Abstract
Beneficial endophytes are key factors in plant productivity and disease control; therefore, research is ongoing to characterize the potential of endophytes as microbial inoculants to promote plant growth and act as biocontrol agents. In this study, we searched for and identified a set of endophytes occurring naturally in a few important crops. Beneficial endophytes were shown to antagonize several important fungi and bacteria, in particular those affecting tomato (Solanum lycopersicum), including Rhizoctonia solani, Alternaria alternata, Clavibacter michiganensis pv. michiganensis, Ralstonia solanacearum, and Xanthomonas vesicatoria. We characterized the most promising as potential inoculants of tomato, aiming to foster plant growth and control the bacterial spot disease caused by Xanthomonas vesicatoria. Among the selected endophytes, we identified a novel streptomycetes, taxonomically related to Streptomyces avermitilis and a pseudomonad, identified as Pseudomonas granadensis. The beneficial effects of these two bacteria, used as single inoculants or as a combination of both, were seen in a significant increase in root and shoot length (approximately 31% and 34% for the streptomycetes and 18% and 16% for the pseudomonad) and dry root biomass (90% for the streptomycetes and 70% for the pseudomonad). Additionally, both inoculants reduced disease progression and severity following inoculation with X. vesicatoria. There was no significant difference between plants treated with single inoculants and plants treated with both. The penetration and efficient colonization of tomato tissues by a green fluorescent protein-tagged culture of the streptomycetes was observed by confocal microscopy, confirming its endophytic nature.
Résumé
Les endophytes bénéfiques sont des facteurs clés de la productivité végétale et de la lutte contre les maladies, en conséquence, les recherches se poursuivent pour caractériser le potentiel des endophytes en tant qu’inoculants microbiens pour promouvoir la croissance végétale et qu’agents de lutte biologique. Dans le cadre de cette étude, nous avons cherché et identifié une série d’endophytes que l’on trouve naturellement dans les principales cultures. Il a été démontré que les endophytes bénéfiques contrent les effets de plusieurs bactéries et champignons importants, particulièrement de ceux s’attaquant à la tomate (Solanum lycopersicum), y compris Rhizoctonia solani, Alternaria alternata, Clavibacter michiganensis pv. michiganensis, Ralstonia solanacearum et Xanthomonas vesicatoria. Nous avons caractérisé les plus prometteurs comme inoculants potentiels pour la tomate, visant à promouvoir la croissance végétale et la lutte contre la tache bactérienne causée par Xanthomonas vesicatoria. Parmi les endophytes sélectionnés, nous avons identifié un nouveau streptomycète taxinomiquement apparenté à Streptomyces avermitilis ainsi qu’une pseudomonade identifiée en tant que Pseudomonas granadensis. Les effets bénéfiques de ces deux bactéries, utilisées individuellement ou conjointement, se sont manifestés par une augmentation significative de la longueur des racines et des plantules (approximativement de 31% et 34% pour les streptomycètes et de 18% et 16% pour la pseudomonade) et la biomasse racinaire sèche (90% pour les streptomycètes et 70% pour la pseudomonade). De plus, les deux inoculants ont réduit la progression et la gravité de la maladie à la suite de l’inoculation avec X. vesicatoria. Il n’y a pas eu de différence notable entre les plants traités avec un inoculant unique et ceux traités avec les deux. La pénétration et la colonization efficace des tissus de la tomate par une culture de streptomycètes marquée avec une protéine verte fluorescente ont été observées par microscopie confocale, confirmant sa nature endophyte.
Introduction
Pathogenic microorganisms affecting plant health are a major and chronic threat to plant productivity, food production and ecosystem stability worldwide. As agricultural production increased over the past few decades, farmers became more and more dependent on agrochemicals inputs, as a relatively reliable tool for crop protection, therefore supporting economic stability of their operations and rural development. However, the increasing use of chemical inputs may have several negative effects on agricultural eco-systems, among them the development of pathogen resistance to the applied pesticide and impacts on non-target organisms (De Weger et al. Citation1995; Gerhardson Citation2002; Zhang et al. Citation2018). Furthermore, the growing costs of agrochemicals, particularly in less-affluent regions of the world, and consumer demand for safe and pesticide-free food has led to a search for substitutes for these products. Additionally, there is also a number of fastidious diseases, for which chemical solutions are few, ineffective, or non-existent (Gerhardson Citation2002). Therefore, biological control is being considered as a prospective alternative or supplemental way of reducing the use of chemicals in agriculture and, at the same time, ensuring food security and safety (De Weger et al. Citation1995; Gerhardson Citation2002; Postma et al. Citation2003; Welbaum et al. Citation2004).
Currently, microbial endophytic communities are the focus of several studies aimed at unravelling and clarifying their role as plant growth promoters and their involvement in supporting plant health (Vurukonda et al. Citation2018; Khan Citation2019; Verma et al. Citation2019). Several different bacterial species, colonizing plant tissues and vessels, have been identified from the root system up to the stem, leaves, and other plant organs. Most are described as producers of metabolites positively altering plant physiology, for example, by enhancing nutrient acquisition or by stimulating plant defence mechanisms towards pathogens (Brader et al. Citation2014). A number of rhizobacteria and mycorrhizal fungi proved to be very efficient in promoting plant growth and, therefore, crop productivity (Glick et al. Citation1999). Several rhizosphere bacteria are able to enhance nutrient uptake from the rhizosoil by the plants that they colonize: for this reason, they are considered efficient bio-fertilizers. In most cases, such growth-promoting rhizosphere bacteria belong to the following genera: Alcaligenes, Arthrobacter, Azospirillum, Azotobacter, Bacillus, Burkholderia, Enterobacter, Klebsiella, Pseudomonas and Serratia (Okon & Labandera-Gonzalez Citation1994; Leontidou et al. Citation2020). Streptomyces spp. belong to the rhizosphere microbial communities as well and, recently, their ability to act as plant growth promoters has been emphasized (Dias et al. Citation2017). Frequently, rhizobacteria are also found endophytically in roots and other plant tissues, thus showing their ability to penetrate and colonize their hosts. In such cases, their plant-stimulating activity does not cease, but can efficiently continue in the colonized plant tissues (Sturz and Nowak Citation2000; Viaene et al. Citation2016; Vurukonda et al. Citation2018).
Several companies develop and sell microbial inoculants in the form of a single microorganism or a consortium of microbes on the global market. Such products claim to have a role as bio-fertilizers or, in other cases, as enhancers of plant endogenous resistance to biotic stresses (Herrmann and Lesueur Citation2013; Basu et al. Citation2021). In this study, our research was aimed at checking a set of endophytes that might be used to formulate a commercial microbial consortium: therefore, we attempted to determine the functions of such microbes that benefit plant growth and/or protect the crop plant from various phytopathogens. Exploring the functions of bacterial plant endophytes will help us to understand whether a given endophyte would be beneficial to the host plant or not, and what influences the dynamics of the plant--microbiome—pathogen relationship in the soil. Furthermore, these studies will help us to find new and interesting microbes colonizing the rhizosphere, but naturally adapted to the endophytic environment. These microbes have great potential as biological control agents, thereby allowing the development and implementation of effective and innovative biological control strategies for the management of important agricultural pathogens.
