2022 CPDS SECTION EDITORS AND ADDRESSES
INDEX – TITLES AND AUTHORS / TITRES ET AUTEURS
DIAGNOSTIC LABORATORIES / LABORATOIRES DIAGNOSTIQUES 9
V. Joshi, P. Berlakoti & B. Babu. Diseases / symptoms diagnosed on commercial crop samples submitted to the British Columbia Ministry of Agriculture (BCAGRI) Plant Health Laboratory in 2021 9
J.F. Elmhirst. Diseases diagnosed on ornamental nursery and landscape plants submitted to Elmhirst Diagnostics & Research, British Columbia, in 2021 17
H. Fu, Y. Yang, K. Zahr, A. Sarkes, S. Dijanovic & J. Feng. Diseases/symptoms diagnosed on plant samples submitted to the Alberta Plant Health Lab (APHL) in 2021 20
M. Prahdan, V. Bisht, D. Kaminski & P. Bajracharya. 2021 Manitoba Agriculture Crop Diagnostic Centre Laboratory submissions 24
T. Blauel & M.R. McDonald. Diagnoses on plant samples submitted to the Ontario Crops Research Centre, Bradford Diagnostic Laboratory in 2021 28
M. Melzer & X. Shan. Diseases diagnosed on plant samples submitted to the Plant Disease Clinic, University of Guelph in 2021. 30
A.-M. Breton, A. Dionne, D. Hamel, L. Pichette, N. Shallow & J. Vivancos. Maladies et problèmes abiotiques diagnostiqués sur les échantillons de plantes reçus en 2021 au laboratoire d’expertise et de diagnostic en phytoprotection (LEDP) du MAPAQ 38
M.T. Tesfaendrias. Diseases diagnosed on plant samples submitted to the New Brunswick Department of Agriculture, Aquaculture and Fisheries, Plant Disease Diagnostic Laboratory in 2021 52
M.M. Clark. Diseases diagnosed on commercial crop samples submitted to the PEI Analytical Laboratories Plant Disease Diagnostic Services (PDDS) in 2021 55
CEREALS / CÉRÉALES 59
V. Fetterley, R.K. Bamrah, B. Yadav, K. Uloth & G.S. Brar. Survey of cereal crop diseases in British Columbia during the 2020 and 2021 growing seasons 59
B. Wei, M. Zid & R. Aboukhaddour. Stripe rust of cereals in Alberta, 2021 61
M.W. Harding, T.K. Turkington, S. Waterman, H. Klein-Gebbinck, R. Aboukhaddour, N. Rauhala, B. Wei, M. Zid, G.C. Daniels & M.A. Kennedy. Wheat disease survey in Alberta, 2021 63
N. Rauhala, T.K. Turkington, J. Busaan, S. Waterman, H.W. Klein-Gebbinck, M.W. Harding, G.C. Daniels, M.A. Kennedy, R. Aboukhaddour, M. Zid & H. Spence. 2021 barley disease survey in Alberta 66
T.K. Turkington, M.A. Henriquez & B. McCallum. 2021 wheat disease survey in Alberta, Saskatchewan and Manitoba 68
T. Islam, E. Boots, A. Karstens & H.R. Kutcher. Leaf spot diseases of oat and barley in Saskatchewan in 2021 70
M.R. Fernandez, N. Waelchli, C. Kenny, F. Waelchli, A. Akhavan, C. Peru & S. Hartley. Leaf spotting diseases of common and durum wheat in Saskatchewan in 2021 72
B. Olson, A. Akhavan, T. Blois, B. Ernst, M. Japp, S. Junek, H.R. Kutcher & T. Prasad. Seed-borne fusarium on cereal crops in Saskatchewan in 2020 75
M. Beyene, M. Banik & X. Wang. Barley and oat leaf spot diseases in Manitoba, 2021 80
M.A. Henriquez, D. Kaminski, A. Kirk, J. Doherty, D. Miranda & O. Gruenke. Fusarium head blight of spring wheat and winter wheat in Manitoba in 2021. 81
M.A. Henriquez, D. Kaminski, A. Kirk, J. Doherty, D. Miranda & O. Gruenke. Leaf spot diseases of spring wheat and winter wheat in Manitoba in 2021 83
B. McCallum, W. McNabb & E. Reimer. Leaf and stripe rust of wheat in Manitoba in 2021 85
J.G. Menzies, A.G. Xue, S. Deceuninck, Z. Popovic & H. Derksen. Crown rust of oat in Manitoba and Ontario in 2020 86
A.G. Xue & Y. Chen. Diseases of barley in Ottawa, Ontario in 2021 88
A.G. Xue & Y. Chen. Diseases of oat in Ottawa, Ontario in 2021 91
A.G. Xue & Y. Chen. Diseases of spring wheat in Ottawa, Ontario in 2021 93
A.G. Xue & Y. Chen. Diseases of winter wheat in Ottawa, Ontario in 2021 95
X. Zhu, A.Z. Kebede & T. Woldemariam. Status of corn diseases in eastern Ontario, 2021 crop season 97
E. Johnstone, R. Matters & A. Foster. Survey of fusarium head blight and leaf diseases of spring wheat on Prince Edward Island in 2021. 100
OILSEEDS, PULSES, FORAGES AND SPECIAL CROPS/OLÉAGINEUX, PROTÉAGINEUX, PLANTES FOURRAGÈRES ET CULTURES SPÉCIALES 103
B. Yadav, K. Uloth, M. Abbasi, V. Fetterley, R. Bamrah & G.S. Brar. Oat and forage disease survey in British Columbia in 2021 103
M.W. Harding, K. Kopec, G.C. Daniels, S. Chatterton & B. Alexander. Blossom blight and stem rot in irrigated alfalfa seed fields in Alberta in 2021 105
J.D. Reich, B. Groenenboom & S. Chatterton. Survey of white mould of dry bean in southern Alberta in 2020 and 2021 108
M.W. Harding, G.C. Daniels, T.B. Hill, M.A. Kennedy, A. Sarkes, Y. Yang & J. Feng. Canola disease survey in Alberta, 2021. 110
H. Yu, J. Cordero-Elvia, K.F. Chang, C.X. Yang, R. Fredua-Agyeman, G.D. Turnbull, S.F. Hwang & S.E. Strelkov. Canola disease survey in central and northern Alberta, 2021. 114
S.E. Strelkov, V.P. Manolii, Y. Aigu, M. Marchal, R. Mignot, G.C. Daniels, M.W. Harding & S.F. Hwang. The occurrence and spread of clubroot on canola in Alberta in 2021. 117
M.W. Harding, G.C. Daniels, T.B. Hill & M. Kennedy. A survey for pea diseases in Alberta, 2021. 120
A. Akhavan, C. Peru, A. Dolatabadian, D. Fernando, J. Giroyed, B. Esau, C. Jacob, J. Ippolito, K. Kindrachuk, S. Miller, S. Chant, K. Boere, A. Noble, L. Cowell, S. Friesen, K. Stonehouse, M. Struthers, M. Brown, M. O’Connor, K. Anderson, K. Makohoniuk, J. Kwasnicki, C. Neuberger, C. Fennig, B. Johnson & L. Roszell. Survey of canola diseases in Saskatchewan, 2021. 124
K. Nabetani, T. Islam, H.R. Kutcher, C. Peru, M. Beaith, A. Akhavan, C. Jacob, S. Roberts, M. Brown, A. Noble, K. Stonehouse, A. Fransoo, M. Cott & D. Froese. Diseases of flax in Manitoba and Saskatchewan in 2021 129
A. Akhavan, C. Peru, J. Ippolito, K. Kindrachuk, S. Chant, S. Friesen, M. Brown, D. Risula, K. Boere, K. Makohoniuk, J. Kwasnicki, C. Neuberger, L. Roszell, C. Fennig & B. Johnson. 2021 survey of lentil diseases in Saskatchewan. 131
M. Hubbard & Z. Hossain. Factors influencing anthracnose of lentil in Saskatchewan in 2020 and 2021. 135
A. Akhavan, C. Peru, J. Ippolito, K. Kindrachuk, K. Boere, S. Chant, S. Friesen, M. Brown, A. Noble, D. Risula, K. Stonehouse, K. Makohoniuk, J. Kwasnicki, C. Neuberger, C. Fennig, L. Roszell & B. Johnson. 2021 survey of field pea diseases in Saskatchewan. 139
B.D. Olson, A. Akhavan, S. Banniza, T. Blois, B. Ernst, S. Junek, S. Phelps, T. Prasad & D. Risula. Seed-borne pathogens of pulse crops in Saskatchewan in 2020. 141
A. Akhavan, C. Peru, J. Kwasnicki, S. Miller & S. Roberts. 2021 survey of soybean diseases in Saskatchewan. 146
Y.M. Kim, E. Sari, A. Hou, M.J. Thompson & W.C. Penner. Diseases of dry bean in Manitoba in 2021. 148
Y.M. Kim, D. Kaminski, J. Graham, M. Pradhan, E. Bargen, A Brackenreed, T. Buss, N. Clouson, J. Cornelsen, T. Cummer, A. Farooq, J. Frey, D. Froese, N. Ort, T. Henderson, M.J. Thompson, L. Kaskiw, D. Lange & R. Picard. Survey of canola diseases in Manitoba in 2021 151
Y.M. Kim, T.L. Henderson, M.J. Thompson, S.F. Hwang, K.F. Chang, S. Chatterton, C. Tkachuk, L. Schmidt, N. Clouson, D. Lange & A. Farooq. Field pea diseases in Manitoba in 2021. 155
Y.M. Kim, E. Sari, D. Kaminski, S. Phelps, B.D. Gossen, C. Tkachuk, L. Schmidt, D. Lange, A. Farooq, N. Clouson, A. Akhavan, S. Roberts, C. Peru, W.C. Penner, T.L. Henderson & M.J. Thompson. Soybean root rot and phytophthora rot in Manitoba and Saskatchewan in 2021 158
FRUITS & BERRIES/FRUITS, FRUITS À ÉCALE ET BAIES 161
J.F. Elmhirst, L.A. Wegener, S. Sveinson-Dyer, D. Frost & D. Henderson. Surveys for Phytophthora fragariae on strawberry in the British Columbia Fraser Valley, 2002 and 2017 161
K. Grigg-McGuffin, K. Goldenhar & E. Debrouwer. Survey of apple tree viruses in Ontario, 2021 167
VEGETABLES/LÉGUMES 170
M. Gray, M. Dessureault & H. Meberg. Presence of verticillium wilt in potatoes grown in the British Columbia Fraser Valley, 2021 170
M.W. Harding, G.C. Daniels, P. Ragan & J. Feng. Monitoring of fungal diseases and aster yellows on dry bulb onion in Alberta, 2021 172
V. Bisht, M. Tenuta & S. Graham. Incidence of verticillium wilt (V. dahliae) in potato in Manitoba, 2021 crop 175
FOREST TREES/ARBRES FORESTIERS 179
N. Feau, P. Herath, N. Sullivan, S. Zeglen, H.H. Kope & R. Hamelin. Monitoring and surveillance of poplar leaf spot and canker disease (Sphaerulina musiva) in British Columbia 179
GENERAL CROP SURVEY/ENQUÊTE GÉNÉRALE SUR LES CULTURES 183
R. J. Howard & K. Ferris. A survey of field and horticultural crop diseases in the Yukon territory in 2021. 183
DIAGNOSTIC LABORATORIES / LABORATOIRES DIAGNOSTIQUES
DISEASES/SYMPTOMS DIAGNOSED ON COMMERCIAL CROP SAMPLES SUBMITTED TO THE BRITISH COLUMBIA MINISTRY OF AGRICULTURE, FOOD AND FISHERIES (BCMAFF), PLANT HEALTH LABORATORY IN 2021.
CROP: Commercial Crops – Plant Health Laboratory ReportLOCATION: British ColumbiaNAMES AND AGENCY:V. JOSHI, P. BURLAKOTI & B. BABU
Plant Health Laboratory, Plant and Animal Health Branch, B.C. Ministry of Agriculture, Food and Fisheries, Abbotsford Agriculture Centre, 1767 Angus Campbell Road, Abbotsford, BC V3G 2M3Telephone: (778) 666-0581; Facsimile: (604) 556-3010; E-mail: [email protected]Web page: https://www2.gov.bc.ca/gov/content/industry/agriculture-seafood/animals-and-crops/plant-health/plant-health-laboratory
ABSTRACT: The British Columbia Ministry of Agriculture, Food and Fisheries (BCMAFF) Plant Health Laboratory (PHL) provides diagnoses of diseases and disorders caused by fungi, bacteria, viruses, and insect pests of agricultural crops grown in British Columbia. The PHL also makes assessments of plant parasitic nematode damage and possible abiotic factors affecting plant health. Between January 1 and Nov. 15, 2021, the PHL received 1211 samples including cannabis, Christmas trees, field crops, greenhouse vegetable and floriculture crops, forest nursery seedlings, herbaceous and woody ornamentals, small fruits, tree fruits, nuts, turfgrass and specialty crops for diagnosis. The PHL started receiving cannabis samples for disease diagnoses in April 2021. The majority of cannabis samples were submitted for detection of Hop Latent Viroid which was detected in 18% of total (389) cannabis samples. Blueberry Fruit Drop-associated Virus was confirmed (PCR followed by sequencing) for the first time in the PHL.
METHODS: The BCMAFF Plant Health Laboratory provides diagnoses of diseases caused by fungi, bacteria, viruses, and insect pests of agricultural crops grown in British Columbia. Samples are submitted to the laboratory by ministry staff, growers, agri-business representatives, crop insurance personnel, municipalities, and master gardeners. Diagnoses were accomplished by visual and microscopic examination, culturing onto microbial media, biochemical identification of bacteria using BIOLOG® and serological testing of viruses, fungi, and bacteria with micro-well and membrane based (Agdia’s ImmunoStrip test) enzyme linked immuno-sorbent assay (ELISA). Molecular techniques (polymerase chain reaction (PCR)- conventional and/or real time) were used for some genus level and species-specific diagnoses. General primer PCRs (for fungi, virus, and bacteria) were followed by sequencing to identify the organism involved. Some specimens were referred to other laboratories for identification or confirmation of the diagnosis.
RESULTS AND COMMENTS: Overall in 2020, British Columbia had mild winter weather conditions in January followed by mild temperatures and lots of rain. The long, mild, wet spring was normal for B.C., and was followed by a very dry summer starting in late June. Extreme heat with significant high temperatures was experienced for a couple of months. These conditions created a significant stress on plants, as even the night time temperatures hit above 20-250C. Many ornamentals and berry crops experienced drought-like conditions resulting in stressed plants, small berries, and poor-quality fruit. Fire blight incidence was higher than usually seen in tree fruit, as well as woody ornamental crops. Dry weather in August was followed by very wet conditions starting in September which continued late into November bringing atmospheric rivers resulting in widespread flood conditions in parts of the Fraser Valley and some tree fruit growing areas. Damage to the plants associated with excess moisture and flooded conditions is yet to be seen in the spring of 2022.
Summaries of diseases and their causal/ associated agents diagnosed on crop samples submitted to the laboratory are presented in to , organized by crop category. Between January 1 and Nov. 15, 2021, the PHL received 1211 samples for diagnosis. Crop categories comprising most of the samples in 2021 included cannabis (32.17%), followed by field vegetables (14.47%), berry crops (13.48%), woody ornamentals (8.18%), and tree fruit and grape (6.69%). All other crop categories comprised a combined 25% of the total submissions. The PHL started receiving cannabis samples for disease diagnoses in April 2021. The majority (389) of cannabis samples were submitted for detection of Hop Latent Viroid (HLVd) which was detected in 18% of total cannabis samples (). Blueberry Fruit Drop-associated Virus (BFDaV) was confirmed (PCR followed by sequencing) in one sample submitted to the lab. BFDaV was first described in British Columbia in 2016 (Diaz-Lara and Martin), but this is the first sample confirmed in the PHL (). Diagnoses not listed include abiotic disorders caused by suspected nutritional stress, pH imbalance, water stress, drought stress, adverse growing/cultural conditions, genetic abnormalities, environmental and chemical stresses including damage from herbicides, fruit abortion due to lack of pollination, insect-related injury, or damage where no conclusive causal factor was identified. More than one disease-causing agent was identified on many samples and are listed under individual disease/symptom. The frequency of diseases diagnosed in the lab does not reflect their prevalence in the field in B.C.
Table 1. Diseases/symptoms detected in cannabis samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 2. Diseases/symptoms detected in field crop samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 3. Diseases/symptoms detected in floriculture samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 4. Diseases/symptoms detected in forest nursery samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 5. Diseases/symptoms detected in greenhouse vegetable samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 6. Diseases/symptoms detected in herbaceous perennial samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 7. Diseases/symptoms detected in berry and nut crop samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 8. Diseases/symptoms detected in specialty crop samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 9. Diseases/symptoms detected in tree fruit and grape samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 10. Diseases/symptoms detected in turfgrass (golf course, lawn, sports field, and sod) samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 11. Diseases/symptoms detected in field vegetable samples submitted to the BCMAFF Plant Health Laboratory in 2021.
Table 12. Diseases/symptoms detected in woody perennial samples submitted to the BCMAFF Plant Health Laboratory in 2021.
REFERENCE
- Diaz-Lara A, Martin RR. 2016. Blueberry fruit drop-associated virus: a new member of the family Caulimoviridae isolated from blueberry exhibiting fruit-drop symptoms. Plant Dis. 100(11):2211–2214.
DISEASES DIAGNOSED ON ORNAMENTAL NURSERY AND LANDSCAPE PLANTS SUBMITTED TO ELMHIRST DIAGNOSTICS & RESEARCH, BRITISH COLUMBIA, IN 2021
CROP: Diagnostic Laboratory Report LOCATION: British Columbia NAME AND AGENCY:J. F. ELMHIRST
Elmhirst Diagnostics & Research, 5727 Riverside St., Abbotsford, BC V4X 1T6 Telephone: 604-832-9495; E-mail: [email protected]
ABSTRACT: Diseases of ornamental nursery and landscape plants in south coastal British Columbia submitted to Elmhirst Diagnostics & Research in 2021 and identified causal agents are listed. Rainy weather throughout the months of May and June led to high levels of botrytis on many crops. This was followed by extreme heat and drought in July-August which damaged many crops and landscape plants. This is the first report of Myrothecium roridum causing leaf rot of Peperomia ferryrae and the first report in British Columbia of downy mildew of foxglove (Digitalis purpurea) caused by Peronospora digitalidis. The northern root knot nematode (Meloidogyne hapla) was found to be causing root galls on clematis.
METHODS: Elmhirst Diagnostics & Research (EDR) provides diagnosis of diseases of commercial horticultural crops in British Columbia caused by fungi, bacteria, viruses, plant parasitic nematodes, arthropod and mite pests as well as abiotic factors. Laboratory diagnostic services are provided in conjunction with on-site diagnostic consultations. Diagnosis is performed primarily by association of known symptoms with the presence of a pathogen known to cause these symptoms and identified by microscopic examination. If further identification or confirmation is needed, fungal and bacterial pathogens are isolated in pure culture for further examination of morphological characteristics or plant tissue or cultured specimens are sent to other laboratories for identification by ELISA, PCR or DNA sequencing. Problems caused by abiotic factors such as nutrient or pH imbalance, water stress, physiological response to growing conditions, genetic abnormalities and environmental and chemical stresses including herbicide damage are not included. The frequency of diseases diagnosed does not reflect their prevalence in the field.
RESULTS AND COMMENTS: A summary of disease diagnoses and causal or associated agents on ornamental nursery and landscape crops is presented in . Spring 2021 was cool and wet through to the end of June, resulting in a high level of botrytis blight on many crops. This was followed by extreme heat and drought in July-August which damaged many crops and landscape plants. This is the first report of Myrothecium roridum causing leaf and stem rot of Peperomia ferryrae, although it has been previously reported causing leaf and stem rot of other peperomia species in Korea and the United States (Han et al. Citation2014) and a leaf spot of impatiens in British Columbia in 2000 (Joshi Citation2001). This is the first report in British Columbia of downy mildew of foxglove (Digitalis purpurea) caused by Peronospora digitalidis, first reported in the U.S. (California) in 2002 (Tjosvold and Koike Citation2004), Oregon in 2017 (Wallace and Crouch Citation2018), and in the nursery and landscape in Washington State (Pacific Northwest Plant Disease Management Handbook Citation2021). Both M. roridum and P. digitalidis were identified by microscopic examination of pathogen morphology in comparison to published descriptions. The northern root knot nematode (Meloidogyne hapla) was found to be causing root galls on clematis.
Table 1. Diseases diagnosed on ornamental nursery and landscape plants submitted to Elmhirst Diagnostics & Research in 2021.
REFERENCES
- Foxglove (Digitalis spp.)-Downy Mildew. In : Pscheidt JW and Ocamb CM, senior editors. 2021. Pacific Northwest Plant Disease Management Handbook [online]. Corvallis (OR): Oregon State University [accessed 2022 Mar 8]. https://pnwhandbooks.org/plantdisease/host-disease/foxglove-digitalis-spp-downy-mildew
- Joshi V. 2001. Diseases diagnosed on commercial crops submitted to the BCMAFF Plant Diagnostic Laboratory in 2000. Can Plant Dis Surv. 81:7–15.
- Han K-S, Choi S-K, Kim H-H, Lee S-C, Park J-H, Cho M-R, Park M-J. 2014. First report of Myrothecium roridum causing leaf and stem rot disease on Peperomia quadrangularis in Korea. Mycobiology 42(2):203–205.
- Tjosvold SA, Koike ST. 2004. First occurrence of downy mildew on Digitalis purpurea (common foxglove), caused by Peronospora digitalidis, in California and the United States. Plant Dis. 86(10):1176.
- Wallace EC, Crouch JA. 2018. First report of Peronospora digitalidis causing downy mildew disease on foxglove in Oregon. Plant Dis. 102(4):827.
DISEASES/SYMPTOMS DIAGNOSED ON PLANT SAMPLES SUBMITTED TO THE ALBERTA PLANT HEALTH LAB (APHL) IN 2021
CROP: All Crops – Plant Health Laboratory Report LOCATION: Alberta NAMES AND AGENCIES: H. FU, Y. YANG, K. ZAHR, A. SARKES, S. DIJANOVIC & J. FENG
Alberta Plant Health Lab, Alberta Agriculture, Forestry and Rural Economic Development, Edmonton, AB T5Y 6H3 Telephone: (780)-644-3436; E-mail: [email protected]
ABSTRACT: The Alberta Plant Health Lab (APHL) provides plant pest diagnosis and expertise to Alberta’s agricultural industry. The lab accepts samples exclusively from agricultural fieldmen, academic institutions, applied research associations and municipal pest management departments, at no cost. A total of 375 samples were processed for diagnosis in year 2021. No Dutch elm disease was identified in elm tree samples this year. Needle cast pathogens still account for largest portion of the conifer samples received. No fusarium head blight caused by Fusarium graminearum was detected in cereals. Clubroot was found in turnip and canola samples. The fire blight pathogen, Erwinia amylovora, was detected on a crab apple sample.
METHODS: Samples were submitted to APHL by agricultural fieldsmen, academic institutions, applied research associations and municipal pest management departments. Diagnoses were based on a combination of visual examination of symptoms and signs, microscopic observation, culturing on artificial media, PCR/qPCR, DNA barcoding and use of commercial diagnostic kits. Fungal barcoding was performed by sequencing DNA fragments of internal transcribed spacers (ITS) (White et al. Citation1990) and/or elongation factor −1α (EF1) (Stielow et al. Citation2015) and/or β-tubulin (Stukenbrock et al. Citation2012). Bacteria were usually identified based on DNA sequencing of 16S ribosomal RNA gene (Klindworth et al. Citation2013) and/or cpn60 (Links et al. Citation2012). The diagnosis of clubroot was done by qPCR according to Zahr et al. (Citation2021). PCR identification of Fusarium graminearum from submitted cultures was performed following methodology from Zuzak et al. (Citation2018). For identification of Fusarium species from plant tissues, protocols described by Demeke et al. (Citation2005) were used. Phytoplasma were detected by PCR using the primer pairs P1/Tint and R16MF2n/R16MR2n (Smart et al. Citation1996). Confirmation of late blight on potato and tomato was done using the Agdia ImmunoStrip kit for Phytophthora species (http://www.agdia.com). For diagnosis of all other diseases, when PCR techniques were used, quantitative PCR (qPCR) preceded conventional PCR and probe-based qPCR preceded SYBR Green based qPCR. The primers and protocols were chosen from the most recent literature and verified by APHL using positive and negative controls.
RESULTS AND COMMENTS: A total of 373 samples were processed for disease diagnosis between January 1 and December 31, 2021. Pathogens associated with disease samples comprised fungi, oomycetes, protists, bacteria and viruses. More than one potential causal agent was identified in majority of samples. Summaries of symptoms and associated pathogens diagnosed are provided in to 7, by crop category.
Table 1. Diseases diagnosed on cereal crop samples submitted to the Alberta Plant Health Lab in 2021.
Fusarium species were commonly isolated from various cereal crop samples (). However, no F. graminearum was isolated from any of the cereal samples. There was a substantial increase in cereal leaf samples diagnosed with bacterial infection associated with Xanthomonas translucens. Pythium arrhenomanes was found to be associated with one wheat root rot sample. In addition, a phytoplasma, the causal agent of aster yellows disease was detected in two barley samples.
Thirty-two canola samples were infected with the clubroot pathogen, Plasmodiophora brassicae (). Twenty samples tested positive for the blackleg pathogen, Leptosphaeria maculans. Root rots caused by Fusarium tricinctum, F. culmorum, F. avenaceum and a Phoma sp. were observed in one canola sample. No Verticillium sp. was detected in nine samples that showed wilt symptoms.
Table 2. Diseases diagnosed on canola samples submitted to the Alberta Plant Health Lab in 2021.
An increase in potato tuber samples with virus was detected this year (). Ten of the tuber samples were infected with Potato Mop Top Virus (PMTV) and three were infected with Tobacco Rattle Virus (TRV). Powdery scab (Spongospora subterranea), common scab (Streptomyces acidiscabies, S. scabies and S. turgidiscabies), soft rot (Pectobacterium atrosepticum and P. carotovorum), dry rot (Neonectria candida, Fusarium avenaceum and F. sambucinum) and tuber rot (Erwinia atroseptica and F. sambucinum) were also identified in submitted tuber samples. Wilting caused by Colletotrichum coccodes, Globisporangium ultimum and Verticillium dahliae was observed in one potato sample.
Table 3. Diseases diagnosed on potato samples submitted to the Alberta Plant Health Lab in 2021.
Only three legume samples were received for disease diagnosis this year (). Two pea samples had root rot symptoms associated with Fusarium equiseti, F. oxysporum, F. solani and F. avenaceum. The pathogens Rhizoctonia sp., Globisporangium sp., F. avenaceum, F. redolens and F. solani were isolated from one dry bean root rot sample.
Table 4. Diseases diagnosed on legume samples submitted to the Alberta Plant Health Lab in 2021.
Sixteen elm samples were submitted for Dutch elm disease (DED) testing; however, none of them were positive (). Plenodomus tracheiphilus was identified from most of the diseased elm samples. Other pathogens isolated from elm samples were Cytospora sp., Verticillium dahliae, Fusarium spp., Cryptosphaeria sp., Didymella sp. and Alternaria sp. The fungus Sydowia polyspora, causal agent of needle cast and needle blight, was identified in nine spruce and three pine samples. Rhizosphaera kalkhoffii, the causal agent of rhizosphaera needle cast, was isolated from one of the spruce samples. Cytospora canker caused by Cytospora sp. was found on cotoneaster, crab apple, elm, lilac, maple, poplar and spruce samples. Fire blight was detected in one crab apple sample. Fusarium spp. were isolated from apple, elm, maple, poplar and strawberry samples.
Table 5. Diseases diagnosed on trees and fruits submitted to the Alberta Health Lab in 2021.
Nineteen vegetable samples were received for disease diagnosis (). The clubroot pathogen, Plasmodiophora brassicae, was found in one turnip sample. Streptomyces scabies was found associated with scab of beet. Dickeya zeae, Pseudomonas putida and Pseudomonas sp. caused soft rot of carrot. Two garlic samples tested positive for phytoplasma. Pathogens including Fusarium proliferatum, F. acuminatum, F. oxysporum, F. solani, Alternaria embellisia, Stemphylium vesicarium, Pantoea agglomerans and Pseudomonas fluorescens were found associated with bulb rot of garlic. Botrytis aclada was found causing neck rot of onion.
Table 6. Diseases diagnosed on vegetable crops submitted to the Alberta Plant Health Lab in 2021.
Neoascochyta exitialis and Pseudomonas coronafaciens were found associated with a leaf spot of timothy (). Fusarium sp. and Microdochium sp. were isolated from one brome leaf spot sample. Pseudomonas syringae caused discolouration of green foxtail in one sample.
Table 7. Diseases diagnosed on other crops submitted to the Alberta Plant Health Lab in 2021.
REFERENCES
- Demeke T, Clear RM, Patrick SK, Gaba D. 2005. Species-specific PCR-based assays for the detection of Fusarium species and a comparison with the whole seed agar plate method and trichothecene analysis. Inter J Food Microbiol. 103:271–284.
- Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. 2013. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41: e1.
- Links MG, Dumonceaux TJ, Hemmingsen SM, Hill JE., Neufeld, J. 2012. The chaperonin-60 universal target is a barcode for bacteria that enables de novo assembly of metagenomic sequence data. PLoS One. 7: e49755.
- Smart CD, Schneider B, Blomquist CL, Guerra LJ, Harrison NA, Ahrens U, Lorenz KH, Seemüller E, Kirkpatrick BC. 1996. Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Appl Environ Microbiol. 62:2988–2993.
- Stielow JB, Lévesque CA, Seifert KA, Meyer W, Irinyi L, Smits D, Renfurm R, Verkley GJ, Groenewald M, Chaduli D, et al. 2015. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35:242–263.
- Stukenbrock EH, Quaedvlieg W, Javan-Nikhah M, Zala M, Crous PW, McDonald BA. 2012. Zymoseptoria ardabiliae and Z. pseudotritici, two progenitor species of the septoria tritici leaf blotch fungus Z. tritici (synonym: Mycosphaerella graminicola). Mycologia 104:1397–1407.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes MA, Gelfand DH, Sninsky JJ, White TJ. (editors). PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press, p. 315–322.
- Zahr K, Sarkes A, Yang Y, Ahmed H, Zhou Q, Feindel D, Harding M, Feng J. 2021. Plasmodiophora brassicae in its environment – effects of temperature and light on resting spore survival in soil. Phytopathology 111:1743–1750.
- Zuzak K, Zahr K, Yang Y, Sarkes A, Feindel D, Daniels G, Harding MW, Feng J. 2018. A duplex PCR method for identification of cultures of Fusarium graminearum from infected wheat grain without DNA extraction. Can J. Plant Pathol. 40:417–422.
2021 MANITOBA AGRICULTURE CROP DIAGNOSTIC CENTRE LABORATORY SUBMISSIONS
CROP: Diagnostic Laboratory Report LOCATION: Manitoba NAMES AND AGENCIES: M. PRADHAN1, V. BISHT2, D. KAMINSKI2, P. BAJRACHARYA2
1Manitoba Agriculture, Crop Diagnostic Centre, 545 University Crescent, Winnipeg, MB R3T 5S6 Telephone: (204) 792-8001; Facsimile: (204) 945-4327; E-mail: [email protected] 2Manitoba Agriculture, Crops Industry Branch, Box 1149, Carman, MB R0G 0J0
ABSTRACT: This report summarizes the diseases and disorders diagnosed on plant samples submitted to and analyzed by the Manitoba Agriculture Crop Diagnostic Centre in 2021. Samples received by the laboratory included field crops as well as ornamentals, grasses and trees grown in Manitoba.
METHODS: The Manitoba Agriculture Crop Diagnostic Centre provides diagnoses and control recommendations for disease problems of agricultural crops, both field and horticultural crop plants. Manitoba Agriculture Crop Industry Branch Specialists, extension and other departmental personnel, farmers, agri-business representatives and the public, submit samples to the laboratory. Diagnostic methods used included visual examination for symptoms, microscopy, moist chamber incubation, culturing onto artificial media (general and pathogen specific), Agdia ImmunoStrips®, ELISA and molecular (PCR) testing.
RESULTS: Summaries of diseases diagnosed on plants in different crop categories are presented in to and cover the period from January 1 to December 23, 2021.
Table 1. Summary of diseases diagnosed on cereal crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 2. Summary of diseases diagnosed on vegetable crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 3. Summary of diseases diagnosed on potato crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 4. Summary of diseases diagnosed on shelterbelt trees and woody ornamental plants submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 5. Summary of diseases diagnosed on oilseed crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 6. Summary of diseases diagnosed on fruit crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 7. Summary of diseases diagnosed on special crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 8. Summary of diseases diagnosed on pulse crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
Table 9. Summary of diseases diagnosed on forage legume crop samples submitted to the Manitoba Agriculture Crop Diagnostic Centre in 2021.
DIAGNOSES ON PLANT SAMPLES SUBMITTED TO THE ONTARIO CROPS RESEARCH CENTRE – BRADFORD DIAGNOSTIC LABORATORY IN 2021
CROP: Diagnostic Laboratory Report LOCATION: Bradford/Holland Marsh, Ontario NAMES AND AGENCY: T. BLAUEL AND M.R. MCDONALD
Ontario Crops Research Centre – Bradford, Dept. of Plant Agriculture, University of Guelph, 1125 Woodchoppers Lane, King, ON L7B 0E9 Telephone: (905) 775-3783; E-mail: [email protected]; Website: https://bradford-crops.uoguelph.ca/
ABSTRACT: The Integrated Pest Management (IPM) program provided by the Ontario Crops Research Centre – Bradford (OCRC-B) offers diagnostic services to support the vegetable growers of the Holland Marsh and surrounding area. In 2021, 84 plant samples were submitted to the diagnostic laboratory for identification and management recommendations. The plant samples submitted had symptoms of infectious disease (71%), insect damage (7%) and abiotic disorders (22%).
INTRODUCTION AND METHODS: In addition to the scouting and forecasting services provided by the Integrated Pest Management (IPM) program, the diagnostic laboratory at the Ontario Crops Research Centre – Bradford (OCRC-B), formerly the Muck Crops Research Station, provides diagnostic services and management recommendations for plant diseases, insect feeding damage, abiotic disorders and weeds to vegetable growers in and around the Holland Marsh. In 2021, plant samples were submitted to the OCRC-B diagnostic laboratory by growers, IPM scouts and industry representatives. Diagnosis of plant symptoms and issues involved visual and microscopic assessment and culturing of pathogens on growth media. These samples were analysed in addition to the regular identification and quantification of diseases and insect pests provided by the pest management scouts as part of the IPM program.
RESULTS AND DISCUSSION: The diagnostic laboratory received 84 samples between May 14 and October 20, 2021. Of these, 70% were diagnosed with infectious diseases (59 samples), 6% with insect issues (5 samples) and 24% were diagnosed with an abiotic disorder (20 samples). The percentage of samples submitted comprising each crop is as follows: onion (42%), carrot (32%), celery (11%) and other crops (15%). The 2021 growing season began with fair weather conditions, some rain which delayed seeding, changing to high temperatures and dry conditions in May and June which resulted in some wind erosion and delayed crop emergence. Conditions changed to warmer temperatures and increased precipitation resulting in long leaf wetness periods in July and August which were conducive for the development of many diseases such as stemphylium leaf blight of onion. Onion downy mildew development was sporadic and there was no serious outbreak. Botrytis leaf blight was also identified but did not cause any serious damage. The Holland Marsh received large amount of rain in September which flooded the fields resulting in many carrots lost to waterlogging. The diseases and/or disorders diagnosed on crop samples submitted to the OCRC-B diagnostic laboratory in 2021 are presented in .
Table 1. Plant samples and associated diseases submitted to the OCRC-B diagnostic laboratory in 2021.
ACKNOWLEDGEMENTS: Funding was provided in part by the Bradford Cooperative Storage Ltd., agrochemical companies and growers participating in the Ontario Crops Research Centre – Bradford IPM program.
DISEASES DIAGNOSED ON PLANT SAMPLES SUBMITTED TO THE PLANT DISEASE CLINIC, UNIVERSITY OF GUELPH IN 2021
CROPS: Commercial Crops - Diagnostic Laboratory Report LOCATION: Ontario NAMES AND AGENCY: M. MELZER & X. SHAN
Plant Disease Clinic, Laboratory Services Division, University of Guelph, 95 Stone Road W, Guelph, ON N1H 8J7 Telephone: (519) 823-1268; Facsimile: (519) 767-6240; Email: [email protected] Web page: www.guelphlabservices.com
ABSTRACT: Diseases and their causal agents diagnosed on plant samples received by the Plant Disease Clinic, University of Guelph in 2021 are summarized in this report. Samples included greenhouse vegetables, annual and perennial ornamental plants, field crops, berry crops, tree fruits, turfgrass and trees.
METHODS: The Plant Disease Clinic of the University of Guelph provides plant pest diagnostic services to growers, agri-businesses, provincial and federal governments and the general public across Canada. Services include plant disease diagnosis, plant parasitic nematode identification and enumeration, pathogen detection from soil and water, and insect identification. The following data are for samples received by the laboratory for disease diagnosis in 2021. Diagnoses were accomplished using microscopic examination, culturing on artificial media, biochemical identification of bacteria using BIOLOG®, enzyme linked immunosorbent assay (ELISA), polymerase chain reaction (PCR) based techniques including DNA multiscan®, PCR and RT-PCR, and DNA sequencing.
RESULTS AND COMMENTS: In 2021, the Plant Disease Clinic received samples representing over 90 plant genera for disease diagnosis. Results are presented in to . For various reasons, the frequency of diseases diagnosed on samples submitted to the laboratory does not reflect the prevalence of diseases of various crops in the field. Problems caused by plant parasitic nematodes, insects and abiotic factors are not listed. Most diseases identified in 2021 are commonly diagnosed on the respective plant hosts.
Table 1. Diseases diagnosed on vegetable samples (including greenhouse vegetables) submitted to the University of Guelph Plant Disease Clinic in 2021.
Table 2. Diseases diagnosed on fruit samples submitted to the University of Guelph Plant Disease Clinic in 2021.
Table 3. Diseases diagnosed on herbaceous ornamental samples submitted to the University of Guelph Plant Disease Clinic in 2021.
Table 4. Plant diseases diagnosed on woody ornamental samples submitted to the University of Guelph Plant Disease Clinic in 2021.
Table 5. Diseases diagnosed on field crop samples submitted to the University of Guelph Plant Disease Clinic in 2021.
Table 6. Diseases diagnosed on herb and specialty crop samples submitted to the University of Guelph Plant Disease Clinic in 2021.
MALADIES ET PROBLÈMES ABIOTIQUES DIAGNOSTIQUÉS SUR LES ÉCHANTILLONS DE PLANTES REÇUS EN 2021 AU LABORATOIRE D’EXPERTISE ET DE DIAGNOSTIC EN PHYTOPROTECTION DU MINISTÈRE DE L’AGRICULTURE, DES PÊCHERIES ET DE L’ALIMENTATION DU QUÉBEC
CULTURES: Échantillons reçus en 2021 au Laboratoire d’expertise et de diagnostic en phytoprotection (LEDP), regroupant de nombreuses cultures RÉGION: Québec NOMS ET ORGANISME: A.-M. BRETON, A. DIONNE, D. HAMEL, L. PICHETTE, N. SHALLOW ET J. VIVANCOS
LEDP, ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ), Complexe scientifique, 2700, rue Einstein, D.1-200h, Québec, QC G1P 3W8 Téléphone: 418-643-5027, poste 2700; Télécopieur: 418-646-6806; Courriel: [email protected] Sites Internet: http://www.mapaq.gouv.qc.ca/fr/Productions/Protectiondescultures/diagnostic/Pages/diagnostic.aspxhttp://www.agrireseau.qc.ca/lab/
RÉSUMÉ: Malgré les deux périodes de confinement dues à la pandémie mondiale, la quantité d’échantillons reçus au laboratoire de phytopathologie est demeurée proportionnelle à celles des années précédentes. Du 1er janvier au 23 novembre 2021, 2631 échantillons ont été traités dans la section phytopathologie du LEDP. En ordre d’importance, les échantillons reçus comprennent des plantes maraîchères (serres et champs), des arbres et arbustes fruitiers, des petits fruits, des grandes cultures et céréales, des plantes à usage industriel, des plantes ornementales herbacées, des plantes fourragères, des arbres et arbustes ornementaux (serres et pépinières) ainsi que des plantes aromatiques et médicinales.
MÉTHODES: Le laboratoire de phytopathologie du LEDP offre des services de diagnostic et de détection des maladies parasitaires aux conseillers agricoles, aux producteurs, aux particuliers et aux instances gouvernementales. Les données présentées ci-dessous concernent les maladies identifiées sur les échantillons de plantes reçus en 2021. Les échantillons reçus font d’abord l’objet d’un examen visuel, généralement suivi d’examens au stéréomicroscope et au microscope photonique. Selon les symptômes observés, un ou plusieurs tests diagnostiques sont réalisés dans le but de détecter ou d’identifier l’agent ou les agents phytopathogènes.
Voici les principaux tests de laboratoire réalisés afin d’appuyer le diagnostic: les nématodes vermiformes sont extraits du sol et des tissus végétaux par la méthode de l’entonnoir de Baermann, tandis que les nématodes à kystes sont extraits du sol à l’aide d’un appareil de Fenwick. Leur identification (au genre et, lorsque possible, à l’espèce) est réalisée par un examen microscopique des caractères morphologiques et par des techniques de biologie moléculaire. Ditylenchus sp. est détecté dans la plante par réaction en chaîne par polymérase (PCR). Les champignons et oomycètes sont isolés sur des milieux de culture gélosés et identifiés selon leurs caractéristiques morphologiques et/ou par le séquençage de certains gènes. Plusieurs espèces sont détectées dans la plante par des outils de biologie moléculaire (PCR, qPCR). Les bactéries sont isolées sur des milieux de culture gélosés, puis identifiées à l’aide de tests biochimiques BiologR et/ou par le séquençage de certains gènes. Comme pour les nématodes et les champignons, certaines espèces de bactéries sont détectées dans la plante par des outils de biologie moléculaire (PCR, qPCR). Les phytoplasmes sont détectés par des techniques de biologie moléculaire (PCR nichée et séquençage d’ADN). Les virus, quant à eux, sont détectés par des tests sérologiques ELISA ou par RT-PCR, PCR ou RT-qPCR.
RÉSULTATS ET DISCUSSIONS: Les présentent le sommaire des maladies identifiées sur les échantillons de plantes reçues, quelle que soit leur origine (champ, serre ou entrepôt). Notez que le nombre de maladies rapportées ne correspond pas au nombre d’échantillons réellement reçus et traités durant l’année puisque plus d’un problème peut être identifié sur un même échantillon (plante reçue) et que le diagnostic de certains cas n’a pas été inclus dans ce rapport. Cela concerne notamment les causes indéterminées, les causes incertaines ou hypothétiques, les détections négatives et les données potentiellement nominatives. Étant donné que les problèmes abiotiques (non parasitaires) diagnostiqués sur les échantillons sont, en majorité, de nature hypothétique, ils ont rarement été cités dans ce rapport; ces diagnostics sont établis en fonction de l’observation des symptômes, du résultat de certains tests de laboratoire et d’informations obtenues à la suite de discussions avec les clients.
Tableau 1. Sommaire des maladies diagnostiquées parmi les plantes maraîchères reçues au LEDP en 2021.
Tableau 2. Sommaire des maladies diagnostiquées parmi les arbres fruitiers et petits fruits reçus au LEDP en 2021.
Tableau 3. Sommaire des maladies diagnostiquées parmi les grandes cultures/céréales et cultures industrielles reçues au LEDP en 2021.
Tableau 4. Sommaire des maladies diagnostiquées parmi les plantes fourragères reçues au LEDP en 2021.
Tableau 5. Sommaire des maladies diagnostiquées parmi les arbres et arbustes ornementaux ou d’utilisation industrielle reçus au LEDP en 2021.
Tableau 6. Sommaire des maladies diagnostiquées parmi les plantes herbacées ornementales reçues au LEDP en 2021.
Tableau 7. Sommaire des maladies diagnostiquées parmi les plantes aromatiques et médicinales reçues au LEDP en 2021.
REMERCIEMENTS: Les auteurs remercient Marion Berrouard, Dominic Lafleur, Kariane Pouliot, Chantal Malenfant, Jaëlle Falardeau, Ludovic Jacques, Carolle Fortin et Annie-Pier Hachey pour leur assistance technique ainsi que les étudiants, Louis Robitaille, Paul-Émile Gareau, Carlos-Mario Jimenez et India-Jane Tremblay.
DISEASES DIAGNOSED ON PLANT SAMPLES SUBMITTED TO THE NEW BRUNSWICK DEPARTMENT OF AGRICULTURE, AQUACULTURE AND FISHERIES PLANT DISEASE DIAGNOSTIC LABORATORY IN 2021
CROP: Diagnostic Laboratory Report LOCATION: New Brunswick NAME AND AGENCY: M.T. TESFAENDRIAS
New Brunswick Department of Agriculture, Aquaculture and Fisheries1350 Regent Street, Fredericton, NB E3C 2G6 Telephone: (506) 453-3478; Facsimile: (506) 453-7978; E-mail: [email protected]
ABSTRACT: The New Brunswick Department of Agriculture, Aquaculture and Fisheries (NBDAAF) Plant Disease Diagnostic Laboratory provides diagnostic services and disease management recommendations to growers and the agricultural industry in New Brunswick. In 2021, a total of 157 plant tissue samples were submitted to the diagnostic laboratory for problem identification and possible control recommendations.
INTRODUCTION AND METHODS: The NBDAAF Plant Disease Diagnostic Laboratory located in Fredericton, NB, provides diagnostic services and control recommendations for diseases of various crops to growers and the agricultural industry in New Brunswick as part of an integrated pest management (IPM) service. Samples are submitted to the diagnostic laboratory by IPM scouts, growers, agribusiness representatives, crop insurance agents and NBDAAF crop specialists and extension officers. Disease diagnoses are based on a combination of visual examination of symptoms, microscopic observations, and culturing onto growth media.
RESULTS AND COMMENTS: From February 1 to November 30, 2021, the Plant Disease Diagnostic Laboratory received 157 diseased plant samples for diagnosis. Samples diagnosed during scouting and field visits are not included in this report. Summaries of diseases and causal agents diagnosed on plant tissue samples submitted to the NBDAAF Plant Disease Diagnostic Laboratory in 2021 are presented in to by crop category.
