Abstract
Grey mould, caused by Botrytis cinerea, is an increasingly destructive disease of ginseng (Panax ginseng) in China. However, the extent of variation in the morphological characteristics of fungal colonies, genetic background and pathogenicity of different strains of this pathogen remains unknown. In this study, we isolated and identified 102 isolates of B. cinerea from ginseng. Among these, the sclerotial phenotype was dominant, followed by the mycelial and conidial phenotypes, with the proportion of each phenotype in the pathogen population showing no obvious variance across three provinces. Isolates were separated into three groups with differing pathogenicity, based on the diameter of the lesions they caused in pathogenicity tests; moderate-pathogenicity isolates were dominant, followed by strong- and weak-pathogenicity isolates. Variation was observed in the pathogenicity of isolates from three provinces. The Nei’s genetic diversity index and the Shannon’s information index of isolates from Jilin province was highest, followed by those from Liaoning, while isolates from Heilongjiang displayed the lowest genetic diversity. Furthermore, most of the genetic variance was found within the geographically defined B. cinerea populations; the low-genetic variance among populations was probably counteracted by gene flow. Results from this study provide a base understanding of genetic and pathogenic variation in B. cinerea populations from the main ginseng-growing regions of China, which will be helpful for further research and control of grey mould of ginseng.
Résumé: La pourriture grise, causée par Botrytis cinerea, est une maladie de plus en plus destructrice du ginseng (Panax ginseng) en Chine. Toutefois, l’ampleur de la variation sur le plan des caractéristiques morphologiques des colonies fongiques, du bagage génétique et de la pathogénicité des différentes souches de cet agent pathogène est toujours inconnue. Dans cette étude, nous avons isolé et identifié 102 isolats de B. cinerea provenant du ginseng. Parmi ceux-ci, le phénotype des sclérotes dominait, suivi des phénotypes du mycélium et des conidies, la proportion de chaque phénotype dans la population d’agents pathogènes n’affichant aucune variance évidente dans trois provinces. Les isolats ont été divisés en trois groupes de pathogénicité différente, en se basant sur le diamètre des lésions qu’ils causaient au cours de tests de pathogénicité: les isolats dont la pathogénicité était modérée étaient les plus nombreux, suivis des isolats à pathogénicité élevée, puis à faible pathogénicité. Une variation a été observée dans la pathogénicité des isolats de trois provinces. L’indice de diversité génétique de Nei et l’indice de Shannon des isolats de la province de Jilin étaient les plus élevés, suivis des isolats de la province du Liaoning, tandis que ceux du Heilongjiang ont affiché le degré de diversité génétique le plus faible. En outre, le plus fort pourcentage de variance génétique a été trouvé dans les populations de B. cinerea géographiquement définies; le faible pourcentage de variance génétique des populations a probablement été atténué par le flux génique. Les résultats de cette étude établissent les fondements de la compréhension des variations génétiques et pathogéniques dans les populations de B. cinerea dans les principales régions productrices de ginseng de Chine, ce qui contribuera à la poursuite de recherches plus approfondies ainsi qu’à la lutte contre la pourriture grise du ginseng.
Introduction
Plant disease results from the interaction of hosts and pathogens through complex molecular pathways (Soltis et al. Citation2019). Pathogens typically possess a narrow range of hosts. However, the airborne necrotrophic fungus Botrytis cinerea (teleomorph: Botryotinia fuckeliana) causes grey mould on more than 586 plant species worldwide (AbuQamar et al. Citation2016; Korolev and Elad Citation2016). Under conditions of moderate temperature and high-relative humidity, B. cinerea infects fruits, leaves, stems, and other plant organs, rapidly producing large amounts of asexual conidia on infected tissues (O’Neill et al. Citation1997). Multiple cycles of infection-sporulation-dissemination will usually occur in a single growing season (Decognet et al. Citation2009).
Botrytis cinerea has become a model organism for understanding the complexity of fungal pathogenesis, owing to its wide host range, adaptability and great economic impact. Unfortunately, despite much effort, the origins, diversity and dispersion of B. cinerea are still unclear. Non-host specificity, wide adaptability, frequent variations in virulence, and multiple infection modes and survival forms make it very difficult to control the diseases caused by B. cinerea (Williamson et al. Citation2007). Furthermore, this fungus usually infects hosts and then remains quiescent, causing disease when environmental conditions become appropriate and hosts experience physiological deterioration (Van Kan Citation2006).
Variability in phenotype, morphology, pathogenicity and fungicide resistance provides B. cinerea with strong adaptability. Genetic studies have revealed that this fungus is extremely diverse (Fournier et al. Citation2003). Botrytis cinerea was initially separated into two sympatric sibling species (Giraud et al. Citation1997), but was later partitioned into group I and group II, with group II showing higher genetic diversity and being the predominant infectious agent (Fournier et al. Citation2005; Fekete et al. Citation2012).