Material and methods
Bacterial isolates
Root samples and rhizosphere soil from multiple important agricultural plants (olive, grapevine, kiwifruit, tomato, rice) were collected from a number of sites, located in north-western Italy, from the hills of Piedmont to the Po river valley in Emilia Romagna. Host plants that were healthy, but grown in fields severely affected by plant diseases were selected. For bacterial isolation, 2–5 g of fresh roots were washed under running tap water and, later, surface sterilized in 2% NaOCl for 1 min. After three additional washings with sterilized distilled water (SDW), the root samples were blot dried and ground with a sterilized mortar and pestle. Serial dilutions were prepared from the ground roots, and 100 μL aliquots from each dilution were spread onto King’s B medium (King et al. Citation1954), Nutrient Agar (NA) and ISP agar plates (Shirling and Gottlieb Citation1966) and, then, incubated for 2–7 days at 28 ± 2°C. Morphologically distinct bacterial colonies were selected for further purification. The purified isolates were then stored, pending their phenotypical characterization by morphological and microscopic observations (Islam et al. Citation2016).
Plant growth promoting features
Assays were carried out to check whether the isolates could be prospective plant growth promoters and/or microbial antagonists. Such tests were: Phosphorus (P) solubilization, production of siderophores, synthesis of indole-acetic acid (IAA), ammonia and hydrogen cyanide production, chitinase production.
Semi quantitative estimations of P solubilization and chitinase production were done by observing a halo around the bacterial colony growing in Pikovskaya medium (containing insoluble P) and chitin medium (containing colloidal chitin), respectively (Mehta and Nautiyal Citation2001). Production of siderophores was estimated on chrome-azurol S-agar medium by observing the development of orange colour around the bacterial colony (Schwyn and Neilands Citation1987). Quantitative estimation of IAA was assessed by observing the development of a pink colour in the culture supernatant 30 min after the addition of o-phosphoric acid (H3PO4); the absorbance values were then read at λ = 535 nm, using an UV/visible spectrophotometer. The IAA production was calculated from the regression equation of a standard curve and the results were expressed as μg mL−1 (Gordon and Weber Citation1951). Bacterial strains were tested for ammonia production in peptone broth according to Cappuccino and Sherman (Citation1992). Briefly, freshly grown cultures were inoculated in 10 mL peptone broth and incubated at 28 ± 2°C for 48–72 h; then, Nessler’s reagent was added to each tube after incubation. The development of faint yellow to dark brown colour indicated the ammonia production. For hydrogen cyanide (HCN) production, bacterial isolates were streaked onto nutrient agar medium (NA) supplemented with 0.4% of glycine. The production of cyanide was then detected by placing a piece of Whatman filter paper No.1 soaked in a solution of 0.5% picric acid in 2% sodium carbonate, placed on the underside of the Petri dish lids. The development of an orange colour after three days of incubation indicated HCN production (Bakker and Schipper Citation1987).
In vitro antagonistic activity
The antimicrobial activity of selected endophytes () was tested in vitro against a set of important phytopathogenic fungi and bacteria in dual culture on potato dextrose agar (PDA) or NA plates. Briefly, 15 µL of a suspension containing the putative antagonist at a concentration of 108 CFU mL−1, spectrophotometrically adjusted, was spot inoculated on one side of the plate (1 cm from the edge) 48 h before pathogen inoculation. Later, a fungal disc (4 mm diameter) of a five days old mycelium developed by the phytopathogenic fungi was placed at the opposite side of the same plate (1 cm from the edge).
Table 1. List of phytopathogenic bacteria and fungi used in the experiments
Plates inoculated with the fungi in the same way, but at the centre, served as a control. Plates were incubated at 27 ± 2°C for 5 days. After incubation, the diameter of the inhibition zone was measured (in mm) and colony growth inhibition (%) was calculated by using the formula: PI = (C − T)/C × 100, where PI is the percentage inhibition, C is the colony growth of the pathogen in control plates, and T is the colony growth of the pathogen in dual culture. All isolates were tested in triplicate (Shrivastava et al. Citation2017).
To test the possible antibacterial activity, a 15 µL droplet of a bacterial suspension containing the putative antagonist at a concentration of 108 CFU mL−1, spectrophotometrically adjusted, was pipetted at the centre of a Petri dish containing NA medium and then incubated for 48 hours at 27°C to obtain a macrocolony. Later, the inoculated Petri dishes were taken out of the incubator, opened inside a sterile hood and sprayed with a suspension of the selected pathogenic bacteria containing 106 CFU mL−1. The Petri dishes were then closed and placed again into the incubator at the same temperature as above for an additional two days to allow bacterial growth. The inhibition activity of the putative bacterial antagonists against the phytopathogenic bacteria was measured by calculating the average inhibition area (AIA) around the macrocolony as AIA = (R2 × 3.14) – (r2 × 3.14), where R is the diameter of the inhibition area and r is the diameter of the macrocolony. All the experiments in vitro were done in triplicate (Tontou et al. Citation2016).
Molecular identification of isolates
Based on their in vitro PGP traits and their antimicrobial activity, the most promising isolates were selected for identification. Gram grouping was assessed using the assay proposed by Wada et al. (Citation2012), with minor modifications. For Gram negative bacteria, total genomic DNA was extracted and purified according Chen and Kuo (Citation1993) and for Gram positive bacteria DNA was extracted and purified using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Amplification of the 16S rRNA gene was performed on the putative antagonists using the 16S rRNA gene universal primers 1525 R (5′- AAGGAGGTGATCCAGCC-3′) and 27 F (5′-AGAGTTTGATCCTGGCTCAG-3ʹ) (Wawrik et al. Citation2005) for strain PT65 and primers pair strepB (5′-ACAAGCCCTGGAAACGGGGT-3ʹ) strepE (5′-CACCAGGAATTCCGATCT-3ʹ) (Ramazani et al. Citation2013) for strain SA51, with target fragments of 1500 bp and 520 bp respectively. The amplification was carried out in a 25 µL volume. PCR amplifications were performed with final concentration of 1x GoTaq buffer, 0.8 μM each of the forward and reverse primers, 0.2 mM dNTP’s mix, 1.25 mM of MgCl2, 2 μL DNA template, 1.25 U Taq Polymerase, and the remaining volume added with nuclease-free water. The conditions of PCR were those of Marchesi et al. (Citation1998). The PCR products were migrated in a 1.2% agarose gel by electrophoresis at 65 V for 40 minutes and visualized under UV light. Amplicons were then purified and sequenced and the 16S rRNA genes sequences were analysed using BLAST online at http://blast.ncbi.nlm.nih.gov/Blast.cgi (Tamura et al. Citation2007).
In planta studies
Evaluation of selected isolates for growth promotion
Pot experiments were carried out using tomato (S. lycopersicum L. var. Leader F1, ISI Sementi, Italy) as a test plant under greenhouse conditions. Seeds were surface-sterilized with 0.5% NaClO, followed by 70% ethanol dips for 1 min and 5–6 times washings with sterile distilled water: after their sterilization, seeds were sown in surface-sterilized plastic pots filled with 300 g sterile peat soil. After seedling emergence, 1 mL of bacterial culture containing approximately 108 CFU mL−1 was applied to the soil around each seedling. Soil moisture was maintained during the experiment by daily sprinkling with sterile distilled water. After five consecutive treatments (a treatment every 10 days) plants were measured for root and shoot length, and dry biomass was recorded according to standard protocols (Vurukonda et al. Citation2016). Each treatment consisted of a pot of 12 plants (replicates) and experiments were repeated three times.