Table 1. Summary of diseases diagnosed on fruit tree crop samples submitted to the NBDAAF Plant Disease Diagnostic Laboratory in 2021.
Table 2. Summary of diseases diagnosed on berry and grape crop samples submitted to the NBDAAF Plant Disease Diagnostic Laboratory in 2021.
Table 3. Summary of diseases diagnosed on vegetable (field and greenhouse) crop samples submitted to the NBDAAF Plant Disease Diagnostic Laboratory in 2021.
Table 4. Summary of diseases diagnosed on cereal and legume field crop samples submitted to the NBDAAF Plant Disease Diagnostic Laboratory in 2021.
Table 5. Summary of diseases diagnosed on trees, shrubs and ornamental plant samples submitted to the NBDAAF Plant Disease Diagnostic Laboratory in 2021.
DISEASES DIAGNOSED ON COMMERCIAL CROP SAMPLES SUBMITTED TO THE PEI ANALYTICAL LABORATORIES PLANT DISEASE DIAGNOSTIC SERVICE (PDDS) IN 2021
CROP: Diagnostic Laboratory Report - All Crops LOCATION: Prince Edward Island NAMES AND AGENCIES: M.M. CLARK
PEI Department of Agriculture and Land, PEI Analytical Laboratories, Plant Disease Diagnostic Laboratory, 23 Innovation Way, Charlottetown, PE C1E 0B7 Telephone: (902) 368-5261; Facsimile: (902) 368-6299; E-mail: [email protected] Web page: https://www.princeedwardisland.ca/en/information/agriculture-and-land/pei-analytical-laboratories-peial
ABSTRACT: The Plant Disease Diagnostic (PDD) Laboratory section of PEI Analytical Laboratories, Prince Edward Island Department of Agriculture and Land, provides diagnosis and surveillance of disease problems caused by fungi, bacteria, viruses, and insect pests of commercial crops produced on PEI. In 2021, a total of 256 diagnoses were completed on a total of 135 samples processed. Among potato foliar diseases, brown spot and early blight were detected more frequently in 2021 than in the previous two seasons. No potato late blight was observed. Forty-five apple samples were submitted and diseases identified included black rot and sudden apple decline (SAD) which was associated with Cylindrocarpon sp., Fusarium sp., Phytophthora sp. and Pythium sp. Six samples tested for fire blight were negative. The fusarium head blight fungus, Fusarium graminearum, was detected in barley, Puccinia striiformis (stripe rust) was observed on winter wheat, and Plasmodiophora brassicae (clubroot) was isolated from canola roots.
METHODS: Samples for disease diagnosis are submitted to the PDD laboratory by agriculture extension staff, researchers, producers, greenhouse growers, agri-business representatives, crop insurance agents, and the general public. Diagnoses are based on a combination of investigative work, visual examination of symptoms, microscopic observations, and culturing onto artificial media. When additional confirmation is necessary, culture samples may be sent to other laboratories with producer’s consent for identification of associated pathogens by other means such as polymerase chain reaction (PCR).
RESULTS: A total of 256 diagnoses were completed on a total of 135 samples processed during the period of June 7 to October 29, 2021. A summary of the diseases and disorders diagnosed per crop on samples submitted in 2021 is provided in . The diagnoses reported do not necessarily reflect the major disease problems encountered in the field during the season, but rather those most prevalent within the samples submitted. Samples submitted to the diagnostic laboratory which were associated with insect damage are not included in this report. Categories of samples received were potatoes (49.83%), fruit crops (33.45%), cereal crops (5.57%), cole crops (4.88%) and other crops (6.27%).
Table 1. Diseases and disorders diagnosed on commercial crop samples submitted to the PEI Analytical Laboratories, Plant Disease Diagnostic Service in 2021.
The 2021 year had optimum growing conditions for various crops including potato, corn, cereals and fruit. No potato late blight infections were reported or identified since the level of inoculum was very low and growers continued to carry out improved management practices along with their spray regimes correlated with spore trapping surveillance. Among potato foliar diseases, brown spot and early blight were detected more frequently in 2021 than in the previous two seasons. The Fusarium oxysporum fungus was isolated more frequently from potato seed that were breaking down due to fusarium dry rot, and from plant stems with wilt infection. Other Fusaria isolates involved in seed piece decay included Fusarium solani, Fusarium graminearum and Fusarium culmorum. Potato blackleg infections continued to be observed this season and were found in ‘Russet Burbank’, ‘Satina’ and ‘Colomba’ varieties. Blackleg is primarily a seed-borne disease that is often associated with cool, wet soil conditions, but new strains can develop under dry conditions. The physiological disorder, pinkeye, was identified this season in the ‘Clearwater Russet’ and ‘Alverstone’ varieties. Pinkeye symptoms appear at or before the end of the harvest period and usually disappear in storage as the tuber dries up. However, this tissue is vulnerable to invasion by soft rot bacteria such as Pseudomonas sp. and Pectobacterium.
A total of 45 apple samples were submitted to the PDD laboratory; diseases identified included black rot and sudden apple decline (SAD). Microorganisms involved in the SAD disease included Cylindrocarpon sp., Fusarium sp., Phytophthora sp. and Pythium sp. Six samples tested for fire blight were negative. The PEI PDD continues to be involved in a regional research survey project for SAD.
Finally, in barley crops, the fusarium head blight fungus, Fusarium graminearum, was detected in glumes that had no typical salmon-coloured symptoms, the Puccinia striiformis (stripe rust) fungus was observed on leaves of winter wheat, and the clubroot organism, Plasmodiophora brassicae, was isolated from canola roots.
Diagnoses were also completed this season on new crops such as luffa, turf grass, garlic, and watermelon.
CEREALS/CÉRÉALESSURVEY OF CEREAL CROP DISEASES IN BRITISH COLUMBIA DURING THE 2020 AND 2021 GROWING SEASONS
CROPS: Wheat and Barley LOCATION: British Columbia Peace River Region NAMES AND AGENCIES: V. FETTERLEY1, R.K. BAMRAH1, B. YADAV1, K. ULOTH2 & G.S. BRAR1
1The University of British Columbia, 2357 Main Mall, Vancouver, BC V6T 1Z4 Telephone: (604) 827-5274; Facsimile: (604) 822-6394; E-mail: [email protected] 2BC Pest Monitoring Network, Dawson Creek, BC
ABSTRACT: Cereal crops were surveyed in the Peace River Region of British Columbia for foliar disease in July during the 2020 and 2021 growing season. In total, 22 fields were surveyed in 2020, and 43 in 2021 (). In both years, disease pressure was low. In 2021, the low disease pressure was likely attributable to the warm and dry weather experienced throughout the 2021 growing season in western Canada. Stripe rust was observed on wheat in 2020. Loose smut was observed on wheat in 2021, and on barley in the two years surveyed.
Fig. 1 Location of fields surveyed in 2020 (red) and 2021 (blue), in the Peace River region of British Columbia.
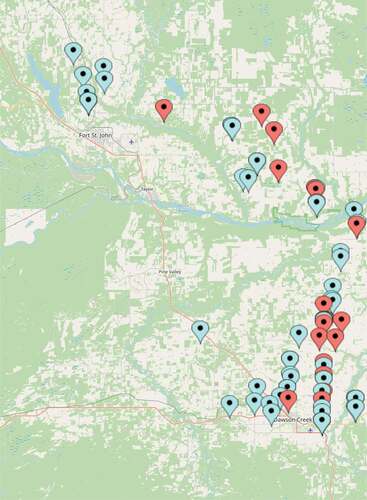
INTRODUCTION AND METHODS: During the 2020 growing season, crops were surveyed on July 16 and 17. In total, three barley and 19 wheat fields were assessed for foliar diseases. In 2021, 15 barley and 28 wheat fields were surveyed. All the fields surveyed were selected at random around Fort St. John and Dawson Creek with an aim to cover the whole BC Peace Regional Agricultural District. Crops between the late booting and hard dough stage were assessed for foliar disease. A 5-category scale was used for stripe rust to assess disease severity in each field during the 2020 growing season: clean (no visible symptoms); trace (<3% leaf area affected); light (3-15%); moderate (>15-20%); and severe (>20%).
RESULTS AND COMMENTS: Of the 15 barley fields surveyed in 2021, three were free of any disease. Scald was observed in trace amounts on lower leaves in seven fields. Low incidences of net blotch and loose smut were observed in three of the surveyed barley fields and one field had severe levels of loose smut. Signs of bacterial leaf streak were observed in two of the 15 surveyed barley fields and fusarium head blight (FHB) was observed on one spike. During the 2020 growing season, trace levels of leaf spot were observed in two fields of 2-row barley. The only 6-row barley field surveyed had a moderate to high severity of loose smut (10 smutted heads in 6 m of row). The same field had 0-5% incidence of barley scald on the flag and penultimate leaves.
During the 2020 growing season, a total of 19 wheat fields were surveyed and 11 had no disease except trace levels of leaf spots on the lower canopy. Three fields surveyed showed light to moderate leaf spot severity on flag leaves and the lower canopy. Two wheat fields had trace to light levels of stripe rust.
Among the 28 wheat fields surveyed in 2021, 20 showed no signs of any disease. Loose smut was observed in three fields, but only one had severe levels. Silver heads, caused by the wheat stem maggot, were observed in five fields. Both years, stripe rust was observed on wild foxtail barley (Hordeum jubatum) growing in and around two wheat fields, but no sign of rust was observed in cereal crops in the 2021 growing season.
In summary, more than 50% of the barley and wheat fields surveyed in Peace River region of British Columbia were free of disease in both years. In the 2021 growing season, diseases were generally found in trace amounts, although severe levels of loose smut were observed in one barley and one wheat field. The 2021 growing season was marked by very dry weather, explaining the low disease incidence observed. Wheat and barley plants at the booting stage were more affected by the drought and were shorter than crops past the anthesis stage. Although warmer weather tends to result in lower disease pressure on crops, such conditions can severely impact the yield of non-irrigated fields.
ACKNOWLEDGEMENTS: We acknowledge the financial support provided by the BC Peace River Grain Industry Development Council (BC-GIDC), the Peace Region Forage Seed Association, as well as the BC Hydro Agricultural Compensation Fund.
STRIPE RUST OF CEREALS IN ALBERTA, 2021
CROPS: Wheat, Barley LOCATION: Alberta NAMES AND AGENCY: B. WEI, M. ZID & R. ABOUKHADDOUR
Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada, 5403 1st Avenue South, Lethbridge, AB T1J 4B1 Telephone: (403) 317-2222; Facsimile: (403) 382-3156; E-mail: [email protected]
ABSTRACT: During the 2021 growing season, a lack of stripe rust infection was remarkable in southern Alberta. In total, 28 cereal fields were surveyed for stripe rust and leaf spot incidence and severity. Data were collected from nine barley fields and 19 wheat fields comprising six spring wheat and 13 winter wheat fields. The survey area was bounded by Highway #522 in the north, Township Road 40 in the south, Highway 2 in the west and Range Road 145 in the east. Stripe rust was found in only one barley field at trace level, and no stripe rust infection was reported on wheat. Leaf spots were found in eight barley fields and 12 wheat fields. The most severe leaf spot infection was found in Lethbridge, with a severity rating of over 50%. High temperatures and record dry weather in spring and summer resulted in solar damage on several cereal fields and hindered development of stripe rust and leaf spot disease.
INTRODUCTION AND METHODS: Commercial cereal fields, including winter wheat, spring wheat and barley, were surveyed in southern Alberta from early July to early August, 2021. Data were collected from nine barley fields and 19 wheat fields comprising six spring and 13 winter wheat fields. The survey area was bounded by Highway #522 in the north, Township Road 40 in the south, Highway 2 in the west, and Range Road 145 in the east. Fields were evaluated for both disease incidence and severity in a ‘W’ pattern until 10 sites separated by approximately 25 m were assessed. Incidence was assessed as the number of diseased plants within 1-m2. The severity of leaf spot diseases was rated as the average percentage of the total flag leaf surface area covered with lesions. The severity of stripe rust (Puccinia striiformis Westend.) was evaluated as the average percentage of the total flag leaf surface area covered with stripes, per plant. Based on the disease severity level, fields were classified as clean (0%), trace (1-3%), light (3-5%), moderate (6-19%) or severe (20-100%).
RESULTS AND COMMENTS: In total, 28 commercial fields were surveyed, consisting of 19 wheat fields and nine barley fields (, ). Among the nine barley fields, one field was clean but with solar damage on leaves and the other eight fields were infected with leaf spots pathogens. Three infected barley fields were rated as severe, three as moderate, and one as a light disease level. One barley field was affected by a complex of foliar diseases, including leaf blotch, net blotch and stripe rust. Among the surveyed wheat fields, 10 fields were rated as having a severe infection level, consisting of four winter wheat and all six spring wheat fields. Two winter wheat fields were moderately affected by leaf spots. Seven winter wheat fields were clean. Solar damage on leaves was occasionally observed in two winter wheat fields. Hail damage was found in one spring wheat field in Spring Coulee. Due to the high temperatures and dry conditions this spring and summer, there was no stripe rust infection observed in Alberta and leaf spots were found less frequently compared to previous years.
Fig. 1 Map showing surveyed fields in 2020; blue pins indicate wheat fields and pink pins indicate barley fields.
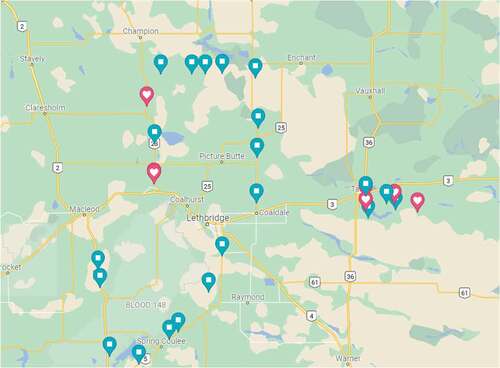
Table 1. Number of wheat and barley fields surveyed and the corresponding stripe rust severity levels recorded in southern Alberta during the summers of 2021 to 2016, and 2011.
REFERENCES
- Aboukhaddour R, Gourlie R, Despins T, Harding M, Klein-Gebbinck HW, Feng J, McCallum B. 2021. Stripe (yellow) rust of cereal in Alberta, 2020. Can. Plant Dis Surv. 101:99–101. In, Can J Plant Pathol. 43:sup1.
- Aboukhaddour R, Ghanbarnia K, McCormack K. 2020. Stripe (yellow) rust of cereal in Alberta, 2019. Can Plant Dis Surv. 100:100–101. In, Can J Plant Pathol. 42:sup1.
- Aboukhaddour R, Ghanbarnia K, Xi K, Kumar K, Harding M, Klein-Gebbinck H. 2019. Stripe (yellow) rust of cereals in Alberta. Can Plant Dis Surv. 99:112–113. In, Can J Plant Pathol. 41:sup1.
- Aboukhaddour R, Amundsen E, Randhawa H, Gaudet D. 2018. Stripe rust in southern Alberta, 2015–2016. Can Plant Dis Surv. 98:142–143.
- Aboukhaddour R, Amundsen E. 2018. Stripe rust in southern Alberta, 2016–2017. Can Plant Dis Surv. 98:144–145.
WHEAT DISEASE SURVEY IN ALBERTA, 2021
CROP: Wheat LOCATION: Alberta NAMES AND AGENCIES: M.W. HARDING1, T.K. TURKINGTON2, S. WATERMAN3, H. KLEIN-GEBBINCK4, R. ABOUKHADDOUR5, N. RAUHALA2, B. WEI5, M. ZID5, G.C. DANIELS1 & M.A. KENNEDY1
1Crop Diversification Centre South, Alberta Agriculture, Forestry and Rural Economic Development, 301 Horticulture Station Rd. E., Brooks, AB T1R 1E6 Telephone: (403) 362-1338; Facsimile: (403) 362-1326; E-mail: [email protected] 2Lacombe Research and Development Centre, Agriculture and Agri-Food Canada, 6000 C&E Trail, Lacombe, AB T4L 1W1 3Field Crop Development Centre, Olds College, 5030 50th St., Lacombe, AB T4L 1W8 4Beaverlodge Research Farm, Agriculture and Agri-Food Canada, Beaverlodge, AB T0H 0C0 5Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada, 5403 1st Ave. S., Lethbridge, AB T1J 4B1
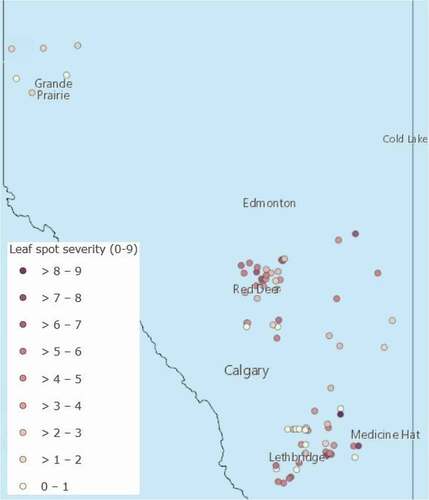
ABSTRACT: Wheat disease symptoms were evaluated in 79 fields (66 spring wheat and 13 winter wheat), representing 21 counties in Alberta. Foliar fungal diseases such as tan spot, speckled leaf blotch, and stagonospora leaf blotch were rated together as a leaf spot complex. Stripe rust, powdery mildew, bacterial leaf streak and wheat streak mosaic virus symptoms were also evaluated. Finally, other root and head diseases (take all, ergot, smut) were noted. Symptoms of leaf spots were reported in 91% of fields, however the hot, dry conditions that persisted across many regions of Alberta during the summer of 2021 prevented significant disease development. Overall, leaf spots were either absent, or had caused light severity in over 60% of fields, while stripe rust was not observed on wheat in Alberta in 2021.
INTRODUCTION AND METHODS: Foliar diseases on wheat can reduce yield and quality of grain due to the energy drain by the pathogen, and limiting photosynthetic capacity. Some infections can also cause rupturing of the leaf epidermis and subsequent moisture loss, while some leaf pathogens can directly affect the head and developing kernels.
Wheat fields were surveyed between the late milk and hard dough ages from June 20 to August 5, 2021. Seventy-nine fields in 21 counties were evaluated (). Disease severity and percent leaf area damaged (PLAD) were recorded. In-field assessments were performed at five points along a ‘diamond-shaped’ sampling pattern with the first point a minimum of 25 m into the field, and remaining points 25 m apart. Disease symptoms at each sampling point were rated for leaf spot severity using a 0-9 scale modified from Saari and Prescott (Citation1975), and stripe rust severity using a 0-100% scale (Chen [date unknown]). For other diseases (wheat streak mosaic, ergot, smut, bacterial leaf streak and take-all), a 0-3 rating (0=absent, 1=trace, 2=moderate, 3=severe) was recorded. Finally, 10 to 12 flag leaves were randomly collected into labelled paper bags at each of the five sampling points in 72 fields, and then air dried for 24-48 hours in a cool dry room and stored at 4°C. The collected leaves were assessed for percent leaf area damaged (PLAD), and will be used for determination of causal agents.
RESULTS AND COMMENTS: Fungal leaf spot symptoms were present in 91% of fields and generally at light to moderate severity (). The average severity of disease was 3.3 on the 0-9 scale and severity values ranged from 0 to 9. The average PLAD was 11.3% and ranged from 0% to 70% (). Stripe rust was not observed in any of the wheat fields surveyed in Alberta in 2021. Sixty of the fields were scored for the presence of five other diseases and bacterial leaf streak was reported in 16.7% fields at trace to moderate levels (). Fields with bacterial leaf streak were located almost exclusively in southern Alberta. Ergot was seen in 5% of fields at trace levels, while wheat streak mosaic and smut were observed at trace levels in one field each. Take-all symptoms were not reported in any of the fields surveyed.
Table 1. Fungal leaf spot severity and percent leaf area damaged (PLAD) for 79 wheat fields surveyed during the 2021 growing season in Alberta.
Table 2. Occurrence of five wheat diseases in 60 wheat fields surveyed during the 2021 growing season in Alberta.
REFERENCES
- Saari EE, Prescott JM. 1975. A scale for appraising the foliar intensity of wheat diseases. Plant Dis Rep. 59:377–380.
- Chen X. [date unknown]. Stripe rust severity index. United States Department of Agriculture Agricultural Research Service (USDA-ARS) and Washington State University (WSU). [accessed 2022 Apr 27]. https://striperust.wsu.edu/disease-management/control/stripe-rust-severity-index/
2021 BARLEY DISEASE SURVEY IN ALBERTA
CROP: Barley LOCATION: Alberta NAMES AND AGENCIES: N. RAUHALA1, T.K. TURKINGTON1, J. BUSAAN1, S. WATERMAN2, H.W. KLEIN-GEBBINCK3, M.W. HARDING4, G.C. DANIELS4, M.A. KENNEDY4, R. ABOUKHADDOUR5, M. ZID5 & H. SPENCE3
1Lacombe Research and Development Centre, Agriculture and Agri-Food Canada, 5030 50th St., Lacombe, AB T4L 1W8 Telephone: (403) 782-8100; Facsimile: (403) 782-6120; E-mail: [email protected]; [email protected] 2Field Crop Development Centre, Olds College, 5030 50th St., Lacombe, AB T4L 1W8 3Beaverlodge Research Farm, Agriculture and Agri-Food Canada, Beaverlodge, AB T0H 0C0 4Crop Diversification Centre South, Alberta Agriculture, Forestry and Rural Economic Development, 301 Horticulture Station Rd. E, Brooks, AB T1R 1E6 5Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada, 5403 1st Ave. S, Lethbridge, AB T1J 4B1
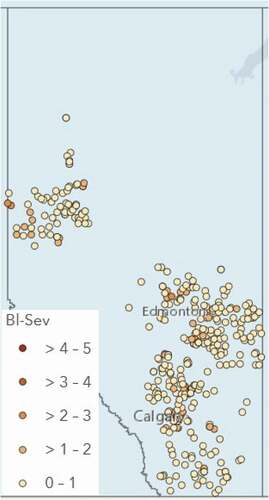
ABSTRACT: In 2021, 71 random commercial barley crops were surveyed for disease levels in Alberta. Disease assessments were typically done using two approaches, the first based on a 0-9 rating scale where 0 was no disease development in the lower, middle and upper canopy, and 9 was >50% leaf area diseased. The second approach for leaf spots involved rating penultimate leaf samples for percentage leaf area diseased. Ratings for other diseases were based on incidence and/or presence or absence, especially where only trace levels were found. Overall, leaf disease levels were much lower than in previous years due to the hot, dry growing conditions across the province. No stripe rust was observed in 2021, while loose smut was only noted in 15 fields, and suspected bacterial leaf streak was observed in one field.
INTRODUCTION AND METHODS: A survey to document diseases of barley was conducted in 71 fields across Alberta from July 20 – August 5, 2021, with evaluations being done at the late milk to soft dough stage. The fields were typically traversed in a diamond pattern starting at least 25 m in from the field edge, with visual assessments made at each of five locations at least 25 m apart. Disease symptoms at each sampling point were rated for leaf spot severity using a 0-9 scale modified from Saari and Prescott (Citation1975). A rating of 0 was no disease development in the lower, middle and upper canopy, and 9 was >50% leaf area diseased. In addition, penultimate leaves were collected from 54 fields and rated for the percentage leaf area diseased (PLAD) due to scald, net-form net blotch and other leaf spots (a combination of symptoms due to spot-form net blotch (Pyrenophora teres f. maculata), spot blotch (Cochliobolus sativus), and physiological leaf spotting). Other diseases (loose smut, stripe rust, bacterial leaf streak, and root rots where stunting and/or premature ripening of plants occurred and were associated with root or stem base symptoms) were rated based on incidence and/or presence or absence, especially where only trace levels were found. Following the survey, a representative tissue sub-sample of diseased plant parts collected at each location was cultured in the laboratory for pathogen isolation and identification.
RESULTS AND COMMENTS: Weather conditions across the region were generally hot and dry for the entire growing season. Survey results are presented in for 61 fields rated using the 0-9 rating scale. Based on the 0-9 assessments, scald (Rhynchosporium commune) was present in 30 of the 61 fields with 25 of those fields having low levels with ratings from >0-3, another five fields having levels of 4-6, while no fields had higher levels of 7-9 on a 0-9 rating scale (). Net-form net blotch (Pyrenophora teres f. teres) was found in 21 of the 61 fields, although some of these fields only had trace levels (). Sixteen fields had low levels with ratings from >0-3 and another five fields had levels of 4-6 on the 0-9 scale. Other leaf spots were found in 53 of the 61 fields with 35 of those fields having ratings from >0-3 and another 18 fields having ratings from 4-6 (0-9 scale).
Table 1. Disease prevalence and severity in 61 commercial barley fields in Alberta, 2021.
For the penultimate leaves, there were a total of 71 fields sampled; however, in three fields the leaves were too senesced to rate, while in five fields only the 0-9 scale was used to rate disease. Finally, nine fields reported only ranges in incidence and severity. As a result, PLAD is reported for 54 fields (). Based on PLAD assessments, scald was absent in 21 fields, but was present in 33 of 54 fields, with 29 of those fields having low levels with ratings from >0-3 and another four fields having levels of 4-7 (). Net-form net blotch was found in 16 of 54 fields, with 12 of those fields having a low PLAD from >0-3, one field with 9.5 PLAD, and three fields with 14-37.5 PLAD. Other leaf spots were found in 47 of 54 fields, with 30 of those fields having a low PLAD from >0-3, 14 fields having 3-10.6 PLAD, and three fields having ≥20 PLAD. Overall, total leaf disease severity averaged 5.5 PLAD with 100% of fields with symptoms and a range of 0.02-38 PLAD (). These other leaf spots were identified in the laboratory and Cochliobolus sativus, the causal agent of spot blotch, was isolated from four of the 54 fields, while P. teres f. maculata (spot-form net blotch) was isolated from 10 of the 54 fields, and Septoria spp. were isolated from six fields. The saprophytic fungi Alternaria spp. and Epicoccum spp. were isolated from 38 and 13 of the 54 fields, respectively. The presence of these saprophytes is often associated with dead plant tissues resulting from physiological issues and/or disease-related damage.
Table 2. Disease prevalence and percentage leaf area diseased (PLAD) assessments on penultimate leaf samples collected from 54 commercial barley fields in Alberta, 2021.
In the nine fields where ranges were reported, the leaf spot complex (one or more of net-form net blotch, spot-form net blotch/spot blotch, scald) was present in most fields at low to moderate severities (percentage leaf area affected ranged from 0 to 60%), although one field without symptoms was suspected of having sun damage.
Loose smut (Ustilago nuda) was found at trace levels in 15 of the 61 barley fields surveyed in 2021, while one field in Alberta had moderate levels.
No stripe rust (Puccinia striiformis) was found in any of the 61 commercial barley fields surveyed. Low levels of bacterial leaf streak were found in one field, while 27 fields had trace to moderate levels of root rot, and two fields had trace levels of ergot.
Overall barley leaf disease levels in 2021 were much lower than in the previous year (Rauhala and Turkington Citation2021).
ACKNOWLEDGEMENTS: The authors would like to acknowledge the support of summer students, graduate students and technicians Avy Lamb, Kathleen McHugh, Lexie Herspiegel, Bohan Wei, and Jeremy Hodges. The generous funding of the Canadian Barley Research Coalition, Barley Council of Canada, Alberta Barley, Saskatchewan Barley Commission, Manitoba Crop Alliance, and the Brewing and Malting Barley Research Institute under the Canadian Agricultural Partnership and the Canadian National Wheat Cluster is graciously acknowledged. The in-kind support of Alberta Agriculture, Forestry and Rural Economic Development is also gratefully acknowledged.
REFERENCES
- Rauhala NE, Turkington TK. 2021. 2020 barley disease survey in central Alberta. Can Plant Dis Surv. 101:61–62. In, Can J Plant Pathol 43:sup1.
- Saari EE, Prescott JM. 1975. A scale for appraising the foliar intensity of wheat diseases. Plant Dis Rep. 59:377–380.
2021 WHEAT DISEASE SURVEY IN ALBERTA, SASKATCHEWAN AND MANITOBA
CROP: Wheat LOCATION: Alberta, Saskatchewan and Manitoba NAMES AND AGENCIES: T.K. TURKINGTON1, M. A. HENRIQUEZ2 & B. MCCALLUM2
1Lacombe Research and Development Centre, Agriculture and Agri-Food Canada, 5030 50th St., Lacombe, AB T4L 1W8 Telephone: (403) 782-8100; Facsimile: (403) 782-6120; E-mail: [email protected]; [email protected] 2Morden Research and Development Centre, Agriculture and Agri-Food Canada, Morden, MB R6M 1Y5
ABSTRACT: In 2021, producer-collected, wheat flag leaf samples were obtained from 11 commercial wheat fields in the Prairie region. These were kindly mailed by participating producers to the Lacombe Research and Development Centre, Agriculture and Agri-Food Canada (AAFC). Disease assessments were done by AAFC staff by rating the percentage area of the flag leaf that was diseased by the leaf spot complex. Overall, leaf disease levels were generally low and this was likely due to the hot, dry growing conditions across the province. No stripe or leaf rust were observed in the samples collected in 2021.
INTRODUCTION AND METHODS: A survey to document leaf diseases of wheat was conducted in 11 fields across Alberta, Saskatchewan and Manitoba in August 2021, with leaf collections being done by volunteer producers at the late milk to soft dough stage. Participating farmers were each sent a kit with survey instructions and materials to randomly collect five flag leaves at each of five sampling sites along a ‘diamond-shaped’ sampling pattern, for a total of twenty-five leaves per field. In addition to the sampling kit, a questionnaire was included to collect cropping practice information related to rotation, tillage practices, variety, etc. Leaf samples, along with answers to the questionnaire, were returned to AAFC Lacombe by mail for rating, assessment of causal agents, and tabulation of questionnaire results. Leaf samples were rated for wheat leaf disease symptoms, including tan spot (Pyrenophora tritici-repentis), the septoria complex (Zymoseptoria tritici and Parastagonospora nodorum), spot blotch (Bipolaris sorokiniana) and physiological leaf spotting, and were checked for the presence of leaf rust (Puccinia triticina) and stripe rust (Puccinia striiformis). Each leaf was rated for percentage leaf area affected and averages were calculated for each field. Other issues, such as bacterial leaf streak, were also noted and rated if present. Representative leaf samples from each field were placed in moist chambers and incubated for up to 48-72 hours to promote pathogen sporulation. Causal agents and other saprophytic fungi were identified based on fruiting structures and/or spore morphology.
RESULTS AND COMMENTS: Sampling kits were prepared and producers contacted in late June/early July with kits being sent out to volunteer producers over the following weeks. Unfortunately, the summer of 2021 was challenging for wheat producers given the wide spread occurrence of hot dry weather conditions. This resulted in much more rapid crop development and as a consequence, in some cases, crops were too advanced for producers to sample. Based on our experience in 2021, the PBN will be contacting volunteer producers in late spring followed by distribution of kits before the end of June 2022.
In total, samples from 11 wheat fields were sent back for rating and tabulation of cropping information. Samples from single fields were submitted from Alberta (AB) and Saskatchewan (SK), and from a total of nine Manitoba (MB) fields. Overall, the average proportion of leaf area affected was 5.7%, with average values of 2.9%, 1.1%, and 6.6% for AB, SK, and MB, respectively (). No stripe rust or leaf rust was observed in the samples collected. Assessment of causal agents indicated that symptoms in four of 11 fields were associated with spot blotch (Bipolaris sorokiniana), while the causal agents of tan spot and the septoria complex were not observed in these fields. In one field, the causal agents of the septoria complex were noted, while the causal agents of tan spot and spot blotch were not observed. The causal agent of tan spot was not observed in any of these fields. Overall, the main fungi observed from all fields were the saprophytes Epicoccum spp. and Alternaria spp. It is suspected that a significant proportion of symptoms may have been due to stress responses, i.e., physiological leaf spotting, especially in fields where the causal agents of tan spot, the septoria complex and spot blotch were not observed. Saprophytes don’t cause damage to leaf tissue, but infect after the leaf has already been damaged due to a pathogen, or heat stress, drought, hail damage, etc. There was diversity in the varieties grown and sampled, which included AAC Brandon (2); AAC Elie (1); AAC Leroy (1); AAC Redberry (1); AAC Starbuck (2); AAC Wheatland (2); Cardale (1); and Prosper (1). Given the small number of samples it is challenging to associate and compare the disease levels observed with the cropping practices indicated.
Table 1. Prairie Biovigilance Network (PBN) wheat leaf disease survey results in Alberta, Saskatchewan and Manitoba, 2021.
ACKNOWLEDGEMENTS: The authors would like to acknowledge the support of participating farmers and the assistance of the Manitoba Crop Alliance, Alberta Barley, and Saskatchewan Barley Commission with identifying potential collaborators. The current report is part of the AAFC Prairie Biovigilance Network (PBN) and A-base funding from AAFC is graciously acknowledged. We would also like to thank Noryne Rauhala and Kristy Vogelzang, AAFC Lacombe for their assistance with kit assembling and shipment.
LEAF SPOT DISEASES OF OAT AND BARLEY IN SASKATCHEWAN IN 2021
CROP: Oat and Barley LOCATION: Saskatchewan NAMES AND AGENCY: T. ISLAM, E. BOOTS, A. KARSTENS & H.R. KUTCHER
Crop Development Centre, University of Saskatchewan, 51 Campus Drive, Saskatoon, SK S7N 5A8 Telephone: (306) 966-8661; Facsimile: (306) 966-5015; Email: [email protected]
ABSTRACT: In 2021, 30 oat fields and 30 barley fields were surveyed across Saskatchewan. Stagonospora avenae and Pyrenophora avenae were found in 26% of the oat crops surveyed. In barley, Pyrenophora teres f. teres was the most prevalent pathogen, found in 27% of the crop surveyed.
INTRODUCTION AND METHODS: In 2021, 30 oat fields and 30 barley fields were surveyed in Saskatchewan. Surveys were conducted by the Saskatchewan Crop Insurance Corporation between late July and early August. Samples were collected when crops were at early milk to soft dough stage. The overall severity of leaf spots in the upper canopy was assessed and rated as trace (1-2% leaf area affected), light (3-15%), moderate (16-40%), or severe (41-100%). Ten flag leaves were collected from each crop surveyed. Leaves were cut into 1 cm-long pieces for plating. Leaves were sterilized in 70% ethanol and triple rinsed with sterile distilled water, then dried before being plated onto acidified potato dextrose agar. Plates were incubated under light at room temperature and pathogens were identified after four days based on morphology (Zillinsky Citation1983). Kernels were harvested from 30 panicles of oats. Panicles were threshed and kernels sterilized in 5% bleach solution before triple rinsing with sterile water. Kernels were plated on acidified potato dextrose agar and left for five days under light at room temperature before pathogens were identified according to morphology (Zillinsky Citation1983, Leslie and Summerell Citation2006). Disease prevalence was defined as the percentage of fields affected by each disease out of all fields surveyed.
RESULTS AND COMMENTS: Oat leaf pathogens were identified in 57% of oat crops surveyed, which was higher than the previous year (29% in 2020). The overall disease severity in the upper leaf canopies was trace (1-2% leaf area affected) to light (3-15% leaf area affected). Pathogens detected in the oat leaf samples were Stagonospora avenae (cause of septoria blotch; 26% of the crops surveyed), Pyrenophora avenae (cause of leaf blotch; 26% of the crops surveyed) and Cochliobolus sativus (cause of spot blotch; 17% of the crops surveyed). The frequency of the individual pathogens expressed as a percentage of total pathogens isolated was 37%, 34% and 29%, respectively (). In addition to leaf pathogens, Alternaria spp. were detected in 100% of the crops surveyed and Epicoccum spp. were detected in 17% of the crops surveyed. In oat grain samples, four different Fusarium spp. (F. poae, F. avenaceum, F. graminearum and F. nivale) were identified, which are causal agents of fusarium head blight (FHB). Prevalence (% of fields surveyed with the pathogen) was 43%, 13%, 10% and 3%, respectively (), which was lower than last year. In 2020, prevalence of F. poae, F. avenaceum and F. graminearum was 76%, 41% and 16%, respectively.
Table 1. Prevalence and incidence of leaf spot pathogens in oat and barley in Saskatchewan in 2021.
Table 2. Prevalence and frequency of Fusarium species isolated from infected oat kernels in Saskatchewan in 2021.
In barley, trace to light levels of disease severity were observed in the upper leaf canopies. Leaf pathogens were identified in eight (27%) of the 30 crops surveyed. Only Pyrenophora teres f. teres (cause of net blotch) and Cochliobolus sativus (cause of spot blotch) were identified in leaf samples. Pyrenophora teres f. teres was the most frequently identified pathogen with a prevalence of 27% and frequency of 65%. The prevalence of Cochliobolus sativus was only 3% and the frequency was 35%. Two saprophytes, Alternaria spp. and Epicoccum spp. were also detected at a prevalence of 100% and 77%, respectively.
ACKNOWLEDGEMENTS: This survey was supported by the leaf samples submitted by the Saskatchewan Crop Insurance Corporation. We thank our colleagues from the province and the members of the Cereal and Flax Pathology Group (CFPATH) – University of Saskatchewan.
REFERENCES
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell.
- Zillinsky FJ. 1983. Common diseases of small grain cereals: a guide to identification. Mexico: CIMMYT.
LEAF SPOTTING DISEASES OF COMMON AND DURUM WHEAT IN SASKATCHEWAN IN 2021
CROP: Common and durum wheat LOCATION: Saskatchewan NAMES AND AGENCIES: M.R. FERNANDEZ1, N. WAELCHLI1, C. KENNY2, F. WAELCHLI3, A. AKHAVAN4, C. PERU4 & S. HARTLEY5
1Agriculture and Agri-Food Canada, Swift Current Research and Development Centre, P.O. Box 1030, Swift Current, SK S9H 3X2 Telephone: (306) 770-4459; E-mail: [email protected] 2Agriculture and Agri-Food Canada, Saskatoon Research and Development Centre, 107 Science Place, Saskatoon, SK S7N 0X2 3Saskatchewan Crop Insurance Corporation, Box 3000, 484 Prince William Dr., Melville, SK S0A 2P0 4Saskatchewan Ministry of Agriculture, Crops and Irrigation Branch, 3085 Albert St., Regina, SK S4S 0B1 5Saskatchewan Ministry of Agriculture, Crop Protection Laboratory, 1610 Park St., Regina, SK S4N 2G1
ABSTRACT: The leaf spot (LS) disease complex was evaluated in common and durum wheat crops across Saskatchewan in 2021. Disease severity was compared in relation to wheat species, soil zone, crop district and cultivar. Mean LS severity was lower than in the previous three years, which was attributed to dry conditions throughout most of the province. The highest mean LS severity for common wheat was in the Black/Gray soil zone, while for durum wheat it was in the Brown soil zone. Pyrenophora tritici-repentis (tan spot) was the predominant LS pathogen.
INTRODUCTION AND METHODS: A survey for leaf spot (LS) diseases of common and durum wheat in Saskatchewan was conducted between the milk and dough growth stages in 2021. A total of 92 common and durum crops were sampled in 14 crop districts (CD) in the three soil zones (, ). Among the crops sampled, 59 were identified as common wheat and 33 as durum wheat. Information on the agronomic practices employed was obtained from the producers for most fields sampled.
Table 1. Incidence and severity of leaf spotting diseases in common and durum wheat crops surveyed in Saskatchewan in 2021.
In each field, 50 flag leaves were collected at random and air-dried at room temperature. The percentage of leaf area affected by LS (severity) was recorded for each leaf, and a mean percentage leaf area with LS was calculated for each crop and CD. For crops with LS severity of ≥5%, 1 cm2 surface-disinfested leaf pieces were plated on water agar for identification and quantification of the causal LS pathogens.
Cultivars were identified in 24 of the common and 31 of the durum wheat samples. The most popular cultivars (grown in at least five fields) were ‘AAC Brandon’ (10) for common wheat, and ‘Transcend’ (13) and ‘AAC Stronghold’ (5) for durum wheat.
For common wheat, of the 46 samples with a crop rotation history, 28 had been preceded by an oilseed crop, 13 by a cereal, and 5 by a pulse; while the most frequently-grown crop two years previously was a cereal (21), an oilseed (8), or a pulse (3). For durum wheat, of the 35 samples with a crop rotation history, 10 had been preceded by an oilseed crop, 19 by a pulse, 3 by a cereal, and 2 by fallow; while the most frequently-grown crop two years previously was a cereal (24), a pulse (6) or an oilseed (3).
Tillage systems were classified as conventional-, minimum-, or zero-till. Of the samples with tillage information, for common wheat, 21 were under zero-till, 14 under minimum-till, and 12 under conventional-till, while for durum wheat, 14 were under zero-till, 7 under minimum-till, and 9 under conventional-till.
RESULTS AND COMMENTS: Most of the province experienced dry to very dry conditions in the spring of 2021, and summer was drier overall than in the previous few years (). Some of the samples collected were too dry to be properly evaluated for leaf spots. Assessed samples were from 23 fields in the Brown soil, 27 in the Dark Brown soil, and 42 in the Black/Grey soil zone (). LS symptoms were observed in 40 of the 59 common, and 23 of the 33 durum, wheat crops. In individual samples, percentage flag leaf area affected ranged from zero to 5% for common wheat, and zero to 7.5% for durum wheat. Samples with ≥5% of the flag leaf area affected constituted 0% of the common wheat, and 3% of the durum wheat samples. The overall mean percentage of spotting on the flag leaf was 0.8%, which was numerically lower than in the previous three years: 2018 at 1.9%, 2019 at 2.6%, and 2020 at 1.1% (Fernandez et al. Citation2019, Citation2020, Citation2021).
Fig. 2 Percent of average precipitation in the Canadian Prairies from early May to late July of 2021. Normal precipitation based on 1981-2010. (Agriculture and Agri-Food Canada Citation2021).
Mean LS severity was somewhat lower for common (0.7%) than durum (1.0%) wheat (). For common wheat, the highest mean LS severity was in the Black/Gray soil zone at 0.9%, with the highest values being observed in Crop Districts 1A (2.1%) and 8A (1.5%). For durum wheat, the Brown soil zone had the highest mean LS severity at 1.2%, with the highest value being observed in Crop Districts 3BS/3BN (3.1%). These LS severities were all lower than in the last few years.
Overall, for the most frequently-grown cultivars, the mean LS for the common wheat ‘AAC Brandon’ was 1.2%, while for durum wheat it was 1.1% for ‘Transcend’ and 0.4% for ‘AAC Stronghold’.
In all affected samples, leaf spots were caused primarily by Pyrenophora tritici-repentis (tan spot), followed, in common wheat, by the septoria leaf spot complex at around 10% of fungal isolations.
REFERENCES
- Agriculture and Agri-Food Canada. 2021. Maps of historic agroclimate conditions. [ accessed 2022 Apr 26] https://www.agr.gc.ca/DW-GS/historical-historiques.jspx?null&lang=eng&jsEnabled=true
- Fernandez MR, Abdellatif L, Waelchli N, Kenny C, Waelchli F, Akhavan A, Peru C, Hartley S. 2021. Leaf spotting diseases of common and durum wheat in Saskatchewan in 2020. Can. Plant Dis. Surv. 101:83–86. In, Can J Plant Pathol. 43:sup 1.
- Fernandez MR, Abdellatif L, Waelchli N, Kenny C, Waelchli F, Ziesman B, Peru C, Hartley S. 2020. Leaf spotting diseases of common and durum wheat in Saskatchewan in 2019. Can. Plant Dis. Surv. 100:82–85. In, Can J Plant Pathol. 42:sup 1.
- Fernandez MR, Kenny C, Abdellatif L, Lokuruge P, Waelchli F, Ziesman B, Peru C, Hartley S. 2019. Leaf spotting diseases of common and durum wheat in Saskatchewan in 2018. Can. Plant Dis. Surv. 99:126–130. In, Can J Plant Pathol. 41:sup 1.
SEED-BORNE FUSARIUM ON CEREAL CROPS IN SASKATCHEWAN IN 2020
CROP: Cereal crops (Wheat, Durum, Barley and Oats) LOCATION: Saskatchewan NAMES AND AGENCIES: B. OLSON1, A. AKHAVAN2, T. BLOIS3, B. ERNST4, M. JAPP5, S. JUNEK6, H.R. KUTCHER7 & T. PRASAD8
1Box 88, Hazlet, SK S0N 1E0 Telephone: (306) 774-5643; E-mail:[email protected] 2Saskatchewan Ministry of Agriculture, 3085 Albert St., Regina, SK S4S 0B1 320/20 Seed Labs Inc., 507-11th Ave., Nisku, AB T9E 7N5 4Prairie Diagnostic Seed Lab, 1105 Railway Ave., Weyburn, SK S4H 3H 5SaskBarley, Bay 6A-3602 Taylor St. E., Saskatoon, SK S7H 5H9 6Discovery Seed Labs Ltd., 450 Melville St., Saskatoon, SK S7J 4M2 7Crop Development Centre, Univ. of Saskatchewan, 51 Campus Dr., Saskatoon, SK S7N 5A8 8Lendon Seed Lab, 147 Hodsman Rd., Regina, SK S4N 5W5
ABSTRACT: Commercial test results from four seed labs for seed-borne Fusarium graminearum and total Fusarium spp. are summarized. Results of 1950 wheat, 922 durum, 814 barley, and 297 oat samples were reported in 2020. Compared to 2019, the combined frequency of F. graminearum-free samples increased to 66.5% and the mean percent infection rate was down slightly at 1.5%. Total Fusarium spp. frequency and severity decreased slightly compared to those reported in 2019.
INTRODUCTION AND METHODS: The results of 3983 tests from four commercial seed testing laboratories were acquired and combined. The tests were conducted from September of 2020 through May 2021 and are assumed to be from the 2020 crop. The tests were conducted by agar-plating or quantitative polymerase chain reaction (PCR) techniques. In the case of PCR tests, the presence or absence of DNA of all Fusarium spp. or of F. graminearum allowed calculation of percent infection. No attempt was made to select fusarium-damaged kernels (FDK) so the samples can be considered random. The percent frequency of all Fusarium spp. including F. graminearum (total Fusarium), and the percent frequency of F. graminearum alone, were calculated. The mean percent infection was calculated for both total Fusarium spp. and F. graminearum. Individual Fusarium spp. other than F. graminearum are not reported, as not all labs provided that information. The results of 1950 wheat, 922 durum, 814 barley, and 297 oat samples are reported by Saskatchewan crop district and provincial means determined.
