Abstract
Impatiens balsamina (garden balsam) is an annual herb and widely grown for ornamental and medicinal use in China. In 2020 and 2021, powdery mildew symptoms were found on I. balsamina in Xinxiang City, Henan Province, China. The pathogenic fungus was identified based on its morphological characteristics and molecular analysis. The internal transcribed spacer (ITS) region was amplified and sequenced. The sequence obtained for the fungus showed 100% identity with the previously reported Podosphaera xanthii. The virulence of the pathogen on I. balsamina was confirmed by utilizing Koch’s postulates. Therefore, we identified the causal organism of this disease as P. xanthii. This is the first report of powdery mildew caused by P. xanthii on I. balsamina in China. The identification of P. xanthii on I. balsamina will support efforts towards its future control and management.
Résumé: Impatiens balsamina (balsamine des jardins) est une herbe annuelle cultivée extensivement en Chine pour ses propriétés tant ornementales que médicinales. En 2020 et 2021, des symptômes du blanc ont été décelés chez I. balsamina à Xinxiang, dans la province du Henan. Le champignon pathogène a été identifié en fonction de ses caractéristiques morphologiques et par analyse moléculaire. La région de l’espaceur transcrit interne (ITS) a été amplifiée et séquencée. La séquence obtenue pour le champignon a affiché 100% d’identité avec le champignon Podosphaera xanthii précédemment rapporté. La virulence de l’agent pathogène à l’égard de I. balsamina a été confirmée en vérifiant les postulats de Koch. En conséquence, nous avons identifié l’organisme causal de cette maladie comme étant P. xanthii. Il s’agit de la première mention du blanc causé par P. xanthii chez I. balsamina en Chine. L’identification de P. xanthii chez I. balsamina contribuera à appuyer les futurs efforts de lutte et de gestion.
Introduction
Impatiens balsamina L., garden balsam, is an annual plant that is cultivated widely in home gardens due to its value as an ornamental. In China, the flowers of this plant are traditionally utilized for preparing a red dye to paint fingernails. Recently, Impatiens balsamina was found to be a suitable plant for the bioremediation of contaminated soils due to its phytostabilization of polychlorinated biphenyls, lubricant oil and lead (Gamage et al. Citation2021; Liu et al. Citation2021). In addition, it has been extensively used in traditional Chinese medicine to treat various ailments (Ding et al. Citation2008). Previous studies demonstrated that various components isolated from multiple I. balsamina organs showed antimicrobial, antitumor and anti-inflammatory activities (Sakunphueak and Panichayupakaranant Citation2012; Szewczyk Citation2018; Lee et al. Citation2020).
Common fungal diseases of Impatiens include mainly brown spot (Cercosporafu mshian), powdery mildew (Sphaerotheca balsaminae = Podosphaera balsaminae), wilt (Rhizoctonia solani), ring spot (Ascochyta phaseolorum), downy mildew (Plasmopara obduce), and anthracnose (Phytophthora nicotianae) (Zhou et al. Citation2015). Powdery mildew was repeatedly reported as a disease on I. balsamina and severely affected plant health (Farr and Rossman Citation2020). Previously, various powdery mildew fungi were demonstrated to infect I. balsamina. For example, Golovinomyces biocellatus and P. balsaminae were reported to cause powdery mildew on I. balsamina in Argentina, China, Germany, India, Japan and Korea (Hanlin and Amano Citation1986; Akram Citation1995; Wolcan Citation2004; Choi et al. Citation2021), while Podosphaera xanthii was reported as the cause of powdery mildew on I. balsamina in India (Mulpuri et al. Citation2016) and Thailand (Meeboon Citation2016). Generally, powdery mildew infection of I. balsamina resulted in senescence, colour changing, the loss of leaves and even plant death.
In October 2020 and July 2021, powdery mildew-like signs and symptoms were observed on leaves of I. balsamina grown in home gardens in Henan Province, China. White to greyish powder-like masses were abundant on both sides of the leaves and covered up to 90% of the leaf area (). Some of infected leaves were chlorotic or senesced. More than 90% of the examined plants exhibited these signs and symptoms. However, the causal organism of the pathogen was unclear. Therefore, examination of morphological characteristics and molecular analysis were used to identify the pathogenic fungus.