Antagonistic activity in planta
The two most prospective beneficial microorganisms (later identified as Streptomyces sp., strain SA51 and Pseudomonas sp., strain PT65) were tested individually and as co-inoculants against X. vesicatoria, the causal agent of the bacterial spot disease, using tomato as a host plant. Twenty-one day old tomato plantlets were transplanted into pots and the roots were inoculated with a 108 CFU mL−1 microbial suspension of each antagonist (as a single inoculant or as a combo of both) every 10 days (3 treatments). Twenty-four hours after the third treatment with the antagonists, X. vesicatoria was spray-inoculated on the canopy at a concentration of 108 CFU mL−1. Each inoculated plant was then sealed in a polythene bag (PE) overnight, which was removed early next morning. Phytopathometric measurements started with the first appearance of symptoms and were carried out weekly for three additional weeks. Disease severity on the tomato plants affected by X. vesicatoria was evaluated using a descriptive scale ranging from 0 to 4 as follows: 0 = no symptoms; 1 = 1–10 spots on 1–3 leaves; 2 = 11–30 spots on 4–10 leaves; 3 = more than 30 spots and some confluent necrosis on 5–20 leaves; 4 = confluent necrosis on more than 20 leaves or branch desiccation. Statistical analyses were done using the MaxStats software (https://maxstat.de/en/home-en/) as proposed by Giovanardi et al. (Citation2015). The plant disease index (DI) was calculated according to the following formula: DI = [∑ (Ni × i)/(N × 4)] × 100, where i means a 0–4 disease level, and Ni means the plant number of reaction i (Cao et al. Citation2020).
Fluorescence labelling of Streptomyces sp. strain SA51
Streptomyces sp., strain SA51, was chosen to examine its possible penetration into the host plant and the tissue colonization pattern. The strain was tagged with the gfp marker genes using the transformation protocols described by Ali et al. (Citation2018). Briefly, the wild-type strain SA51 was grown in 5 mL of ISP medium at 28°C, until the optical density at λ = 600 nm reached 0.6. The bacterial cells were then pelleted by centrifugation at 10 000 x g for 5 min at 4°C, resuspended in 5 mL of cold sterile water, and centrifuged again as described above. Finally, the supernatant was discarded and the bacterial pellet was washed twice with 10% glycerol and centrifuged at 5000 x g for 5 min. Transformation was performed by electroporation, using a Gene Pulser Xcell™ electroporation system (Bio-Rad, USA) in an electroporation cuvette (0.2 cm) containing 100 μL of a competent cell suspension added with 2 μL of plasmid DNA (100 ng μL−1 final concentration). The following pulse conditions were applied: 12.5 kV cm−1, 25 μF, and 200 Ω (Choi et al. Citation2006; Krzyzanowska et al. Citation2012). After transformation, 1 mL of LB broth was added, the mixture was then incubated for 1 h at 28°C, plated onto LB agar supplemented with kanamycin (50 μg mL−1) and incubated again for 48 h at 28°C. The identification and selection of clones carrying the gfp gene was carried out under UV light.
Specimen preparation for confocal microscopy
The penetration and the endophytic colonization of strain SA51 in tomato plants were studied using a confocal laser scanning microscopy (CLSM). Different specimens (root, stem, petiole and leaf) were prepared by scratching an area of the tissue’s surface, around 1 cm in length, with a sterile blade. All specimens were gently rinsed with sterile distilled water prior to dipping them into phosphate-buffered saline (PBS) for microscopic observation. Observations were done with an Axio Imager 2 system using a Carl Zeiss LSM 780 confocal microscope (Zeiss, Zaventem, Belgium). GFP fluorescence was recorded by using an excitation Argon laser of 488 nm and collecting the emission at λ = 500–600 nm; an excitation with Ne-He laser (λ = 561 nm) was also used and the emission band of 538–624 nm was collected. Images were acquired and reconstructed with the Zen 2012 Software provided by Zeiss.
Results
Characterization of bacterial isolates
Microbial isolation from selected plant species led to a collection of 42 bacterial isolates. Six isolates were chosen for further characterization, as they showed features indicative of plant growth promotion. Phenotypically, the selected bacterial isolates varied widely, as shown in . All the isolates produced round and conical shaped colonies, the elevation was either raised or convex, had smooth or undulate margins with the colour ranging from white to pink. Microscopic and agar plate observations were performed to investigate additional characteristics of the isolates, such as cell shape, morphology, etc. All isolates were motile; the Gram-negative cells were mostly rod-shaped, whereas Gram-positive bacteria were spore forming filamentous hyphae.
Table 2. Morphological and microscopic characteristics of isolates used in this study
Screening for potential PGP and biocontrol abilities
Plant growth promoting (PGP) traits of six selected isolates were extensively studied. All six isolates produced IAA, solubilized P and produced ammonia. However, significant differences in PGP traits were observed among the isolates (). Isolate SA51 produced a significant amount of IAA (25.26 ± 2.01 µg mL−1), closely followed by isolate PN53 (23.90 ± 1.34 µg mL−1) and isolate PT65 (22.78 ± 1.18 µg mL−1). The P solubilizing activity was highest for isolate AR39 (271), followed by isolate PT65 (233) and isolate SA51 (209). Hydrogen cyanide production was observed in only one isolate (PT65) and siderophore production was observed in three isolates: SA51, PT65 and PN53 ().
Table 3. Plant Growth Promoting (PGP) traits observed among the bacterial isolates
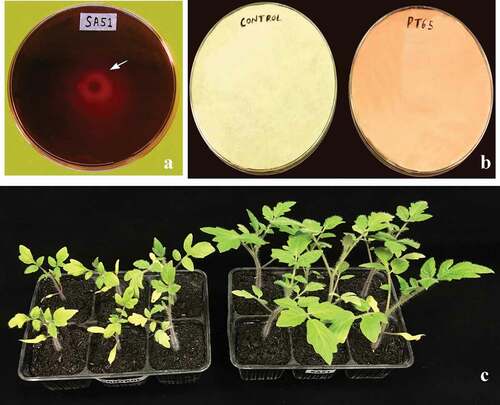
Fig. 1 Plant growth promoting traits: A, Siderophore production by strain SA51 orange halo (arrow) indicates positive reaction, B, HCN production by strain PT65, C, In planta plant growth promotion by strain SA51 inoculated on tomato plantlets compared with control plantlets, inoculated plantlets developed broader leaves.
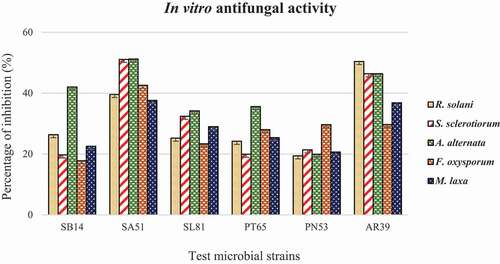
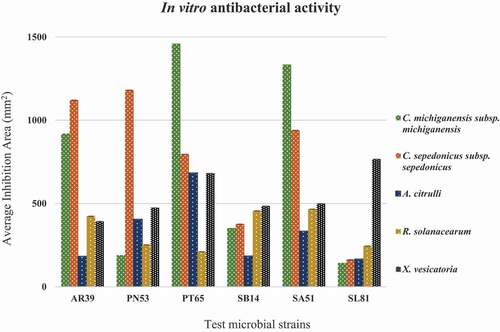
The biocontrol activity of the selected isolates against phytopathogens was checked in vitro by measuring the area of the inhibition halo for bacteria and the inhibition of mycelial growth for fungi (). All isolates were able to inhibit the growth of phytopathogens significantly. The antibacterial activity was more pronounced in isolates PT65 and SA51: in particular, the clavibacters were highly sensitive to both antagonists, whereas the phytopathogens R. solanacearum and A. citrulli showed a more resilient behaviour, towards most antagonists. The antagonistic isolate SL81 showed the best inhibition potential against X. vesicatoria, whereas its performance against other phytopathogenic bacteria was reduced when compared with other antagonists (). A pronounced antifungal activity was assessed for any of the six isolates tested: all bacterial isolates reduced the mycelial growth in the range of 20–50%, according to the bacterialfungus combination (). Isolates SA51 and AR39 were the most active against fungi, in particular against R. solani, A. alternata and S. sclerotiorum, where mycelial growth was consistently inhibited by 45–55%. Alternaria alternata was the fungus showing the highest sensitivity to almost all antagonists, whereas F. oxysporum f. sp. lycopersici and M. laxa showed the lowest percentage of inhibition. Strain SA51 was particularly active against both bacterial and fungal pathogens, giving an inhibition halo ranging from 334 mm2 against A. citrulli, up to 1334 mm2 against C. michiganensis pv. michiganensis, whereas most fungi showed an inhibition of their mycelial growth by almost 50%.
