Abstract
Plants of the cannabis (Cannabis sativa L., marijuana) strain ‘Chronic Ryder’, grown outdoors in the Fraser Valley of British Columbia (BC), displayed symptoms of powdery mildew in late July 2019. The disease progressed rapidly under cool, wet conditions to infect leaves, stems, shoot tips and inflorescences by early September. To identify the pathogen, DNA was extracted from healthy and diseased leaves and subjected to PCR using eukaryotic universal primers that amplified the ITS1-5.8S-ITS2 region of rDNA. A 650 bp band present only in the diseased plants, showed 99.45% sequence homology to Podosphaeria macularis, the powdery mildew pathogen that infects hop plants. In greenhouse-grown cannabis infected with the powdery mildew pathogen Golovinomyces ambrosiae, a 700 bp size band was present. Conidial morphology also distinguished the two pathogens – P. macularis produced ovoid-oval conidia with fibrosin bodies, while G. ambrosiae produced cylindrical-oblong conidia with no fibrosin bodies. A survey of powdery mildew-infected hop fields and indoor cannabis growing facilities in different geographical locations in BC, showed that plants were infected exclusively by either P. macularis or G. ambrosiae, respectively. Inoculations using P. macularis-infected leaf segments placed on cannabis ‘Pink Kush’ leaves under high humidity at 22–26°C gave rise to small mildew colonies after 24–35 days, confirmed to be P. macularis by PCR. Development of P. macularis in cannabis plants was slower compared with G. ambrosiae. To date, only one outdoor location for cannabis has been confirmed to have P. macularis; the potential for spread to other sites or to indoor cultivation facilities is unknown.
Résumé
Tard en juillet 2019, les plants de cannabis (Cannabis sativa, marijuana) de la souche ‘Chronic Ryder’ cultivés à l’extérieur dans la vallée du Fraser en Colombie-Britannique (C.-B.) ont affiché des symptômes du blanc. La maladie a progressé rapidement par temps frais et humide, ayant infecté, dès le début de septembre, les feuilles, les tiges, l’extrémité des pousses et les inflorescences. Afin d’identifier l’agent pathogène, l’ADN a été extrait de feuilles saines et infectées et soumis à une PCR utilisant des amorces universelles eukariotes qui ont amplifié la région de l’ITS1-5.8-ITS2 de l’ADNr. Une bande de 650 bp, présente seulement chez les plants infectés, a affiché une homologie de séquence de 99,45% avec Podosphaeria macularis, l’agent pathogène qui infecte les plants de houblon. Chez le cannabis cultivé en serre infecté avec l’agent pathogène du blanc Golovinomyces ambrosiae, une bande de 700 bp est apparue. La morphologie des conidies a également différencié les deux agents pathogènes: P. macularis a produit des conidies ovoïdes ou ovales avec corpuscules de fibrosine, tandis que G. ambrosiae a produit des conidies cylindriques ou oblongues sans corpuscules de fibrosine. Une étude menée dans différentes régions de la C.-B., dans les champs de houblon et dans les serres de cannabis infectés par le blanc, a montré que les plants étaient infectés exclusivement par P. macularis ou G. ambrosiae, respectivement. Des feuilles de cannabis ‘Pink Kush’ ont été inoculées avec des portions de feuilles infectées par P. macularis, dans des conditions d’humidité élevée et à une température de 22 à 26°C, ce qui, au bout de 24 à 35 jours, a produit de petites colonies de blanc de P. macularis: cela a été confirmé par PCR. Le développement de P. macularis sur les plants de cannabis était plus lent comparativement à G. ambrosiae. À ce jour, on confirme qu’une seule culture extérieure de cannabis est infectée par P. macularis; la possibilité qu’il se propage à d’autres sites de production extérieure ou intérieure reste inconnue.
Introduction
Hops (Humulus lupulus) are long-lived perennial flowering plants that belong to the Cannabaceae family and are cultivated throughout the Pacific Northwest region that includes British Columbia, Washington, Idaho and Oregon. Hops are also grown in California and in eastern parts of Canada and the USA, including Ontario, Quebec, New York, and Massachusetts. The plants produce male and female flowers on separate plants (dioecious). The cones on female plants are harvested and used during the beer-brewing process to provide an important source of flavouring agents, which also help to preserve the beer (Schönberger and Kostelecky Citation2012). Powdery mildew, caused by Podosphaeria macularis (formerly Sphaerotheca humuli) is an important disease that infects young shoot tips, leaves, and cones of hop plants and can cause necrosis of infected tissues (Ocamb and Gent Citation2020). The pathogen overwinters within infected buds that form on underground rhizomes and subsequent mycelial growth emerges on shoot tips the following spring. Large numbers of conidia are produced that are disseminated to initiate secondary infections on leaves throughout the growing season. Optimal environmental conditions for disease development are temperatures of 18–21°C with intermittent periods of rainfall (Gent et al. Citation2008). Pathogen races that infect hop plants are not reported to infect other plant species (Ocamb and Gent Citation2020).
