Abstract
Crown gall, caused by Agrobacterium tumefaciens (syn. Rhizobium radiobacter), is characterized by gall formation on crowns, stems or roots on many plant species. We describe the occurrence of this disease for the first time on cannabis (Cannabis sativa L. marijuana) plants in two licenced production facilities in British Columbia. Galls were observed on crowns, stems and roots of several genotypes, including ‘White Rhino’, ‘Pink Kush’ and ‘Sour Kush’. The affected plants displayed no other visible symptoms. The incidence of crown gall was extremely low, estimated at 0.01% of total plants. Isolations made from root and stem galls on MacConkey medium (selective for Gram -ve bacteria) and on D1 medium (selective for Agrobacterium spp.) yielded a range of bacterial species, which did not include Agrobacterium sp. However, the presence of Agrobacterium in gall tissues was confirmed following PCR amplification with primers for the indole-acetic acid (iaa) H gene on the T-DNA region of Agrobacterium spp. and showed > 99% similarity to A. tumefaciens. Artificial inoculations were conducted using an A. tumefaciens strain from ATCC (Rhizobium radiobacter, strain designation TT134 [6-1-2, CIP 104 336], cat. 15955) which resulted in gall formation on five cannabis genotypes tested. These galls contained the iaaH gene as determined by PCR and the pathogen was successfully re-isolated on MacConkey agar medium at 4 and 10 weeks post-inoculation. Our findings confirm the occurrence of A. tumefaciens on naturally infected and artificially infected cannabis plants grown under greenhouse conditions and demonstrate reproducible gall symptoms on several cannabis genotypes following A. tumefaciens inoculation.
Résumé
La galle du collet, causée par Agrobacterium tumefaciens (syn. Rhizobium radiobacter), est caractérisée par la formation de galles sur les collets, les tiges ou les racines de plusieurs espèces de plantes. Nous décrivons l’apparition de cette maladie sur des plants de cannabis (Cannabis sativa L. marijuana) pour la première fois dans deux sites de production autorisés de la Colombie-Britannique. Les galles ont été observées sur les collets, les tiges et les racines de plusieurs génotypes, y compris ‘White Rhino’, ‘Pink Kush’ et ‘Sour Kush’. Les plants touchés n’affichaient aucun autre symptôme. L’incidence de la galle du collet était très faible, étant estimée à 0,01% de tous les plants. Des isolements faits à partir de galles de racines et de collets sur de la gélose MacConkey (milieu sélectif pour isoler les bactéries Gram-négatives) et de l’agarose D1 (milieu sélectif pour Agrobacterium spp.) ont permis d’obtenir une gamme d’espèces bactériennes qui n’incluait pas Agrobacterium sp. Toutefois, la présence d’Agrobacterium a été confirmée dans les tissus des galles à la suite d’une amplification par PCR avec des amorces pour le gène H de l’acide indole-acétique (aia) situé sur la région de l’ADN-T d’Agrobacterium spp. et a affiché plus de 99% de similitude avec A. tumefaciens. Des inoculations artificielles ont été effectuées avec une souche d’A. tumefaciens issue d’ATCC (Rhizobium radiobacter, désignation de la souche TT134 [6-1-2, CIP 104336], cat. 15955) qui ont entraîné la formation de galles sur cinq génotypes de cannabis testés. Ces galles contenaient le gène aiaH, comme établi par PCR, et l’agent pathogène a été de nouveau isolé avec succès sur de la gélose MacConkey à 4 et 10 semaines après l’inoculation. Nos résultats confirment l’occurrence d’A. tumefaciens chez les plants de cannabis naturellement et artificiellement infectés, cultivés en serre, et démontrent la reproductibilité des symptômes de la galle sur plusieurs génotypes de cannabis après inoculation avec A. tumefaciens.
Introduction
Crown gall, caused by Agrobacterium tumefaciens (biovar 1, syn. Rhizobium radiobacter) (CABI Citation2021; The Uniprot Consortium Citation2021) induces tumour-like outgrowths or galls on the crowns, roots and stems of plant species in over 93 families (Kado Citation2002). Symptoms on affected plants include stunted growth, chlorotic foliage and reduced vigour (Kado Citation2002). Agrobacterium spp. are Gram-negative, aerobic bacteria that thrive in well-aerated soils and in the rhizosphere of plants and infect via wound sites (Kado Citation2002; Matthysse Citation2006). During infection, the wounded cells release phenolics and sugar compounds that induce transcription of Agrobacterium virulence proteins, followed by transfer of the T (transfer)-DNA region on the Ti (tumour-inducing)-plasmid into plant cells (Gelvin Citation2000, Citation2003). The T-DNA region contains phytohormone genes and opine synthase genes within the left and right borders (LB and RB) (Gelvin Citation2000, Citation2003). The phytohormone genes encode auxins and cytokinins, which induce unregulated plant cell division that results in visible gall formation, while the opine synthase genes produce opines that are used as a nutrient source by the bacterium (Gelvin Citation2000, Citation2003; Kado Citation2002, Citation2014). Under laboratory conditions, Agrobacterium spp. can be differentiated from other microbes using differential growth media, carbon utilization tests, pathogenicity tests, and other biochemical tests (Moore et al. Citation2001; Suzaki et al. Citation2004; Islam et al. Citation2010). More recently, PCR of various housekeeping genes, such as Elongation factor G (fusA), Recombinase A (recA), RNA polymerase sigma factor (rpoD), as well as virulence genes and regions of T-DNA, are used to identify Agrobacterium at the species level (Bini et al. Citation2008; Puławska et al. Citation2016) which provide a more accurate and rapid means of identification.
Previously, classification of Agrobacterium species was based on their pathogenicity and they were separated into biovars depending on host range (Gan and Savka Citation2018). However, this classification system did not account for the fact that tumour-inducing plasmids, which confer pathogenicity or virulence of a strain, can be transferred to non-pathogenic strains of Agrobacterium (Puławska et al. Citation2016). In addition, there exists a close genetic relationship between the genus Agrobacterium and the genus Rhizobium, which has caused some debate as to the correct taxonomic structure of the genus Agrobacterium as well as some reclassification of some biovars in Agrobacterium (Young et al. Citation2001, Citation2003; Farrand et al. Citation2003; Mousavi et al. Citation2014, Citation2015; Gan and Savka Citation2018). Just as the taxonomy of Agrobacterium remains unresolved, the taxonomy of A. tumefaciens (biovar 1) also remains to be clarified. At present, A. tumefaciens is no longer considered to be a taxonomic group but instead comprises a complex of up to 11 different genomospecies (Gan and Savka Citation2018). Until each species has been formally identified, this pathogen will continue to be referred to as the A. tumefaciens genomospecies complex (Lindström and Young Citation2011; Kuzmanović et al. Citation2015; Puławska et al. Citation2016; Mafakheri et al. Citation2019). For the purposes of this study, the pathogen will be referred as to A. tumefaciens.
