Abstract
Red raspberry (Rubus idaeus L.) is an important fruit crop in British Columbia, Canada, and the Pacific Northwest (PNW) region of the USA, as well as in other regions of the world. Root rot and wilting complex (RRWC), primarily caused by Phytophthora rubi, is the most important biotic constraint responsible for declining raspberry production in these regions, causing millions of dollars in losses. Other root-infecting fungal species and the root lesion nematode (Pratylenchus penetrans) may also be found associated with the disease complex. The average lifespan of raspberry plantings in the PNW is 10 to 12 years, which is reduced to 5 years by the disease complex. Phytophthora spp. play a predominant role in the RRWC complex due to the persistent nature of oospores, rapid dispersal of inoculum, and the polycyclic nature of infection, all of which increase disease severity. In this review, we discuss the current understanding of Phytophthora spp. and other pathogens associated with the RRWC, including pathogen biology and the disease cycle, the impact of infection on the plant, as well as current and potential cultural, biological, and chemical options for management. In addition, we discuss breeding efforts for disease resistance, including conventional and molecular approaches to identify sources of resistance, molecular markers linked to potential resistance genes, and their incorporation into elite breeding materials or cultivars. We also present the current gaps in knowledge, unique challenges, and future perspectives in sustainable disease management of this important disease complex.
Résumé
La framboise (Rubus idaeus L.) est une importante culture fruitière en Colombie-Britannique, au Canada, dans la région du nord-ouest du Pacifique (NOP) des États-Unis ainsi que dans d’autres régions du monde. Le complexe pourridié/flétrissement (CPF), maladies causées principalement par Phytophthora rubi, est la contrainte biotique la plus importante responsable de la diminution de la production de framboises dans ces régions, occasionnant des pertes de millions de dollars. Les autres espèces fongiques qui infectent les racines et les nématodes des lésions racinaires (Pratylenchus penetrans) peuvent également être associées à ce complexe de maladies. La durée de vie moyenne d’une plantation de framboisiers dans le NOP est de 10 à 12 ans, durée qui est réduite à 5 ans par le complexe de maladies. Phytophthora spp. joue un rôle important dans le CPF à cause de la persistance des oospores, de la dissémination rapide de l’inoculum et de la nature polycyclique de l’infection, caractéristiques qui accroissent la gravité de la maladie. Dans cet article, nous faisons état de la compréhension actuelle de Phytophthora spp. et des autres agents pathogènes associés au CPF, y compris de la biologie de l’agent pathogène et du cycle de la maladie, des effets de l’infection chez la plante de même que des solutions possibles, culturales, biologiques et chimiques, de gestion de la maladie. De plus, nous traitons des efforts de sélection déployés en vue de la résistance, y compris des approches traditionnelles et moléculaires pour définir des sources de résistance, trouver des marqueurs moléculaires liés aux gènes de résistance potentiels et les incorporer dans les meilleurs matériaux pour la sélection ou dans les cultivars. Nous présentons également les lacunes actuelles dans les connaissances, les défis particuliers à relever ainsi que les perspectives quant à la gestion durable de cet important complexe de maladies.
Introduction
Red raspberry (Rubus idaeus L.) is an important fruit crop belonging to the Rosaceae family with global annual production of more than 870 000 tonnes (FAOSTAT Citation2018). It is widely cultivated in Europe (Kennedy and Duncan Citation1991; Strautina et al. Citation2012), North America (Gigot et al. Citation2013a), and South America (Latorre and Muñoz Citation1993; Wilcox and Latorre Citation2002). In North America, the Pacific Northwest (PNW, inclusive of Oregon, Washington, Idaho and British Columbia) is a major raspberry growing region (Finn et al. Citation2008; Kempler et al. Citation2012a; Gigot et al. Citation2013a). Within Canada, British Columbia (BC) is the largest producer of raspberries, accounting for almost 80% of domestic production (Statistics Canada Citation2020). The majority of raspberry production in BC occurs in the Fraser Valley. In 2019, 83% of BC production was for the processing market and the remainder was sold on the fresh market (Klootwyk Citation2020). The raspberry processing market is globally commoditized, and profitability for BC growers has been declining due to increased disease pressure as well as high costs of production compared to global competitors (Finn et al. Citation2008; Nikolić et al. Citation2008; Burlakoti and Dossett Citation2020).
A range of pathogens, including oomycetes, fungi, viruses and bacteria, cause economically damaging diseases of raspberry, with negative impacts on plant health, yield and fruit quality. The root rot and wilting complex (RRWC) is the most economically important raspberry disease problem worldwide (Wilcox Citation1989; Weiland et al. Citation2018). Although Phytophthora rubi (Wilcox and Duncan) Man in’t Veld (synonym. P. fragariae Hickman var. rubi), an oomycete or ‘water mold’, is reported as a principal causal agent of RRWC of raspberry (Wilcox and Latorre Citation2002; Schlenzig et al. Citation2005; Gigot et al. Citation2013a), multiple species of Phytophthora, other root-infecting fungi, and root lesion nematodes (Vrain et al. Citation1994; Gigot et al. Citation2013a; Weiland et al. Citation2018) are also associated with this disease complex.
In the PNW, RRWC is the greatest challenge to raspberry growers and is responsible for declining raspberry plant vigour and fruit yields, resulting in millions of dollars in losses (Anonymous Citation2013; Gigot et al. Citation2013a; Tabima et al. Citation2018; Burlakoti and Dossett Citation2020). In British Columbia, control measures for RRWC are the single largest non-labour expense for many growers, including costs for fumigation (depending on the product and application method) at the time of planting as well as post-plant fungicide applications. Moreover, the lifespan of raspberry plantings in the PNW has declined from 10–12 years to 5–6 years over recent decades due to yield and vigour reductions associated with this disease complex (Gigot et al. Citation2013a). The abundant rainfall and moderate spring and fall climatic conditions in the PNW provide a favourable environment for inoculum production, pathogen infection, and development of RRWC symptoms (Ellis and Miller Citation1993; Gigot et al. Citation2013a).
To our knowledge, there is no previous comprehensive review on RRWC of red raspberry. Here, we review the current understanding of the biology of the causal agents associated with the disease complex and discuss breeding efforts to develop genetic resistance as well as the cultural, biological, and chemical options available for disease management. In addition, we discuss current gaps, unique challenges, and future perspectives in sustainable disease management.
Global distribution and impact of the disease complex
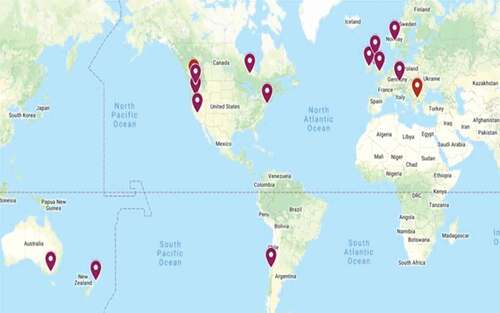
The disease was first reported in England and Scotland in the 1930s as a ‘Dieback disease of raspberry’ (Waterston Citation1937). Root rot was later reported in North America in the 1960s (Converse and Schwartze Citation1968). Phytophthora spp. were identified as the causal agents of raspberry root rot in Canada in 1988 (as reported in Valois et al. Citation1996) and were reported in the 1980s in Australia (Washington Citation1988) and New Zealand (Boesewinkel Citation1982). Currently, RRWC is a widely recognized disease complex in raspberry production regions across the world (), including Canada (Joshi and Jeffries Citation2013, Citation2017; Burlakoti and Sapkota Citation2020), the USA (Gigot et al. Citation2013a; Stewart et al. Citation2014; Weiland et al. Citation2018), Chile (Wilcox and Latorre Citation2002), UK (Jennings et al. Citation2003) and several countries in Europe (Duncan et al. Citation1987; Wilcox et al. Citation1993; Schlenzig et al. Citation2005; Nikolić et al. Citation2008; Weber and Entrop Citation2017). Raspberry root rot is a major production challenge in the United Kingdom, and therefore breeding programmes have placed a heavy emphasis on developing root rot resistant cultivars (Jennings et al. Citation2003).
Phytophthora rubi is a principal species causing RRWC of raspberry, but multiple Phytophthora spp. have been sporadically reported to infect raspberry, such as P. cryptogea Pethyb. and Leth., in New York state (Wilcox Citation1989), Chile (Wilcox and Latorre Citation2002), Australia (Washington Citation1988) and New Zealand (Boesewinkel Citation1982). In addition, P. citricola Sawada was reported from New York state (Wilcox Citation1989), Chile (Latorre and Muñoz Citation1993; Wilcox and Latorre Citation2002) and Bulgaria (Ilieva et al. Citation1995) and P. citrophthora (R.E. Sm. and E. H. Sm.) Leonian from Chile (Latorre and Muñoz Citation1993; Wilcox and Latorre Citation2002) and Bulgaria (Ilieva et al. Citation1995). Finally, P. gonapodyides (Petersen) Buisman and P. megasperma Drechs were reported from Chile (Wilcox and Latorre Citation2002) and New York state (Wilcox Citation1989). A summary of the various species of Phytophthora, fungi and nematodes found in association with the raspberry RRWC from diverse geographic regions across world is presented in .
Table 1. Phytophthora species, fungi and nematodes associated with the root rot and wilting complex of raspberry
The root lesion nematode, Pratylenchus (Pr.) penetrans (Cobb) Filipjev and Schuurmans (Vrain et al. Citation1994; Gigot et al. Citation2013a; Weiland et al. Citation2018), and other potential root-infecting pathogens including Verticillium dahliae Kleb. (Weiland et al. Citation2018), species of Fusarium, Cylindrocarpon and Pythium (Joshi and Jeffries Citation2013; Burlakoti and Sapkota Citation2020) have also been recovered from plants affected by raspberry decline in the PNW. These reports have not always been followed by pathogenicity tests to confirm the ability of these microbes to cause symptoms. P. rubi and Pr. penetrans are two common soil-borne pathogens also present in some red raspberry orchards (Gigot et al. Citation2013a; Zasada et al. Citation2015; Weiland et al. Citation2018) and their population densities depend on soil type and management practices (Gigot et al. Citation2013a). Although Phytophthora spp. are considered to be the principal cause of raspberry root rot, based on their frequency of recovery and pathogenicity studies, it is difficult to separate their effects from other root infections due to chronic damage by Pr. penetrans (Vrain et al. Citation1996; Gigot et al. Citation2013a). Sixteen different genera of soil nematodes have been found in association with raspberry decline in BC (McElroy Citation1977).
Phytophthora identification and genomics
Traditional methods include isolation of Phytophthora by direct plating of infected tissues on selective media and use of baiting techniques that use zoospores-attractive baits, followed by close examination of cultural and morphological features to identify the species (Koprivica et al. Citation2009; Martin et al. Citation2012). These traditional techniques are time and labour intensive and also require mycological expertise for proper identification (Hughes et al. Citation2000; Grote et al. Citation2002). In addition to traditional methods, immunological detection techniques that target specific pathogen antigens (e.g. Agdia ImmunoStrip, Agdia Inc Citation2017) and molecular techniques that target specific DNA regions in the Phytophthora genome (Cooke et al. Citation2007; Martin et al. Citation2012) are available. Information on identification of species of Phytophthora can be found at http://phytophthora-id.org/ (Grünwald et al. Citation2011) and at https://idtools.org/id/phytophthora/index.php (Abad et al. Citation2019). Genetic markers developed for Phytophthora identification target the internal transcribed spacer (ITS) region of nuclear ribosomal DNA (Bonants et al. Citation1997), cytochrome oxidase subunits (I, II), nad1, and nad9 of the mitochondrial genome (Martin et al. Citation2014). Additionally, sequencing of different nuclear polymorphic loci targeting β-tubulin, heat shock protein 90, translation elongation factor 1α, enolase, and NADH dehydrogenase (nad5) were also used to identify species of Phytophthora (Van’t Klooster et al. Citation2000; Kroon et al. Citation2004; Villa et al. Citation2006). ITS-based sequencing has some limitations for accurately distinguishing among species of Phytophthora. For instance, P. rubi and P. fragariae have identical ITS sequences (Cooke et al. Citation2007; Martin et al. Citation2012), making it difficult to distinguish these species without other molecular approaches.