Cannabis (marijuana) and hemp plants (both Cannabis sativa L.) are also members of the family Cannabaceae that are dioecious and are grown for multiple uses, which include for medicinal purposes as extracts (cannabis, hemp), for psychotropic compounds (cannabis), as well as for seed and fibre (hemp). Like hops, only female plants are grown for commercial production, and plants are initiated from seed or vegetative cuttings and planted annually. Both cannabis and hemp are cultivated in many provinces in Canada and in many US states in which legal cultivation of cannabis is permitted. Powdery mildew, caused by Golovinomyces ambrosiae (previously referred to as G. chicoraceraum s. lat. or G. spadiceus, which is now a synonym of G. ambrosiae) (Qiu et al. Citation2020; Punja Citation2021), is a widespread disease on indoor cultivated cannabis (in greenhouses and controlled environment facilities) in Canada (Pépin et al. Citation2018; Punja Citation2018) and has been reported to infect hemp plants in the USA (Cala et al. Citation2019; Gauthier et al. Citation2020; Wiseman et al. Citation2021). Currently, there is an expanding outdoor cultivation of cannabis in Canada, and a number of US states, including California, have been cultivating cannabis (marijuana) outdoors for decades. The diseases affecting cannabis grown indoors have been described (Punja et al. Citation2019), and the emerging diseases affecting field-grown hemp are currently being researched (Gauthier et al. Citation2020; Punja Citation2021). The potential impact of hop production on the emergence of diseases in cannabis and hemp (and vice-versa) is gaining interest. It has recently been reported that Hop latent viroid can infect cannabis plants to cause symptoms of stunting and flower abortion (Bektas et al. Citation2019; Warren et al. Citation2019) while the powdery mildew pathogen of hops was shown to infect hemp leaves following artificial inoculation under laboratory conditions (Cala et al. Citation2019; Weldon et al. Citation2020).
In this study, the occurrence of powdery mildew in field-grown cannabis plants in late July 2019 in British Columbia was studied. The objective was to describe the symptoms of the disease and utilize methods to identify the pathogen using molecular approaches and light and scanning microscopy. The results show that the cannabis plants were infected by the hop powdery mildew pathogen, Podosphaeria macularis. This demonstration of natural infection in cannabis plants by a previously unreported pathogen, presumed to originate from hop plants, could result in potential spread to other geographical regions where hops, cannabis, and hemp are grown in proximity to each other. A preliminary report has been published (Punja Citation2020).
Materials and methods
Sample collection
Symptoms of powdery mildew that included distinct white colonies developing on the leaves of field-grown plants of cannabis ‘Chronic Ryder’ (an autoflower photoperiod-independent strain) were observed in late July 2019 (). The planting of this strain was part of a larger 25 ha licenced production site located in the Fraser Valley of BC, which also included other strains. Transplants (originating from seeds) were transferred from the greenhouse to the field in June 2019 and grown under the prevailing environmental conditions. Initial symptoms were observed in July, and diseased leaf samples were collected in early September and again in early October. In addition, hop, ‘Nugget and Chinook’ leaf samples showing symptoms of powdery mildew were obtained from commercial growers in the Abbotsford region of the Fraser Valley and from Kelowna, BC in the Okanagan Valley during September and October 2019. Samples of greenhouse-grown cannabis leaves infected with powdery mildew were obtained from six production facilities during 2019–2020 and were included for molecular and microscopic comparisons to characterize the pathogen species present.
Fig. 1 Symptoms of powdery mildew infection caused by Podosphaeria macularis on cannabis strain ‘Chronic Ryder’ on field grown plants in the Fraser Valley of British Columbia. Note the development of localized mildew colonies (a-c) and severe infection of young developing shoots (d, e) causing leaf curl. Older mildew colonies have a light beige centre (f).

PCR analysis
The DNA was extracted from 50 mg to 100 mg of symptomatic and healthy leaves originating from all cannabis and hop plants collected (total of 24 samples). DNA was extracted using the QIAGEN DNeasy Plant Mini Kit (cat. No. 69104). Species-level identification was conducted using PCR of the ITS1-5.8S-ITS2 region of ribosomal DNA. The universal eukaryotic primers UN-UP18S42 (5′-CGTAACAAGGTTTCCGTAGGTGAAC-3′) and UN-LO28S576B (5′-GTTTCTTTTCCTCCGCTTATTAATATG-3′) (Schroeder et al. Citation2006) were used to amplify the ITS regions (ITS1 and ITS2) as well as the 5.8S gene of the rDNA to produce a DNA template for sequencing. PCR conditions were as follows: initial denaturation at 94°C for 3 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s and extension at 72°C for 2 min, and a final extension at 72°C for 7 min, followed by a 4°C hold. PCR products were cut from the gel, and DNA was collected using the MinElute Gel Extraction Kit (cat. No. 28604) and 8 uL was collected and sent to Eurofins Genomics (https://www.eurofinsgenomics.com/en/home/) for sequencing. The resulting sequences were compared to the ITS1-5.8S-ITS2 sequences from the National Centre for Biotechnology Information (NCBI) GenBank database to confirm species identity. Multiple sequence alignment of the respective isolates of each species was done using the CLUSTAL W program (http://www.genome.jp/tools/clustalw). The sequences were subsequently included in a phylogenetic analysis using the neighbour-joining (NJ) method (Saitou and Nei Citation1987; Tamura et al. Citation2004) in the software MEGA v. 5 (Tamura et al. Citation2011). A bootstrap consensus tree was inferred from 1000 replicates. Sclerotinia sclerotiorum was used as an outgroup. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The percentages of replicated trees in which the associated taxa are clustered together in the bootstrap test (1000 replicates) are shown next to the branches as described by Felsenstein (Citation1985).
Light and scanning electron microscopy
Leaves from powdery mildew infected cannabis ‘Chronic Ryder’ plants grown under field conditions with sporulating lesions were examined using a light microscope. Clear sticky cellophane tape was firmly pressed onto the leaves and transferred to a microscope slide and examined using a compound microscope at various magnifications for characteristics of conidia and chains. Similar samples were obtained from mildew-infected squash leaves infected by Podosphaeria xanthii (Li and Punja Citation2021) grown under greenhouse conditions, as well as from cannabis leaves with mildew infection from commercial greenhouses, and from hop fields with powdery mildew infection, and examined using a light microscope. Sporulating colonies were also examined using a dissecting microscope for mycelial growth and evidence of necrosis. For scanning electron microscopy, diseased hop leaves and cannabis leaves were prepared as described previously (Punja Citation2018) and examined for the characteristics of the conidia. Comparisons were made to previously published images of the powdery mildew pathogen infecting cannabis (Punja Citation2018; Punja et al. Citation2019).