Nursery and greenhouse crops are particularly susceptible to infection by A. tumefaciens, as the ambient environmental conditions are ideal for pathogen establishment (Schroth et al. Citation1971; Pulawska Citation2010). A range of fruit and nut trees, such as cherry, apple, walnut and almond, are affected by crown gall and disease development has a large economic impact on these hosts (Kado Citation2002, Citation2010, Citation2014; Moore and Putnam Citation2020). Sunflower is highly susceptible to A. tumefaciens and is used to determine the virulence of various Agrobacterium strains (Braun and White Citation1941; Kado Citation2002). Other ornamental hosts, such as roses, Marguerite daisies, chrysanthemum spp., and morning glory can be systemically infected by A. tumefaciens (Kado Citation2002). Crown gall development also occurs on field crops such as tomatoes, beans, alfalfa, and cotton but, unlike fruit and nut trees, the incidence of disease has not been of economic importance.
On Cannabis sativa L, which includes both hemp and high THC-containing cannabis (marijuana), crown gall is a relatively uncommon disease reported to occur on hemp (McPartland Citation1992, Citation2003). The disease has not been previously reported on high THC-containing cannabis. With the expanding cultivation of cannabis in greenhouses across Canada, a range of emerging diseases has been reported (Punja Citation2021), of which bacterial pathogens are still relatively uncommon. In this study, we report the occurrence of symptoms resembling crown gall on the crown, roots, and stems of cannabis plants originating from two licenced production facilities in British Columbia.
The objectives of this research were to: (i) confirm the presence of A. tumefaciens in symptomatic tissues using culture-based and molecular approaches; (iii) compare the isolation frequency and growth of Agrobacterium and other bacterial species on two commonly used isolation media; (iii) evaluate the susceptibility of different tissues (roots, crowns, stems, petioles) of cannabis to infection; and (iv) compare the susceptibility of five different genotypes (strains) of cannabis to artificial inoculation. Symptoms of the disease on cannabis plants are described for the first time.
Materials and methods
Crown gall symptoms and sample source
During 2019–2020, four diseased plant samples (A–D) were obtained from two commercial cannabis licenced production facilities in British Columbia (BC). One sample displayed galls on roots of genotype ‘Sour Kush’ grown in an indoor licenced production facility (sample A) (). The remaining three samples displayed large galls (up to 5 cm in diameter) on the crown of affected plants grown under greenhouse conditions at a second facility (samples B–D) (). The galls in samples B and C were on 6-week-old flowering plants of cannabis genotype ‘White Rhino’ (). Sample D was obtained from a plant of ‘Pink Kush’ at harvest time (10 weeks of age) (). None of the plants showed any other symptoms of disease. In 2021, additional samples of stem cuttings of ‘Pink Kush’ from the propagation room of the second facility showed symptoms, with galls measuring 2–3 mm in diameter (sample E) (). In 2022, a further 16 samples of stem cuttings of genotype ‘Powdered Donuts’ and 40 samples of stem cuttings of ‘Black Cherry’ with gall sizes ranging from 3 mm to 1 cm (sample F) were obtained. All of these samples were included in the isolation and/or molecular analyses described below to verify the presence of Agrobacterium.
Fig. 1 (Colour online) Symptoms of crown gall caused by A. tumefaciens on naturally infected and artificially inoculated cannabis plants. (a) Gall development on roots of genotype ‘Sour Kush’. (b) Close-up of gall shown in (a). (c) Development of galls at the crown and roots of genotype SWD four weeks after artificial inoculation. (d, e) Galls at the crown of plants of genotype WHR resulting from natural infection. (f) Gall at the crown of a plant of genotype ‘Pink Kush’. (g) Galls on the middle stem of plants of genotype CPH following artificial inoculation. (h) Same as (g) but on genotype MBD. (i) Galls on the top of the stems of genotype SWD following artificial inoculation. (j) Same as (i) but on genotype HAP. (k) Same as (i) but on genotype CPH. Photos shown in I-L were taken 3 weeks after inoculation with A. tumefaciens.
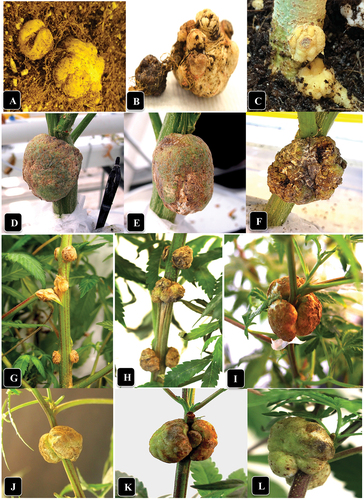
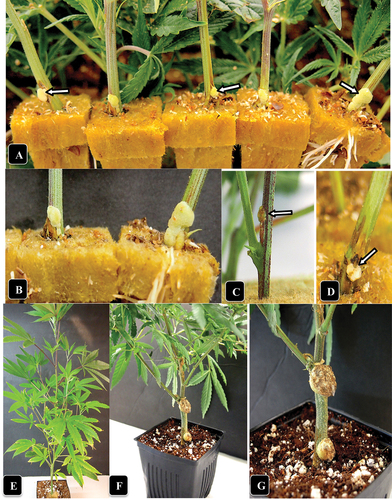
Fig. 2 (Colour online) Symptoms of crown gall caused by A. tumefaciens on naturally infected and artificially inoculated cannabis stem cuttings. (a) Gall development at the base of five cuttings in a propagation room (arrows). (b) Close-up of gall development. (c, d) Galls developing following artificial inoculation with A. tumefaciens after 7 days (c) and 14 days (d). (e) An inoculated cannabis plant shows no foliar symptoms as aresult of gall development at two sites. Photo was taken 4 weeks after inoculation. (f, g) Comparison of gall sizes on a cannabis plant genotype CPH following artificial inoculation with A. tumefaciens. the gall on the middle of the stem section is larger than on the crown.