Molecular markers, including restriction fragment length polymorphisms (RFLP) (Asadollahi et al. Citation2013), random amplified polymorphic DNA (RAPD) (Kumari et al. Citation2014), the presence and absence of transposable elements (Levis et al. Citation1997), amplified fragment length polymorphisms (AFLP) (Moyano et al. Citation2003) and simple sequence repeats (SSR) (Leyronas et al. Citation2015) greatly facilitate the study of fungal genetic diversity, and multilocus profiles generated by molecular markers are highly suitable for studying the genetic diversity and population evolution of complex phytopathogens such as B. cinerea. Inter-simple sequence repeats (ISSR) markers have been applied extensively to population genetic diversity studies in plants (Kumar Citation2018), insects (Sun et al. Citation2015) and fungi (Tiago et al. Citation2016), because they offer stable amplification that is cost- and time-effective and easy to perform.
Panax ginseng grows naturally in China and several other Asian countries and is a valuable traditional Chinese herbal medicine (Jung et al. Citation2014). Its roots are used to bolster immunity, provide nutrition, ameliorate fatigue, and enhance resistance to stress, disease and exhaustion (Yeh et al. Citation2003). Commercial ginseng currently on the market comes almost exclusively from cultivated sources (Dong et al. Citation2018). Grey mould is one of the most important diseases of cultivated ginseng plants (Liu et al. Citation2020; Zhou et al. Citation2020). The presence of severe infection by B. cinerea negatively affects photosynthesis and the accumulation of dry matter including ginsenosides, resulting in significant yield and quality decline (Li and Li Citation2010; Zhou et al. Citation2020).
Fungicide treatment is the most effective strategy for grey mould control, and a mixture of fungicides is usually used (Kim et al. Citation2009; Rashid et al. Citation2014). Unfortunately, fungicide efficacy can be unpredictable because of host diversity, short pathogen life cycle, prolific reproduction and high genetic variability of B. cinerea populations (Bardas et al. Citation2010; Shao et al. Citation2020). There is currently only very limited information available regarding B. cinerea, as the causative agent of ginseng grey mould (Yuan et al. Citation2016; Liu et al. Citation2020; Zhou et al. Citation2020). In the present work, we studied the variability of genetic structure and pathogenicity of B. cinerea isolates from ginseng-growing regions of China. Our objectives were to (1) characterize the genetic and morphological variation among isolates; (2) determine pathogenic variability; and (3) clarify relationships between morphological type and pathogenicity.
Materials and methods
Pathogen collection
In total, 118 ginseng leaves with symptoms of grey mould were sampled from 20 sites across the main ginseng-growing regions of Liaoning, Jilin and Heilongjiang provinces of China from June to August 2018. After transfer to the laboratory in sterilized, self-sealing plastic bags, diseased leaves were kept moist in sterilized Petri dishes without growth medium for 1 to 2 days. Conidia of B. cinerea that developed on the foliar lesions were transferred onto sterile potato dextrose agar (PDA) supplemented with 100 mg·L−1 streptomycin sulphate and incubated at 20°C for 4 days. Pure cultures of B. cinerea were obtained by transferring a small number of spores to fresh PDA Petri dishes using a dissecting needle. Following colony development, mycelia were transferred to fresh PDA dishes in one or two cycles of purification until the phenotypes of all isolates from the same diseased leaf were identical. Pure cultures were maintained on PDA slants and stored in 15% (v/v) glycerol stocks at – 80°C.
Cultural and morphological characteristics observation
All isolates were identified on the basis of morphology and nucleotide sequence. Colony morphology, colour of mycelia, production of sclerotia and its arrangement, colour, and size on PDA was determined. Morphological characteristics of mycelia and conidiophores were observed using a Leica DM2500 optical microscope (Leica Microsystem, Nussloch, Germany).
Molecular identification
Isolates were individually transferred to potato dextrose broth (PDB) and incubated at 20°C with shaking at 130 rpm in the dark for 3 days. Mycelia of B. cinerea isolates were then washed with sterile water three times and dried at 65°C for 4 h. Samples were ground thoroughly under liquid nitrogen, and total genomic DNA was extracted using a fungal DNA Microextraction Kit D3390 (Omega Bio-Tek) according to the manufacturer’s instructions. The yield and integrity of the genomic DNA were checked using 1% (w/v) agarose gel electrophoresis. DNA quality and concentration were determined using a Nanodrop spectrophotometer (Thermo Fisher, MA) and diluted to a final concentration of 30 ng·μL−1.
The universal PCR primers ITS1 and ITS4 (White et al. Citation1990) and specific primers C729+ and 729- (Rigotti et al. Citation2002) were used to identify the purified isolates. Amplification products were visualized using 1% (w/v) agarose gel electrophoresis. Nucleotide sequences of the amplicons were queried against the NCBI database (https://www.ncbi.nlm.nih.gov/) using the BlastN program.