The popularity of next generation sequencing is surging and will likely be a powerful tool in Phytophthora detection and management. The sequence data obtained from these techniques will be valuable for understanding phylogenetic relationships between species and to resolve taxonomic issues. For example, previously there was a controversy regarding the differences between P. rubi and P. fragariae, and both were considered as a single species (Hickman Citation1941). Later, these species were named P. fragariae var. fragariae when Phytophthora root rot of raspberry was reported (Wilcox et al. Citation1993). Subsequently, Man in’t Veld (Citation2007) separated these pathogens as different species – P. rubi and P. fragariae – infecting raspberry and strawberry, respectively, based on mitochondrial cox I loci, which was further supported by genome analysis (Tabima et al. Citation2018).
Different molecular approaches including genotyping by sequencing (GBS) and whole genome sequencing were used to study genomics and population diversity of P. rubi from the PNW (Tabima et al. Citation2017, Citation2018), which provided a new insight into host-pathogen emergence, pathogen migration, etc. The whole genome assembly of P. rubi had 9434 scaffolds, which covered 74 656 193 bp and had a coverage of 92x and an N50 score of 16.8 Kb (Tabima et al. Citation2017). Using GBS, these researchers studied genetic diversity of P. rubi collected from the PNW, and their study revealed a low genetic diversity among P. rubi populations across western US, leading the authors to further hypothesize that P. rubi populations had migrated to Washington from California and Oregon.
Adams et al. (Citation2020) studied genome structure of key races of P. fragariae and also compared three strains of P. rubi from Scotland and Germany. They identified distinct population structures between the two species. Advancements in comparative genomic studies, including whole genome sequencing, have led to identification of putative effectors in several economically important species of Phytophthora, such as P. sojae (Song et al. Citation2015; Huang et al. Citation2017), P. infestans (Haas et al. Citation2009), and P. capsici (Stam et al. Citation2013). Adams et al. (Citation2020) identified PfAvr1, PfAvr2, and PfAvr3 as RxLR effectors from P. fragariae isolated from 11 strains of P. fragariae from strawberry (10 from Canada and one from Scotland). These effectors play critical roles in host—pathogen interactions and effector genes of pathogen can be used for high throughput screening of host genotypes to identify disease resistance genes (Kamoun Citation2006; Vleeshouwers et al. Citation2008; Wang and Jiao Citation2019). To date, effectors of P. rubi have not been discovered.
Phytophthora biology and disease cycle
The principal causal agents of RRWC are Phytophthora spp. (Phylum Oomycota), which affect a broad range of plant species worldwide (Cooke et al. Citation2000; Whisson Citation2010). The disease cycle is complex due to the rapid dispersal of inoculum, the production and long-term survivability of oospores, the polycyclic nature of infection, and their interaction with other plant pathogens. Phytophthora spp. produce both asexual sporangia and zoospores as well as sexual oospores. Mycelia and oospores surviving in infected raspberry tissues and soil are the primary inocula of the disease cycle of the pathogen. Oospores, the long-lived, thick-walled sexual spores, can persist in soil for up to 10 years when environmental conditions are unfavourable (Duncan and Cowan Citation1980; Judelson and Blanco Citation2005; Newton et al. Citation2010). The oospores germinate to form sporangia or hyphae when the soil is wet either from precipitation or irrigation (Duncan Citation1990; Judelson and Blanco Citation2005). The sporangia either infect host tissues by direct germination or by producing small, single-celled biflagellate zoospores (Tyler Citation2002; Judelson and Blanco Citation2005). A few hours of flooding or soil saturation is sufficient for zoospore release and this stage remains motile for up to 24 hours. Zoospores swim through water (Thomson and Allen Citation1976; Judelson and Blanco Citation2005), attach to root tips, newly formed rootlets, or sites of root wounding, such as those caused by plant parasitic nematode feeding (Cooke et al. Citation1995; Laun and Zinkernagel Citation1997; Narayan et al. Citation2010; Hardham and Blackman Citation2018). During attachment to root tissues, the zoospores lose their flagella and form cysts and penetrate plant roots to establish infection (Hardham and Hyde Citation1997; Tyler Citation2002; Tani et al. Citation2004). Thereafter, the pathogen begins to colonize the roots, interfering with water and nutrient movement in the vascular system and leading to root rot. Usually, the infection spreads from the root tips to the crown and then to the base of stems via sporulation and mycelial growth, leading to progression of symptoms from below-ground to above-ground plant organs (Duncan Citation1990; Laun and Zinkernagel Citation1997). Initially, below-ground symptoms appear as discoloured, water-soaked necrotic lesions on the roots and crowns (), which later results in wilting and dieback of the above-ground canes (). Specific symptoms include the production of fewer, weaker rootlets and discoloration of foliage which turns yellow and eventually brown and scorched () (Wilcox Citation1989; Laun and Zinkernagel Citation1997; Stewart et al. Citation2014). Other diagnostic above-ground symptoms include stunted lateral development on the floricanes bearing the crop of the current season and a lack of cane regrowth or wilting and dieback of new primocanes () (Wilcox Citation1989; Laun and Zinkernagel Citation1997; Stewart et al. Citation2014).
Fig. 2 Symptoms of red raspberry root rot complex. (a) Healthy raspberry plants without any disease symptoms. (b-d) Dark reddish-brown lesions at crown/roots with very few new rootlets. (e) Cane wilting with necrosis. (f) Leaf scorching. (g-h) Severely infected fields showing plants with leaf chlorosis and scorching, wilting and completely dead canes.

The movement of infested soil and planting materials are key means of pathogen dispersal between fields or regions (Brasier Citation2008; Nikolić et al. Citation2008). Pathogen inocula are also dispersed from the infected tissues to healthy plant tissues by splashing rainwater while motile zoospores can spread across fields from surface water run off or drainage flows. Soils infested with the pathogen can also be dispersed across fields by animals and humans during farm operations (Brasier and Hansen Citation1992; Ristaino and Gumpertz Citation2000). Soil temperature and moisture, as well as ambient temperature, are key factors affecting pathogen spread, rate of multiplication in host tissues, and development of disease symptoms. Laboratory studies showed that the minimum, optimum and maximum temperatures for mycelial growth of Phytophthora rubi were 4°C, 19 to 22°C, and 25–28°C, respectively (Duncan et al. Citation1991; Wilcox and Latorre Citation2002; Jung et al. Citation2017). Graham et al. (Citation2021) found that P. rubi growth and sporulation was highest at 20°C among the tested temperature range between 5 to 20°C. They reported that P. rubi was most active and caused most severe diseases in PNW when soil temperature ranged from 15 to 20°C. High rainfall is common in the PNW from fall until spring, which enhances pathogen sporulation and spread and promotes infection of host tissues (Duncan and Kennedy Citation1989; Ellis and Miller Citation1993; Gigot et al. Citation2013a).
Approaches and strategies to manage RRWC of raspberry
Effective management of RRWC requires incorporation of multiple management tools as a system approach during both pre- and post-raspberry planting. Early, reliable diagnosis of root infection in fields will help to determine when fungicides should be applied and when other management options should be implemented to suppress the disease. The integration of these techniques with cultural (e.g. proper site selection, drainage improvement, and raised beds), biostimulants (e.g. phosphoric acids), host resistance (i.e. resistant cultivars), potential biocontrol agents and fungicides (e.g. metalaxyl, phosphites) is crucial for RRWC management (Wilcox et al. Citation1999a; Moore et al. Citation2015; Moore and Hoashi-Erhardt Citation2020). Among these, genetic resistance remains the most important factor in an integrated approach because pre-plant fumigation and post-plant cultural and chemical/biological controls can only reduce the onset and severity of the RRWC in the presence of the pathogen (Wilcox et al. Citation1999a). Here, we discuss the range of currently available management options and their strengths and limitations.
Pre-plant fumigation
Management of soil-borne pathogens in raspberry production in the PNW relies on a combination of resistant cultivars and management practices that reduce incidence, spread and severity. Pre-plant fumigation between cropping cycles has historically been a first line of defence since crop rotation is ineffective in reducing Phytophthora inoculum due to the persistence of long-lived oospores and restricted availability of land (Duncan Citation1980; Walters et al. Citation2017).
Fumigating in the fall is effective in reducing Phytophthora populations, giving spring plantings the opportunity to establish vigorous root systems before being potentially challenged by pathogen pressure (Walters et al. Citation2011a). In recent decades, chemical fumigation practices have shifted dramatically due to loss of highly effective products (e.g. methyl bromide), increased costs and decreased efficacy of replacement products, and changes to worker safety and environmental regulations (e.g. buffer zone requirements) that make application more difficult (Walters et al. Citation2017). Fumigation options are limited in BC compared to the USA due to a lack of custom application services and fewer available products. These diminishing options have motivated research into alternative disease management methods throughout the PNW. Polyethylene tarping during fumigation improves efficacy and reduces environmental risks but bears additional costs (Walters et al. Citation2011b). With or without tarping, bed fumigation (via incorporation or through the dripline) reduces buffer zone requirements, amount of fumigant, and cost when compared with broadcast application (Zasada et al. Citation2010). While bed fumigation is effective for controlling plant parasitic nematodes (PPN) and Phytophthora in the short-term, these pathogens are mobile and can recolonize planting beds from the untreated inter-row space (Schneider et al. Citation2008).
Telone C-35 (34.7% chloropicrin, 63.4% 1,3-dichloropropene) is the standard fumigant used in the USA, but Pic-Clor 60 (59.6% chloropicrin, 39% 1,3-dichloropropene) is more effective because chloropicrin is biologically more active on Phytophthora and 1,3-dichloropropene acts primarily on PPN (Walters et al. Citation2017). Of these two chemistries, only chloropicrin is available in Canada but is not generally used due to lack of custom application services. However, metam sodium or potassium products have been used in both Canada and the USA to reduce both Phytophthora and PPN populations (Walters et al. Citation2017). These products are available in liquid (Vapam [sodium N-methyldithiocarbamate] and K-Pam [potassium N-methyldithiocarbamate]) and granular formulations (Dazomet [tetrahydro-3,5-dimethyl-2H-1,3,5-thiadiazine-2-thione]). After application, these products are converted into methyl isothiocyanate, having low vapour pressure, strong affinity to water, and only dispersing short distances in soil (Carlock and Dotson Citation2010). Therefore, these products are not as volatile as traditional fumigants and must be either deeply incorporated into the soil profile using specialized equipment or with application of high volumes of water (Carlock and Dotson Citation2010).
Alternatives to fumigants
Brassica seed meals have been proposed as an alternative to chemical fumigants (Mazzola et al. Citation2007), but their impact on soil-borne diseases and PPNs has been inconsistent, and their use is also cost-prohibitive (Gigot et al. Citation2013b). Anaerobic soil disinfestation (ASD) is another non-chemical fumigation method that involves incorporation of a high carbon feedstock into the soil prior to saturation with irrigation and sealing with a tarp for six weeks during the hottest time of the year, a practice that has shown promise in California strawberry production (Shennan et al. Citation2016). Similarly, soil solarization involves irrigation of soil to field capacity prior to tarping with clear polyethylene to trap the heat of the sun, which reduced Phytophthora populations in strawberry and raspberry production (Pinkerton et al. Citation2002). Compared with shallow-rooted crops (e.g. strawberry) grown in warmer regions (e.g. California), ASD and sun solarization are not used in the PNW because they are less effective for deep-rooted crops like raspberry and in regions where cooler climate conditions restrict the depth of their sterilizing effects and timing of implementation to the middle of the summer (Pinkerton et al. Citation2009).
Steam sterilization of soil has been identified as a potential tool to replace chemical fumigation (Samtani et al. Citation2012; Kim et al. Citation2020), but the process is very slow and expensive, which may prevent it from being widely adopted. Evaluation of the removal of raspberry roots between cropping cycles was motivated by the fact that fumigants tend not to penetrate even small portions of root debris left behind after field renovation and that portions of roots can interfere with the use of tarps in either broadcast or bed fumigation (Rudolph et al. Citation2016). However, mechanically removing roots prior to fumigation has shown little effect on PPN and their effect was not measured directly in suppressing Phytophthora root rot of raspberry (Rudolph et al. Citation2019).