Inoculation studies
The small leaf segments ca. 0.5–1.0 cm2 in size were cut from powdery mildew infected leaves of field-grown cannabis and hop plants, both infected with P. macularis. In addition, leaves from greenhouse-grown cannabis plants of strain ‘White Rhino’ with infection due to G. ambrosiae were collected. Each piece was cut to ensure that it contained at least one sporulating mildew colony. The segments were placed mycelial side down onto the surface of lightly misted leaves of cannabis strains ‘Copenhagen Kush’, ‘Pink Kush’, and ‘Hash Plant’ that were initiated from cuttings and grown in rockwool cubes as described elsewhere (Scott and Punja Citation2020). The plants were placed under a 24 hr photoperiod in a growing room maintained at 22–25°C as described elsewhere (Scott and Punja Citation2020). Hop plants of cultivar ‘Cascade‘ were obtained from a local nursery and grown under greenhouse conditions with natural light and a temperature range of 22–30°C. The following inoculations were performed (i) from cannabis ‘ Chronic Ryder’ to the three cannabis strains described above; (ii) from naturally infected hop plants to cannabis strain ‘Pink Kush’; (iii) from cannabis strain, ‘Chronic Ryder’ to hops cultivar ‘Cascade’; (iv) from cannabis strain ‘Copenhagen Kush’ to hop plants ‘Cascade’ and cannabis strain ‘Copenhagen Kush’. All the inoculated plants were placed inside a plastic dome that maintained 95–100% relative humidity for 2–3 days after which time the leaf segments were removed. The cannabis plants were maintained under a 24-hr photoperiod and 22–25°C ambient temperature and monitored for symptom development daily for 35 days. The hop plants were returned to the greenhouse and grown under ambient conditions (temperature range of 22–30°C) for 2 months. In a final experiment, cannabis plants of strain ‘Copenhagen Kush’ with powdery mildew infections, were placed adjacent to a hop plant ‘Centennial’ grown in a 5 L pot in the greenhouse, and both plants were misted daily for 3 days and left to grow with periodic examination for development of colonies of powdery mildew. In all the above inoculations where disease symptoms were observed, leaf samples were collected for PCR analysis as described above.
Results and discussion
Symptomology
The symptoms of powdery mildew infection on field-grown cannabis strain ‘Chronic Ryder’ were atypical of those observed on indoor or greenhouse-grown cannabis plants reported to be caused by G. chicoracearum s. lat. (now G. ambrosiae) (Punja Citation2018; Punja et al. Citation2019). These symptoms are shown in comparison to the symptoms of powdery mildew infection on greenhouse-grown cannabis plants of strain ‘White Rhino’ are shown in . Powdery mildew symptoms on hop leaves () and on hop cones () collected in this study are also included for comparison. Symptoms on cannabis ‘Chronic Ryder’ in the field included localized spots on leaves that were initially white and subsequently developed a light beige centre (). On young actively growing shoots, powdery mildew growth and sporulation were prolific (), causing the shoot tips and young leaves to curl (). A close-up of the discrete colonies with beige centres is shown in . A comparison of the symptoms of powdery mildew infection on the leaves of cannabis strain ‘Chronic Ryder’ from field-grown plants, cannabis strain ‘White Rhino’ from greenhouse-grown plants and greenhouse and field-grown hop plants ‘Cascade’ is shown in . On ‘Chronic Ryder’, the mildew colonies appeared off-white () and developed beige-coloured centres as the leaves aged (). When examined with a dissecting microscope, some tissue necrosis could be seen below the colonies (). On ‘White Rhino’, powdery mildew colonies were white, with effuse mycelium and abundant sporulation (). There was no development of beige centres, and tissue necrosis was not observed below the colonies. On greenhouse-grown hop leaves, powdery mildew colonies were localized and white () while those on field-grown ‘Cascade’ plants were initially white when young () and they developed a beige centre and the colonies had a blister-like appearance (). On hop cones, white mycelium developed in the early stages (), turning a beige colour with abundant sporulation (), and the diseased tissues became necrotic (). On mature hop leaves, the mildew colonies developed varying shades of beige and formed blisters ().
Fig. 2 Comparison of symptoms of powdery mildew caused by Podosphaeria macularis on leaves of field grown cannabis ‘Chronic Ryder’ (a-c), Golovinomyces ambrosiae on leaves of greenhouse grown cannabis ‘White Rhino’ (d-f) and P. macularis on greenhouse (g) and field-grown hop leaves (h, i). Colonies of P. macularis on cannabis developed a beige-coloured centre and there was evidence of necrosis (c). Colonies of G. ambrosiae were white and effuse (f) while P. macularis on hop leaves developed blister-like colonies (i). Image shown in (g) was provided by Katherine Sims.
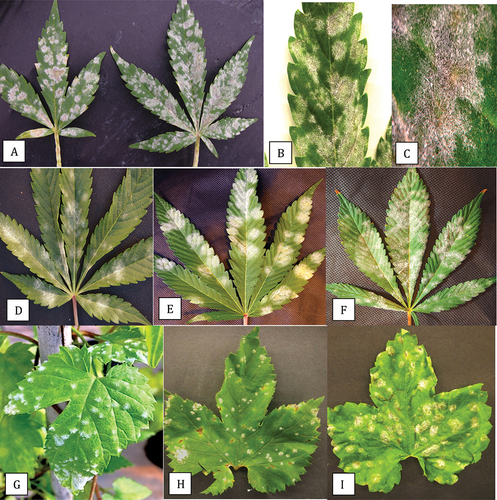
Fig. 3 Powdery mildew symptoms on hop cones and leaves caused by Podosphaeria macularis. On cones, prolific sporulation was observed (a, b) and was accompanied by tissues necrosis of the bracts (c). On leaves, colonies developed various shades of light beige pigmentation (d-f).