Pathogenicity assay
The pathogenicity of B. cinerea isolates was examined on healthy leaves of 4-year-old ginseng seedlings. After sterilizing with 75% (v/v) alcohol, leaves were washed with sterile water three times and dried naturally in a sterile environment. Mycelial plugs 5 mm in diameter were prepared from one PDA Petri dish for each isolate. For each isolate, two pieces of sterile filter paper wetted with 2.5 mL distilled water were placed on the bottom of a sterile Petri dish 9 cm in diameter. One piece of a palmate compound leaf was placed on the filter paper with the adaxial surface up. Leaves were punctured near the centre using a sterile toothpick, and a mycelial plug was placed onto the wound with the mycelial surface down. Sterile PDA plugs were used to inoculate-control leaves. Pathogenicity assay was repeated six times with a 2-days interval; each isolate was inoculated on three leaves each day. After inoculation, the Petri dishes were sealed with parafilm to maintain high relative humidity and incubated at 20°C in the dark for 3 days. Disease severity was evaluated as the mean diameter of lesions developed on the leaves using the crossover method (Zhang et al. Citation2014). Data were analyzed using Statistical Package for the Social Science (SPSS) v. 23.0 (SPSS Inc. Chicago, IL, United States) with the Student’s t-test and the one-way analysis of variance (ANOVA).
Genetic diversity assessment of B. cinerea isolates
Genetic variation among B. cinerea isolates was evaluated using 10 polymorphic ISSR markers designed by the University of British Columbia, Canada (http://www.ubc.ca; Supplementary Table S1).
Amplification reactions were performed in a final volume of 25 µL containing 30 ng of template DNA, 0.4 µmol·L−1 of primer, 0.2 mmol·L−1 of dNTPs (TransGen Biotech), 1× reaction buffer (containing 20 mmol·L−1 of Tris-HCl pH 8.3, 20 mmol·L−1 of KCl, 10 mmol·L−1 of (NH4)2SO4, 2 mmol·L−1 of MgSO4, TransGen Biotech) and 1.25 U Taq DNA polymerase (TransGen Biotech). Polymerase chain reactions were performed in a T100 Thermal Cycler (BIO-RAD, Singapore) with the following program: 1 cycle at 94°C for 5 min, followed by 35 cycles at 94°C for 45s, annealing at 49 to 54°C depending on the primer (see Supplementary Table S1) for 45s and 72°C for 120s; final extension at 72°C for 7 min. Amplicons were separated on 1% (w/v) agarose gel and visualized using a Gel Imager ChampGel 5000 (Zhejiang Biotechnology, China). A binary qualitative data matrix was constructed from banding profiles with scores of ‘1’ indicating the presence and ‘0’ indicating the absence of a band.
Pairwise genetic distances were estimated using Nei’s coefficient (Nei Citation1978). A dendrogram was reconstructed using the unweighted pair-group method with arithmetical averages (UPGMA) algorithm and computation for multivariate analysis using NTSYS-pc version 2.10e (Tang et al. Citation2015). Popgene v. 1.32 was used to analyze the effective number of alleles (Ne), number of alleles observed (Na), Nei’s genetic diversity index (H), Shannon’s information index (I), number of polymorphic loci (NP), percentage of polymorphic loci (PPL), total genetic diversity (Ht), intra-population genetic diversity (Hs), inter-population genetic diversity (Dst), coefficient of genetic differentiation (Gst) and gene flow (Nm) (Yeh et al. Citation1999). SPSS v. 23.0 (Kirkpatrick and Butler Citation2015) and GenALEx v. 6.502 (Peakall and Smouse Citation2012) were used for genetic diversity index calculation and principal coordinate analysis (PCoA).
Results
Botrytis cinerea isolates collected from ginseng-growing regions of China
We successfully purified 102 isolates (Supplementary Table S2), which were all identified as B. cinerea on the basis of colony morphology, conidiogenous structure, spore characteristics, and nucleotide sequence similarity. Colonies of B. cinerea were initially white and gradually turned grey. Hyphae were septate and hyaline, bearing pseudo-dichotomously ramified conidiophores. A 700 bp band was amplified using the ITS1/ITS4 primers and showed the highest (≥99.4%) sequence similarity with sequences (including MN891765, MF405181, KY308184) of B. cinerea in GenBank. The specific 710 bp band amplified by C729+/729- primers confirmed that all of these isolates were B. cinerea.
Cultural and morphological characteristics
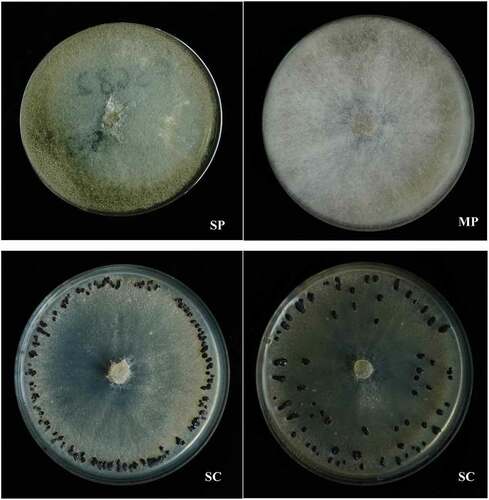
We categorized all 102 B. cinerea isolates into three groups. Twenty isolates (19.6%) were of a mycelia phenotype (MP) with well-developed aerial hyphae, conidia formed only at the centre and periphery, no sclerotia formed; seven isolates (6.9%) were of a conidia phenotype (SP), with strong sporulation, very few aerial hyphae, and no sclerotia formed; 75 isolates (73.5%) were of a sclerotia phenotype (SC), with rich sclerotia arranged regularly towards the periphery or irregularly on the Petri dish and few aerial hyphae formed ().