Fig. 2 Antagonistic property of SA51: A – Antimicrobial activity against Xanthomonas vesicatoria halozone (arrow) indicates zone of inhibition, B, Antifungal activity against Fusarium oxysporum f. sp. lycopersici.
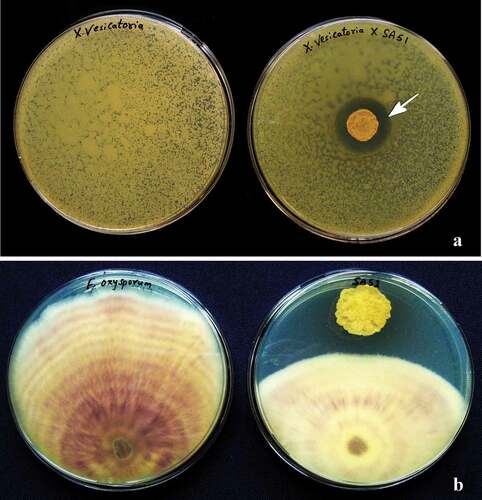
Fig. 3 Antibacterial activity of test isolates against different phytopathogens. Error bars: Mean of ± SD (n = 3).
Plant growth promotion
Considering their stimulating behaviour and their antagonistic activity, isolates SA51 and PT65 were chosen to test their activity in planta, as a single inoculant and as a combination of both. Their possible plant growth promoting activity was studied under greenhouse conditions. Inoculation of tomato seedlings improved shoot formation, root length, and dry biomass of plants, with respect to non-inoculated plants that showed lower values (): this was observed using both isolates as single inoculants and in combination. Root length reached around 18–20 cm in the treated plants compared with approx. 15 cm in the controls. A similar behaviour was observed in shoot development: shoot length reached around 37–43 cm in treated plants, compared with approx. 32 cm in the control plants. Again, root biomass was consistent with the increased development of the respective aerial parts: indeed, inoculated plants developed an average dry root biomass of 1.05–1.16 g per plant, compared with an average dry biomass of 0.61 per non-bacterial inoculated plant. Therefore, strain SA51 as a single inoculant increased root and shoot lengths up to 31% and 34%, respectively, and dry biomass increased by 90%. Strain PT65 increased root and shoot length by 18 and 16%, respectively; additionally, an increase in dry biomass by 70% was also observed. The combined inoculation of both isolates did not produce any better growth of tomato plants; conversely, the PGP activity due to combined inoculant was slightly lower than that of SA51 used as a single inoculant.
Table 4. Plant Growth Promoting (PGP) traits observed in tomato plantlets inoculated with two beneficial bacteria, as single isolate or in combination
Disease control
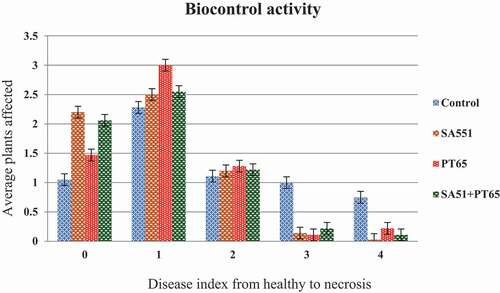
In greenhouse experiments, disease incidence and severity were monitored on tomato plants up to five weeks after challenge inoculation; disease quantity was assessed according the incidence of symptomatic plants and the severity of symptoms observed. Two weeks after inoculation of X. vesicatoria, the development of bacterial spots on leaves was observed in both control and treated plants, together with the typical chlorosis that emerged and spread from older leaves to younger ones. As time progressed, disease quantity increased more rapidly in the control plants compared with treated ones. At the end of phytopathometric evaluations, the number of healthy plants (disease index = 0) was significantly higher in treated replicates than in the control ones; in particular, plants treated with strain SA51 showed the highest number of healthy plants. Also, plants showing a disease index = 1 were more numerous in treated replicates than in control ones: in disease class 1, plants treated with strain PT65 were the most numerous. The most severe disease (disease index = 4) occurred much more frequently among untreated plants. Plants treated with strain SA51 never reached a disease index = 4. The use of both inoculants in combination did not show a higher antagonistic effect against X. vesicatoria, although healthy plants were more numerous than in replicates treated with strain PT65. In all other disease index classes there was no increased protection, when compared to replicates treated with single inoculants.
Endophytic colonization of tomato plants by strain SA51
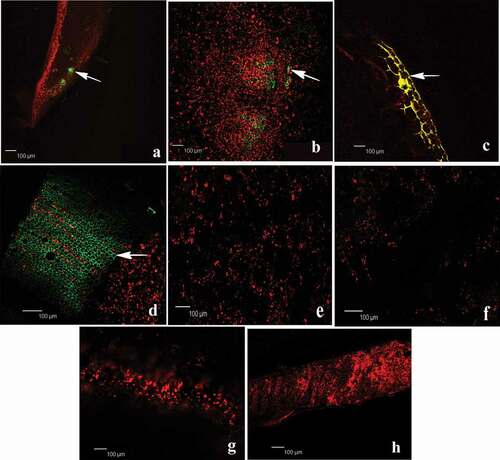
Electroporation enabled us to introduce the gfp gene into the isolate SA51 and to obtain a constitutive GFP expression by the modified bacterial cells. The visualization of the tissue colonization pattern in tomato plant by the GFP-labelled Streptomyces sp. SA51 was done 15 days after its inoculation: different plant parts were prepared on slides and were viewed by CLSM. As shown in , the fluorescence of strain SA51 indicated the presence of high numbers of cells inside root tissues, compared with non-inoculated roots, thus confirming the successful penetration and endophytic colonization. The entire colonization process by an EGFP-tagged SA51 derivative was recorded, from the very earliest stages (bacteria adhesion to roots) to the colonization of the intercellular spaces of stem, leaves and petioles within the cortex of the differentiation zone. The tomato root was rapidly colonized by EGFP-tagged SA51 cells, which were first attached to the plant surface and, later, profusely colonized the apoplast within 15 days. SA51 cells were attached along the whole plant internal parts, mostly at random distribution. clearly shows the abundant colonization of strain SA51 along different tissues, especially in the root and stem, where the internalization of bacteria took place. Plant colonization by tagged strain SA51 clearly proceeded upwards, although the number of fluorescing cells decreased in aerial parts, like petioles and leaves.
Fig. 6 Endophytic localization of gfp gene expression in tomato roots after seedling inoculation with Streptomyces-gfp SA51 strain. Fluorescence microscope images of different parts of tomato plants growing under greenhouse conditions showing green fluorescence due to colonization of Streptomyces-gfp. The photograph shows internal tissue of root, stem, petiole and leaf respectively from left to right. Top line SA51 strain (a-d) arrow indicates colonization of bacteria inside tissues and bottom-line control (un-inoculated) (e-f) of tomato seedlings. Bar represent the scale of measurement.
