.
- Punja ZK. 2021c. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag Sci. (in press). doi:https://doi.org/10.1002/ps.6307
- Punja ZK, Collyer D, Scott C, Lung S, Holmes J, Sutton D. 2019. Pathogens and molds affecting production and quality of Cannabis sativa L. Front Plant Sci. 10:1120. doi:https://doi.org/10.3389/fpls.2019.01120.
- Punja ZK, Li N, Roberts A. 2021. The Fusarium solani species complex infecting cannabis (Cannabis sativa L., marijuana) plants and a first report of Fusarium (Cylindrocarpon) lichenicola causing root and crown rot. Can J Plant Pathol. (in press). doi:https://doi.org/10.1080/07060661.2020.1866672
- Punja ZK, Rodriguez G. 2018. Fusarium and Pythium species infecting roots of hydroponically grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40:498–513. doi:https://doi.org/10.1080/07060661.2018.1535466.
- Punja ZK, Scott C, Chen S. 2018. Root and crown rot pathogens causing wilt symptoms on field-grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40:528–541. doi:https://doi.org/10.1080/07060661.2018.1535470.
- Punja ZK, Yip R. 2003. Biological control of damping-off and root rot caused by Pythium aphanidermatum on greenhouse cucumbers. Can J Plant Pathol. 25:411–417. doi:https://doi.org/10.1080/07060660309507098.
- Robideau GP, De Cock AWAM, Coffey MD, Voglmayr H, Brouwer H, Bala K, Chitty DW, Desaulniers N, Eggertson QA, Gachon CMM, et al. 2011. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol Ecol Resour. 11:1002–1011. doi:https://doi.org/10.1111/j.1755-0998.2011.03041.x
- Rojas JA, Jacobs J, Napieralski S, Karaj B, Bradley CA, Chase T, Esker P, Giesler L, Jardine D, Malvick D, et al. 2017. Oomycete species associated with soybean seedlings in North America. Identification and pathogenicity characterization. Phytopathology. 107:280–292. doi:https://doi.org/10.1094/PHYTO-04-16-0177-R
- Romero G, Estévez de Jensen C, Palmateer AJ. 2012. First report of Pythium dissotocum affecting cilantro in hydroponic systems in Puerto Rico. Plant Health Prog. 13(1):32. doi:https://doi.org/10.1094/PHP-2012-1214-01-BR
- Rose S, Yip R, Punja ZK. 2004. Biological control of Fusarium and Pythium root rots on greenhouse cucumbers grown in rockwool. Acta Hortic. 635:73–78. doi:https://doi.org/10.17660/ActaHortic.2004.635.9.
- Rudsari MK, Okhovat SM, Mirabolfathi M, Jones E, Kafi M. 2015. Identification of Pythium species and their pathogenicity on cool season turfgrass in Tehran province. Biolog Forum. 7:349–356.
- Schroeder KL, Okubara PA, Tambong JT, Lévesque CA, Paulitz TC. 2006. Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology. 96:637–647. doi:https://doi.org/10.1094/PHYTO-96-0637.
- Small E. 2017. Cannabis. A complete guide. Boca Raton (FL): CRC Press.
- Stanghellini M, Kronland WC. 1986. Yield loss in hydroponically grown lettuce attributed to subclinical infection of feeder roots by Pythium dissotocum. Plant Dis. 70:1053–1056. doi:https://doi.org/10.1094/PD-70-1053.
- Stirling GR, Turaganivalu U, Stirling AM, Lomavatu MF, Smith MK. 2009. Rhizome rot of ginger (Zingiber officinale) caused by Pythium myriotylum in Fiji and Australia. Austral Plant Pathol. 38:453–460. doi:https://doi.org/10.1071/AP09023.
- Sutton JC, Sopher CR, Owen-Going TN, Liu W, Grodzinski B, Hall JC, Benchimol RA. 2006. Etiology and epidemiology of Pythium root rot in hydroponic crops: current knowledge and perspectives. Summa Phytopathol. 32:307–321. doi:https://doi.org/10.1590/S0100-54052006000400001.
- Thiessen LD, Schappe T, Cochran S, Hicks K, Post AR. 2020. Surveying for potential diseases and abiotic disorders of industrial hemp (Cannabis sativa L.) production. Plant Health Prog. 21:321–332. doi:https://doi.org/10.1094/PHP-03-20-0017-RS.
- Troth A, Thiessen L. 2019. Pythium root and crown rot of industrial hemp. North Carolina State Extension Publications; [accessed 2020 Mar 24]. https://content.ces.ncsu.edu/pythium-root-and-crown-rot-of-industrial-hemp.
- Utkhede RS, Lévesque CA, Dinh D. 2000. Pythium aphanidermatum root rot in hydroponically grown lettuce and the effect of chemical and biological agents on its control. Can J Plant Pathol. 22:138–144. doi:https://doi.org/10.1080/07060660009500487.
- Wang PH, Chung CY, Lin YS, Yeh Y. 2003. Use of polymerase chain reaction to detect the soft rot pathogen, Pythium myriotylum, in infected ginger rhizomes. Lett Appl Microbiol. 36:116‐120. doi:https://doi.org/10.1046/j.1472-765X.2003.01272.x.
- Wei L, Xue AG, Cober ER, Babcock C, Zhang J, Zhang S, Li W, Wu J, Liu L. 2010. Pathogenicity of Pythium species causing seed rot and damping-off in soybean under controlled conditions. Phytoprotection. 91:3–10. doi:https://doi.org/10.7202/1008539ar.
- Zhang BQ, Yang XB. 2000. Pathogenicity of Pythium populations from corn-soybean rotation fields. Plant Dis. 84:94–99. doi:https://doi.org/10.1094/PDIS.2000.84.1.94.