Fig. 1 Morphological characteristics and sclerotia distributing patterns of Botrytis cinerea isolates. (SP) with strong sporulation, very little aerial hyphae, no sclerotia formed; (MP) with well-developed aerial hyphae, conidia formed only at the centre and periphery, no sclerotia formed; (SC) with rich sclerotia arranging regularly towards the periphery or irregularly on the Petri dish, little aerial hyphae formed.
The SC isolates were predominant, representing 76.2%, 77.8% and 66.7% of B. cinerea isolates from Liaoning, Jilin and Heilongjiang provinces of China, respectively. The MP isolates accounted for 14.3%, 15.6% and 27.8%, respectively. SP was a minor category, accounting for only 9.5%, 6.7% and 5.6% of isolates, respectively.
Genetic diversity of B. cinerea populations
In total, 72 polymorphic bands were amplified using 10 ISSR primers. Each ISSR primer yielded five to nine bands. indicates the genetic diversity among the B. cinerea isolates tested. In general, the effective number of alleles (Ne = 1.5575) was close to the number of alleles observed (Na = 2.0), which indicates that alleles were distributed evenly among B. cinerea isolates from the three provinces. In addition, Nei’s genetic diversity index (H), Shannon’s information index (I), the number of polymorphic loci (NP) and the percentage of polymorphic loci (PPL) were 0.3339, 0.5062, 72, and 100%, respectively, indicating that ISSRs were suitable for analyzing the genetic diversity of the B. cinerea populations.
Table 1. Genetic diversity of Botrytis cinerea isolates from Jilin, Liaoning, and Heilongjiang provinces of China.
We observed genetic variance among B. cinerea isolates from different ginseng-growing regions. The genetic diversity of B. cinerea isolates from Jilin was highest (H = 0.3350, I = 0.5063), followed by Liaoning (H = 0.3139, I = 0.4795), and genetic diversity of isolates from Heilongjiang was the lowest (H = 0.2323, I = 0.3634). The total genetic diversity (Ht), the intra-population genetic diversity (Hs) and the inter-population genetic diversity (Dst) of B. cinerea isolates were 0.3395, 0.2937 and 0.0458, respectively, indicating that most of the genetic variance was from within geographically distributed populations. The mean coefficient of genetic differentiation (Gst) was 0.1348 and the gene flow (Nm) was 3.2084, which indicates that the genetic variance among B. cinerea populations from different geographic regions was counteracted significantly by gene flow, resulting in populations with a similar genetic background.
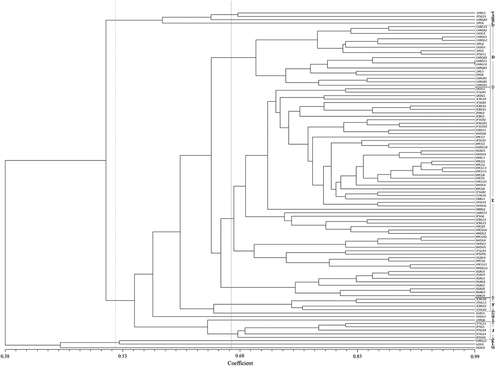
To investigate the relationship among B. cinerea isolates, we calculated a similarity matrix based on the simple matching coefficient and performed multivariate PCoA analysis based on ISSR statistical data. The coefficient of genetic similarity of B. cinerea isolates ranged from 0.38 to 0.99. The resulting dendrogram derived from UPGMA analysis divided the 102 B. cinerea isolates into four groups at a threshold of 0.52: group I (LHRG1, JFSG21, LHRQ42 and JJYG6), group III (LHRG22 and JZJG1), group IV (LKDG4) and group II (the other 95 isolates). At the threshold of 0.65, all isolates were divided into 13 groups (groups A to M); groups A, D, E, F and J contained 2, 18, 61, 4 and 5 isolates, respectively, while each of the other groups had only one isolate. Furthermore, all isolates in group D were from Liaoning and Jilin provinces, and most of the isolates (except LHRG73) in group E were from Heilongjiang and Jilin provinces; all isolates in group F and group J were from Jilin province (). We found no significant correlation between geographic origin and the dendrogram clustering results; however, complex genetic diversity was observed among these B. cinerea isolates, and a few of them, such as LKDG4, LHRG1 and JFSG21, showed obvious genetic variance from the others.
Fig. 2 Unweighted pair-group method with arithmetic means (UPGMA) clustering dendrogram of Botrytis cinerea isolates using Neighbour-Joining similarity estimates. At the threshold of 0.65, all isolates were divided into 13 groups (groups A to M); groups A, D, E, F and J contained 2, 18, 61, 4 and 5 isolates, respectively, while each of the other groups had only one isolate. Furthermore, all isolates in group D were from Liaoning and Jilin provinces of China, and most of the isolates (except LHRG73) in group E were from Heilongjiang and Jilin provinces of China; all isolates in group F and group J were from Jilin province of China.