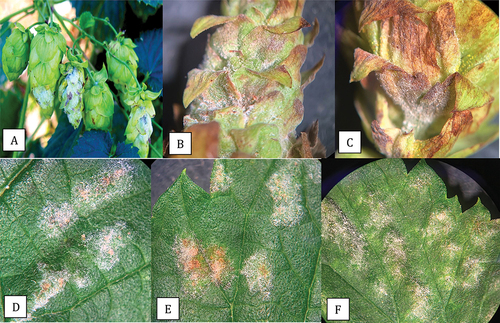
Powdery mildew on hops, caused by P. macularis, was first reported in the Pacific Northwest from Washington state in 1997 (Ocamb et al. Citation1999; Gent et al. Citation2008). The pathogen infects young shoots, leaves, and hop cones, and the disease is initiated by inoculum in overwintering infected crown buds (Ocamb and Gent Citation2020). The pathogen develops on both the upper and lower leaf surfaces, initially forming localized spots and causing blisters, following which entire leaves can be covered by powdery mildew colonies (Ocamb and Gent Citation2020). Sporulation occurs within 10 days following infection, depending on the hop cultivar, giving a white powdery appearance to infected plants. Infection of hop cones causes them to develop necrotic areas, resulting in a significant reduction in quality, which accounts for most of the economic losses due to this pathogen (Gent et al. Citation2008; Marks and Gevans Citation2014; Twomey et al. Citation2015). The symptoms observed in hop leaves and cones in the present study are consistent with those described previously (Gent et al. Citation2008; Marks and Gevans Citation2014; Ocamb and Gent Citation2020). Podosphaeria species also infect apples (P. leucotricha) (OMAFRA Citation2021) and roses (P. pannosa). In these hosts, the pathogen causes infection of young developing shoots, resulting in leaf curl and epinasty. Similar symptoms were observed in cannabis plants ‘Chronic Ryder’. Only one mating type (MAT1-1) of P. macularis has been reported to be present in the Pacific Northwest region (Wolfenbarger et al. Citation2015). Chasmothecia containing asci have therefore not been reported in the Pacific Northwest region but they occur in eastern US hop production sites, such as New England and New York, where both mating types are present (Wolfenbarger et al. Citation2015). The host range of P. macularis includes hops (Ocamb and Gent Citation2020) and this pathogen has been reported to infect red raspberries (Rubus spp.) in the Pacific Northwest region (Pscheidt and Ocamb Citation2021).
PCR analysis
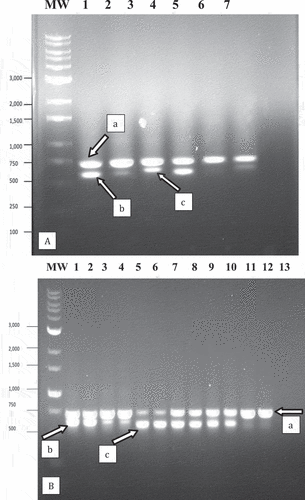
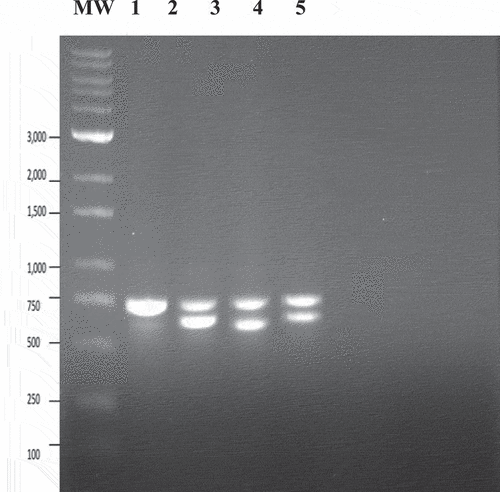
Using the universal eukaryotic primer set UN-UP18S42 and UN-LO28S576B to amplify the ITS regions (ITS1 and ITS2) as well as the 5.8S gene of the rDNA, a PCR band was observed that corresponded to cannabis plant DNA at around 750 bp (). An additional band of either 700 bp or 650 bp that corresponds to fungal DNA was also present. Sequencing of all bands shown in showed the 750 bp band (labelled ‘a’ in ) had a 99.85% sequence homology (with 99% query coverage) to Cannabis sativa subsp. indica (KC292629.1) and has been deposited in Genbank with accession number MW130747. The 700 bp band size (labelled ‘b’ in ) showed 100% sequence homology to ‘G. chicoracearum’ MH782037.1 (now G. ambrosiae) and has been deposited in Genbank under the accession number MN853362. The 650 bp band (labelled ‘c’ in ) showed 99.45% homology to P. macularis MH687414.1 (99% query coverage) and has been deposited under accessions numbers MN853352 and MN853353. A similar banding pattern was observed in DNA extracted from powdery mildew infected hop leaves, with the 750 bp band (in lanes 5 and 6 in ) showing a 99.63% homology (with 100% query coverage) to Humulus lupulus voucher (MN381793.1) and has been deposited in Genbank with accession number MW130749. The 650 bp band (labelled ‘c’ in ) corresponded to P. macularis.