Materials and Methods
Collection and morphological characterization
Mildew-infected plant leaves were collected and transported to the lab. In order to morphologically characterize the fungi, the leaves were bleached, and fungal structures on the leaves were stained with trypan blue and observed under a light microscope (Sunny Optical, EX30, Zhejiang, China) (Zhu et al. Citation2017).
DNA extraction and amplification
To collect fungal conidia, mildew-infected leaves were painted with 5% (w/v) cellulose acetate dissolved in acetone. After evaporation of the acetone, cellulose-acetate strips were harvested. Total fungal genomic DNA was extracted according to a previous method (Zhu et al. Citation2019). The fungal ribosomal ITS segment universal primers, ITS1 (5ʹ-TCCGTAGGTGAACCTGCGG-3ʹ) and ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) (White et al. Citation1990), were used for polymerase chain reaction (PCR) amplification, using the following program: 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 54°C for 30 s, 72°C for 1 min, and final elongation at 72°C for 5 min. PCR products were detected by electrophoresis in 1.5% agarose gels. The amplicon was sequenced and the resulting sequence was deposited in GenBank (NCBI) as accession no. MW301281.
Phylogenetic analysis
To build of a phylogenetic tree, ITS sequences of Podosphaera sp. were retrieved and analyzed with the ITS sequence of the isolated fungus () in MEGA software. The evolutionary tree was constructed using the neighbor-joining method with the following options: 1000 bootstraps replicates, p-distance, 50% site coverage cut off (Zhu et al. Citation2021a). The ITS sequences of Erysiphe sp. and Arthrocladiella mougeotii were included as outgroups (Zhu et al. Citation2020a; Zhu et al. Citation2020b).
Table 1. Podosphaera sp. and their corresponding hosts
Pathogenicity assay
Briefly, three healthy plants were placed in a settling tower and then inoculated by blowing pathogen conidia from diseased leaves with pressurized air into the settling tower (Zhu et al. Citation2021b). Three non-inoculated plants served as controls. The controls and inoculated plants were maintained separately in two growth chambers (temperature 18°C; humidity 60%; light/dark 16 h/8 h) and disease signs and symptoms were checked daily. After signs and symptoms appeared, the morphological characteristics of the powdery mildew on infected leaves were determined to confirm whether these were consistent with the disease on originally infected plants.
Results
Morphological characteristics of the powdery mildew on Impatiens balsamina
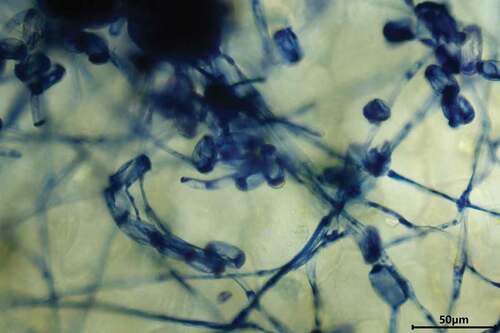
White to greyish powder-like masses were abundant on both sides of I. balsamina leaves and covered up to 90% of the leaf area. Some infected leaves were chlorotic or senesced. More than 90% of plants exhibited these signs and symptoms. Cylindrical conidiophores (n = 50) were 90–220 × 10–13 μm and composed of foot cells, followed by shorter cells and conidia. Foot cells (n = 50) were straight and 40 to 80-μm long. The ellipsoid-ovoid-shaped conidia (n = 50) with conspicuous fibrosin bodies were 26–39 × 15–25 μm with a length/width ratio of 1.6 to 2.0 (). No chasmothecia were observed. Based on these morphological characteristics of fungal structures by microscopy analysis, the pathogen was initially identified as P. xanthii.
Fig. 2 Fungal structures of P. xanthii on host leaf surface. The structures were stained with trypan blue.
Confirmation of the powdery mildew fungus by molecular analysis
BLASTn analysis using the ITS fragment amplified in this study (MW301281) indicated that the sequence was 100% identical (563/563) to P. xanthii on Abelmoschus esculentus. The same results were obtained with the powdery mildew collected in 2021.