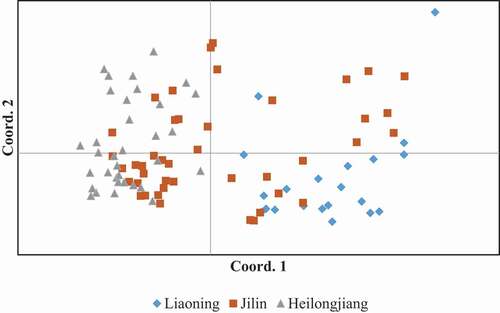
The PCoA plot () presents the genetic distances of B. cinerea isolates from the different ginseng-growing regions. Botrytis cinerea isolates from Liaoning and Heilongjiang provinces can be clearly distinguished, indicating that B. cinerea isolates from these two provinces were genetically distinct. Botrytis cinerea isolates from Jilin province mixed with those from Liaoning and Heilongjiang provinces, indicating that genetic exchange between B. cinerea isolates from Jilin province with those from Liaoning and Heilongjiang provinces occurred. Furthermore, obvious genetic differentiation within B. cinerea populations was observed, especially in the population from Jilin province.
Pathogenicity of B. cinerea isolates
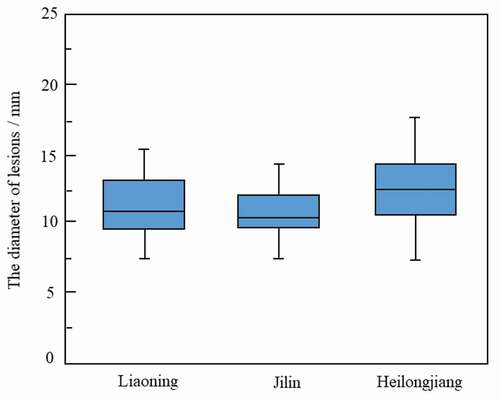
All 102 isolates were pathogenic on ginseng plants. Wet blotch lesions were observed 1 day post-inoculation (dpi), and these lesions enlarged and turned brown gradually at 3 to 5 dpi. However, pathogenicity varied significantly, and the diameter of lesions that developed on ginseng leaves ranged from 3.6 to 20.3 mm, with a mean value of 11.4 mm (Supplementary Table S3). The diameter of lesions produced by isolates from Liaoning ranged from 6.6 to 16.5 mm, with a mean value of 11.3 mm. Lesions produced by isolates from Jilin and Heilongjiang provinces were 3.6 to 16.0 mm, with a mean of 10.7 mm, and 6.5 to 20.3 mm, with a mean of 12.3 mm, respectively (). The pathogenicity of isolates showed no obvious correlation with their geographic origins.
Fig. 4 Pathogenicity of Botrytis cinerea isolates from Jilin, Liaoning, and Heilongjiang provinces of China, as determined by measuring lesion diameter in pathogenicity tests.
We generated three classifications of pathogenicity based on the diameter of lesions: weak pathogenicity (diameter of lesions ˂ 10 mm), moderate pathogenicity (10 mm ≤ diameter of lesions ≤ 15 mm) and strong pathogenicity (diameter of lesions ˃ 15 mm). Isolates with moderate pathogenicity were dominant (77.5%); isolates with strong (16.7%) or weak (5.9%) pathogenicity were comparably infrequent (). Because isolates with strong pathogenicity pose a serious threat to ginseng plants, their accumulation will increase the potential risk of grey mould.
Table 2. Pathogenicity of Botrytis cinerea isolates from Jilin, Liaoning, and Heilongjiang provinces of China.
Isolates with moderate pathogenicity represented 73.3% and 88.9% of all isolates in Jilin and Heilongjiang provinces, respectively (), but only 66.7% of isolates in Liaoning province. In addition, there was a markedly higher proportion of strongly pathogenic isolates from Liaoning province (9.5%) than from Jilin (4.4%) or Heilongjiang (5.6%) provinces; weakly pathogenic isolates from Heilongjiang province (5.6%) were markedly fewer than those from Liaoning (23.8%) or Jilin (22.2%) provinces.
The pathogenicity of B. cinerea isolates showed no substantial association with colony morphology. Moderately pathogenic isolates were predominant accounting for 77.3%, 75.0% and 85.7% of SC, MP and SP isolates, respectively (). Strongly pathogenic SP isolates were not identified, while 5.3% and 10.0% of SC and MP isolates, respectively, were strongly pathogenic. Weakly pathogenic SC, MP and SP isolates comprised 17.3%, 15.0% and 14.3% of these phenotypes, respectively.
Table 3. Pathogenicity of Botrytis cinerea isolates with SC, MP and SP phenotype.