Diseased tissue analysis
Sample A (roots) and samples E and F (stem cuttings) were used for the isolation of putative A tumefaciens. For sterilization, sample A was immersed in 70% EtOH for 1 min, followed by 10% bleach with 0.1% (v/v) Tween 20 for 20 min. Samples E and F, being smaller in size, were immersed in 70% EtOH for 30 s, followed by 10% bleach with 0.1% (v/v) Tween 20 for 1 min. All samples were rinsed in sterile distilled water for 1 min and then blotted dry with sterile blotting paper. For sample A, the gall was cut in half longitudinally with a sterile scalpel and tissue pieces (0.5 cm2) were taken from the region between the centre and the outer surface of the gall and minced into small pieces using a sterile scalpel. The tissue was suspended in 3–5 mL of sterile water and serially diluted up to 10−5. For each dilution, 300 µL was plated onto Petri dishes containing MacConkey agar, a medium selective for Gram-negative bacteria (Islam et al. Citation2010). For sample E, the entire gall was minced and added to 1 mL of sterile distilled water. Serial dilutions were not performed and 300 µL was plated onto MacConkey agar medium. For sample F, aliquots of 50, 100, and 200 µL of the 10−4 and 10−5 dilutions were first plated onto King’s B medium, then replica-plated onto MacConkey agar and finally replica-plated onto D1 medium (Kado and Heskett Citation1970), an Agrobacterium selection medium. For comparison, cultures of Pseudomonas, Enterobacter and Kluyvera spp. as well as Rhizobium radiobacter (Beijerinck and van Delden) Young et al. (A. tumefaciens, strain designation TT134 [6-1-2, CIP 104336], ATCC cat. 15955) were included as controls. Rhizobium radiobacter (cat. 15955) from ATCC was confirmed to be synonymous with Agrobacterium tumefaciens by ATCC and will be referred to as ATCC 15955 for the purposes of this study. The dishes were incubated overnight or up to 72 hr at ambient room temperature (21–23°C). Single bacterial colonies that displayed varying shades of pink on MacConkey agar and pale olive-green colonies on D1 medium (resembling the Agrobacterium control) were subcultured in 3 mL of Luria Broth or YEB medium and shaken overnight at 200 rpm and 26°C. After that, they were transferred to fresh MacConkey or nutrient agar plates and sent to the University of Guelph Agriculture and Food Laboratory for microbial 16S rDNA sequencing and identification to species level. For comparison, a confirmed isolate of A. tumefaciens GV3101 (obtained from Dr. Yu Xiang, Agriculture and Agri-Food Canada, Summerland, BC) was included for identification purposes.
Molecular analyses
Crown gall samples A, C, E (6 samples) and a subset of sample F (8 samples), as well as growing substrate (cocofibre) originating from the root zone of sample C, were used for total DNA extraction using Qiagen’s DNeasy Plant Mini Kit (cat. 69104). To determine the appropriate primers to be used for PCR identification of Agrobacterium, a number were selected based on previous studies that used primers specific to the nuclear genome, T-DNA region and virulence genes on the Ti-plasmid of various Agrobacterium strains (Haas et al. Citation1995; Suzaki et al. Citation2004; Bini et al. Citation2008). Plasmid DNA from ATCC 15955 and A. tumefaciens strain GV3101 was isolated from overnight liquid cultures from single colonies according to the manufacturer’s protocol of the Monarch Plasmid Miniprep Kit (NEB cat. T1010S) and used as positive controls in PCR reactions. DNA samples from healthy cannabis of genotypes ‘White Rhino (WHR)’, ‘Copenhagen Kush (CPH)’, and ‘Sweet Durga (SWD)’ were used as negative controls in PCR reactions. DNA was isolated according to the manufacturer’s protocol of the Qiagen DNeasy Plant Mini Kit (cat. 69104). The Qiagen Taq PCR Core Kit (cat. 201223) was used according to the manufacturer’s directions and PCR reactions were conducted in an Applied Biosystems 2720 Thermocycler (Thermofisher). The results from these PCR reactions were analyzed by gel electrophoresis on a 1% agarose gel.
Following these initial primer analyses, two primer sets were selected: iaaH-F2/iaaH-R1 and iaaH-F10/iaaH-R10. Both are designed to amplify conserved regions in the indole-acetic acid H (iaaH) gene of A. tumefaciens and A. vitis (now Allorhizobium vitis) (Bini et al. Citation2008). When both primer sets were tested, primer set iaaH-F2 (ACATGCATGAGTTATCGTTTGGAAT) and iaaH-R1 (GCATCAAGGTCATCGTAAAAGTAGGT) provided more easily interpreted results (). Primers iaaH-F10 and iaaH-R10 amplified bands from the cannabis DNA controls (), yielding results that were less clear than the iaaH-F2/iaH-R1 primer set. The PCR conditions for the iaaH-F2/iaaH-R1 primers were as follows: 1 cycle of 94°C for 3 min, then 40 cycles of 94°C for 30 s, 50°C for 45 s, 72°C for 1 min and a final extension of 72°C for 4 min. The positive A. tumefaciens (ATCC 15955) control and DNA from healthy cannabis plants were included. The PCR product was visualized on a 1% agarose gel. Bands of interest were extracted and purified using NEB’s Monarch DNA Gel Extraction Kit (cat. T1020S) according to the manufacturer’s instructions. The bands were sent to Eurofins Genomics for sequencing. Sequence identity was compared using NCBI’s BLAST (Zhang et al. Citation2000; Morgulis et al. Citation2008).
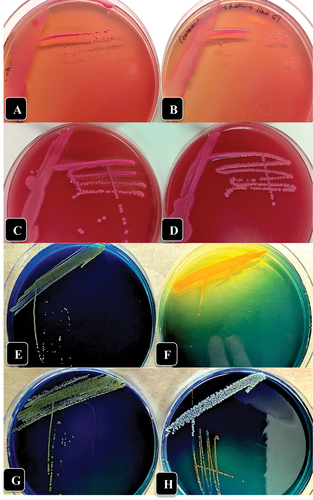
Fig. 3 (Colour online) Growth of three bacterial genera recovered from crown gall tissues in this study on McConkey agar (a-d) and D1 medium (e-h) compared to A. tumefaciens showing colony appearance after 72 hr of incubation at 23°C. (a) A. tumefaciens ATCC 15955. (b) Pseudomonas migulae. (c) Enterobacter sp. (d) Kluyvera cryocrescens. (e) A. tumefaciens ATCC 15955. (f) Enterobacter sp. (g) Pseudomonas migulae. (h) Kluyvera cryocrescens.