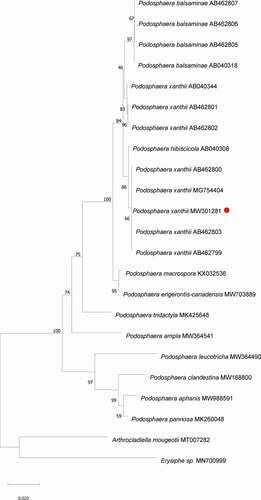
The phylogenetic tree clearly showed that the ITS sequence of the powdery mildew fungus on I. balsamina clustered with P. xanthii on A. esculentus (MG754404) and on I. balsamina (AB462803, AB462799, AB462800) ().
Fig. 3 PCR detection of the powdery mildew on I. balsamina samples collected in 2021 (A) and 2020 (B). M: marker; 1–4: diseased samples.
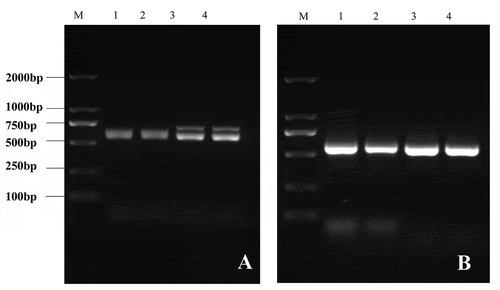
Fig. 4 Phylogenetical analysis of P. xanthii and Podosphaera sp. The neighbour joining tree was constructed in MEGA software with 1000 bootstrap replicates and p-distance method. The bar indicates a distance of 0.020. Red dot highlights P. xanthii. Arthrocladiella mougeotii and Erysiphe sp. were included as outgroup.
Pathogenicity of identified powdery mildew
Pathogenicity assays were performed using an inoculation test. Signs of powdery mildew were visible on the inoculated plants 10 days after inoculation (), while the controls remained healthy. The same results were obtained from the two repeated pathogenicity experiments. Therefore, the pathogen was identified and confirmed as P. xanthii based on morphological and molecular analysis.
Discussion
Impatiens balsamina is an important plant with high ornamental, bioremediation and medical value (Ding et al. Citation2008; Sakunphueak and Panichayupakaranant Citation2012; Szewczyk Citation2018; Lee et al. Citation2020; Gamage et al. Citation2021; Liu et al. Citation2021). However, its quality and survival are significantly affected by fungal pathogens, including powdery mildews. Previously, Golovinomyces biocellatus and P. balsaminae were identified as the causal agents of powdery mildew on I. balsamina in many countries, including China (Tai Citation1979; Hanlin and Amano Citation1986; Akram Citation1995; Wolcan Citation2004). However, P. xanthii was recently reported to cause powdery mildew on I. balsamina in India (Mulpuri et al. Citation2016) and Thailand (Meeboon Citation2016). Podosphaera xanthii is known to cause powdery mildew on many ornamental plants (Farr and Rossman Citation2020) and is one of the most important limiting pathogens in the production of cucurbits (Pérez-García et al. Citation2010). Whether P. xanthii could cause powdery mildew on I. balsamina in China was not known.
In 2020 and 2021, powdery mildew was observed on I. balsamina in Xinxiang City, Henan province, China (). Initially, we microscopically examined the morphology of the fungus () and found that its characteristics were consistent with those of P. xanthii (Braun and Cook Citation2012). To confirm this identification, we used molecular and phylogenetic analysis (). The sequence of our powdery mildew was 100% identical to that of P. xanthii on A. esculentus (Choi et al. Citation2018), and our putative P. xanthii clustered with P. xanthii previously reported on I. balsamina (Ito and Takamatsu Citation2010). Earlier, it was demonstrated that isolates of different powdery mildew species on Impatiens formed distinct clades (Ito and Takamatsu Citation2010). While the ITS sequence of the powdery mildew we identified from I. balsamina also showed 100% identity with the previously reported P. balsaminae on I. balsamina, in agreement with Ito and Takamatsu (Citation2010), our phylogenetic analysis confirmed that the powdery mildew fungus identified in this study was P. xanthii. The pathogenic ability of the powdery mildew was tested with inoculation assays, which showed that P. xanthii successfully infected I. balsamina and that signs of powdery mildew developed 10-days after inoculation (). Therefore, the causal organism of powdery mildew disease on I. balsamina was identified and confirmed as P. xanthii.