Discussion
There is great phenotypic and genotypic diversity among B. cinerea populations from different hosts (Kumari et al. Citation2014). Botrytis cinerea is an important pathogen of P. ginseng and is the causal agent of grey mould (Kim et al. Citation2009, Citation2011; Lu et al. Citation2016). Although valuable work has been carried out on the biological (Li and Li Citation2010) and mycological characteristics (Cho et al. Citation2008) of B. cinerea, as well as on the molecular mechanisms of fungicide resistance, the morphological, genetic and pathogenic variability of B. cinerea populations from the main ginseng-growing regions of China remain unknown. Despite obvious variation in colony morphology, pathogenicity and genetic diversity of B. cinerea populations, the present work revealed no obvious correlation of this variation with the geographical origin of B. cinerea in China. Similar results have also been reported for other host plants infected with B. cinerea (Asadollahi et al. Citation2013; Kumari et al. Citation2014; Bardin et al. Citation2018).
We established that ISSR markers are suitable for genetic diversity analysis of B. cinerea populations. The high Nei’s gene diversity index and Shannon’s informative index indicated obvious genetic differentiation among the ginseng B. cinerea population of China. Similar results have also been reported in other agronomic ecosystems (Asadollahi et al. Citation2013; Kumari et al. Citation2014; Zhang et al. Citation2018). Furthermore, most of the genetic diversity was derived from within B. cinerea populations, as confirmed by the low coefficient of genetic differentiation and high gene flow among B. cinerea populations from different geographical origins. These results were consistent with those of Fekete et al. (Citation2012).
The evolution of a population involves concomitant processes of diversification, differentiation and genetic variation, with the latter being introduced by mutation, recombination and migration (Bardin et al. Citation2014; Kumari et al. Citation2014). A high level of genetic variation suggests that there should be frequent gene mutation within the population and sexual reproduction probably occurs. Although sexual structures of B. cinerea have not been reported in the field, the crossing of B. cinerea isolates has been observed in vitro (Walker et al. Citation2017). Before carrying out this research, we speculated that B. cinerea populations from different ginseng-growing regions would be genetically distant from each other and that the genetic variation would be significant. However, our results were to the contrary, so we deduce that frequent gene flow has diminished the genetic variation among B. cinerea populations from these three provinces. This is probably the result of genetic migration pathways, including the spread of conidia through host bridging by adjacent hosts, or the trans-regional transport of host roots, seeds, fruit and soil across longer distances (Decognet et al. Citation2009; Van Kan et al. Citation2014).
Botrytis cinerea was previously considered a generalist fungus. However, differentiation among transposon genotypes within B. cinerea group II has been found in sympatry on various hosts irrespective of geography (Samuel et al. Citation2012). In the present work, we found that although most isolates showed moderate pathogenicity, isolates with strong pathogenicity were also identified, increasing the risk for controlling grey mould. In addition, no evident correlation between phenotype and pathogenicity was observed, consistent with a previous report by Petrasch et al. (Citation2019). Different phenotypes show no significant barrier to gene flow (Asadollahi et al. Citation2013). Furthermore, we found evidence of gene flow within populations of B. cinerea, which should accelerate the generation of highly pathogenic isolates.
S_Fig._1_Symptom_on_ginseng_leaves.docx
Download MS Word (7.2 MB)S_Table_3_The_diameter_of_lesions_that_inoculated_B.docx
Download MS Word (20.5 KB)S_Table_2_B._cinerea_isolates_from_ginseng_producing_regions.docx
Download MS Word (15.7 KB)S_Table_1_ISSR_primers_used_for_genetic_diversity_analysis.docx
Download MS Word (14.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2022.2067900.
Additional information
Funding
References
- AbuQamar S, Khaled M, Tran LSP. 2016. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit Rev Biotechnol. 37(2):262–274. doi:10.1080/07388551.2016.1271767.
- Asadollahi M, Fekete E, Karaffa L, Flipphi M, Árnyasi M, Esmaeili M, Váczy KZ, Sándor E. 2013. Comparison of Botrytis cinerea populations isolated from two open-field cultivated host plants. Microbiol Res. 168(6):379–388. doi:10.1016/j.micres.2012.12.008.
- Bardas GA, Veloukas T, Koutita O, Karaoglanidis GS. 2010. Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag Sci. 66(9):967–973. doi:10.1002/ps.1968.
- Bardin M, Decognet V, Nicot P. 2014. Remarkable predominance of a small number of genotypes in greenhouse populations of Botrytis cinerea. Phytopathology. 104(8):859–864. doi:10.1094/PHYTO-10-13-0271-R.
- Bardin M, Leyronas C, Troulet C, Morris CE. 2018. Striking similarities between Botrytis cinerea from non-agricultural and from agricultural habitats. Front Plant Sci. 9:1820. doi:10.3389/fpls.2018.01820.
- Cho HS, Jeon YH, Do GR, Cho DH. 2008. Mycological characteristics of Botrytis cinerea causing gray mold on ginseng in Korea. J Ginseng Res. 32(1):26–32.
- Decognet V, Bardin M, Trottin-Caudal Y, Nicot PC. 2009. Rapid change in the genetic diversity of Botrytis cinerea populations after the introduction of strains in a tomato glasshouse. Phytopathology. 99(2):185–193. doi:10.1094/PHYTO-99-2-0185.
