Abstract
Wheat leaf rust, caused by Puccinia triticina Eriks., is a common and destructive disease of wheat worldwide. The durable leaf rust-resistance gene Lr34 is typically described as an adult plant resistance gene, which is not expressed at the seedling stage at normal greenhouse temperatures. However, Lr34 is expressed at the seedling stage when plants with Lr34 are grown at low temperatures, i.e. approximately 8°C. Lines and cultivars with Lr34 demonstrate a range of responses from near immunity to intermediate reactions on the primary leaf. The Thatcher-Lr34 near isogenic lines ‘RL6058ʹ (Thatcher*6/PI58548) and ‘RL6091ʹ (Tc*6/Chinese Spring) were resistant to a number of P. triticina virulence phenotypes at the seedling stage when grown under these cool conditions, but were susceptible at typical greenhouse temperatures. A double haploid population from a cross between ‘Thatcher’ and ‘RL6058ʹ, previously genotyped for Lr34, was grown at 8°C and screened for leaf rust response at the seedling stage. The population is segregated by a seedling resistance gene that corresponds to the presence of a resistance allele of Lr34. After extended incubation at cold temperatures, the seedling progeny lines with Lr34 also developed distinct leaf tip necrosis; this phenotype was absent in susceptible plants, similar to their reaction as adult plants. When uninfected plants from this population were grown to the adult plant stage at 10°C, those with the Lr34 resistance allele had extensive leaf necrosis and shrunken, sometimes sterile, spikes.
Résumé
La rouille brune, causée par Puccinia triticina Eriks., est une maladie courante et destructrice du blé partout dans le monde. Le gène durable de la résistance à la rouille brune, Lr34, est habituellement considéré comme un gène de résistance des plants adultes qui n’est pas exprimé au stade de plantule en serre, aux températures normales de culture. Toutefois, Lr34 est exprimé au stade de plantule quand les plants qui le portent croissent à basses températures, c.-à-d. à environ 8°C. Les lignées et les cultivars possédant Lr34 affichent une gamme de réactions, d’une immunité presque complète à des réactions intermédiaires chez la première feuille. Les lignées presque isogéniques Thatcher-Lr34 RL6058 (Thatcher*6/PI58548) et RL6091 (Tc*6/Chinese Spring) étaient résistantes à un certain nombre de phénotypes de virulence de P. triticina au stade de plantule lorsque cultivées à basses températures, mais y étaient réceptives aux températures normales de culture en serre. Une population dihaploïde issue d’un croisement entre Thatcher et RL6058, préalablement génotypée pour Lr34, a été cultivée à 8°C et criblée pour la réaction à la rouille brune au stade de plantule. La population a ségrégé pour un gène de résistance au stade de plantule qui correspondait à l’allèle de résistance de Lr34. Après une incubation prolongée à basses températures, les lignées de descendance des plantules possédant Lr34 ont aussi développé une nécrose distinctive des extrémités foliaires; ce phénotype n’existait pas chez les plants réceptifs, analogue à leur réaction en tant que plants adultes. Lorsque des plants sains de cette population ont été cultivés jusqu’au stade adulte à 10°C, ceux possédant l’allèle de résistance Lr34 affichaient une nécrose foliaire extensive et des spicules ratatinés, parfois stériles.
Keywords:
Mots clés:
Introduction
Wheat leaf rust, caused by Puccinia triticina Eriks., is one of the most common and destructive diseases of wheat on a worldwide basis. It can be effectively controlled through the use of host genetic resistance, which is more economical and environmentally friendly than the chemical control alternative. One of the most effective and durable leaf rust-resistance genes is Lr34, which was first described by Dyck (Citation1977, Citation1987). It is typically characterized as an adult plant slow-rusting gene. This gene also confers resistance to stripe rust (Yr18; Singh Citation1992a), stem rust (Sr57; Dyck et al. Citation1985), barley yellow dwarf virus (Bdv1, Singh Citation1993) and powdery mildew (Pm38; Spielmeyer et al. Citation2005). It is sometimes referred to as Lr34/Yr18/Sr57 but for simplicity, we refer to it here as Lr34. Lr34 also causes leaf tip necrosis in adult plants (Singh Citation1992b). The cloned Lr34 encodes an ABC transporter (Krattinger et al. Citation2009). Lr34 is common, in wheat germplasm throughout the world (Kolmer et al. Citation2008) and in Canada (McCallum et al. Citation2011) because of its effectiveness and durability.