RESULTS AND COMMENTS: The 2020 crop year was characterized by moisture level concerns (Saskatchewan Ministry of Agriculture Citation2020). Most of the province received minimal or below average rainfall. Hot temperatures and drying winds were common throughout the growing season. This resulted in an early harvest of above average quality. By October 19, 99% of the crop was in the bin compared to the 5-year average of 88% harvested by this date.
Cereal yields were above 10-year averages (Saskatchewan Ministry of Agriculture Citation2020). The average wheat yield was 46 bu/acre compared to the 10-year average of 40 bu/acre. Durum yield was 39 bu/acre compared to the 10-year average of 38 bu/acre. Average barley yield was 67 bu/acre, greater than the 10-year average of 60 bu/acre. Oat yield was 86 bu/acre, up from the 10-year average of 81 bu/acre.
A total of 1950 wheat, 922 durum, 814 barley and 297 oat samples were processed during the period covered by this report. This represented an increase in oat samples (24.3%) and a decrease in durum (10.5%), wheat (17.2%) and barley (9.2%) samples compared to 2019 (Olson et al. Citation2021).
Fusarium graminearum frequency and severity (mean % infection) was calculated for wheat, durum, barley, and oat individually and combined. Frequency and severity of total Fusarium spp. was calculated individually and combined as well (, , , and ). The frequency of F. graminearum in 2020 was 33.5%. This was significantly lower than the 40.6% reported in 2019 (Olsen et al. 2021) but above the 2018 level of 22.0% and the 2017 level of 23.1% (Olson et al. Citation2020a, Citationb). The severity of F. graminearum decreased to levels in line with 2018 and 2017 (). Total Fusarium frequency was 84.2%, which was similar to the previous year (). Total Fusarium severity was 4.2%, also similar to levels reported in 2019 ().
Table 1. Five-year summary of frequency (% PFS) and severity (mean % infection) of Fusarium graminearum and total Fusarium spp. in wheat, durum, barley and oat seeds combined.
Table 2. Number of wheat seed samples tested from September 2020 to May 2021 and levels of infection with Fusarium graminearum and Fusarium spp. in each Saskatchewan crop district.
Table 3. Number of durum seed samples tested from September 2020 to May 2021 and levels of infection with Fusarium graminearum and total Fusarium spp. in each Saskatchewan crop district.
Table 4. Number of barley seed samples tested from September 2020 to May 2021 and levels of infection with Fusarium graminearum and total Fusarium spp. in each Saskatchewan crop district.
Table 5. Number of oat seed samples tested from September 2020 to May 2021 and levels of infection with Fusarium graminearum and total Fusarium spp. in each Saskatchewan crop district.
The combined total of Fusarium-free samples dropped slightly from 17.6% in 2019 to 15.8% in 2020 (). Combined mean infection rates remained low at 4.2%. Combined F. graminearum-free samples increased from 59.4% in 2019 to 66.5% in 2020 and the combined mean % infection rate was down slightly from 2019 at 1.5% ().
Wheat – The percentage of F. graminearum-free samples in 2020 was 65.0% (), up from the 56.1% reported in 2019 (Olson et al. Citation2021). The mean infection rate was 1.6%, down from the 1.8% reported in 2019. Total Fusarium spp.-free samples were 16.4% compared to 15.0% in 2019. The mean percent infection decreased from 2019 to 4.5%.
Durum – Of the 922 samples, 64.4% were found to be F. graminearum-free. Mean percent infection was 1.8% (). In 2019, the frequency of F. graminearum-free samples was 52.0% and the mean percent infection was 2.2% (Olson et al. Citation2021). The total Fusarium spp.-free frequency was 18.8%, down from the 23.0% reported in 2019 and the mean percent infection was 3.4%, down from 3.8% in 2019.
Barley – The percentage of F. graminearum-free samples was 68.8% in 2020, down from 69.9% in 2019 (Olson et al. Citation2021). Mean infection was 1.0% compared to 1.3% in 2019. Total Fusarium spp.-free samples were 14.1%, down from 19.7% in 2019. The total Fusarium spp. mean infection was 4.1%, up from 3.8% in 2019 ().
Oat – Of the 297 samples, 91.2% were found to be F. graminearum-free. This was higher than the 84.5% reported in 2019 (Olson et al. Citation2021). Mean infection was 0.6%, down slightly from 0.8% in 2019. Total Fusarium spp.-free samples were 5.3% down from 13.8% in 2019. The total Fusarium spp. mean infection was 7.5%, up from 6.2% in 2019 ().
ACKNOWLEDGEMENTS: We would like to acknowledge the cooperation of 20/20 Seed Labs Inc., Lendon Seed Lab, Prairie Diagnostic Seed Lab, and Discovery Seed Labs Ltd. in providing the seed testing results that made this report possible. We also wish to acknowledge the funding support of the Saskatchewan Wheat Development Commission, the Saskatchewan Barley Development Commission and the Saskatchewan Oat Development Commission.
REFERENCES
- Olson BD, Blois T, Ernst B, Junek S, Kutcher HR, Ziesman B. 2018. Seed-borne fusarium on cereal crops in Saskatchewan in 2016. Can Plant Dis Surv. 98:88–92.
- Olson BD, Blois T, Ernst B, Japp M, Junek S, Kutcher HR, Prasad T, Ziesman B. 2020a. Seed-borne fusarium on cereal crops in Saskatchewan in 2017. Can Plant Dis Surv. 100:96–99. In, Can J Plant Pathol. 42:sup1.
- Olson BD, Blois T, Ernst B, Japp M, Junek S, Kutcher HR, Prasad T, Ziesman B. 2020b. Seed-borne fusarium on cereal crops in Saskatchewan in 2018. Can Plant Dis Surv. 100:100–103. In, Can J Plant Pathol. 42:sup1.
- Olson BD, Ernst B, Fatima S, Japp M, Junek S, Kutcher HR, Prasad T, Wenaus J. 2021. Seed-borne fusarium on cereal crops in Saskatchewan in 2019. Can Plant Dis Surv. 101:103–106. In, Can J Plant Pathol. 43:sup1.
- Saskatchewan Ministry of Agriculture. 2020. 2020 crop report summary. Regina (SK). [ accessed 2022 Apr 15]. https://pubsaskdev.blob.core.windows.net/pubsask-prod/121410/Crop%252BReport%252B%252B25%252B-%252BFinal%252B-%252BOctober13%252Bto%252B19%252B%252B2020%252B-%252Bcomplete.pdf
BARLEY AND OAT LEAF SPOT DISEASES IN MANITOBA, 2021
CROP: Barley and Oat LOCATION: Manitoba NAMES AND AGENCY: M. BEYENE, M. BANIK & X. WANG
Morden Research and Development Centre, Agriculture and Agri-Food Canada, 101 Route 100, Morden, MB R6M 1Y5 Telephone: (204) 822-7530; Facsimile: (204) 822-7507; E-mail: [email protected]
ABSTRACT: In 2021, 33 barley fields and 20 oat fields were assessed for leaf spot diseases in Manitoba. The severity of leaf spot diseases in barley and oat was low in 2021, partially due to dry weather conditions during the growing season, which were not very conducive to developing leaf spot pathogens. Cochliobolus sativus (spot blotch) and Pyrenophora teres (net blotch) were the principal pathogens found in barley, whereas Pyrenophora avenae was the predominant leaf spot pathogen isolated from commercial oat fields.
INTRODUCTION AND METHODS: In 2021, barley and oat leaf spot diseases in Manitoba were assessed by surveying 33 barley fields and 20 oat fields from July 18 to August 5, when most crops were at the early to soft-dough stages of growth (ZGS 79-82, Zadoks et al. Citation1974). Depending on crop availability, fields were sampled at regular intervals, approximately 20-25 km apart along survey routes. The areas sampled were bounded by Highways # 67, 16 to the north, 12 to the east, 3 to the south, 8 to the north and 83 to the west. To identify the causal agent(s) and disease(s), 10 surface-sterilized pieces of infected leaves were incubated on filter paper in moist chambers for 3-5 days to promote sporulation.
RESULTS AND COMMENTS:
Barley – Cochliobolus sativus (causal agent of spot blotch) and Pyrenophora teres (net blotch) were the principal pathogens isolated from infected leaf tissues and caused the most damage in the fields surveyed. C. sativus was isolated from 17 fields (51.5% of fields), and P. teres was found in 10 fields (33.3% of fields) (). The disease level in 2021 was similar to that found in previous years (Banik et al. Citation2016, Citation2017, Citation2019).
Table 1. Incidence and isolation frequency of leaf spot pathogens of barley in Manitoba in 2021.
Oat – Pyrenophora avenae, the causal agent of pyrenophora leaf blotch, was the most prevalent leaf pathogen in oat (). This pathogen was isolated from 60% of fields, similar to the levels reported in the last few years (Banik et al. Citation2016, Citation2017, Citation2019). C. sativus and S. avenae were isolated from 15.0% and 3.5% fields, respectively ().
Table 2. Incidence and isolation frequency of leaf spot pathogens of oat in Manitoba in 2021.
REFERENCES
- Banik M, Beyene M, Wang X. 2016. Barley and oat leaf spot diseases in 2015 in Manitoba. Can Plant Dis Surv. 96:95–96.
- Banik M, Beyene M, Wang X. 2017. Barley and oat leaf spot diseases in 2016 in Manitoba. Can Plant Dis Surv. 97:100–102.
- Banik M, Beyene M, Wang X. 2019. Barley and oat leaf spot diseases in 2018 in Manitoba. Can Plant Dis Surv. 99:89–90. In, Can J Plant Pathol. 41:sup1.
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research. 14:415–421.
FUSARIUM HEAD BLIGHT OF SPRING WHEAT AND WINTER WHEAT IN MANITOBA IN 2021
CROP: Spring Wheat and Winter Wheat LOCATION: Manitoba NAMES AND AGENCIES: M.A. HENRIQUEZ1, D. KAMINSKI2, A. KIRK2, J. DOHERTY1, D. MIRANDA1 & O. GRUENKE1
1Agriculture and Agri-Food Canada, Morden Research and Development Centre, 101 Route 100, Morden, MB R6M 1Y5 Telephone: (204) 822-7551; Facsimile: (204) 822-7507; E-mail: [email protected] 2Manitoba Agriculture, 65-3rd Avenue NE, Carman, MB R0G 0J0
ABSTRACT: In 2021, fusarium head blight (FHB) incidence and severity were assessed in 113 spring wheat and 19 winter wheat fields in Manitoba. In spring and winter wheat the disease occurred in 12% and 5.3% of the wheat fields surveyed, respectively, with a provincial mean FHB severity (FHB Index) of 0.005% for spring wheat. The most prevalent Fusarium species in spring wheat were F. graminearum and F. poae.
INTRODUCTION AND METHODS: Spring wheat and winter wheat in Manitoba were surveyed for fusarium head blight (FHB) at 113 and 19 field locations, respectively. The survey for FHB was conducted from late July to late August for spring wheat, and July for winter wheat when most of the crops were at growth stage ZGS 73 – 85. In contrast to other disease surveys conducted in Manitoba, the fields were not surveyed at random. Instead, information on their location was obtained from producers. The proportion of infected spikes per field (incidence) and the proportion of infected spikelets in each spike (severity) were recorded from five heads (main stems) at 10 sites along a W-pattern in the field, while avoiding sampling tillers. An FHB Index (overall severity) was determined for each field surveyed by [(average % incidence X average % severity)/100].
Fifty spikes were processed for pathogen isolation and identification in the laboratory. Kernels from each field surveyed were surface-sterilized in a laminar flow bench and then placed on potato dextrose agar media plates (PDA, 25% strength + streptomycin). Identification of Fusarium species involved microscopic examination and morphological characterization using the criteria of Leslie and Summerell (Citation2006).
RESULTS AND COMMENTS: According to the Manitoba Agricultural Services Corporation’s Variety Market Share Report (MASC 2021), there were approximately 2,470,659 million acres of spring wheat seeded in Manitoba in 2021. The top five cultivars, based on seed acreage, were ‘AAC Brandon’ (51.6%), ‘AAC Starbuck’ (11.0%), ‘AAC Viewfield’ (8.3%), ‘AAC Wheatland’ (5.0%), ‘AAC Redberry’ (5.0%). ‘AAC Brandon’ was the predominant spring wheat cultivar grown in the fields sampled in this survey. FHB disease levels were lower in 2021 (0.005%) than the levels observed in 2020 (0.2%). Provincially, FHB was detected in 13 fields for a prevalence of 12%. The average incidence of the disease across all fields was 0.43%. The average severity (measured as the percentage of spikelets with infection) was 0.06% across all fields. The provincial mean FHB severity (FHB Index) was 0.005%.
According to the Manitoba Agricultural Services Corporation’s Variety Market Share Report (MASC 2021), there were approximately 35,390 acres of commercial winter wheat seeded in Manitoba for 2021. The top cultivars, based on their seed acreage, were ‘Emerson’ (34.7%). ‘AAC Gateway’ (32.6%), and ‘AAC Elevate’ (15.5%). FHB disease levels were lower in 2021 than the levels observed in 2020. FHB was detected only one of the 19 fields surveyed for a prevalence of 5.3%. The average incidence of the disease across all fields was 2.0%. The average severity (measured as the percentage of spikelets with infection) was 0.15% across all fields.
The results from spring wheat kernels plated on 25% PDA + streptomycin media showed that Fusarium graminearum and F. poae were the most frequently isolated pathogen species, accounting for 33.3% of isolations for both species, followed by F. sporotrichioides at 27.8% ( and ). The frequency of Fusarium graminearum was lower in 2021 than in 2020 (66.1%), while F. poae was higher in 2021 than in 2020 (11.6%). Fusarium isolates from winter wheat were not identified to species.
Table 1. Fusarium head blight (FHB) incidence and severity in spring wheat fields in Manitoba in 2021.
Table 2. Fusarium species isolated from kernels in FHB-affected spring wheat fields in Manitoba in 2021.
ACKNOWLEDGEMENTS: We gratefully acknowledge the participation of Manitoba Agriculture Farm Production Extension Specialists, as well as Dr. Henriquez’s summer students.
REFERENCES
- Henriquez MA, Kaminski D, Doherty J, Miranda D, Gruenke O. 2021. Fusarium head blight of spring wheat and winter wheat in Manitoba in 2020. Can Plant Dis Surv. 101:77–78. In, Can J Plant Pathol. 43:sup1.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell.
- MASC: 2021 variety market share report. Manitoba Agricultural Services Corporation. [ accessed 2022 Apr 19] https://www.masc.mb.ca/masc.nsf/sar_varieties_2021.pdf
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research. 14:415–421.
LEAF SPOT DISEASES OF SPRING WHEAT AND WINTER WHEAT IN MANITOBA IN 2021
CROP:Spring Wheat and Winter Wheat LOCATION: Manitoba NAMES AND AGENCIES: M.A. HENRIQUEZ1, D. KAMINSKI2, A. KIRK2, J. DOHERTY1, D. MIRANDA1 & O. GRUENKE1
1Agriculture and Agri-Food Canada, Morden Research and Development Centre, 101 Route 100, Morden, MB R6M 1Y5 Telephone: (204) 822-7551; Facsimile: (204) 822-7507; E-mail: [email protected] 2Manitoba Agriculture, 65-3rd Avenue NE, Carman, MB R0G 0J0
ABSTRACT: In 2021, leaf spot (LS) diseases were assessed in 107 spring wheat and 17 winter wheat fields in Manitoba. Leaf spot diseases were observed in all spring wheat fields surveyed at a provincial mean severity of 6.0%. The most prevalent LS species was Parastagonospora nodorum followed by Pyrenophora tritici-repentis. Leaf spot diseases were observed in 17 winter wheat fields surveyed at a provincial mean severity of 2.0%; the most prevalent species was Parastagonospora nodorum.
INTRODUCTION AND METHODS: A survey for leaf spot (LS) diseases was conducted between the milk and dough growth stages in 2021 (ZGS 73 – 85; Zadoks et al. Citation1974). A total of 107 spring wheat and 17 winter wheat fields were sampled. In contrast to other disease surveys conducted in Manitoba, the fields were not surveyed at random. Instead, information on their location was obtained from producers. In each field, 50 flag leaves were collected at random and percentage of leaf area affected by LS (severity) was recorded using a scale from 1 (slightly affected) to 50 (leaves dead) (Fernandez Citation1998).
From each field, 1-cm2 surface-disinfested leaf pieces were plated on water agar to promote pathogen sporulation for disease identification. Identification of LS pathogens involved microscopic examination and morphological characterization.
RESULTS AND COMMENTS: According to the Manitoba Agricultural Services Corporation’s Variety Market Share Report (MASC 2021), there were approximately 2,470,659 million acres of spring wheat seeded in Manitoba in 2021. The top five cultivars, based on seed acreage, were ‘AAC Brandon’ (51.6%), ‘AAC Starbuck’ (11.0%), ‘AAC Viewfield’ (8.3%), ‘AAC Wheatland’ (5.0%), ‘AAC Redberry’ (5.0%). ‘AAC Brandon’ was the predominant spring wheat cultivar grown in the fields sampled in this survey.
Leaf spot diseases were observed in all spring wheat fields surveyed. The provincial mean LS severity was 6.0% (). This severity was lower than 2020 (6.7%) (Henriquez et al. Citation2021). Unlike previous years (Henriquez et al. Citation2019a, Citation2020a, Citation2021), Parastagonospora nodorum was the most prevalent and widespread LS pathogen in Manitoba in 2021, accounting for 48.8% of isolations and detected in 48.1% of surveyed fields. This was followed by Pyrenophora tritici-repentis (tan spot) found in 43.1% of isolations and detected in 39.8% of surveyed fields ().
Table 1. Leaf spot (LS) severity in spring wheat fields in Manitoba in 2021.
Table 2. Prevalence and isolation frequency of leaf spot pathogens in spring wheat fields in Manitoba in 2021.
According to the Manitoba Agricultural Services Corporation’s Variety Market Share Report (MASC 2021), there were approximately 35,390 acres of commercial winter wheat seeded in Manitoba for 2021. The top cultivars, based on their seed acreage, were ‘Emerson’ (34.7%), ‘AAC Gateway’ (32.6%), and ‘AAC Elevate’ (15.5%). Leaf spot diseases were observed in all 17 fields surveyed. The provincial mean LS severity was 2.0%. In previous reports (Henriquez et al. Citation2019b, Citation2020b, Citation2021), Pyrenophora tritici-repentis (tan spot) was the most prevalent and widespread LS pathogen of winter wheat in Manitoba. However, in 2021, Parastagonospora nodorum was the most prevalent LS pathogen isolated in Manitoba with a frequency of 100% and prevalence of 26.3%.
ACKNOWLEDGEMENTS: We gratefully acknowledge the participation of Manitoba Agriculture Farm Production Extension Specialists, as well as Dr. Henriquez’s summer students.
REFERENCES
- Fernandez MR. 1998. Percentage leaf spot infection. Laboratory protocols. Semi-Arid Prairie Agricultural Research Centre. Swift Current (SK): Agriculture and Agri-Food Canada.
- Henriquez MA, Kaminski D, Doherty J, Miranda D, Gruenke O. 2021. Leaf spot diseases of spring wheat and winter wheat in Manitoba in 2020. Can Plant Dis Surv.101:79–80. In, Can J Plant Pathol. 43:sup1.
- Henriquez MA, Kaminski D, Doherty J, Miranda D, Gruenke O. 2020a. Leaf spot diseases of spring wheat in Manitoba in 2019. Can Plant Dis Surv. 100:75–76. In, Can J Plant Pathol. 42:sup1.
- Henriquez MA, Kaminski D, Doherty J, Miranda D, Gruenke O. 2020b. Leaf spot diseases of winter wheat in Manitoba in 2019. Can Plant Dis Surv. 100:77–78. In, Can J Plant Pathol. 42:sup1.
- Henriquez MA, Derksen H, Doherty J, Miranda DE, Gruenke O. 2019a. Leaf spot diseases of spring wheat in Manitoba in 2018. Can Plant Dis Surv. 99:99. In, Can J Plant Pathol. 41:sup1.
- Henriquez MA, Derksen H, Doherty J, Miranda DE, Gruenke O. 2019b. Leaf spot diseases of winter wheat in Manitoba in 2018. Can Plant Dis Surv. 99:98. In, Can J Plant Pathol. 41:sup1.
- MASC: 2021 variety market share report. Manitoba Agricultural Services Corporation. [ accessed 2022 Apr 19] https://www.masc.mb.ca/masc.nsf/sar_varieties_2021.pdf
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Res. 14:415–421.
LEAF AND STRIPE RUST OF WHEAT IN MANITOBA IN 2021
CROP: Spring and Winter Wheat LOCATION: Manitoba and Eastern Saskatchewan NAMES AND AGENCY: B. MCCALLUM, W. MCNABB & E. REIMER
Morden Research and Development Centre, Agriculture and Agri-Food Canada, 101 Route 100, Morden, MB R6M 1Y5 Telephone: (204) 294-1837; Facsimile: (204) 822-7507; E-mail: [email protected]
ABSTRACT: The annual survey for leaf and stripe rust of wheat was conducted during early to mid-July for winter wheat and from late July to early September for spring wheat. The number of infected wheat leaf samples collected for analysis was greatly reduced when compared to recent years. A total of nine leaf samples were collected from the winter wheat and there were 46 from spring wheat.
INTRODUCTION AND METHODS: The goals of this survey were to determine the prevalence and severity of leaf and stripe rust, as well as to collect infected leaf samples from which the causal organisms were isolated to determine the virulence structure of the leaf rust fungus (Puccinia triticina Erikss.), and to collect samples of (Puccinia striiformis Westend.). Most samples were collected from sites such as trap nurseries which did not receive fungicide applications and offered a greater probability of finding plants with leaf or stripe rust. The survey of commercial fields was less extensive as most wheat fields were sprayed with fungicide to control disease. Trials of winter wheat were surveyed for rust at trap nurseries in Manitoba during early to mid-July. Trap nurseries, research trial sites and commercial fields of spring wheat in Manitoba and eastern Saskatchewan were examined for the incidence and severity of rust from late July to early September, 2021.
RESULTS AND COMMENTS: The wheat crop in Manitoba was challenged by drought conditions which persisted throughout most of the growing season. By the end of the cereal growing season, the Canadian Drought Monitor map (Agriculture and Agri-Food Canada Citation2021) had recorded exceptional drought in much of the central and Interlake regions of Manitoba and extreme drought in the western and eastern regions. Low levels of precipitation and elevated temperatures were not conducive to the development of rust diseases.
Winter wheat was surveyed at six Manitoba Crop Variety Evaluation Trial (MCVET) locations. Only trace levels of leaf rust were present at one of the sites and both leaf and stripe rust were undetectable at the other locations.
Spring wheat was surveyed at 16 research locations which included seven MCVET sites, eight Uniform Rust Nursery (URN) locations and one Demonstration trial site. Leaf rust symptoms were present at trace levels on very few entries at six of the MCVET locations. Leaf rust was observed at all of the URN trials in Manitoba and Saskatchewan which include only highly susceptible cultivars. The highest levels of infection were present at St. Adolphe and Portage la Prairie, Manitoba where the susceptible variety Morocco had 30-40% disease severity. Stripe rust was found only at trace levels at one location, Portage La Prairie.
Five commercial fields of winter wheat and 20 fields of spring wheat were inspected in the central and western regions of Manitoba. All fields were found to be free of leaf and stripe rust symptoms.
REFERENCE
- Agriculture and Agri-Food Canada. 2021. Canadian Drought Monitor: conditions as of March 31, 2021. [ accessed 2022 Apr 26]. https://www.agr.gc.ca/atlas/maps_cartes/canadianDroughtMonitor/monthlyAssessments/en/2021/cdm_2103_mn_en.pdf
CROWN RUST OF OAT IN MANITOBA AND ONTARIO IN 2020
CROP: Oat LOCATION: Manitoba, Ontario NAMES AND AGENCIES: J.G. MENZIES1, A.G. XUE2, S. DECEUNINCK1, Z. POPOVIC1 & H. DERKSEN1
1Agriculture and Agri-Food Canada, Morden Research and Development Centre, 101 Route 100, Morden, MB R6M 1Y5. Telephone: (204) 822-7522; Facsimile: (204) 822-7507; E-mail: [email protected] 2Agriculture and Agri-Food Canada, Ottawa Research and Development Centre, K.W. Neatby Building, 960 Carling Avenue, Ottawa, ON K1A 0C6.
ABSTRACT: In 2020, 36 fields with wild oats and 51 fields of common oats were surveyed for the incidence and severity of crown rust (Puccinia coronata var. avenae f. sp. avenae) in Manitoba. Crown rust-infected plants were found in 92% and 86% of wild and common oat fields at mean incidences of 37% and 15%, respectively, and mean severities of 2MR-MS and 4MR-MS. Virulence was observed to all resistance genes in 151 single pustule isolates from Manitoba. There was no virulence expressed to 10 of the 24 differential oat lines for the 4 spi tested from Ontario.
INTRODUCTION AND METHODS: Surveys for the incidence and severity of oat crown rust, caused by Puccinia coronata var. avenae f. sp. avenae (Urban and Marková), were conducted in Manitoba from August 2nd to August 18th, 2020. The areas surveyed were in Manitoba crop districts 1, 2, 3, 7, 8, 9, and 11. Incidence was considered to be the percentage of leaves infected with rust in a given field, and the severity was the mean percentage leaf area with pustules. Crown rust collections were obtained from wild oat (Avena fatua L.) and common oat (A. sativa L.) in the fields, and susceptible and resistant oat lines and cultivars grown in uniform rust nurseries. The nurseries were located at Indian Head and Saskatoon, SK, Emerson, MB, and Casselman and Ottawa, ON. Samples from fields in Ontario were collected in July and crown rust samples from the eastern prairie region were also generously provided by Dr. T. Zegeye-Gebrehiwot (Morden Research and Development Centre). For virulence studies, single-pustule isolates (spi) were established from the rust collections. Races were identified using 16 standard oat crown rust differentials () as described by Chong et al. (Citation2000, Citation2008). In addition, single Pc-gene lines with Pc91, Pc94, Pc96, temp_pc97, temp_Pc98, Pc101, Pc103-1, and Pc104 were used as supplemental differentials.
Table 1. Frequencies (%) of virulence of Puccinia coronata f. sp. avenae isolates from the eastern prairie region and eastern Canada on 16 standard and eight supplemental crown rust differential oat lines in 2020.
RESULTS AND COMMENTS: Thirty-six fields with wild oats and 51 fields of common oat lines were surveyed in Manitoba in 2020. Oat plants infected with P. coronata f. sp. avenae were found in 33 (92%) of the wild oat fields and 44 (86%) of the common oat fields.
Crown rust incidence on wild oats ranged from 0 to 100%, with a mean incidence of 37%. The severity of crown rust on wild oats ranged from 0 to 10S with a mean severity of 2MR-MS. Crown rust incidence on commercial oats ranged from 0 to 100%, with a mean incidence of 15%. The severity of crown rust on common oats ranged from 0 to 100S with a mean severity of 4MR-MS. The incidence and severity of crown rust was greatest in Manitoba crop districts 1 and 8.
Forty-seven spi were obtained from wild oat collections from Manitoba, and 34 races were identified. Twenty-seven (79%) races were each represented by only one spi. The most common races were JTQG and GTQG-91,104, represented by 3 spi, and GTQG-91, represented by two spi. Virulence was observed to all the Pc genes in the spi from wild oat, except Pc96 (). Virulence was less than 5% for Pc96, Pc97, and Pc98, and was observed in 85% or more of the spi from wild oat for Pc38, Pc39, Pc45, Pc48, Pc51, Pc52, Pc56, Pc68 and Pc91.
One hundred four spi were made from common oat collections from Manitoba, with 62 races identified. Forty-six (74%) races were represented by only one spi, with 10 and four races represented by two and three spi, respectively. Two races (JTQG-91 and JTQG-91,104) were represented by 13 spi each. Virulence was observed to all Pc genes (). Virulence to Pc62, Pc97 and Pc98 was observed in 5% or less of the spi. Virulence to Pc genes was observed in 85% or more of the spi for Pc38, Pc39, Pc45, Pc48, Pc51, Pc52, Pc56, Pc68 and Pc91.
Three spi were obtained from collections from the Uniform Rust Nursery and three races identified. Virulence to the different Pc genes ranged from 0 to 100%, but too few spi were assessed for further comment ().
Overall, in Manitoba for the wild oat and common oat spi, virulence was observed to all Pc genes, but less than 5% of the spi possessed virulence to Pc62, Pc96, Pc97 and Pc98.
Four spi were made from the eastern Canada collections, and four races identified. As with the Uniform Rust Nursery spi, virulence to the different Pc genes ranged from 0 to 100%, but too few spi were assessed for further comment ().
Greater than 85% of all Canadian spi from the 2020 collections possessed virulence to resistance genes Pc38, Pc39, Pc45, Pc48, Pc51, Pc52, Pc68 and Pc91, while virulence was observed at 5% or less to Pc62, Pc96, Pc97, and Pc98 ().
REFERENCES
- Chong J, Gruenke J, Dueck R, Mayert W, Woods S. 2008. Virulence of oat crown rust (Puccinia coronata f. sp. avenae) in Canada during 2002-2006. Can. J. Plant Pathol. 30:115–123.
- Chong J, Leonard KJ, Salmeron JJ. 2000. A North American system of nomenclature for Puccinia coronata f. sp. avenae. Plant Dis. 84:580–585.
DISEASES OF BARLEY IN ONTARIO IN 2021
CROP / CULTURE: Barley LOCATION: Ontario NAMES AND AGENCIES: A.G. XUE & Y. CHEN
Ottawa Research and Development Centre, Agriculture and Agri-Food Canada, 960 Carling Avenue, Ottawa, ON K1A 0C6 Telephone: (613) 759-1513; Facsimile: (613) 759-1926; E-mail: [email protected]
ABSTRACT: Seventeen barley fields located in the major production areas in Ontario were surveyed for diseases in 2021. Of the 14 diseases observed, spot blotch, barley yellow dwarf and net blotch were the most prevalent, having moderate to severe levels of infection in eight, four, and two fields, respectively. Fusarium head blight (FHB) was observed in 12 fields at low severities. Fusarium sporotrichioides and F. poae were the predominant species isolated from the FHB-infected kernels.
INTRODUCTION AND METHODS: A survey for barley diseases was conducted in Ontario during the fourth week of July 2021 when plants were at the soft dough stage of development. Seventeen fields consisting of two 2-row and 15 6-row barley were chosen at random in central and eastern regions where most of the barley crops were grown in Ontario. Foliar disease severity was determined on 10 flag and penultimate leaves sampled at each of three random sites per field, using a rating scale of 0 (no disease) to 9 (severely diseased). The diagnosis was based on visual symptoms. Average severity scores of <1, <3, <6 and ≥6 were considered as trace, slight, moderate, and severe disease levels, respectively. The severity of covered smut, ergot, leaf stripe, loose smut, and take-all was rated as the percentage of plants infected at each of the three random sites per field. FHB was rated for incidence (% infected spikes) and severity (% infected spikelets in the affected spikes) based on approximately 200 spikes at each of the three sites per field. FHB index [(% incidence x % severity)/100] was determined for each field. The percentage of infected plants or FHB index values of <5, <10, <20 and ≥20% were considered as slight, moderate, severe, and very severe disease levels, respectively.
Determination of the causal species of FHB was based on 50 infected spikes collected from each affected field. The spikes were air-dried at room temperature and subsequently threshed. Fifty discoloured kernels per sample were chosen at random, surface sterilized in 1% NaOCI for 60s and plated in 9-cm diameter Petri dishes on modified potato dextrose agar (10 g dextrose per liter amended with 50 ppm of streptomycin sulphate). The plates were incubated for 10-14 days at 22-25°C and a 14-hour photoperiod using fluorescent and long wavelength ultraviolet tubes. Fusarium species isolated from kernels were identified by microscopic examination using standard taxonomic keys.
RESULTS AND COMMENTS: A total of 14 diseases or disease complexes were observed (). Spot blotch (Cochliobolus sativus), barley yellow dwarf (BYDV), and net blotch (Pyrenophora teres) were the most common foliar diseases and were found in all of the surveyed fields at average severities of 3.1, 2.5 and 2.0 respectively. Moderate to severe levels of the three diseases were observed in eight, four, and two fields, respectively. Yield reductions due to these diseases were estimated to have averaged <10% in affected fields. Other foliar diseases observed included leaf rust (Puccinia hordei), powdery mildew (Blumeria graminis f.sp. hordei), scald (Rhynchosporium secalis), septoria complex [including speckled leaf blotch (Septoria passerinii) and leaf blotch (Stagonospora nodorum)], and stem rust (Puccinia graminis f. sp. tritici or f. sp. secalis); they were observed in 13, two, six, 15 and seven fields at mean severities of 3.1, 3.5, 1.2, 2.3 and 1.3, respectively. Severe levels of these diseases were not observed, but moderate levels of septoria complex, leaf rust and powdery mildew were found in two, one, and one field, respectively. None of these diseases would have resulted in substantive damage to the crop.
Table 1. Prevalence and severity of barley diseases in Central and Eastern Ontario in 2021.
Covered smut (Ustilago hordei), ergot (Claviceps purpurea), leaf stripe (Pyrenophora graminea), loose smut (Ustilago nuda) and take-all (Gaeumannomyces graminis) were observed in four, four, six, 11, and 16 fields at mean severities of 1.0, 1.0, 1.0, 2.2 and 2.3%, respectively. These diseases occurred at the trace to slight levels, except for two fields with moderate to severe levels of loose smut and two fields with moderate levels of take-all. Yield reductions due to loose smut and take-all were estimated at <5% in affected fields.
FHB was observed in 12 fields at a mean FHB index of 0.1% (range 0.01% to 0.3%) (). Moderate to severe FHB infection was not observed. Yield and quality reductions due to FHB were estimated at <1%. Six Fusarium species were isolated from putatively infected kernels (). Fusarium sporotrichioides and F. poae predominated and occurred in 100 and 94% of the affected fields, isolated from 13.9 and 9.3% of infected kernels, and represented 37.7 and 25.2% of the pathogen population, respectively. Fusarium equiseti and F. graminearum were less common, occurring in 65-71% of fields and 4.8-6.6% of kernels, and representing 13.1-17.9% of the pathogen population. Fusarium acuminatum and F. avenaceum were the least common, occurring in 12-18% of fields and 0.6-1.6% of kernels, and collectively representing <7% of the pathogen population.
Table 2. Prevalence of Fusarium species isolated from putatively infected barley kernels from 17 fields in Central and Eastern Ontario in 2021.
The 14 diseases observed on barley in Ontario in 2021 were the same as those recorded in 2019 and 2020 (Xue and Chen Citation2020, Citation2021). Overall, the incidence and severity of these diseases were generally greater in 2021 than in 2019 and 2020. More frequent rain events in June and July in 2021 compared with 2019 and 2020 in Central and Eastern Ontario were likely responsible for the increased disease severities observed.
ACKNOWLEDGEMENTS: This work was made possible by funding from the Agriculture and Agri-Food Canada A-base project ‘Toward a Prairie Biovigilance Network’, AAFC-STB-MRS Project No. J-002517.
REFERENCES
- Xue AG, Chen Y. 2020. Diseases of barley in Ontario in 2019. Can Plant Dis Surv. 100:58–59. In, Can J Plant Pathol. 42:sup1.
- Xue AG, Chen Y. 2021. Diseases of barley in Ottawa, Ontario in 2020. Can Plant Dis Surv. 101:65–67. In, Can J Plant Pathol. 43:sup1.
DISEASES OF OAT IN ONTARIO IN 2021
CROP: Oat LOCATION: Ontario NAMES AND AGENCIES: A.G. XUE &Y. CHEN
Ottawa Research and Development Centre, Agriculture and Agri-Food Canada, 960 Carling Avenue, Ottawa, ON K1A 0C6 Telephone: (613) 759-1513; Facsimile: (613) 759-1926; E-mail: [email protected]
ABSTRACT: Twenty oat fields located in the major production areas in Ontario were surveyed for diseases in 2021. Of the 10 diseases observed, crown rust, and barley yellow dwarf were most prevalent, having moderate to severe levels of infection in 15 and seven fields, respectively. Fusarium head blight (FHB) was observed in all surveyed fields at low severities. Fusarium poae and F. sporotrichioides were the predominant species isolated from the FHB infected kernels.
INTRODUCTION AND METHODS: A survey to document diseases in Ontario oat crops was conducted during the fourth week of July 2021 when plants were at the soft dough stage of development. Twenty fields were chosen at random in central and eastern regions where most oat crops were grown in Ontario. Foliar disease severity was determined on 10 flag and penultimate leaves sampled at each of three random sites per field, using a rating scale of 0 (no disease) to 9 (severely diseased). Disease diagnosis was based on visual symptoms. Average severity scores of <1, <3, <6 and ≥6 were considered as trace, slight, moderate and severe disease levels, respectively. The severity of loose smut and take-all was based on the percentage of plants infected at each of the three random sites per field. FHB was rated for incidence [% infected panicles (heads)] and severity (% infected spikelets in the affected panicles) based on approximately 200 panicles at each of the three sites per field. FHB index [(% incidence x % severity)/100] was determined for each field. The percentage of infected plants or FHB index values of <5, <10, <20 and ≥20% were considered as slight, moderate, severe and very severe disease levels, respectively.
Determination of the causal species of FHB was based on 50 infected panicles collected from each affected field. The panicles were air-dried at room temperature and subsequently threshed. Fifty discoloured kernels per sample were chosen at random, surface sterilized in 1% NaOCI for 60s and plated in 9-cm diameter petri dishes on modified potato dextrose agar (10 g dextrose per liter amended with 50 ppm of streptomycin sulphate). The plates were incubated for 10-14 days at 22-25°C and a 14-hour photoperiod using fluorescent and long wavelength ultraviolet tubes. Fusarium species isolated from kernels were identified by microscopic examination using standard taxonomic keys.
RESULTS AND COMMENTS: Ten diseases were identified (). Crown rust (Puccinia coronata f. sp. avenae) and barley yellow dwarf (BYDV) were the most prevalent foliar diseases and were found in all of the surveyed fields at average severities of 5.2 and 3.1, respectively. Moderate to severe levels of infection from the two diseases were observed in 15 and seven fields, respectively. Yield reductions due to these diseases were estimated to be <25% in affected fields. Other foliar diseases observed were halo blight (Pseudomonas syringae pv. coronafaciens), pyrenophora leaf blotch (Pyrenophora avenae), spot blotch (Cochliobolus sativus), stagonospora leaf blotch (Stagonospora avenae f. sp. avenaria), and stem rust (Puccinia graminis f. sp. tritici); they were observed in 12, 19, 16, 14 and four fields at mean severities of 2.1, 1.7, 1.3, 1.4 and 1.8, respectively. These diseases occurred at the trace to slight levels, except for one field with severe levels of halo blight and one field with severe levels of pyrenophora leaf blotch. Collectively, these diseases would have resulted in <5% yield reduction.
Table 1. Prevalence and severity of oat diseases in Central and Eastern Ontario in 2021.
Loose smut (Ustilago nuda) and take-all root rot (Gaeumannomyces graminis var. avenae) were observed in three and 16 fields at incidence levels of 2.3 and 2.0%, respectively (). Each of these two diseases was found at moderate to severe levels in one field. Yield reductions due to these diseases were estimated at <5% in affected fields.
Fusarium head blight occurred in 18 fields at a mean FHB index of 0.2% (range 0.01-1.2%) (). Moderate to severe FHB infection was not observed. Yield and quality reductions due to FHB were estimated at <1%. Five Fusarium species were isolated from putatively infected kernels (). Fusarium poae and F. sporotrichioides predominated and occurred in 90 and 55% of the affected fields, isolated from 13.7 and 10.8% of infected kernels, and represented 47.1 and 37.1% of the pathogen population, respectively. Fusarium equiseti and F. graminearum were less common, occurring in 20-55% of fields and 1.8-2.6% of kernels, and representing 6.2-8.9% of the pathogen population. Fusarium acuminatum was the least common, occurring in 5% of fields and 0.2% of kernels, and representing 0.7% of the pathogen population.
Table 2. Prevalence of Fusarium species isolated from putatively infected oat kernels of 20 fields in Central and Eastern Ontario in 2021.
The 10 diseases observed on oat in Ontario in 2021 were the same as those recorded in 2019 and 2020 (Xue and Chen Citation2020, Citation2021). Overall, the incidence and severity of these diseases were generally greater in 2021 than in 2019 and 2020. More frequent rain events in June and July in 2021 compared with 2019 and 2020 in Central and Eastern Ontario were likely responsible for the increased disease severities observed.
ACKNOWLEDGEMENTS: This work was made possible by funding from the Agriculture and Agri-Food Canada A-base project ‘Toward a Prairie Biovigilance Network’, AAFC-STB-MRS Project No. J-002517.
REFERENCES
- Xue AG, Chen Y. 2020. Diseases of oat in Ontario in 2019. Can Plant Dis Surv. 100:65–66. In, Can J Plant Pathol. 42:sup1.
- Xue AG, Chen Y. 2021. Diseases of oat in Ottawa, Ontario in 2020. Can Plant Dis Surv. 101:70–72. In, Can J Plant Pathol. 43:sup1.
DISEASES OF SPRING WHEAT IN ONTARIO IN 2021
CROP: Spring wheat LOCATION: Ontario NAMES AND AGENCIES: A.G. XUE & Y. CHEN
Ottawa Research and Development Centre, Agriculture and Agri-Food Canada, 960 Carling Avenue, Ottawa ON, K1A 0C6 Telephone: (613) 759-1513; Facsimile: (613) 759-1926; E-mail: [email protected]
ABSTRACT: Nineteen spring wheat fields located in the major production areas in Ontario were surveyed for diseases in 2021. Of the 11 diseases observed, bacterial leaf blight, tan spot and take-all were the most prevalent, having moderate to severe levels of infection in five, three and seven fields, respectively. Fusarium head blight (FHB) was observed in nine fields at low severities. Fusarium graminerum and F. sporotrichioides were the predominant species isolated from the FHB-infected kernels.
INTRODUCTION AND METHODS: A survey for spring wheat diseases was conducted in Ontario during the fourth week of July 2021 when plants were at the soft dough stage of development. Nineteen fields were chosen at random in central and eastern regions where most of the spring wheat crops were grown in Ontario. Foliar disease severity was determined on 10 flag and penultimate leaves sampled at each of the three random sites per field, using a rating scale of 0 (no disease) to 9 (severely diseased). Disease diagnosis was based on visual symptoms. Average severity scores of <1, <3, <6 and ≥6 were considered as trace, slight, moderate and severe disease levels, respectively. The severity of ergot, loose smut and take-all was based on the percentage of plants infected at each of the three random sites per field. FHB was rated for incidence (% infected spikes) and severity (% infected spikelets in the affected spikes) based on approximately 200 spikes at each of the three sites per field. FHB index [(% incidence x % severity)/100] was determined for each field. The percentage of infected plants or FHB index values of <5, <10, <20 and ≥20% were considered as slight, moderate, severe and very severe disease levels, respectively.
Determination of the causal species of FHB was based on 50 infected spikes collected from each affected field. The spikes were air-dried at room temperature and subsequently threshed. Fifty discoloured kernels per sample were chosen at random, surface sterilized in 1% NaOCI for 60s and plated in 9-cm diameter Petri dishes on modified potato dextrose agar (10 g dextrose per liter amended with 50 ppm of streptomycin sulphate). The plates were incubated for 10-14 days at 22-25°C and a 14-hour photoperiod provided by fluorescent and long wavelength ultraviolet tubes. Fusarium species isolated from kernels were identified by microscopic examination using standard taxonomic keys.
RESULTS AND COMMENTS: Eleven diseases or disease complexes were observed (). Bacterial leaf blight (Pseudomonas syringae pv. syringae), tan spot (Pyrenophora tritici-repentis), and septoria/stagonospora leaf blotch complex (normally associated with the pathogens Septoria tritici and Stagonospora spp.) were the most important foliar diseases and were found in 18, 19 and 19 fields at average severities of 2.2, 2.0 and 1.7 respectively. Severe infections from these diseases were not observed, but moderate levels of infection were found in five, three and three fields, respectively. Yield reductions due to these diseases were estimated to have averaged <10% in affected fields. Other foliar diseases observed included leaf rust (Puccinia triticina), powdery mildew (Blumeria graminis f.sp. tritici), spot blotch (Cochliobolus sativus), and stem rust (Puccinia graminis). These diseases were found in six, nine, 18 and two fields at average severities of 1.8, 2.3, 1.2 and 1.5, respectively. No moderate or severe levels of infection were observed and these diseases likely caused little to no yield reduction.
Table 1. Prevalence and severity of spring wheat diseases in Central and Eastern Ontario in 2021.
Ergot (Claviceps purpurea), loose smut (Ustilago tritici) and take-all root rot (Gaeumannomyces graminis var. tritici) were observed in eight, one and 19 fields at incidence levels of 0.2, 5.0 and 3.2%, respectively (). Ergot occurred at trace to slight levels only while moderate and severe levels of loose smut and take-all were found in one and seven fields, respectively. Yield reductions due to loose smut and take-all were estimated at <5% in affected fields.
Fusarium head blight occurred in nine fields at a mean FHB index of 0.3% (range 0.01-2.0%) (). Moderate to severe FHB infection was not observed. Yield and quality reductions due to FHB were estimated at <1%. Six Fusarium species were isolated from putatively infected kernels (). Fusarium graminearum and F. sporotrichioides predominated and occurred in 37 and 63% of the affected fields, isolated from 5.9 and 3.6% of infected kernels, and represented 35.4 and 21.5% of the pathogen population, respectively. Fusarium avenaceum, F. equiseti and F. poae were less common, occurring in 32-53% of fields and 1.9-2.6% of kernels, and representing 11.4-15.8% of the pathogen population. Fusarium acuminatum was the least common, occurring in 11% of fields and 0.4% of kernels, and representing 2.5% of the pathogen population.
Table 2. Prevalence of Fusarium species isolated from putatively infected spring wheat kernels of 19 fields in Central and Eastern Ontario in 2021.
The 11 diseases observed on spring wheat in Ontario in 2021 were the same as those recorded in 2019 and 2020 (Xue and Chen Citation2020, Citation2021). Overall, the incidence and severity of these diseases were generally greater in 2021 than in 2019 and 2020. More frequent rain events in June and July in 2021 compared with 2019 and 2020 in Central and Eastern Ontario were likely responsible for the increased disease severities observed.
ACKNOWLEDGEMENTS: This work was made possible by funding from the Agriculture and Agri-Food Canada A-base project ‘Toward a Prairie Biovigilance Network’, AAFC-STB-MRS Project No. J-002517.
REFERENCES
- Xue AG, Chen Y. 2020. Diseases of spring wheat in Ontario in 2019. Can. Plant Dis. Surv. 100:92–93. In, Can J Plant Pathol. 42:sup1.