Fig. 4 PCR analysis of samples originating from cannabis and hop leaf tissues infected with powdery mildew using universal eukaryotic primers for the ITS1-5.8S-ITS2 region of ribosomal DNA. (a) Cannabis leaf tissues infected by Podosphaeria macularis (lane 1) showing amplification of plant DNA (band at ca. 750 bp labelled ‘a’) and pathogen DNA at ca. 650 bp (labelled ‘b’). Lanes 2 and 3 = Banding pattern from leaf tissues infected by Golovinomyces ambrosiae showing a band at ca. 700 bp corresponding to fungal DNA (labelled ‘c’). Lane 4 = Leaf tissues infected by P. macularis. Lane 5 = Healthy leaf tissues. Lane 6 = Leaf tissues infected by G. ambrosiae. Lane 7 = control (no DNA). (b) Lanes 1 and 2 = Young leaf tissues infected by G. ambrosiae. Lanes 3 and 4 = Old leaf tissues infected by G. ambrosiae. Lanes 5 and 6 = Hop leaves infected by P. macularis showing band at ca. 650 bp corresponding to pathogen DNA (labelled ‘c’). Lanes 7–10 = Old cannabis leaves heavily infected by P. macularis. Lanes 11 and 12 = Young cannabis leaves with small colonies of P. macularis. Lane 13 = control (no DNA).
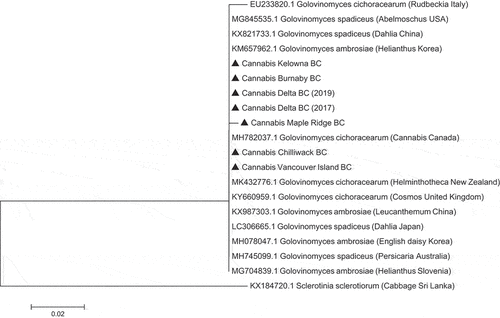
Phylogenetic analysis that included the P. macularis sequence from cannabis ‘Chronic Ryder’ as well as two sequences from hop plants in BC and three sequences from Genbank that represented P. macularis isolates from hops in Washington state, New England, and the United Kingdom showed that all P. macularis were placed in a clade that was distinct from G. chicoracearum (now G. ambrosiae) from cannabis (). Phylogenetic analysis using seven sequences of isolates of G. chicoracearum s. lat. (now G. ambrosiae) on cannabis from different regions within BC showed they were clustered together in a clade with G. ambrosiae and G. spadiceus from a range of hosts and could not be distinguished from them. A recent taxonomic revision of the Golovinomyces ambrosiae/spadiceus/latisporus complex indicates that the species G. ambrosiae encompasses G. spadiceus as synonym and therefore the species designation G. ambrosiae accurately reflects the powdery mildew pathogen affecting cannabis and hemp (Qiu et al. Citation2020; Wiseman et al. Citation2021).
Fig. 5 Phylogenetic analysis of 3 isolates of Podosphaeria macularis originating from cannabis and hop plants in this study from British Columbia (BC) (marked with▲) using ITS1-ITS2 sequences compared to isolates from hops in different geographic regions (GenBank numbers are shown). The evolutionary history was inferred using the Neighbour-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the distances used to infer the phylogenetic tree. The distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. The analysis involved eight nucleotide sequences. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA7 (Kumar et al. Citation2016). The outgroup used was Sclerotinia sclerotiorum.
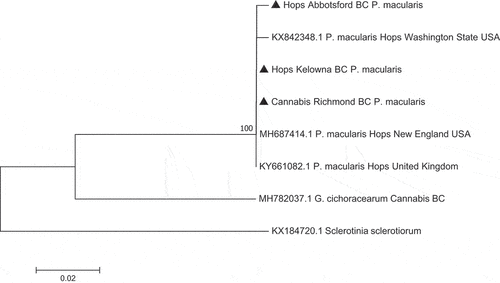
Fig. 6 Phylogenetic analysis of seven isolates of Golovinomyces ambrosiae originating from cannabis plants in this study from different geographic regions in British Columbia (BC) (marked with▲) using ITS1-ITS2 sequences compared to isolates from different hosts and geographic regions (GenBank numbers are shown). The evolutionary history was inferred using the Neighbour-Joining method. The tree is drawn to scale, with branch lengths in the same units as those of the distances used to infer the phylogenetic tree. The distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. The analysis involved 20 nucleotide sequences. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA7 (Kumar et al. Citation2016). The outgroup used was Sclerotinia sclerotiorum.
Microscopic examination of conidia
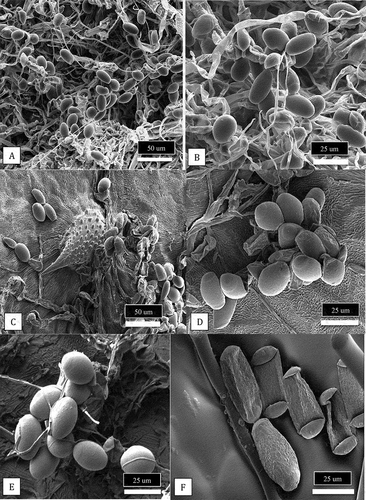
Light microscopy. Using the scotch tape method of mounting spores and examination in a compound microscope, the conidia from leaves of field-grown cannabis strain ‘Chronic Ryder’ (infected with P. macularis) were produced in abundance () and were observed to form in long chains; the conidia were ellipsoid to ovoid (). Similar mounts made from powdery mildew infected squash leaves (P. xanthii) showed almost identical spore morphology (). On field-grown hop leaves infected with P. macularis, prolific production of ellipsoid to ovoid-shaped spores with mycelium was observed () but the chains had broken apart (). On greenhouse-grown cannabis leaves infected with G. ambrosiae, conidia were produced in large numbers () and were cylindrical to barrel-shaped and were formed in chains (). The formation of spores in chains that do not readily break apart is a characteristic of Podosphaeria species (Braun and Cook Citation2012) and was observed in P. macularis and P. xanthii in this study. In addition, fibrosin bodies were observed in fresh conidia of P. macularis that were absent in G. ambrosiae (unpublished).