There have been attempts to detect this gene at the seedling stage. Dyck and Samborski (Citation1982) reported that the expression of Lr34 varied depending on temperature and light. Singh and Gupta (Citation1992) found a slight effect of Lr34 on seedlings grown at 12–13°C but this effect was not seen when plants were grown from 14°C to 23°C. Higher expression of Lr34 leaf rust resistance was observed at lower temperatures of 13–17°C compared to 25–30°C as characterized by a longer latent period, few uredinia per cm−2 and smaller uredinia on adult plants (Drijepondt and Pretorius Citation1989). Dyck and Samborski (Citation1982) reported a slight effect of Lr34 on seedlings below normal greenhouse temperatures. Drijepondt and Pretorius (Citation1991) were able to detect Lr34 in seedlings of ‘RL6058ʹ (Thatcher-Lr34) grown at 7°C but not at higher temperatures, expressed as a lower infection type, when compared with ‘Thatcher’. Rubiales and Niks (Citation1995) found that Lr34 significantly increased the latent period and reduced the infection frequency in seedlings grown at 8°C but not at higher temperatures. Agarwal et al. (Citation2003) reported that a resistant seedling infection type of ‘0;’ or ‘;’ could be observed on ‘Thatcher’ near-isogenic lines with Lr34 at 14.5°C, whereas they were susceptible at 20°C. Alternatively, Singh et al. (Citation2007) found a slight reduction in the seedling infection type for ‘Thatcher+Lr34ʹ (‘23ʹ) compared with ‘Thatcher’ (‘3+’) when seedlings were grown at 5–7°C but concluded that this temperature regime was not practical for routine screening. Risk et al. (Citation2012) reported enhanced resistance at the seedling stage for ‘Thatcher-Lr34ʹ and transgenic lines with Lr34res when plants were grown at either 4°C or 10°C.
A number of authors have reported a degree of seedling resistance associated with wheat lines that have a resistant allele of Lr34 that increases as the incubation temperature is lowered. The longer-term effects of Lr34 when plants are incubated at cold temperatures are not known. The objective of this study was to analyse the longer-term effects of cold temperature incubation on seedlings with Lr34, in terms of leaf rust resistance, leaf tip necrosis, and plant viability, within a population that segregated for this resistance gene. This knowledge can be applied to develop better seedling-stage tests for Lr34 and should lead to a better understanding of the function of this important resistance gene.
Materials and methods
Lr34 seedling assay for characterized lines
Initially, well-characterized wheat lines were used to develop a seedling assay for Lr34. These lines were the leaf rust-susceptible check ‘Thatcher’ (Tc), ‘RL6058ʹ, which is the near isogenic line (NIL) Thatcher-Lr34 (Tc*6/PI58548), ‘RL6091ʹ a different Thatcher-Lr34 NIL (Tc*6/Chinese Spring) and the Canadian cultivar ‘Glenlea’ that has Lr34 (Dyck et al. Citation1985). Five to eight seeds of each line were seeded in three small flats, and the plants were grown at typical greenhouse temperatures (15–25°C) for 14 days (d). For all trial plants were fertilized by watering weekly with a dilute solution of 20:20:20 N:P:K (0.5 g L−1). At 14d, the plants were inoculated at the two leaf stages (Zadoks growth stage 12 [Zadoks et al. Citation1974]) with Puccinia triticina isolates 06-1-1 TDBG, 12–3 MBDS and 77–2 TJBJ. The latter codes are as defined by Long and Kolmer (Citation1989). Urediniospores were suspended in lightweight mineral oil (Bayol, Esso Canada, Toronto, ON) and sprayed onto the leaves of the plants using compressed air (McCallum and Seto-Goh Citation2009). Plants were incubated in a humidity cabinet for 12 h then transferred to a growth cabinet set at 8°C with a light intensity of 3.0–3.5 kLux. Infected leaves were sampled and scanned at 26, 30, 38, 42 d after inoculation (dai). The cold temperature delayed the development of the plants compared to normal growth temperature. Plants were approximately at the Zadoks growth stage 13 at 26 and 30 dai and stage 14 at 38 and 42 dai. The experiment was repeated using isolate 06-1-1 TDBG because all isolates initially tested produced similar results.