- Xue AG, Chen Y. 2021. Diseases of spring wheat in Ottawa, Ontario in 2020. Can. Plant Dis. Surv. 101:87–89. In, Can J Plant Pathol. 43:sup1.
DISEASES OF WINTER WHEAT IN ONTARIO IN 2021
CROP: Winter wheat LOCATION: Ontario NAMES AND AGENCIES: A.G. XUE & Y. CHEN
Ottawa Research and Development Centre, Agriculture and Agri-Food Canada, 960 Carling Avenue, Ottawa, ON K1A 0C6 Telephone: (613) 759-1513; Facsimile: (613) 759-1926; E-mail: [email protected]
ABSTRACT: Seventeen barley fields located in the major production areas in Ontario were surveyed for diseases in 2021. Of the nine diseases observed, powdery mildew, bacterial leaf blight, and the septoria/stagonospora leaf blotch complex were the most prevalent, having moderate to severe levels of infection in seven, four, and three fields, respectively. Fusarium head blight (FHB) was observed in 15 fields at low severities. Fusarium sporotrichioides and F. equiseti were the predominant species isolated from the FHB infected kernels.
INTRODUCTION AND METHODS: A survey for winter wheat diseases was conducted in Ontario during the first week of July 2021 when plants were at the soft dough stage of development. Twenty-four fields were chosen at random in central and eastern regions where most of the winter wheat crops were grown in Ontario. Foliar disease severity was determined on 10 flag and penultimate leaves sampled at each of three random sites per field, using a rating scale of 0 (no disease) to 9 (severely diseased). Disease diagnosis was based on visual symptoms. Average severity scores of <1, <3, <6 and ≥6 were considered as trace, slight, moderate and severe disease levels, respectively. The severity of ergot and take-all was based on the percentage of plants infected at each of the three random sites per field. FHB was rated for incidence (% infected spikes) and severity (% infected spikelets in the affected spikes) based on approximately 200 spikes at each of the three sites per field. An FHB Index [(% incidence x % severity)/100] was determined for each field. The percentage of infected plants or FHB Index values of <5, <10, <20, and ≥20% were considered as slight, moderate, severe, and very severe disease levels, respectively.
Determination of the causal species of FHB was based on 50 infected spikes collected from each affected field. The spikes were air-dried at room temperature and subsequently threshed. Fifty discoloured kernels per sample were chosen at random, surface sterilized in 1% NaOCI for 60s plated in 9-cm diameter Petri dishes on modified potato dextrose agar (10 g dextrose per liter amended with 50 ppm of streptomycin sulphate). The plates were incubated for 10-14 days at 22-25°C and a 14-hour photoperiod provided by fluorescent and long wavelength ultraviolet tubes. Fusarium species isolated from kernels were identified by microscopic examination using standard taxonomic keys.
RESULTS AND COMMENTS: Nine diseases or disease complexes were observed (). Powdery mildew (Blumeria graminis f. sp. tritici), bacterial leaf blight (Pseudomonas syringae pv. syringae), and septoria/stagonospora leaf blotch complex (normally associated with the pathogens Septoria tritici and Stagonospora spp.) were the most important foliar diseases and were found in 19, 23 and 24 fields at average severities of 3.0, 2.5 and 2.1, respectively. Moderate to severe levels of infection from the three diseases were observed in seven, four and three fields, respectively. Yield reductions due to these diseases were estimated to have averaged <20% in affected fields. Other foliar diseases observed included tan spot (Pyrenophora tritici-repentis), leaf rust (Puccinia triticina), and spot blotch (Cochliobolus sativus). These diseases were found in 21, five and six fields at average severities of 1.3, 1.6 and 1.2, respectively. No moderate or severe levels of infection were observed and these diseases likely caused little to no measurable yield reduction.
Table 1. Prevalence and severity of winter wheat diseases in Central and Eastern Ontario in 2021.
Ergot (Claviceps purpurea) and take-all root rot (Gaeumannomyces graminis var. tritici) were observed in five and 10 fields at incidence levels of 0.4 and 1.8%, respectively (). These diseases occurred at trace to slight levels, except for one field with a moderate level of take-all. Yield reductions due to ergot and take-all were estimated at <1% in affected fields.
Fusarium head blight occurred in 15 fields at a mean FHB Index of 0.2% (range 0.01-2.0%) (). Moderate to severe FHB infection was not observed. Yield and quality reductions due to FHB were estimated at <1%. Five Fusarium species were isolated from putatively infected kernels (). Fusarium sporotrichioides and F. equiseti predominated and occurred in 63 and 25% of the affected fields, isolated from 2.8 and 0.8% of infected kernels, and represented 64.7 and 19.6% of the pathogen population, respectively. Fusarium acuminatum, F. graminearum and F. poae were less common, occurring in 8-17% of fields and 0.2-0.3% of kernels, and representing 3.9-7.8% of the pathogen population.
Table 2. Prevalence of Fusarium species isolated from putatively infected winter wheat kernels of 24 fields in Central and Eastern Ontario in 2021.
The nine diseases observed on winter wheat in Ontario in 2021 were the same as those recorded in 2020 (Xue and Chen Citation2021). Overall, the incidence and severity of these diseases were generally greater in 2021 than in 2020. More frequent rain events in June and July in 2021 compared with 2020 in Central and Eastern Ontario were likely responsible for the increased disease severities observed.
ACKNOWLEDGEMENTS: This work was made possible by funding from the Agriculture and Agri-Food Canada A-base project ‘Toward a Prairie Biovigilance Network’, AAFC-STB-MRS Project No. J-002517.
REFERENCE
- Xue AG, Chen Y. 2021. Diseases of winter wheat in Ottawa, Ontario in 2020. Can Plant Dis Surv. 101: 90–91. In, Can J Plant Pathol. 43:sup1.
STATUS OF CORN DISEASES IN EASTERN ONTARIO, 2021 CROP SEASON
CROP / CULTURE: Corn LOCATION / REGION: Eastern Ontario NAMES AND AGENCIES: X. ZHU, A. Z. KEBEDE & T. WOLDEMARIAM
Agriculture and Agri-Food Canada, Ottawa Research and Development Centre, Ottawa, ON K1A 0C6 Telephone: (613) 759-1616; Facsimile: (613) 952-9295; E-mail: [email protected]
ABSTRACT: There were 61 corn fields surveyed in Eastern Ontario during the 2021 crop season. The most frequently detected leaf and stalk diseases were anthracnose leaf blight (48, 78.7%) and top-dieback (49, 80.3%), caused by the same pathogen, Colletotrichum graminicola (Ces.) G.W. Wilson. Leaf diseases with mean severities of 1.8±0.4, 1.2±0.4, 1.2±0.4, 1.3±0.4, 1.1±0.3, 1.7±0.5, and 1.1±0.3 were recorded for anthracnose leaf blight, eyespot, gray leaf spot, northern corn leaf blight, northern leaf spot, physoderma brown spot, and rust, respectively. All leaf diseases were much less frequent and severe than in previous years because of a drier growing season. One field was found with 60% top dieback and two fields with 27% and 70% pythium stalk rot. Three fields had common smut ranging from 2-10% incidence and two fields had head smut with lower incidence. Ear rot was found at much lower levels than in previous years. Grey leaf spot was found in five of seven counties surveyed. Since 2018, it has spread over a wider range and has the potential to become an important leaf disease in Eastern Ontario. Stewart’s bacterial wilt, Goss’s bacterial wilt, and tar spot were not detected.
INTRODUCTION AND METHODS: The corn disease survey provides vital information on population dynamics of endemic pathogens and allows for scouting of new invasive pathogens, such as tar spot of corn (Phyllachora maydis Maubl.) which has spread quickly in Southern Ontario and grey leaf spot (GLS) (Cercospora zeae-maydis Tehon & E.Y. Daniels) which was found in Eastern Ontario in 2018 (Jindal et al. Citation2019). To document the occurrence of various corn diseases of Eastern Ontario, a survey was conducted from September 8 to 10, 2021. The location of the 61 corn fields surveyed based on GPS coordinates is presented in . The diseases surveyed included anthracnose leaf blight (ALB) [Colletotrichum graminicola (Ces.) G.W. Wilson]; eyespot [Aureobasidium zeae (Narita & Hiratsuka) Dingley]; GLS; northern corn leaf blight (NCLB) [Exserohilum turcicum (Pass.) K.J. Leonard and E.G. Suggs]; northern corn leaf spot (NLS) [Bipolaris zeicola (G.L. Stout) Shoemaker]; physoderma brown spot (PBS) [Physoderma maydis Miyabe (Miyabe)]; southern corn leaf blight [Bipolaris maydis (Y. Nisik. & C. Miyake) Shoemaker]; tar spot; rust (Puccinia sorghi Schwein.); southern rust (P. polyspora Underw.); common smut [Ustilago maydis (DC.) Corda]; head smut [Sphacelotheca reiliana (Kuhn) G.P. Clinton]; ear rot (Fusarium spp.), stalk rot (Fusarium spp., Pythium spp.); top dieback (C. graminicola); Stewart’s bacterial wilt (Pantoea stewartii); and Goss’s bacterial wilt and blight (Clavibacter michiganensis subsp. nebraskensis).
Leaf disease severity was rated on a scale of 1-7 based on percent area diseased, where 1 = no disease and 7= severely diseased (Reid and Zhu Citation2005). For ear and stalk diseases, disease incidence was recorded based on the number of plants with a particular disease symptom. Leaf samples showing typical NCLB symptoms (long, elliptical, 2-15 cm, tan or greyish-green, necrotic lesions) were collected from each field visited for E. turcicum race identification and distribution patterns. Additional symptomatic plant parts were also collected for subsequent laboratory analysis, especially for unknown or suspected Goss’s bacterial wilt, Stewart’s bacterial wilt, and tar spot.
RESULTS AND COMMENTS: The year 2021 was an unusual corn growing season in Eastern Ontario. There was significantly less rainfall from May 1 to September 4, than average. For example, at the Central Experimental Farm in Ottawa, it rained only 9.1, 64.8, 49.3, and 35.5 mm in May, June, July, and August, respectively, which was much less than the 20-year averages of 39.7, 90.2, 81.9, and 85.3 mm, respectively. However, drought symptoms were not significant because longer periods of sunny days were usually followed by heavy rainfall. The longest period of sunshine was 14 days, which was followed by 18 mm rainfall on August 13th. For most of the corn fields, the pollination period ended in mid-August. Thus, the accumulated corn heat units (3242 CHU) up to August 31st were 160 CHU greater than the 20-year average. Less rain and more CHU caused most corn to flower and mature 10-14 days earlier than in most years, with much less leaf and ear disease, although stalk diseases were slightly higher.
Leaf diseases: ALB (48 fields, 78.8%) and PBS (46 fields, 75.4%) were the two most frequent leaf diseases in Eastern Ontario in 2021, followed by NCLB (16 fields, 26.2%), GLS (15 fields, 24.6%), eyespot (13 fields, 21.3%), rust (7 fields, 11.5%), and NLS (7 fields, 11.5%) (). Except for one field in Carleton-Ottawa which had a severity rating of 2.5 for eyespot (diseased leaf area approximately 3%), all other fields had severity ratings ≤2 (diseased leaf area <1%). Average disease severities were 1.8±0.4, 1.2±0.4, 1.2±0.4, 1.3±0.4, 1.1±0.4, 1.7±0.5, and 1.1±0.3 for ALB, eyespot, GLS, NCLB, NLS, PBS, and rust, respectively (). GLS has the potential to become an important leaf disease in Eastern Ontario. In 2018, GLS was found in 12 fields spread across three counties (Jindal et al. Citation2019); in 2021 it was found in 15 fields over five counties (). Southern corn leaf blight, tar spot, Stewart’s bacterial wilt, or Goss’s bacterial wilt and blight were not found.
Table 1. Disease occurrence in Eastern Ontario corn crops in 2021 grouped by county.
Ear diseases: At the time of the survey, ear rot was found in 8 (13.1%) of sampled fields with average incidence of 0.4±2.2; most had insect or bird damage. Gibberella/Fusarium ear rots appeared in one field in Carleton-Ottawa with incidence up to 17%. This field had 22.8% ear damage caused by European corn borer. Head smut was found in two sampled fields with incidence <1%. In Leeds and Grenville, one field with tree shade showed a different incidence in different rows, incidences were 40%, 8% and 0% for the 1st (shortest plants), 3rd and 5th rows (normal height) close to the trees, respectively.
Stalk diseases: Top dieback was found in 49 (80.3%) of sampled fields. One field in Renfrew had incidence up to 60%. Pythium stalk rot was found in 16 (26.2%) of sampled fields with average incidence of 1.8±9.4 (). One field in Renfrew had incidence of 27% and another field in Carleton-Ottawa had incidence up to 70%. Common smut was found in 39 (63.9%) sampled fields with average incidence of 0.8±1.3 (). Unlike other years, in 2021 most common smut was found on the stalk node above the aerial root. Three fields in Carleton-Ottawa had incidence of 2% and 10%.
Others: Corn rootworm, a mixture of northern corn rootworm [Diabrotica longicornis (Say)] and western corn rootworm (D. virgifera); grasshoppers (Melanoplus spp.), European corn borer [Ostrinia nubilalis (Hübner)], leaf miner (Agramyza parvicornis Loew), Japanese beetle (Popillia japonica Newman), and corn flea beetle (Chaetocnema pulicaria Melsheimer) were found in 40 (65.6%), 47 (77%), 18 (29.5%), 26 (42.6%), 36 (22%), 1 (1.6%), and 1 (1.6%) of fields, respectively. Ear damage caused by European corn borer was found on non-GMO corn with incidence 22.8%. Spider mites (Tetranychus urticae Koch) were found in 22 (36.2%) of fields. Fourteen fields had bird and other animal damage. In three counties, Leeds and Grenville, Carleton-Ottawa, and Stormont, Dundas and Glengarry, 17 fields showed drought symptoms and were dried at survey time.
ACKNOWLEDGEMENTS: This survey was supported in part by the AAFC Growing Forward Partnership with the Canadian Field Crop Research Alliance (CFCRA) and GFO through funding from the ‘Canadian Agricultural Partnership (CAP)’ a federal-provincial-territorial initiative which is administered by the Agricultural Adaptation Council. We would also like to thank farmers, our grower co-operators, various seed companies (Bayer, Corteva, Horizon Seeds, Hyland Seeds, Maizex Seeds, and Pride Seeds) and the Ontario Corn Committee (OCC) for access to their fields.
REFERENCES
- Jindal K, Reid LM, Tenuta AU, Woldemariam T, Zhu X. 2019. Status of corn diseases in Ontario, 2018 crop season. Can Plant Dis Surv. 99:117–122. In, Can J Plant Pathol. 41:sup1.
- Reid LM, Zhu X. 2005. Screening corn for resistance to common diseases in Canada. Agriculture and Agri-Food Publication No. A42-103/2005E-PDF, Ottawa (ON).
- Zhu X, Reid LM, Kebede AZ, Tenuta AU, Woldemariam T. 2020. Status of corn diseases in Ontario, 2019 crop season. Can Plant Dis Surv. 100:108–112. In, Can J Plant Pathol. 42:sup1.
SURVEY OF FUSARIUM HEAD BLIGHT AND LEAF DISEASES OF SPRING WHEAT ON PRINCE EDWARD ISLAND, 2021
CROP: Spring Wheat LOCATION: Prince Edward Island NAMES AND AGENCIES: E. JOHNSTONE, R. MATTERS & A. FOSTER
Agriculture and Agri-Food Canada, Charlottetown Research and Development Centre, 440 University Avenue, Charlottetown, PE C1A 4N6 Telephone: (902) 370-1397; Facsimile: (902) 370-1444; e-mail: [email protected]
ABSTRACT: In 2021, Prince Edward Island (PE) experienced a growing season with ample rainfall and moderate temperatures which promoted Fusarium head blight (FHB) and leaf disease of spring wheat. A total of 12 fields were sampled this year. Powdery mildew and septoria blotch were observed across the province, while stripe rust was observed in Harrington. From each sample, 20 wheat spikes were cultured and a total of 28 Fusarium spp. cultures were isolated with 5% of total spikes infected. Fusarium spp. were most often isolated from samples collected in Kings County, with no isolates from Prince County samples despite high disease incidence. The most abundant causal species of FHB in 2021 was F. graminearum.
INTRODUCTION AND METHODS: In 2021, producers on Prince Edward Island experienced relief from the drought of 2020, with just below average rainfall in June and above average rainfall in July. The Environment Canada weather station at the Agriculture and Agri-Food Canada (AAFC) Harrington Research Farm (46°20ʹ37.020” N, 63°10ʹ11.050” W) recorded 83.2 mL rainfall in June and 142.7 mL in July with average temperatures of 17.4°C and 17.5°C, respectively. Twelve fields of spring wheat were surveyed for Fusarium head blight in PE in 2021 and leaf diseases were rated visually (). Flag leaves of wheat plants were observed for leaf disease and an overall severity score was assigned on a scale of 0-9, where 0 represented no signs of disease and 9 represented >90% of the leaf covered or destroyed by disease. Powdery mildew (Blumeria graminis f. sp. tritici) was visually identified in the field by the appearance of white-grey pustules on the leaf surface. Septoria blotch (Mycosphaerella graminicola) was identified in the field by small yellow-brown spots and lesions on the leaf surface which were brown with yellow edges. Visual signs and symptoms of FHB, including the presence of orange to pink sporodochia or black perithecia and blighted or bleached spikelets, were observed. An overall severity score was determined on a scale of 0-9, where 0 represented no signs of disease and 9 represented >90% of the wheat spike infected. The FHB index was calculated as the product of incidence and severity ratings.
Fig. 1 Google maps screen capture of Prince Edward Island, county lines drawn approximately, stars indicate survey locations.
To identify the causal species of FHB, 30 wheat spikes were collected from each field post-anthesis (ZGS 70-82) from July 23 to August 9. Twenty spikes were randomly selected from the collection and remaining stem and awns were removed to allow the spike to fit in a 100-mm petri plate. Prior to plating, spikes were surface sterilized with 70% ethanol for 30s before plating on potato dextrose agar (PDA) amended with 5 mg mL−1 pentachloronitrobenzene (PCNB), 50 μg mL−1 tetracycline, 50 μg mL−1 streptomycin and 50 μg mL−1 cefotaxime. Spikes on PDA were incubated at room temperature for seven to 10 days. Suspect Fusarium spp. colonies with phenotypes expressing red, pink and purple colours with white cottony mycelium were sub-cultured onto PDA amended with tetracycline, streptomycin and cefotaxime, as above. The sub-cultures were incubated at room temperature for four days. Pure isolates retaining Fusarium characteristics were sub-cultured again onto amended PDA plates. Isolates were identified to species by morphological classification indicators as defined in FusKey (Seifert Citation1996) and the Fusarium Laboratory Manual (Leslie and Summerell Citation2006). Isolates were further characterized by colony PCR where mycelium from pure cultures was scraped into 2-mL tubes each containing 200 µl of AE buffer (Qiagen) and 45 mg of acid-washed silicon dioxide sand, then processed at 3 m s−1 for 45s using a Fisherbrand bead mill 24 homogenizer. Homogenized mycelium solution was used as template for PCR identification with PrimeTime Gene Expression Master Mix (Integrated DNA Technologies) in a duplex reaction with FgrF4 and FgrR4 primers and FgrP4 probe to identify F. graminearum (Hafez et al. unpublished) and SpoF and SpoR primers and SpoPr probe to identify F. sporotrichioides (Zitnick-Anderson et al. Citation2018). Representative isolates for each phenotype were further confirmed to species by sequencing of the TEF1α gene using primers EF1 and EF2 primers (O’Donnell et al. Citation1998). PCR products were sequenced by Eurofins Genomics (Toronto, ON) with results compared to NCBI translation elongation factor 1 (TEF) databases.
RESULTS AND COMMENTS: Powdery mildew and septoria blotch leaf diseases were observed across the island this season supported by cool and damp field conditions. The most severe leaf disease was recorded in Kings and Queens counties (). Stripe rust (Puccinia striiformis) was also recorded earlier in the season on spring wheat varieties at the AAFC Harrington Research Farm. Growers reported the application of fungicides at all sites except Harrington and New Glasgow.
Table 1. Severity of leaf disease visually identified at survey locations.
Visual symptoms of FHB were observed at all sites across PE in 2021. Disease incidence ranged from 10-80%, with 10-40% severity (). The highest FHB index was observed at the Montague site where a moderate level of septoria blotch was also recorded. Low levels of FHB were observed at sites in all three counties and may reflect local weather conditions or differences in FHB management practices.
Table 2. Incidence of visual FHB and number of Fusarium spp. isolated from wheat spikes in PE in 2021.
F. graminearum was the most abundant species with 23 isolates identified from 240 spikes plated (). Other species identified were three isolates of F. poae, and one each of F. sporotrichioides and F. avenaceum. Seventy-five percent of all isolates were collected from the field at the Montague site in Kings County where 80% of sampled spikes were infected. The remaining 25% of survey isolates were identified from Queens County fields. No Fusarium spp. were isolated from Prince County fields despite reporting up to 60% incidence, possibly due to spikes being sampled too early post-anthesis. This year’s survey results contrast from the drought conditions of 2020 where a total of 61 isolates were identified and the most abundant causal species of FHB was F. sporotrichioides (Johnstone et al. Citation2021). Unlike 2020, F. cerealis was not isolated this season. It is suspected that a more representative collection of Fusarium spp. isolates may be cultured from grain post-harvest.
ACKNOWLEDGEMENTS: This work was made possible by funding from the Canadian Agricultural Partnership (CAP) AgriScience project led by the Atlantic Grains Council (Project ID: ASP-008).
REFERENCES
- Johnstone E, Matters R, Foster A. 2021. Causal species of fusarium head blight of spring wheat and winter wheat in Prince Edward Island in 2020. Can Plant Dis Surv. 101: 92–94. In, Can J Plant Pathol. 43:sup1.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell.
- Seifert K. 1996. Fuskey-Fusarium interactive key. Ottawa (ON): Agriculture and Agri-Food Canada.
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci. 95(5):2044–2049.
- Zitnick-Anderson K, Simons K, Pasche JS. 2018. Detection and qPCR quantification of seven Fusarium species associated with the root rot complex in field pea. Can J Plant Pathol. 40(2):261–271.
OILSEEDS, PULSES, FORAGES AND SPECIAL CROPS/OLÉAGINEUX, PROTÉAGINEUX, PLANTES FOURRAGÈRES ET CULTURES SPÉCIALES OATS AND FORAGE DISEASE SURVEY IN BRITISH COLUMBIA IN 2021
CROP: Oats and Forages LOCATION: British Columbia NAMES AND AGENCIES: B. YADAV1, K. ULOTH2, M. ABBASI1, V. FETTERLEY1, R. BAMRAH1 & G.S. BRAR1
1Faculty of Land and Food Systems, 2357 Main Mall, The University of British Columbia, Vancouver, BC V6T 1Z4 2British Columbia Pest Monitoring Network, Dawson Creek, BC, Canada Telephone: (604) 827-5274; Facsimile: (604) 822-6394; E-mail: [email protected]
ABSTRACT: Oat and forage fields were assessed for various common diseases in the Peace River Region of British Columbia and Alberta in 2021. Fungal leaf spots were observed in four fields of oats with some traces of barley yellow dwarf virus. In forage fields, stem eyespot was the most common observation. Overall, disease pressure in 2021 was low for both oats and forages due to unusual dry and warm weather conditions in the region.
INTRODUCTION AND METHODS: Crops were surveyed from June 29 to July 29, 2021 near Fort St. John, Sweetwater, Beaverlodge (AB), Rose Prairie, and Dawson Creek agricultural districts of the Peace River Region with an aim to assess the prevalence of foliar diseases on cereal crops and forages. In total, seven fields of oats (Avena sativa), and 33 fields of forages were assessed for disease (). A five-category scale was used to measure typical disease severity in each field: clean (no obvious symptoms), trace (3% leaf area affected), light (3-15%), moderate (>15-20%), and severe (>20%). Four to five leaf samples were collected in glassine bags from the infected fields, which fairly represented the disease symptoms. In the lab, samples were observed under a stereo-microscope and were characterized through culturing on potato dextrose agar (PDA) medium and kept under the ambient light up to 20 days at room temperature.
Fig. 1 Locations of fields surveyed for oat (red) and forages (green) in 2021, in the Peace River region of British Columbia (left) and Alberta (right).
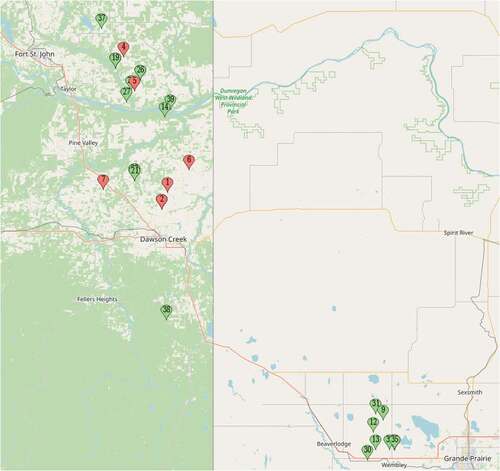
RESULTS AND COMMENTS: The seven oat fields that were surveyed were mostly clean and healthy. The crops were between milking and hardening dough stage. Up to 15% of oat plants in two fields had purple leaves in the lower canopy which is characteristic of barley yellow dwarf virus. We identified fungal leaf spots in all oat fields at trace severity. Due to hot and dry weather in B.C. during the 2021 growing season, major disease issues, such as oat crown rust, were not observed in the region.
In forages, stem eyespot was observed in all 33 fields: 29 fields of creeping red fescue (Festuca rubra), one field each of pubescent wheatgrass (Agropyron cristatum), perennial ryegrass, and two fields of crested wheat grass. To confirm the identify of the disease, the samples collected from the fields of pubescent wheatgrass and perennial ryegrass have been cultured and DNA will be extracted for future genomic analysis. Anthracnose was observed on two fields each of creeping red fescue and perennial wheatgrass and one field of crested wheatgrass. Two fields of timothy (Phleum pratense) with purple spots were observed and one field had traces of purple black spot, European skippers, and silver heads. One field of mixed forage had up to 5% traces of powdery mildew (Blumeria graminis). One field of crested wheatgrass was infected with stem smut, (Tranzscheliella hypodytes), which was identified by spore morphology as in Vanky (2012).
Stem and leaf parts from all collected samples were cultured on PDA medium after surface disinfection. An unknown fungus with white hyphae and Cladosporium cf. herbarum were isolated from creeping red fescue. Two distinct morphologies of Cladosporium were isolated from timothy, with thick and thin-walled spores, respectively, likely representing two different species. The year 2021 was characterized by unusually dry and hot weather (Environment and Climate Change Canada Citation2022), which explains the low disease incidence seen throughout this growing season in the Peace River Region. The dry growing season had a significant impact on crop quality. Crops seeded earlier appeared healthier than later seeded crops. Oat crops in the booting stage were drier and shorter than those that had completed anthesis.
ACKNOWLEDGEMENTS: We acknowledge the financial support offered by the B.C. Peace River Grain Industry Development Council, B.C. Hydro Agricultural Compensation Fund, and the Peace Region Forage Seed Association.
REFERENCES
- Environment and Climate Change Canada. 2022. Monthly climate summaries. [ accessed 2022 Feb 23]. https://climate.weather.gc.ca/prods_servs/cdn_climate_summary_e.html
- Vánky K. 2012. Smut fungi of the world. St. Paul (MN): APS Press.
BLOSSOM BLIGHT AND STEM ROT IN IRRIGATED ALFALFA SEED FIELDS IN ALBERTA, 2021
CROP: Alfalfa LOCATION: Alberta NAMES AND AGENCIES: M.W. HARDING1, K. KOPEC2, G.C. DANIELS1, S. CHATTERTON3 & B. ALEXANDER2
1Alberta Agriculture, Forestry and Rural Economic Development, Crop Diversification Centre South, 301 Horticulture Station Road E., Brooks, AB T1R 1E6 Telephone: (403) 362-1338; Facsimile: (403) 362-1326; E-mail: [email protected] 2Alfalfa Seed Commission Alberta, P.O. Box 2158, Brooks, AB, T1R 1C8 3Agriculture and Agri-Food Canada, Lethbridge Research and Development Centre, P. O. Box 3000, Lethbridge, AB T1J 4B1
ABSTRACT: Five irrigated seed alfalfa fields in southern Alberta were evaluated for symptoms of blossom blight and stem rot between July 6 and August 4, 2021. Disease ratings were recorded every two weeks between late flower and late seedpod stages in each field. Racemes were collected and florets plated on semi-selective media for recovery of Botrytis spp. and Sclerotinia sclerotiorum. Conditions were very hot and extremely dry during the sampling period, and disease levels were very low for most field visits. Heat blast of florets was evident in every field. The incidence of blossom blight ranged from 0 to 26%, with an overall average of 3.1%, while stem rot incidence ranged from 0 to 0.5% with an overall average of 0.1%. Botrytis spp. were recovered from 89.3% of florets and S. sclerotiorum was recovered from 8.5% of florets. The presence of Botrytis spp. and the incidence of blossom blight increased as the season progressed while the presence of S. sclerotiorum decreased with time and stem rot symptoms were generally absent.
INTRODUCTION AND METHODS: Blossom blight and stem rot can reduce alfalfa seed yields. Blossom blight is frequently caused by Botrytis spp. and Sclerotinia sclerotiorum Lib. de Bary (Gossen et al. Citation1998; Huang et al. Citation2000), and S. sclerotiorum can also cause stem rot (Huang et al. Citation2000). Five alfalfa seed fields were surveyed and sampled at two-week intervals between July 6 (late flower) and August 4, 2021 (late seedpod). Disease ratings were collected by moving into the field, away from the field margin, and evaluating 20 plants at each of ten randomly selected sample points approximately 10-20 m apart (total of 200 plants/field each sampling date). Each alfalfa plant was rated for blossom blight using a 0-4 scale, and for stem rot using a 0-5 scale (). At each location, three racemes were collected and placed in a labelled paper bag (30 racemes/field), and kept in an insulated cooler on ice and stored at 4°C for three to seven days until they could be processed in the lab. The presence of Botrytis spp. and S. sclerotiorum was detected by plating florets on semi-selective, differential media as described in Reich et al. (Citation2017). Briefly, 100 florets per field were sampled randomly from the racemes and surface-sterilized by placing in 0.5% NaClO + 2-3 drops Tween 20 for 1 min, and rinsing three times with sterile distilled water. Fifty florets from each field were then plated on Sclerotinia semi-selective medium (SSM) and 50 florets on Botrytis semi-selective medium (BSM) for each of the three sample dates. Plates were incubated at room temperature (~23°C) in the dark for three to five days and growth on the plates was evaluated, and results recorded.
Table 1. Disease severity rating scales for blossom blight and stem rot on alfalfa.
RESULTS AND COMMENTS: Disease levels were low in 2021, likely due to hot, dry conditions during the growing season in most of southern Alberta. Heat blast of alfalfa florets was common in all fields. Blossom blight was absent on July 6, but reached 26% in one field by August 4 (not shown). The overall average incidence of blossom blight was 3.1%, (). Stem rot was rarer with incidence levels less than 1% throughout the season. Average disease severities for both blossom blight and stem rot were very low (< 1) throughout the season (). However, despite the lack of disease symptoms, the pathogens were detected on blossoms at every sampling date and in every field (with one exception on the July 6 sampling date). Botrytis spp. were present on between 72% and 100% of florets (not shown) with an overall average incidence of 89.3% (). Sclerotinia sclerotiorum was present on 0% to 19.3% of florets (not shown) with an overall average incidence of 8.5% (). These data support the hypothesis that the environmental conditions were conducive to the production and discharge of spores by Botrytis spp. and S. sclerotiorum, but were limiting to disease initiation and/or progression during most of the 2021 growing season. Furthermore, the results showed that the incidence of Botrytis spp. colonizing alfalfa florets increased as the season progressed through flowering and pod maturation while the levels of S. sclerotiorum decreased over the same period (). These results were consistent with those observed by Reich et al. (Citation2017).
Table 2. Incidence and severity of blossom blight and stem rot on alfalfa in Alberta, 2021.
Fig. 1 Incidence of Botrytis spp. and S. sclerotiorum on alfalfa florets, and the incidence of blossom blight and stem rot symptoms in five alfalfa fields (lower) at three sampling dates.
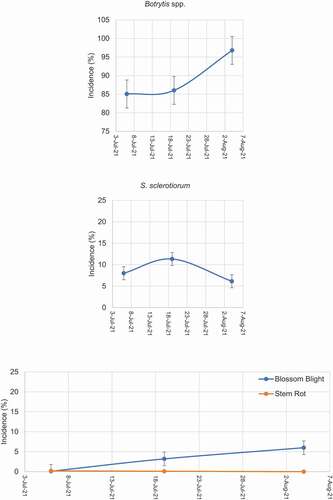
ACKNOWLEDGEMENTS: The authors acknowledge the support of the Alfalfa Seed Commission Alberta, Alberta Agriculture Forestry and Rural Economic Development, and Agriculture and Agri-Food Canada. Special thanks to the five cooperating alfalfa seed producers who facilitated this research on their farms.
REFERENCES
- Gossen BD, Holley JD, Harrison LM, Smith SR. 1998. Distribution of blossom blight of alfalfa in western Canada and impact on seed yield. Can J Plant Pathol. 20:122.
- Huang, HC, Acharya SN, Erickson RS. 2000. Etiology of alfalfa blossom blight caused by Sclerotinia sclerotiorum and Botrytis cinerea. Plant Pathol Bull. 9:11–16.
- Reich J, Chatterton S, Johnson D. 2017. Temporal dynamics of Botrytis cinerea and Sclerotinia sclerotiorum in seed alfalfa fields of Southern Alberta, Canada. Plant Dis. 101:331–343.
SURVEY OF WHITE MOULD OF DRY BEAN IN SOUTHERN ALBERTA IN 2020 AND 2021
CROP: Field bean (Phaseolus vulgaris L.) LOCATION: Southern Alberta NAMES AND AGENCY J.D. REICH, B. GROENENBOOM & S. CHATTERTON
Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada, P. O. Box 3000, 5403 1 Ave. S., Lethbridge, AB T1J 4B1 Telephone: (403) 317-2226; E-mail: [email protected]
ABSTRACT: Forty-five commercial dry bean fields in southern Alberta were surveyed at early pod formation (R6 to R7, mid-August) in 2021. Despite another dry year in the region, disease pressure remained high in many fields and was comparable to 2020. Overall, pinto bean fields had greater incidences of white mould than any other variety, although sample sizes for cultivars in the red and black market classes were limited.
INTRODUCTION AND METHODS: White mould, caused by the fungal pathogen Sclerotinia sclerotiorum (Lib.) de Bary, is one of the main production constraints of dry bean (Phaseolus vulgaris L.) in southern Alberta (Balasubramanian et al. Citation2014). In 2020 and 2021, surveys of 41 and 45 commercial dry bean fields, respectively, were performed to assess the prevalence, incidence, and severity of white mould in dry bean fields in the production centres of southern Alberta (the regions of Bow Island, Cranford and Rolling Hills). Surveys were performed at the early to mature pod stages (R6 to R7, August 9 to 13) (Osorno et al. Citation2013). Ten sites in each field were assessed. Sites were >20 m apart from each other and from the field border and formed a U-shape pattern within each field. At each site, ten plants were rated for disease incidence and severity where, 1 = no white mould, 2 = white mould present on one branch, 3 = white mould present on two branches, and 4 = white mould present on main stem (Balasubramanian et al. Citation2014).
RESULTS AND DISCUSSION: 2020 was a much wetter year than 2021 in southern Alberta (; Alberta Agriculture and Forestry (AAF) Citation2021). Despite differences in precipitation, white mould disease incidence and severity were, on average, the same in both years surveyed ( and ).
Table 1. Total precipitation from June 1 to August 31 over the last 14 years in the regions of southern Alberta where disease surveys were performed in commercial fields in 2021.
Table 2. White mould prevalence, incidence, and severity in dry bean fields in southern Alberta in 2020.
Table 3. White mould prevalence, incidence, and severity in dry bean fields in southern Alberta in 2021.
The prevalence of white mould was lower in 2020 than in 2021 (68% vs 96%, respectively), but a handful of fields exhibited much more extreme disease symptoms in 2020. A few pinto bean fields surveyed in 2020 showed 98 to 100% of plants infected with severities around 3.9, whereas the fields with the greatest disease in 2021 had 62 to 69% incidences. Even though 2021 was one of the driest growing seasons in the last 14 years, incidences and severity of white mould were high in many fields surveyed and were comparable to 2020 levels. As in previous years, this finding indicates that, because dry bean fields are irrigated in southern Alberta, the disease pressure from S. sclerotiorum remains high regardless of the regional climate.
In both years, certain cultivars had more disease than others. Overall, pinto bean cultivars had greater disease incidence (32 to 34%) and severity (1.6 to 1.7) than any other cultivar. A handful of great northern bean fields had high incidences (>50%) of white mould in 2021, but most fields of these cultivars remained below the 25% incidence level in both years.
ACKNOWLEDGEMENTS: Funding for these surveys was provided by the Alberta Pulse Growers Commission and Agriculture and Agri-Food Canada through the CAP Pulse Cluster.
REFERENCES
- Alberta Agriculture and Forestry (AAF). 2021. Historical weather data. [ accessed 2021 Nov 13]. http://agriculture.alberta.ca/acis/township-data-viewer.jsp
- Balasubramanian PM, Conner RL, McLaren DL, Chatterton S, Hou A. 2014. Partial resistance to white mould in dry bean. Can J Plant Sci. 94:683–691.
- Harding MW, Daniels GC, Burke DA, Pugh CA, Nielson JM. 2017. White mould on dry bean in Alberta in 2016. Can Plant Dis Surv. 97:185–187.
- Osorno J, Endres G, Ashley R, Kandel H, Berglund D. 2013. Dry bean production guide – A1133-20 (revised Dec 2019). Kandel H, Endres G, editors. Fargo (ND): North Dakota State University (NDSU) Extension Service. [accessed 2022 Feb 21]. https://www.ag.ndsu.edu/publications/crops/dry-bean-production-guide#section-2
CANOLA DISEASE SURVEY IN ALBERTA, 2021
CROP: Canola LOCATION: Alberta NAMES AND AGENCIES: M.W. HARDING1, G.C. DANIELS1, T.B. HILL1, M.A. KENNEDY1, A. SARKES2, Y. YANG2 & J. FENG2
1Alberta Agriculture, Forestry and Rural Economic Development, Crop Diversification Centre South, 301 Horticulture Station Road E., Brooks, AB T1R 1E6 Telephone: (403) 362-1338; Facsimile: (403) 362-1326; E-mail: [email protected] 2Alberta Agriculture, Forestry and Rural Economic Development, Crop Diversification Centre North, 17507 Fort Road NW, Edmonton, AB T5Y 6H3
ABSTRACT: Diseases can negatively affect canola production, primarily due to yield reduction. Blackleg, sclerotinia stem rot and verticillium stripe were evaluated in 359 fields representing approximately 1% of canola acres within each county in Alberta in 2021. Blackleg was found in 89.4% of fields and on 18.6% of the stems rated. Blackleg severity averaged 0.39 on a 0 to 5 scale. Sclerotinia stem rot symptoms on lower main stems were observed in 25.4% of fields on 0.8% of stems. Finally, although verticillium stripe-like symptoms were seen in approximately 2.8% of fields, the presence of Verticillium spp. was not confirmed in any of the samples submitted to the Alberta Plant Health Lab.
INTRODUCTION AND METHODS: Canola (Brassica napus L.) was produced on 6.7 million acres in Alberta in 2021 (Statistics Canada Citation2021). Blackleg of canola, caused by Leptosphaeria maculans (Sowerby) P. Karst., is evaluated annually on canola crops in Alberta in approximately 1% of canola fields in each county/municipality. In total, 379 canola fields were surveyed in 52 counties and municipal districts across the province with the primary objective of characterizing the blackleg disease situation on canola. Twenty of the fields visited or sampled were unable to be rated for blackleg, so a grand total of 359 fields are reported. Sclerotinia stem rot and verticillium stripe symptoms were also recorded, but disease severity was not rated. The survey was performed between August 4 and October 18, 2021. Surveyors were encouraged to visit fields near swathing time, or within 7 days post-swathing and collect 10 stems at each of 10 locations along a W-shaped survey transect (100 stems/field). Sampling locations were ≥ 20 m from one another, and from field margins. The lower stems (bottom 6 to 12 in) were collected and shipped directly to the Crop Diversification Centre South (Brooks, AB) where they were evaluated for the presence of blackleg symptoms such as stem cankers, lesions with pycnidia and internal stem blackening. Blackleg prevalence was calculated as percentage of fields with symptoms. Blackleg incidence was calculated as percentage of stems showing blackleg symptoms. Blackleg severity was estimated using a 0-5 scale for rating vascular discolouration (WCC/RRC Citation2009; ).
Table 1. A rating scale to estimate blackleg severity on canola (WCC/RCC 2009).
Three other diseases were also evaluated on the roots and lower main stems. Sclerotinia stem rot (Sclerotinia sclerotiorum Lib. de Bary) infections occurring on lower main stems were recorded when they were soft and would shred when twisted, and/or when sclerotia were observed inside the stem. Prevalence was calculated as percentage of fields with stem rot and incidence as percentage of stems showing symptoms. Stem rot severity was not calculated. Symptoms of verticillium stripe [Verticillium longisporum (C. Stark) Karapapa, Bainbr. & Heale (Citation1997)] were also recorded when discolouration of the internal stem tissues appeared in a ‘starburst’ pattern, rather than sectoring, or when unilateral striping on stems was observed. Verticillium stripe prevalence and incidence were calculated in the same manner as sclerotinia stem rot. Colonization of symptomatic host tissues by V. longisporum was confirmed by probe-based qPCR using the primers/probe set: GGGAGGACTCACAGATCGAA/CCGTGAATTCAGAGGCAGAT/6FAM-TCACGACCTCTGGTCATGAC-IABkFQ. When the test for V. longisporum was negative, the same DNA sample was tested by qPCR for Verticillium albo-atrum and V. dahliae using the primer/probe sets as described by Maurer et al. (Citation2013), for Leptosphaeria maculans using primer/probe set: CCTCACACTCTCGACCCCTA/GCATGTTCTTGAACCGCTAC/HEX/CACAGCCATATCATCCTGCA/IABkFQ, and for L. biglobosa using the primer/probe set: GAAGAATGGCAAAATCACAGG/AGCTCTGCGCGACCTTTT/6FAM/AGGAAGAAGCAGCCATAGGC/IABkFQ. For all qPCR tests, DNA from healthy canola plant, the corresponding fungal culture and known infected plant samples were included as control. Finally, clubroot symptoms were rated and recorded using a separate protocol and methodology, and the clubroot results will be presented in a separate report.
RESULTS AND COMMENTS: Of the 359 canola fields evaluated, 321 had blackleg symptoms for an overall prevalence of 89.4% (). The average incidence of blackleg on canola was 18.6%, while overall average severity was 0.39 on a 0 to 5 scale. Sclerotinia stem rot was observed in 25.4% of fields with a mean disease incidence of 0.8% (). Finally, symptoms of verticillium stripe were seen in nearly 3% of fields, but PCR analyses of all samples gave negative test results for the presence of Verticillium longisporum, V. albo-atrum and V. dahliae (not shown).
Table 2. Blackleg prevalence, incidence and severity in canola fields in Alberta in 2021.
Table 3. Prevalence and incidence of sclerotinia stem rot on canola lower main stems in Alberta, 2021.
Blackleg and sclerotinia diseases in 2021 were more common and more severe compared with the levels in 2019 and 2020 (Harding et al. Citation2020, Citation2021). This was surprising considering the above average temperatures, and below average rainfall, in Alberta for most of the 2021 growing season. However, environmental conditions suitable for blackleg disease initiation were present in early-mid June, and there were approximately 1.2 million acres of irrigated crop production in southern Alberta where a microclimate for disease may have been present throughout the season.
ACKNOWLEDGEMENTS: We gratefully acknowledge the significant contributions of the Alberta Association of Agricultural Fieldmen, and their staff, for assistance with collecting canola stems from across the province. Finally, we express appreciation for the landowners and producers for access to their fields.
REFERENCES
- Harding MW, Daniels GC, Burke DA, Pugh CA, Hill TB, Zahr K, Sarkes A, Feng J. 2021. Canola and mustard disease survey in Alberta, 2020. Can Plant Dis Surv. 101:109–113. In Can J Plant Pathol. 43: sup1.
- Harding MW, Daniels GC, Burke DA, Pugh CA, Hill TB, Zahr K, Sarkes A, Feng J. 2020. A survey for blackleg and sclerotinia stem rot on canola in Alberta in 2019. Can Plant Dis Surv. 100:113–116. In Can J Plant Pathol. 42:sup1.
- Karapapa VK, Bainbridge BW, Heale JB. 1997. Classical and molecular characterization of Verticillium longisporum comb, nov., pathogenic to oilseed rape. Mycol Res. 101:1281–1294.
- Maurer KA, Radišek S, Berg G, Seefelder S. 2013. Real-time PCR assay to detect Verticillium albo-atrum and V. dahliae in hops: development and comparison with a standard PCR method. J Plant Dis Protect. 120:105–114.
- Statistics Canada. 2021. Principal field crop areas, June 2021. [ accessed 2021 Nov 25]. https://www150.statcan.gc.ca/n1/daily-quotidien/210629/dq210629b-eng.htm
- Western Canada Canola/Rapeseed Recommending Committee (WCC/RRC) Incorporated. 2009. Procedures of the Western Canada Canola/Rapeseed Recommending Committee for the evaluation and recommendation for registration of canola/rapeseed candidate cultivars in western Canada.
CANOLA DISEASE SURVEY IN CENTRAL AND NORTHERN ALBERTA IN 2021
CROP: Canola LOCATION: Central and northern Alberta NAMES AND AGENCIES: H. YU, J. CORDERO-ELVIA, K.F. CHANG, C.X. YANG, R. FREDUA-AGYEMAN, G.D. TURNBULL, S.F. HWANG & S.E. STRELKOV
Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, AB T6G 2P5 Telephone: (780) 492-6693; Facsimile: (780) 492-4265; E-mail: [email protected]
ABSTRACT: Root and lower stem samples were collected from 35 canola crops across central and northern Alberta from late August to early October, 2021, and cultured on potato dextrose agar to test for the presence of soilborne pathogens. A total of 524 root and 402 stem samples were assessed. Fusarium spp. were recovered most frequently from the roots at all locations, occurring at an average incidence of 85.0%, followed by Alternaria spp. (incidence of 13.9%), Rhizoctonia spp. (11.3%) and Rhizopus spp. (6.1%). Fusarium spp. also were the predominant pathogen recovered from the lower stems, occurring at an average incidence of 81.7%, followed by Alternaria spp. (40.6%) and Rhizoctonia spp. (7.1%).