Fig. 7 Light microscopic images of spores of Podosphaeria and Golovinomyces from cannabis, squash and hop plants. Clear sticky cellophane tape was gently pressed onto the leaves and transferred to a microscope slide which was examined under a compound microscope at various magnifications for characteristics of conidia and chains. (a, b) Conidia and chains of P. macularis from cannabis plants ‘Chronic Ryder’. (c, d) Conidia and chains of P. xanthii from squash plants. (e, f) Conidia of P. macularis from hop leaves. (g, h) Conidia of G. ambrosiae from cannabis leaves.
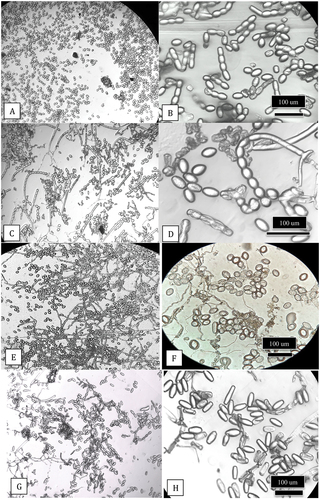
Scanning electron microscopy. Under the scanning microscope, conidia from the leaves of cannabis ‘Chronic Ryder’ infected with P. macularis appeared ovoid in shape () and were very similar in appearance to the conidia produced on powdery mildew infected hop leaves (). In contrast, the spores of the powdery mildew pathogen on greenhouse-grown cannabis leaves were larger and cylindrical in shape ().
Inoculation studies
From the various combinations of host-pathogen inoculations, powdery mildew colonies developed on cannabis leaves ‘Coppenhagen Kush’ inoculated with leaf segments originating from mildew-infected ‘Coppenhagen Kush’ plants (G. ambrosiae), and on leaves of ‘Pink Kush’ inoculated with leaf segments originating from mildew-infected ‘Chronic Ryder’ plants (P. macularis) (). None of the cannabis plants inoculated with powdery mildew inoculum from hop leaves or hop plants inoculated with powdery mildew inoculum from cannabis leaves, developed symptoms. Greenhouse inoculations, where mildew-infected cannabis plants and hops were grown right next to one another, did not give rise to infections on the hop plant (). The presence of P. macularis on the leaves of ‘Pink Kush’ was confirmed by PCR (). From controlled inoculation studies, Turechek et al. (Citation2001) reported that the number of colonies and infection area on the leaves of the susceptible hop cultivar ‘Symphony’, inoculated with P. macularis was significantly reduced at temperatures above 21°C, while minimal disease development was observed at 27°C and none at 30°C. In addition, leaf age had a significant effect on infection frequency, with young expanding leaves being most susceptible to infection and older leaves (>12 days) having significantly reduced or no infection. Disease progress in hop fields in Washington was most rapid from May to July and was lower in August (Turechek et al. Citation2001). The absence of infection in the present study when inoculum from hop leaves was placed on cannabis leaves and plants incubated at 22–25°C, and on greenhouse grown hop plants exposed to inoculum of powdery mildew from cannabis leaves, may be due to the higher ambient temperatures at which inoculated plants were placed, as well as a lack of moist conditions under which the pathogen is likely to develop (Ocamb and Gent Citation2020). In addition, the inoculated hop leaves were older and less likely to be susceptible to infection.
Fig. 9 (a-d) Symptoms on cannabis plants ‘Pink Kush’ inoculated with powdery mildew originating from cannabis ‘Chronic Ryder’ (Podosphaeria macularis). Progression of the disease was observed from 12 to 35 days following inoculation. (a) At 12 days, colonies (arrows) can be seen. (b) Mildew colony at 18 days after inoculation. (c) Mycelial growth with strands developing at 24 days. (d) Spread of mycelium and darkening of affected area after 35 days. Images a-d were taken from two symptomatic plants. The presence of P. macularis was confirmed by PCR (see Fig. 10). (e) Method used for transmission of powdery mildew from a diseased cannabis plant to a hop plant. The cannabis plant with powdery mildew colonies (arrow) was placed under the canopy of a hop plant. (f) The same hop plant after two months of growth in the greenhouse. Infection was not observed on the hop plant.

Fig. 10 PCR analysis using universal eukaryotic primers for the ITS1-5.8S-ITS2 region of ribosomal DNA of samples of cannabis leaves infected with powdery mildew caused by Golovinomyces ambrosiae or Podosphaeria macularis following artificial inoculation. Lane 1 – healthy control showing amplification of plant DNA (band at ca. 750 bp). Lanes 2 and 4 = Leaf infected with G. ambrosiae showing a band at ca. 700 bp corresponding to fungal DNA. Lane 3 = Leaf infected by P. macularis showing a band at ca. 650 bp corresponding to fungal DNA . Lane 5 – control (no DNA).