Cultural practices
Even where it may be possible to implement pre-plant fumigation, it does not provide long-term suppression of Phytophthora. Therefore, site selection is critical since the incidence and severity of the RRWC are high in sites with heavier soils and poor drainage (Turner and Ken Citation1985). Waterlogging exacerbates rotting of raspberry roots in the presence of Phytophthora species (Duncan and Kennedy Citation1989). Therefore, cultural practices that reduce standing water and keep the root zone from being overly saturated are important. Subsoiling and installation of drainage tiles as well as annual sub-soiling to minimize compaction are essential to ensuring proper drainage between rows. In most cases, use of raised planting beds permits drainage and aeration of the root zone, reduces root rot severity, and improves plant growth (Heiberg Citation1995, Citation1999; McGregor and Franz Citation2002). For example, field trials of ‘Titan’ raspberry in New York state demonstrated that raised beds (36 cm high and 91 cm wide) resulted in a more than two-fold increase in yield compared with a flat-planted control (Maloney et al. Citation1993). Similarly, the percentage of Phytophthora infected canes was 50% lower and yield was three times greater for plants on raised beds (30 cm high and 100 cm wide) compared with flat beds (Wilcox et al. Citation1999a). In contrast, another field trial showed raised beds (25 cm high and 75 cm wide) did not increase yield significantly in the second year but did increase cane height and diameter by 15% and 26%, respectively (Maloney et al. Citation2005). The effects of raised beds on RRWC have not been tested in BC production.
Substituting the bare-root standard planting stock with disease-free tissue culture (TC) plug plants in the PNW can provide more rapid field establishment, since plug plants have the benefit of being free of most pests or diseases. Planting TC plugs through polyethylene (PE) mulch has many benefits: it allows for better moisture control in the planting bed, reduces Phytophthora severity, increases cane productivity, eliminates weed competition, and controls the physical space in which primocanes emerge to reduce the need for primocane thinning (Heiberg Citation1996, Citation1999). More than a decade of ongoing research in the PNW found that the use of polythene and biodegradable mulches in raspberry beds improved establishment of raspberry plantings (Goldberger et al. Citation2019; Zhang et al. Citation2020). In contrast, research in the eastern USA found that straw mulches increased soil moisture and Phytophthora root rot severity (Wilcox et al. Citation1999a). Lastly, preplant granular application of gypsum for two years (CaSO4: 13.5 t ha−1) in a Phytophthora infested field of ‘Heritage’ raspberry increased yield by 48% (Maloney et al. Citation2005). This may be due to calcium-mediated plant defence mechanisms (inhibiting fungal polygalacturonase activity) and/or inhibition of Phytophthora sporulation (Messenger et al. Citation2000; Stasikowski et al. Citation2014).
Post-plant fungicide applications
Fungicide applications are a key management tool for the RRWC as most widely-grown cultivars are moderately to highly susceptible to Phytophthora infection. The first chemical control method utilized Bordeaux mixture, comprised of copper sulphate and lime (Schwinn and Margot Citation1991). While copper-based products have been used in combination with other products to control root rot in raspberry (Duncan and Kennedy Citation1989), disease management evolved to include Fungicide Resistance Action Committee (FRAC) group 33, 4, 45, 21, and M1 chemistries (). Across global cropping systems with Phytophthora disease challenges, chemical controls included broad-spectrum products, such as dithiocarbamates, chlorothalonil, and phthalimides, which impact fungal organisms as well as oomycetes (Whisson Citation2010). Afterwards, there was a shift towards oomycete-specific chemistries, including phenylamides, carbamates, cyanoactamide oxaimes, phosphonates, acylpicolides, and carboxylic acid amides (Whisson Citation2010).
Table 2. Registered fungicides for the control of the root rot and wilting complex on raspberry
Systemic fungicides such as phenylamide (apoplastic, xylem translocated, FRAC group 33) and phosphonates (symplastic, xylem and phloem translocated, FRAC group 4) are particularly effective in controlling oomycete pathogens (Guest and Grant Citation1991). Canadian and US registered uses of fungicides to control the RRWC include foliar application of FRAC group 33 fungicides, such as fosetyl-Al and the mono-and di-basic salts of phosphoric acid, as well as soil application of metalaxyl (FRAC group 4), oxathiapiprolin (FRAC group 45), and cyazofamid (FRAC group 21) (). Metalaxyl is the most widely used product to control the RRWC of raspberry, the roots absorbing soil-drenched product and translocating it via the vascular system to provide systemic control of the oomycete (Carris and Bristow Citation1987). Due to its highly specific mode of action that involves inhibition of nucleic acid metabolism, there are no reports on effects on other pathogenic or beneficial fungal organisms (Davidse et al. Citation1983; Whisson Citation2010).
Field experiments demonstrated that metalaxyl controls Phytophthora in both waterlogged and non-waterlogged conditions (Duncan and Kennedy Citation1989), and it is a major fungicide in controlling the RRWC (Maloney et al. Citation1993; Wilcox et al. Citation1999a). However, over-reliance on a single chemistry can result in development of pathogen resistance, and rotation with other effective chemical groups is advised. Phosphorous acid (i.e. phosphonic acids) – fosetyl-Al being the most common – includes a range of compounds based on diprotic phosphorous acid (i.e. H3PO3) compounds that do not provide nutritional phosphorus to plants but are used as highly effective controls for Phytophthora in various cropping systems (Bristow and Windom Citation1992; McGregor and Franz Citation2002). On raspberry grown in Australia, Washington (Citation1988) found metalaxyl, phosphorous acid, or fosetyl-Al controlled Phytophthora while Maloney et al. (Citation2005) reported that phosphorous acid applications increased yield in the presence of Phytophthora spp. in New York state.
Development of insensitivity of Phytophthora spp. to fungicides may become problematic for managing the RRWC in raspberries when pre- and post-plant chemical control options become more restricted, highlighting the need for additional fungicides. In studies on other crops, screening of 184 isolates of P. cryptogea from various floriculture crops in North Carolina showed partial to full insensitivity to metalaxyl (Hwang and Benson Citation2005). Also, phosphite insensitivity was reported for isolates of P. cinnamomi Rands from various trees in Australia (Wilkinson et al. Citation2001), isolates of P. capsici Leonian from pepper in India (Veena et al. Citation2010), and isolates of P. citrophthora, P. parasitica, and P. syringae Kleb. from citrus in California, USA (Adaskaveg et al. Citation2017).
Biostimulants and biological control agents
The importance of biostimulants in improving horticultural crop yields, quality, and resistance to stresses has been recognized for decades (reviewed by Drobek et al. Citation2019), yet the promise of biostimulants has not been translated to beneficial field practices for raspberry production. For example, while acting as disease control products, phosphorous acids are considered biostimulants, but documentation of their direct impact on plant growth and fruit yield or quality is limited. Currently, biofungicides or biocontrol agents (BCAs) registered for Phytophthora root rot in the USA (Environmental Protection Agency, EPA) and Canada (Pest Management Regulatory Agency, PMRA) are mostly based on filamentous endophytic fungi, including Trichoderma harzianum Rifai, T. virens Pers. and Gliocladium virens Mill, Giddens and Foster, or bacteria such as Bacillus subtilis Ehrenberg (). Various species of Trichoderma have demonstrated potential in numerous assessments under controlled conditions, but field efficacy trials have had mixed results. For example, dipping strawberry roots in a suspension of Trichoderma spp. prior to transplanting reduced P. cactorum (Lebert And Cohn) Schrot. populations in the field (Porras et al. Citation2007), whereas trials with nursery containers did not demonstrate a significant effect of T. asperellum Samuels, Lieckf. and Nirenberg on Phytophthora populations (Funahashi and Parke Citation2016). Additionally, various glucanolytic actinomycete strains are antagonistic to P. rubi due to their glucan-hydrolysing enzymes that have been shown under laboratory conditions to degrade the cell wall and lyse cells of Phytophthora (Valois et al. Citation1996).
Table 3. Registered and potential biofungicides and other products for control of soil-borne diseases in raspberry and other crops
Utilization of host resistance
One of the reasons the RRWC is a pervasive problem is due to the use of susceptible parents with exceptional fruit quality as founders of the current germplasm. For example, Dale et al. (Citation1993) demonstrated that ‘Lloyd George’, a cultivar which is extremely susceptible to Phytophthora infection, was present in the genetic background of 87% of new cultivars with known pedigrees released from 1960–1992 in Europe and North America and had an average genetic contribution of 22% in these cultivars. This susceptibility to Phytophthora has been a major driving factor in the development and adoption of substrate production systems around the world. Development of host resistance is a more economically and environmentally sustainable option for combatting the RRWC because it reduces reliance on expensive cultural and chemical control practices. Efforts were made to identify sources of resistance and incorporate them into commercial raspberry cultivars.
Screening methodologies for the RRWC
Since the 1970s, raspberry breeders have been searching for new sources of resistance to Phytophthora root rot and attempting to study inheritance of resistance. To achieve these goals, various screening methodologies have been developed. These include using potted plants in the greenhouse (Wilcox Citation1989; Kennedy and Duncan Citation1991; Laun and Zinkernagel Citation1993; Hoashi-Erhardt et al. Citation2008; Knight and Fernández Fernández Citation2008; Graham et al. Citation2011; Stewart et al. Citation2014) as well as hydroponic and semi-hydroponic systems (Pattison et al. Citation2004, Citation2007; Pattison and Weber Citation2005; Kempler et al. Citation2012b). While the use of potted plants may better reflect growing conditions in the field, the advantage of the hydroponic systems is greater ease in assessing root damage as one of the phenotypic criteria. Different inoculation methods have been used in screening assays. These include inoculation with mycelial plugs in pots (Kennedy and Duncan Citation1991; Graham et al. Citation2011), adding mycelium suspensions or zoospores to the root zone (Laun and Zinkernagel Citation1993; Pattison et al. Citation2004; Hoashi-Erhardt et al. Citation2008; Knight and Fernández Fernández Citation2008; Kempler et al. Citation2012b), and growing seedlings in a mixture of vermiculite (containing pathogen inoculum) and potting mix (1:10 ratio) (Wilcox Citation1989; Laun and Zinkernagel Citation1993; Stewart et al. Citation2014). Generally, most studies comparing greenhouse screening techniques with field responses have shown good agreement between the two approaches (Laun and Zinkernagel Citation1993; Wilcox et al. Citation1999b; Hoashi-Erhardt et al. Citation2008). While greenhouse screening (Wilcox Citation1989; Kennedy and Duncan Citation1991; Graham et al. Citation2011) and hydroponic trials (Pattison et al. Citation2004, Citation2007) can provide consistent results, the source of plant materials, experimental conditions, and growth conditions may not be representative of field conditions, in which symptoms manifest after several years where soil and growing conditions are heterogenous. For example, while the cultivar ‘Cowichan’ showed some resistance to root rot in the greenhouse, field screening showed it to be susceptible (Kempler et al. Citation2007). Similarly, Hoashi-Erhardt et al. (Citation2008) reported two cultivars, ‘Cascade Delight’ and ‘Cascade Dawn’, which had high root and shoot tolerance ratings in the greenhouse but showed only partial resistance in the field. The presence of other microorganisms in field soil may contribute to differing results obtained in greenhouse and field experiments. Consequently, for rapid assessment of resistance or susceptibility responses, greenhouse trials can be used initially but should be followed up with field trials. These types of studies are urgently needed to identify resistance to the RRWC.
Sources of resistance to the RRWC
Red raspberry is diploid (2n) and has seven pairs of chromosomes. It easily hybridizes with black raspberry to produce purple raspberry (Foster et al. Citation2019). For many years, wild North American red raspberry (Rubus strigosus Michx) germplasm has been recognized as a source of resistance to root rot while wild red raspberry from Europe (R. idaeus) has been generally considered to be largely susceptible, but few studies surveying wild germplasm have been conducted to confirm this. Dossett et al. (Citation2014) surveyed 64 R. strigosus accessions from across North America and found populations ranged from very susceptible to highly resistant, with more variability in western populations. In contrast, Graham et al. (Citation2009) surveyed 12 wild populations from Scotland and found them to be uniformly susceptible. Bristow et al. (Citation1988) screened seedlings of several Rubus species for their reaction to P. erythroseptica Pethyb. and Laff. and found variation in the percentage of resistant seedlings from wild R. strigosus across North America. In addition, a higher percentage of resistant seedlings were identified in wild R. idaeus seedlings from Norway when the seed was collected from mother plants growing in low-lying, wet areas compared to those collected from locations with better drainage. Finally, resistance was also found in other related species, including R. spectabilis Pursch. and R. parviflorus L. Kempler et al. (Citation2012b) reported Phytophthora resistance in selections derived from introgression of R. strigosus, R. innominatus Moore, R. niveus Thunb, R. lasiostyles Focke, and R. coreanus Miq.