Identification and characterization of strains PT65 and SA51
The most interesting isolates PT65 and SA51, selected based on their in vitro PGP traits and biocontrol activity, were morphologically characterized and taxonomically identified. Microscopic studies revealed that strain PT65 is a Gram negative and rod-shaped bacterium, motile by one polar flagellum. On King’s B, isolate PT65 developed creamy, smooth, shiny, circular, convex colonies, with bluish pigmentation under ultra-violet light. Strain SA51 is a Gram positive bacterium and produced colonies on ISP medium that appear antique pink in colour, round and cottony. Based on their 16S rRNA gene sequence and Blast analysis, strain PT65 was identified as a pseudomonad closely related to the species Pseudomonas granadensis (P. fluorescens lineage), with sequence homology of 99%; strain SA51 was identified as Streptomyces sp., showing a sequence homology of 96% with P. avermitilis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Interestingly, strain SA51 did not completely fit in one specific taxonomic group, but its identity confirmed its relatedness with the species S. avermitilis. Further studies are under way to assign strain SA51 to a new species of the genus Streptomyces (Vurukonda et al. Citation2020).
Discussion
Plant–microbe interactions are critical to the integrity, function, and long-term sustainability of agro-ecosystems (Nannipieri et al. Citation2003). Plant-associated, beneficial bacterial communities sustain many important ecosystem processes, such as nutrient uptake, decomposition of organic matter and waste, nutrient availability, degradation of pesticides, stability of soil structure, and plant growth and health. An understanding of the plant-associated bacterial community is important, considering their potential significance in plant growth promotion, protection against biotic and abiotic stresses, source of novel bio-molecules, and agents in bioremediation and determinants of soil and environmental health (Nelson Citation2004; Sessitsch et al. Citation2004; Lugtenberg and Kamilova Citation2009).
In ths study, we observed that several microbes, naturally present in different crop plants as endophytes, such as strains AR39, PT65, PN53, SB14, SA51 and SL81, were able to produce IAA and siderophores, solubilize phosphate and produce HCN. Siderophores produced by bacteria can promote plant growth through enhanced direct iron availability to plants under iron deficiency or by inhibiting the availability of iron to plant pathogens (Ahmad et al. Citation2008). In addition, phosphorus is one of the major macronutrients necessary for plant growth and development; therefore, phosphate-competent bacteria are capable of solubilizing the insoluble phosphates present in soil, thus increasing soil quality and enhancing plant growth (Rodriguez and Fraga Citation1999). It is well understood that such properties have a crucial role in plant growth promotion, and some researchers have previously reported these capabilities in other strains belonging to the same genus identified in this work. Vurukonda et al. (Citation2016) reported P. putida strains with plant growth promotion and their activity was associated with the production of IAA, siderophores and the solubilization of phosphate. Pseudomonas putida AKMP-7, showing the ability for IAA and siderophore production, significantly increased the root weight and enhanced the growth of wheat plants under stress condition (Ali et al. 2011). Recent reports state that endophytic bacteria have similar effects on maize (Sandhya et al. Citation2017). Among the tested isolates, only strain PT65 was able to produce HCN; this feature might be present in deleterious bacteria able to reduce plant yields through HCN production (Bakker and Schipper Citation1987). However, HCN production is also reported to be a biological control mechanism used by several microbial agents against various phytopathogens (Kumar et al. Citation2012). Beneficial plant growth promoting bacteria (PGPB) produce such secondary metabolites that are recognized as biological control agents against fungal and bacterial plant pathogens. In addition, HCN can indirectly increase availability of phosphorus and iron to plants, resulting in increased plant growth (Rijavec and Lapanje Citation2016). Our results show that different bacterial species may exert different levels of antagonism, and differing degrees of antagonism may occur inside the same species. Indeed, strains SB14, SL81 and SA51 described in this study belong to the same Streptomyces group (data not published).
PGPB have been applied to a wide range of agricultural plant species for the purpose of growth enhancement, including increased rate of seed germination, crop yields, stress tolerance and disease control (Kloepper et al. Citation1980; Gururani et al. Citation2013). For example, yield increases between 10% and 20% with bio-fortification: this has been well documented for several agricultural crops (Dimkpa et al. Citation2008, Citation2009). To determine the plant growth promotion by bacteria, bacterial suspensions of SA51 and PT65 were applied to tomato seedlings as root inoculants. Four weeks after regular inoculations, the most common plant growth parameters, such as root and shoot length, and root dry weight, showed a significant increase. These results indicate that both isolates, particularly SA51, may be used to enhance and support plant growth of tomato plants. The combined use of both inoculants did not produce better growth promotion than that ascribed to a single inoculant: this indicates that a single microorganism is able to prime plant growth at the seedling stage, and the addition of other microbial stimulants did not stimulate the host plant any further. This fact is also true when using two inoculants belonging to very distant taxa (SA51 is a streptomycete and PT65 is a pseudomonad) where, supposedly, they may have a different behaviour inside their host plant and may produce very different stimuli.
Plant growth physiological parameters varied according to individual bacterial inoculation, and the effect was observed to be positive. It was clear that bacterial inoculation has a significant effect on plant size in tomato. Huang et al. (Citation2017) proposed that this parameter alone is not enough to assess the PGPB effects, although other studies considered the increase in plant height as a marker of the effect of PGPB (Kang et al. Citation2007; Muthukumar et al. Citation2010; Xia et al. Citation2015). In our study, the root and shoot length along with root dry biomass in bacterized plants were observed to increase by 24–26% and 50%, respectively. Similarly, Xia et al. (Citation2015) stated that different endophytic bacteria increased growth parameters by 25% in tomato. Such significant increase may be considered the result of changes in the plant nutrition and hormonal balances. This situation leads to the conclusion that the ACC deaminase enzyme produced by PGPB may prevent harm by disintegrating the ACC that is the precursor of ethylene (Glick Citation2014). However, we did not characterize the isolates for ACC deaminase activity. Ribaudo et al. (Citation2016) explained that the effects of endophytic bacteria on tomato growth may be due to the IAA they produce. Similarly, Khan et al. (Citation2012) showed that endophytic bacteria that produce IAA have an additional advantage in terms of nitrogen fixation ability, so to increase the growth, flowering and yield of many plants, including tomato. In most of the findings, the endophytic Pseudomonas spp. (Muthukumar et al. Citation2010; Qessaoui et al. Citation2019), Bacillus spp. and Serratia spp. (Amaresan et al. Citation2014) were able to improve root and shoot growth via secondary metabolites production like IAA, siderophore and inorganic phosphate solvent enzymes, etc. These findings were very similar to our results, since we confirmed that strains SA51 and PT65 were successful in production of IAA, siderophore and phosphate solubilization. Therefore, we consider that the increase observed in plant development parameters and tolerance may be due to these metabolites, thus confirming recent results by Ahmet et al. (Citation2018). The identification of strain SA51 as a member of the S. avermitilis group may suggest that our streptomycete has the capability of producing the avermectin, which is known to act as an antimicrobial and an insecticide (Cheng et al. Citation2018; Choi et al. Citation2018; Rath et al. Citation2019). Likewise, Riera et al. (Citation2017) found that P. granadensis exerts its beneficial effect on plants by expressing PGP traits, like P-solubilization and siderophore production.
Antagonism of endophytic bacteria in vitro by a dual culture test is used extensively as an in vitro test for a preliminary screening, when searching for biological control agents (Desai et al. Citation2002; Kim et al. Citation2008). In our study, the six best performing isolates were tested in the same way and confirmed their antagonistic behaviour against a number of important phytopathogenic fungi and bacteria. Two taxonomically distant endophytes selected in this study (the streptomycete SA51 and the pseudomonad PT65) were used to protect tomato plants from Xanthomonas vesicatoria, the causal agent of the tomato spot disease. As strains SA51 and PT65 significantly inhibited the growth of X. vesicatoria in vitro, thus indicating that some antibacterial molecule is produced and released in the medium, the same antibacterial substance may be produced in colonized plant tissue, thus explaining disease reduction in tomato. These data support other similar results (Linares et al. Citation2006; Montesinos Citation2007). Plant defence priming is also an event initiated and supported by beneficial endophytes (Pieterse et al. Citation2014). Therefore, we cannot exclude that both our inoculants, once penetrated into the tomato plants, exerted positive effects in the interplay between microbes and the host plant. As already observed in case of the increased growth promotion, both antagonists inoculated in combination did not result in a significantly better protection from the disease. As such, we may again assume that just a single inoculant is sufficient to mobilize any targeted biochemical pathway available to induce plant protection.