INTRODUCTION: Canola (Brassica napus L.) is an important cash crop in Alberta, with 2.4 million ha seeded in 2020 (Statistics Canada Citation2020). Unfortunately, soilborne diseases can negatively affect canola yield and quality. A survey of 35 canola crops was conducted in central and northern Alberta between August and October 2021, with root and stem samples collected to evaluate the incidence of soil-borne pathogens.
METHODS: Thirty-five canola fields were sampled near Edmonton, St. Albert, Namao, Morinville, Gibbons, Josephsburg, Villeneuve, Redwater, Opal and Bruderheim, Alberta. The samples were collected along W-shaped transects in each field, with approximately 50 m between sampling points. All plants within a 1-m2 quadrat were examined at each of five points along each transect. The incidence of clubroot, blackleg, root rot, and white mould was noted at each sampling point. Lower stem and root samples were also collected at random from low-lying areas in the fields. Samples showing symptoms of root rot and/or yellowing stems were taken to the laboratory, where they were analysed for the presence of fungal pathogens. The outer layer of the root and lower stem surfaces was peeled off, and only those samples with inside surfaces showing discoloured tissues were sectioned. This method excluded most saprophytic microorganisms from the isolation process, thereby allowing detection of only endophytic pathogenic fungi. The plant material was surface-sterilized in a 1% bleach solution for 2 min, rinsed in sterile distilled water, and then incubated on potato dextrose agar for 10-12 days under ambient light at room temperature. The fungal isolates obtained were sub-cultured for purification and identified visually. The percentage of pathogen-free samples and the mean percent incidence of each pathogen was calculated for the root samples from each location.
RESULTS AND DISCUSSION: A total of 926 canola lower stem and root samples were collected at random from the W-shaped transects and low-lying areas in the surveyed fields. Despite dry weather through most of the summer (Alberta Government Citation2021), a number of plants with root rot symptoms were observed. The incidence of root rot was highest at Edmonton, with a mean incidence of 4.1%, although 50% of the roots were infected at one sampling point (). At Morinville and Redwater, the overall incidence of root rot was also > 4%. Blackleg incidence was greatest at Morinville, Opal and Bruderheim. Some canola crops near Edmonton were heavily infected with clubroot, with a mean incidence of 7.2%, including one sampling point where 95% of the roots were infected. Most of the other areas showed little to no clubroot infection. A small amount of white mould was observed at Edmonton, St. Albert, Morinville and Gibbons.
Table 1. Incidence (%) of disease in canola plants collected in central Alberta, 2021.
Fusarium spp. were recovered most frequently from the root samples, occurring at an incidence of 85% across all locations (). The next most common fungi associated with the roots were Alternaria spp. (incidence of 13.9%), Rhizoctonia spp. (11.3%) and Rhizopus spp. (6.1%). A total of five unknown isolates were detected in canola roots collected at Morinville. During processing, evidence of damage resulting from insect feeding was observed on some roots. These damaged areas likely served as colonization access points for invasion of the roots by pathogenic fungi. In the lower stems, Fusarium spp. also were the predominant pathogenic fungi, occurring at an incidence of 81.7%, followed by Alternaria spp. (incidence of 40.6%), Rhizoctonia spp. (7.1%) and Rhizopus spp. (2.1%) (). The incidence of Fusarium spp. was greater than reported in the previous year (Yang et al. Citation2021). Other fungi including Penicillium spp. were detected occasionally in both infected root and stem samples. The high incidence of Fusarium, Alternaria and Rhizoctonia in both belowground and aboveground tissues suggests an interaction of these fungi during infection of canola. In many cases, Fusarium was found in association with Rhizoctonia or Alternaria spp. Possible synergistic effects among these pathogens should be investigated further.
Table 2. Incidence of fungi recovered from diseased canola roots collected in central and northern Alberta, 2021.
Table 3. Incidence of fungi recovered from diseased canola stems collected in central and northern Alberta, 2021.
ACKNOWLEDGEMENTS: This survey was supported financially by the Alberta Canola Producers Commission and Results Driven Agriculture Research (RDAR), with in-kind support from the University of Alberta.
REFERENCES
- Alberta Government. 2021. Agricultural moisture situation update. [ accessed 2021 Nov 19]. https://open.alberta.ca/publications/moisture-situation-update
- Statistics Canada. 2020. Production of principal field crops, July 2020. [ accessed 2021 Nov 12]. https://www150.statcan.gc.ca/n1/daily-quotidien/200831/dq200831a-eng.htm
- Yang C, Hwang SF, Chang KF, Fredua-Agyeman R, Strelkov SE. 2021. Soilborne pathogens of canola in central and northern Alberta in 2020. Can Plant Dis Surv. 101:118–120. In, Can J Plant Pathol. Vol. 43:sup 1.
THE OCCURRENCE AND SPREAD OF CLUBROOT ON CANOLA IN ALBERTA IN 2021
CROP: Canola LOCATION: Alberta NAMES AND AGENCIES: S.E. STRELKOV1, V.P. MANOLII1, Y. AIGU1, M. MARCHAL1, R. MIGNOT1, G.C. DANIELS2, M.W. HARDING2 & S.F. HWANG1
1Department of Agricultural, Food and Nutritional Science, University of Alberta, 410 Agriculture/Forestry Centre, Edmonton, AB T6G 2P5 Telephone: (780) 492-1969; Facsimile: (780) 492-4265; E-mail: [email protected] 2Alberta Agriculture, Forestry and Rural Economic Development, Crop Diversification Centre South, 301 Horticultural Station Road East, Brooks, AB T1R 1E6
ABSTRACT: Five hundred and ninety-seven canola (Brassica napus) crops were surveyed across Alberta for the occurrence and severity of clubroot (Plasmodiophora brassicae) in 2021. Despite drought-like conditions in much of the province, the disease was identified in 103 of the crops, with infections ranging from mild (81 crops), to moderate (15), to severe (7). Another 191 cases of clubroot were recorded during independent inspections by county and municipal personnel, for a total of 294 confirmed field infestations in 2021.
METHODS: Five hundred and ninety-seven canola (Brassica napus L.) crops were surveyed across Alberta for the occurrence and severity of clubroot, caused by Plasmodiophora brassicae Wor., in 2021. The crops were generally visited after swathing, and selected either randomly or because there had been reports of clubroot in the area. Approximately 50-100 canola roots from a 20-30 m2 area near the entrance to each field were examined visually for symptoms of clubroot. If no symptoms were observed, the crop was not inspected further, as clubroot occurs most frequently near field approaches (Cao et al. Citation2009). If clubroot symptoms were found on any plants near the entrance, then the entire crop was surveyed more extensively by examining the roots of all plants within a 1-m2 area at each of 10 points along a ‘W’ sampling pattern. The roots were evaluated for clubroot symptom severity on a 0-3 scale following Kuginuki et al. (Citation1999) where 0 = no galling, 1 = a few small galls, 2 = moderate galling and 3 = severe galling. The ratings on the individual plants were used to calculate a disease severity index (DSI) for each crop according to the formula of Horiuchi and Hori (Citation1980) as modified by Strelkov et al. (Citation2006):
DSI (%) = [∑ ((n × 0) + (n × 1) + (n × 2) + (n × 3))/(N × 3)] × 100, where 0, 1, 2 and 3 are the symptom severity classes, n is the number of plants in each class, and N is the total plant number.
Whenever possible, field visits were coordinated with the agricultural fieldman in each county or municipal district. Survey information from independent inspections conducted by the fieldmen also was collected when available.
RESULTS AND COMMENTS: Clubroot symptoms (root galling) were identified in 103 (17%) of the 597 canola crops inspected (). These included 7 severely (DSI > 60%) infected crops, 15 moderately (DSI = 10-60%) infected crops, and 81 mildly (DSI < 10%) infected crops. An additional 191 cases of the disease were documented in independent inspections conducted by agricultural fieldmen, for a total of 294 confirmed clubroot infestations in 2021 (). This number was perhaps greater than expected, given the drought-like conditions prevalent across much of Alberta in summer 2021 (Government of Alberta Citation2021). Clubroot incidence and severity increase with increasing moisture (Hamilton and Crête Citation1978; Dobson Citation1982), while the optimum air temperatures for disease development range from 20°C to 25°C (Sharma et al. Citation2011; Gossen et al. Citation2012). It is likely that localized rainfall events in some regions enabled infection and the appearance of symptoms, despite the generally hot and dry conditions.
Table 1. Distribution of Plasmodiophora brassicae-infected canola (Brassica napus) crops identified in Alberta in 2021.
Clubroot has been diagnosed in at least 5045 canola crops from 2005 to 2021, although in some cases, these crops were grown in the same field over multiple years (). Hence, the total number of individual fields infested with P. brassicae is somewhat lower. As of 2020, these diagnoses at the crop level represented a minimum of 3398 unique fields, but possibly as many as 3561 (since no geo-coordinates or legal land descriptions were available for 163 reports over this period, precluding the possibility of crosschecking for duplicate field counts). The 294 novel clubroot diagnoses made in 2021 will add to this total, and an analysis to determine the most current number of individual infested fields is underway.
Fig. 1 Cumulative diagnoses of clubroot in canola crops in Alberta from 2005-2021. Clubroot has been diagnosed in at least 5045 canola crops from 2005 to 2021, although in some cases, these crops were grown in the same field over multiple years.
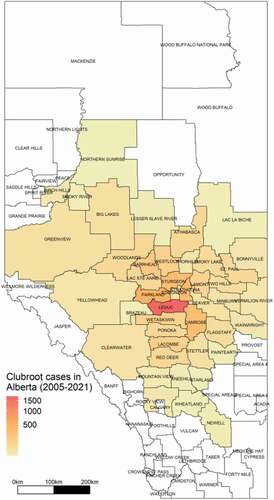
ACKNOWLEDGEMENTS: The authors would like to thank T. Adolf, J. Aitken, J. Babcock, M. Bates, Q. Beaumont, J. Benson, J. Boden, H. Brook, D. Burlock, C. Dowhan, L. Duncan, T. Eleniak, C. Erichsen-Arychuk, D. Fortin, T. Green, R. Hrywkiw, S. Kaus, A. Kihn, K. King, K. Kornelsen, K. Kozdroski, K. Langlois, K. MacDonald, S. Majec, J. Makus, M. Marquis, M. Martinson, T. McGinn, C. McIntosh, D. Medcke, K. Meunier, S. Oracheski, D. Orchard, A. Ouellett, L. Poile, J. Schwindt, J. Stambulic, S. Steffen, A. Stewart, J. Tigert, D. Ullery, A. Van Beers, C. Verpy, B. Weeks, T. Wilson, M. Winchell and C. Wolf for their assistance with the survey. The financial support provided by research grants from Results Driven Agriculture Research (RDAR) and Alberta Canola is gratefully acknowledged, as is the in-kind support from the counties and municipal districts, Alberta Agriculture, Forestry and Rural Economic Development, and the University of Alberta.
REFERENCES
- Government of Alberta. 2021. Agricultural moisture situation update [2021]. [ accessed 2022 Feb 22]. https://open.alberta.ca/publications/moisture-situation-update-2021
- Cao T, Manolii VP, Hwang SF, Howard RJ, Strelkov SE. 2009. Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada. Can J Plant Pathol. 31:321–329.
- Dobson R. 1982. Soil water matric potential requirements for root-hair and cortical infection of chinese cabbage by Plasmodiophora brassicae. Phytopathology 72:1598–1600.
- Gossen BD, Adhikari KKC, McDonald MR. 2012. Effects of temperature on infection and subsequent development of clubroot under controlled conditions. Plant Pathol. 61:593–599.
- Hamilton H, Crête R. 1978. Influence of soil moisture, soil pH, and liming sources on the incidence of clubroot, the germination and growth of cabbage produced in mineral and organic soils under controlled conditions. Can J Plant Sci. 58:45–53.
- Horiuchi S, Hori M. 1980. A simple greenhouse technique for obtaining high levels of clubroot incidence. Bull. Chugoku Natl Agric Exp Stn E (Environ Div). 17:33–55.
- Kuginuki Y, Hiroaki Y, Hirai M. 1999. Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinensis). Eur J Plant Pathol. 105:327–332.
- Sharma K, Gossen BD, McDonald MR. 2011. Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms. Plant Pathol. 60:830–838.
- Strelkov SE, Tewari JP, Smith-Degenhardt E. 2006. Characterization of Plasmodiophora brassicae populations from Alberta, Canada. Can J Plant Pathol. 28:467–474.
A SURVEY FOR PEA DISEASES IN ALBERTA, 2021
CROP: Pea LOCATION: Alberta NAMES AND AGENCIES: M.W. HARDING, G.C. DANIELS, T.B. HILL & M. KENNEDY
Alberta Agriculture, Forestry and Rural Economic Development, Crop Diversification Centre South, 301 Horticulture Station Road E., Brooks, AB T1R 1E6 Telephone: (403) 362-1338; Facsimile: (403) 362-1326; E-mail: [email protected]
ABSTRACT: A survey of pea diseases in Alberta was conducted in 2021. One hundred and fifteen fields, representing approximately 1% of pea acres in each county, were evaluated for root, stem and foliar disease symptoms. Hot, dry conditions in much of July were unfavourable for disease initiation or development. Drought stress was a factor in many fields, and was often a more significant production issue than disease. Root rot was the most prevalent disease in pea, and was found in 90.4% of fields while mycosphaerella blight prevalence was down significantly to 45.2% of fields. Bacterial blight was reported in 4.3% of fields, but white mould and downy mildew were not observed in 2021.
INTRODUCTION AND METHODS: One hundred and fifteen pea fields, in 52 Alberta counties (), were surveyed for root rot (Aphanomyces euteiches Dreschs., Fusarium spp.), mycosphaerella blight [Mycosphaerella pinodes (Berk. & Blox.) Vestergr., Ascochyta pisi Lib.], white mould [Sclerotinia sclerotiorum (Lib) de Bary], downy mildew [Peronospora viciae (Berk.) Casp.], and bacterial blight [Pseudomonas syringae pv. pisi (Sackett) Dye & Wilkie]. Field visits occurred between June 30 and July 21, 2021. Five sample locations were visited in each field along a W-shaped transect with each location >20 m apart and from the field margin. Ten plants were rated at each location for a total of 50 plants/field. Whole plants were rated for disease severity of root rot and mycosphaerella blight. Root rots were rated on a 1-7 disease intensity scale based on root discolouration and root mass reduction as described by Chatterton et al. (Citation2019). Mycosphaerella blight was rated using a 1-7 scale modified from Liu et al. (Citation2013). The presence or absence of sclerotinia white mould, downy mildew and bacterial blight were noted for each plant. Prevalence was calculated as the percent of fields with disease symptoms and incidence was calculated as the percent of plants with symptoms. Sclerotinia white mould, downy mildew and bacterial blight were rated for presence/absence such that prevalence and incidence were calculated, but not severity. Root samples with root rot symptoms were washed and frozen so identification of A. euteiches and/or Fusarium spp. could be performed. The identities of root rot pathogens will be presented in a separate report.
Fig. 1 Survey locations for Alberta’s 2020 pea disease survey. RR = root rot (left panel) and Asc = Ascochyta (right panel).
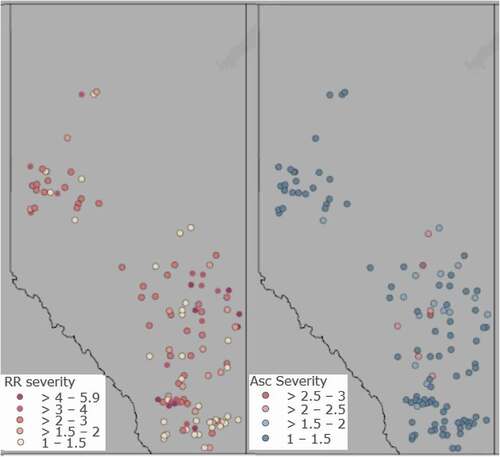
RESULTS AND COMMENTS: Hot, dry conditions were observed in many regions of Alberta in late June and throughout July during the 2021 growing season. Drought was a primary issue in many fields and represented a much greater production constraint than pests or diseases. However, some diseases, such as root rot, mycosphaerella blight and bacterial blight were present (), albeit at reduced prevalence, incidence and severity when compared to disease levels in 2020 (). Root rots had the highest prevalence, incidence and severity followed by mycosphaerella blight and bacterial blight. White mould and downy mildew were not reported. Survey results for each county are presented in .
Table 1. Summary of prevalence, incidence and severity of pea diseases in Alberta, 2021.
Table 2. Comparison of prevalence, incidence and severity of pea disease in Alberta in 2020 and 2021.
Table 3. Pea disease prevalence, incidence and severity for each municipality/county surveyed in Alberta in 2021.
ACKNOWLEDGEMENTS: The authors acknowledge support of Alberta Agriculture, Forestry and Rural Economic Development and express appreciation to landowners and producers that allowed access to their fields.
REFERENCES
- Chatterton S, Harding MW, Bowness R, McLaren DL, Banniza S, Gossen BD. 2019. Importance and causal agents of root rot on field pea and lentil on the Canadian Prairies, 2014-2017. Can J Plant Pathol. 41:98–114.
- Liu J, Cao T, Feng J, Chang KF, Hwang SF, Strelkov SE. 2013. Characterization of fungi associated with ascochyta blight of field pea in Alberta, Canada. Crop Prot. 54:55–64.
SURVEY OF CANOLA DISEASES IN SASKATCHEWAN, 2021
CROP: Canola LOCATION: Saskatchewan NAMES AND AGENCIES: A. AKHAVAN1, C. PERU1, A. DOLATABADIAN2, D. FERNANDO2, J. GIROYED3, B. ESAU3, C. JACOB1, J. IPPOLITO1, K. KINDRACHUK1, S. MILLER1, S. CHANT1, K. BOERE1, A. NOBLE1, L. COWELL4, S. FRIESEN1, K. STONEHOUSE1, M. STRUTHERS1, M. BROWN1, M. O’CONNOR1, K. ANDERSON5, K. MAKOHONIUK6, J. KWASNICKI6, C. NEUBERGER6, C. FENNIG6, B. JOHNSON6 & L. ROSZELL6
1Saskatchewan Ministry of Agriculture, 3085 Albert St., Regina, SK S4S 0B1 Telephone: (306) 787-4671; Facsimile: (306) 787-0428; E-mail: [email protected] 2Dept. of Plant Science, University of Manitoba, Winnipeg, MB, R3T 2N2 3Prairie Co-op, Meadow Lake, SK S9X 1A0 4Nutrien, Saskatoon, SK S7K 7G3 5Bayer Crop Science Inc., Saskatoon, SK S7K 4B5 6Saskatchewan Association of Rural Municipalities, Regina, SK S4V 3A4
ABSTRACT: The annual survey in Saskatchewan covered 212 canola fields across six large regions of the province. Blackleg was the most prevalent disease in 66.5% of crops surveyed with a mean disease incidence of 6.9% (ranging from 3.1% to 11%). Sclerotinia stem rot was observed with symptoms in 34.9% of the crops surveyed. The mean incidence of sclerotinia stem rot among all canola crops surveyed in Saskatchewan was 2.2% but ranged from 0.5% to 4.9% among regions. Verticillium stripe with typical symptoms and pathogen signs was also confirmed for the first time in Saskatchewan.
METHOD: A total of 212 canola crops were surveyed between July 28 and September 16 in the major canola growing regions of Saskatchewan. In 2021, the number of surveyed crops was highest in the East-central with 51 out of 212 fields being in this region. The distribution of surveyed crops across the rest of the province was as follows: 38 (Northeast), 33 (West-central), 31 (Southwest), 30 (Southeast) and 29 (Northwest). Crops were surveyed before swathing while plants were between growth stages 5.2 and 5.5 (Harper and Berkenkamp Citation1975). Disease assessments were made by examining 20 plants from each of five sites in each field. Individual sample sites were located at least 20 m from the field edge and separated from each other by at least 20 m. Fields were assessed for both prevalence (percent of fields with symptoms of the disease) and incidence (percent of plants surveyed with symptoms of the disease per field). The diseases assessed include: sclerotinia stem rot (Sclerotinia sclerotiorum), blackleg (Leptosphaeria maculans), aster yellows (AY phytoplasma), foot rot (Rhizoctonia spp., Fusarium spp.), alternaria black spot (Alternaria brassicae, A. raphani), fusarium wilt (F. oxysporum f. sp. conglutinans), verticillium stripe (Verticillium longisporum), powdery mildew (Erysiphe cruciferarum), downy mildew (Peronospora parasitica), white rust (Albugo candida), grey stem (Pseudocercosporella capsellae), bacterial pod spot (Pseudomonas syringae pv. maculicola) and clubroot (Plasmodiophora brassicae). Severity ratings were also conducted for both sclerotinia stem rot and blackleg. For sclerotinia stem rot, each plant (100 per field) was rated for severity according to a rating scale of 0 to 5 described by Kutcher and Wolf (Citation2006). For blackleg, plant stems were cut and then scored for basal stem cankers severity using a rating scale ranging from 0 to 5 (WCC/RRC Citation2009) ( and ). Average severity values for blackleg and sclerotinia stem rot in each field were calculated as the sum of the severity ratings divided by the total number of plants examined. Stem lesions were recorded when observed on the upper stem or when associated with a basal canker. The prevalence of alternaria black spot (A. brassicae, A. raphani) in the field was recorded. For all of the diseases assessed, prevalence and average disease incidence or severity values were calculated across the entire province and separately for each of six regions within Saskatchewan.
Table 1. Sclerotinia rating scale (Kutcher and Wolf Citation2006).
Table 2. Blackleg rating scale (WCC/RRC 2009).
RESULTS AND COMMENTS: In 2021, approximately 4,903,800 hectares (12,117,600 acres) was seeded to canola in Saskatchewan. As of late-January 2022, 4,862,200 hectares (12,014,800 acres) of canola were harvested in Saskatchewan (Statistics Canada Citation2022). The Saskatchewan Crop Report (Saskatchewan Ministry of Agriculture Citation2021) estimated that 98% of the Saskatchewan canola crop had been harvested by October 4, 2021.
Sclerotinia stem rot was observed in 34.9% of the canola crops surveyed. The average incidence in the province was 2.2% (6.3% in infested crops) (). The incidence of sclerotinia stem rot was significantly lower in 2021 than 2020 (16.8% in all crops and 21.2% in infested crops) and 2019 (13% in all crops and 16% in infested crops) (). Incidence was highest in the Southwest region (4.9%) and lowest in the Southeast region (0.5%). The average severity of sclerotinia stem rot in canola crops in Saskatchewan was very low at 0.03. The severity of sclerotinia stem rot ranged from 0.01 to 0.07 in different regions ().
Table 3. Mean disease incidence and severity of sclerotinia stem rot of canola in Saskatchewan in 2021.
Table 4. Mean disease incidence and severity of blackleg basal cankers in Saskatchewan in 2021.
Table 5. Prevalence of alternaria pod spot, aster yellows, and foot rot of canola fields surveyed in Saskatchewan in 2021.
Table 6. Mean disease incidence and sclerotinia severity reported as both, the average severity across infected plants and the average severity across all plants surveyed per field from 2011-2021 (Akhavan et al. Citation2021).
Symptoms of blackleg basal infection (rated after cutting of lower stems) were present in 66.5% of the Saskatchewan canola crops included in the survey (). The average incidence in the province was 6.9% (10.4% in infested crops). The levels of blackleg were lower than those in 2020 (81.2% prevalence), and 2019 (69% prevalence) (). In 2021, the average incidence was highest in the East-central region (11%) and lowest in the Northeast and Southwest regions (3.1% and 3.6%, respectively). The average severity of blackleg basal cankers in the province was 0.09. Average severity was highest in the East-central region (0.15) and lowest in the Southwest regions (0.04). Blackleg stem lesions were present in 28.8% of canola crops with an average incidence of 2% (data not shown). The highest average blackleg stem lesion incidence occurred in the East-central region (3.8%). The lowest incidence was in the Northeast region (0.3%). Both blackleg basal cankers and stem lesions were present on the same plant in 1.4% of crops across the province (data not shown).
Table 7. Mean blackleg canker severity reported as both, the average severity across infected plants and the average severity across all plants surveyed per field from 2011-2021 (Akhavan et al. Citation2021).
Aster yellows had a prevalence of 6.6% () with an average incidence of 0.4% (5.3% in infested fields). The average incidence is lower than in 2020 when the average incidence in Saskatchewan was 0.7% (Akhavan et al. Citation2021). The highest prevalence of aster yellows in 2021 was in the Northwest region (24.1%) with an average incidence of 2.2% (). Province-wide, aster yellows was observed in 19.3% of surveyed canola fields (this includes observations in surveyed fields where infected plants were seen outside of the 100 plant sample) (Data not shown).
Foot rot was recorded in 1.4% of canola crops in the province. The highest incidence was in the Northwest region (3.5%). Foot rot was not detected in the Northwest, Southeast and Southwest regions of Saskatchewan (). In 2021, alternaria pod spot was assessed in all regions in the province with the highest prevalence observed in the Southwest region (64.5%) ().
Symptoms suggesting verticillium stripe were found in four samples during the 2021 survey which were submitted to Dr. Dilantha Fernando and Dr. Aria Dolatabadian at the University of Manitoba for confirmation. Three samples were confirmed as Verticillium dahliae and one as V. longisporum, lineage A1/D3. In 2021, outside of the survey, two additional samples were found with typical symptoms and pathogen signs of verticillium stripe, one at swathing and one shortly after, which were also sent to Fernando and Dolatabadian at the University of Manitoba for confirmation. Both samples were confirmed to be caused by V. longisporum, one each of lineage A1/D1 and lineage A1/D2. This is the first confirmed report from Saskatchewan of the occurrence of verticillium stripe with typical symptoms and pathogen signs. Also, to the best of our knowledge, it is the first report of V. longisporum A1/D2 and A1/D3 lineages from Canada.
REFERENCES
- Akhavan A, Peru C, Cubbon D, Giroyed J, Esau B, Darcy O, Jacob C, Ippolito J, Kindrachuk K, Campbell E, et al. 2021. Survey of canola diseases in Saskatchewan, 2020. Can Plant Dis Surv. 101:132–136. In Can J Plant Pathol. 43:sup1.
- Harper FR, Berkenkamp B. 1975. Revised growth-stage key for Brassica campestris and B. napus. Can J Plant Sci. 55:657–658.
- Kutcher HR, Wolf TM. 2006. Low-drift fungicide application technology for sclerotinia stem rot control in canola. Crop Prot. 25:640–646.
- Saskatchewan Ministry of Agriculture. 2021. Saskatchewan Crop Report for the period September 28 to October 4, 2021. [ accessed 2022 Jan 19]. https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/market-and-trade-statistics/crops-statistics/crop-report
- Statistics Canada. 2022. Table 32-10-0359-01- Estimated areas, yield, production, average farm price and total farm value of principal field crops, in metric units, annual, CANSIM database. [ accessed 2022 Jan 19]. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210035901
- Western Canada Canola/Rapeseed Recommending Committee (WCC/RRC) Incorporated. 2009. Procedures of the Western Canada Canola/Rapeseed Recommending Committee for the evaluation and recommendation for registration of canola/rapeseed candidate cultivars in western Canada.
DISEASES OF FLAX IN MANITOBA AND SASKATCHEWAN IN 2021
CROP: Flax LOCATION: Manitoba and Saskatchewan NAMES AND AGENCIES: K. NABETANI1, T. ISLAM1, H. R. KUTCHER1, C. PERU2, M. BEAITH3, A. AKHAVAN2, C. JACOB2, S. ROBERTS4, M. BROWN4, A. NOBLE4, K. STONEHOUSE4, A. FRANSOO4, M. COTT5 & D. FROESE6
1Crop Development Centre, University of Saskatchewan, 51 Campus Drive, College of Agriculture and Bioresources, Saskatoon, SK S7N 5A8 Telephone: (306) 966–8661; Facsimile: (306) 966–5015; Email: [email protected] 2Crops and Irrigation Branch, Saskatchewan Ministry of Agriculture, 3085 Albert Street, Regina, SK S4S 0B1 3Saskatchewan Flax Development Commission, 8-3815 Thatcher Avenue, Saskatoon, SK S7R 1A3 4Regional Services Branch, Saskatchewan Ministry of Agriculture, 3085 Albert Street, Regina, SK S4S 0B1 5Manitoba Crop Alliance, 38 4th Avenue NE, Box 188, Carman, MB R0G 0J0 6Manitoba Agriculture and Resource Development, 65-3rd Ave NE, Carman, MB R0G 0J0
ABSTRACT: Among 84 crops surveyed in 2021, pasmo was the most prevalent disease found in 63% of crops surveyed in Saskatchewan and 32% in Manitoba. Alternaria blight was found in 32% of crops surveyed in Saskatchewan and 4% in Manitoba and was the second most prevalent disease. Fusarium wilt was observed in 17% of crops in Saskatchewan and 24% in Manitoba. Aster yellows was observed in 12% of crops in Saskatchewan and 20% in Manitoba. No cases of flax rust or powdery mildew were reported in 2021.
METHODS: In 2021, 84 flax crops were surveyed, of which 59 were in Saskatchewan and 25 were in Manitoba. Of the flax crops surveyed in Saskatchewan, nine were at Linseed Coop trial plots situated within multiple research farms throughout Saskatchewan. All surveys were conducted between August 3rd and September 16th. Maturity and stand establishment were measured on a scale of 1 to 5, where 1 was very poor/early and 5 was excellent/mature. Disease prevalence was measured as the percentage of fields affected by each disease out of all crops surveyed. One hundred flax plants in each crop were examined. Disease incidence was determined as the percentage of flax plants affected by each disease, and pasmo severity as the percentage of stem area covered by pasmo symptoms averaged over 100 plants.
RESULTS AND COMMENTS: Of all 84 flax crops surveyed, 69% (76% in Manitoba, 66% in Saskatchewan) were at the yellow to brown or brown boll stages at the time of the survey. Twenty-eight percent of flax crops were at the green to yellow or yellow boll stages (20% in Manitoba, 31% in Saskatchewan) and only 4% of all flax crops; 4% in Manitoba and 3% in Saskatchewan; were at the green boll stage. The majority of flax crops, 54% (56% in Manitoba, 53% in Saskatchewan), had excellent stand; however, the rate was lower than in 2019 or 2020 (Islam et al. Citation2020; Nabetani et al. Citation2021). This could be attributed to the unusually hot and dry conditions during the 2021 growing season. Lodging was reported in only one flax crop. The low incidence of lodging could be due to the shorter plant heights observed in many flax crops under the drought conditions. Drought or very dry conditions were reported in 20% of the flax crops. Grasshopper infestation was reported in 30% of flax crops and ranged from less than threshold (two grasshoppers /m2) to visible crop damage.
Despite the hot, dry conditions in 2021, the prevalence of pasmo was 54% among the 84 flax crops surveyed (32% in Manitoba, 63% in Saskatchewan); pasmo was the most prevalent disease of flax in both provinces (). Trace to 10% incidence of pasmo was seen in 25% of all flax crops surveyed. Eight percent of flax crops showed 11-30% pasmo incidence and 11% had 31-60% incidence. Only 10% of flax crops had a pasmo incidence greater than 60%. The average incidence was 15.5% for all crops and 28.9% for diseased crops. Pasmo severity in most infected crops ranged from trace to low. Of the 84 flax crops surveyed, trace to 5% pasmo severity was found in 14% of crops and 6-25% severity in 25% of crops (). Pasmo severity between 25% and 75% was observed in 13% of flax crops, which were all located in southeast Saskatchewan. One crop with very high (>76%) severity occurred in Saskatchewan. The average pasmo severity was 10.4% for all crops and 19.4% for diseased crops. High pasmo incidence and severity was observed mainly in flax crops grown in south east and south-central regions of Saskatchewan where there were higher moisture levels during the growing season than the rest of the province in 2021. The low incidence and severity of pasmo in Manitoba likely was due to the severe drought conditions.
Table 1. Pasmo incidence and severity in 84 flax crops, comprising 59 crops in Saskatchewan and 25 crops in Manitoba in 2021.
Alternaria blight was the second most prevalent disease of flax and was observed in 24% of all surveyed flax crops (4% in Manitoba, 32% in Saskatchewan). It was less prevalent than in 2018, 2019 or 2020 (Rashid et al. Citation2019; Islam et al. Citation2020; Nabetani et al. Citation2021). Orange flax bolls which were darker than normal were observed in several flax crops in Saskatchewan during the survey. Since Alternaria spp. were recovered from some orange boll samples, alternaria blight was likely to be the cause. Boll filling did not seem to be affected by the blight, based on observation. The third most prevalent disease was fusarium wilt, which was found in 19% of flax crops (24% in Manitoba and 17% in Saskatchewan). The prevalence of fusarium wilt in 2021 was higher than reported in the surveys of 2018, 2019 or 2020 (Rashid et al. Citation2019; Islam et al. Citation2020; Nabetani et al. Citation2021). This could be attributed to the dry conditions that many regions in Manitoba and Saskatchewan experienced. It is also possible that flax plants could have been more prone to show wilt symptoms due to lack of moisture, on top of fusarium infection. Aster yellows was found in 14% of flax crops (20% in Manitoba and 12% in Saskatchewan). No powdery mildew or flax rust was observed during the survey.
REFERENCES
- Islam T, Kutcher HR, Lee S, Beaith M, Peru C, Ziesman B, Jacob C, Hicks L, Noble A. 2020. Diseases of flax in Saskatchewan in 2019. Can Plant Dis Surv. 100:136. In, Can J Plant Pathol. 42:sup1.
- Nabetani K, Islam T, Kutcher HR, Reza M, Peru C, Beaith M, Jacob C, Roberts S, Campbell E, Ippolito J, et al. 2021. Diseases of flax in Manitoba and Saskatchewan in 2020. Can Plant Dis Surv. 101:156. In, Can J Plant Pathol. 43:sup1.
- Rashid KY, Ziesman B, Jacob C, Hicks L, Kindrachuk K, Peru C, Kutcher HR, Islam T, Cholango, Martinez LP, Cabernel T, et al. 2019. Diseases of flax in Manitoba and Saskatchewan in 2018. Can Plant Dis Surv. 99:180–181. In, Can J Plant Pathol. 41:sup1.
2021 SURVEY OF LENTIL DISEASES IN SASKATCHEWAN
CROP: Lentil LOCATION: Saskatchewan NAMES AND AGENCIES: A. AKHAVAN1, C. PERU1, J. IPPOLITO1, K. KINDRACHUK1, S. CHANT1, S. FRIESEN1, M. BROWN1, D. RISULA1, K. BOERE1, K. MAKOHONIUK2, J. KWASNICKI2, C. NEUBERGER2, L. ROSZELL2 C. FENNIG2 & B. JOHNSON2
1Saskatchewan Ministry of Agriculture, 3085 Albert St., Regina, SK S4S 0B1 Telephone: (306) 787-4671; Facsimile: (306) 787-0428; E-mail: [email protected] 2Saskatchewan Association of Rural Municipalities, 2301 Windsor Park Rd., Regina, SK S4V 3A4
ABSTRACT: A total of 67 lentil crops were surveyed in Saskatchewan in 2021. Root rot, anthracnose, and stemphylium blight were the most prevalent diseases observed in the survey, though variation in the prevalence of these diseases was found across lentil-growing regions in Saskatchewan. Sclerotinia stem and pod rot and botrytis stem and pod rot were not found in lentil crops in 2021.
INTRODUCTION AND METHODS: A total of 67 lentil fields were surveyed for the presence and incidence of diseases in Saskatchewan in 2021. The survey was conducted between July 05 and July 27 when crop stage ranged from R1 (early bloom, one open flower at any node on 50% of the plants in the field) to R7 (physiological maturity). The number of surveyed crops was highest in Southwest Saskatchewan, with 31 of the 67 crops surveyed located in this region. The distribution of the surveyed crops across the rest of the province was as follows: 15 (West-central), 14 (Southeast), five (East-central) and two (Northwest). Disease assessments were made by visually examining 10 plants from each of 10 sites along a W-pattern in each field. Individual sites were located at least 50 m from the field edge and at least 30 m apart. Crops were assessed for the incidence of anthracnose (Colletotrichum truncatum), ascochyta blight (Ascochyta lentis), sclerotinia stem and pod rot (Sclerotinia sclerotiorum), botrytis stem and pod rot (Botrytis cinerea) and stemphylium blight (Stemphylium spp.) and the prevalence of root rot complex (Fusarium spp./Pythium spp./Rhizoctonia solani/Aphanomyces euteiches) and all previously listed diseases.
Prevalence of each of these diseases (percentages of the crops surveyed showing symptoms) was calculated for each region surveyed (-), as well as provincial totals () and compared to totals from the previous five years (Akhavan et al. Citation2021). The average incidence of anthracnose, ascochyta blight and stemphylium blight was calculated and averaged for each region and on a provincial level ().
Table 1. Prevalence of plant diseases in lentil crops surveyed in West-central Saskatchewan, 2012-2021.
Table 2. Prevalence of plant diseases in lentil crops surveyed in Southwest Saskatchewan, 2012-2021.
Table 3. Prevalence of plant diseases in lentil crops surveyed in Southeast Saskatchewan, 2012-2021.
Table 4. Prevalence of plant diseases in lentil crops surveyed in East-central Saskatchewan, 2012-2021.
Table 5. Prevalence of plant diseases in lentil crops surveyed in Northeast Saskatchewan, 2012-2021.
Table 6. Prevalence of plant diseases in lentil crops surveyed in Northwest Saskatchewan, 2012-2021.
Table 7. Prevalence of plant diseases in lentil crops surveyed in Saskatchewan, 2012-2021.
Table 8. Average disease incidence in Saskatchewan lentil crops surveyed in 2021.
RESULTS AND COMMENTS: Approximately 1.5 million hectares (3.7 million acres) of lentils were seeded in Saskatchewan in 2021. This is similar to the 1.5 million hectares (3.8 million acres) seeded in 2020 (Statistics Canada Citation2022). As of January 2022, 1.5 million hectares (3.7 million acres) of lentils were harvested in Saskatchewan (Statistics Canada Citation2022). The Saskatchewan Crop Report (Saskatchewan Ministry of Agriculture Citation2021) estimated that 100% of the Saskatchewan lentil crop had been harvested by October 4, 2021.
Root rot complex symptoms were present in 85.1% of the surveyed crops. Across the regions, prevalence ranged from 80% to 100% of fields in the 2021 survey (). The highest prevalence was found in the Northwest (100%) followed by the Southwest (87.1%) region. Root rot was present in 85.7% of the fields surveyed in the Southeast region and 80% in the East-central and West-central regions in 2021. Root rot has been a notable issue in pea and lentil crops in recent years, with a number of potential pathogenic agents (Fusarium spp./Pythium spp./Rhizoctonia solani/Aphanomyces euteiches). No sampling or further testing was performed to confirm causal pathogens.
Anthracnose (Colletotrichum lentis) was observed in 58.2% (39 fields) of fields surveyed in 2021 (). The highest prevalence was found in the Southwest (71%) region followed by the West-central (66.7%) region. The average incidence of anthracnose was 6.4% when averaged across the province (). The incidence of anthracnose was highest in Southwest Saskatchewan (7.6%) followed by the West-central and Southeast (6.1%).
Ascochyta blight symptoms (Ascochyta lentis) were observed in 7.5% of crops surveyed in 2021 (). Among regions, the prevalence ranged from 0% in the Northwest and Southeast to 40% in the East-central, while the average incidence ranged from 0% in the Northwest and Southeast to 3.2% in the East-central. It is important to note that diagnosis was only based on visual symptoms in the field. Plants having visual symptoms that were consistent with ascochyta blight were not confirmed with additional testing.
Stemphylium blight (Stemphylium spp.) was found in 37.3% of lentil fields surveyed. This is a lower level of prevalence compared to previous years (). The highest prevalence was observed in the Southwest region (48.4%) followed by the West-central (33.3%), Southeast (28.6%), and East-central (20%) regions. The average incidence of stemphylium blight was 3.1% across all fields surveyed in 2021 ().
Sclerotinia stem and pod rot (Sclerotinia sclerotiorum) and botrytis stem and pod rot (Botrytis cinerea) were not noted in fields surveyed in 2021.
REFERENCES
- Akhavan A, Peru C, Ippolito J, Kindrachuk K, Chant S, Tetland S, Brown M, Marcino S, Risula D, Campbell E, et al. 2021. 2020 survey of lentil diseases in Saskatchewan. Can. Plant Dis Surv. 101:137–140. In, Can J Plant Pathol. 43:sup1.
- Saskatchewan Ministry of Agriculture. 2021. Saskatchewan crop report for the period September 28 to October 4, 2021. [ accessed 2021 Jan 17]. https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/market-and-trade-statistics/crops-statistics/crop-report
- Statistics Canada. 2022. Estimated areas, yield, production, average farm price and total farm value of principal field crops, in metric units, annual, CANSIM database: Table 32-10-0359-01. [ accessed 2021 Jan 17]. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210035901
FACTORS INFLUENCING ANTHRACNOSE OF LENTIL IN SASKATCHEWAN IN 2020 AND 2021
CROP: Lentil (Lens culinaris) LOCATION: Saskatchewan NAMES AND AGENCIES: M. HUBBARD & Z. HOSSAIN
Agriculture and Agri-Food Canada, Swift Current Research and Development Centre, 1 Airport Road, Swift Current, SK S9H 3X2 Telephone: (306) 772-0470; E-mail: [email protected]; [email protected]
ABSTRACT: Anthracnose is arguably the most important disease of lentil in Saskatchewan. Interest in this disease has increased in recent years because of reports of insensitivity to Fungicide Resistance Action Committee (FRAC) group 11 fungicides, which are a frequently used management tool. Surveys were conducted in 2020 and 2021. Anthracnose was much more severe and common in 2020 than in 2021. In 2020, disease incidence differed among crop districts. Application of the fungicide Dyax (pyraclostrobin, group 11; fluxapyroxad, group 7) and the absence of hail damage were linked to increased disease, while application of a group 3 or group 7 fungicide did not lead to reduced disease. In 2021, no crop production or environmental factors were found to significantly impact anthracnose, possibly due to very low disease pressure.
INTRODUCTION AND METHODS: Anthracnose of lentil is caused by the fungal pathogen Colletotrichum lentis Damm (Damm et al. Citation2014). This disease can result in significant yield losses and is frequently managed by the application of strobilurin fungicides, also known as quinone outside inhibitors (QoI), or Fungicide Resistance Action Committee (FRAC) group 11 fungicides (Buchwaldt et al. Citation2018). However, insensitivity to group 11 fungicides has been reported in Saskatchewan (Bogdan Citation2020; Hoffmeister et al. Citation2021). Thus, understanding what influence factors other than strobilurin fungicide application may have on disease severity and/or incidence has value.
Lentil disease data was collected from 130 fields in the summer of 2020 and 29 fields in 2021, by surveyors from the Saskatchewan Ministry of Agriculture and the pulse industry, and sent to the pulse pathology laboratory at the Swift Current Research and Development Centre, Agriculture and Agri-Food Canada. Ten sites per field, distributed in a W-pattern, were surveyed. The slope position of each site was recorded as a low point, mid-slope or flat, or a high point. Ten randomly-selected plants at each site were rated as a group. In 2020, disease severity was rated on a 0-9 rating scale, based on the percentage of plant area impacted by disease, where 0= no symptoms; 1= <2% plant area affected (PAA); 2= 2-5% PAA; 3= 5-10% PAA; 4= 10-25% PAA; 5= 25-50% PAA; 6= 50-75% PAA, 7= 75-90% PAA, lesions coalescing with stem girdling; 8= >90% PAA, stem girdling or breakage; 9= plants dead. In 2021, a 0-5 scale was used, where 0= no lesions; 1= <5% of plant area diseased (lower canopy), a few superficial lesions at stem base; 2= 5-25% of stems and leaves affected (lower canopy), lesions, some leaf drop; 3= 25-50% of stems and leaves (lower and mid canopy), lesions and leaf drop; 4= 50-75% of stems and leaves affected (lower, mid and upper canopy), lesions, leaf drop, shoot die-back; 5= >75% of stems and leaves affected (lower, mid and upper canopy), lesions, leaf drop, severe shoot die-back. Incidence was calculated as a percentage of the plants with symptoms based on the same ten plants used to assess severity. In 2021, root rot was rated as present or absent from each site. Agronomic and environmental data collected from some fields included location, rotation (2020), herbicides (2020), drought stress, hail damage and insect damage. Statistical analysis was conducted in SAS Studio via a Kruskal-Wallis test, followed by a Dwass, Steel, Critchlow-Fligner (DSCF) multiple means comparison test if required.
RESULTS AND COMMENTS: In 2020, the average disease severity and incidence were 4.1 ± 0.30 and 6.2 ± 3.50, respectively. In 2021, these values were 0.54 ± 0.14 and 1.08 ± 0.34, respectively.
In 2020, disease incidence was lower in crop districts 3AS and 3BS than in 2B (). The drier conditions in the southwest and south-central regions (Government of Saskatchewan Citation2020) likely contributed to the lower disease incidence values in 3AS and 3BS. However, it was also dry in the Regina region, including district 2B. Potentially, factors other than moisture, such as pathogen population or application of the fungicide Dyax, could account for the higher incidence in this district. The majority of the fields in the survey that were reported to have been sprayed with Dyax were located in district 2B (). This may, at least in part, be due to more fungicides being applied in fields with higher disease pressure. Indeed, the application of Dyax was associated with higher anthracnose incidence and severity (). However, the number of fungicide applications per field (0, N=5, 1, N=47 or 2, N=12) did not impact incidence or severity. The fungicides reported in the survey were Delaro (175 g/L prothioconazole and 150 g/L trifloxystrobin, N=26), Dyax (pyraclostrobin, 50% and fluxapyroxad, 50%) (N=21), Elatus (250 g/L azoxystrobin and 100 g/L benzovindiflupyr, N=26), Priaxor (167 g/L fluxapyroxad and 333 g/L pyraclostrobin, N=4) and Proline (480 g/L prothioconazole, N=4). All of these products, except for Proline, contain a group 11 active ingredient. Dyax, Elatus and Priaxor all include a group 7 fungicide, while Delaro contains a group 3. Despite the likely existence of insensitivity to group 11 fungicides, neither the application of a group 3 nor a group 7 fungicide (the two alternative modes of action documented in the survey) was associated with a statistically significant reduction in disease incidence or severity. The limited sample size and nature of the survey make a complete assessment difficult. The smaller samples sizes (number of fields) in crops districts 6B and 7B, which were generally wetter in 2020, may have precluded statistically significant differences in incidence being detected between these two districts and 3AS and/or 3BS. For the 2020 growing season, Akhavan et al. (Citation2021) reported higher anthracnose incidence in west-central Saskatchewan, which includes 7A and 7B, than in the southwest, southeast, east-central or northwest.