The earliest sources of disease resistance in cultivated raspberry germplasm originate from cultivars such as ‘Latham’ and ‘Newman’ that contain a high proportion of R. strigosus in their ancestry. In particular, ‘Latham’s descendants ‘Chief’ and ‘Boyne’, and ‘Newman’s descendants ‘Newburgh’ and ‘Taylor’ were used extensively in breeding programmes, especially in eastern North America, through much of the 1900s until the 1970s because they contributed improved winter hardiness and resistance to Raspberry bushy dwarf virus. The lineage of most cultivars developed over the last 40 years in North America (and many from Europe) that carry root rot tolerance can be traced to these sources, although it is not always clear if other sources of resistance also play a role. To help highlight the extent of ‘Latham’s and ‘Newman’s contribution to this trait, we have indicated below when their progeny are described in studies related to genetics and inheritance of Phytophthora resistance. In Norway, ‘Asker’ (syn. ‘Winklers Sämling’) has also been an important source of breeding for resistance. While its origin is uncertain, the presence of glandular hairs and spines on the canes are indicative of R. strigosus ancestry, similar to ‘Latham’ and ‘Newman’. The sources of root rot resistance in recent popular cultivars are also shown in .
Table 4. Raspberry cultivars available in the Pacific Northwest and Europe with their relative degree of resistance or susceptibility to the red raspberry disease complex
Kennedy and Duncan (Citation1993) identified ‘Latham’ and ‘Autumn Bliss’ as having resistance to root rot among six raspberry genotypes evaluated by inoculating with 11 isolates of P. rubi in a controlled environment. Levesque and Daubeny (Citation1999) screened 48 cultivars and breeding selections for root rot resistance and identified ‘Amity’, ‘Summit’, and ‘Autumn Bliss’ as being resistant to P. rubi in greenhouse studies. These same cultivars also showed resistance to root rot under field conditions (Daubeny and Anderson Citation1993). ‘Newman’ derivatives ‘Newburgh’ (‘Newman’ × ‘Herbert’) and ‘Taylor’ (‘Newman’ × ‘Lloyd George’) feature prominently in the pedigree of all three of these cultivars and are likely where much of their resistance came from. ‘Autumn Bliss’, in addition to having ‘Newburgh’ and ‘Taylor’ in its pedigree, is also descended from R. arcticus L., for which the resistance potential is unknown.
Breeding need and efforts for resistance to the RRWC
The shift towards substrate production systems in fresh market raspberries has reduced the need for genetic resistance to the RRWC in many areas, and there has consequently been less of a focus in many breeding programmes compared to 30 years ago. However, the need still exists, particularly where production is more focused on the processed market. Breeding programmes in BC, Washington and Oregon still consider Phytophthora root rot as one of the major biotic issues, and identification of resistance sources and incorporating them in raspberry cultivars has been a major focus (Daubeny Citation2002; Finn et al. Citation2008; Hoashi-Erhardt et al. Citation2008). Many of the breeding selections evaluated by Levesque and Daubeny (Citation1999) were descendants of R. strigosus clones found by Bristow et al. (Citation1988) to have Phytophthora resistance and have subsequently been used in the BC breeding programme. Breeding efforts in BC in the late 1980s and early 1990s focused on further developing and utilizing these sources of resistance in addition to ‘Latham’ and ‘Newburgh’ as parent materials in the cultivar development programme (Daubeny and Anderson Citation1993). Because the original seedlings evaluated by Bristow et al. (Citation1988) came from wild-collected seed, it has taken several generations to improve yield and fruit quality to commercially acceptable levels. To date, none of the recent cultivars released from the BC programme have had particularly strong root rot tolerance, although the most recent, ‘Squamish’, has held up better than many others (M. Dossett, personal obs.). Two selections (BC 93-16-43 and BC 90-19-34) descended from R. strigosus have been the subject of recent germplasm releases from the programme (Dossett et al. Citation2013) and are also currently being used in breeding and to map loci associated with resistance to root rot. Recent popular raspberry cultivars that are currently available are shown in along with their relative degree of resistance or susceptibility to the RRWC.
A high degree of root rot tolerance has been identified in the breeding programme at Washington State University because many of the fields used for evaluating seedlings have inherent disease pressure. ‘Cascade Bounty’ (Moore and Finn Citation2007) has exceptional root rot tolerance from its mother, ‘Chief’, a ‘Latham’ progeny, but it lacks the fruit quality necessary to be widely adopted by growers in the PNW. ‘Cascade Delight’ and ‘Cascade Dawn’ (Moore Citation2004, Citation2006), released around the same time, also have a degree of tolerance, but only ‘Cascade Delight’ has gained traction amongst growers, filling a gap in the mid-late fresh market season. More recently, ‘Cascade Harvest’ (Moore et al. Citation2015) and ‘WSU 2166ʹ (i.e. Cascade Premier) (Moore et al. Citation2019) have shown a degree of tolerance to Phytophthora root rot at several trial sites, with good quality and adaptation to mechanical harvest. ‘Cascade Harvest’ may have derived some of its tolerance from its mother ‘Cascade Dawn’ as well as its paternal grandmother ‘Newburgh’, although it is less clear where resistance in ‘WSU 2166ʹ originated. Many cultivars released from the PNW are adapted British Columbia and less so in eastern North America, but have found success in different raspberry growing areas around the world, including Chile and European countries (Daubeny and Anderson Citation1993; Kempler and Daubeny Citation1999) including Serbia (Nikolić et al. Citation2008) and Latvia (Strautina et al. Citation2012).
Inheritance, genetic mapping, and marker development
Once resistance has been identified, the next challenge is to understand the pattern of inheritance. Nestby and Heiberg (Citation1995) applied two different mating schemes to combine susceptible and resistant parents. They measured percent survival, plant height, and disease index of raspberry seedlings. They reported broad-sense heritability for disease index and percent survival to be very high (H2 = 0.88–0.94), indicating a large portion of the phenotypic variance was due to genetic effects. Narrow-sense heritabilities estimated in their study were modest (h2 = 0.21 for disease index and h2 = 0.38 for percent survival), indicating that while quantitative variation for resistance was present, it was much less important than dominance effects in the germplasm studied. The parents in the study with the highest combining ability for these traits included ‘Festival’(which has ‘Newman’ as a both paternal and maternal grandmother), ‘Sentry’ (grandchild of ‘Latham and ‘Newman’), and ‘Boyne’ (grandchild of ‘Latham’) as well as ‘Asker’.
Pattison et al. (Citation2007) studied resistance in ‘Latham’ using a hydroponic growing system and found that the disease index caused by P. rubi showed quantitative variation while the presence or absence of petiole lesions followed a dominant two-gene model explaining most of the variance in disease phenotype, with additional additive variation. Linkage maps and quantitative trait loci (QTL) analysis supported the dominant two-gene model from generational means analysis; however, the maps lacked utility for marker-assisted breeding and future comparative studies because the associated random amplified polymorphic DNA (RAPD) bands were never sequenced or anchored to a physical map (Pattison et al. Citation2007). Graham et al. (Citation2011) also identified two QTLs related to root rot tolerance in ‘Latham’. Their published map was anchored, which should make it valuable for comparison with other sources of resistance in the future. One of the QTLs identified may be directly related to plant resistance while the other may function more generally on plant vigour (J. Graham, pers. comm.). The microsatellite locus Rub118b has been useful for identifying seedlings as potentially resistant. Breeders are interested in developing molecular maps and markers to identify QTLs and major genes linked to root rot resistance that could save breeding cost and time to cultivar release (Pattison et al. Citation2007; Jennings et al. Citation2016).
Current gaps and future research perspectives
Pathogen biology, genomics, and diagnosis
Multiple species of Phytophthora are reported to cause the RRWC from diverse geographical areas across the world. However, species diversity of Phytophthora associated with the RRWC in BC and other Canadian provinces is presently unknown. In addition, root-infecting fungi such as V. dahliae Kleb. were reported in association with the RRWC in Washington (Weiland et al. Citation2018); however, the role of other fungi in the RRWC in BC has not been studied, and potential interactions of these fungi with Phytophthora spp. are also unknown. Both P. rubi and Pr. penetrans are commonly recovered from raspberry samples in fields showing the RRWC in the PNW; however, the interaction between these two pathogens and their impact on raspberry decline has not been researched. In addition, while the general life cycle of P. rubi associated with the RRWC is known, there are very limited comprehensive field studies to understand the epidemiology of the RRWC. A better understanding of the environmental and agronomic factors associated with disease epidemiology, including oospore survival and disease epidemics within and between seasons, are critical to designing management strategies that reduce pathogen inoculum.
The development of rapid, effective, and resource-friendly diagnostic tools is urgently needed to assess the presence of Phytophthora spp. in raspberry fields. Although the availability of Agdia Phytophthora ImmunoStrip (Agdia Inc. Citation2017) can detect infection by Phytophthora, it has limited sensitivity and is prone to cross-reactivity with Pythium species (Cooke et al. Citation2007; Martin et al. Citation2012). Moreover, it cannot diagnose the species of Phytophthora and isolation of the pathogen is required to verify the results of the ImmunoStrip assay. Few species-specific DNA markers are available to identify the species associated with the RRWC; however, these markers cannot distinguish between closely related species such as P. rubi and P. fragariae. Additional research is required to develop multiplex PCR assays that can simultaneously detect multiple species of Phytophthora associated with the RRWC. Other advanced molecular techniques, such as Next Generation Sequencing, could be powerful tools to detect Phytophthora spp. and to understand the phylogenetic relationships among the strains of Phytophthora spp.
Using whole genome and genotype by sequencing (GBS) techniques, genetic diversity of strains of P. rubi from red raspberry from western USA were studied (Tabima et al. Citation2017, Citation2018). Adams et al. (Citation2020) also studied comparative genomics of three strains of P. rubi from Europe and few key races of P. fragariae. However, comprehensive studies on the genomics and genetics of P. rubi and other species associated with the RRWC in Canada are required to understand phylogenetic relationship, population composition, and mechanisms or genes responsible for pathogenicity and virulence. Similarly, research is required to assess the comparative genomics of P. rubi strains collected from Canada and other geographical areas across the world, which may lead to a better understanding of pathogenesis-related genes and their expression as well as to identification of putative avirulence genes/effectors and understanding of the mechanisms of effector regulation.
Breeding for resistance to the RRWC
In general, sources of root rot resistance in raspberry germplasm have tended to combine with unacceptable fruit quality, inferior yield, susceptibility to other major diseases or a combination thereof. As a result, several generations of breeding and selection are needed to successfully develop cultivars with the necessary agronomic qualities to be adopted by the industry. At present, the biggest challenge to breeders aiming to improve genetic resistance to the RRWC is the lack of tools available to apply selection pressure on this trait. In the absence of reliable selection pressure (i.e. uniform and consistent disease development), breeders have selected for vigour and improvements in growth and other highly heritable agronomic traits while assuming that Phytophthora resistance is also carried through in some of these selections. Knight (Citation1991) found a high level of resistance to Phytophthora infection in F1 hybrids between R. spectabilis and red raspberry but noted that the level of resistance was reduced in subsequent generations. Similar results have been found by many breeders in their unsuccessful attempts to introgress various sources of root rot resistance into raspberry lines with commercial quality (M. Dossett, personal communication).