Seedling assay for leaf rust resistance and leaf tip necrosis in a Thatcher/Thatcher-Lr34 population
A double haploid population (03B10B) of 510 lines was developed from the cross ‘Thatcher’/’RL6058ʹ (Thatcher-Lr34 NIL) using the maize pollination method (Thomas et al. Citation1997). This population was phenotyped and genotyped previously due to the presence of either the resistant or susceptible allele at the Lr34 locus in each progeny line as described by Dakouri et al. (Citation2010). Forty-five lines with Lr34 (containing the resistant allele of Lr34) and 45 lines without Lr34 (containing the susceptible allele of Lr34) were selected from this population. These lines and the parental lines ‘Thatcher’ and ‘RL6058ʹ were seeded in clumps of five to eight seeds. All the plants were inoculated at 14 d with P. triticina isolate 06-1-1 TDBG, then incubated in 100% humidity as described by McCallum and Seto-Goh (Citation2009). These plants were then grown in a growth chamber at 8°C. Symptoms started to appear at 22 dai and the plants were rated for leaf rust at 29 days after inoculation (Zadoks stage 13–14). The infection reactions were scored as described by McCallum and Seto-Goh (Citation2009). Leaves from these plants were sampled and scanned at 48 dai (Zadoks stage 14). The complete experiment was repeated at a later date.
The effects of extended cold temperature exposure to lines with Lr34
From the original 90 selected lines from the 03B10B population described above, 76 lines were randomly selected, 39 with the resistant allele of Lr34 and 37 with the susceptible allele as space and seed for some lines was limited, and all the lines with the resistant allele reacted similarly in the previous experiments as did all the lines with the susceptible allele. These lines were seeded in 11.5 cm diameter pots and initially grown at standard greenhouse temperatures of 15–25°C. The checks ‘Thatcher’, ‘RL6058ʹ (Thatcher-Lr34) and ‘Glenlea’ were also included. At 13 d after seeding, approximately at the two leaf stages (Zadoks stage 12), the plants were thinned to a single seedling per pot and transferred into a growth cabinet set at 10°C day/15°C night with 16-hr days and 8-hr nights. The plants were not inoculated with leaf rust but were scored for leaf tip necrosis (present or absent) at 8 weeks after seeding. At 24 weeks after seeding, the lines were scored a second time for leaf tip necrosis, and at 28 weeks the flag leaves of the plants were photographed recording the level of leaf tip necrosis (approximately Zadoks stage 65).
Results and discussion
When wheat seedlings inoculated with leaf rust were grown at 8°C, symptoms of infection started to appear at 22 dai on ‘Thatcher’ and at 24 dai on ‘RL6058ʹ (Tc-Lr34), ‘RL6091ʹ (Tc-Lr34) and ‘Glenlea’. Pustules appeared on each of the four lines, but remained relatively small on ‘RL6058ʹ and ‘RL6091ʹ whereas they developed into larger pustules over time on ‘Thatcher’ and ‘Glenlea’. By 42 dai, the reaction on each of the lines was as follows: ‘Thatcher’ ‘3’, ‘RL6058ʹ “;1-“, ‘RL6091ʹ “;11-“, and ‘Glenlea’ “; 123-“ and fewer pustules were produced on the Lr34+ lines than on ‘Thatcher’ (). Each of the three P. triticina isolates used produced similar results (). Pustule development was delayed in all the lines, with Lr34 resulting in much smaller and fewer pustules, particularly on ‘RL6058ʹ and ‘RL6091ʹ. The results were very similar when the experiment was repeated using only isolate 06-1-1 TDBG. These results differ from Singh et al. (Citation2007) who reported a ‘23ʹ seedling infection type for Thatcher-Lr34 plants grown at 5–7°C, which was only slightly more resistant than ‘Thatcher’ which displayed an infection type of ‘3+’. Risk et al. (Citation2012) compared seedling leaf rust resistance and expression of Lr34res in Thatcher, Thatcher-Lr34, and transgenic lines with Lr34res and hypothesized that cold-induced resistance in seedlings was caused by the slower growth of the fungus or the accumulation of a Lr34res-dependent inhibitory substance.