Many researchers investigated the efficacy of different endophytes on a variety of pathosystems and obtained neutral or different levels of positive effects (Kang et al. Citation2007; Romero et al. Citation2016). Similarly, Streptomyces and Pseudomonas species were seen to have different levels of antagonism on bacterial spot disease and plant growth in tomato (Naue et al. Citation2014) and pepper plants (Mirik et al. Citation2008). Focussing on tomato as a host plant, other pathogenic bacteria were also controlled using the same approach, as in the case of P. syringae pv. tomato, the causal agent of the bacterial speck (Romero et al. Citation2016).
Three endophytes, strains SA51, PT65 and AR39, showed the strongest antagonism in vitro and strain SA51 was chosen to produce fluorescing GFP mutants to study its penetration and its colonization pattern inside the tomato plant. Indeed, endophytic colonization is a requirement to ensure an intimate association with the plant, thus supporting efficacy in promoting plant growth (Marasco et al. Citation2012). Establishment of internal colonization by endophytes is dependent on the surface characteristics of the bacteria and plant polymer degrading enzymes. Therefore, the production of cellulases, chitinases, proteases and pectinases play a significant role (Compant et al. Citation2005; Carro and Menendez Citation2020). To evaluate the ability of Streptomyces sp. strain SA51 to colonize internal parts of the tomato plants, endophytic colonization studies were performed. The results showed that plants were rapidly, efficiently and abundantly colonized by GFP-labelled Streptomyces sp. strain SA51, even after 15 days of inoculation, and showed profuse colonization in roots, collar and aerial parts of tomato plants. This indicates that strain SA51 not only penetrated the tomato plants, but also abundantly multiplied in its tissues, from roots to leaves. Finally, this popular genetic manipulation technique confirmed its robustness to study the plant-Streptomyces interactions related to plant tissue colonization, as it has been used to monitor epi- and/or endophytic colonization by other beneficial bacteria inside different host plants (Gamalero et al. Citation2004; Chi et al. Citation2005; Götz et al. Citation2006).
In conclusion, in this study, we isolated and studied a group of endophytes that are culturable, non-pathogenic, plant specific, and possess multiple PGP traits; therefore, due to their nature, they might be regarded as promising biocontrol and plant growth-promoting agents. The selected isolates are currently being used to develop bacterial formulations as single inoculants since, according our results, combinations of more microbes in the same product deserve additional studies to be proposed as prospective co-inoculants of beneficial bacteria. Finally, we demonstrated an efficient endophytic colonization of tomato plants by the most promising strain (SA51). This may lead to a possible intimate and long-lasting plant-microbe interaction resulting in an increased productivity.
Acknowledgements
The authors are thankful to CCS Aosta Srl, Quart (AO) for the financial support of a Ph.D. grant devoted to study the role and use of Actinobacteria in agricultural systems (symbiotic agriculture). The authors kindly acknowledge the EU COST Action 16107: ‘Integrating science on Xanthomonadaceae for integrated plant disease management in Europe’ (www.EuroXanth.eu) for providing an international network of scientists devoted to increase knowledge and use of endophytes in plant production.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmad F, Ahmad I, Khan MS. 2008. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 163:173–181. doi:https://doi.org/10.1016/j.micres.2006.04.001.
- Ahmet A, Kamuran C, Husseini A. 2018. Effects of endophytic bacteria on disease and growth in plants under biotic stress. Yyu J Agr Sci. 28(2):200–208.
- Ali SKZ, Sandhya V, Vurukonda SSP. 2018. Transcriptomic profiling of maize (Zea mays L.) seedlings in response to Pseudomonas putida stain FBKV-2 inoculation under drought stress. Ann Microbiol. 68(6):331–349.
- Amaresan N, Jayakumar V, Thajuddin N. 2014. Isolation and characterization of endophytic bacteria associated with chilli (Capsicum annuum) grown in coastal agricultural ecosystem. Indian J Biotechnol. 13:247–255.
- Bakker AW, Schipper B. 1987. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas sp. mediated plant growth stimulation. Soil Biol Biochem. 19:451–457. doi:https://doi.org/10.1016/0038-0717(87)90037-X.
- Basu A, Prasad P, Das SN, Kalam S, Sayyed RZ, Reddy MS, El Enshasy H. 2021. Plant Growth Promoting Rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability. 13(3):1140. doi:https://doi.org/10.3390/su13031140.
- Brader G, Compant S, Mitter B, Trognitz F, Sessitsch A. 2014. Metabolic potential of endophytic bacteria. Curr Opin Biotechnol. 27:30–37. doi:https://doi.org/10.1016/j.copbio.2013.09.012.
- Cao P, Li C, Wang H, Yu Z, Xu X, Wang X, Zhao J, Xiang W. 2020. Community structures and antifungal activity of root-associated endophytic actinobacteria in healthy and diseased cucumber plants and Streptomyces sp. HAAG3-15 as a promising biocontrol agent. Microorganisms. 8(2):236. doi:https://doi.org/10.3390/microorganisms8020236.
- Cappuccino JC, Sherman N. 1992. Negative staining. In: Cappuccino JC, Sherman N, editors. Microbiology: a laboratory manual. Redwood City: Benjamin/Cummings; p. 125–179.
- Carro L, Menendez E. 2020. Knock, knock-let the bacteria in: enzymatic potential of plant associated bacteria. In: Sharma V, Salwan R, LK TA-A, editors. Molecular aspects of plant beneficial microbes in agriculture. London, UK: Elsevier Inc; p. 169–178.
- Chen WP, Kuo TT. 1993. A simple and rapid method for the preparation of Gram- negative bacteria genomic DNA. Nucleic Acids Res. 21(9):2260. doi:https://doi.org/10.1093/nar/21.9.2260.
- Cheng Y, Yang R, Lyu M, Wang S, Liu X, Wen Y, Song Y, Li J, Chen Z. 2018. IdeR, a DtxR-family iron-response regulator, controls iron homeostasis, morphological differentiation, secondary metabolism, and oxidative stress response in Streptomyces avermitilis. Appl Environ Microbiol AEM. 01503:18.
- Chi F, Shen SH, Cheng HP, Jing YX, Yanni YG, Dazzo FB. 2005. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl Environ Microbiol. 71:7271–7278. doi:https://doi.org/10.1128/AEM.71.11.7271-7278.2005.
- Choi HY, Van Minh N, Choi JM, Hwang JY, Seo ST, Lee SK, Kim WG. 2018. Enzymatic synthesis of avermectin B1a glycosides for the effective prevention of the pine wood nematode Bursaphelenchus xylophilus. Appl Microbiol Biotechnol. 102(5):2155–2165. doi:https://doi.org/10.1007/s00253-018-8764-4.
- Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods. 64:391–397. doi:https://doi.org/10.1016/j.mimet.2005.06.001.
- Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Barka EA. 2005. Endophytic colonization of Vitis vinifera L. by a plant growth-promoting bacterium, Burkholderia sp. strain PsJN. Appl Environ Microbiol. 71(4):1685–1693. doi:https://doi.org/10.1128/AEM.71.4.1685-1693.2005.