Artificial inoculation studies
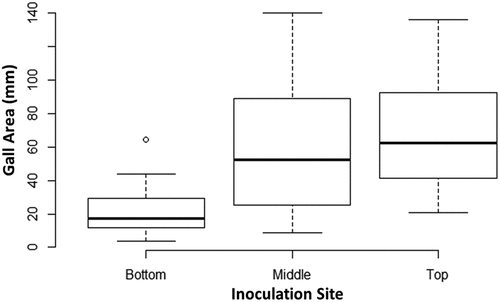
To assess the ability of A. tumefaciens to induce gall formation on cannabis plants, strain ATCC 15955 was used. Unrooted cuttings of genotype ‘Pink Kush’ (PNK) and rooted cuttings of cannabis genotypes ‘Copenhagen Kush’ (CPH), ‘Hash Plant’ (HAP), ‘Moby Dick’ (MBD), and ‘Sweet Durga’ (SWD) obtained as described by Punja et al. (Citation2021) were used. Unrooted cuttings were inserted into rockwool or peat plugs while rooted cuttings were transferred to potting medium composed of cocofibre and perlite (3:1, v/v) and grown under two Sunblaster brand 54-watt 6400k T5HO lights with a 24 h photoperiod. Plants were watered as needed with a solution of 1 mL/L Sensi Grow Coco pH Perfect A+B and 1 mL/L General Hydroponics Calimagic adjusted to a pH of 5.8–6.2 using Advanced Nutrients pH-Down (Scott and Punja Citation2021). After 2 weeks, plants were inoculated with an overnight culture of strain ATCC 15 955 grown in nutrient broth with an estimated O.D.600 reading of 1.1. Five to 10 plants of CHP, HAP, MBD and SWD were inoculated at three wound sites made along the main stem (at the bottom near the crown, in the centre, and towards the top) of each plant as well as on two petioles/plant (for SWD and MBD genotypes). Unrooted cuttings of PNK were only inoculated at the base of the cuttings. The inoculation sites were first wiped with 70% ethanol and a sterile syringe was inserted into the stem. A 10 µL droplet of the inoculum was placed on the wound and a piece of Parafilm was wrapped around the inoculation site. Control plants/cuttings received 10 µL of nutrient broth on the inoculation site. The plants were grown at ambient temperature (21–23°C) under a 24-hr photoperiod for four weeks while the inoculated cuttings were left in a humid chamber for 2 weeks. To determine whether A. tumefaciens could infect the roots of cannabis plants, roots of SWD near the surface of the potting medium were wiped with 70% ethanol and wound-inoculated as described above. A 10 µL drop of overnight culture was applied to the wound and covered with soil. Photos of galls were taken 3 weeks post-inoculation and the circular tool in ImageJ (Schneider et al. Citation2012) was used to measure the area of each gall. The gall sizes at each inoculation site on the four strains were analyzed for differences using ANOVA and means separation was achieved post-hoc using Tukey’s HSD test (p = 0.05) (). Gall area was also compared between inoculation sites (bottom, middle, top) of all strains using ANOVA and means separation achieved post-hoc using Tukey’s HSD test (p = 0.05).
Table 1. Comparison of crown gall development on four genotypes of cannabis (Cannabis sativa L.) plants at 3 weeks post-inoculation at three inoculation sites.
For re-isolation of A. tumefaciens to fulfill Koch’s postulates, two galls each on CPH plants inoculated at the top of the stem after 4 weeks and at 10 weeks were assayed. The galls were surface sterilized in 70% EtOH for 1 min, followed by 10% bleach with 0.1% (v/v) Tween 20 for 15 min, rinsed twice in sterile double distilled water for 1 min and dried on sterile blotting paper. The outer layer of tissues surrounding the gall was removed using a sterile scalpel and the inner tissues were minced into small pieces, which were suspended in 10 mL of sterile distilled water for 15 min and then 100–300 µL was plated onto Petri dishes containing MacConkey agar. Dishes were incubated for 48–72 h at ambient room temperature (21–23°C). Single colonies that were light pink in colour were transferred to Luria Broth medium and grown overnight at 26°C with shaking at 225 rpm. A sterile inoculation loop was used to streak out the cell culture onto fresh MacConkey agar plates. Cultures were sent for microbial identification to the Agriculture and Food Laboratory, University of Guelph.
Results
Crown gall symptoms and diseased tissue analysis
The symptoms observed on cannabis plants that displayed crown and root galls are shown in . On genotype ‘Sour Kush’, galls measuring 1–1.5 cm in diameter were observed on roots below the soil surface (), and when removed from the soil, the root gall (sample A) measured up to 2 cm in diameter (). Following artificial inoculations made on roots and the crown tissue at the soil line of genotype SWD using strain ATCC 15955, similar-appearing galls were formed within 4 weeks that were yellowish-white in colour and measured 1–1.5 cm in diameter. On the crowns of plants of genotype ‘White Rhino’, galls measuring 4–5 cm in diameter were observed () (representing samples B, C); on genotype ‘Pink Kush’, similar-sized galls were observed () (sample D). On naturally infected stem cuttings of ‘Pink Kush’, galls were creamy-white in appearance and measured 2–3 mm in diameter (representing sample E) (). They all formed galls at a nodal site where a leaf had been removed close to surface of the rockwool (). Identical-appearing galls were observed on sample F.
Root gall sample A and stem cutting gall samples E and F were surface-sterilized and tissues were minced using a sterile scalpel and plated onto agar media to isolate A. tumefaciens. Single bacterial colonies that displayed varying shades of pinkish-red on MacConkey agar and light olive-green colonies on D1 medium (all resembling the Agrobacterium controls) () were subcultured for identification using 16S rDNA sequencing. The following microbes that displayed varying shades of pink on MacConkey agar were identified: Pseudomonas spp. (P. canadensis, P. fluorescens, P. simiae, or P. poae), Kluyvera cryocrescens, Enterobacter spp. (E. hormaechei or E. cloacae) and Raoultella terrigena (). The microbes that displayed light olive-green colonies on medium D1 were identified primarily as Pseudomonas spp. (P. migulae, P. tensinigenes, P. umsongensis, P. veronii or P. fildesensis, P. extremaustralis, or P. lutea) (). The Enterobacter spp. (E. mori, E. ludwigii, E. roggenkampi,i E. asburiae) produced a distinct yellow halo and could be distinguished from the other bacteria on D1 medium (). Agrobacterium tumefaciens was not identified from any of these samples. The positive control A. tumefaciens was confirmed to be able to grow on both MacConkey agar and D1 medium, producing pink colony morphologies that overlapped with Pseudomonas spp. and Enterobacter spp. on MacConkey agar and pale green colonies on D1 medium that overlapped with Pseudomonas spp.