Cala et al. (Citation2019) inoculated leaves placed inside Petri dish chambers to demonstrate the ability of P. macularis to infect the hemp cultivar ‘Anka’. Cross-inoculation of the hemp powdery mildew pathogen G. ambrosiae (= G. spadiceus) onto hop leaves resulted in poor development of the pathogen. Weldon et al. (Citation2020) similarly conducted cross–inoculation studies using detached hemp and Japanese hop leaves and demonstrated the infectivity of P. macularis onto hemp ‘Anka’ and ‘Wild Horse’ leaves, while G. spadiceus did not show significant development on hop ‘Symphony’ or ‘Zeus’ leaves. In hemp seedlings and potted plants, G. spadiceus developed compatible infections, while on hop plants, there was infrequent infection by G. spadiceus and it was considered to be non-pathogenic (Weldon et al. Citation2020). Our results are similar to those described in these studies, except that infection due to P. macularis was demonstrated on cannabis leaves for the first time, in addition to the observation of natural infection on field-grown cannabis ‘Chronic Ryder’. The conditions that favour development of P. macularis in hops in the spring (Ocamb and Gent Citation2020) suggest that disease development in cannabis or hemp plants grown outdoors may be favoured by more moderate conditions encountered later in the season closer to harvest. Severe infection in cannabis plants in this study was observed between September and October 2019, during which cool damp conditions prevailed. In Oregon, Podosphaeria infection was observed only on outdoor grown hemp plants, while indoor grown plants showed a high incidence of Golovinomyces species (Garfinkel Citation2020). Mahaffee et al. (Citation2003b) showed that infection of hop leaves was reduced by short exposure (>2 hr) to temperatures greater than 30°C, which also reduced the susceptibility of leaves to infection. Disease incidence and severity usually decline in southern Idaho and Washington during extended heat waves (Mahaffee et al. Citation2003a; Gent et al. Citation2008). Therefore, it is unlikely that P. macularis would become a problem in greenhouse grown cannabis plants as temperatures generally exceed 25°C at most times, providing a less conducive environment for infection and pathogen establishment.
Sampling conducted in this study at different locations in British Columbia showed that G. ambrosiae was present exclusively in greenhouse grown cannabis plants and P. macularis was identified in hop plants grown outdoors. It remains to be seen to what extent the hop powdery mildew pathogen can spread and establish on outdoor plantings of cannabis or hemp in BC. In this study, a possible source of inoculum of P. macularis was an ornamental nursery located 2.8 km from the cannabis field, in which two large hop plants were infected with powdery mildew. The distance over which conidia can travel between infected hop plants to cannabis or hemp fields is unknown, but localized dispersal of inoculum within hop yards occurs within a 2 km distance (Gent et al. Citation2019). Alternatively, the inoculum of P. macularis could originate from diseased raspberry plantings, which are common in the Fraser Valley of BC. The potential for the spread and establishment of P. macularis as a pathogen of concern in C. sativa needs to be established from additional surveys conducted at different times during the growing season and in different geographic regions where hop and raspberry production may occur in proximity to hemp and cannabis.
Acknowledgements
Technical assistance provided by Li Ni, S. Chen, Xin Zhang and Cameron Scott is gratefully acknowledged. Several licenced cannabis producers generously provided access to facilities for sampling and documenting the presence of pathogens. I thank S. Sabaratnam and M. Lanthier for providing samples of powdery mildew infected hop leaves. This work makes use of the 4D LABS Scanning Electron Microscopy facility supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF), Western Economic Diversification Canada (WD), and Simon Fraser University (SFU).
Additional information
Funding
References
- Bektas A, Hardwick KM, Waterman K, Kristof J. 2019. Occurrence of hop latent viroid in Cannabis sativa with symptoms of Cannabis stunting disease in California. Plant Dis. 103(10):2699. doi:https://doi.org/10.1094/PDIS-03-19-0459-PDN
- Braun U, Cook RTA. 2012. Taxonomic manual of the Erysiphales (powdery mildews). CBS Biodivers Ser. 11:1–707.
- Cala A, Portilla N, Weldon B, Gura W, King N, Stack G, Toth J, Carlson C, Gadoury D, Sapkota S, et al. 2019. Evaluating disease resistance in CBD hemp cultivars. Hop hemp powdery mildew handout. [accessed 2020 Oct 26]. http://hemp.cals.cornell.edu
- Felsenstein J. 1985. Phylogenies and the comparative method. Amer Nat. 125(1):1–15. doi:https://doi.org/10.1086/284325
- Garfinkel A. 2020. Multiple Botrytis and powdery mildew species associated with industrial hemp in Oregon. (abstr.). Amer Phytopath Soc Ann Meet. https://apsnet.confex.com/apsnet/2020/meetingapp.cgi/Paper/16975.
- Gauthier N, Leonberger K, Bowers K, editors. 2020. Science of hemp: production and pest management. Proceedings of the first annual scientific conference. Lexington: Publication SR-112 College of Agriculture, Food and Environment, University of Kentucky. [accessed 2021 Apr 20]. https://plantpathology.ca.uky.edu/files/sr112.pdf.
- Gent DH, Bhattacharyya S, Ruiz T. 2019. Prediction of spread and regional development of hop powdery mildew: a network analysis. Phytopathology. 109(8):1392–1403. doi:https://doi.org/10.1094/PHYTO-12-18-0483-R
- Gent DH, Nelson ME, George AE, Grove GG, Mahafee WF, Ocamb CM, Barbour JD, Peetz A, Turechek WW. 2008. A decade of hop powdery mildew in the Pacific Northwest. Plant Health Prog. 9(1):33. doi:https://doi.org/10.1094/PHP-2008-0314-01-RV
- Schroeder K L, Okubara P A, Tambong J T, Lévesque CA, Paulitz TC. 2006. Identification and quantification of pathogenic pythium spp. from soils in Eastern Washington using real-time polymerase chain reaction. Phytopathology. 96(6):637–647. doi:https://doi.org/10.1094/PHYTO-96-0637
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874. doi:https://doi.org/10.1093/molbev/msw054
- Mahaffee WF, Thomas CS, Turechek WW, Ocamb CM, Nelson ME, Fox A, Gubler WD. 2003a. Responding to an introduced pathogen: Podosphaeria macularis (hop powdery mildew) in the Pacific Northwest. Plant Health Progress. doi:https://doi.org/10.1094/PHP-2003-1113-07-RV
- Mahaffee WF, Turechek WW, Ocamb CM. 2003b. Effect of variable temperature on infection severity of Podosphaera macularis on hops. Phytopathology. 93(12):1587–1592. doi:https://doi.org/10.1094/PHYTO.2003.93.12.1587
- Marks M, Gevans A. 2014. Hop powdery mildew. Identification and management. University of Wisconsin-Extension Bulletin (A4053–02). UW Cooperative Extension Publisher.