To the best of our knowledge, this is the first record of P. xanthii causing powdery mildew on I. balsamina in China. The sudden outbreak of the disease caused by P. xanthii may adversely affect plant health and thus reduce the ornamental and medical value of I. balsamina. Therefore, the identification and confirmation of P. xanthii infecting I. balsamina provides additional insights into the hosts of this pathogen in China and will support disease control efforts in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akram M. 1995. Impatiens balsamina: a natural host of Sphaerotheca fuliginea. Plant Disease. 79(7):754. doi:https://doi.org/10.1094/pd-79-0754a.
- Braun U, Cook RTA. 2012. Taxonomic Manual of Erysiphales (Powdery Mildews). Vol. 11. Utrecht (Netherlands): CBS Biodiversity Series 11. (CBS Biodiversity Series 11.
- Choi IY, Ju HJ, Shin HD. 2021. First report of powdery mildew caused by Podosphaera xanthii on New Guinea impatiens in Korea. New Dis Rep. 43. doi:https://doi.org/10.1002/ndr2.12017.
- Choi IY, Kim JH, Uhm MJ, Cho SE, Shin HD. 2018. First report of powdery mildew caused by Podosphaera xanthii on okra in Korea. Plant Dis. 102(8):1663. doi:https://doi.org/10.1094/PDIS-01-18-0028-PDN.
- Cornejo J, Elfar K, Latorre B. 2019. First report of powdery mildew caused by Podosphaera pannosa on Mandarin ‘W. Murcott’in Chile. Plant Dis. 103(10):2690. doi:https://doi.org/10.1094/PDIS-04-19-0714-PDN.
- Ding Z-S, Jiang F-S, Chen N-P, Gui-Yuan L, Zhu C-G. 2008. Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina. Molecules. 13(2):220–229. doi:https://doi.org/10.3390/molecules13020220.
- Ellingham O, Denton G, Denton J, Robinson R. 2016. First report of Podosphaera macrospora on Heuchera in the United Kingdom. New Dis Rep. 33. doi:https://doi.org/10.5197/j.2044-0588.2016.033.023.
- Farr D, Rossman A. 2020. Fungal Databases, US National Fungus Collections, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/” after the word ”USDA
- Gamage SSW, Masakorala K, Brown MT, Gamage SMKW. 2021. Comparative phytoremediation potentials of Impatiens balsamina L. and Crotalaria retusa L. for soil contaminated with used lubricating oil. Env Adv. 5:100095. doi:https://doi.org/10.1016/j.envadv.2021.100095
- Garibaldi A, Gilardi G, Gullino M. 2005. First report of powdery mildew caused by Podosphaera leucotricha on Photinia× fraserii in Italy. Plant Dis. 89(12):1362. doi:https://doi.org/10.1094/PD-89-1362D.
- Hanlin RT, Amano K. 1986. Host range and geographical distribution of the powdery mildew fungi. Mycologia. 82(4):533. doi:https://doi.org/10.2307/3760031.
- Hirata T, Cunnington JH, Paksiri U, Limkaisang S, Shishkoff N, Grigaliunaite B, Sato Y, Takamatsu S. 2000. Evolutionary analysis of subsection Magnicellulatae of Podosphaera section Sphaerotheca (Erysiphales) based on the rDNA internal transcribed spacer sequences with special reference to host plants. Can J Bot. 78(12):1521–1530. doi:https://doi.org/10.1139/b00-124.
- Ito M, Takamatsu S. 2010. Molecular phylogeny and evolution of subsection Magnicellulatae (Erysiphaceae: podosphaera) with special reference to host plants. Mycoscience. 51(1):34–43. doi:https://doi.org/10.1007/s10267-009-0005-3.
- Lee TH, Suh WS, Subedi L, Kim SY, Choi SU, Lee KR, Kim CS. 2020. Three new oleanane-type triterpenoidal glycosides from Impatiens balsamina and their biological activity. Plants. 9(9):1083. doi:https://doi.org/10.3390/plants9091083.
- Liu W, Wu J, Lian J, Zhang X, Zeb A, Zhou Q, Sun Y. 2021. Potential use of Impatiens balsamina L. for bioremediation of lead and polychlorinated biphenyl contaminated soils. Land Degrad Dev. 32(13):3773–3784. doi:https://doi.org/10.1002/ldr.3857.