Molecular analyses
DNA was extracted from gall sample A (on roots), sample C (on crown), sample E (6) and sample F (8) (on stem cuttings) and PCR was conducted with primers iaaH-F2 and iaaH-R1. A band positioned between 250 bp and 500 bp was amplified from the following samples: gall sample C (, lane 2), ATCC 15955 (, lane 3), and galls of genotypes CPH and SWD artificially inoculated with ATCC 15955 (, lanes 5 and 7). The band in gall sample C was faint (, lane 2) compared to the bright bands seen in the positive Agrobacterium controls (, lanes 3, 5, and 7). No band of the expected size was obtained from root sample A (, lane 1) or sample F (8) (data not shown). Positive bands were also obtained from tissue sample E (small galls on cuttings 1–6) ( lanes 3–14) as well as from ATCC 15 955 (, lane 1), and galls of genotype CPH artificially inoculated with ATCC 15 955 (, lane 2). The bands from samples C and E were extracted, purified and sequenced and aligned to produce a consensus sequence of 390 bp in size, which showed 99.74% similarity (and 100% query coverage) to A. tumefaciens accession CP033030.1 (strain A6 plasmid pTiA6), accession CP032920.1 (strain 15 955 plasmid pTi15955), accession CP026926.1 (strain 1D1609 plasmid pTiD1609), accession KY000061.1 (strain CFBP2413 plasmid pTi_CFBP2413), and A. fabacearum accession KY000030.1 (strain CFBP5767 plasmid pTi_CFBP5767). These findings confirm the presence of A. tumefaciens in naturally infected samples C and E but not in A.
Fig. 4 PCR analysis for presence of Agrobacterium in cannabis tissue samples using primers iaaH-F2 and iaaH-R1. (a) Lane L = 1 kb Plus DNA ladder; lane 1 = root gall sample A; lane 2 = crown gall sample C; lane 3 = ATCC 15955 positive control; lane 4 = cannabis genotype WHR negative control; lane 5 = artificially inoculated gall using ATCC 15955 on genotype CPH; lane 6 = cannabis genotype CPH negative control; lane 7 = artificially inoculated gall using ATCC 15955 on genotype SWD; lane 8 = cannabis genotype SWD negative control. Red arrows indicate sequences that were identified as Agrobacterium using NCBI BLAST. (b) Lane L = 1 kb Plus DNA ladder; lane 1 = ATCC 15955 positive control; lane 2 = artificially inoculated gall using ATCC 15955 on genotype CPH; lanes 3–8 = galls on stems from 6 different cuttings of genotype PNK; lane 9 = artificially inoculated gall using ATCC 15955 on genotype SWD. (c) PCR analysis using primers iaaH-F10 and iaaH-R10. Lanes 1 and 2 = root gall sample A; lane 3 = crown gall sample C of genotype WHR; lanes 4 and 5 = ATCC 15955 positive control; lanes 6 and 7 = cannabis genotype WHR negative control; lanes 8 and 9 = artificially inoculated gall using ATCC 15955 on genotype CPH; lanes 10 and 11 = cannabis genotype CPH negative control; lanes 12 and 13 = artificially inoculated gall using ATCC 15955 on genotype SWD; and lanes 14 and 15 = cannabis genotype CPH negative control. The red arrows indicate the size of highly amplified bands between positive and negative samples.
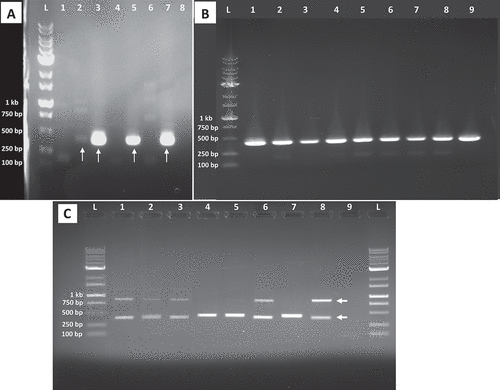
The sequences were analyzed using NCBI’s Conserved Domain Database and domains identified as indoleacetamide hydrolase (PRK07488 super family, Accession# cl30060) were confirmed for the bands isolated from gall sample C (, lane 2), stem cutting sample E (, lane 4) and the positive Agrobacterium controls (, lanes 3, 5, and 7). For the negative controls of cannabis DNA, namely CPH and SWD, the iaaH-F2 and iaaH-R1 primers produced faint bands between 100 bp and 250 bp in size (, lanes 4 and 6). These bands were extracted and sequenced and the sizes of the query sequences were 205 bp (lane 4) and 251 bp (lane 6). NCBI BLAST did not show any significant sequence similarity to these bands. However, when the bands were aligned using Geneious Prime software (2021.2.2) to a draft cannabis genome from strain ‘Purple Kush’ (canSat3, available on The Cannabis Genome Browser at http://genome.ccbr.utoronto.ca), they were shown to be present in the ‘Purple Kush’ genome, but their identity is unknown. No domains were identified in gall sample A (, lane 1) or in the cannabis DNA controls (, lanes 4 and 6) using NCBI’s Conserved Domain Database.
PCR using primers iaaH-F10 and iaaH-R10 resulted in strong amplification of two bands of sizes ~ 750 bp and > 500 bp in cannabis DNA samples (). These bands were also amplified in the A. tumefaciens controls and infected gall tissues (, lanes 4, 5, 8, 9, 12, 13); however, the ~ 750 bp band was very faint compared to that in the cannabis DNA samples (, lanes 1, 2, 6, 7, 10, 11, 14, 15). Using NCBI BLAST, the ~ 750 bp band in cannabis DNA samples showed ~ 98–99% similarity to Cannabis sativa UBP1-associated protein 2A (Accession XM_030648587.1, XM_030648586.1 and XM_030648585.1). The > 500 bp band showed ~ 98–99% similarity to Cannabis sativa early endosome antigen 1 (Accession XR_004008807.1, XM_030629599.1, XM_030629594.1 and XM_030629590.1). No domains were identified in these bands using NCBI’s Conserved Domain Database. The > 500 bp band in the WHR cannabis DNA sample (, lanes 6 and 7) showed chimeric identity, where 365 bp (88%) of the query sequence showed 98% identity to Cannabis sativa early endosome antigen 1 while 34 bp (8%) towards the 3’ end of the query sequence showed 100% identity to various A. tumefaciens Ti plasmids. The ~ 750 bp band in Agrobacterium positive controls (, lanes 4, 5, 8, 9, 12, 13) was too faint to isolate; however, the > 500 bp band showed ~ 98–99% identity with various A. tumefaciens Ti plasmids (Accession CP033030.1, CP032920.1 and CP026926.1). The sequences were analyzed using NCBI’s Conserved Domain Database and indoleacetamide hydrolase (PRK07488 super family, Accession# cl30060) was identified in the > 500 bp band of the A. tumefaciens controls only (, lanes 4, 5, 8, 9, 12, 13). Domains were not identified in the other cannabis DNA sequences.