Screening methodologies to assess root rot resistance are not sufficiently developed to handle large plant numbers and have not always provided consistent results. For example, Daubeny (Citation1996) reported BC 86-41-15 as an example of a resistant genotype in a greenhouse pot trial, produced from a second backcross generation derivative of R. strigosus. Three years later, Levesque and Daubeny (Citation1999) found this same genotype to be relatively susceptible when it was screened using the same methodology. Similarly, ‘Meeker’ was considered to have some level of resistance to Phytophthora root rot in the early 1990s after its adoption, but it is now considered susceptible (Burlakoti and Dossett Citation2020). Another cultivar, ‘Chilliwack’, was reported (Daubeny and Anderson Citation1993) to have root rot resistance at the time of its release. However, after a few years, Levesque and Daubeny (Citation1999) reported the same cultivar to be susceptible. Adding to the difficulty in identifying resistance to the RRWC is the fact that field screening requires a considerable amount of space and access to sites with appropriate disease pressure, often requiring three or more years to reliably identify resistant genotypes (Hoashi-Erhardt et al. Citation2008).
Among the currently available raspberry cultivars grown in BC, only a few, such as ‘Cascade Harvest’, ‘Cascade Dawn’, and ‘Squamish’, have shown some level of field resistance to root rot, although the level of resistance has often been inconsistent. These inconsistencies may be due to inadequate testing prior to release, differences in microclimate and environmental factors related to infection in different years and at different field sites, build-up of pathogen populations that overcome resistance over time on the same site, and evolution of pathogen populations and shifts of pathogen species composition over time. In addition, association of other pathogens may affect the disease complex. Further research is required to understand the population dynamics and diversity of Phytophthora spp. and to screen new sources of resistance in breeding programmes.
Molecular markers associated with root rot tolerance in raspberry are also quite limited. At present, the only marker available comes from ‘Latham’. Though this locus is likely to be shared with other R. strigosus genotypes, the marker allele does not transfer well for use in germplasm where resistance cannot be directly traced to ‘Latham’ (M. Dossett, pers. obs.). Additional sources of resistance from other Rubus species, including those identified by Bristow et al. (Citation1988) and Kempler et al. (Citation2012b), are being introgressed into elite breeding lines and may carry novel resistance loci and mechanisms, but no markers currently exist to assist with selection of resistant progeny. There has been a lot of recent interest in generating genomics tools for Rubus. Integration of knowledge and data generated from different genomic approaches, such as genome sequencing, transcriptomic, and associated single nucleotide polymorphisms (SNPs) and linkage maps, will provide valuable information for further development of marker assisted breeding in the future (Foster et al. Citation2019; Jarret et al. Citation2020).
Other soil-borne pathogens associated with the RRWC have generally not received as much attention as Phytophthora spp. Root lesion nematodes are known to be a major problem in raspberry, but screening methods have many of the same limitations as those identified for Phytophthora, requiring lots of time and replications, which limit the feasibility of screening large numbers of seedlings. While only a very limited number of clones have been studied, no sources of strong resistance to Pr. penetrans have been identified, suggesting limited utility of marker-assisted selection. Nonetheless, the studies that have been performed (Bristow et al. Citation1980; Vrain and Daubeny Citation1986; Vrain et al. Citation1994; Zasada and Moore Citation2014) indicate that some quantitative variation in both colonization rates and tolerance to Pr. penetrans exist. If this is the case, it may mean that genomic selection could be a useful tool for realizing genetic gain for these traits, if an affordable genotyping platform can be developed for implementation.
The availability of raspberry genome sequences and advances in genomic tools may help in identifying molecular basis of desirable traits and facilitate breeding new cultivars with disease resistance traits (Foster et al. Citation2019; Wight et al. Citation2019). Micro propagation is a common propagation technique in raspberry breeding programmes and nurseries worldwide to obtain healthy planting materials, and rapid multiplication or maintenance of germplasms (Hall et al. Citation2009). The advent in biotechnology has further increased the possibilities of developing disease resistant raspberry cultivars using gene editing methods and Agrobacterium-mediated transformation (Hall et al. Citation2009; Foster et al. Citation2019).
Integrated disease management
The challenges to disease management lie in the fact that most common raspberry cultivars are susceptible or have only a low degree of tolerance to the RRWC and that chemical management options are relatively limited in number and efficacy, as some of the products are registered for suppression of disease. In addition, multiple pathogens could be associated with RRWC and single products may not control all associated pathogens. Development of resistance to metalaxyl and phosphites were reported in strains of Phytophthora causing diseases of other fruits and vegetable crops although the status of fungicide insensitivity of Phytophthora populations associated with RRWC in BC is unknown. In this regard, more studies are needed to understand the fungicide sensitivity of diverse strains of Phytophthora associated with the RRWC in Canada. In addition, further field studies are needed to identify proper application timing for fungicide products with different modes of action. The raspberry industry also needs a fungicide resistance management strategy or plan.
If association of other vascular fungi with the RRWC is confirmed in Canada, additional groups of fungicides may be required to manage the disease complex since fungicides registered for oomycetes do not inhibit fungi. Host resistance may not be effective against all Phytophthora species. For example, some red raspberry cultivars with resistance to P. rubi show susceptibility to P. megasperma (Wilcox and Latorre Citation2002). Therefore, to attain durable and robust genetic resistance, knowledge of the biological variation in Phytophthora species is needed to long-term implementation of an integrated management approach. Pr. penetrans is also associated with the RRWC in the PNW and nematode infestations in roots provide avenues for Phytophthora infection (Daubeny and Anderson Citation1993; Weiland et al. Citation2018). Understanding the mechanism of interaction between P. rubi and Pr. Penetrans is requisite to management of the RRWC in raspberry fields.
Several cultural practices suggested for managing the RRWC are either impractical or not cost effective on a field scale. In the past, chemical soil fumigation was used to control soil-borne diseases, but this practice is now less common. Dominus (allyl isothiocyanate) is a synthetic version of the natural compound found in various Brassica species, which is chemically related to metam products and can reduce buffer zone requirements during field applications (Hoffmann et al. Citation2020). However, this product is very expensive. Brassica cover crops that are used to add organic matter and improve soil health can be used as biofumigants. Brassica crops contain glucosinolates that degrade into isothiocyanates once incorporated into the soil (Kirkegaard and Sarwar Citation1998) and these compounds have antifungal and nematicidal properties (Brown and Morra Citation1997). The ability of brassica cover crops to suppress Phytophthora root rot of raspberry is unknown. Further, the use of non-chemical soil fumigation practices, such as anaerobic soil fumigation and steam sterilization, can be applied before planting; however these approaches are difficult to apply for managing the RRWC after plants are established. Use of biostimulants and products that improve soil microbial diversity and abundance (i.e. improving soil health) present an opportunity to enhance plant resistance (Calvo et al. Citation2014), but these opportunities have yet to be evaluated to manage RRWC. Similarly, plant growth promoting rhizobacteria demonstrate impacts on raspberry yield under field conditions (Orhan et al. Citation2006); however, their impact on the RRWC per se is unknown. Information on the potential use of BCAs to manage RRWC in raspberries is limited primarily to the laboratory context and to studies that are not necessarily specific to the Phytophthora spp. affecting raspberry. Moreover, most BCAs work better under laboratory conditions than in the field where interactions with the native microbial ecosystem and other extraneous factors are more likely to impact their efficacy. Therefore, none of these biological products are currently recommended to control the RRWC in the PNW. Since BCAs have one or more diverse modes of action (FRAC Citation2020), including competition, antibiosis, mycoparasitism, and cell disruption, more studies are required to evaluate and elucidate the host-pathogen-biocontrol interactions for controlling the RRWC.
In summary, integrated disease management of the RRWC requires a combination of all possible approaches, including field establishment with disease-free tissue culture plants, proper site selection and water management, planting less susceptible raspberry cultivars, and appropriate use of pre-plant and post-plant protections chemicals. Applying the knowledge gained on pathogen biology, host-pathogen interactions, breeding efforts, and genomic resources will also help in developing and implementing effective disease management options.
Acknowledgements
We thank the Canadian Agricultural Partnership (CAP) AgriScience Programme and the Raspberry Industry Development Council for funding support (CAP– AgriScience Project No. ASP-007).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abad G, Burgess T, Bienapfl JC, Redford AJ, Coffey M, Knight L. 2019. IDphy: molecular and morphological identification of Phytophthora based on the types. PH2019499. https://idtools.org/id/phytophthora/index.php.
- Adams TM, Armitage AD, Sobczyk MK, Bates HJ, Tabima JF, Kronmiller BA, Tyler BM, Grunwald NJ, Dunwell JM, Nellist CE, et al. 2020. Genomic investigation of the strawberry pathogen Phytophthora fragariae indicates pathogenicity is associated with transcriptional variation in three key races. Front Microbiol. 11:1–17. doi:https://doi.org/10.3389/fmicb.2020.00490.
- Adaskaveg JE, Förster H, Hao W, Gray M. 2017. Potassium phosphite resistance and new modes of action for managing Phytophthora diseases of citrus in the United States. In: Deising HB, Fraaije B, Mehl A, Oerke EC, Sierotzki H, Stammler G, editors. Modern fungicides and antifungal compounds. Braunschweig (Germany): Deutsche Phytomedizinische Gesellschaft; VIII. p. 205−210.
- Agdia Inc. 2017. User guide Phytophthora ImmunoStrip. [accessed 2020 Aug 7]. https://orders.agdia.com/agdia-immunostrip-for-phyt-isk-92601.
- Anonymous. 2013. Crop profile for raspberry in Canada. Pesticide risk reduction program, pest management center agriculture and agri-food Canada. [accessed 2019 Feb 13]. http://publications.gc.ca/site/eng-/9.810691/publication.html.
- Boesewinkel HJ. 1982. A list of 142 new plant disease recordings from New Zealand and short notes on three diseases. Australas Plant Pathol. 11:40–43. doi:https://doi.org/10.1071/APP9820040.
- Bonants P, Hagenaar-de Weerdt M, van Gent-pelzer M, Lacourt I, Cooke D, Duncan J. 1997. Detection and identification of Phytophthora fragariae Hickman by the polymerase chain reaction. Eur J Plant Pathol. 103:345–355. doi:https://doi.org/10.1023/A:1008640227432.
- Brasier CM. 2008. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 57:792–808. doi:https://doi.org/10.1111/j.1365-3059.2008.01886.x.
- Brasier CM, Hansen EM. 1992. Evolutionary biology of Phytophthora part II: phylogeny, speciation, and population structure. Annu Rev Phytopathol. 30:173–200. doi:https://doi.org/10.1146/annurev.py.30.090192.001133.
- Bristow PR, Barritt BH, McElroy FD. 1980. Reaction of red raspberry clones to the root lesion nematode. Acta Hortic. 112:39–46.
- Bristow PR, Daubeny HA, Sjulin TM, Pepin HS, Nestby R. 1988. Evaluation of Rubus germplasm for reaction to root rot caused by Phytophthora erythroseptica. J Am Soc Hortic Sci. 113:588–591.
- Bristow PR, Windom GE. 1992. The effect of sodium tetrathiocarbonate and fosetyl-Al in controlling Phytophthora root rot of red raspberry in the Pacific Northwest. Phytopathology. 82:1132.
- Brown PD, Morra MJ. 1997. Control of soil-borne plant pests using glucosinolate-containing plants. Adv Agron. 61:167–231.
- Burlakoti RR, Dossett M. 2020. Past efforts and future perspectives of managing major diseases of red raspberries in British Columbia. Acta Hortic. 1277:397–402. doi:https://doi.org/10.17660/ActaHortic.2020.1277.56.
- Burlakoti RR, Sapkota S. 2020. Root rot and wilting disease complex of red raspberry in the Fraser Valley of British Columbia in 2018 and 2019. Canadian plant disease survey, volume 100: disease highlights 2019. Can J Plant Pathol. 42(sup1):1–175. doi:https://doi.org/10.1080/07060661.2020.1752524.
- Calvo P, Nelson L, Kloepper JW. 2014. Agricultural uses of plant biostimulants. Plant Soil. 383:3–41.
- Carlock LL, Dotson TA. 2010. Metam-sodium. In: Krieger R, editor. Hayes’ handbook of pesticide toxicology. Academic Press; p. 2293–2306.
- Carris LM, Bristow PR. 1987. Absorption and translocation of metalaxyl in cabbage, red raspberry, and strawberry. J Agric Food Chem. 35:851–855. doi:https://doi.org/10.1021/jf00078a001.
- Converse RH, Schwartze CD. 1968. A root rot of red raspberry caused by Phytophthora erythroseptica. Phytopathology. 58:56–59.
- Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM. 2000. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet Biol. 30:17–32. doi:https://doi.org/10.1006/fgbi.2000.1202.