Fig. 1 Leaf rust infection types on wheat leaves inoculated with Puccinia triticina isolate 06-1-1 TDBG grown at 8°C. Infection types observed at (a) 26, (b) 30, (c) 38, and (d) 42 days after inoculation. From left to right within each panel, the lines are Thatcher (Tc), Tc-Lr34 (RL6058), Tc-Lr34 (RL6091) and Glenlea
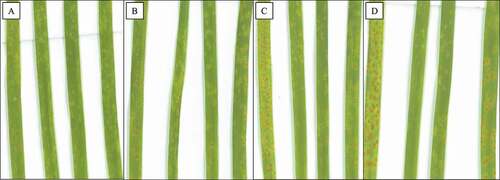
Fig. 2 Symptoms of wheat leaf rust on plants grown at 8°C for 42 days after inoculation. Puccinia triticina isolates were (a) 12–3 MBDS, (b) 06-1-1 TDBG, and (c) 77–2 TJBJ. From left to right within each panel, the lines are Thatcher (Tc), Tc-Lr34 (RL6058), Tc-Lr34 (RL6091) and Glenlea
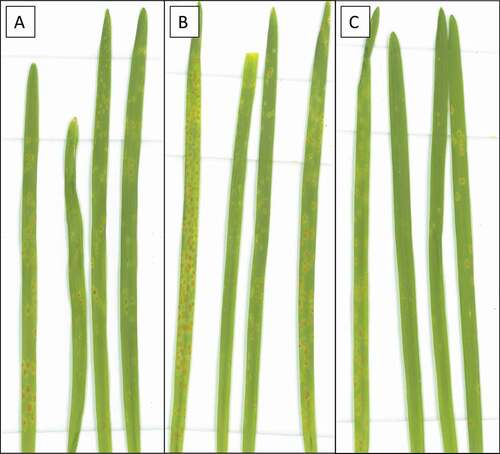
When the 90 progeny lines from the Thatcher/RL6058 (03B10B) were inoculated with leaf rust isolate 06-1-1 TDBG, infection was again evident at approximately 22 days after inoculation. Lines were rated for leaf rust at 29 dai. Two phenotypes were observed, the 45 lines with the susceptible allele of Lr34- were rated as “3-“, similar to ‘Thatcher’, and the 45 lines with the resistant allele of Lr34+ had a reaction type ‘;1 = ’, similar to ‘RL6058ʹ. At 36 dai, leaf tip necrosis, and chlorosis started to appear on ‘RL6058ʹ and the lines with Lr34, but not on ‘Thatcher’ or the lines without Lr34. The leaf tip necrosis progressed, was rated at 48 dai as the presence or absence of necrosis; then, infected leaves were sampled, and scanned (). The results of the second complete trial of the experiment confirmed those of the first. This analysis demonstrated that both seedling leaf rust resistance and leaf tip necrosis co-segregated with the resistance allele of Lr34 in a segregating population.
Fig. 3 Parental and progeny lines from the doubled haploid population 03B10B inoculated with Puccinia triticina isolate 06-1-1 TDBG and grown for 48 days at 8°C. From left to right, the plants are parental line Thatcher (Tc) and Tc-Lr34 (RL6058), followed by 30 progeny lines that carried either the resistant (+) or susceptible (-) allele of Lr34.
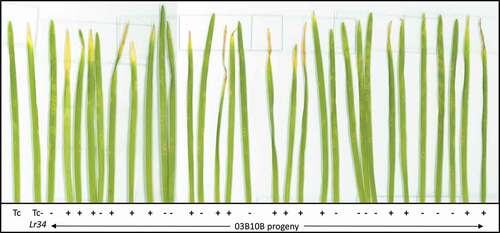
A subset of these 90 lines was then grown for an extended period of time at low temperatures (10°C), without pathogen inoculation. Plants were initially rated for leaf tip necrosis at 8 weeks after seeding, and the results were the same as in the previous experiment: lines with Lr34 showed extensive leaf tip necrosis whereas those without did not. By 10 weeks after seeding, the first leaves on the plants with Lr34 were rapidly dying back, whereas those without Lr34 retained these first leaves longer. At 14 weeks after seeding, leaf tip necrosis was apparent on the flag and penultimate leaf tip of lines with Lr34. Leaf tip necrosis was rated for a second time at 24 weeks after seeding, and the plants were photographed at 28 weeks to document the extent of leaf tip necrosis. Along with extensive leaf tip necrosis, some plants with Lr34 had shrivelled and sometimes sterile spikes, compared to the relatively normal appearance of plants without Lr34 (). Of the 39 lines with the Lr34-resistant allele five lines did not have a spike that emerged from the boot, eight lines produced completely sterile white spikes, similar to ‘RL6058ʹ () and three lines were partially sterile with a portion of the Spike White. The ‘Glenlea’ plants also had leaf tip necrosis, but to a lesser extent than ‘RL6058ʹ or progeny lines with Lr34 (). This again demonstrated that both extensive leaf tip necrosis and shrivelled, sometimes sterile spikes, co-segregated with the resistance allele of Lr34 in this population.