Artificial inoculation studies
The symptoms that developed on cannabis genotypes which were wound-inoculated with strain ATCC 15955 are shown in . The galls on stems of genotypes CPH and MBD at the middle inoculation site are shown in . The galls that formed at the top of the stems were larger compared to those further down and can be seen for genotypes MBD (), SWD (), HAP () and CPH (). The galls that formed on stem cuttings of genotype PNK are shown in . To determine if there were any differences in crown gall development among cannabis genotypes, the gall area sizes were compared 3 weeks after inoculation at each of the inoculation sites (). The ANOVA analysis was not significant (p > 0.05), indicating that gall area was not affected by genotype. To assess gall development by inoculation site, gall area was compared between inoculation sites using ANOVA and post-hoc Tukey’s HSD test. Gall area was significantly affected by inoculation site (ANOVA, p = 6.71e−06) (), with galls at the middle and top inoculation sites being similar but significantly different from the crown (bottom) inoculation site (p = 2.512e−04 and p = 1.36e−05, respectively). A size comparison of the galls that developed at the crown and middle of the stem on genotype CPH is shown in . These galls appeared to have no effect on the growth of the cannabis plant 8 weeks after inoculation. However, when galls developed on stems at the top of the plant, some were seen to cause girdling and death of the affected stem (unpublished observations). To confirm that these galls were the result of A. tumefaciens infection and not a physical response to wounding through callus development, samples of galls were taken from genotype CPH at 4 and 10 weeks after inoculation with strain ATCC 15955 and crushed and plated onto MacConkey medium as described previously. Pinkish-colour colonies recovered were sent for sequencing and were positively identified as A. tumefaciens (99% identity). In addition, the control plants that were wounded and received nutrient broth medium did not show any gall/callus development.
Fig. 5 Comparison of gall area caused by A. tumefaciens at various inoculation sites. Gall area was compared between each of the 3 inoculation sites (n = 22) using the data from all genotypes. The box of each dataset represents the interquartile range (IQR) which contains the 3rd quartile (Q3 - top side of the box), the median value of all data (the middle line), and the first quartile (Q1 - bottom side of the box). The bars represent the maximum (Q3 + 1.5 × IQR) and minimum (Q1–1.5 × IQR) of the data. Outliers are represented by data points below or above the minimum and maximum, respectively. An ANOVA resulted in a p > 0.05 and a Tukey’s HSD test showed significant differences between top/bottom gall areas and middle/bottom gall areas. No significant difference was found between top/middle gall areas.
Discussion
Crown gall development on plants infected by A. tumefaciens is the result of insertion of the transfer DNA (T-DNA) on the Ti-plasmid into plant cells, where it integrates into the host genome (Chilton et al. Citation1977; Stachel et al. Citation1986; Gelvin Citation2000, Citation2003; Kado Citation2002). The expression of genes encoding auxins, cytokinins and opines from the T-DNA causes plant cells to continue to proliferate without the presence of Agrobacterium and on tissue culture media without phytohormones (Braun and White Citation1941). The successful recovery of A. tumefaciens from crown gall tissues on culture media can be affected by several factors (Cubero et al. Citation1999; Kado Citation2002). Frequently, these tissues harbour small numbers of the pathogen inside tumours, depending on the plant species. Conventional disease diagnosis based on the recovery of A. tumefaciens on non-selective or selective media, followed by inoculation into herbaceous hosts, e.g., tomato or tobacco, is time-consuming (Cubero et al. Citation1999). Therefore, PCR methods utilizing primers that amplify specific regions within the T-DNA or that target virulence genes on the Ti-plasmid or the nuclear genome are used to confirm presence of A. tumefaciens in plant galls compared to bacterial isolation on selective media (Cubero et al. Citation1999). In this study, PCR primers targeting the T-DNA region were used to confirm A. tumefaciens presence in both naturally infected and artificially infected cannabis gall tissues which was absent in healthy controls. Among two T-DNA primer sets evaluated in this study, the iaaH-F2 and iaaH-R1 primers were more reliable. Primers iaaH-F10 and iaaH-R10 produced bands in both A. tumefaciens and cannabis plant DNA controls. When sequenced, a similarity to early endosome antigen 1 was found with these primers in cannabis DNA while in A. tumefaciens, sequences showed indoleacetamide hydrolase conserved domains. These primers were not utilized further.
The difficulty in recovering A. tumefaciens from several crown gall samples in this study, such as those on roots (sample A) and on stem cuttings (samples E, F) when plated onto MacConkey agar and D1 medium, was likely due to the presence of competing micro-organisms. Gall age and tissue condition can influence the success of recovery of A. tumefaciens from putative crown gall tissues (Cubero et al. Citation1999; Kado Citation2002). From the stem cuttings, gall tissues were observed to contain a range of bacterial species belonging to the genera Pseudomonas, Enterobacter and Kluyvera. These bacteria likely outcompeted the slower-growing A. tumefaciens on the isolation plates. Their colony appearance on both McConkey agar and on D1 medium were similar to those of colonies of Agrobacterium, making it difficult to select representative colonies. In particular, colonies of Pseudomonas spp. were almost identical in morphology and colour to Agrobacterium on these isolation media. However, when artificially inoculated galls on plants grown in autoclaved potting medium under laboratory conditions, which likely contained fewer competitive microbes, were plated onto MacConkey agar, A. tumefaciens was recovered, confirming that this isolation medium was appropriate. As well, the control strain ATCC 15955 was confirmed to grow on both MacConkey and D1 media. Both media therefore supported growth of A. tumefaciens in pure cultures but not from naturally infected gall tissue that contained other competing bacterial species. Gram-negative bacteria belonging to the genera Pseudomonas and Enterobacter have been previously isolated from plant gall tissues (Vasanthakumar and McManus Citation2004; Harmon et al. Citation2018; Vuletin Selak et al. Citation2022). Pseudomonas spp. are commonly identified as a component of the core community of root colonizers of cannabis plants and represented a major component of the cannabis rhizosphere, phyllosphere and endosphere communities (Balthazar et al. Citation2022). Many Pseudomonas spp. have been demonstrated to have biocontrol activity against several pathogens of cannabis (Balthazar et al. Citation2022).
In the root gall sample A, the PCR result was negative using the iaaH primers for A. tumefaciens, suggesting this gall was the result of callus growth from a wound site on the roots or caused by a different organism. In contrast, gall sample C showed a positive weak band for A. tumefaciens T-DNA. Furthermore, we confirmed presence of the iaaH gene in young galls produced on stem cuttings of PNK (sample E) that displayed strong PCR bands, confirming the presence of A. tumefaciens. In the absence of an isolate of A. tumefaciens from cannabis plants, we selected an A. tumefaciens strain (ATCC cat. 15955) to demonstrate pathogenicity to cannabis plants. We observed that cannabis plants are highly susceptible to A. tumefaciens infection regardless of genotype. Gall size was larger higher up the stem of inoculated plants compared to the crown, perhaps because of greater translocation of nutrients and increased growth rate of these plant tissues.