- Cooke DEL, Duncan JM, Unkles S. 1995. Diagnosis and detection of Phytophthora fragariae in raspberry and strawberry 1. EPPO Bull. 25:95–98. doi:https://doi.org/10.1111/j.1365-2338.1995.tb01443.x.
- Cooke DEL, Schena L, Cacciola SO. 2007. Tools to detect, identify and monitor Phytophthora species in natural ecosystems. J Plant Pathol. 89:13–28.
- Dale A, Moore PP, McNicol RJ, Sjulin TM, Burmistrov LA. 1993. Genetic diversity of red raspberry varieties throughout the-world. J Am Soc Hortic Sci. 118:119–129. doi:https://doi.org/10.21273/JASHS.118.1.119.
- Daubeny H. 2002. Raspberry breeding in the 21st century. Acta Hortic. 585:69–72.
- Daubeny HA. 1987. ‘Chilliwack’ and ‘Comox’ red raspberries. HortScience. 22:1343–1345.
- Daubeny HA. 1996. Brambles. Fruit Breed. 2:109–190.
- Daubeny HA, Anderson A. 1991. ‘Tulameen’ red raspberry. HortScience. 26:1336–1338. doi:https://doi.org/10.21273/HORTSCI.26.10.1336.
- Daubeny HA, Anderson AK. 1993. Achievements and prospects-the British Columbia red raspberry breeding program. Acta Hortic. 352:285–293.
- Daubeny HA, Kempler C. 1995. ‘Qualicum’ red raspberry. HortScience. 30:1470–1472. doi:https://doi.org/10.21273/HORTSCI.30.7.1470.
- Davidse LC, Hofman AE, Velthuis GC. 1983. Specific interference of metalaxyl with endogenous RNA polymerase activity in isolated nuclei from Phytophthora megasperma f. sp. medicaginis. Exp Mycol. 7:344–361. doi:https://doi.org/10.1016/0147-5975(83)90019-1.
- Dossett M, Forge T, Koch C, Kempler C. 2014. Resistance to Phytophthora rubi in wild North American red raspberry germplasm. HortScience. 49:S299.
- Dossett M, Kempler C, Daubeny H. 2013. BC 90-19-34 and BC 93-16-43 red raspberries. HortScience. 48:664–667. doi:https://doi.org/10.21273/HORTSCI.48.5.664.
- Drobek M, Frąc M, Cybulska J. 2019. Plant biostimulants: importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress-a review. Agronomy. 9:10–3390. doi:https://doi.org/10.3390/agronomy9060335.
- Duncan JM. 1980. Persistence of mycelium of Phytophthora fragariae in soil. Trans Br Mycol Soc. 75:383–387. doi:https://doi.org/10.1016/S0007-1536(80)80117-3.
- Duncan JM. 1990. Phytophthora species attacking strawberry and raspberry 1. EPPO Bull. 20:107–115. doi:https://doi.org/10.1111/j.1365-2338.1990.tb01186.x.
- Duncan JM, Cowan JB. 1980. Effect of temperature and soil moisture content of persistence of infectivity of Phytophthora fragariae in naturally infested field soil. Trans Br Mycol Soc. 75:133–139. doi:https://doi.org/10.1016/S0007-1536(80)80203-8.
- Duncan JM, Kennedy DM. 1989. The effect of waterlogging on Phytophthora root rot of red raspberry. Plant Pathol. 38:161–168. doi:https://doi.org/10.1111/j.1365-3059.1989.tb02129.x.
- Duncan JM, Kennedy DM, Scott PH., et al 1991. Relationships between non-papillate, soilborne species of Phytophthora: root rot of raspberry. In: Lucas JA, Shattock RC, Shaw DS, and Cooke LR, editors. Phytophthora. Cambridge (UK): Cambridge University Press; p. 129−147.
- Duncan JM, Kennedy DM, Seemuller E. 1987. Identities and pathogenicities of Phytophthora spp. causing root rot of red raspberry. Plant Pathol. 36:276–289. doi:https://doi.org/10.1111/j.1365-3059.1987.tb02235.x.
- Ellis MA, Miller SA. 1993. Using a Phytophthora-specific immunoassay kit to diagnose raspberry Phytophthora root rot. HortScience. 28:642–644. doi:https://doi.org/10.21273/HORTSCI.28.6.642.
- Ezziyyani M, Requena ME, Egea‐Gilabert C, Candela ME. 2007. Biological control of Phytophthora root rot of pepper using Trichoderma harzianum and Streptomyces rochei in combination. J Phytopathol. 155:342–349. doi:https://doi.org/10.1111/j.1439-0434.2007.01237.x.
- FAOSTAT. 2018. Food and Agriculture Organization of the United Nations. FAOSTAT Database. [accessed 2020 Sep 21]. http://www.fao.org/faostat/en/#data/QC.
- Finn CE, Kempler C, Moore PP. 2008. Raspberry cultivars: what’s new? What’s succeeding? Where are breeding programmes headed? Acta Hortic. 777:33–40.
- Foster TM, Bassil NV, Dossett M, Worthington ML, Graham J. 2019. Genetic and genomic resources for Rubus breeding: a roadmap for the future. Hortic Res. 6:1–9. doi:https://doi.org/10.1038/s41438-019-0199-2.
- [FRAC] Fungicide Resistance Action Committee. 2020. FRAC code list: fungicides sorted by mode of action. [accessed 2020 May 4]. http://www.frac.info.
- Funahashi F, Parke JL. 2016. Effects of soil solarization and Trichoderma asperellum on soilborne inoculum of Phytophthora ramorum and Phytophthora pini in container nurseries. Plant Dis. 100:438–443. doi:https://doi.org/10.1094/PDIS-04-15-0453-RE.
- Gigot JA, Zasada IA, Walters TW. 2013b. Integration of brassicaceous seed meals into red raspberry production systems. Appl Soil Ecol. 64:23–31. doi:https://doi.org/10.1016/j.apsoil.2012.10.013.
- Gigot J, Walters TW, Zasada IA. 2013a. Impact and occurrence of Phytophthora rubi and Pratylenchus penetrans in commercial red raspberry (Rubus idaeus) fields in Northwestern Washington. Int J Fruit Sci. 13:357–372. doi:https://doi.org/10.1080/15538362.2013.748373.
- Goldberger JR, Devetter LW, Dentzman KE. 2019. Polyethylene and biodegradable plastic mulches for strawberry production in the United States: experiences and opinions of growers in three regions. HortTechnology. 29:619–628. doi:https://doi.org/10.21273/HORTTECH04393-19.
- Graham J, Hackett CA, Smith K, Woodhead M, Hein I, McCallum S. 2009. Mapping QTLs for developmental traits in raspberry from bud break to ripe fruit. Theor Appl Genet. 118:1143–1155. doi:https://doi.org/10.1007/s00122-009-0969-6.
- Graham J, Hackett CA, Smith K, Woodhead M, MacKenzie K, Tierney I, Cooke D, Bayer M, Jennings N. 2011. Towards an understanding of the nature of resistance to Phytophthora root rot in red raspberry. Theor Appl Genet. 123:585–601. doi:https://doi.org/10.1007/s00122-011-1609-5.
- Graham KA, Beck BR, Zasada IA, Scagel CF, Weiland JE. 2021. Growth, sporulation, and pathogenicity of the raspberry pathogen Phytophthora rubi under different temperature and moisture regimes. Plant Dis. 9:1–7.
- Grote D, Olmos A, Kofoet A, Tuset JJ, Bertolini E, Cambra M. 2002. Specific and sensitive detection of Phytophthora nicotianae by simple and nested-PCR. Eur J Plant Pathol. 108:197–207. doi:https://doi.org/10.1023/A:1015139410793.
- Grünwald NJ, Martin FN, Larsen MM, Sullivan CM, Press CM, Coffey MD, Hansen EM, Parke JL. 2011. Phytophthora-ID. org: a sequence-based Phytophthora identification tool. Plant Dis. 95:337–342. doi:https://doi.org/10.1094/PDIS-08-10-0609.
- Guest D, Grant B. 1991. The complex action of phosphonates as antifungal agents. Biol Rev. 66:159–187. doi:https://doi.org/10.1111/j.1469-185X.1991.tb01139.x.
- Haas BJ, Kamoun S, Zody MC, Jiang RHY, Handsaker RE, Cano LM, Grabherr M, Kodira CD, Raffaele S, Torto-Alalibo T, et al. 2009. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature. 461:393–398. doi:https://doi.org/10.1038/nature08358.
- Hall HK, Kummer KE, Jamieson AR, Jennings SN, Weber CA. 2009. Raspberry breeding. Plant breeding reviews. Vol. 32. Portland (OR):Timber Press, Inc; p. 39–353.
- Hardham AR, Blackman LM. 2018. Phytophthora cinnamomi. Mol Plant Pathol. 19:60–285. doi:https://doi.org/10.1111/mpp.12568.
- Hardham AR, Hyde GJ. 1997. Asexual sporulation in the oomycetes. Adv Bot Res. 24:353–398. Academic Press.
- Heiberg N. 1995. Control of root rot of red raspberries caused by Phytophthora fragariae var. rubi. Plant Pathol. 44:153–159.
- Heiberg N. 1996. Effects of black plastic mulching in red raspberry. Norw Agric Res. 10:15–23.
- Heiberg N. 1999. Effects of raised beds, black soil mulch and oxadixyl on root rot (Phytophthora fragariae var. rubi) in red raspberry. Acta Hortic. 505:249–255. doi:https://doi.org/10.17660/ActaHortic.1999.505.32.
- Heiberg N, Semb L, Duncan JM, Kennedy DM. 1989. Raspberry root rot in Norway. Acta Hortic. 262:189–192.
- Hickman CJ. 1941. The red core root disease of the strawberry caused by Phytophthora fragariae n. sp. J Pomol Hort Sci. 18:89–118.
- Hoashi-Erhardt WK, Moore PP, Windom GE, Bristow PR. 2008. Field and greenhouse response of red raspberry genotypes to root rot. HortScience. 43:1367–1370. doi:https://doi.org/10.21273/HORTSCI.43.5.1367.
- Hoffmann M, Ajwa HA, Westerdahl BB, Koike ST, Stanghellini M, Wilen C, Fennimore SA. 2020. Multitactic preplant soil fumigation with allyl isothiocyanate in cut flowers and strawberry. HortTechnology. 1:1–8.
- Huang J, Gu LF, Zhang Y, Yan TX, Kong GH, Kong L, Guo BD, Qiu M, Wang Y, Jing MF, et al. 2017. An oomycete plant pathogen reprograms host pre-mRNA splicing to subvert immunity. Nat Commun. 8:1–15. doi:https://doi.org/10.1038/s41467-017-02233-5.
- Hughes KJD, Inman AJ, Cooke DEL. 2000. Comparative testing of nested PCR‐based methods with bait‐plant tests for detecting Phytophthora fragariae var. fragariae in infected strawberry roots from fruit crops in the UK. EPPO Bull. 30:533–538. doi:https://doi.org/10.1111/j.1365-2338.2000.tb00942.x.
- Hwang J, Benson DM. 2005. Identification, mefenoxam sensitivity, and compatibility type of Phytophthora spp. attacking floriculture crops in North Carolina. Plant Dis. 89:185–190. doi:https://doi.org/10.1094/PD-89-0185.
- Ilieva E, Arulappan FX, Pieters R. 1995. Phytophthora root and crown rot of raspberry in Bulgaria. Eur J Plant Pathol. 101:623–626. doi:https://doi.org/10.1007/BF01874866.
- Jarret D, Jennings N, Williams D, Graham J. 2020. Development and use of genetic tools in Rubus and Ribes breeding at James Hutton Institute/Limited. Acta Hortic. 1277:1–10.
- Jennings DL. 1982. New raspberry cultivar Glen Moy. Scottish Crop Research Institute for 1981. p. 71–72.
- Jennings SN, Brennan RM, Gordon SC. 2003. Commercial breeding for pest and disease resistance in cane and bush fruits. IOBC WPRS Bull. 26:67–72.
- Jennings SN, Graham J, Ferguson L, Young V. 2016. New developments in raspberry breeding in Scotland. Acta Hortic. 1133:23–28.