- Ni L, Punja ZK. 2021. Management of powdery mildew on greenhouse cucumber (Cucumis sativus L.) plants using biological and chemical approaches. Can J Plant Pathol. 43(1):35–42. doi:https://doi.org/10.1080/07060661.2020.1746694
- Ocamb CM, Gent DH. 2020. Hop (Humulus lupulus) – powdery mildew. In: Pscheidt JW, Ocamb CM, editors. Pacific Northwest pest management handbook. Corvallis: Oregon State University. [accessed 2021 Mar 30]. https://pnwhandbooks.org/plantdisease/host-disease/hop-humulus-lupulus-powdery-mildew.
- Ocamb CM, Klein R, Barbour J, Griesbach J, Mahaffee W. 1999. First report of hop powdery mildew in the Pacific Northwest. Plant Dis. 83(11):1072. doi:https://doi.org/10.1094/PDIS.1999.83.11.1072A
- Ontario Ministry of Agriculture, Food and Rural Affairs. Powdery mildew. Integrated pest management for apples. Publication 310. [accessed 2021 May 5]. http://www.omafra.gov.on.ca/english/crops/facts/powderymil.htm.
- Pépin N, Punja ZK, Joly DL. 2018. First report of powdery mildew caused by Golovinomyces cichoracearum sensu lato on Cannabis sativa in Canada. Plant Dis. 102(12):2644. doi:https://doi.org/10.1094/PDIS-04-18-0586-PDN
- Pscheidt JW, Ocamb CM, Editors. 2021. Pacific Northwest plant disease management hand book [online]. Corvallis (OR): Oregon State University. [accessed 2021 Jul 10]. https://pnwhandbooks.org/plantdisease.
- Punja ZK. 2018. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40(4):514–527. doi:https://doi.org/10.1080/07060661.2018.1535467
- Punja ZK. 2020. First report of the hops powdery mildew pathogen Podosphaeria macularis on naturally infected marijuana (Cannabis sativa L.) plants in the field. (abstr.). Amer Phytopath Soc Ann Meet. https://apsnet.confex.com/apsnet/2020/meetingapp.cgi/Paper/16065.
- Punja ZK. 2021. Emerging diseases of Cannabis sativa and sustainable management. Pest Manage Sci. doi:https://doi.org/10.1002/ps.6307
- Punja ZK, Collyer D, Scott C, Lung S, Holmes J, Sutton D. 2019. Pathogens and molds affecting production and quality of Cannabis sativa L. Front Plant Sci. 10:1120. doi:https://doi.org/10.3389/fpls.2019.01120.
- Qiu PL, Liu SY, Bradshaw M, Rooney-Latham S, Takamatsu S, Bulgakov TS, Tang SR, Feng J, Jin DN, Aroge T, et al. 2020. Multi-locus phylogeny and taxonomy of an unresolved, heterogeneous species complex within the genus Golovinomyces (Ascomycota, Erysiphales), including G. ambrosiae, G. circumfusus and G. spadiceus. BMC Microbiol. 20(1):1–16. doi:https://doi.org/10.1186/s12866-020-01731-9
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Schönberger C, Kostelecky T. 2012. 125th anniversary review: the role of hops in brewing. J Inst Brew. 117(3):259–267. doi:https://doi.org/10.1002/j.2050-0416.2011.tb00471.x
- Scott C, Punja ZK. 2020. Evaluation of disease management approaches for powdery mildew on Cannabis sativa L. (marijuana) plants. Can J Plant Pathol. 43(3):394–412. doi:https://doi.org/10.1080/07060661.2020.1836026
- Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Nat Acad Sci USA. 101(30):11030–11035. doi:https://doi.org/10.1073/pnas.0404206101
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 28(10):2731–2739. doi:https://doi.org/10.1093/molbev/msr121
- Turechek WW, Mahafee WF, Ocamb CM. 2001. Development of management strategies for hop powdery mildew in the Pacific Northwest. Plant Health Prog. 2(1):8. doi:https://doi.org/10.1094/PHP-2001-0313-01-RS
- Twomey MC, Wolfenbarger SN, Woods JL, Gent DH. 2015. Development of partial ontogenic resistance to powdery mildew in hop cones and its management implications. PLoS ONE. 10(3):e0120987. doi:https://doi.org/10.1371/journal.pone.0120987
- Warren JG, Mercado J, Grace D. 2019. Occurrence of hop latent viroid causing disease in Cannabis sativa in California. Plant Dis. 103(10):2699. doi:https://doi.org/10.1094/PDIS-03-19-0530-PDN
- Weldon WA, Ullrich MR, Smart LB, Smart CD, Gadoury DM. 2020. Cross-infectivity of powdery mildew isolates originating from hemp (Cannabis sativa) and Japanese hop (Humulus japonicus) in New York. Plant Health Prog. 21(1):47–53. doi:https://doi.org/10.1094/PHP-09-19-0067-RS
- Wiseman MS, Bates T, Garfinkel A, Ocamb CM, Gent DH. 2021. First report of powdery mildew caused by Golovinomyces ambrosiae on Cannabis sativa in Oregon. Plant Dis. (in press). doi:https://doi.org/10.1094/PDIS-11-20-2455-PDN
- Wolfenbarger SN, Twomey MC, Gadoury DM, Knaus BJ, Grünwald NJ, Gent DH. 2015. Identification and distribution of mating-type idiomorphs in populations of Podosphaera macularis and development of chasmothecia of the fungus. Plant Pathol. 64(5):1094–1102. doi:https://doi.org/10.1111/ppa.12344