Table 1. Number of lentil fields with and without application of the fungicide Dyax (group 11 and 7) and overall number of fungicide applications by crop district in 2020, in crop districts with three or more fields.
Fig. 1 Anthracnose incidence in 2020 in crop districts with data from three or more lentil fields. Bars with the same letter are not significantly difference (Kruskal-Wallis, followed by DSCF multiple mean comparison test, p ≤ 0.05).
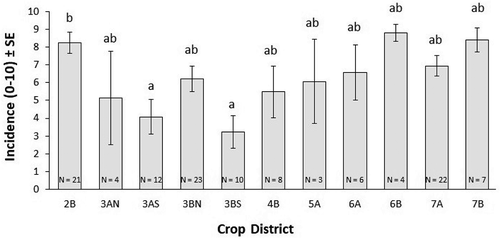
Fig. 2 Anthracnose severity and incidence in 2020 in lentil fields with or without Dyax fungicide application. Within a panel, bars with the same letter are not significantly difference (Kruskal-Wallis, p ≤ 0.05).
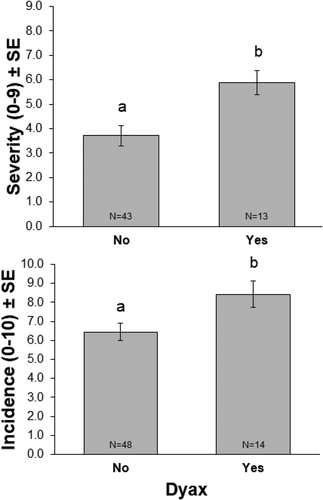
In 2020, slope position did not have a significant impact on anthracnose severity or incidence. Herbicides from groups 1, 2, 4, 5, 9 or 14 were not linked to any significant differences in disease incidence or severity. Crop rotation did not impact anthracnose incidence or severity. Neither insect damage nor drought stress altered disease. Hail damage, surprisingly, was associated with reduced anthracnose incidence, but did not influence severity (). However, this may be explained by hail occurring in regions that had lower disease pressure. Districts 3AS and 3BS, which had lower incidence, also had a higher percentage of fields in which hail damage was noted ( and ).
Fig. 3 Impact of hail on disease severity and incidence in 2020 and 2021. Within a panel, bars with the same letter are not significantly difference (Kruskal-Wallis, p ≤ 0.05).
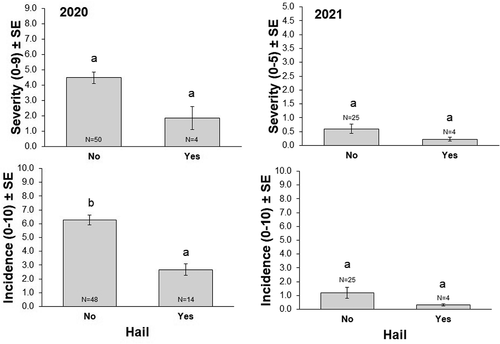
Table 2. Number of lentil fields with and without hail in 2020 in crop districts with three or more fields.
Table 3. Number of lentil fields with and without hail in 2021 in crop districts with three or more fields.
In 2021, there were no statistically significant differences between crop districts, slope position, presence of root rot, drought, hail or insect damage in terms of anthracnose severity or incidence. Potentially, the smaller survey size and lower disease pressure in 2021, as compared to 2020, made it harder to detect factors influencing disease.
The results suggest that research into regional differences in anthracnose populations in Saskatchewan, sensitivity of C. lentis isolates to group 3 or group 7 fungicide active ingredients, as well as the influence of Dyax application or hail injury on anthracnose, could be of merit.
REFERENCES
- Akhavan A, Peru C, Ippolito J, Kindrachuk K, Chant S, Tetland S, Brown M, Marcino S, Risula D, Campbell E, et al. 2021. 2020 survey of lentil disease in Saskatchewan. Can Plant Dis Surv. 101:137–140. In, Can J Plant Pathol. 43:sup1.
- Bogdan J. 2020. Fungicide insensitivity in pulse crops. Saskatoon (SK): Saskatchewan Pulse Growers. [ accessed 2022 Feb 25]. https://saskpulse.com/files/technical_documents/200706_Fungicide_insensitivity_in_pulse_crops.pdf
- Buchwaldt L, Dzananovic E, Durkin J. 2018. Lentil anthracnose: epidemiology, fungicide decision support system, resistance and pathogen races. Can J Plant Pathol. 40(2):189–198.
- Damm U, O’Connell RJ, Groenewald JZ, Crous PW. 2014. The Colletotrichum destructivum species complex – hemibiotrophic pathogens of forage and field crops. Stud Mycol. 79:49–84.
- Government of Saskatchewan. 2020. Cumulative rainfall report, April 1 to October 19, 2020. [ accessed 2022 Feb 25] https://publications.saskatchewan.ca/#/products/108083
- Hoffmeister M, Martens G, Yarnell L, Blain N, Wieja B, Riediger N, Stammler G. 2022. Method for QoI sensitivity monitoring in Colletotrichum lentis by a species-specific pyrosequencing assay. Can J Plant Pathol. 44(1):128–135.
2021 SURVEY OF FIELD PEA DISEASES IN SASKATCHEWAN
CROP: Field pea LOCATION: Saskatchewan NAMES AND AGENCIES: A. AKHAVAN1, C. PERU1, J. IPPOLITO1, K. KINDRACHUK1, K. BOERE1, S. CHANT1, S. FRIESEN1, M. BROWN1, A. NOBLE1, D. RISULA1, K. STONEHOUSE1, K. MAKOHONIUK2, J. KWASNICKI2, C. NEUBERGER2, C. FENNIG2, L. ROSZELL2 & B. JOHNSON2
1Saskatchewan Ministry of Agriculture, 3085 Albert St., Regina, Saskatchewan S4S 0B1 Telephone: (306) 787-4671; Facsimile: (306) 787-0428; E-mail: [email protected] 2Saskatchewan Association of Rural Municipalities, 2301 Windsor Park Rd., Regina, SK S4V 3A4
ABSTRACT: A total of 48 field pea crops were surveyed in Saskatchewan in 2021. Root rot complex and mycosphaerella/ascochyta blight were the most prevalent diseases in 2021 and were present in 91.7% and 83.3% of the surveyed crops. Symptoms consistent with bacterial blight were identified in 22.9% of crops. White mould was not present in surveyed fields this year.
INTRODUCTION AND METHODS: In total, 48 field pea crops were surveyed in Saskatchewan in 2021. The distribution of fields across the six regions in the province is described in . The highest number of surveyed crops were located in West-central and Southwest Saskatchewan with 13 and 11 of the surveyed field located in these two regions. The survey was conducted between June 28 and July 22 when crop growth stage ranged from R1 (flower bud) to R5 (beginning maturity). Disease assessments were made by examining 10 plants from each of five sites along a W-pattern with at least 20 m between sampling sites. Crops were assessed for the incidence of root rot complex (Aphanomyces euteiches, Fusarium spp., Rhizoctonia spp., and Pythium spp.), mycosphaerella/ascochyta complex [Peyronellaea (Mycosphaerella) pinodes, Ascochyta pisi and Phoma medicaginis f.sp. pinodella], downy mildew (Peronospora viciae), white mould (Sclerotinia sclerotiorum) and bacterial blight (Pseudomonas syringae pv. pisi). The severity of the root rot complex and mycosphaerella/ascochyta blight complex was assessed for each plant using the rating scales described below ( and ). The presence of bacterial blight (Pseudomonas syringae pv. pisi) was also assessed. All disease assessments were made based on visual symptoms in the field. No additional testing was conducted to confirm diagnosis.
Table 1. Prevalence of root rot complex, mycosphaerella/ascochyta complex, white mould and bacterial blight in Saskatchewan field pea crops in 2021.
Table 2. Incidence and severity of field pea diseases in Saskatchewan in 2021.
Table 3. Severity scale for root rot complex of field pea (Chatterton et al. Citation2019).
Table 4. Severity rating scale for mycosphaerella/ascochyta blight of field pea (modified from Liu et al. Citation2013).
RESULTS AND COMMENTS: Approximately 849,600 hectares (2.1 million acres) of field pea were seeded in Saskatchewan in 2021. This is lower than 939,600 hectares (2.3 million acres) seeded in 2020 (Statistics Canada Citation2022). As of mid-January, 813,700 hectares (2 million acres) of field pea were harvested (Statistics Canada Citation2022) in Saskatchewan. The Saskatchewan Crop Report (Saskatchewan Ministry of Agriculture Citation2021) estimated that 100% of the Saskatchewan field pea crop had been harvested by October 4, 2021.
Root rot complex was present in 91.7% of the surveyed field pea crops with an average incidence of 51.4% across the province ( and ). Average disease incidence ranged from 29.1% (East-central) to 74.0% (Northwest). Disease severity was generally low with an average severity of 2.3 across the province.
Mycosphaerella/Ascochyta complex was present in 83.3% of surveyed fields and was assessed based on percent of plant affected. Average incidence was 33% and ranged from 18% (West-central) to 62% (Southeast) and average severity was 1.4 across the province.
Symptoms consistent with bacterial blight were present in 22.9% of crops. Bacterial streaming test was conducted with a microscope on representative samples, but no additional testing was performed to identify/confirm the causal organism. Presence of this disease may be influenced by crop damage due to adverse weather in these regions. Bacterial blight was not observed in Northeast, Northwest and West-central Saskatchewan.
Downy mildew was observed in a single field in the East-central region. White mould was not present in surveyed fields this year.
REFERENCES
- Chatterton S, Harding MW, Bowness R, McLaren DL, Banniza S, Gossen BD. 2019. Importance and causal agents of root rot on field pea and lentil on the Canadian prairies, 2014–2017. Can J Plant Pathol. 41:98–114.
- Liu JF, Cao TS, Feng J, Chang KF, Hwang SF, Strelkov SE. 2013. Characterization of the fungi associated with ascochyta blight of field pea in Alberta, Canada. Crop Prot. 54:55–64.
- Saskatchewan Ministry of Agriculture. 2021. Saskatchewan crop report for the period September 28 to October 4, 2021. [ accessed 2021 Jan 14]. https://www.saskatchewan.ca/crop-report
- Statistics Canada. 2022. Estimated areas, yield, production, average farm price and total farm value of principal field crops, in metric units, annual, CANSIM database: Table 32-10-0359-01. [ accessed 2021 Jan 29]. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210035901
SEED-BORNE PATHOGENS OF PULSE CROPS IN SASKATCHEWAN IN 2020
CROP: Pulse crops (Pea, Lentil, Chickpea) LOCATION: Saskatchewan NAMES AND AGENCIES: B. D. OLSON1, A. AKHAVAN2, S. BANNIZA3, T. BLOIS4, B. ERNST5, S. JUNEK6, S. PHELPS7, T. PRASAD8 & D. RISULA2
1Box 88, Hazlet, SK S0N 1E0Telephone: (306) 774-5643; Facsimile: n/a; Email: [email protected] 2Saskatchewan Ministry of Agriculture, 3085 Albert St., Regina, SK S4S 0B1 3Crop Development Centre, University of Saskatchewan, 51 Campus Dr., Saskatoon, SK S7N 5A8 420/20 Seed Labs Inc., 507 – 11th Ave., Nisku, AB T9E 7N5 5Prairie Diagnostic Seed Lab, 1105 Railway Ave., Weyburn, SK S4H 3H5 6Discovery Seed Labs Ltd., 450 Melville St., Saskatoon, SK S7J 4M2 7Saskatchewan Pulse Growers, 207 – 116 Research Drive, Saskatoon, SK S7N 3R3 8Lendon Seed Lab, 147 Hodsman Road, Regina, SK S4N 5W5
ABSTRACT: Results of commercial plate tests for seed-borne pathogens of 1111 field pea, 1066 lentil and 152 chickpea samples are summarized. The percentage of pathogen-free samples in most crops and for most pathogens continues to be very high. Ascochyta blight levels continue to be elevated in field peas and chickpeas.
METHODS: Commercial agar plate tests for pathogens in seed samples of field pea, lentil, and chickpea across Saskatchewan were conducted and results summarized by four companies during the fall of 2020 through early spring of 2021. All samples were assumed to be from the 2020 crop year. Seeds were assessed for the presence of the following pathogens:
Ascochyta (Mycosphaerella/Peyronellaea) pinodes, Didymella (Ascochyta) pisi and Phoma medicaginis var. pinodella (Ascochyta pinodella) complex, which causes ascochyta blight of field pea;
Didymella (Ascochyta) lentis, causal agent of ascochyta blight of lentil;
Didymella (Ascochyta) rabiei, causal agent of ascochyta blight of chickpea;
Colletotrichum lentis, causal agent of anthracnose of lentil;
Botrytis spp. which causes botrytis stem and pod rot (gray mould) of field pea, lentil and chickpea;
Sclerotinia sclerotiorum, causal agent of sclerotinia stem and pod rot (white mould) of field pea, lentil and chickpea.
A total of 2329 samples were tested for ascochyta blight pathogens, 2301 samples were tested for Botrytis spp., 1081 samples for C. lentis and 2301 samples for S. sclerotiorum. The mean incidence (%) of seed infection in diseased samples and the percentage of pathogen-free samples were calculated for each crop district and a provincial average was determined.
RESULTS AND COMMENTS: The 2020 growing season was characterized by moisture level concerns over most of the province. Hot temperatures and drying winds were prevalent throughout most of the province. This resulted in an early harvest with above average quality.
Lentil production in Saskatchewan increased from 3.4 million acres in 2019 to 3.8 million acres in 2020. The 5-year average is 3.9 million acres. The average 2020 lentil yield was 1451 lbs/acre, down from the average of 1570 lbs/acre in 2019, but above the 5-year average (2016-2020) of 1360 lbs/acre (Government of Saskatchewan Citation2020).
Field pea acreage in 2020 was 2.3 million acres, which is considerably above the 5-year average of 2.1 million acres. The average yield in 2020 was reported as 39.5 bu/acre, up slightly from the 37.0 bu/acre reported in 2019. The 5-year average yield (2016-2020) was reported to be 35.3 bu/acre (Government of Saskatchewan Citation2020).
Chickpea seeded acres fell from 331800 acres in 2019 to 252300 acres in 2020. This remains above the 5-year average of 223700 acres. The decline in seeded acres was likely related to a world-wide surplus of chickpeas and resulting lower prices. Yields in 2020 were reported at 1558 lbs/acre, which was up slightly from the 1522 lbs/acre reported in 2019 and in line with the 5-year average of 1557 lbs/acre (Government of Saskatchewan Citation2020).
A total of 1111 field pea, 1066 lentil and 152 chickpea samples were processed during the period covered by this report. This represents a decrease of 49% in chickpea samples compared to 2019 (Olson et al. Citation2021). Chickpea total sample numbers remained relatively low compared to lentil and pea samples. Based on the location of chickpea production, more samples would be expected to originate from southern and south-western crop districts but seed testing labs from these areas are under-represented in this report. Lentil samples decreased by 15% and field peas by 5% compared to 2019. The high number of samples received by some seed labs was indicative of producers’ continued concerns related to disease.
A 5-year summary of pathogen incidence in pulse seed samples tested from 2016-2020 (Olsen et al. 2021, 2020, 2019a, 2019b) is presented in and individual crop test results for 2020 in , and .
Table 1. Summary of pathogens in pulse seed samples tested from 2016 to 2020 in Saskatchewan.
Table 2. Number of field pea samples tested from September 2020 to May 2021 and levels of infection with Ascochyta spp., Botrytis spp. and Sclerotinia sclerotiorum for each Saskatchewan Crop District.
Table 3. Number of lentil seed samples tested from September 2020 to May 2021 and levels of infection with Ascochyta lentis, Colletotrichum lentis, Botrytis spp., and Sclerotinia sclerotiorum for each Saskatchewan Crop District.
Table 4. Number of chickpea seed samples tested from September 2020 to May 2021 and levels of infection with Ascochyta rabiei, Botrytis spp., and Sclerotinia sclerotiorum for each Saskatchewan Crop District.
Pea – The percentage of Ascochyta-free samples was 33.9%, down from the 36.3% reported in 2019 (). The mean percent infection was 2.8% (). The percentage of Botrytis-free samples was 93.6%, unchanged from the previous year (). A mean percent infection level of 0.8% is only a slight decrease from the 1.0% reported in 2019. The percentage of S. sclerotiorum-free samples was 99.1%, in line with the previous year. The provincial mean percent infection was 0.6%. This is the same as reported in 2019 ().
Lentil – The percentage of A. lentis-free samples was 97.9% (), up from the previous year of 95.5% (). The provincial mean percent infection was 0.5% (), down from the 0.8% reported for 2019 (). The percentage of C. lentis-free samples was 85.3% with a mean percent infection of 1.2% (), compared to 89.2% and 0.9% in 2019 (). The frequency of Botrytis-free samples was 95.9% (), up slightly from 93.2% in 2019 (). The mean percent infection was 0.8% (), a slight increase from 1.0% in 2019 (). The frequency of S. sclerotiorum-free samples remained high at 97.8% (), compared to 96.0% in 2019 (). The mean percent infection rate was 0.5% (), unchanged from 0.5% in 2019 ().
Chickpea – Chickpea production in Saskatchewan is largely centered in the southern and south-western regions of the province where small numbers of samples were evaluated by the four contributing companies. The overall percentage of Ascochyta rabiei-free samples was 44.1% (), down from 51.3% in 2019 (). The mean infection percentage was 3.2% (), unchanged from 2019 (). The frequency of Botrytis-free samples was 87.1% (), up from 86.6% in 2019 (). The mean infection rate was 1.1% (), unchanged from the 1.1% reported in 2019 (). The frequency of S. sclerotiorum-free samples was 99.3% (), up from the 89.8% reported in 2019 (). The mean percent infection was 0.3% ().
ACKNOWLEDGEMENTS: We would like to acknowledge the cooperation of 20/20 Seed Labs Inc., Lendon Seed Lab, Prairie Diagnostic Seed Lab, and Discovery Seed Labs Ltd. for providing seed testing results. We also wish to recognize the financial support of the Saskatchewan Pulse Growers.
REFERENCES
- Government of Saskatchewan. 2020. Specialty Crop Report. Regina (SK) [accessed 2022 Feb 18]. https://pubsaskdev.blob.core.windows.net/pubsask-prod/125055/Specialty%252BCrop%252BReport%252B2020.pdf
- Olson B, Banniza S, Ernst B, Fatima S, Junek S, Phelps S, Prasad T, Risula D, Wenaus J. 2021. Seed-borne pathogens of pulse crops in Saskatchewan in 2019. Can Plant Dis Surv. 101:144–147. In, Can J Plant Pathol. Vol. 43:sup1.
- Olson B, Banniza S, Blois T, Ernst B, Junek S, Phelps S, Prasad T, Ziesman B. 2020. Seed-borne pathogens of pulse crops in Saskatchewan in 2018. Can Plant Dis Surv. 100:130–133. In, Can J Plant Pathol. Vol. 42:sup 1.
- Olson B, Banniza S, Blois T, Ernst B, Junek S, Phelps S, Prasad T, Ziesman B. 2019a. Seed-borne pathogens of pulse crops in Saskatchewan in 2017. Can Plant Dis Surv. 99:150–154. In, Can J Plant Pathol. Vol. 41:sup 1.
- Olson B, Banniza S, Blois T, Ernst B, Junek S, Phelps S, Ziesman B. 2019b. Seed-borne pathogens of pulse crops in Saskatchewan in 2016. Can Plant Dis Surv. 99:145–149. In, Can J Plant Pathol. Vol. 41:sup 1.
2021 SURVEY OF SOYBEAN DISEASES IN SASKATCHEWAN
CROP: Soybean LOCATION: Saskatchewan NAMES AND AGENCIES: A. AKHAVAN1, C. PERU1, J. KWASNICKI2, S. MILLER1 & S. ROBERTS1
1Saskatchewan Ministry of Agriculture, 3085 Albert St., Regina, SK S4S 0B1 Telephone: (306) 787-4671; Facsimile: (306) 787-0428; E-mail: [email protected] 2Saskatchewan Association of Rural Municipalities, 2301 Windsor Park Rd., Regina, SK S4V 3A4
ABSTRACT: A total of 15 soybean crops were surveyed in Saskatchewan in 2021. Brown spot and bacterial blight were the most prevalent diseases in 2021 soybean crops with 100% and 93.3% of survey crops showing symptoms, respectively. Downy mildew was present in 13.3% of crops with an overall average incidence of 11%. Frogeye leaf spot symptoms were observed in 6.7% of surveyed crops with an overall average incidence of 16%. Average severity in fields with symptoms present was 1.2, 1.9 and 0.1 for brown spot, bacterial blight and downy mildew, respectively. White mould, anthracnose, soybean rust and charcoal rust were not present in any of the 15 surveyed soybean crops in 2021.
INTRODUCTION AND METHODOLOGY: The 15 Saskatchewan soybean crops were surveyed between August 11th and 25th while the crops were in growth stages R4 (full pod) to R7 (beginning maturity). Fields were located across the major soybean production area in Saskatchewan. Disease assessments were made by examining 10 plants from each of five sites along a “W” pattern in each field (a total of 50 plants per field). Each of the 50 plants was assessed for the presence of the following diseases: brown spot (Septoria glycines), bacterial blight (Pseudomonas savastanoi pv. glycinea), downy mildew (Peronospora manshurica), white mould (Sclerotinia sclerotiorum), pod and stem blight (Diaporthe sojae), anthracnose (Colletotrichum spp.), frogeye leaf spot (Cercospora sojina) and phytophthora root rot (Phytophthora spp.). Disease severity was also assessed for brown spot, bacterial blight and downy mildew using a 0 to 5 rating scale, where 0 = no disease present and 5 = severe symptoms with defoliation. The prevalence of iron chlorosis, sudden death syndrome (Fusarium virguliforme), soybean rust (Phakopsora meibomiae and P. pachyrhizi), charcoal rot (Macrophomina phaseolina), northern stem canker (Diaporthe caulivora) and soybean cyst nematode (Heterodera glycines) was estimated by recording their presence or absence in the field. All disease assessments were made based on visual symptoms in the field.
RESULTS AND COMMENTS: In 2021, approximately 34,400 hectares (85,000 acres) were seeded to soybean in Saskatchewan. This is lower than the 51,300 hectares (126,700 acres) seeded in 2020 (Statistics Canada Citation2022). As of mid-January 2022, 34,400 hectares (85,000 acres) of soybean were harvested in Saskatchewan (Statistics Canada Citation2022). The Saskatchewan Crop Report (Saskatchewan Ministry of Agriculture Citation2021) estimated that 98% of the Saskatchewan soybean crop had been harvested by October 4, 2021.
The most prevalent disease in Saskatchewan was bacterial blight which was present in 14 of the 15 fields surveyed with an average incidence of 85.6% when averaged across infected fields (). The average disease severity of infected plants in fields with symptoms present was 2.1 on a 0 to 5 rating scale.
Table 1. Prevalence, incidence and severity of bacterial blight, brown spot and downy mildew in Saskatchewan soybean fields in 2021.
Symptoms consistent with brown spot were observed in all the surveyed crops with an average incidence of 79.5% and the average severity of infected plants in fields with symptoms present was 1.5.
Frogeye leaf spot symptoms were observed in one of the surveyed crops with an incidence of 16% in that field.
Downy mildew was present in two fields with an average incidence of 11% in infected fields.
Symptoms consistent with phytophthora root rot were found outside of the survey area in three fields and were submitted to Dr. Yong Min Kim for further analysis.
Symptoms suggesting sudden death syndrome were observed in one crop, but was not confirmed at the Saskatchewan Crop Protection Lab.
Iron chlorosis was also observed in one of the fields surveyed. No additional diseases including white mould, anthracnose, soybean rust, northern stem canker and charcoal rust were observed.
REFERENCES
- Statistics Canada. 2022. Estimated areas, yield, production, average farm price and total farm value of principal field crops, in metric units, annual, CANSIM database: Table 32-10-0359-01. [accessed 2022 Jan 18] https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210035901
- Saskatchewan Ministry of Agriculture. 2021. Saskatchewan crop report for the period September 28 to October 4, 2021. [accessed 2022 Jan 18]. https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/market-and-trade-statistics/crops-statistics/crop-report
DISEASES OF DRY BEAN IN MANITOBA IN 2021
CROP: Dry bean LOCATION: Manitoba NAMES AND AGENCIES: Y.M. KIM1, E. SARI2, A. HOU2, M.J. THOMPSON1 & W.C. PENNER2
1Agriculture and Agri-Food Canada (AAFC), Brandon Research and Development Centre, 2701 Grand Valley Rd., Brandon, MB R7A 5Y3 Telephone (204) 578-6691; Facsimile (204) 578-6524 E-mail: [email protected] 2AAFC, Morden Research and Development Centre, Unit 101, Route 100, Morden, MB R6M 1Y5
ABSTRACT: A total of 40 bean crops in Manitoba were surveyed for root and foliar diseases in 2021. Fusarium root rot was the most prevalent root disease and common bacterial blight the most widespread foliar disease throughout the province. Halo blight and white mould were also observed. In 2021, rhizoctonia root rot, rust and anthracnose were not detected in any of the surveyed dry bean crops.
METHODS: Crops of dry bean in Manitoba were surveyed for root and foliar diseases at 40 different locations in 2021. The survey for root diseases was conducted from mid- to late July when most plants were at the early flowering to beginning seed stage. The majority of the crops surveyed were selected at random from regions in southern Manitoba where most of the dry bean crops are grown, with 10% of the crops located outside of the traditional bean growing regions. During the root disease survey, the severity of halo blight (Pseudomonas syringae pv. phaseolicola) also was assessed. When the plants were starting to mature during mid-August, the foliar disease survey was carried out on the same fields assessed for root rot.
For the root diseases, at least 10 plants were sampled at each of three random sites in each crop. Root diseases were rated on a scale of 0 (no disease) to 9 (death of plant). Fifteen symptomatic roots were collected from each of the 40 crops for fungal isolation and identification. Identification of Fusarium species involved visual assessment, microscopic examination and morphological characterization using the criteria of Leslie and Summerell (Citation2006). Fifteen roots from each of the 40 crops surveyed were frozen for future PCR analysis of root rot pathogens. Foliar diseases were identified by their symptoms. Common bacterial blight (CBB) (Xanthomonas axonopodis pv. phaseoli) was assessed based on the percent incidence of leaf infection and on a severity scale of 0 (no disease) to 5 (50-100% of the leaf area covered by lesions). Anthracnose (Colletotrichum lindemuthianum), rust (Uromyces appendiculatus), white mould (Sclerotinia sclerotiorum) and halo blight (Pseudomonas syringae pv. phaseolicola) severity were assessed as percentages of infected plant tissue.
RESULTS AND COMMENTS: In early May, cool and dry soils slowed seeding and crop emergence with a provincial seeding progress of 18%, similar to the 4-year average (Manitoba Agriculture and Resource Development [MARD] Citation2021a). In many areas, conserving existing soil moisture remained a top priority. Rain over the first weekend of May provided a little seedbed moisture but dissipated quickly. By June 1st, seeding of the dry bean crop was 95% completed (MARD Citation2021b). Frost occurred in all areas of agro-Manitoba throughout several nights in the last week of May. However, damage from frost was relatively limited, considering the severity and duration of cold temperatures.
Six percent of harvest was completed by September 14 compared to the 5-year (2016-2020) average of 19% completed by the same date (MARD Citation2021c). In 2019, harvest was slowed down due to widespread rainfall with 69% of the crop harvested by October 29th (Manitoba Agriculture Citation2019a). In 2020, 99% of the crop was harvested by October 6 (MARD Citation2020a) compared with 90% of the crop harvested by October 5, 2021 (MARD Citation2021d).
Harvest of early and late maturing types of edible beans was completed by early October. Reported yields ranged from 1,000 to 1,200 lbs/acre in moisture-limited areas and up to 1,600 lbs/acre in areas with better moisture conditions. In 2020, average yields ranged from 1800 to 2200 lbs/acre (MARD Citation2020b) compared with the 2019 yields of 1000 to 1500 lbs/acre (Manitoba Agriculture Citation2019b).
Fusarium root rot was observed in all of the 40 dry bean crops surveyed (), with severity ratings ranging from 3.0 to 5.9, and a mean of 4.5. It has remained the most prevalent root disease of dry bean for a number of years (Conner et al. Citation2011; Henriquez et al. Citation2013; Kim et al. Citation2020, Citation2021). A number of Fusarium spp. including F. redolens, F. oxysporum, F. acuminatum and F. avenaceum were isolated from symptomatic root tissue. Rhizoctonia root rot (Rhizoctonia solani) and pythium root rot (Pythium spp.) were not detected in any of the crops surveyed based on microscopic examination and morphological characterization. Thirty-three crops (83%) had average root rot severity ratings >4 (i.e., symptoms were present on 50% of the root system and plants were stunted) and this would have had a detrimental effect on yield. These results were higher than those of 2019 (35%) and 2020 (43%). There were more surveyed crops with average root rot ratings >4 in 2018 (68%) and 2017 (63%). However, in 2016, a much wetter year, 93% of bean crops had severity ratings >4, which represents the highest percentage of bean crops surveyed with yield-robbing root rot severity ratings over the past six years. In 2021, halo blight was assessed in the 40 crops surveyed and was observed in two (5%) crops with an average of 0.2% leaf area infected.
Table 1. Prevalence and severity of root diseases and halo blight in 40 crops of dry bean in Manitoba in mid- to late July in 2021.
Two foliar diseases were observed during the survey in August (). Common bacterial blight symptoms were observed in 98% (39/40) of crops. The incidence of CBB leaf infection ranged from 0.3 to 23.3% with a mean of 8.6%, while severity ranged from 0.3 to 2.7, with a mean of 1.3. Anthracnose was not detected from 2014 to 2021, unlike many years prior to this period. Rust was not observed in any of the crops surveyed in 2021. White mould symptoms were detected in 2.5% (1/40) of the crops with 0.3% of tissue infection in 2021. Seasonal precipitation in many of the bean growing regions of Manitoba was above normal in 2016, but below normal in 2017, 2018 and 2019 which would have contributed to the reduced risk of yield losses due to white mould in the latter three years. In 2021, most of the surveyed fields had no white mould disease impact at all due to below average growing season precipitation.
Table 2. Prevalence and severity of foliar diseases in 40 crops of dry bean in Manitoba in August in 2021.
REFERENCES
- Conner RL, McLaren DL, Penner WC, Kerley TJ. 2011. Diseases of field bean in Manitoba in 2010. Can Plant Dis Surv. 91:107–108.
- Henriquez MA, McLaren DL, Conner RL, Penner WC, Kerley TJ. 2013. Diseases of field bean in Manitoba in 2012. Can Plant Dis Surv. 93:143–144.
- Kim YM, McLaren DM, Conner RL, Penner WC, Kerley TJ. 2020. Diseases of dry bean in Manitoba in 2019. Can Plant Dis Surv. 100:153–154. In, Can J Plant Pathol. 42:sup1.
- Kim YM, McLaren DM, Hou A, Vachon N, Penner WC. 2021. Diseases of dry bean in Manitoba in 2020. Can Plant Dis Surv. 101:150–151. In, Can J Plant Pathol. 43:sup1.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell.
- Manitoba Agriculture. 2019a Oct 29. Crop report #27. [accessed 2021 Nov 30]https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- Manitoba Agriculture. 2019b Nov 12. Crop report summary. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- Manitoba Agriculture and Resource Development (MARD). 2020a Oct 6. Crop report #23. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2020b Oct 20. Crop report summary. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021a May 4. Crop Report #2. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021b June 1. Crop Report #6. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021c Sep 14. Crop Report #21. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021d Oct 5. Crop Report #24. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
SURVEY OF CANOLA DISEASES IN MANITOBA IN 2021
CROP: Canola LOCATION: Manitoba NAMES AND AGENCIES: Y.M. KIM1, D. KAMINSKI2, J. GRAHAM3, M. PRADHAN4, E. BARGEN2, A. BRACKENREED5, T. BUSS2, N. CLOUSON2, J. CORNELSEN5, T. CUMMER2, A. FAROOQ2, J. FREY2, D. FROESE2, N. ORT5, T. HENDERSON1, M.J. THOMPSON1, L. KASKIW2, D. LANGE2 & R. PICARD2
1Agriculture and Agri-Food Canada, Brandon Research and Development Centre, 2701 Grand Valley Rd., Brandon, MB R7A 5Y3 Telephone: (204) 578-6691; Facsimile: (204) 578-6524; E-mail: [email protected] 2Manitoba Agriculture and Resource Development, Carman, MB R0G 0J0 381 Ellesmere Ave, Winnipeg, MB R2M 0G5 4Manitoba Agriculture and Resource Development, Crop Diagnostic Centre, 201-545 University Crescent, Winnipeg, MB R3T 5S6 5Canola Council of Canada, 400-167 Lombard Ave, Winnipeg, MB R3B 0T6
ABSTRACT: A total of 135 canola crops were surveyed in Manitoba for the prevalence and incidence or severity of sclerotinia stem rot, blackleg, alternaria pod spot, aster yellows, verticillium stripe, foot rot and clubroot. Blackleg (basal canker) was the most prevalent disease throughout the province. No canola plants collected from the 135 surveyed canola crops were confirmed to have clubroot. Verticillium stripe was identified in four canola samples submitted to the Manitoba Crop Diagnostic Centre.
METHODS: A total of 135 canola crops were surveyed in the Southwest (62), Northwest (23), Eastern/Interlake (15) and Central (35) regions of Manitoba between July 22 and September 8, 2021. All crops were Brassica napus and the majority were surveyed before swathing while plants were between growth stages 5.1 and 5.5 (Harper and Berkenkamp Citation1975). In each canola crop, 100 plants were selected in a regular pattern starting at a corner of the field or at a convenient access point. The edges of the fields were avoided. Twenty plants were removed from each of five points of a “W” pattern in the field. Points of the “W” were at least 20 paces apart. All plants were pulled up, removed from the field and examined for the presence of diseases. For soil collection from 51 fields, samples were obtained from each of the five points of the “W”, or if the field entrance was identifiable, they were collected at five points near the entrance.
Canola crops were assessed for the prevalence (percent crops infested) and incidence (percent plants infected per crop) of sclerotinia stem rot (Sclerotinia sclerotiorum), aster yellows (Candidatus Phytoplasma asteris), foot rot (Fusarium spp. and Rhizoctonia sp.), blackleg (Leptosphaeria maculans), verticillium stripe (Verticillium longisporum) and clubroot (Plasmodiophora brassicae). For sclerotinia stem rot, each plant was also scored based on the possible impact of infection on yield using a disease severity scale of 0 (no symptoms) to 5 (main stem lesion with potential effects on seed formation and filling of entire plant) (Kutcher and Wolf Citation2006). Blackleg lesions that occurred on the upper portions of the stem were assessed separately from basal stem cankers. Stem lesions were recorded as present or absent. Basal stem cankers were scored using a disease severity scale of 0 to 5, based on the area of diseased tissue in a stem cross-section where 0 = no diseased tissue visible and 5 = diseased tissue occupying 100% of the cross-section and plant dead (WCC/RRC Citation2009). If present, clubroot symptoms were rated using a scale of 0 to 3, where 0 = no galling and 3 = severe galling (Kuginuki et al. Citation1999). The prevalence and percent severity of alternaria pod spot (Alternaria spp.) (Conn et al. Citation1990) were also determined. When diseases were observed in the crop, but not in the sample of 100 plants, they were recorded as “trace” for incidence and counted as 0.1%. Mean disease incidence or severity values were calculated for each region. In addition to the visual assessment of diseases, soil samples were collected from approximately 51 of the surveyed canola fields in Manitoba to test for the presence of the clubroot pathogen by DNA analysis (Cao et al. Citation2007). Canola samples symptomatic for verticillium stripe were submitted to the Manitoba Agriculture Crop Diagnostic Centre for confirmation of the disease.
RESULTS: A number of diseases were present in each of the four regions of Manitoba. However, no clubroot symptoms were observed in the 135 Manitoba canola crops surveyed in 2021. Information on the monitoring and occurrence of clubroot in Manitoba in previous years is provided by Froese et al. (Citation2019), Kubinec et al. (Citation2014) and Derksen et al. (Citation2013). A map of clubroot distribution in Manitoba (2009-2020) is available online (Manitoba Agriculture and Resource Development Citation2021a). The map will be updated for any soil samples positive for clubroot DNA once analyses are completed.
Sclerotinia stem rot was prevalent in 1.5% of the crops surveyed, ranging from a high of 6.7% in the Eastern/Interlake region to 0% in the Central and Southwest regions with a provincial mean of 1.5% (). Mean disease incidence averaged across all crops was 0.02% and ranged from 0.13% in the Eastern/Interlake region to 0% in the Central and Southwest regions. For infested crops only, mean disease incidence was 1.5%. Throughout the province, mean severity of sclerotinia stem rot was 0.02 and ranged from 0 in the Central and Southwest regions to 0.13 in the Eastern/Interlake region.
Table 1. Mean prevalence, incidence and severity of sclerotinia stem rot and blackleg in Manitoba in 2021.
Aster yellows was observed in 10% of canola crops in Manitoba with an average disease incidence of 3.4% in these crops (). The prevalence of this disease was substantially less than in 2012 (95%) when record high levels of aster yellows were observed in all regions of Manitoba. Contributing factors to the high level of aster yellows in 2012 included drought in the midwestern United States, the early arrival of aster leafhoppers from the southern U.S., and the higher-than-normal percentage of infected individuals (10-15%) in the leafhopper population. The reduced risk of aster yellows in recent years has been due to lower numbers of leafhoppers and a percentage of infected aster leafhoppers that is normally 2-3% in Manitoba. In 2021, there was little concern over aster yellows in Manitoba (Gavloski 2017, Citation2021; Manitoba Agriculture and Resource Development Citation2021b).
Table 2. Mean prevalence and incidence or severity of alternaria pod spot, aster yellows, verticillium stripe and foot rot in Manitoba in 2021.
Blackleg basal cankers occurred in 84% of the crops surveyed in 2021 (), with prevalence ranging from 100% in the Eastern/Interlake region to 74% in the Northwest region. The mean incidence of basal cankers averaged across all crops was 10%, while the mean incidence in infested crops was 12%. The severity of blackleg basal cankers was similar in recent years with mean ratings of approximately 2 or less. A value of “2” indicates that 26-50% of the basal stem cross-section area was diseased. The mean prevalence of blackleg stem lesions in 2021 was 50%. In previous years, 54% and 47% and 53% of crops had stem lesions in 2018, 2019 and 2020 respectively (McLaren et al. Citation2019, Citation2020, Citation2021). The average incidence of blackleg stem lesions was 7% in infested crops and 4% in all crops.
The mean prevalence of alternaria pod spot in 2021 was 7% and ranged from 20% in the Eastern/Interlake region to 0% in the Central region (). The mean severity of alternaria pod spot was 1.1% in infested crops.
Verticillium stripe was observed in 30% of canola crops surveyed in Manitoba, with a mean incidence of 15% in diseased fields (). Four survey samples submitted to the Manitoba Crop Diagnostic Centre were confirmed positive for verticillium stripe. For several years, some surveyors may have incorrectly reported the stem symptoms of verticillium stripe as fusarium wilt.
Foot rot occurred in 1% of canola crops surveyed with a provincial mean disease incidence of <1%. Foot rot was observed in the Southwest (1.6%) region only (). White rust (Albugo candida) has not been confirmed in any crop of B. napus since 2011 (McLaren et al. Citation2012).
The distribution of disease incidence (sclerotinia, blackleg, aster yellows, verticillium stripe and foot rot) and severity (alternaria pod spot) classes in 135 crops of Brassica napus in Manitoba in 2021 is presented in .
Table 3. Distribution of incidence (sclerotinia, blackleg, aster yellows, verticillium stripe and foot rot) and severity (alternaria pod spot) classes in 135 crops of Brassica napus in Manitoba in 2021.
ACKNOWLEDGEMENTS: We thank Manitoba canola producers for their continued support of this survey work and both the Manitoba Canola Growers Association and the Canola Council of Canada for their financial assistance.
REFERENCES
- Cao T, Tewari J, Strelkov SE. 2007. Molecular detection of Plasmodiophora brassicae, causal agent of clubroot of crucifers in plant and soil. Plant Dis. 91(1):80–87.
- Conn KL, Tewari JP, Awasthi RP. 1990. A disease assessment key for alternaria blackspot in rapeseed and mustard. Can Plant Dis Surv. 70:19–22.
- Derksen H, Kubinec AM, McLaren DL. 2013. Detection of Plasmodiophora brassicae in Manitoba, 2017. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/insects/pubs/insect-summary-2017.pdf
- Gavloski J. 2021. Summary of insects on crops in Manitoba in 2021. Manitoba Agriculture. November 2021. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/insects/pubs/insect%20summary-2021.pdf
- Harper FR, Berkenkamp B. 1975. Revised growth-stage key for Brassica campestris and B. napus. Can J Plant Sci. 55(2):657–658.
- Kubinec AM, Derksen H, Desjardins M, McLaren DL. 2014. Monitoring and occurrence of clubroot in Manitoba in 2013. Can Plant Dis Surv. 94:184–185.
- Kuginuki Y, Yoshikawa H, Hirai M. 1999. Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of chinese cabbage (Brassica rapa L. ssp. pekinensis). Eur J Plant Pathol. 105(4):327–332.
- KutcherHR, WolfTM.2006. Low-drift fungicide application technology for sclerotinia stem rot control in canola. Crop Prot. 25(7):640–646.
- Manitoba Agriculture and Resource Development. 2021a. Clubroot distribution in Manitoba: cumulative testing 2009-2020. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/plant-diseases/clubroot-distribution-in-manitoba.html
- Manitoba Agriculture and Resource Development. 2021b. Aster leafhoppers and aster yellows. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/insects/aster-leafhoppers-yellows.html
- McLaren DL, Platford RG, Kubinec A, Kutcher HR, Bisht V, Derksen H, Kristjanson I, Phillips K, Henderson TL, Hausermann DJ, et al. 2012. Survey of canola diseases in Manitoba in 2011. Can Plant Dis Surv. 92:130–132.
- McLaren DL, Derksen H, Graham J, Ariyaratne I, Arnott S, Bargen E, Berthelette C, Bonser G, Brackenreed A, Buss T, et al. 2019. Survey of canola diseases in Manitoba in 2018. Can Plant Dis Surv. 99:175–178. In, Can J Plant Pathol. 41:sup1.
- McLaren DL, Kaminski D, Graham J, Bargen E, Buss T, Clouson N, Cummer T, Farooq A, Forbes B, Froese D, et al. 2020. Survey of canola diseases in Manitoba in 2019. Can Plant Dis Surv. 100:149–152. In, Can J Plant Pathol. 42:sup1.
- McLaren DL, Kaminski D, Graham J, Pradhan M, Bargen E, Brackenreed A, Buss T, Clouson N, Cornelsen J, Cummer T, et al. 2021. Survey of canola diseases in Manitoba in 2020. Can Plant Dis Surv. 101:152–155. In, Can J Plant Pathol. 43:sup1.
- Western Canada Canola/Rapeseed Recommending Committee (WCC/RCC). 2009. Procedures for evaluation and recommendation for registration of canola/rapeseed candidate cultivars in western Canada. Appendix B. Disease testing protocols, p. 11.
FIELD PEA DISEASES IN MANITOBA IN 2021
CROP: Field pea LOCATION: Manitoba NAMES AND AGENCIES: Y.M. KIM1, T.L. HENDERSON1, M.J. THOMPSON1, S.F. HWANG2, K.F. CHANG3, S. CHATTERTON4, C. TKACHUK5, L. SCHMIDT5, N. CLOUSON6, D. LANGE7 & A. FAROOQ8
1Agriculture and Agri-Food Canada (AAFC), Brandon Research and Development Centre, 2701 Grand Valley Road, Brandon, MB R7A 5Y3 Telephone: (204) 578-6691; Facsimile: (204) 578-6524; E-mail: [email protected] 2Department of Agricultural, Food and Nutritional Science, University of Alberta, 410 Agriculture/Forestry Centre, Edmonton, AB T6G 2P5 3Alberta Agriculture and Forestry, Crop Diversification Centre North, 17507 Fort Road NW, Edmonton, AB T5Y 6H3 4AAFC, Lethbridge Research and Development Centre, P.O. Box 3000, Lethbridge, AB T1J 4B1 5Manitoba Pulse and Soybean Growers, P.O. Box 1760, Carman, MB R0G 0J0 6Manitoba Agriculture and Resource Development, P.O. Box 370, Swan River, MB R0L 1Z0 7Manitoba Agriculture and Resource Development, Altona, MB R0G 0B0 8Manitoba Agriculture and Resource Development, Hamiota, MB R0M 0T0
ABSTRACT: A total of 46 and 41 pea crops were surveyed in Manitoba for root and foliar diseases, respectively. Fusarium root rot was the most prevalent root disease and mycosphaerella blight the most widespread foliar disease throughout the province. Diseases that were less frequently observed included downy mildew and bacterial blight. White mould, rust, powdery mildew and anthracnose were not observed in any of the crops surveyed in 2021. Root samples collected from a total of 142 pea fields in 2016 (30), 2017 (30), 2018 (40) and 2019 (42) indicated that Aphanomyces euteiches was present in 77%, 47%, 56% and 83% of these fields, respectively. The 2020 (46) and 2021 (46) PCR results for A. euteiches from 92 crops were not available at the time of this report.
METHODS: Field pea crops were surveyed for root and foliar diseases at 46 and 41 different locations, respectively, in Manitoba. The crops surveyed were randomly chosen from regions in south-central and southwest Manitoba, where field pea is commonly grown. For the root disease survey, three of the 46 crops were from the Swan River area, where the area seeded to field pea increased from 2,925 ha in 2016 to 4,840 ha in 2018. In Manitoba, field pea acreage has increased in recent years from approximately 22,000 and 26,000 ha in 2014 and 2015, respectively (Manitoba Pulse and Soybean Growers Citation2015). In 2016, the area sown to field pea more than doubled to 66,000 ha based on an increased demand for peas (Manitoba Agriculture and Agrifood Statistics Citation2017). However, in 2017, the seeded area dropped to 26,200 ha mainly as a result of wet, unfavourable growing conditions for peas during the 2016 field season, which deterred many growers from seeding peas in the following year (Manitoba Agriculture Citation2017). The area seeded to field pea in Manitoba increased for four consecutive years to 34,000 ha, 45,620 ha and 63,076 ha in 2018, 2019 and 2020, respectively (Manitoba Agricultural Services Corporation [MASC] Citation2018, Citation2019, Citation2020) with 96,090 ha seeded in 2021 (Manitoba Agriculture and Resource Development [MARD] Citation2021a).