The occurrence of crown gall on cannabis plants is considered to be of minor economic importance during production, given the low frequency of affected plants observed (0.01%) and the fact that affected plants did not show any foliar symptoms. The source of initial inoculum is unknown, but infection occurred early during propagation of stem cuttings under high humidity conditions and were seen at leaf removal sites. The crop preceding cannabis in this particular licenced facility was tomatoes, which are known to be susceptible to A. tumefaciens (Kado Citation2002); whether this served as a potential source of the pathogen remains to be determined.
The high susceptibility of various genotypes of cannabis to infection by A. tumefaciens, as demonstrated in this study, highlights the opportunity to conduct genetic transformation studies to introduce agronomically important genes using A. tumefaciens as a vector, e.g., to enhance disease resistance (Holmes and Punja Citation2021). In the first report of successful Agrobacterium-mediated transformation, Feeney and Punja (Citation2003) transformed suspension culture cells of hemp with A. tumefaciens strain EHA101 containing a phosphomannose isomerase (PMI) gene. Transgenic calli displayed a pink colour when induced with the appropriate substrate. Recently, Deguchi et al. (Citation2020) developed a method for Agroinfiltration to transiently express β-glucuronidase in flowers, leaves, stem, and roots of hemp cultivars. This approach was also used in gene silencing where the phytoene desaturase gene was silenced via transient hairpin RNA expression, resulting in an albino phenotype (Deguchi et al. Citation2020). Ahmed et al. (Citation2021) developed a nanoparticle-based transient gene transformation approach for C. sativa in which gold nanoparticles coated with various plasmid constructs were introduced through syringe infiltration. Zhang et al. (Citation2021) used Agrobacterium-mediated transformation to overexpress homologous developmental genes to promote callogenesis and shoot production in C. sativa. These recent studies highlight the ongoing interest in conducting genetic transformation experiments with cannabis and hemp. However, at the present time, there are no reports of the successful recovery of transgenic plants of either cannabis or hemp, due in part to the difficulty of regenerating plantlets from transformed cells in tissue culture (Feeney and Punja Citation2003). Recent advances in tissue culture methods should accelerate progress towards the recovery of such transgenic plants in the future (Andre et al. Citation2016; Holmes et al. Citation2021; Page et al. Citation2021). In conclusion, the results from this study demonstrate the occurrence of A. tumefaciens in cannabis production facilities, causing gall development on crown and stem tissues but with no other apparent visible symptoms such as reduced growth or vigour of affected plants.
Acknowledgments
We thank Dr. Yu Xiang for providing A. tumefaciens strain GV3101 and Cameron Scott for maintaining cannabis plants for this study. Funding was provided from the Natural Sciences and Engineering Research Council of Canada (NSERC), through a Collaborative Research and Development (CRD) Grant and an NSERC Alliance Grant, with matching industry funding from Agrima Botanicals and Pure Sunfarms.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed S, Gao X, Jahan A, Adams M, Wu N, Kovinich N. 2021. Nanoparticle-based genetic transformation of Cannabis sativa. J Biotech. 326:48–51. doi: 10.1016/j.jbiotec.2020.12.014.
- Andre CM, Hausman JF, Guerriero G. 2016. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 7:19. doi: 10.3389/fpls.2016.00019.
- Balthazar C, Joly DL, Filion M. 2022. Exploiting beneficial Pseudomonas spp. cannabis production. Front Microbiol. 12. doi: 10.3389/fmicb.2021.833172.
- Balthazar C, Novinscak A, Cantin G, Joly DL, Filion M. 2022. Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis cinerea and other cannabis fungal pathogens. Phytopathology. 112(3):549–560. doi: 10.1094/PHYTO-03-21-0128-R.
- Bini F, Kuczmog A, Putnoky P, Otten L, Bazzi C, Burr TJ, Szegedi E. 2008. Novel pathogen-specific primers for the detection of Agrobacterium vitis and Agrobacterium tumefaciens. Vitis. 47(3):181–189. doi: 10.5073/vitis.2008.47.181-189.
- Braun AC, White PR. 1941. Crown gall production by bacteria-free tumor tissues. Science. 94(2436):239–241. doi: 10.1126/science.94.2436.239.
- CABI. 2021. Rhizobium radiobacter (crown gall). Invasive species compendium. Wallingford (UK): CAB International. www.cabi.org/isc.
- Chilton MD, Drummond MH, Merlo DJ, Sclaky D, Montoya AL, Gordon MP, Nester EW. 1977. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 11(2):263–271. doi: 10.1016/0092-8674(77)90043-5.
- Cubero J, Martínez MC, Llop P, López MM. 1999. A simple and efficient PCR method for the detection of Agrobacterium tumefaciens in plant tumours. J App Microbiol. 86(4):591–602. doi: 10.1046/j.1365-2672.1999.00700.x.
- Deguchi M, Bogush D, Weeden H, Spuhler Z, Potlakayala S, Kondo T, Zhang ZJ, Rudrabhatla S. 2020. Establishment and optimization of a hemp (Cannabis sativa L.) agroinfiltration system for gene expression and silencing studies. Sci Rept. 10(1):3504. doi: 10.1038/s41598-020-60323-9.
- Farrand SK, van Berkum PB, Oger P. 2003. Agrobacterium is a definable genus of the family Rhizobiaceae. Int J Syst Evol Microbiol. 53(5):1681–1687. doi: 10.1099/ijs.0.02445-0.
- Feeney M, Punja ZK. 2003. Tissue culture and Agrobacterium-mediated transformation of hemp (Cannabis sativa L.). Vitro Cell Dev Biol Plant. 39(6):578–585. doi: 10.1079/IVP2003454.
- Gan HM, Savka MA. 2018. One more decade of Agrobacterium taxonomy. Curr Topics Microbiol Immunol. 418:1–14. doi: 10.1007/82_2018_81.
- Gelvin SB. 2000. Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol. 51(1):223–256. doi: 10.1146/annurev.arplant.51.1.223.
- Gelvin SB. 2003. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 67(1):16–37. doi: 10.1128/MMBR.67.1.16–37.2003.
- Haas JH, Moore LW, Ream W, Manulis S. 1995. Universal PCR primers for detection of phytopathogenic Agrobacterium strains. App Environ Microbiol. 61(8):2879–2884. doi: 10.1128/aem.61.8.2879-2884.1995.
- Harmon CL, Timilsina S, Bonkowski J, Jones DD, Sun X, Vallad GE, Sepulveda LR, Bull C, Jones JB. 2018. Bacterial gall of Loropetalum chinense caused by Pseudomonas amygdali pv. loropetali pv. nov. Plant Dis. 102(4):799–806. doi: 10.1094/PDIS-04-17-0505-RE.