Fig. 4 Parental and progeny lines from the 03B10B population (Thatcher/Thatcher-Lr34) kept at 10°C for 28 weeks. From left to right the plants are Thatcher, Thatcher-Lr34 (RL6058), three 03B10B progeny lines, the first with the susceptible Lr34 allele (Lr34-) and the other two with the Lr34 resistant allele (Lr34+), followed by Glenlea

Although Lr34 is known as an adult plant resistance gene, it can be effective at the seedling stage when the plants are grown at low temperatures (8–10°C in this study) (Risk et al. Citation2012). The infection types produced in seedlings are similar to those observed in adult plants, with few pustules and a range of pustule sizes. However, in adult plants there is a pattern of pustule formation on flag leaves, with larger pustules more frequently found at the base of the flag leaf graduating to smaller and less-frequent pustules formed towards the tip of the leaf. This pattern was not evident on seedling leaves. While the cultivar, Glenlea, had some level of seedling resistance, the Thatcher-Lr34 NILs had a much stronger expression of seedling resistance. These results confirme previous findings that Lr34 can be effective at the seedling stage at low temperatures although this is dependent on the genetic background.
Seedling leaf tip necrosisalso co-segregated with the resistant allele of Lr34 in the ‘Thatcher’/’RL6058ʹ population. This trait was observed in all lines with the resistant allele of Lr34 whether these lines were inoculated with the pathogen or not. Lines with the resistant allele of Lr34 from this population also had extensive leaf tip necrosis on mature plants at 28 weeks after seeding. Nearly half of these lines (16/39) either failed to produce a spike or the spike was partially or fully sterile and many of the remaining lines had some portion of spikelets that failed to form. Seedling leaf tip necrosis was also observed when the Lr34 gene was transformed into barley (Risk et al. Citation2013). This effect was evident throughout the life of the transformed plants and resulted in stunted, less vigorous plants compared to the controls. However, the transformed plants had superior resistance to a number of biotrophic pathogens of barley (Risk et al. Citation2013).
Rinaldo et al. (Citation2017) transformed Stewart durum wheat with the resistant allele of Lr34 and found it was effective throughout the life of the plant, and resistance was enhanced by cold temperatures (10°C) at the seedling stage. However, they found no associated seedling leaf tip necrosis. When they inoculated ‘RL6058ʹ (Thatcher-Lr34) with Puccinia striiformis f. sp. tritici they found a four-fold induction of Lr34 expression at 10°C but not at 22°C. Based on their results, and those of other studies, Rinaldo et al. (Citation2017) proposed two models for the relationship between resistance and leaf tip necrosis associated with Lr34, one in which they were part of the same pathway and a second on independent pathways. Both models showed the growth and seedling resistance at 10°C as very short duration that did not include the associated leaf tip necrosis. Our results support the single pathway model proposed by Rinaldo et al. (Citation2017).
Rajagopalan et al. (Citation2020) found that the flavonoid-rich metabolites were nearly identical between ‘Thatcher’ and ‘RL6058ʹ except that a phenylpropanoid diglyceride, 1-Op-coumaroyl-3-O-feruloylglycerol (CFG), accumulated in ‘RL6058ʹ but not in ‘Thatcher’. Krattinger et al. (Citation2019) identified abscisic acid (ABA) as a substrate for the Lr34 ABC transporter and transgenic rice plants with Lr34 had altered expression of ABA induced stress response genes. Transgenic rice and barley plants with a high expression of Lr34res had severe reductions in plant vigour and yield (Krattinger et al. Citation2019), similar to the effects seen in the current study on wheat plants grown at low temperatures for extended durations.
In wheat, Johnston et al. (Citation2017) found a positive effect of Lr34 on yield under natural growing conditions in New Zealand, because of its positive effects on disease control, but Lr34 had a negative effect on yield when diseases were under control through fungicide use. They indicated that Lr34 has been absent from wheat cultivars released in New Zealand since 2006, likely due to the negative appeal of leaf tip necrosis and possibly because of its associated yield loss in full-fungicide disease control management. The relatively low temperatures during the growing season for wheat in New Zealand may result in a larger yield penalty than is seen in areas where the growing season is warmer. However, Singh and Huerta-Espino (Citation1997) also reported a small (5%) but significant yield reduction associated with Lr34 under fungicide protection in Mexico.