- Joshi V, Jeffries M. 2013. Diseases diagnosed on commercial crops submitted to the British Columbia Ministry of Agriculture plant health laboratory in 2012. Can Plant Dis Surv. 93:7–17.
- Joshi V, Jeffries M. 2017. Diseases/symptoms diagnosed on commercial crop samples submitted to the British Columbia Ministry of Agriculture plant health laboratory in 2016. Can Plant Dis Surv. 98:6–16.
- Judelson HS, Blanco FA. 2005. The spores of Phytophthora: weapons of the plant destroyer. Nat Rev Microbiol. 3:47–58. doi:https://doi.org/10.1038/nrmicro1064.
- Jung T, Jung MH, Scanu B, Seress D, Kovács GM, Maia C, Pérez-Sierra A, Chang TT, Chandelier A, Heungens K, et al. 2017. Six new Phytophthora species from ITS Clade 7a including two sexually functional heterothallic hybrid species detected in natural ecosystems in Taiwan. Pers: Mol Phylog Evol Fungi. 38:100–135.
- Kamoun S. 2006. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol. 44:41–60. doi:https://doi.org/10.1146/annurev.phyto.44.070505.143436.
- Kempler C, Daubeny HA. 1999. Development of fresh market raspberry cultivars. Acta Hortic. 505:121–128.
- Kempler C, Daubeny HA. 2000. ‘Malahat’ red raspberry. HortScience. 35:783–785. doi:https://doi.org/10.21273/HORTSCI.35.4.783.
- Kempler C, Daubeny HA, Frey L, Walters T. 2006. ‘Chemainus’ red raspberry. HortScience. 41:1364–1366. doi:https://doi.org/10.21273/HORTSCI.41.5.1364.
- Kempler C, Daubeny HA, Harding B, Baumann T, Finn CE, Moore PP, Walters T. 2007. ‘Saanich’ red raspberry. HortScience. 42:176–178. doi:https://doi.org/10.21273/HORTSCI.42.1.176.
- Kempler C, Daubeny HA, Harding B, Kowalenko G. 2005. ‘Cowichan’ red raspberry. HortScience. 40:1916–1918.
- Kempler C, Hall H, Finn CE. 2012a. Raspberry. In: Badenes ML, Byrne DH, editors. Fruit breeding. Boston (MA): Springer; p. 263−304.
- Kempler C, Muehlchen AM, Forge TA. 2012b. Screening for resistance to Phytophthora root rot in raspberries: identifying new sources of resistance. Acta Hortic. 926:59–64.
- Kennedy DM, Duncan JM. 1991. Methods for assessing the resistance of raspberry genotypes to Phytophthora root rot. Plant Pathol. 40:387–394. doi:https://doi.org/10.1111/j.1365-3059.1991.tb02395.x.
- Kennedy DM, Duncan JM. 1993. Occurrence of races in Phytophthora fragariae var rubi on raspberry. Acta Hortic. 352:555–562.
- Kim DS, Hoffmann M, Kim S, Scholler BA, Fennimore SA. 2020. Integration of steam with allyl-isothiocyanate for soil disinfestation. HortScience. 55:920–925. doi:https://doi.org/10.21273/HORTSCI14600-20.
- Kirkegaard JA, Sarwar M. 1998. Biofumigation potential of brassicas: I. variation in glucosinolate profiles of diverse field-grown brassicas. Plant Soil. 201:71–89. doi:https://doi.org/10.1023/A:1004364713152.
- Klootwyk B. 2020. Raspberry 2020 processed market outlook summary. In: Kabaluk T, Frey L, editors. Proceedings of the Lower Mainland Horticulture Improvement Association 62th Annual Horticulture Growers’ Short Course; January 30–February 1; Abbotsford, BC. p. 29–31.
- Knight VH. 1991. Use of the salmonberry, Rubus spectabilis Pursh., in red raspberry breeding. J Hortic Sci. 66:575–581. doi:https://doi.org/10.1080/00221589.1991.11516186.
- Knight VH, Fernández Fernández F. 2008. Screening for resistance to Phytophthora fragariae var. rubi in Rubus germplasm at East Malling. Acta Hortic. 777:353–360.
- Koprivica M, Dulic-Markovic I, Jevtic R, Cooke DE. 2009. Methods for detection of Phytophthora fragariae var. rubi on raspberry. Pestic Phytomed (Belgrade). 24:177–184.
- Kroon LPNM, Bakker FT, Van Den Bosch GBM, Bonants PJM, Flier WG. 2004. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet Biol. 41:766–782. doi:https://doi.org/10.1016/j.fgb.2004.03.007.
- Latorre BA, Muñoz R. 1993. Root rot of red raspberry caused by Phytophthora citricola and P. citrophthora in Chile. Plant Dis. 77:715–718. doi:https://doi.org/10.1094/PD-77-0715.
- Laun N, Zinkernagel V. 1993. Methods of screening raspberries for resistance to Phytophthora root rot. Acta Hortic. 352:569–578. doi:https://doi.org/10.17660/ActaHortic.1993.352.83.
- Laun N, Zinkernagel V. 1997. A comparison of the resistance against Phytophthora fragariae var. rubi, the causal agent of a raspberry root rot. J Phytopathol. 145:197–204. doi:https://doi.org/10.1111/j.1439-0434.1997.tb00386.x.
- Levesque CA, Daubeny HA. 1999. Variation in reaction to Phytophthora fragariae var. rubi in raspberry genotypes. Acta Hortic. 505:231–235. doi:https://doi.org/10.17660/ActaHortic.1999.505.29.
- Maloney KE, Wilcox WF, Sanford JC. 1993. Raised beds and metalaxyl for controlling phytophthora root rot of raspberry. HortScience. 28:1106–1108. doi:https://doi.org/10.21273/HORTSCI.28.11.1106.
- Maloney K, Pritts M, Wilcox W, Kelly MJ. 2005. Suppression of Phytophthora root rot in red raspberries with cultural practices and soil amendments. HortScience. 40:1790–1795. doi:https://doi.org/10.21273/HORTSCI.40.6.1790.
- Man in’t Veld WA. 2007. Gene flow analysis demonstrates that Phytophthora fragariae var. rubi constitutes a distinct species, Phytophthora rubi comb. nov. Mycologia. 99:222–226.
- Martin FN, Abad ZG, Balci Y, Ivors K. 2012. Identification and detection of Phytophthora: reviewing our progress, identifying our needs. Plant Dis. 96:1080–1103. doi:https://doi.org/10.1094/PDIS-12-11-1036-FE.
- Martin FN, Blair JE, Coffey MD. 2014. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet Biol. 66:19–32. doi:https://doi.org/10.1016/j.fgb.2014.02.006.
- Mazzola M, Brown J, Izzo AD, Cohen MF. 2007. Mechanism of action and efficacy of seed meal-induced pathogen suppression differ in a brassicaceae species and time-dependent manner. Phytopathology. 97:454–460. doi:https://doi.org/10.1094/PHYTO-97-4-0454.
- McElroy FD. 1977. Distribution of stylet-bearing nematodes associated with raspberries and strawberries in British Columbia. Can Plant Dis Surv. 57:3–8.
- McGregor GR, Franz P. 2002. Field management of root rot in raspberries caused by Phytophthora spp. Acta Hortic. 585:293–297. doi:https://doi.org/10.17660/ActaHortic.2002.585.47.
- Messenger BJ, Menge JA, Pond E. 2000. Effects of gypsum soil amendments on avocado growth, soil drainage, and resistance to Phytophthora cinnamomi. Plant Dis. 84:612–616. doi:https://doi.org/10.1094/PDIS.2000.84.6.612.
- Moore PP. 2004. ‘Cascade Delight’ red raspberry. HortScience. 39:185–187. doi:https://doi.org/10.21273/HORTSCI.39.1.185.
- Moore PP. 2006. ‘Cascade Dawn’ red raspberry. HortScience. 41:857–859. doi:https://doi.org/10.21273/HORTSCI.41.3.857.
- Moore PP, Daubeny HA. 1993. ‘Meeker’ red raspberry. Fruit Var J. 47:2–4.
- Moore PP, Finn CE. 2007. ‘Cascade Bounty’ red raspberry. HortScience. 42:93–396. doi:https://doi.org/10.21273/HORTSCI.42.2.393.
- Moore PP, Hoashi-Erhardt W, Finn CE, Martin RR, Dossett M. 2015. ‘Cascade Harvest’ red raspberry. HortScience. 50:624–627. doi:https://doi.org/10.21273/HORTSCI.50.4.624.
- Moore PP, Hoashi-Erhardt W, Finn CE, Martin RR, Dossett M. 2019. ‘WSU 2166ʹ red raspberry. HortScience. 541:564–567.
- Moore PP, Hoashi-Erhardt WK. 2020. Comparison of selection for root rot tolerance and machine harvestability. Acta Hortic. 1277:149–154.
- Narayan RD, Blackman LM, Shan W, Hardham AR. 2010. Phytophthora nicotianae transformants lacking dynein light chain 1 produce non-flagellate zoospores. Fungal Genet Biol. 47:663–671. doi:https://doi.org/10.1016/j.fgb.2010.04.008.
- Nestby R, Heiberg N. 1995. Genetic variation for resistance to Phytophthora fragariae var. rubi in red raspberries. Euphytica. 81:143–149. doi:https://doi.org/10.1007/BF00025426.
- Newton AC, Duncan JM, Augustin NH, Guy DC, Cooke DEL. 2010. Survival, distribution, and genetic variability of inoculum of the strawberry red core pathogen, Phytophthora fragariae var. fragariae, in soil. Plant Pathol. 59:472–479. doi:https://doi.org/10.1111/j.1365-3059.2010.02273.x.
- Nikolić M, Ivanović M, Milenković S, Milivojević J, Milutinović M. 2008. The state and prospects of raspberry production in Serbia. Acta Hortic. 777:243–249.
- Orhan E, Esitken A, Ercisli S, Turan M, Sahin F. 2006. Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci Hortic. 111:38–43. doi:https://doi.org/10.1016/j.scienta.2006.09.002.
- Pattison JA, Samuelian SK, Weber CA. 2007. Inheritance of Phytophthora root rot resistance in red raspberry determined by generation means and molecular linkage analysis. Theor Appl Genet. 115:225–236. doi:https://doi.org/10.1007/s00122-007-0558-5.
- Pattison JA, Weber CA. 2005. Evaluation of red raspberry cultivars for resistance to Phytophthora root rot. J Am Pomol Soc. 59:50–56.
- Pattison JA, Wilcox WF, Weber CA. 2004. Assessing the resistance of red raspberry (Rubus idaeus L.) genotypes to Phytophthora fragariae var. rubi in hydroponic culture. HortScience. 39:1553–1556. doi:https://doi.org/10.21273/HORTSCI.39.7.1553.
- Pinkerton JN, Bristow PR, Windom GE, Walters TW. 2009. Soil solarization as a component of an integrated program for control of raspberry root rot. Plant Dis. 93:452–458. doi:https://doi.org/10.1094/PDIS-93-5-0452.
- Pinkerton JN, Ivors KL, Reeser PW, Bristow PR, Windom GE. 2002. The use of soil solarization for the management of soilborne plant pathogens in strawberry and red raspberry production. Plant Dis. 86:645–651. doi:https://doi.org/10.1094/PDIS.2002.86.6.645.
- Porras M, Barrau C, Arroyo FT, Santos B, Blanco C, Romero F. 2007. Reduction of Phytophthora cactorum in strawberry fields by Trichoderma spp. and soil solarization. Plant Dis. 91:142–146. doi:https://doi.org/10.1094/PDIS-91-2-0142.
- Ristaino JB, Gumpertz ML. 2000. New frontiers in the study of dispersal and spatial analysis of epidemics caused by species in the genus Phytophthora. Annu Rev Phytopathol. 38:541–576. doi:https://doi.org/10.1146/annurev.phyto.38.1.541.
- Rudolph RE, Zasada IA, Hesse C, DeVetter LW. 2019. Brassicaceous seed meal, root removal, and chemical fumigation vary in their effects on soil quality parameters and Pratylenchus penetrans in a replanted floricane raspberry production system. Appl Soil Ecol. 133:44–51. doi:https://doi.org/10.1016/j.apsoil.2018.08.024.