- Dong LL, Xu J, Li Y, Fang HL, Niu WH, Li XW, Zhang YJ, Ding WL, Chen SL. 2018. Manipulation of microbial community in the rhizosphere alleviates the replanting issues in Panax ginseng. Soil Biol Biochem. 125(10):64–74. doi:10.1016/j.soilbio.2018.06.028.
- Fekete É, Fekete E, Irinyi L, Karaffa L, Árnyasi M, Asadollahi M, Sándor E. 2012. Genetic diversity of a Botrytis cinerea cryptic species complex in Hungary. Microbiol Res. 167(5):283–291. doi:10.1016/j.micres.2011.10.006.
- Fournier E, Giraud T, Albertini C, Brygoo Y. 2005. Partition of the Botrytis cinerea complex in France using multiple gene genealogies. Mycologia. 97(6):1251–1267. doi:10.1080/15572536.2006.11832734.
- Fournier E, Levis C, Fortin D, Leroux P, Giraud T, Brygoo Y. 2003. Characterization of Bc-hch, the Botrytis cinerea homolog of the Neurospora crassa het-c vegetative incompatibility locus, and its use as a population marker. Mycologia. 95:251–261. doi:10.2307/3762036.
- Giraud T, Fortini D, Levis C, Leroux P, Brygoo Y. 1997. RFLP markers show genetic recombination in Botryotinia fuckeliana (Botrytis cinerea) and transposable elements reveal two sympatric species. Mol Biol Evol. 14:1177–1185. doi:10.1093/oxfordjournals.molbev.a025727.
- Jung J, Kim KH, Yang K, Bang KH, Yang TJ. 2014. Practical application of DNA markers for high-throughput authentication of Panax ginseng and Panax quinquefolius from commercial ginseng products. J Ginseng Res. 38:123–129. doi:10.1016/j.jgr.2013.11.017.
- Kim JH, Kim GH, Kim HT. 2011. Sensitivity of Botrytis cinerea isolated from infected leaves of ginseng to tolyfluanid. Korean J Pestic Sci. 15(2):188–193.
- Kim JH, Min JY, Bae YS, Kim HT. 2009. Molecular analysis of Botrytis cinerea causing ginseng grey mold resistant to carbendazim and the mixture of carbendazim plus diethofencarb. Plant Pathol J. 25(4):322–327. doi:10.5423/PPJ.2009.25.4.322.
- Kirkpatrick LA, Butler L. 2015. A simple guide to IBM SPSS Statistics - Version 23.0. United States: CENGAGE Learning Custom Publishing.
- Korolev N, Elad Y. 2016. Vegetative incompatibility in Botrytis. In: Fillinger S, Elad Y, editors. Botrytis – the fungus, the pathogen and its management in agricultural systems. Cham: Springer International Publishing; p. 55–70.
- Kumar M. 2018. Assessment of genetic diversity and population structure in gladiolus (Gladiolus hybridus Hort.) by ISSR markers. Physiol Mol Biol Plants. 24(3):493–501. doi:10.1007/s12298-018-0519-2.
- Kumari S, Tayal P, Sharma E, Kapoor R. 2014. Analyses of genetic and pathogenic variability among Botrytis cinerea isolates. Microbiol Res. 169(11):862–872. doi:10.1016/j.micres.2014.02.012.
- Levis C, Fortini D, Brygoo Y. 1997. Flipper, a mobile Fot1-like transposable element in Botrytis cinerea. Mol Gen Genet. 254:674–680. doi:10.1007/s004380050465.
- Leyronas C, Bryone F, Duffaud M, Troulet C, Nicot PC. 2015. Assessing host specialization of Botrytis cinerea on lettuce and tomato by genotypic and phenotypic characterization. Plant Pathol. 64(1):119–127. doi:10.1111/ppa.12234.
- Li XY, Li Y. 2010. Biological characteristics of ginseng Botrytis cinerea Pers. Plant Dis Pests. 1(5):10–14.
- Liu K, Li Y, Wang R, Chen BW, Wang TY, Ding WL. 2020. Preliminary report of the resistance of Botrytis cinerea isolates from ginseng producing area to four fungicides in China. Plant Prot. 46(2):196–198.
- Lu XH, Jiao XL, Hao JJ, Chen AJ, Gao WW. 2016. Characterization of resistance to multiple fungicides in Botrytis cinerea populations from Asian ginseng in northeastern China. Eur J Plant Pathol. 144(3):467–476. doi:10.1007/s10658-015-0786-5.
- Moyano C, Alfonso C, Gallego J, Raposo R, Melgarejo P. 2003. Comparison of RAPD and AFLP marker analysis as a means to study the genetic structure of Botrytis cinerea populations. Eur J Plant Pathol. 109(5):515–522. doi:10.1023/A:1024211112831.
- Nei M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 89:583–590. doi:10.1093/genetics/89.3.583.
- O’Neill TM, Shtienberg D, Elad Y. 1997. Effect of some host and microclimate factors on infection of tomato stems by Botrytis cinerea. Plant Dis. 81(1):36–40. doi:10.1094/PDIS.1997.81.1.36.