- Meeboon J. 2016. Notes on powdery mildews (Erysiphales) in Thailand I. Podosphaera sect. Sphaerotheca. Plant Pathol Quar. 6(2):142–174. doi:https://doi.org/10.5943/ppq/6/2/5.
- Mulpuri S, Soni PK, Gonela SK. 2016. Morphological and molecular characterization of powdery mildew on sunflower (Helianthus annuus L.), alternate hosts and weeds commonly found in and around sunflower fields in India. Phytoparasitica 44(3):353–367. doi:https://doi.org/10.1007/s12600-016-0531-4.
- Pérez-García A, Romero D, Fernández-Ortuño D, López-Ruiz F, Vicente AD, Torés J. 2010. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol Plant Pathol. 10(2):153–160. doi:https://doi.org/10.1111/j.1364-3703.2008.00527.x.
- Sakunphueak A, Panichayupakaranant P. 2012. Comparison of antimicrobial activities of naphthoquinones from Impatiens balsamina. Nat Prod Res. 26(12):1119–1124. doi:https://doi.org/10.1080/14786419.2010.551297.
- Smith RL, May TW, Kaur J, Sawbridge TI, Mann RC, Pascoe IG, Edwards J. 2021. Re-evaluation of the Podosphaera tridactyla species complex in Australia. J Fungi. 7(3):171. doi:https://doi.org/10.3390/jof7030171.
- Solano-Báez AR, Leyva-Mir SG, Camacho-Tapia M, Victoria AA, Rodríguez-Bautista G, Sánchez-Rosas CS, Márquez-Licona G. 2021. First Report of Podosphaera aphanis causing powdery mildew on wild blackberry species (Rubus spp.) in Mexico. Plant Dis. doi:https://doi.org/10.1094/PDIS-05-21-0932-PDN
- Szewczyk K. 2018. Phytochemistry of the genus Impatiens (Balsaminaceae): a review. Biochem Syst Ecol. 80:94–121. doi:https://doi.org/10.1016/j.bse.2018.07.001
- Tai FL. 1979. Sylloge Fungorum Sinicorum. Science Press Peking.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications. 18(1):315–322.
- Wolcan S. 2004. Podosphaera balsaminae on Impatiens balsamina and Impatiens× hawked. Australas Plant Pathol. 33(1):133–134. doi:https://doi.org/10.1071/AP03075.
- Zhou S, Dong X, Guo W, Liu J, Li Y. 2015. Cultivation points of Impatiens and control of diseases and insect Pests. Special Economic Animal and Plant. 18(11):4.
- Zhu M, Ji J, Duan X, Li Y. 2021a. First Report of Golovinomyces cichoracearum causing powdery mildew on Zinnia elegans in China. Plant Dis. 105(4):1213. doi:https://doi.org/10.1094/pdis-11-20-2333-pdn.
- Zhu M, Ji J, Shi W, Li Y. 2021b. Occurrence of powdery mildew caused by Blumeria graminis f. sp. poae on Poa pratensis in China. Plant Dis. 105(4):1212. doi:https://doi.org/10.1094/pdis-09-20-2051-pdn.
- Zhu M, Ji J, Zhao M, Chai J, Li YF. 2020a. First report of powdery mildew caused by an Erysiphe sp. on Aristolochia debilis in China. Plant Dis. 104:7. doi:https://doi.org/10.1094/PDIS-01-20-0007-PDN
- Zhu M, Riederer M, Hildebrandt U. 2017. Very-long-chain aldehydes induce appressorium formation in ascospores of the wheat powdery mildew fungus Blumeria graminis. Fungal Biol. 121(8):716–728. doi:https://doi.org/10.1016/j.funbio.2017.05.003.
- Zhu M, Riederer M, Hildebrandt U. 2019. UV-C irradiation compromises conidial germination, formation of appressoria, and induces transcription of three putative photolyase genes in the barley powdery mildew Fungus, Blumeria graminis f. sp. hordei. Fungal Biol. 123(3):218–230. doi:https://doi.org/10.1016/j.funbio.2018.12.002.
- Zhu M, Zhao M, Ji J, Yang C, Li YF. 2020b. First report of Arthrocladiella mougeotii causing powdery mildew on Lycium chinense Mill. in Henan, China. Plant Dis. 104(11):1. doi:https://doi.org/10.1094/PDIS-02-20-0445-PDN.