- Rudolph R, Zasada I, Devetter L. 2016. Soil quality and cover crops in red raspberry in the Pacific Northwest. Whatcom Ag Monthly. [accessed 2020 Jan 13]. http://whatcom.wsu.edu/ag/edu/sfc/documents/sfc2015/Rudolph_SFC2015.pdf.
- Samtani JB, Gilbert C, Weber JB, Subbarao KV, Goodhue RE, Fennimore SA. 2012. Effect of steam and solarization treatments on pest control, strawberry yield, and economic returns relative to methyl bromide fumigation. HortScience. 47:64–70. doi:https://doi.org/10.21273/HORTSCI.47.1.64.
- Schlenzig A, Cooke DEL, Chard JM. 2005. Comparison of a baiting method and PCR for the detection of Phytophthora fragariae var. rubi in certified raspberry stocks. EPPO Bull. 35:87–91. doi:https://doi.org/10.1111/j.1365-2338.2005.00806.x.
- Schneider SM, Ajwa HA, Trout TJ, Gao S. 2008. Nematode control from shank- and drip-applied fumigant alternatives to methyl bromide. HortScience. 43:1826–1832. doi:https://doi.org/10.21273/HORTSCI.43.6.1826.
- Schwinn FJ, Margot P. 1991. Control with chemicals. Adv Plant Pathol. 7:225–265.
- Shennan C, Muramoto J, Baird G, Zavatta M, Toyama L, Mazzola M, Koike ST. 2016. Anaerobic soil disinfestation (ASD): a strategy for control of soil borne diseases in strawberry production. Acta Hortic. 1137:113–119. doi:https://doi.org/10.17660/ActaHortic.2016.1137.16.
- Smith VL, Wilcox WF, Harman GE. 1990. Potential for biological control of Phytophthora root and crown rots of apple by Trichoderma and Gliocladium spp. Phytopathology. 80:880–885. doi:https://doi.org/10.1094/Phyto-80-880.
- Song TQ, Ma ZC, Shen DY, Li Q, Li WL, Su LM, Ye TY, Zhang MX, Wang YC, Dou DL. 2015. An oomycete CRN effector reprograms expression of plant HSP genes by targeting their promoters. PLoS Pathog. 11:1–30. doi:https://doi.org/10.1371/journal.ppat.1005348.
- Stam R, Jupe J, Howden AJ, Morris JA, Boevink PC, Hedley PE, Huitema E. 2013. Identification and characterization CRN effectors in Phytophthora capsici shows modularity and functional diversity. PloS One. 8:1–13. doi:https://doi.org/10.1371/annotation/90bd45cb-33a7-426f-a928-9ddc351b08cc.
- Stasikowski PM, McComb JA, Scott P, Paap T, O’Brien PA, Hardy GSJ. 2014. Calcium sulphate soil treatments augment the survival of phosphite-sprayed Banksia leptophylla infected with Phytophthora cinnamomi. Australas Plant Pathol. 43:369–379. doi:https://doi.org/10.1007/s13313-014-0303-x.
- Statistics Canada. 2020. Table 32-10-0364-01 area, production and farm gate value of marketed fruits. doi:https://doi.org/10.25318/3210036401-eng.
- Stephens MJ, Enfield JR, Hall HK. 2012. ‘Wakefield’ red raspberry. HortScience. 47:1556–1558. doi:https://doi.org/10.21273/HORTSCI.47.10.1556.
- Stewart JE, Kroese D, Tabima JF, Larsen MM, Fieland VJ, Press CM, Zasada IA, Grünwald NJ. 2014. Pathogenicity, fungicide resistance, and genetic variability of Phytophthora rubi isolates from raspberry (Rubus idaeus) in the western United States. Plant Dis. 98:1702−. 1708. doi:https://doi.org/10.1094/PDIS-11-13-1130-RE.
- Strautina S, Krasnova I, Kalnina I, Kampuss K. 2012. Results of red raspberry breeding in Latvia. Acta Hortic. 946:171–176.
- Tabima JF, Coffey MD, Zazada IA, Grünwald NJ. 2018. Populations of Phytophthora rubi show little differentiation and high rates of migration among states in the western United States. Mol Plant Microbe Interact. 31:614–622. doi:https://doi.org/10.1094/MPMI-10-17-0258-R.
- Tabima JF, Kronmiller BA, Press CM, Tyler BM, Zasada IA, Grünwald NJ. 2017. Whole genome sequences of the raspberry and strawberry pathogens Phytophthora rubi and P. fragariae. Mol Plant Microbe Interact. 30:767–769. doi:https://doi.org/10.1094/MPMI-04-17-0081-A.
- Tani S, Yatzkan E, Judelson HS. 2004. Multiple pathways regulate the induction of genes during zoosporogenesis in Phytophthora infestans. Mol Plant Microbe Interact. 17:330–337. doi:https://doi.org/10.1094/MPMI.2004.17.3.330.
- Thomson SV, Allen RM. 1976. Mechanisms of survival of zoospores of Phytophthora parasitica in irrigation water. Phytopathology. 66:1198–1202. doi:https://doi.org/10.1094/Phyto-66-1198.
- Turner D, Ken M. 1985. The handbook of soft fruit growing. London (UK): Croom Helm Ltd.
- Tyler BM. 2002. Molecular basis of recognition between Phytophthora pathogens and their hosts. Annu Rev Phytopathol. 40:137–167. doi:https://doi.org/10.1146/annurev.phyto.40.120601.125310.
- Valois D, Fayad K, Barasubiye T, Garon M, Dery C, Brzezinski R, Beaulieu C. 1996. Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var. rubi, the causal agent of raspberry root rot. Appl Environ Microbiol. 62:1630–1635. doi:https://doi.org/10.1128/aem.62.5.1630-1635.1996.
- Van’t Klooster JW, van Den Berg VG, van West P, Govers F. 2000. tef1, a Phytophthora infestans gene encoding translation elongation factor 1α. Gene. 249:145–151. doi:https://doi.org/10.1016/S0378-1119(00)00151-7.
- Veena SS, Anandaraj M, Sarma YR. 2010. Variability in the sensitivity of Phytophthora capsici isolates to potassium phosphonate. Indian Phytopathol. 63:71–75.
- Villa NO, Kageyama K, Asano T, Suga H. 2006. Phylogenetic relationships of Pythium and Phytophthora species based on ITS rDNA, cytochrome oxidase II and β-tubulin gene sequences. Mycologia. 98:410–422. doi:https://doi.org/10.3852/mycologia.98.3.410.
- Vleeshouwers VG, Rietman H, Krenek P, Champouret N, Young C, Oh SK, Wang M, Bouwmeester K, Vosman B, Visser RG, and Jacobsen E. 2008. Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS One. 3:e2875.
- Vrain TC, Daubeny HA. 1986. Relative resistance of red raspberry and related genotypes to the root lesion nematode. HortScience. 21:1435–1437.
- Vrain TC, Daubeny HA, Hall JW, DeYoung RM, Anderson AK. 1994. Inheritance of resistance to root lesion nematode in red raspberry. HortScience. 29:1340–1341. doi:https://doi.org/10.21273/HORTSCI.29.11.1340.
- Vrain TC, Pepin HS. 1989. Effect of Pratylenchus penetrans on root rot of red raspberry caused by Phytophthora erythroseptica. Acta Hortic. 262:231–240.
- Vrain T, DeYoung R, Hall J, Freyman S. 1996. Cover crops resistant to root-lesion nematodes in raspberry. HortScience. 31:1195–1198. doi:https://doi.org/10.21273/HORTSCI.31.7.1195.
- Walters T, Gigot J, Zasada I. 2011b. Preplant soil fumigation and alternatives for berry production. Washington State University Extension Fact Sheet FS064E.
- Walters T, Particka M, Zasada I, Pinkerton JN. 2011a. Productivity and economics of methyl bromide alternatives for raspberry nursery. Proceedings of Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, Orlando, FL, USA.
- Walters TW, Bolda M, Zasada IA. 2017. Alternatives to current fumigation practices in western states raspberry. Plant Health Prog. 18:104–111. doi:https://doi.org/10.1094/PHP-RS-16-0068.
- Wang W, and Jiao F. 2019. Effectors of Phytophthora pathogens are powerful weapons for manipulating host immunity. Planta. 250:413−425.
- Washington WS. 1988. Phytophthora cryptogea as a cause of root rot of raspberry in Australia; resistance of raspberry cultivars and control by fungicides. Plant Pathol. 37:225–230. doi:https://doi.org/10.1111/j.1365-3059.1988.tb02068.x.
- Waterston JM. 1937. A note on the association of a species of Phytophthora with a “Die-Back” disease of the raspberry. Trans Bot Soc Edinburgh. 32:251–259. doi:https://doi.org/10.1080/13594863709441518.
- Weber RW, Entrop AP. 2017. Dactylonectria torresensis as the main component of the black root rot complex of strawberries and raspberries in northern Germany. Erwerbs-Obstbau. 59:157–169. doi:https://doi.org/10.1007/s10341-017-0343-9.
- Weiland JE, Benedict C, Zasada IA, Scagel CR, Beck BR, Davis A, Graham K, Peetz A, Martin RR, Dung JK, et al. 2018. Late-summer disease symptoms in Western Washington red raspberry fields associated with co-occurrence of Phytophthora rubi, Verticillium dahliae, and Pratylenchus penetrans, but not raspberry bushy dwarf virus. Plant Dis. 102:938–947. doi:https://doi.org/10.1094/PDIS-08-17-1293-RE.
- Whisson SC. 2010. Phytophthora. Encyclopedia of life sciences. Chichester: John Wiley and Sons, Ltd. p. 1–9.
- Wight H, Zhou J, Li M, Hannenhalli S, Mount S, Liu Z. 2019. Draft genome assembly and annotation of red raspberry Rubus idaeus. BioRxiv:546135.
- Wilcox WF. 1989. Identity, virulence, and isolation frequency of seven Phytophthora spp. causing root rot of raspberry in New York. Phytopathology. 79:93–101. doi:https://doi.org/10.1094/Phyto-79-93.
- Wilcox WF, Latorre BA. 2002. Identities and geographic distributions of Phytophthora spp. causing root rot of red raspberry in Chile. Plant Dis. 86:1357–1362. doi:https://doi.org/10.1094/PDIS.2002.86.12.1357.
- Wilcox WF, Nevill JR, Burr JA. 1999b. Susceptibility of red, black and purple raspberry cultivars to three Phytophthora species under greenhouse and field conditions. Acta Hortic. 505:241–248.
- Wilcox WF, Pritts MP, Kelly MJ. 1999a. Integrated control of Phytophthora root rot of red raspberry. Plant Dis. 83:1149–1154. doi:https://doi.org/10.1094/PDIS.1999.83.12.1149.
- Wilcox WF, Scott PH, Hamm PB, Kennedy DM, Duncan JM, Brasier CM, Hansen EM. 1993. Identity of a Phytophthora species attacking raspberry in Europe and North America. Mycol Res. 97:817–831. doi:https://doi.org/10.1016/S0953-7562(09)81157-X.
- Wilkinson CJ, Shearer BL, Jackson TJ, Hardy GSJ. 2001. Variation in sensitivity of Western Australian isolates of Phytophthora cinnamomi to phosphite in vitro. Plant Pathol. 50:83–89. doi:https://doi.org/10.1046/j.1365-3059.2001.00539.x.
- Zasada IA, Moore PP. 2014. Host status of Rubus species and hybrids for the root lesion nematode, Pratylenchus penetrans. HortScience. 49:1128–1131. doi:https://doi.org/10.21273/HORTSCI.49.9.1128.
- Zasada IA, Weiland JE, Han Z, Walters TW, Moore P. 2015. Impact of Pratylenchus penetrans on establishment of red raspberry. Plant Dis. 99:939–946. doi:https://doi.org/10.1094/PDIS-09-14-0980-RE.
- Zasada I, Pinkerton JN, Walters T, Particka M. 2010. Methyl bromide alternatives trials in raspberry nurseries. Proceedings of Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions; Orlando (FL). p. 1–5.
- Zhang H, Flury M, Miles C, Liu H, Devetter L. 2020. Soil-biodegradable plastic mulches undergo minimal in-soil degradation in a perennial raspberry system after 18 months. Horticulturae. 6:1–15. doi:https://doi.org/10.3390/horticulturae6030047.