Detecting Lr34 phenotypically in adult plants is relatively easy under field conditions, but can be challenging in the greenhouse (Dyck and Samborski Citation1982). A low-temperature seedling test could be a quicker and more reliable option for phenotyping wheat plants indoors in the presence of Lr34. However, the perfect marker based on the gene sequence of Lr34 is very rapid and reliable (Lagudah et al. Citation2009; Dakouri et al. Citation2010). The genetic background appears to influence the effect of Lr34 at the seedling stage. The deleterious effects of Lr34 in wheat also appear to be much stronger at low temperatures. This may be a problem for winter wheat when grown at relatively cool temperatures compared to spring wheat, which normally grows at higher temperatures.
Acknowledgements
The authors thank Pat Seto-Goh for excellent technical assistance and Madeleine Lévesque-Lemay for editing the suggestions.
References
- Agarwal S, Sharma AK, Saini RG. 2003. Seedling reaction of Thatcher Triticum aestivum L. near isogenic lines with adult plant leaf rust resistance genes Lr34 and Lr37. Wheat Inf Ser. 97:21–217.
- Dakouri A, McCallum B, Walichnowski A, Cloutier S. 2010. Fine-mapping of the leaf rust Lr34 locus in Triticum aestivum (L.) and characterization of large germplasm collections support the ABC transporter as essential for gene function. Theor Appl Genet. 121(2):373–384. doi:https://doi.org/10.1007/s00122-010-1316-7
- Drijepondt SC, Pretorius ZA. 1989. Greenhouse evaluation of adult-plant resistance conferred by the gene Lr34 to leaf rust of wheat. Plant Dis. 73(8):669–671. doi:https://doi.org/10.1094/PD-73-0669
- Drijepondt SC, Pretorius ZA. 1991. Expression of two wheat leaf rust resistance gene combinations involving Lr34. Plant Dis. 75(5):526–528. doi:https://doi.org/10.1094/PD-75-0526
- Dyck PL. 1977. Genetics of leaf rust reaction in three introductions of common wheat. Can J Genet Cytol. 19(4):711–716. doi:https://doi.org/10.1139/g77-077
- Dyck PL. 1987. The association of a gene for leaf rust resistance with the chromosome 7D suppressor of stem rust resistance in common wheat. Genome. 29(3):467–469. doi:https://doi.org/10.1139/g87-081
- Dyck PL, Samborski DJ. 1982. The inheritance of resistance to Puccinia recondita in a group of common wheat cultivars. Can J Genet Cytol. 24(3):273–283. doi:https://doi.org/10.1139/g82-029
- Dyck PL, Samborski DJ, Martens JW. 1985. Inheritance of resistance to leaf rust and stem rust in the wheat cultivar Glenlea. Can J Pl Pathol. 7:351–354.