The survey of root diseases was conducted during late June to early July when most plants were at the late vegetative to mid-reproductive stage. At least 10 plants were sampled from each of three random sites in each crop surveyed. Root diseases were rated on a scale of 0 (no disease) to 9 (death of plant) (Xue Citation2000). To confirm the visual disease identification, 15 symptomatic roots were collected from each crop for fungal isolation and identification. Identification of Fusarium species involved visual assessment, microscopic examination and morphological characterization using the criteria of Leslie and Summerell (Citation2006). Fifteen roots from each of the 46 pea crops were frozen for future PCR analysis of the root rot pathogens. Soil samples from each of 46 fields were taken in late June and early July of 2021 during the root rot survey and will be assessed for Aphanomyces euteiches using PCR assays (Gangneux et al. Citation2014).
Foliar diseases were assessed from mid- to late July when most plants were at the intermediate to round pod stage. A minimum of 30 plants (10 plants from each of three sites) was assessed in each of the 41 fields. Foliar diseases were identified based on their symptoms. The severity of mycosphaerella blight, white mould and anthracnose was estimated using a scale of 0 (no disease) to 9 (whole plant severely diseased). Powdery mildew, downy mildew, rust and bacterial blight were rated as the percentage of foliar area diseased.
RESULTS AND COMMENTS: A lack of overwinter snow accumulation and early snowmelt resulted in dry soils in March. Cool and dry soils slowed the beginning of seeding operations to late April in many areas of the province where field peas are produced (MARD Citation2021b). Due to lack of rainfall, germination and timely emergence were a concern in many crops. However, seeding of field peas was completed in most regions in Manitoba (MARD Citation2021c) with 95% complete in the northwest region by May 18. By the week of August 2nd, some pea crops were harvested in areas of lighter soil zones (MARD Citation2021d). Field pea harvest was completed by September 6 with yields ranging from 25 to 65 bu/ac throughout the province (MARD Citation2021e).
Two diseases were identified based on laboratory assessment of the roots collected from 46 pea crops (). Fusarium root rot was the most prevalent as in previous years (McLaren et al. Citation2020, Citation2021) with a number of Fusarium spp. including F. acuminatum, F. redolens and F. avenaceum isolated from symptomatic root tissue in 2021. Of all crops surveyed, root rot severity ratings ranged from 1.6 to 6.2 with a mean of 3.1. Rhizoctonia root rot (Rhizoctonia solani) was not detected in any of the crops sampled. Six (13%) pea crops had average root rot severity ratings >4 (i.e., symptoms were present on 50% of the root system) and this would have had a detrimental effect on crop yield. Fusarium oxysporum, an efficient root colonizer known to cause wilt of pea, was detected in 14 of the 46 crops sampled for fungal isolation and identification. Assessment of frozen samples for root pathogens using PCR is planned for the near future.
Table 1. Prevalence and severity of root diseases in 46 crops of field pea in Manitoba in 2021.
Aphanomyces euteiches was detected in root samples collected from 77% (23/30), 47% (14/30) and 56% (25/40) of pea fields in 2016, 2017 and 2018, respectively (Gangneux et al Citation2014). Aphanomyces root rot is favoured by wet, poorly drained soils and is most severe under flooded soil conditions. Seasonal precipitation in many of the pea growing regions of Manitoba in 2016 was above normal, which would have contributed to the increased incidence of aphanomyces root rot. Drier conditions prevailed in 2017 and 2018. In 2019, A. euteiches was confirmed in 83% (35/42) of pea fields. Assessment of the 2020 and 2021 samples for A. euteiches is ongoing and results are pending at this time.
Three foliar diseases were observed (). Mycosphaerella blight (Mycosphaerella pinodes) was the most prevalent, as in previous years (McLaren et al. Citation2020, Citation2021), and was present in all the crops surveyed. Disease severity ranged from 2.2 to 6.4 with a mean of 3.5. Downy mildew (Peronospora viciae) was detected in 12% (5/41) of the crops surveyed and the percentage of leaf area infected ranged from <0.1% to 2%. Bacterial blight (Pseudomonas syringae pv. pisi) was observed in 7% of the crops (3/41) surveyed with the percentage of foliar area infected ranging from <0.1% to 2%.
Table 2. Prevalence and severity of foliar diseases in 41 crops of field pea in Manitoba in 2021.
Powdery mildew (Erysiphe pisi) was not observed in any of the surveyed crops. All newly registered pea cultivars are required to have resistance to powdery mildew, so the absence of this disease could be mainly attributed to the use of new cultivars by growers or that the early seeded crops escaped infection. Symptoms of white mould (Sclerotinia sclerotiorum), rust (Uromyces spp.) and anthracnose (Colletotrichum pisi) were not observed in any of the crops surveyed in 2021.
REFERENCES
- Gangneux C, Cannesan M-A, Bressan M, Castel L, Moussart A, Vicré-Gibouin M, Driouich A, Trinsoutrot-Gattin I, Laval K. 2014. A sensitive assay for rapid detection and quantification of Aphanomyces euteiches in soil. Phytopathology 104(10):1138–1147.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell.
- Manitoba Agriculture. 2017 Oct 16. Crop report #25. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- Manitoba Agriculture and Agrifood Statistics. 2017. Farm – Crop – Livestock – Food. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/markets-and-statistics/pubs/mb-agriculture-statistics-factsheet-2019.pdf
- Manitoba Agriculture and Resource Development (MARD). 2021a Oct 5. Crop report #24. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021b. Crop report #1. April 27. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021c. May 18. Crop report #4. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021d Aug 3. Crop report #15. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- MARD. 2021e Sep 7. Crop report #20. [accessed 2021 Nov 30]. www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/index.html
- Manitoba Agricultural Services Corporation (MASC). 2018. Seeded acreage report (2018). [accessed 2021 Nov 30] www.masc.mb.ca/masc.nsf/mmpp_index.html
- MASC. 2019. Seeded acreage report (2019). [accessed 2021 Nov 30]. www.masc.mb.ca/masc.nsf/mmpp_index.html
- MASC. 2020. Seeded acreage report (2020). [accessed 2021 Nov 30]. www.masc.mb.ca/masc.nsf/mmpp_index.html
- Manitoba Pulse and Soybean Growers. 2015. Pulses and soybeans in Manitoba. [accessed 2021 Nov 30] https://www.manitobapulse.ca/pulses-in-manitoba/
- McLaren DL, Kim YM, Henderson TL, Vachon NJ, Kerley TJ, Hwang SF, Chang KF, Chatterton S, Tkachuk C, Klippenstein S, Clouson N. 2020. Field pea diseases in Manitoba in 2019. Can Plant Dis Surv. 100:155–157. In, Can J Plant Pathol. 42:sup1.
- McLaren DL, Kim YM, Henderson TL, Vachon NJ, Hwang SF, Chang KF, Chatterton S, Tkachuk C, Schmidt L, Clouson N, et al. 2021. Field pea diseases in Manitoba in 2020. Can Plant Dis Surv. 101:158–160. In, Can J Plant Pathol. 43:sup1.
- Xue AG. 2000. Effect of seed-borne Mycosphaerella pinodes and seed treatments on emergence, foot rot severity, and yield of field pea. Can J Plant Pathol. 22(3):248–253.
SOYBEAN ROOT ROT AND PHYTOPHTHORA ROT IN MANITOBA AND SASKATCHEWAN IN 2021
CROP: Soybean LOCATION: Manitoba and Saskatchewan NAMES AND AGENCIES: Y.M. KIM1, E. SARI2, D. KAMINSKI3, S. PHELPS4, B.D. GOSSEN5, C. TKACHUK6, L. SCHMIDT6, D. LANGE7, A. FAROOQ8, N. CLOUSON9, A. AKHAVAN10, S. ROBERTS10, C. PERU10, W.C. PENNER2, T.L. HENDERSON1 & M.J. THOMPSON1
1Agriculture and Agri-Food Canada (AAFC), Brandon Research and Development Centre, 2701 Grand Valley Road, Brandon, MB R7A 5Y3 Telephone: (204) 578-6691; Facsimile: (204) 578-6524; E-mail: [email protected] 2AAFC, Morden Research and Development Centre, Unit 101, Route 100, Morden, MB R6M 1Y5 3Manitoba Agriculture and Resource Development, Box 1149, Carman, MB R0G 0J0 4Saskatchewan Pulse Growers, 207-116 Research Drive, Saskatoon, SK S7N 3R3 5AAFC, Saskatoon Research and Development Centre, 107 Science Place, Saskatoon, SK S7N 0X2 6Manitoba Pulse and Soybean Growers, Box 1760, Carman, MB R0G 0J0 7Manitoba Agriculture and Resource Development, Box 969, Altona, MB R0G 0B0 8Manitoba Agriculture and Resource Development, 1129 Queens Ave., Brandon, MB R7A 1L9 9Manitoba Agriculture and Resource Development, 120-6th Ave. N, Swan River, MB R0L 1Z0 10Saskatchewan Ministry of Agriculture, 3085 Albert St., Regina, SK S4S 0B1
ABSTRACT: In 2021, 60 and 15 soybean crops were surveyed in Manitoba and Saskatchewan, respectively, for root diseases. Samples from all fields were rated for root rot severity. Root samples from 40 and 15 fields in Manitoba and Saskatchewan, respectively, were assessed in the laboratory for root pathogens and fusarium root rot was the most prevalent root disease. Sixty-seven Manitoba soybean crops were assessed for phytophthora rot, along with 15 Saskatchewan crops. In 2021, Phytophthora sojae was not detected in any of the soybean plant samples that were received from Manitoba and Saskatchewan.
INTRODUCTION: For the first time in a decade, Manitoba soybean production was lower in 2018 than in 2017 with 2.29 million acres and 1.89 million acres seeded in 2017 and 2018, respectively (Soy Canada Citation2021). Soybean production in Manitoba decreased for three consecutive years (2018-2020) to 1.15 million acres in 2020, but increased to 1.31 million acres in 2021. Favourable yields and attractive soybean prices contributed to the increase in area seeded to soybean in Manitoba in 2021. The seeded area in Saskatchewan increased from 240,000 acres in 2016 to 850,000 acres in 2017, but declined in 2018, 2019 and 2020 to 407,500, 150,000 and 126,700 acres, respectively (Soy Canada Citation2021). In 2021, soybean production in Saskatchewan decreased further to 85,000 acres. The 2018, 2019 and 2020 field seasons were difficult for soybean growers – dry weather with lower yields in some cases may have influenced producers to reduce the soybean acreage in 2021 in Saskatchewan.
Root rot has been a problem in Manitoba and Saskatchewan and is a constraint also in other areas of Canada where soybean production has been established (Chang et al. Citation2013; Nyandoro et al. Citation2019; OMAFRA Citation2011). This disease complex may become more of an issue in Manitoba and Saskatchewan with the increased development and cultivation of early maturing soybean varieties as well as the association of Fusarium species with numerous field crops. Phytophthora root rot has been identified in Manitoba and Saskatchewan soybean crops and an annual survey for P. sojae is of utmost importance in order to characterize these isolates and provide information on the pathotype diversity that now exists.
METHODS: Soybean crops were surveyed for root diseases at 60 different locations in Manitoba in 2021. Areas of the crop survey were expanded to include not only randomly chosen fields from regions in south-central and southwest Manitoba, where soybean is commonly grown, but fields from non-traditional soybean areas into which the crop is expanding.
The survey for root diseases was conducted during mid-July to mid-August with at least ten plants uprooted at each of three random sites in each crop surveyed. Root diseases were rated on a scale of 0 (no disease) to 9 (death of plant) for all 60 fields. For 40 crops, 15 symptomatic roots were collected for fungal isolation and identification. For Fusarium spp., identification involved visual assessment, microscopic examination and morphological characterization of fungal colonies on growth media using the criteria of Leslie and Summerell (Citation2006). Fifteen roots from each of the 40 soybean crops surveyed were frozen for future PCR analysis of root rot pathogens.
In Manitoba, the 60 crops that were surveyed for root rot and seven additional crops were assessed in mid-July to early September for phytophthora rot. Soybean plants were collected by staff at AAFC-Brandon, AAFC-Morden, Manitoba Agriculture, and the Manitoba Pulse and Soybean Growers. In Saskatchewan during early August to early September, soybean plants were collected from 15 crops by employees at the Saskatchewan Pulse Growers and the Saskatchewan Ministry of Agriculture. All plants were shipped to Manitoba, rated for root rot, and those that were symptomatic for phytophthora disease were identified at AAFC-Brandon for further assessment in the laboratory. Approximately 93 and 32 stems from Manitoba and Saskatchewan soybean plants, respectively, were placed on selective media to identify Phytophthora spp. based on their morphological characteristics (Gallegly and Hong Citation2008).
RESULTS AND COMMENTS: A drier than normal summer with extreme heat occurred in Manitoba and Saskatchewan, with above normal rains for the month of August in much of Manitoba. Weather was favourable throughout the fall for harvesting many crops including soybean. The percentage of soybean harvest completed in Manitoba and Saskatchewan was 96% by October 13 (Manitoba Agriculture and Resource Development Citation2021) and 98% by October 4 (Saskatchewan Agriculture Citation2021). Yields were variable depending on moisture conditions during the growing season, but were reported to range from 15 to 60 bu/ac throughout Manitoba with a mean yield of 27 bu/ac for soybean in Saskatchewan.
Root rot was observed in all 60 and 15 soybean crops surveyed in Manitoba and Saskatchewan, respectively. Root rot severity ratings ranged from 3.2 to 5.5 with a mean of 4.6 (Manitoba) and 4.0 to 6.1 with a mean of 4.5 (Saskatchewan). The microorganisms most frequently isolated from roots of infected plants from all 75 crops belonged to Fusarium spp. (). Rhizoctonia root rot (Rhizoctonia solani) was not detected in any of the 40 Manitoba crops surveyed in 2021. Pythium root rot was not detected in any of the soybean crops surveyed. Assessment of frozen samples for root pathogens using PCR is planned for the near future. Phytophthora rot was not identified in any of the 67 Manitoba fields and was not detected in any plant samples received from Saskatchewan in 2021 ().
Table 1. Prevalence of phytophthora rot (PRR) and prevalence and severity of root rot in 60 (67 for PRR) and 15 crops of soybean in Manitoba and Saskatchewan, respectively, in 2021.
ACKNOWLEDGEMENTS:
We gratefully acknowledge the support of Kiah Simpson and Sheila Miller with the Saskatchewan Ministry of Agriculture and Joanne Kwasnicki with the Saskatchewan Association of Rural Municipalities.
REFERENCES
- Chang KF, Nyandoro R, Howard RJ, Hwang SF, Turnbull GD, Laflamme P, Strelkov SE, McLaren DL. 2013. Occurrence of soybean root rot in southern Alberta, Canada in 2011 and 2012. Can Plant Dis Surv. 93:170–173.
- Gallegly ME, Hong C. 2008. Phytophthora: identifying species by morphology and DNA fingerprints. St. Paul (MN): APS Press.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell.
- Manitoba Agriculture and Resource Development. 2021. Crop report seasonal summary. October 13, 2021. [accessed 2021 Nov 30]. https://www.gov.mb.ca/agriculture/crops/seasonal-reports/crop-report-archive/pubs/seasonal-summary-10-13-2021.pdf
- Nyandoro R, Chang KF, Hwang SF, Ahmed HU, Turnbull GD, Strelkov SE., Willenborg, C. 2019. Management of root rot of soybean in Alberta with fungicide seed treatments and genetic resistance. Can J Plant Sci. 99(4):499–509.
- Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA). 2011. Agronomy guide for field crops: soybean diseases. Publication #811. [accessed 2021 Nov 30]. http://www.omafra.gov.on.ca/english/crops/pub811/pub811.pdf
- Saskatchewan Agriculture. 2021. Final crop report: September 28 to October 4, 2021. [ accessed 2021 Nov 30]. https://publications.saskatchewan.ca/api/v1/products/114999/formats/130012/download
- Soy Canada. 2021. Canadian soybean seeded acres (1980 to current). [ accessed 2021 Nov 30]. https://soycanada.ca/statistics/seeded-area-acres/
FRUITS & BERRIES/FRUITS, FRUITS À ÉCALE ET BAIESSURVEYS FOR PHYTOPHTHORA FRAGARIAE ON STRAWBERRY IN THE BRITISH COLUMBIA FRASER VALLEY, 2002 AND 2017
CROP: Strawberry LOCATION: Fraser Valley, British Columbia NAMES AND AGENCIES: J. F. ELMHIRST1, L. A. WEGENER2, S. SVEINSON-DYER1, D. FROST1 & D. HENDERSON2
1Elmhirst Diagnostics & Research, 5727 Riverside St., Abbotsford, BC V4X 1T6 Telephone: (604) 832-9495; E-mail: [email protected] 2Institute for Sustainable Horticulture, Kwantlen Polytechnic University, 20901 Langley Bypass, Langley, BC V3A 8G9
ABSTRACT: In the 1990’s and early 2000’s, red stele root rot caused by Phytophthora fragariae Hickman was a major disease of cultivated strawberry in the Fraser Valley, British Columbia. In a survey of nine commercial strawberry fields in 2002, isolates of P. fragariae were recovered from symptomatic roots in five of the fields sampled, representing three different cultivars. Two of the isolates were highly resistant to metalaxyl (RIDOMIL GOLD 480EC) in vitro. From the mid-2000’s on, the number of reports of red stele disease and P. fragariae in Fraser Valley strawberries have declined. In 2017, a survey of 22 fields or plantings representing nine cultivars and 10 growers recovered zero isolates of P. fragariae. The decline of this pathogen in the BC Fraser Valley can be attributed primarily to a change in strawberry varieties and cultural practices.
INTRODUCTION: In the 1990ʹs and early 2000ʹs, red stele root rot caused by Phytophthora fragariae Hickman was a major disease of cultivated strawberry in the British Columbia Fraser Valley and growers had begun to report poorer control of the disease with labelled fungicides such as RIDOMIL GOLD 480EC (metalaxyl-m and s-isomer). Thus, a field survey was conducted in 2002 to determine whether there was resistance to metalaxyl within the pathogen population. More recently, from the mid-2000ʹs on, reports by growers and industry of red stele in Fraser Valley strawberries have declined, as have submissions to the provincial Plant Diagnostic Laboratory of strawberry plants/roots infected with P. fragariae (provincial government and industry representatives, personal communications). Thus, 15 years later in 2017, a follow-up survey was conducted to determine the incidence of P. fragariae in the same area and the degree of resistance to metalaxyl.
MATERIALS AND METHODS: In 2002, nine fields representing three cultivars and six commercial strawberry growers in Chilliwack, Abbotsford and Langley, plus the Agriculture and Agri-Food Canada (AAFC) Berry Research Station in Abbotsford, were surveyed for the presence of P. fragariae. In each field, eight to ten plants of the same cultivar and planting date were dug from low areas where the plants exhibited lower leaf necrosis and stunting, and the roots combined. Samples were collected from Dec. 30, 2001 to April 5, 2002 (). One field in Abbotsford-Matsqui was sampled twice on Dec. 30 and April 5 for a total of 10 samples.
Table 1. Strawberry crops surveyed for the presence of Phytophthora fragariae in the Fraser Valley, BC and their resistance to RIDOMIL GOLD 480EC in 2002.
In the lab, new (current year) aspring were washed under running/spring were washed under running water for one hour, brushed to remove adhering soil particles and biofilms containing fungal and bacterial contaminants, surface-sterilized in 10% bleach, rinsed in sterile water, then roots with necrotic tips or red steles were cut and plated onto a semi-selective pea medium adapted from George and Milholland Citation1986 by Paul Reeser, Oregon State University (personal communication). The medium was prepared by autoclaving 75 g frozen peas in 500 mL DH2O for 20 min, straining out the peas, topping up the filtrate to 500 mL, adding 7.5 g agar, and 6 mg BOTRAN 75W fungicide (dicloran 75%) dissolved in 3 mL of 95% ethanol, autoclaving again for 20 min and cooling to 45oC followed by the addition of 10 mg pimaricin or Delvocid (50% natamycin) in 5 mL sterile DH2O, 0.11 g Na-ampicillin in 5 mL sterile DH2O, 5 mg rifampicin in 1-1.5 mL dimethyl sulfoxide (DMSO) and 0.125 g hymexazol. Plates were incubated for 5-7 days in the dark at 19oC and colonies with Phytophthora-like mycelium (slow-growing, aseptate) were transferred to the pea medium again, then to plates of a V8 agar medium containing 75 mL clarified V8 juice + CaCO3 (85 mL V8 +1.25 g CaCO3, centrifuged or filtered), 7.5 g agar and 15 mg Β-cholesterol dissolved in 3-5 mL hot 95% ethanol per 500 mL. After incubating under light at room temperature, colonies were examined microscopically and sub-cultured again, if necessary, to eliminate non-target organisms. To confirm the identity of the remaining Phytophthora-like colonies, small sections of likely aseptate mycelium were cut out, placed in a Petri plate and covered with a triple-filtered soil water extract from a strawberry field for four days, with daily water changes, to stimulate production of sporangia. Several Mortierella, Pythium and other Oomycetes were eliminated at this stage by production of distinctive sporangia or chlamydospores.
Isolates identified as Phytophthora fragariae by morphological characteristics were analyzed by amplification with specific PCR (polymerase chain reaction) primers for Phytophthora fragariae (Bonants et al. Citation1997). DNA was extracted from pure cultures using the BIO 101 FastDNA Kit with the standard DNA extraction protocol (Qbiogene Inc., Carlsbad, CA 92008). Following extraction, 5.0 µl of template DNA was used in 25.0 µl PCR reactions, each containing one Ready-to-GoTM PCR bead (Amersham Biosciences Inc., Quebec, Canada), 19.0 µl sterile distilled water, 0.5 µl forward primer DC1 (5’-ACTTAGTTGGGGGCCTGTCT-3’) and 0.5 µl reverse primer B5 (5’-TGAGATGCCACCCGCAGCA-3’). Amplifications were carried out using a Peltier PTC-200 Thermal Cycler (MJ Research, Watertown, MA). Thermal cycling consisted of one cycle at 94°C for 2 min, 35 cycles at 95°C for 30sec, 56°C for 30sec and 72°C for 1 min, and a final extension step at 72°C for 10 min. Products of PCR were analyzed by electrophoresis on a 2% agarose gel containing ethidium bromide in TBE buffer at 100 volts for 1 hour. Gels were photographed on a UV-transilluminator. A 1-kb ladder (Invitrogen) was used to estimate sizes of PCR products. The expected size of the amplified PCR product for P. fragariae was 750bp and a known isolate of P. fragariae from the American Type Culture Collection (Gaithersburg, MD) was used as the positive control in the PCR procedure.
To determine the degree of resistance to metalaxyl-m, the isolates of P. fragariae recovered from BC strawberries were grown on plates of V8 agar + CaCO3 + Β-cholesterol amended with various concentrations of metalaxyl. To achieve the desired concentration, a commercial sample of RIDOMIL GOLD 480EC (metalaxyl-m and s-isomer, 480 g/L) was diluted in a known volume of sterile distilled water, vacuum-filtered using a 0.2 µ micropore filter to remove possible fungal and bacterial contaminants and added to the V8 medium after autoclaving. In each experiment, a single 10 mm diameter plug of each isolate grown on V8 media was placed in the centre of each of two replicate plates of the same medium amended with each concentration of metalaxyl. The plates were incubated in the dark at 19oC. Mycelial growth (colony diameter) was measured at 5, 10 and 15 days after incubation.
Initially, all five isolates (from fields #1, 2, 3, 5 and 8) were plated on the V8 medium amended with metalaxyl-m and s-isomer at concentrations of 0, 1, 2, 5, 10 and 20 ppm. Isolates #1 and 5 which were not inhibited at these concentrations were then plated on the medium amended with metalaxyl at 0, 10, 25, 50, 100 and 200 ppm. Isolates #2, 3 and 8 which were completely inhibited at 1 ppm were plated on the medium amended with metalaxyl at 0, 1x10−7, 1x10−5, 1x10−3, 1x10−1 and 10 ppm to determine the lowest concentration at which their growth was inhibited. Each experiment was repeated twice with duplicate plates for a total of four plates per concentration. Bivariate regression analysis was performed for mean colony growth in mm (response) versus concentration of metalaxyl (factor) at 15 days after plating using the statistical software JMP 5.0.1, SAS Institute Inc., Cary, NC.
Sporangial production by the two resistant isolates (Isolates # 1 and 5) was compared to that of a sensitive isolate (#3), at various concentrations of metalaxyl. A 10 mm diameter mycelial plug from a colony of each of isolate grown on V8 agar was placed in a sterile Petri dish and 10 mL of filtered soil water extract amended with varying concentrations of metalaxyl m- and s-isomer was added to just cover the plug. Solutions were changed daily for the duration of the experiment. At 3, 5 and 7 days a small section of mycelial growth at the edge of each plug was plucked with tweezers and examined under the microscope for sporangia and zoospore production. The number of healthy sporangia with differentiating zoospores was counted in five fields at 250X magnification (each field approximately 600 µm diameter) and averaged for each concentration of metalaxyl.
In 2017, 25 strawberry samples were collected from 22 individual fields or plantings, representing eight commercial farms in Chilliwack, Abbotsford and Langley, plus one research field (Chilliwack-Cultus Lake) and one nursery greenhouse. The pathogen was isolated following the same methods as in 2002.
RESULTS AND DISCUSSION: In 2002, five isolates of P. fragariae were recovered from five out of nine strawberry fields surveyed in the BC Fraser Valley. Isolates were recovered from three different cultivars: ’Totem’ and ‘Rainier’ (June-bearing) and the day-neutral ‘Diamante’ (). The isolates were confirmed as P. fragariae by amplification of a 750 bp DNA fragment of the pathogen with specific PCR primers (). Two of the isolates, #1 and #5 from cv. ‘Totem’ in Abbotsford and Chilliwack, respectively, exhibited a high degree of resistance to metalaxyl in vitro (). Mycelial growth of these isolates was reduced at 100 ppm but continued up to 200 ppm (). Bivariate regression analysis () demonstrated a linear response of colony growth to varying concentrations of metalaxyl in resistant isolates #’s 1 and 5 (r2 = 0.814 and 0.915, respectively). Isolate #5 was slightly more sensitive than isolate #1, as evidenced by a lower mean growth response to concentrations of metalaxyl (25.1 mm), and a lower parameter intercept for concentration (ppm) versus colony growth (mm) of 29.8, compared to isolate #1 which had a mean growth response of 27.6 mm to concentrations of metalaxyl and parameter intercept of 34.2. In contrast, mycelial growth of the metalaxyl-sensitive isolates #2 and #3 was completely inhibited at 0.1 ppm (). Isolate #8 from cv. ‘Diamante’ was slightly less sensitive, producing a small amount of mycelial growth at 0.1 ppm at 15 and 21 days.
Fig. 1 Agarose gel of PCR products amplified from five isolates of Phytophthora fragariae using primers flanking the ITS region. Lanes 1 to 5, Phytophthora fragariae isolates from fields 1, 2, 3, 5 and 8, respectively; Lane 6, negative control (sterile distilled water); Lane 7, Phytophthora fragariae positive control (ATCC isolate); Lane 8, DNA 100 kb ladder (Invitrogen).
Table 2. Mean colony diameter (mm) of five isolates of Phytophthora fragariae at 5 and 10 days after plating on V8 media amended with various concentrations of metalaxyl-m and s-isomer (RIDOMIL GOLD 480EC).
Table 3. Mean colony diameter (mm) of “metalaxyl-resistant” Isolates 1 and 5 and “metalaxyl-sensitive” Isolate 8 of Phytophthora fragariae at 5, 10 and 15 (24) days after plating on V8 media amended with various concentrations of metalaxyl-m and s-isomer (RIDOMIL GOLD 480EC).
Table 4. Bivariate (regression) analysisa: concentration of metalaxyl (ppm) versus mean colony diameter (mm) for Phytophthora fragariae resistant isolates 1 and 5 at 15 days after plating.
Table 5. Mean colony growth (mm) of “metalaxyl-sensitive” Isolates 2, 3 and 8 of Phytophthora fragariae at 5, 10 and 15 days after plating on V8 media amended with various concentrations of metalaxyl-m and s-isomer (RIDOMIL GOLD 480EC).
When culture plugs were floated on water amended with metalaxyl, sporangial production by resistant Isolate #1 was completely inhibited at 25 ppm, while resistant Isolate #5 formed some small sporangia which released zoospores at 50 and even 100 ppm (). In contrast, sporangial production by the metalaxyl-sensitive Isolate #3 was abundant at concentrations up to 0.001 ppm but was completely inhibited at 0.1 ppm. This isolate also produced oospores in vitro at concentrations of zero to 0.001 ppm, but none at 0.1 ppm. No oospores were observed in the resistant isolates.
Table 6. Mean sporangial (Sp) and oospore (Oo) production per date by three isolates of Phytophthora fragariae in soil extract water amended with various concentrations of metalaxyl-m and s-isomer (RIDOMIL GOLD 480EC).
In 2017, no isolates of P. fragariae were obtained from 25 samples representing 22 fields or plantings, 10 farms and nine cultivars (). By 2017, several fields surveyed in 2002 were no longer in strawberries and some growers were no longer producing this crop. Other plantings were in ground not previously planted to strawberries. Nevertheless, the results confirmed the anecdotal observations of growers and industry representatives that P. fragariae was no longer a common pathogen of strawberry in the BC Fraser Valley.
Table 7. Strawberry crops surveyed for Phytophthora fragariae in the BC Fraser Valley in 2017.
It is possible that P. fragariae was present in some of the samples from which an isolate was not obtained in culture. P. fragariae is slow-growing and a recovery rate of 50% or less from infected roots is not uncommon (Paul Reeser, Oregon State University, personal communication). P. fragariae enters into a dormant phase in summer and, in the Pacific Northwest, it is most successfully isolated from young adventitious roots in November to February (P.R., OSU, pers. comm.) In the 2002 survey, no isolates were obtained after March 15. Thus, in 2017, no samples were collected after the first week of March.
The authors do not suspect the decline of red stele disease in BC strawberries is related to climate change. P. fragariae requires cool, wet weather for infection. Despite increasing average temperatures over the past two decades, these conditions still occur in the Fraser Valley from November to January-February. The closely related species, P. rubi, remains a common root rot pathogen of raspberry crops in the BC Fraser Valley (Burlakoti and Sapkota Citation2020). Thus, it seems more likely that the declining incidence of P. fragariae in commercial strawberries in the BC Fraser Valley over the last 15 years is the result of changes in strawberry varieties and cultural production practices.
Commercial strawberry fields in British Columbia are usually re-planted every two to three years. In 2002, most strawberry transplants were one-year-old bare root plants purchased from suppliers in WA and OR and grown primarily in California. The majority of the crop consisted of June-bearing varieties, such as ‘Totem’, grown in matted rows on flat ground or raised beds with overhead irrigation. Around the mid-2000ʹs, the majority of the industry began to switch to day-neutral varieties grown on raised beds with plastic mulch and drip irrigation. By the time of our 2017 survey, this was the standard production method and the day-neutral cultivar ‘Albion’ was the most common variety. ‘Albion is reported to be resistant to phytophthora crown rot (OMAFRA Citation2016), but it is not known whether it is resistant to one or more races of P. fragariae. The recovery of P. fragariae from day-neutral ’Diamante’ (one of the parents of ‘Albion’) in the 2002 survey shows that day-neutral varieties can be affected by P. fragariae when grown on raised beds with plastic mulch, although the incidence of the disease tends to be less with the higher soil temperatures and improved drainage. Another recent innovation has been the use of tissue culture plants for transplanting, which have less risk of infection than soil-grown, bare-root plants.
ACKNOWLEDGEMENTS: Funding for the 2002 survey and resistance testing was provided by the BC Fraser Valley Strawberry Growers Association (FVSGA), Lower Mainland Horticultural Improvement Association (LMHIA) and the BC Investment Agriculture Foundation (BCIAF) under IAF Projects # 358 and 359 and the results were reported to the funding agencies. The 2017 survey was funded by Elmhirst Diagnostics & Research with use of laboratory facilities contributed by the Institute for Sustainable Horticulture, Kwantlen Polytechnic University, Langley, BC. Opinions expressed in this article are those of the authors alone and not necessarily those of the BC provincial government or funding agencies.
REFERENCES
- Bonants P, Hagenaar-de Weerdt M, van Gent-Pelzer M, Lacourt I, Cooke D, Duncan J. 1997. Detection and identification of Phytophthora fragariae Hickman by the polymerase chain reaction. Eur J Pl Path. 103:345–355.
- Burlakoti RR, Sapkota S. 2020. Root rot and wilting disease complex of red raspberry in the Fraser Valley of British Columbia in 2018 and 2019. Can Plant Dis Surv. 100:166–167. In, Can J Plant Pathol. 42:sup 1.
- George SW, Milholland RD. 1986. Growth of Phytophthora fragariae on various clarified natural media and selected antibiotics. Plant Dis. 70:1100–1104.
- Ontario Ministry of Agriculture and Food (OMAFRA). 2016. June-bearing & day-neutral strawberry varieties. Last Modified February 2021. [ accessed 2022 Jan 14]. http://www.omafra.gov.on.ca/english/crops/facts/strawvar.htm
SURVEY OF APPLE TREE VIRUSES IN ONTARIO, 2021
CROP: Apple, cv. ‘Ambrosia’ LOCATION: Ontario NAMES & AGENCIES: K. GRIGG-MCGUFFIN1, K. GOLDENHAR2 & E. DEBROUWER1
1Ontario Ministry of Agriculture, Food and Rural Affairs, Box 587, Blueline Road & Highway # 3, Simcoe, ON N3Y 4N5 2Ontario Ministry of Agriculture, Food and Rural Affairs, 1 Stone Road West, Guelph, ON N1G 4Y2 Telephone: 519-835-5792, E-mail: [email protected]
ABSTRACT: In March 2021, apple trees cv. ‘Ambrosia’ were sampled to investigate the incidence and distribution of known apple viruses across Ontario. Of the 40 trees randomly selected from 10 farms in different locations, 65% had apple chlorotic leaf spot virus (ACLSV), 52.5% had apple stem pitting virus (ASPV), 20% had apple mosaic virus (ApMV), and 22 trees (55%) had multiple viruses. These incidence rates are very high and of significant concern. All samples where virus was detected were from M9 rootstock. Virus was not detected in M106 rootstock. While the full impact of viral infections in apples is not known, viruses can contribute to reduced tree vigor and long-term yield loss. Increasing awareness of viral infections present in apple tree production in Ontario is important for growers in selecting sources of propagative material.
INTRODUCTION: Viral infections are one of many factors that can negatively affect apple production. Viruses have been shown to reduce vigour, limiting the productive lifespan and reducing yields by 8-67% (Fuchs et al. Citation2018). The Canadian Food Inspection Agency has identified several viruses, including apple chlorotic leaf spot virus (ACLSV), apple stem pitting virus (ASPV), apple stem groove virus (ASGV), tomato ring spot virus (ToRSV) and tobacco ring spot virus (TRSV) as having the potential to play a role in reducing overall tree health and the ability of a grower to manage viruses once in the orchard (Canadian Food Inspection Agency Citation2021)
In 2016 and 2017, a survey of 2620 apple trees in New York showed that over 54% had a single viral infection, with ASPV being the most frequent at 33% followed by ACLSV at 12% (Fuchs et al. Citation2018). For co-infections (two or more viruses in the same tree), the most common combination (8% of trees) was ASPV and ACLSV. Both ASPV and ACLSV are latent viruses that are currently being investigated at Cornell University for their potential role in rapid apple decline.
Ontario has been dealing with rapid collapse of apple trees since 2016. To date, no single organism or abiotic agent has been shown to cause the significant collapse in orchards. We wanted to know the presence of known apple viruses across the province of Ontario in randomly selected trees showing no symptoms of decline. In 2020, several growers reported the cultivar ‘Ambrosia’ showing irregular chlorosis on the leaves which was positively identified with apple mosaic virus (ApMV), as well as ACLSV and ASPV. To investigate this further, a small survey was conducted in March of 2021 in ‘Ambrosia’ trees aged five to 12 years.
METHODS: Samples were collected before bud break, from March 2 to 17, 2021. Dormant bud sticks were collected at the base of 1-year-old wood. Samples close to the leader were used where possible. One shoot from each of 10 major scaffold limbs was taken per tree and packaged together in a zip lock bag. Pruners were sanitized in between each tree and each tree was a separate sample. Four cv. ‘Ambrosia’ trees were sampled randomly throughout a block at 10 locations, two from each of the five apple-growing districts in the province (Ontario Apple Growers Citation2022). Trees at nine of the 10 locations were on ‘Malling 9’ (M9) rootstock while the other site was on ‘Malling 106’ (M106). One orchard that had tested positive for ApMV, ACLSV and ASPV in 2020 was used as a positive check.
Samples were stored at 4-6°C before sending to the Plant Disease Clinic at the University of Guelph to be tested for ACLSV, ASPV, ApMV, apple luteovirus 1 (ALV-1), TRSV and ToRSV. Enzyme linked immunosorbent assay (ELISA) tests were used to detect TRSV and ToRSV and reverse transcription polymerase chain reaction (RT-PCR) tests were used for ACLSV, ASPV, ALV-1 and ApMV.
RESULTS AND DISCUSSION: Nine out of 10 locations had at least one tree that was infected with a virus (). At five locations, all trees were infected with at least one virus. No virus was detected in the M106 rootstock.
Table 1. Incidence of apple chlorotic leaf spot virus (ACLSV), apple stem pitting virus (ASPV), apple mosaic virus (ApMV), apple luteovirus 1 (ALV-1), tobacco ringspot virus (TRSV) and tomato ringspot virus (ToRSV) in 40 ‘Ambrosia’ apple trees from 10 Ontario orchards in March 2021.
The common viruses detected were:
26 trees (65%) were infected with ACLSV
21 trees (52.5%) were infected with ASPV
8 trees (20%) were infected with ApMV
Only ACLSV was detected alone in 4 trees (10%); all other viruses were detected as a co-infection
ALV-1 was only detected once with three other viruses
Multiple viruses were detected in 22 trees (55%):
ACLSV was found in all co-infected trees
14 trees (35%) were infected with ACLSV and ASPV
6 trees (15%) had ACLSV, ASPV and ApMV
1 tree (2.5%) had ACLSV and ApMV
1 tree (2.5%) had ACLSV, ASPV, ApMV and ALV-1
ACLSV was the most common virus detected. In this survey, 10% of the trees were only infected with ACLSV, and it was found in all co-infections (22 of 40 trees, 55%). This was also the most common virus found in a New York survey (Fuchs et al. Citation2018). ACLSV can infect pome and stone fruit. It is considered a latent virus, often showing no discernable symptoms, but can be impactful when present with other latent viruses on susceptible cultivars and certain rootstock combinations. The extent of damage that it can cause is not known but as much as a 30% yield loss has been estimated (Cieniewicz and Fuchs Citation2016a).
ASPV was not found as a single infection but was the most common co-infection with ACLSV (52.5% of samples). ASPV can infect pome fruit. It is considered a latent virus, meaning symptoms are unlikely or asymptomatic, leading to undetected infection. Fruit yield has been shown to decrease when co-infected with ACLSV. Pits can be seen in the woody cylinder when infection is severe which can lead to reduced tree vigour (Cieniewicz and Fuchs Citation2016b).
ApMV was detected in one co-infection with ACLSV and six co-infections with ACLSV and ASPV. ApMV is named for its characteristic mosaic-like symptoms on leaves. It has a wide host range, including pome, stone fruit, berries, hops and hazelnuts. Most common apple cultivars will remain asymptomatic after infection; however, some will produce symptomatic leaves that may be confined to a certain limb or erratically throughout the tree. Reduced growth in ApMV infected trees has been shown leading to reduced lifespan and yields. The main method of transmission of this virus is grafting (Grimova et al. Citation2016).
ALV-1 was only detected in one tree that was co-infected with ACLSV, ASPV and ApMV. ALV-1 is a recently discovered virus in North America. It was found in trees exhibiting apple decline but was also detected in asymptomatic trees. There is ongoing research at Agriculture and Agri-Food Canada on this virus in apples, as its impact is not known.
TRSV and ToRSV were tested for in this survey and not found. These have been shown to infect apples in other regions but have not been commonly detected in Ontario.
Apple stem grooving virus (ASGV) was not included in this survey due to lack of a reliable diagnostic test. This virus is often detected in trees infected with ACLSV and ASPV. It is likely present in Ontario orchards as well as, and along with, the other viruses detected and can result in economic losses.
There are no management options once viruses are in an established orchard. It is important in managing these viruses to use budwood and rootstock free of known viruses. It is highly recommended to not collect budwood from the surveyed orchards where viruses were detected (nine out of 10 locations) or others where virus is also suspected. Further research is needed to determine the impact of multiple and latent viral infections.
ACKNOWLEDGEMENTS: We would like to thank the growers who participated in this survey.
REFERENCES
- Canadian Food Inspection Agency. 2021. List of pests regulated by Canada. [ accessed 2021 Nov 30] https://inspection.canada.ca/plant-health/invasive-species/regulated-pests/eng/1363317115207/1363317187811
- Cieniewicz E, Fuchs M. 2016a. Apple chlorotic leaf spot virus factsheet, New York State IPM Program. [ accessed 2021 Nov 30] https://ecommons.cornell.edu/handle/1813/43944
- Cieniewicz E, Fuchs M. 2016b. Apple stem pitting virus factsheet, New York State IPM Program. [ accessed 2021 Nov 30] https://ecommons.cornell.edu/handle/1813/43945
- Fuchs M, Kahke C, Donahue D, Wallis A, Basedow M. 2018. Distribution of viruses in New York apple orchards. Fruit Quarterly 26(4):5–8. [accessed 2021 Nov 30]. https://nyshs.org/wp-content/uploads/2019/05/Fuchs-Pages-from-NYFQ-BOOK-WInter-2018.1-27-19-3.pdf
- Grimova L, Winkowska L, Konrady M, Rysanek P. 2016. Apple mosaic virus. Phytopathol Mediterr. 55(1):1–19.
- Ontario Apple Growers. 2022. OAG districts and directors. [ accessed 2021 Nov 30]. https://onapples.com/mobile/oag-districts-and-directors
VEGETABLES/LÉGUMESPRESENCE OF VERTICILLIUM WILT IN POTATOES GROWN IN THE BRITISH COLUMBIA FRASER VALLEY, 2021
CROP: Potato LOCATION: British Columbia Fraser Valley NAMES AND AGENCY: M. GRAY, M. DESSUREAULT & H. MEBERG
E.S. Cropconsult Ltd., 6145 171A St., Surrey, BC V3S 5S1 Telephone: 604-841-0764; E-mail: [email protected], [email protected], [email protected]
ABSTRACT: A total of 172 potato fields were monitored by E.S. Cropconsult Ltd. (ESC) during the 2021 growing season in the Fraser Valley, British Columbia. It was a hot season, including a record heat wave from June 25 to July 1 with temperatures reaching ≥40oC, that affected many crops, including potatoes. Foliar symptoms of verticillium wilt were noticed after this time in the sampling regions of Delta, Surrey, Abbotsford and Chilliwack. It is suspected that 30 to 40 potato fields across these regions were infected with Verticillium spp. based on reports by ESC field scouts. Of these, three samples sent for laboratory diagnosis returned positive for Verticillium spp.
INTRODUCTION: In 2019, British Columbia (BC) produced 97,296 tonnes of potatoes in 2,671 hectares of harvested area (Agriculture and Agri-Food Canada Citation2020). E.S. Cropconsult Ltd. (ESC) has been monitoring potato fields since 1988, recording information on insect, mite, disease, and other field observations with a primary focus on Phytophthora infestans and Epitrix tuberis. Historically, verticillium wilt, or early dying caused by a combination of Verticillium spp. and root lesion nematodes (Pratylenchus penetrans), has not been a common disease in South Coastal BC, including the Fraser Valley. It has been more prevalent in the Southern Interior where summer temperatures are higher. Growers and field scouts from ESC noticed a relatively high incidence of wilting and yellowing in Fraser Valley potato crops during the hot and dry summer of 2021. In 2020, similar symptoms in two fields were found to be associated with the presence of Verticillium dahliae and/or root lesion nematodes in soil samples (J. Elmhirst, personal communication). There could be a trend towards an increased incidence of verticillium wilt as climate change continues to lead to hotter and drier summers in BC. In 2021, ESC conducted a review of weekly scouting reports to document this trend.
METHODS: A total of 172 potato fields were monitored weekly by ESC during the 2021 growing season in the BC Fraser Valley. Symptoms of verticillium wilt were identified on potato foliage in several fields throughout Delta, Surrey, Abbotsford and Chilliwack in scouting reports. Symptoms were identified as foliar leaf tissue yellowing or browning on one side of leaf while other side remained green (Ontario Ministry of Agriculture, Food & Rural Affairs Citation2009). The stems were cut lengthwise at the base and no noticeable symptoms were observed within the stem (i.e., no browning of vascular tissue). Two whole representative plant samples from Abbotsford were sent to the BC Ministry of Agriculture, Food and Fisheries Plant Health Laboratory (BCMAFF-PHL) on August 17, 2021, to confirm the presence of Verticillium spp.
Reports were sent to each grower on a weekly basis with recommendations, pest levels, and field observations, including wilt symptoms. This reporting was written either on a per-field basis or in the case of wilt symptoms across many fields, was denoted as “older/senescing fields showing signs of verticillium wilt”.
RESULTS AND COMMENTS: Symptoms of verticillium wilt were first noticed after a record heat wave from June 25 to July 1, 2021 with temperatures reaching more than 40oC that affected many crops in the BC Fraser Valley, including potatoes. Temperatures were over 30°C on several other days, including July 28-30 and August 13-15, 2021. Two representative symptomatic plants sent to the BCMAFF-PHL by ESC were reported positive for Verticillium spp. by incubation and microscopic examination. The species was not identified; however, it was suspected by the lab to be V. dahliae.