- Peakall R, Smouse PE. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics. 28(19):2537–2539. doi:10.1093/bioinformatics/bts460.
- Petrasch S, Knapp SJ, Van Kan JAL, Blanco-Ulate B. 2019. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol Plant Pathol. 20(6):877–892. doi:10.1111/mpp.12794.
- Rashid MH, Hossain MA, Kashem MA, Kumar S, Rafii MY, Latif MA. 2014. Efficacy of combined formulations of fungicides with different modes of action in controlling Botrytis gray mold disease in Chickpea. Sci World J. 2014:639246. doi:10.1155/2014/639246.
- Rigotti S, Gindro K, Richter H, Viret O. 2002. Characterization of molecular markers for specific and sensitive detection of Botrytis cinerea Pers.: fr. in strawberry (Fragaria × ananassa Duch.) using PCR. FEMS Microbiol Lett. 209(2):169–174. doi:10.1111/j.1574-6968.2002.tb11127.x.
- Samuel S, Veloukas T, Papavasileiou A, Karaoglanidis G. 2012. Differences in frequency of transposable elements presence in Botrytis cinerea population from several hosts in Greece. Plant Dis. 96(9):1286–1290. doi:10.1094/PDIS-01-12-0103-RE.
- Shao WY, Zhao YF, Ma ZH. 2020. Advances in Understanding Fungicide Resistance in Botrytis cinerea in China. Phytopathology. 111(3):455–463. doi:10.1094/PHYTO-07-20-0313-IA.
- Soltis NE, Atwell S, Shi G, Fordyce R, Gwinner R, Gao D, Shafi A, Kliebenstein DJ. 2019. Interactions of tomato and Botrytis cinerea genetic diversity: parsing the contributions of host differentiation, domestication and pathogen variation. Plant Cell. 31:502–519. doi:10.1105/tpc.18.00857.
- Sun W, Dong H, Gao YB, Su QF, Qian HT, Bai HY, Zhang ZT, Cong B. 2015. Genetic variation and geographic differentiation among populations of the nonmigratory agricultural pest Oedaleus infernalis (Orthoptera: acridoidea) in China. J Insect Sci. 15(1):150. doi:10.1093/jisesa/iev132.
- Tang YJ, Cao WJ, Wu KL. 2015. Genetic diversity analysis and molecular identification card construction of Chinese cymbidium germplasms based on SRAP markers. Sci Agr Sinica. 48(9):1795–1806.
- Tiago PV, Medeiros LV, Leão MPC, Santos ACDS. 2016. Polymorphisms in entomopathogenic fusaria based on inter simple sequence repeats (ISSR). Biocontrol Sci Technol. 26(10):1–20. doi:10.1080/09583157.2016.1210084.
- Van Kan JAL. 2006. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends in Plant Sci. 11(5):247–253. doi:10.1016/j.tplants.2006.03.005.
- Van Kan JAL, Shaw MW, Downton RG. 2014. Botrytis species: relentless necrotrophic thugs or endophytes gone rogue? Mol Plant Pathol. 15:1–5. doi:10.1111/mpp.12086.
- Walker AS, Ravigné V, Rieux A, Ali S, Carpentier F, Fournier E. 2017. Fungal adaptation to contemporary fungicide applications: the case of Botrytis cinerea populations from Champagne vineyards (France). Mol Ecol. 26:1919–1935. doi:10.1111/mec.14072.
- White TJ, Bruns T, Lee SW, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods & Applications. New York: Academic Press; p. 315–322.
- Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL. 2007. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol. 8(5):561–580. doi:10.1111/j.1364-3703.2007.00417.x.
- Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. 2003. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 26:1277–1294. doi:10.2337/diacare.26.4.1277.
- Yeh FC, Yang R, Boyle T, Ye Z, Mao JX. 1999. POPGENE, version 1.32: the user-friendly software for population genetic analysis. Edmonton (AB, Canada): Molecular Biology and Biotechnology Centre, University of Alberta.
- Yuan Y, Zhou RJ, Fu JF, Lu ZH, Shi XQ, Li ZB. 2016. Comparison of the biological characteristics and pathogenicity of Botrytis cinerea isolates. J Shenyang Agric Univ. 47(3):271–277.
- Zhang YJ, Li XH, Shen FY, Xu HP, Li YN, Liu DQ. 2018. Characterization of Botrytis cinerea isolates from grape vineyards in China. Plant Dis. 102(1):40–48. doi:10.1094/PDIS-01-17-0062-RE.
- Zhang XK, Wu DX, Duan YB, Ge CY, Wang JX, Zhou MG, Chen CJ. 2014. Biological characteristics and resistance analysis of the novel fungicide SYP-1620 against Botrytis cinerea. Pestic Biochem Physiol. 114:72–78. doi:10.1016/j.pestbp.2014.06.012.
- Zhou M, Lu BH, Liu LP, Bai QR, Gao J. 2020. Phenotypic and genotype diversity of Botrytis cinerea on ginseng. J Huazhong Agric Univ. 39(3):45–53.