- Johnston PA, Munro C, Butler RC, Browne J, Gibbs A, Shorter S. 2017. The future of Lr34 in modern, high-input wheat breeding programs. Crop Sci. 57(2):671–680. doi:https://doi.org/10.2135/cropsci2016.03.0158
- Kolmer JA, Singh RP, Garvin DF, Viccars L, William HM, Huerta-Espino J, Ogbonnaya FC, Raman H, Orford S, Bariana HS, et al. 2008. Analysis of the Lr34/Yr18 rust resistance region in wheat germplasm. Crop Sci. 48(5):1841–1852. doi:https://doi.org/10.2135/cropsci2007.08.0474
- Krattinger SG, Kang J, Bräunlich S, Boni R, Chauhan H, Selter LL, Robinson MD, Schmid MW, Wiederhold E, Hensel G, et al. 2019. Abscisic acid is a substrate of the ABC transporter encoded by the durable wheat disease resistance gene Lr34. New Phytol. 223(2):853–866. doi:https://doi.org/10.1111/nph.15815
- Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B. 2009. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science. 323(5919):1360–1363. doi:https://doi.org/10.1126/science.1166453
- Lagudah ES, Krattinger SG, Herrera-Foessel S, Singh RP, Huerta-Espino J, Spielmeyer W, Brown-Guedira G, Selter LL, Keller B. 2009. Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor Appl Genet. 119(5):889–898. doi:https://doi.org/10.1007/s00122-009-1097-z
- Long DL, Kolmer JA. 1989. A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology. 79(5):525–529. doi:https://doi.org/10.1094/Phyto-79-525
- McCallum BD, Humphreys DG, Somers DJ, Dakouri A, Cloutier S. 2011. Allelic variation for the rust resistance gene Lr34/Yr18 in Canadian wheat cultivars. Euphytica. 183(2):261–274. doi:https://doi.org/10.1007/s10681-011-0519-6
- McCallum BD, Seto-Goh P. 2009. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2006. Can J Pl Pathol. 31(1):80–87. doi:https://doi.org/10.1080/07060660909507575
- Rajagopalan N, Lu Y, Burton IW, Monteil-Rivera F, Halasz A, Reimer E, Tweidt R, Brûlé-Babel A, Kutcher HR, You FM, et al. 2020. A phenylpropanoid diglyceride associates with the leaf rust resistance Lr34res gene in wheat. Phytochem 178:112456. doi:https://doi.org/10.1016/j.phytochem.2020.112456
- Rinaldo A, Gilbert B, Boni R, Krattinger SG, Singh D, Park RF, Lagudah E, Ayliffe M. 2017. The Lr34 adult plant rust resistance gene provides seedling resistance in durum wheat without senescence. Plant Biotechnol J. 15(7):894–905. doi:https://doi.org/10.1111/pbi.12684
- Risk JM, Selter LL, Chauhan H, Krattinger SG, Kumlehn J, Hensel G, Viccars LA, Richardson TM, Buesing G, Troller A, et al. 2013. The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol J. 11(7):847–854. doi:https://doi.org/10.1111/pbi.12077
- Risk JM, Selter LL, Krattinger SG, Viccars LA, Richardson TM, Buesing G, Herren G, Lagudah ES, Keller B. 2012. Functional variability of the Lr34 durable resistance gene in transgenic wheat. Plant Biotechnol J. 10(4):477–487. doi:https://doi.org/10.1111/j.1467-7652.2012.00683.x
- Rubiales D, Niks RE. 1995. Characterization of Lr34, a major gene conferring nonhypersensitive resistance to wheat leaf rust. Plant Dis. 79(12):1208–1212. doi:https://doi.org/10.1094/PD-79-1208
- Singh D, Park RF, McIntosh RA. 2007. Characterisation of wheat leaf rust resistance gene Lr34 in Australian wheats using components of resistance and the linked molecular marker csLV34. Aust J Ag Res. 58(11):1106–1114. doi:https://doi.org/10.1071/AR07002
- Singh RP. 1992a. Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathology. 82(8):835–838. doi:https://doi.org/10.1094/Phyto-82-835
- Singh RP. 1992b. Association between gene Lr34 for leaf rust resistance and leaf tip necrosis in wheat. Crop Sci. 32(4):874–878. doi:https://doi.org/10.2135/cropsci1992.0011183X003200040008x
- Singh RP. 1993. Genetic association of gene Bdv1 for tolerance to barley yellow dwarf virus with genes Lr34 and Yr18 for adult plant resistance to rusts in bread wheat. Plant Dis. 77(11):1103–1106. doi:https://doi.org/10.1094/PD-77-1103
- Singh RP, Gupta AK. 1992. Expression of wheat leaf rust resistance gene Lr34 in seedlings and adult plants. Plant Dis. 76(5):489–491. doi:https://doi.org/10.1094/PD-76-0489
- Singh RP, Huerta-Espino J. 1997. Effect of leaf rust resistance gene Lr34 on grain yield and agronomic traits of spring wheat. Crop Sci. 37(2):390–395. doi:https://doi.org/10.2135/cropsci1997.0011183X003700020014x
- Spielmeyer W, McIntosh RA, Kolmer J, Lagudah ES. 2005. Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor Appl Genet. 111(4):731–735. doi:https://doi.org/10.1007/s00122-005-2058-9
- Thomas J, Chen Q, Howes N. 1997. Chromosome doubling of haploids of common wheat with caffeine. Genome. 40(4):552–558. doi:https://doi.org/10.1139/g97-072
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Res. 14(6):415–421. doi:https://doi.org/10.1111/j.1365-3180.1974.tb01084.x