In reports sent to growers from ESC between July 22 and August 17, there were five growers where wilt symptoms were reported in older or senescing fields. There were 20 incidences of specific fields having foliar wilt symptoms diagnosed across the sampling region (). Six additional reports were sent to different growers indicating verticillium wilt symptoms were present in two or more of their fields. It is therefore suspected that approximately 30 to 40 fields were affected by verticillium wilt out of the 172 potato fields monitored this season.
Table 1. Location of potato fields with verticillium wilt and date of diagnosis based on foliar symptoms observed in 2021.
Further, a plant sample from a field in Chilliwack submitted to the BCMAFF-PHL by ESC on the same day as the representative verticillium plant samples in this report, was also reported positive for Verticillium spp. It was submitted for potential black dot (Colletotrichum coccodes) diagnosis due to the presence of dark brown to black speckling on the stems of many plants in the field. The full crop had symptoms of heat stress (wilting/dehydration, yellowing, browning) but no visible identifying symptoms of verticillium wilt. The plant was reported negative for black dot in the lab.
It is not known what affect the extreme heat and flooding events in the Fraser Valley in 2021 will have on future potato production and the incidence of verticillium wilt and early dying.
ACKNOWLEDGEMENTS: Thank you to the ESC staff for their in-field scouting and to the participating growers whose fields were accessed.
REFERENCES
- Agriculture and Agri-Food Canada. 2020. Potato market information review. [ accessed 2022 Feb 26]. https://agriculture.canada.ca/sites/default/files/legacy/resources/prod/doc/pdf/potato_market_review_revue_marche_pomme_terre_2019a-eng.pdf
- Ontario Ministry of Agriculture, Food & Rural Affairs. 2009. Verticillium wilt. Ontario CropIPM. [ accessed 2022 Feb 26]. http://www.omafra.gov.on.ca/IPM/english/potatoes/diseases-and-disorders/verticillium.html#advanced
MONITORING OF FUNGAL DISEASES AND ASTER YELLOWS ON DRY BULB ONION IN ALBERTA, 2021
CROP: Onion LOCATION: Alberta NAMES AND AGENCIES: M.W. HARDING1, G.C. DANIELS1, P. RAGAN2 & J. FENG3
1Alberta Agriculture, Forestry and Rural Economic Development, Crop Diversification Centre South, 301 Horticulture Station Road E., Brooks, AB T1R 1E6 Telephone: (403) 362-1338; Facsimile: (403) 362-1326; E-mail: [email protected] 2Paul Ragan Horticultural Consulting, 72 Sixmile Road South, Lethbridge, AB T1K 5S6 3Alberta Agriculture, Forestry and Rural Economic Development, Crop Diversification Centre North, 17507 Fort Road NW, Edmonton, AB T5Y 6H3
ABSTRACT: Fungal diseases can negatively affect onion production. A bi-weekly assessment of fungal pathogens at a commercial vegetable farm in 2021 was undertaken to determine the predominant fungal pathogens on two cultivars of onion. The survey revealed that in 2021, at the location sampled, the most commonly occurring fungal pathogen was Penicillium spp., followed by Botrytis spp. and Fusarium spp. The incidence of these pathogens started at 5% to 25% in mid-June, dropped to near zero in early-to-mid August, and then increased to >10% in September. One cultivar had a lower incidence of Fusarium spp. and Penicillium spp. than the other, but Botrytis spp. incidence was essentially equal on the two cultivars. The aster yellows pathogen Candidatus Phytoplasma asteris was not detected on any of the samples.
INTRODUCTION AND METHODS: Onion (Allium cepa L.) production in Alberta occurs in many small market gardens that supply local fresh market consumption, and a few larger commercial production/packaging operations under irrigation in southern Alberta. Fungal diseases routinely have a negative impact on onion production with pathogens such as Fusarium oxysporum Schlechtendal and F. avenaceum (Corda) Saccardo causing basal plate rot, Sclerotium cepivorum Berk. causing white rot, Alternaria alternata (Fries) Keissler and A. embellesia Woudenb. & Crous causing skin blotch, and Botrytis porri (van Beyma) Whetzel causing neck rot. Aster yellows caused by Candidatus Phytoplasma asteris (Lee, Gundersen-Rindal, Davis, Bottner, Marcone & Seemüller) can be a significant issue as well. These pathogens have been reported on onion and garlic in Alberta plantings in previous years (Harding et al. 2013, Citation2015, Citation2016, Citation2021).
In 2021, the same onion field was sampled every two weeks beginning on July 16 and ending on September 16. Three to five whole plants of each of two cultivars were collected randomly and stored at 4°C until processing. Cultivar names and field locations were not reported in order that the producer remained anonymous. Fungi were cultured from bulb and neck tissues by dissecting ~1-cm tissue pieces with a clean scalpel, and surface sterilizing the pieces in 1% NaOCl for 30s, followed by a tap water rinse and blotting dry on sterile paper towel. Nine to 12 surface-sterilized neck and bulb tissue pieces of each cultivar were plated on a Botrytis semi-selective medium (BSM) (Gutierrez and Shew Citation1998) and duplicated on potato dextrose agar (PDA). Plates were incubated for 1 wk at 20°C in the dark after which the presence of Botrytis spp. was recorded based on fungal morphology and colour change on BSM, and other fungi on PDA were recorded based on fungal colony and spore morphology visualized with a phase contrast microscope at 400x.
Past surveys have shown aster yellows to be an important disease on onion and garlic in Alberta. To monitor for the presence of the causal agent, Candidatus Phytoplasma asteris, onion neck and bulb tissues were rubbed onto Whatman FTA cards as described by Karavina and Gubba (Citation2017). Samples were collected biweekly on the same dates as above and stored at room temperature until the conclusion of the survey. DNA was extracted from the cards following the manufacturer’s instructions and PCR amplification conducted using specific primer sets for phytoplasma (P1/Tint, Smart et al. Citation1996).
RESULTS AND COMMENTS: Above average temperatures and below average rainfall characterized the 2021 growing season. The heat and drought in 2021 made it very challenging to produce any crop unless irrigation was available. The survey results presented here were from an irrigated field and the yield and quality of the crop was average to above average. Isolates recovered from onion samples included fungi in the genera Botrytis, Fusarium and Penicillium. The general trend was that fungi were present in mid-June but dropped to near zero in August and then increased in September (). Fusarium and Penicillium species were recovered from 25% of samples in mid-July, while Botrytis incidence was lower in July. Neither the aster yellows phytoplasma nor Alternaria spp. were observed. The incidence of Botrytis spp. did not differ between the two cultivars however, ‘Cultivar 2’ had a significantly lower incidence of Fusarium and Penicillium species (). It is anticipated that observations of fungal incidence in onion fields over time may help to inform growers of seasonal disease risks both in season and going into storage.
Fig. 1 Percent incidence of fungal genera in tissue samples from an onion field in Alberta between July 16 and September 16, 2021. Error bars represent the standard error of the mean.
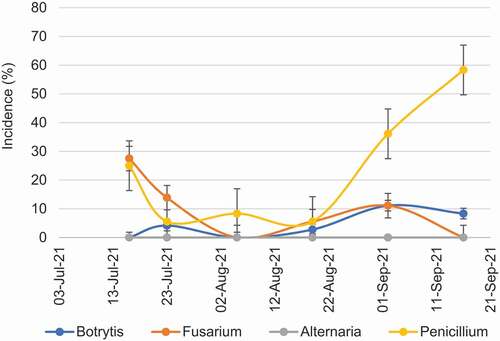
Fig. 2 Percent incidence of fungal genera in tissue samples from two cultivars from an onion field in Alberta between July 16 and September 16, 2021. Error bars represent the standard error of the mean.
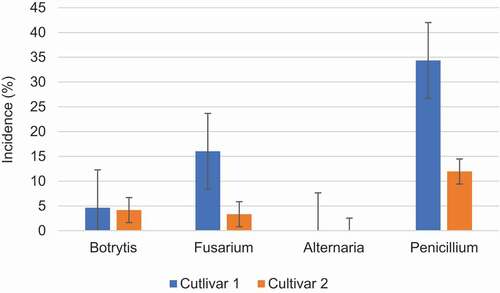
REFERENCES
- Gutierrez WA, Shew HD. 1998. Identification and quantification of ascospores as the primary inoculum for collar rot of greenhouse-produced tobacco seedlings. Plant Dis. 82:485–490.
- Harding MW, Spencer RCJ, Lange RM, Yang J, Howard RJ. 2014. Diseases of garlic in Alberta in 2013. Can Plant Dis Surv. 94:214–216.
- Harding MW, Spencer RCJ, Lange RM, Yang J, Broatch J, Lisowski SLI, Howard RJ. 2015. Diseases of onion and garlic in Alberta in 2014. Can Plant Dis Surv. 95:188–190.
- Harding MW, Daniels GC, Spencer RCJ, Morton D, Feng J, Broatch J, Lisowski SLI, Howard RJ. 2016. Diseases of garlic and onion in Alberta in 2015. Can Plant Dis Surv. 96:203–206.
- Harding MW, Forge T, Feng J, Daniels GC, Neeser-Carazo R, Zhou Q, Munro P, Spencer RCJ. 2021. A survey of onion and garlic diseases in Alberta in 2019 and 2020. Can Plant Dis Surv. 101:109–113. In Can J Plant Pathol. Vol. 43:sup 1.
- Karavina C, Gubba A. 2017. Detection and characterization of tomato spotted wilt virus infecting field and greenhouse-grown crops in Zimbabwe. Eur J Plant Pathol. 149:933–944.
- Smart CD, Schneider B, Blomquist CL, Guerra LJ, Harrison NA, Ahrens U, Lorenz KH, Seemüller E, Kirkpatrick BC. 1996. Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Appl Environ Microbiol. 62:2988–2993.
INCIDENCE OF VERTICILLIUM WILT (V. DAHLIAE) IN POTATO IN MANITOBA, 2021 CROP
CROP: Potato LOCATION: Manitoba NAMES AND AGENCY: V. BISHT1, M. TENUTA2 & S. GRAHAM3
1Manitoba Agriculture & Resource Development, 65 – 3rd Avenue NE, Carman, MB R0G 0J0 Telephone: (204) 745-0260; Fax: (204) 745-5690; Email: [email protected] 2Department of Soil Science, University of Manitoba, 13 Freedman Crescent, Winnipeg, MB R3T 2N2 3J.R. Simplot Co., Highway #1 and Simplot Road, Portage la Prairie, MB R1N 3A4
ABSTRACT: The occurrence of potato early dying in relation to verticillium wilt (Verticillium dahliae) was assessed in 26 potato fields by selecting plants and rating the disease in the cross-section of stems one inch from the soil line. There was wide variability in verticillium wilt in the Manitoba fields, ranging from 3 to 100% incidence and 0.6 to 60.6 on the Verticillium Severity Index. Generally, the fields with a high V. dahliae copy number as determined by qPCR of soil samples in fall 2020 also had a high number of verticillium-affected plants in the 2021 crop.
INTRODUCTION AND METHODS: Twenty-six potato fields were selected for the survey in western, central and south-eastern potato growing areas of Manitoba, based on field history and growers’ understanding of the high or low incidence of disease in their fields. Of the 26 fields, twenty-two were selected based on soil Verticillium dahliae (Vd) densities in fall of 2020 before the 2021 potato crop; fields were classified for Vd inoculum density as low (0 to <5000), medium (5000 to 30,000), or high (>30,000) copies of the rDNA-IGS gene per gram of soil based on qPCR testing of the soils. Four fields were surveyed without known levels of Vd inoculum.
A 100 g subsample of each fall-collected soil sample (from fields to be planted to potato in 2021) was air-dried for seven days at room temperature and q-PCR was used to determine soil densities of Verticillium dahliae expressed as copies rDNA-IGS per gram of soil. The 100 g air-dried subsample of soil was passed through a 2-mm sieve. A portion of air-dried soil was then pulverized with a sterilized mortar & pestle and then passed through a #60 mesh sieve. The resultant pulverized soil was used for DNA extraction using the Qiagen DNeasy Powersoil Kit according to the manufacturer’s protocol, with some modifications – 0.5g soil was extracted, homogenization was accomplished using a Mini-Beadbeater 16 instead of a vortex adaptor and 50 µL instead of 100 µL. Primers used were VdF929-947 and VdR1076-1094 with Vdhrc FAM probe (Bilodeau et al. Citation2012). A standard curve for log copy # in rxn to Cq was generated using a tenfold dilution series of plasmid DNA, dilutions ranging from 0.1 ng/ µL to 10−8 µL. Copy numbers were calculated using the following formula: Copy # /g soil = 10(Cq-intercept/slope) * (µL DNA extract/µL DNA in rxn)/g soil extracted.
Disease rating was done from August 26 to September 15, 2021; plants were randomly selected for rating at ten sites within a field, based on soil EC profile or topography (sampling site included good and bad looking spots). At each site in a field, five to ten plants were assessed for verticillium discolouration/browning in a cross-section of the main stem cut at 2-3 cm above ground level. Disease incidence was defined as the percentage of plants sampled with discolouration. A rating scale of 0 to 5 (Alkher et al. Citation2009) was used for disease severity assessment, where 0 = no discolouration in cross-section or no disease, 1 = trace to ≤ 9% discolouration, 2 = 10 to 24% discolouration, 3 = 25 to 49% discolouration, 4 = 50 to 74% discolouration and 5 = ≥ 75% dark brown discolouration, stem nearly dead or dying. A reference photo guide of the 0-5 rating scale was prepared for use in the field (). A Verticillium Severity Index (VSI) was calculated as the sum of the product of % incidence and severity ratings within each category, expressed as a percentage of the maximum, following the formula: VSI = [((n0*0) + (n1*1) + (n2*2) + (n3*3) + (n4*4) + (n5*5))/(N*5)) *100], where n is the number of plants within each rating category 0 to 5, and N is the total number of plants rated (Wheeler Citation1969; Lu et al. Citation2008; Safi et al. Citation2020).
Fig. 1 Visual rating scale (0-5) of verticillium wilt severity in potato stems based on Alkher et al. (Citation2009); (photos by Vikram Bisht).

Before the stems were cut, a general visual yellowing and wilt rating of whole plants for potato early dying (PED) symptoms was also made at each site, on a 0-5 scale, where 0 = all green plants, 1 = up to 10% plants with some yellowing, 2 = 10 to 25% plants yellow /wilting, 3 = 25 to 50% plants yellowing/wilting and yellowing more pronounced; 4 = 50 to 75% plants yellowing /wilted and a few dead plants; 5 = >75% plants have strong yellowing and severe wilting, along with many dead plants. There was one visual plant rating for each site.
Data on the incidence, VSI and PED in the fields were analysed by using F-test and Single-Factor ANOVA using Statistix 10.0 (Analytical Software, Tallahassee, FL). Treatment means were compared by LSD-Pairwise comparisons at P=0.05. Data from fields with low, medium and high Vd copy numbers were analyzed as separate groups.
RESULTS AND COMMENTS: There were six fields with low Vd (0 to <5000 copies/g soil), eight fields with medium (5000 to 30,000), and six with high (>30,000) copies of Vd per gram of soil in fall 2020. Plants with verticillium wilt were found in all fields surveyed. There was a wide range of disease incidence observed in fields based on the rating of discolouration of the stems in cross-section. The average incidence of verticillium wilt in fields surveyed was 63.4%, and ranged from 3% to 100%, while the average Verticillium Severity Index (VSI) was 28.9, and ranged from 0.6 to 60.6 (). The average visual early dying (PED) rating was 1.6, with a range of 0.10 to 2.80 on a 0-5 scale.
Table 1. Disease incidence (%), Verticillium Severity Index (VSI) and visual rating of potato early dying (PED) symptoms in Manitoba potato fields in 2021, classified as low, medium or high density based on Verticillium dahliae (Vd) copy number (rDNA-IGS gene) as determined by a qPCR test in soil test in in fall 2020.
The percent disease incidence (%) and verticillium severity index (VSI) in 2021 were significantly higher in fields with higher Vd copy number densities in soil in the fall of 2020 ( and ). Data analysis showed a high correlation (r=0.91) between the visual rating for PED and the VSI (analysis not shown). This indicated that the visual PED rating could be a good guide for agronomists in estimating field disease levels. However, there were a few sandy spots in the fields where early dying did not appear to be related to verticillium wilt.
Table 2. Mean % disease incidence, Verticillium Severity Index (VSI) and visual rating of potato early dying (PED) symptoms of verticillium wilt in Manitoba potato fields in 2021 with varying levels of soil inoculum (Vd soil density) based on PCR soil tests in fall, 2020.
Under similar conditions of high levels of soil inoculum (Field 6 and 7 are in same field circle), cultivar ‘Clearwater’ had significantly lower levels of verticillium wilt than ‘Russet Burbank’ (). Yield data was obtained from the agronomist of the grower for both varieties.
Table 3. Comparison between ‘Russet Burbank’ and ‘Clearwater’ varieties, with respect to verticillium wilt disease under similar high soil inoculum density in Manitoba potato Fields ‘6’ and ‘7’ in 2021.
The results suggest wide variability in verticillium wilt incidence and severity in Manitoba fields, as in 2020 (Bisht et al. Citation2021). Soil inoculum levels as determined by qPCR testing specifically for Verticillium dahliae appeared to be a good indicator of potential verticillium disease level in a field and could be used in assessing disease management options, including the use of disease tolerant cultivars. However, other factors including soil characteristics may confound the qPCR verticillium copy count and disease expression in some fields.
ACKNOWLEDGEMENTS: Field help from various agronomists, working with the farms, is greatly appreciated for this survey.
REFERENCES
- Alkher H, El Hadrami A, Rashid KY, Adam LR, Daayf F. 2009. Cross-pathogenicity of Verticillium dahliae between potato and sunflower. Eur J Plant Pathol. 124:505–519.
- Bilodeau GJ, Koike ST, Uribe P, Martin FN. 2012. Development of an assay for rapid detection and quantification of Verticillium dahliae in soil. Phytopath. 102:331–343.
- Bisht V, Tenuta M, Graham S. 2021. Incidence of verticillium wilt (V. dahliae) in potato in Manitoba, 2020 crop. Can Plant Dis Surv 101:169. In, Can J Plant Pathol 43:sup1.
- Lu G, Jian W, Zhang J, Zhou Y, Cao J. 2008. Suppressive effect of silicon nutrient on Phomopsis stem blight development in asparagus. Hort Science 43(3):811–817.
- Safi H, Hussain MS, Nazir M. 2020. Incidence and severity of early blight of tomato in Peshawar, Mardan and Malakand Divisions and variability amongst the isolates of Alternaria solani. Int J Ag Enviro & Biotech. 13(2):175–183.
- Sorensen LH, Schneider AT, Davis JR. 1991. Influence of sodium polygalacturonate sources and improved recovery of Verticillium species from soil. (Abstr.). Phytopathology 81:1347.
- Wheeler BEJ. 1969. An introduction to plant diseases. London (UK): Wiley.
FOREST TREES/ARBRES FORESTIERSMONITORING AND SURVEILLANCE OF POPLAR LEAF SPOT AND CANKER DISEASE (SPHAERULINA MUSIVA) IN BRITISH COLUMBIA
CROP: Poplar (Populus spp.) LOCATION: British Columbia NAMES AND AGENCIES: N. FEAU1,2, P. HERATH2, N. SULLIVAN2, S. ZEGLEN3, H. H. KOPE4 & R.C. HAMELIN2,5
1Present address: Pacific Forestry Centre, Canadian Forest Service, Natural Resources Canada, Victoria, BC V8Z 1M5 Telephone: 604-317-6134; E-mail: [email protected] 2Department of Forest and Conservation Sciences, University of British Columbia, Vancouver, BC V6T 1Z4 3British Columbia Ministry of Forests, Lands and Natural Resource Operations and Rural Development, Nanaimo, BC V9T 6E9 4British Columbia Ministry of Forests, Lands and Natural Resource Operations and Rural Development, Victoria, BC V8W 9C2 5Faculté de Foresterie et Géomatique, Institut de Biologie Intégrative et des Systèmes (IBIS), Université Laval, Québec, QC G1V 0A6
ABSTRACT: Since the first discovery in 2006 of the fungal agent, Sphaerulina musiva, which is responsible for a leaf-spot and lethal canker on poplars in British Columbia, surveillance and monitoring have been an important part of pest-management for this disease. Herein, we report the results of the 2020 survey of this disease on P. trichocarpa and hybrid poplars in four regions in B.C. Frequency of S. musiva on native P. trichocarpa trees was similar to what was observed in previous surveys (<1.5%), indicating that the spread of the pathogen is very limited on P. trichocarpa and the small number of infections detected likely originated from hybrid poplar plantations in the area that provided inoculum. In nurseries and plantations of hybrid clones in the North Okanagan region, the disease was more abundant but seems to be remaining under control, indicating that the pest-containment and phytosanitary measures that have been taken to prevent spread are successful.
INTRODUCTION: It has been recognized that the high selection pressure exerted through breeding, coupled with high productivity levels, have led hybrid poplars (Populus genus) to become vulnerable to multiple biotic agents. One such biotic agent, the ascomycete pathogen Sphaerulina musiva (Peck), was originally reported as an endemic pathogen in eastern North America causing leaf spots on poplar. In 2006, S. musiva was found for the first time in British Columbia on hybrid poplar clones located in the upper Fraser Valley (Callan et al. Citation2007); less than ten years later, the pathogen was found on hybrid poplar clones in the Okanagan Valley. This non-native invasive fungal pathogen causes a leaf spot disease and stem cankers that often lead to breakage and mortality of susceptible poplar trees (Feau et al. Citation2010). The presence of this pathogen in B.C. could threaten the black cottonwood (P. trichocarpa Torr. & A. Gray), a keystone species naturally occurring in riparian ecosystems. Due to the similarity of symptoms on leaves and overlapping morphometric microscopic characters, S. musiva can easily be misdiagnosed as its sister species S. populicola (Peck), which is native to B.C. and induces only a minor leaf spot disease on P. trichocarpa.
Since its first discovery in B.C., S. musiva has been under constant monitoring. The pathogen has spread to several plantations of hybrid poplar clones within the Fraser Valley and has also been found on local black cottonwoods (Herath et al. Citation2016). Recently, the fungus has been detected in a nursery that produces hybrid poplars for phytoremediation in the North Okanagan area (Tabima et al. Citation2020). Such monitoring and surveillance effort is of particular interest to inform a pest management program that includes chemical control in infected poplar nurseries and cultural control by removing susceptible clones and planting tolerant ones (British Columbia Ministry of Forests, Lands and Natural Resource Operations and Rural Development, Citation2019).
This report presents the results of the 2020 survey conducted in four sites located in different regional districts in B.C. Two types of poplars were targeted: native black cottonwoods and hybrid poplar clones. We collected leaves on P. trichocarpa trees located in the vicinity of hybrid poplar plantations in the upper Fraser Valley; additional P. trichocarpa trees were sampled in the north-eastern part of the province, close to an area in Alberta where S. musiva had been reported on balsam poplars (P. balsamifera L.) and hybrid poplars (Leboldus et al. Citation2009). In addition, we targeted two hybrid poplar stands to evaluate the efficiency of pest-containment measures. The first site included a nursery and a plantation located in the North Okanagan at which chemical and cultural control has been applied since the first discovery of the disease in 2014; the second stand, located in north-west B.C. near Hazelton, was a soil-remediation site planted in early 2020 with poplar cuttings from two hybrid clones collected in the nursery at the first site.
METHODS: Field sampling in the Fraser Valley and the Peace River region consisted of roadside collections of natural poplar foliage (). Five leaves with Sphaerulina-like leaf spots were collected from each of 231 trees in August in the upper Fraser Valley and 50 trees in the Peace River region near Dawson Creek. Sampling was also conducted in early September on hybrid poplars in the North Okanagan region near Armstrong at the Community Research Program site (CRP) in a stool-bed (three clones) and an adjacent poplar plantation (two clones) (). Two of the hybrid clones sampled at the CRP sites were also sampled in a phytoremediation field planting (WMF) established in early 2020 near Hazelton in the Kitimat-Stikine region (). Leaf samples were dried, pressed and stored at 4°C until further processed. For each tree, DNA from five leaf spots (when possible, from five different leaves) was extracted together and species identification was conducted using the S. musiva and S. populicola real-time polymerase chain reaction (PCR) assays and the experimental protocol described in Herath et al. (Citation2016). Positive samples for S. musiva were confirmed with a second DNA extraction and real-time PCR reaction. In addition, leaves from 24 random trees of the upper Fraser Valley sampling were directly tested during field collection with a point-of-use real-time PCR system as described in Capron et al. (Citation2020).
Fig. 1 Survey of Sphaerulina musiva and S. populicola infections on Populus spp. in four B.C. regions in 2020. A, location of the different sampling sites; blue, hybrid poplar stands; red, natural P. trichocarpa. B, Dawson Creek, Peace-River region. C, Upper Fraser Valley region.
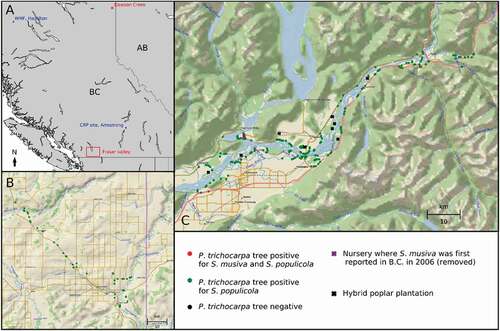
Table 1. Hybrid poplar samples from the Community Research Program (CPR) and WMF Phytoremediation sites tested for Sphaerulina musiva and S. populicola in this survey.
RESULTS AND DISCUSSION: Results of S. musiva positive detection on P. trichocarpa in the upper Fraser Valley and the Peace River region are presented in Fig.’s 1B and 1C. The native species, S. populicola, was detected at a high frequency in these two areas (99.6%; only one negative sample in the upper Fraser Valley), while the non-native pathogen, S. musiva, was found only in the upper Fraser Valley with three detections out of 231 trees tested. Sphaerulina musiva was not detected in the Peace River region. The low frequency of the pathogen on P. trichocarpa in the upper Fraser Valley (1.3%) was close to the value reported in a similar survey conducted in 2011 in the same area (1.4% on P. trichocarpa) (Zeglen et al. Citation2011), suggesting that, although still present, the spread of S. musiva on black cottonwood is limited. However, even though the nursery where S. musiva was reported for the first time in B.C. was removed in 2016, the pathogen is still present in this exact area. This is likely due to the presence of few >15-year-old hybrid poplar plantations that can act as inoculum reservoir for infecting black cottonwoods (Herath et al. Citation2016).
Since the first report of S. musiva in 2014 at the CRP site in North Okanagan, pest containment measures such as annual sprays of fungicides (excepted in 2019 and 2020) and removal of the most susceptible poplar clones have been applied. However, the fungus was still detectable in the nursery located at this site. The lower frequency of detection (16.7%), in comparison with the values reported for hybrid poplar plantations in the Upper Fraser Valley (50.0% or more; Herath et al. Citation2016) is likely the result of these containment measures. In addition, latent infection in vegetative cuttings and asymptomatic plant material contaminated with conidia may both constitute a source of inoculum as new plantations are established (Abraham et al. Citation2018; Ostry Citation1987).
The presence of the pathogen was also assessed at a newly established phytoremediation site in northwestern B.C. (WMF; ). The site was planted with material collected in the infected nursery of the CRP site in North Okanagan. The reduction of the inoculum load in the nursery with the pest containment measures discussed above and the phytosanitary protocol followed (dipping of cuttings in fungicide) before moving the planting material north, may explain the absence of positive detections of S. musiva in this site. However, it seems also difficult to rule out the “positive” role of the local environment in this area, which might be simply not conducive to successful development and spread of the disease.
ACKNOWLEDGEMENTS: This work was supported by the British Columbia Ministry of Forests, Lands and Natural Resource Operations and Rural Development.
REFERENCES
- Abraham ND, Chitrampal P, Keriö S, LeBoldus JM. 2018. Multiplex qPCR for detection and quantification of Sphaerulina musiva in Populus stems. Plant Pathol. 67:1874–1882.
- British Columbia Ministry of Forests, Lands and Natural Resource Operations and Rural Development. 2019. Guidance on the use of hybrid poplar clones for reforestation on crown land. Memorandum April 26, 2019. [accessed 2022 Jan 18]. https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/forestry/forest-health/forest-health-docs/septoria_guidance.pdf
- Callan BE, Leal I, Foord B, Dennis J, Van Oosten C. 2007. Septoria musiva isolated from cankered stems in hybrid poplar stool beds, Fraser Valley, British Columbia. Pacific Northwest Fungi 2:1–9.
- Capron A, Stewart D, Hrywkiw K, Allen K, Feau N, Bilodeau G, Tanguay P, Cusson M, Hamelin RC. 2020. In situ processing and efficient environmental detection (iSPEED) of tree pests and pathogens using point-of-use real-time PCR. PLoS ONE 15:e0226863.
- Feau N, Mottet M-J, Périnet P, Hamelin RC, Bernier L. 2010. Recent advances related to poplar leaf spot and canker caused by Septoria musiva. Can J Plant Pathol. 32:122–134.
- Herath P, Beauseigle S, Dhillon B, Ojeda DI, Bilodeau G, Isabel N, Gros-Louis M-C, Kope H, Zeglen S, Hamelin R, Feau N. 2016. Anthropogenic signature in the incidence and distribution of an emerging pathogen of poplars. Biol. Invasions 18:1147–1161.
- LeBoldus JM, Blenis PV, Thomas BR, Feau N, Bernier L. 2009. Susceptibility of Populus balsamifera to Septoria musiva: A field study and greenhouse experiment. Plant Dis. 93:1146–1150.
- Ostry ME. 1987. Biology of Septoria musiva and Marssonina brunnea in hybrid Populus plantations and control of Septoria canker in nurseries. For Pathol. 17:158–165.
- Tabima JF, Søndreli KL, Keriö S, Feau N, Sakalidis ML, Hamelin RC, LeBoldus JM. 2020. Population genomic analyses reveal connectivity via human-mediated transport across Populus plantations in North America and an undescribed subpopulation of Sphaerulina musiva. MPMI 33:189–199.
- Zeglen S, Kope HH, Beauseigle S, Hamelin RC. 2011. Preliminary distribution surveys for Septoria musiva on poplar in the upper Fraser Valley. Can Plant Dis Surv. 91:155–157.
GENERAL CROP SURVEY/ENQUÊTE GÉNÉRALE SUR LES CULTURES A SURVEY OF FIELD AND HORTICULTURAL CROP DISEASES IN THE YUKON TERRITORY IN 2021
CROPS: Cereals (Wheat), Oilseeds (Canola), Forage Grasses (Timothy and Bromegrass), Fruits (Haskaps), and Vegetables (Cabbage and Potato) LOCATION: Southern Yukon (Whitehorse area) NAMES AND AGENCIES: R.J. HOWARD1 & K. FERRIS2
1RJH Ag Research Solutions Ltd., Box 1456, Brooks, AB T1R 1C3 Telephone: (403) 362-9564; Facsimile: (403) 501-5753; Email: [email protected] 2Agriculture Branch, Energy, Mines and Resources, PO Box 2703 K-20A, Whitehorse, Yukon Y1A 2C6
ABSTRACT: Several kinds of field and horticultural crops growing on six commercial farms in the Whitehorse area of the southern Yukon Territory were surveyed for diseases in summer 2021 by staff of the Agriculture Branch of the Yukon Government. The crops included canola, wheat, bromegrass, timothy, haskaps, cabbage and potato. Fields were visited two or more times during June, July and August, 2021. The incidence and severity of diseases were visually assessed on a crop-by-crop basis and plant samples were collected for laboratory analysis of the pathogens present, if any. Both infectious and non-infectious diseases were present on most crops. The infectious diseases were caused by various species of plant pathogenic bacteria and fungi, most of which have been previously reported on these crops in other areas of Canada.
INTRODUCTION AND METHODS: The 2021 field and horticultural crop disease survey was the second campaign of its kind on agricultural crops in the Territory. The findings of the inaugural survey were published in 2021 (Howard and Ferris Citation2021). The objectives of the 2021 survey were: 1) to determine the kinds and levels of diseases on selected Yukon crops, 2) to identify the major pathogen species attacking Yukon crops, and 3) to use the results to plan future surveillance activities aimed at helping producers to improve their current disease management programs.
All of the fields included in the 2021 survey were situated on six commercial farms, which were designated as Farms 1 to 6, in the Whitehorse area in the southern Yukon. The crops surveyed included cereals (wheat), oilseeds (canola), forage grasses (brome and timothy), fruits (haskaps) and vegetables (cabbage and potatoes). Fields were visited one or more times in the early, mid- and/or late growing season (June, July and August) at a time when damage from diseases was most noticeable. Symptoms were visually assessed on a crop-by-crop basis by determining their incidence and severity. Incidence was represented by the percentage of plants, leaves, heads, fruit, kernels, etc., damaged in the target crop, while severity was estimated to be the proportion of the leaf, fruit, head, root/canopy area, etc., affected by a specific disease on a 0-4 rating scale as follows: 0 = no disease symptoms, 1 = 1-10% of the crop canopy showing symptoms, 2 = 11-25% showing symptoms, 3 = 26-50% showing symptoms, and 4 = >50% showing symptoms. Photographs of affected plants were taken and some were sent to plant pathologists across Western Canada for confirmation or for their opinions on causation. Where possible, representative samples of plants with disease symptoms were packaged and sent to the Alberta Plant Health Lab (APHL) in Edmonton, AB for diagnostic analyses. Background information, such as the general cultural practices and cropping history, was obtained from the producers wherever possible. GPS coordinates were obtained for each field to enable future mapping.
RESULTS AND COMMENTS:
Cereals: A 22-ac field of ‘AAC Redberry’, a Canada Western Red Spring Wheat cultivar was surveyed at Farm #1. Plant samples were taken along a W-shaped transect for a total of five sampling points. The first visit, which occurred on June 17, and subsequent visits (July 1, 13 and 29), involved only visual inspections of foliar diseases and none were seen. By the second visit, a few plants showed purpling of the nodes and some leaf browning, both of which appeared to be physiological disorders. A relatively small number of plants had brown or yellow spots, which did not seem to increase in severity over the remainder of the season and also appeared to be a physiological disorder. On July 29, the awn tips on many plants were bleached, which appeared to have been caused by an early frost. In addition, some of the florets at the tips of the heads failed to produce seed, which may also have been due to frost damage.
Forage Grasses: At Farm #2, a 60-ac field of timothy hay was surveyed on June 16 and 29. Plants were rated at five stops along a W-shaped transect. The crop was just starting to head out when the field was first visited, and the stand generally appeared healthy; however, several bare spots, which appeared to the result of elk feeding, were noticeable. In addition, some plants showed purpling of the leaf tips, which may have been caused by a temporary phosphorus deficiency. A few plants also showed a reddish-yellow leaf colouration, which might also have been caused by a nutritional problem. By June 29, the crop was fully headed out and dark-coloured lesions were noticed on the leaves of several plants, but the average disease incidence (<2%) and severity (<0.1 on a scale of 0-4) were very low. The crop was harvested before a final field visit could be made. Symptomatic leaves were collected on June 29 and sent to the APHL for analysis. Three genera of potential fungal pathogens were isolated, but they were not characterized to the species level, nor inoculated back onto their respective hosts to prove pathogenicity. One isolate, Neoascochyta exitialis, has been reported to cause leaf spots on members of the Poaceae family, which includes timothy. A second isolate was identified as Phaeosphaeria nodorum (syn. Parastagonospora nodorum), which is a common pathogen of wheat in the Prairie Provinces, where it causes glume blotch disease. An isolate of Cladosporium sp. was also found, but the APHL was unable to confirm whether it was C. phlei, the cause of purple eyespot disease of timothy. The lab also identified the bacterium Pseudomonas coronafaciens, which has been reported to cause leaf spots on timothy and some other grass species in other parts of Canada.
At Farm #3, a 7-ac field of bromegrass hay was surveyed on June 15 and 28 and on July 7 using the same surveying procedure described above for timothy. The second nodes were visible on the plants on the first survey date and the crop generally appeared healthy; a few plants showed leaf spotting symptoms, but the average disease incidence and severity ratings were very low. By June 28, the crop was fully headed out and more dark-coloured lesions were noticed on some of the leaves, but the mean disease incidence (2%) and severity (0.02 on a scale of 0-4) were still very low. On the final sampling date (July 7), the stand was close to maturity and the average leaf spot incidence had increased slightly to 8%, whereas the mean disease severity remained very low at 0.07. Some plants were dug up and the roots washed and examined. Some root and crown rot symptoms were evident, but disease incidence and severity ratings were not done. Symptomatic leaves were collected on June 28 and July 7 and sent to the APHL. For the June 28 samples, three genera of fungi were isolated, i.e., Cladosporium, Epicoccum and a Pezizomycete, but they were not characterized to the species level. Cladosporium phlei is known to cause purple spot of timothy and has been reported from the Peace Region of Alberta. Epicoccum is sometimes found as a contaminant in grass seed. Pezizomycetes have not been reported to attack bromegrass and may have been present in the sample as a saprophyte. The July 7 samples yielded Epicoccum, Fusarium, Microdochium and Arthrinium upon lab analysis. Fusarium species are common pathogens on forage grasses and can cause diseases such as head blight and crown and root rots. Microdochium nivale is a widespread snow mould pathogen on many species of cereals and grasses. Arthrinium has not been recorded as a pathogen on bromegrass in Canada; however, it has been reported as an endophyte on timothy and a few other grass species. Epicoccum has occasionally been detected as a contaminant on grass seed.
Oilseeds: An 80-ac field of ‘Synergy’ Polish canola at Farm #1 was surveyed for foliar diseases on June 17, July 1, 15 and 27, and on August 12 following the same procedure used for cereal crops. On the first survey date (June 7), the crop was at the 2-4 leaf stage and appeared healthy, apart from some insect feeding damage. By July 1, by which time the crop was starting to flower, the plants were still exhibiting signs of insect damage, but no symptoms of infectious diseases were apparent. By July 15, most plants were at the late flowering stage and looked healthy, except for a purple colouration that was seen on the lower stems of several plants, which appeared to be a physiological disorder. On July 21, the crop was at the late flowering stage and the bottom leaves on some plants were wilting and turning yellow, which appeared to be the result of dry soil conditions. Once again, no symptoms of infectious diseases were visible in the crop at the various sampling points in the field. On the last (July 27) sampling date, the crop growth stages ranged from late flowering to early pod ripening. The plants remained free of infectious disease symptoms; however, evidence of frost damage, which had occurred on July 16, was seen as dieback of the top florets on some plants. The frost event severely damaged blossoms on the main racemes of many plants and some of them attempted to reflower. Two subsamples of isolated symptomatic plants were collected on July 27 and sent to the APHL. The leaf lesions on sample #1 yielded two genera of presumed saprophytic fungi, Epiccocum and Cladosporium, and one potential pathogen, Alternaria, which can cause black spot, a leaf and pod disease. The lab recovered Fusarium tricinctum, F. culmorum, F. avenaceum, Alternaria, Phoma and Ulocladium from the roots of a stunted plant in sample #2. Various Fusarium species are known to cause root rot of canola. Phoma lingam causes blackleg of canola, but the sample submitted to the APHL did not have any of the characteristic symptoms of that disease. The Alternaria and Ulocladium isolates were likely saprophytes.
Fruits: A 20-ac producing haskap orchard at Farm #4, where the trees were about 4 years old, was surveyed on June 10 (early bloom), June 23 (full bloom), July 5 (developing fruit) and July 20 (mature fruit). On each date, the surveyor walked up and down the length of the orchard between two rows of trees stopping every 75 paces to examine the trees at each stop for a total of ten stops. The leaves, branches and fruit were rated for disease incidence (% leaves, berries or branches affected) and severity (proportion of the leaves, berries or branches showing damage based on a 0 to 4 scale, where 0 = no disease and 4 = severe disease). Most, if not all, of the visual symptoms observed on the haskap bushes resembled the types of damage caused by physiological stresses. A heavy frost early in the season caused obvious visual damage to the trees and may ultimately have been responsible for common symptoms such as leaf puckering and a rusty discolouration on the leaves. Subsequent weathering may have aggravated these symptoms. A dead branch observed on one tree during the June 23 survey appeared to be mechanical damage and may have been caused during harvesting or by heavy snow during the winter of 2020-21. No symptoms of infectious diseases were observed.
Vegetables: The vegetable plantings surveyed were situated on Farms #1, #5 and #6 and mainly included cabbages and potatoes.
Cabbage (Farm #1): A 2-ac field of green and red cabbages was surveyed on June 24, July 8 and 22, and on August 8. At each date, the surveyor walked up and down the length of the field between two rows of cabbages stopping every few paces to examine the plants at each stop for a total of ten stops. The plants were examined for both infectious and non-infectious diseases. On the first survey date (June 24), the crop was growing well and had no obvious disease symptoms; however, many eggs of the cabbage root maggot were observed to have been laid at the stem bases of most of the plants examined. No symptoms of infectious diseases were seen in the crop on the three subsequent survey dates; however, occasional symptoms of intumescence/edema (water congestion in the leaf tissues), tipburn (calcium deficiency) and leaf variegation (chimera; a genetic disorder) were observed on a few isolated plants.
Cabbage (Farm #5): A 50-ft row of napa cabbage and a 150-ft row of green cabbage were surveyed following the same procedure used for Farm #1. On the first survey date (June 23), both the green and napa cabbages appeared to be disease-free. By July 6, the green cabbages still looked healthy, but one out of six napa plants at one sampling site had symptoms of basal stem rot. On July 20, both the green and napa cabbages were still symptomless. By August 3, the napa cabbages were significantly affected by tipburn, a physiological condition, whereas the green cabbages appeared healthy. On the final sampling date (Aug. 17), 100% of the napa cabbages were affected by tipburn and the severity rating was 4. By contrast, none of the green cabbages had any disease symptoms. The napa cabbages at this farm were severely affected by tipburn and what appeared to be secondary bacterial soft rot. Two napa plants with root decay were collected in mid-August and sent to the APHL for diagnosis. The lab isolated the plant pathogenic bacterium Lelliotta amnigena, which has been reported to cause soft rot diseases of carrot, lettuce, onion and several other horticultural crops internationally.
Cabbage and Miscellaneous Vegetables (Farm #6): A variety of organic cruciferous vegetables and some red beets were examined at this location. The survey procedure used was similar to the one used at the Farms #1 and #5. The cruciferous vegetables included bok choy, broccoli, and napa, red and green cabbages. These crops were surveyed on four dates, i.e., June 22, July 7, July 21 and August 4. The survey procedure used was similar to the one used at Farm #1 and #5. Injury from foliar-feeding caterpillars and cabbage root maggots was the primary pest issue in the cruciferous vegetables at this farm throughout the growing season. No specific disease symptoms were seen on any of the four survey dates. The beets also exhibited foliage damage caused by leaf mining insects and one plant was observed with leaf spot symptoms resembling those caused by Phoma betae. This disease was previously observed during the 2020 vegetable disease survey in the Yukon.
Potatoes (Farm #1): A 25-ac field with four varieties was surveyed for foliar diseases on June 22, July 8 and 21, and August 3. The survey method was similar to that used for the cabbages at this farm. The plants were examined for both infectious and non-infectious diseases four times during the growing season. On the first survey date (June 22), the crop had just begun to emerge and appeared very healthy, apart from some minor yellowing and crinkling of the leaf tips on the occasional plant and some insect feeding damage. On July 8, by which time the crop was just starting to flower, the plants generally looked very healthy. By July 21, most of the crop was at the early flowering stage and most plants showed evidence of light frost damage, i.e., yellowing and browning of the upper leaves. On August 3, the final sampling date, the crop was at full bloom and there was widespread evidence of frost damage to the foliage throughout the field. The most typical symptoms were a brown to black discolouration or spotting on the leaves, especially on the youngest ones, as well as on some of the flowers. In addition, a recent windstorm had caused much of the crop to lodge. There were no distinctive symptoms of any infectious diseases on the foliage on any of the survey dates.
Farm #5: Two rows with a total length of 390 ft and comprised of two varieties were surveyed on June 23, July 2 and 20 and August 3. The survey method was similar to that used for the cabbages surveyed at this farm. The potatoes were grown under row covers for most of the growing season and showed no symptoms of infectious diseases on the foliage. The vine growth was heavy and some of the stems had snapped under their own weight and because of strong winds. No symptoms of infectious diseases were noted.
A summary of the diseases and potential causal agents observed during surveys of cereal, oilseed, forage, fruit, vegetable and potato crops in the Yukon Territory in 2021 is given in . Physiological (abiotic) diseases were much more common and damaging compared to infectious diseases. Most of the presumptive pathogens isolated from infected plants have previously been reported on their respective host crops or on related species elsewhere in Canada (Conners Citation1967; CPNFMPC 1975). The majority of the diseases encountered in the 2021 survey occurred at low levels and did not appear to be causing significant damage; however, assessing actual levels of yield and quality losses caused was beyond the scope of this survey.
Table 1. Summary of diseases observed during surveys on cereal, forage, oilseed, fruit and vegetable crops in the Yukon Territory in 2021.
ACKNOWLEDGEMENTS: The authors wish to thank the following individuals and organizations for their assistance with the 2021 crop disease survey: Mr. Randy Lamb, Mr. Brad Barton, Ms. Tawni Drinnan and Ms. Temesha Debler, Agriculture Branch, Energy Mines and Resources, Whitehorse, YT; Dr. Jie Feng, Ms. Snezana Dijanovic, and Messrs. Alain Starkes, Yalong Yang and Kher Zahr, Alberta Plant Health Laboratory, Alberta Agriculture, Forestry and Rural Economics, Edmonton, AB; Mr. Jason Casselman, Canola Council of Canada, Fairview, AB; Dr. Henry Klein-Gebbinck, Beaverlodge Research Farm, Agriculture and Agri-Food Canada, Beaverlodge, AB; Dr. Mary Ruth McDonald, University of Guelph, Guelph, ON; Mr. Travis Cranmer, Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph, ON; and Mr. Robert Spencer, Spencer Horticultural Solutions, Stettler, AB.
REFERENCES
- Comité Permanent de Nomenclature Française des Maladies des Plantes au Canada [CPNFMPC]. 1975. Noms des maladies des plantes au Canada/Names of plant diseases in Canada. Québec (QC): Ministère de l’Agriculture du Québec.
- Conners IL. 1967. An annotated index of plant diseases in Canada and fungi recorded on plants in Alaska, Canada and Greenland. Ottawa (ON): Research Branch, Canada Dept Agric Publ. 1251.
- Howard RJ, Ferris K. 2021. An inaugural survey of cereal, oilseed and vegetable crop diseases in the Yukon territory in 2020. Can Plant Dis Surv. 101:179–182. In, Can J Plant Pathol. 43:sup1.