- De Weger LA, van der Bij AJ, Dekkers LC, Simons M, Wijffelman CA, Lugtenberg BJJ. 1995. Colonization of the rhizosphere of crop plants by plant-beneficial pseudomonads. FEMS Microbiol Ecol. 17(4):221–228. doi:https://doi.org/10.1111/j.1574-6941.1995.tb00146.x.
- Desai S, Reddy MS, Kloepper JW. 2002. Comprehensive testing of biocontrol agents. In: Gnanamanickam SS, editor. Biological control of crop diseases. NY (Basel (USA)): Marcel Dekker, Inc; p. 387–420.
- Dias MP, Bastos MS, Xavier VB, Cassel E, Astarita LV, Santarém ER. 2017. Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol Biochem. 118:479–493. doi:https://doi.org/10.1016/j.plaphy.2017.07.017.
- Dimkpa C, Merten D, Svatos A, Buchel G, Kothe E. 2009. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol. 107(5):1687–1696. doi:https://doi.org/10.1111/j.1365-2672.2009.04355.x.
- Dimkpa C, Svatos A, Merten D, Buchel G, Kothe E. 2008. Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can J Microbiol. 54(3):163–172. doi:https://doi.org/10.1139/W07-130.
- Gamalero E, Lingua G, Giusy Capra FG, Fusconi A, Berta G, Lemanceau P. 2004. Colonization pattern of primary tomato roots by Pseudomonas fluorescens A6RI characterized by dilution plating, flow cytometry, fluorescence, confocal and scanning electron microscopy. FEMS Microbiol Ecol. 48(1):79–87. doi:https://doi.org/10.1016/j.femsec.2003.12.012.
- Gerhardson B. 2002. Biological substitutes for pesticides. Trends Biotechnol. 20(8):338–343. doi:https://doi.org/10.1016/S0167-7799(02)02021-8.
- Giovanardi D, Biondi E, Ignjatov M, Gašić K, Ferrari M, Perez S, Jevtić R, Stefani E. 2015. Seed transmission of Xanthomonas vesicatoria and Clavibacter michiganensis subsp. michiganensis in tomato and Xanthomonas euvesicatoria in pepper and implementation of seed disinfection methods. In: Marčić D, Glavendekić M, Nicot P, editors. Proceedings of the 7th Congress on Plant Protection “Integrated Plant Protection – a Knowledge-Based Step Towards Sustainable Agriculture, Forestry and Landscape Architecture”; Nov 24–28, 2014; Zlatibor, Serbia. p. 65–70.
- Glick BR. 2014. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 169(1):30–9. doi:https://doi.org/10.1016/j.micres.2013.09.009.
- Glick BR, Patten CL, Holguin G, Penrose DM. 1999. Biochemical and genetic mechanisms used by plant growth promoting bacteria. London (UK): Imperial College Press; p. 1–13.
- Gordon SA, Weber RP. 1951. Colorimetric estimation of indoleacetic acid. Plant Physiol. 26(1):192–195. doi:https://doi.org/10.1104/pp.26.1.192.
- Götz M, Gomes NCM, Dratwinski A, Costa R, Berg G, Peixoto R, Mendonça-Hagler L, Smalla K. 2006. Survival of gfp-tagged bacteria in the rhizosphere of tomato plants and their effects on the indigenous bacterial community. FEMS Microbiol Ecol. 56:207–218. doi:https://doi.org/10.1111/j.1574-6941.2006.00093.x.
- Gururani MA, Upadhyaya CP, Baskar V, Venkatesh J, Nookaraju A, Park SW. 2013. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul. 32(2):245–258. doi:https://doi.org/10.1007/s00344-012-9292-6.
- Herrmann L, Lesueur D. 2013. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol. 97(20):8859–8873. doi:https://doi.org/10.1007/s00253-013-5228-8.
- Huang P, De-bashan L, Crocker T, JW K, Bashan Y. 2017. Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting (rhizo) bacteria on growth promotion of crop plants. Biol Fertil Soils. 53(2):199–208. doi:https://doi.org/10.1007/s00374-016-1160-2.
- Islam S, Akanda AM, Prova A, Islam MT, Hossain MM. 2016. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 6:1360.
- Kang SH, Cho H, Cheong H, Ryu C, Kim JF, Park S. 2007. Two bacterial entdophytes eliciting both plant growth promotion and plant defense on pepper (Capsicum annuum L.). J Microbiol Biotechnol. 17(1):96–103.
- Khan AAH. 2019. Plant-bacterial association and their role as growth promoters and biocontrol agents. In: Sayyed R, editor Plant growth promoting rhizobacteria for sustainable stress management. Microorganisms for Sustainability, vol 13. Singapore: Springer; p. 389–419.
- Khan Z, Guelich G, Phan H, Redman R, Doty S. 2012. Bacterial and yeast endophytes from poplar and willow promote growth in crop plants and grasses. ISRN Agron. 2012:890280. doi:https://doi.org/10.5402/2012/890280.
- Kim WG, Weon HY, Lee SY. 2008. In vitro antagonistic effects of Bacilli isolates against four soilborne plant pathogenic fungi. Plant Pathol J. 24(1):52–57. doi:https://doi.org/10.5423/PPJ.2008.24.1.052.
- King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 44:301–307.
- Kloepper JW, Leong J, Teintze M, Schroth MN. 1980. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature. 286(5776):885–886. doi:https://doi.org/10.1038/286885a0.
- Krzyzanowska D, Obuchowski M, Mariusz M, Bikowski M, Rychlowski M, Jafra S. 2012. Colonization of potato rhizosphere by GFP-tagged Bacillus subtilis MB73/2, Pseudomonas sp. P482 and Ochrobactrum sp. A44 shown on large sections of roots using enrichment sample preparation and confocal laser scanning microscopy. Sensors. 12:17608–17619. doi:https://doi.org/10.3390/s121217608.
- Kumar P, Dubey RC, Maheshwari DK. 2012. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res. 167(8):493–499. doi:https://doi.org/10.1016/j.micres.2012.05.002.
- Leontidou K, Genitsaris S, Papadopoulou A, Kamou N, Bosmali I, Matsi T, Madesis P, Vokou D, Karamanoli K, Mellidou I. 2020. Plant growth promoting rhizobacteria isolated from halophytes and drought-tolerant plants: genomic characterisation and exploration of phyto-beneficial traits. Sci Rep. 10(1):14857. doi:https://doi.org/10.1038/s41598-020-71652-0.
- Linares JF, Gustafsson I, Baquero F, Martinez JL. 2006. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci USA. 103(51):19484–19489. doi:https://doi.org/10.1073/pnas.0608949103.
- Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 63(1):541–556. doi:https://doi.org/10.1146/annurev.micro.62.081307.162918.
- Marasco R, Rolli E, Ettoumi B, Vigani G, Mapelli F, Borin S, Abou-Hadid AF, El-Behairy UA, Sorlini C, Cherif A, et al. 2012. A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS One. 7(10):e48479. doi:https://doi.org/10.1371/journal.pone.0048479.
- Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Dymock D, Wade WG. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 64(2):795–9. doi:https://doi.org/10.1128/AEM.64.2.795-799.1998.
- Mehta S, Nautiyal CS. 2001. An efficient method for qualitative screening of phosphate solubilizing bacteria. Curr Microbiol. 43:51–56. doi:https://doi.org/10.1007/s002840010259.
- Mirik M, Aysan Y, Cinar O. 2008. Biological control of bacterial spot disease of pepper with Bacillus strains. Turk J Agric. 32:381–390.
- Montesinos E. 2007. Antimicrobial peptides and plant disease control. FEMS Microbiol Lett. 270(1):1–11. doi:https://doi.org/10.1111/j.1574-6968.2007.00683.x.
- Muthukumar A, Nakkeeran S, Eswarana SG. 2010. In vitro efficacy of bacterial endophytes against the chilli damping-off pathogen Pythium aphanidermatum. Phytopath Medit. 49:179–186.
- Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G. 2003. Microbial diversity and soil functions. Eur J Soil Sci. 54(4):655–670. doi:https://doi.org/10.1046/j.1351-0754.2003.0556.x.
- Naue CR, Rocha DJA, Moura AB. 2014. Biological control of tomato bacterial spot by seed microbiolization. Tropical Plant Pathol. 39: 413–416.
- Nelson LM. 2004. Plant growth promoting rhizobacteria (PGPR): prospects for new inoculants. Online. Crop Manag. 3:1–7. doi:https://doi.org/10.1094/CM-2004-0301-05-RV.
- Okon Y, Labandera-González C. 1994. Agronomic applications of azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol Biochem. 26(12):1591–1601. doi:https://doi.org/10.1016/0038-0717(94)90311-5.
- Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA. 2014. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 52(1):347–375. doi:https://doi.org/10.1146/annurev-phyto-082712-102340.
- Postma JM, Montanari PHJF, Ph VDB. 2003. Microbial enrichment to enhance the disease suppressive activity of compost. Eur J Soil Biol. 39:157–163. doi:https://doi.org/10.1016/S1164-5563(03)00031-1.
- Qessaoui R, Bouharroud R, Furze JN, El Aalaoui M, Akroud H, Amarraque A, Vaerenbergh JV, Tahzima R, Mayad EH, Chebli B, et al. 2019. Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Sci Rep. 9(1):12832. doi:https://doi.org/10.1038/s41598-019-49216-8.
- Ramazani A, Moradi S, Sorouri R, Javani S, Garshasbi M. 2013. Screening for antibacterial activity of Streptomyces species isolated from Zanjan province, Iran. Int J Pharm Chem Biol Sci. 3(2):342–349.
- Rath S, Fostier AH, Pereira LA, Dioniso AC, de Oliveira Ferreira F, Doretto KM, Maniero L, Viera A, de Oliveira Neto OF, Dal Bosco SM. 2019. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils. Chemosphere. 214:111–122. doi:https://doi.org/10.1016/j.chemosphere.2018.09.083.
- Ribaudo CM, Riva DS, Gori JI, Zaballa JI, Molina C. 2016. Identification of endophytic bacteria and their characterization as biocontrol agents against tomato southern blight disease. J Appl Microbiol. 2:1000123. doi:https://doi.org/10.4172/2471-9315.1000123.
- Riera N, Handique U, Zhang Y, Dewdney MM, Wang N. 2017. Characterization of antimicrobial-producing beneficial bacteria isolated from Huanglongbing escape citrus trees. Front Microbiol. 8:2415. doi:https://doi.org/10.3389/fmicb.2017.02415.
- Rijavec T, Lapanje A. 2016. Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front Microbiol. 7:1785. doi:https://doi.org/10.3389/fmicb.2016.01785.
- Rodriguez H, Fraga R. 1999. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 17:319–339. doi:https://doi.org/10.1016/S0734-9750(99)00014-2.
- Romero FM, Marina M, Pieckenstain FL. 2016. Novel components of leaf bacterial communities of field grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res Microbiol. 167(3):222–233. doi:https://doi.org/10.1016/j.resmic.2015.11.001.
- Sandhya V, SkZ A, Sai Shiva Krishna Prasad V, Manjari S. 2017. Plant growth promoting endophytes and their interaction with plants to alleviate abiotic stress. Curr Biotechnol. 6:252–263.
- Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 160(1):47–56. doi:https://doi.org/10.1016/0003-2697(87)90612-9.
- Sessitsch A, Reiter B, Berg G. 2004. Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Can J Microbiol. 50(4):239–249. doi:https://doi.org/10.1139/w03-118.
- Shirling EB, Gottlieb D. 1966. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 16(3):313–340. doi:https://doi.org/10.1099/00207713-16-3-313.
- Shrivastava P, Kumar R, Yandigeri MS. 2017. In vitro biocontrol activity of halotolerant Streptomyces aureofaciens K20: a potent antagonist against Macrophomina phaseolina (Tassi) Goid. Saudi J Biol Sci. 24(1):192–199. doi:https://doi.org/10.1016/j.sjbs.2015.12.004.
- Sturz AV, Nowak J. 2000. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl Soil Ecol. 15(2):183–190. doi:https://doi.org/10.1016/S0929-1393(00)00094-9.
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular Evolutionary Genetics Analysis (MEGA) Software version 4.0. Mol Biol Evol. 24(8):1596–1599. doi:https://doi.org/10.1093/molbev/msm092.
- Tontou R, Giovanardi D, Ferrari M, Stefani E. 2016. Isolation of bacterial endophytes from Actinidia chinensis and preliminary studies on their possible use as antagonists against Pseudomonas syringae pv. actinidiae. J Berry Res. 6(4):395–406. doi:https://doi.org/10.3233/JBR-160118.
- Verma PP, Shelake RM, Das S, Sharma P, Kim JY. 2019. Plant Growth-Promoting Rhizobacteria (PGPR) and Fungi (PGPF): potential biological control agents of diseases and pests. In: Singh D, Gupta V, Prabha R, editors. Microbial interventions in agriculture and environment. Singapore: Springer; p. 281–311.
- Viaene T, Langendries S, Beirinckx S, Maes M, Goormachtig S, Muyzer G. 2016. Streptomyces as a plant’s best friend? FEMS Microbiol Ecol. 92(8):fiw119. doi:https://doi.org/10.1093/femsec/fiw119.
- Vurukonda SSKP, Giovanardi D, Stefani E. 2018. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int J Mol Sci. 19(4):E952. doi:https://doi.org/10.3390/ijms19040952.
- Vurukonda SSKP, Mandrioli M, D’Apice G, Stefani E. 2020. Draft genome sequence of plant growth-promoting Streptomyces sp. strain SA51 isolated from olive trees. Microbiol Resour Announce. 9(1):e00768–19.
- Vurukonda SSKP, Vardharajula S, Shrivastava M, Shaik Zulfikar A. 2016. Multifunctional Pseudomonas putida strain FBKV2 from arid rhizosphere soil and its growth promotional effects on maize under drought stress. Rhizosphere. 1:4–13. doi:https://doi.org/10.1016/j.rhisph.2016.07.005.
- Wada A, Kono M, Kawauchi S, Takagi Y, Morikawa T, Funakoshi K. 2012. Rapid discrimination of Gram-positive and Gram-negative bacteria in liquid samples by using NaOH-sodium dodecyl sulfate solution and flow cytometry. PloS One. 7(10):e47093. doi:https://doi.org/10.1371/journal.pone.0047093.
- Wawrik B, Kerkhof L, Zylstra GJ, Kukor JJ. 2005. Identification of unique type II polyketide synthase genes in soil. Appl Environ Microbiol. 71(5):2232–2238. doi:https://doi.org/10.1128/AEM.71.5.2232-2238.2005.
- Welbaum G, Sturz AV, Dong Z, Nowak J. 2004. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit Rev Plant Sci. 23(2):175–193. doi:https://doi.org/10.1080/07352680490433295.
- Xia Y, DeBolt S, Dreyer J, Scott D, Williams MA. 2015. Characterization of culturable bacterial endophytes and their capacity to promote plant growth from plants grown using organic or conventional practices. Front Plant Sci. 6:490. doi:https://doi.org/10.3389/fpls.2015.00490.
- Zhang L, Yan C, Guo Q, Zhang J, Ruiz-Menjivar J. 2018. The impact of agricultural chemical inputs on environment: global evidence from informetrics analysis and visualization. Int J Low-Carbon Technol. 13(4):338–352. doi:https://doi.org/10.1093/ijlct/cty039.