- Holmes JE, Lung S, Collyer D, Punja ZK. 2021. Variables affecting shoot growth and plantlet recovery in tissue cultures of drug-type Cannabis sativa L. Front Plant Sci. 12:12. doi:10.3389/fpls.2021.732344.
- Holmes JE, Punja ZK. 2021. Development of a tissue culture-based Agrobacterium-mediated transformation system for Cannabis sativa L. (marijuana). Vitro Cell Dev Biol Plant Proceed. Annual Meeting (abstract).
- Islam MS, Akter M, Rahman MA, Rahman MM, Akhtar MM, Alam MF. 2010. Isolation of Agrobacterium tumefaciens strains from crown gall samples of dicots plants in Bangladesh. Curr Res Bacteriol. 3(1):27–36. doi: 10.3923/crb.2010.27.36.
- Kado CI. 2002. Crown gall. Plant Health Instructor. [accessed 2021 Aug 20]. doi: 10.1094/PHI-I-2002-1118-01.
- Kado CI. 2010. Plant bacteriology. St. Paul (MN): APS Press.
- Kado CI. 2014. Historical account on gaining insights on the mechanism of crown gall tumorigenesis induced by Agrobacterium tumefaciens. Front Microbiol. 5(340):1–15. doi: 10.3389/fmicb.2014.00340.
- Kado CI, Heskett MG. 1970. Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology. 60(6):969–976. doi: 10.1094/phyto-60-969.
- Kuzmanović N, Puławska J, Prokić A, Ivanović M, Zlatković N, Jones JB, Obradović A. 2015. Agrobacterium arsenijevicii sp. nov., isolated from crown gall tumors on raspberry and cherry plum. Syst App Microbiol. 38(6):373–378. doi: 10.1016/j.syapm.2015.06.001.
- Lindström K, Young JPW. 2011. International committee on systematics of ProkaryotesSubcommittee on the taxonomy of Agrobacterium and Rhizobium: minutes of the meeting, 7 September 2010, Geneva, Switzerland. Int J Syst Evol Microbiol. 61(12):3089–3093. doi: 10.1099/ijs.0.036913-0.
- Mafakheri H, Taghavi SM, Puławska J, De Lajudie P, Lassalle F, Osdaghi E. 2019. Two novel genomospecies in the Agrobacterium tumefaciens species complex associated with rose crown gall. Phytopathology. 109(11):1859–1868. doi: 10.1094/PHYTO-05-19-0178-R.
- Matthysse AG. 2006. The genus Agrobacterium. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K Stackebrandt E, editors. The prokaryotes. New York (NY): Springer; p. 91–114. doi: 10.1007/0-387-30745-1_5.
- McPartland JM. 1992. A review of cannabis diseases. J Intern Hemp Assoc. 3:19–23.
- McPartland JM. 2003. Diseases of hemp (Cannabis sativa L.). American Phytopathological Society. www.apsnet.org/edcenter/resources/commonnames/Pages/Hemp.aspx.
- Moore LW, Bouzar H, Burr T. 2001. II. Gram-negative bacteria. In: Schaad N, Jones J Chun W, editors Laboratory guide for identification of plant pathogenic bacteria. 3rd ed. St. Paul (MN): APS Press; p. 17–35.
- Moore LW, Putnam ML. 2020. Crown gall disease of nursery crops. Pacific Northwest Pest Management Handbooks. [accessed 2021 Aug 26]. https://pnwhandbooks.org/plantdisease/pathogen-articles/pathogens-common-many-plants/bacteria-other-prokaryotes/crown-gall.
- Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agarwala R, Schäffer AA. 2008. Database indexing for production MegaBLAST searches. Bioinformatics. 24(16):1757–1764. doi: 10.1093/bioinformatics/btn322.
- Mousavi SA, Osterman J, Wahlberg N, Nesme X, Lavire C, Vial L, Paulin L, de Lajudie P, Lindstrom K. 2014. Phylogeny of the Rhizobium–Allorhizobium–Agrobacterium clade supports the delineation of Neorhizobium gen. nov. Syst Appl Microbiol. 37(3):208–215. doi: 10.1016/j.syapm.2013.12.007.
- Mousavi SA, Willems A, Nesme X, de Lajudie P, Lindström K. 2015. Revised phylogeny of Rhizobiaceae: proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst Appl Microbiol. 38:84–90. doi:10.1016/j.syapm.2014.12.003.
- Page SRG, Monthony AS, Jones AMP. 2021. DKW basal salts improve micropropagation and callogenesis compared with MS basal salts in multiple commercial cultivars of Cannabis sativa. Botany. 99(5):269–279. doi: 10.1139/cjb-2020-0179.
- Pulawska J. 2010. Crown gall of stone fruits and nuts, economic significance and diversity of its causal agents: tumorigenic Agrobacterium spp. J Plant Pathol. 92:S1.87–S1.98.
- Puławska J, Warabiedaa W, Ismail E. 2016. Identification and characterization of bacteria isolated from crown galls on stone fruits in Poland. Plant Pathol. 65(6):1034–1043. doi: 10.1111/ppa.12482.
- Punja ZK. 2021. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag Sci. 77(9):3857–3870. doi: 10.1002/ps.6307.
- Punja ZK, Scott C, Lung S. 2021. Several Pythium species cause crown and root rot on cannabis (Cannabis sativa L., marijuana) plants grown under commercial greenhouse conditions. Can J Plant Pathol. 44(1):66–81. doi: 10.1080/07060661.2021.1954695.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat Method. 9(7):671–675. doi: 10.1038/nmeth.2089.
- Schroth M, Weinhold A, McCain A, Hildebrand D, Ross N. 1971. Biology and control of Agrobacterium tumefaciens. Hilgardia. 40(15):537–552. doi: 10.3733/hilg.v40n15p537.
- Scott C, Punja ZK. 2021. Evaluation of disease management approaches for powdery mildew on Cannabis sativa L. (marijuana) plants. Can J Plant Pathol. 43(3):394–412. doi: 10.1080/07060661.2020.1836026.
- Stachel SE, Nester EW, Zambryski PC. 1986. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci U S A. 83:379–383. doi:10.1073/pnas.83.2.379.
- Suzaki K, Yoshida K, Sawada H. 2004. Detection of tumorigenic Agrobacterium strains from infected apple saplings by colony PCR with improved PCR primers. J Gen Plant Pathol. 70:342–347. doi:10.1007/s10327-004-0133-8.
- The UniProt Consortium. 2021. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49:D1. doi: 10.1093/nar/gkaa1100.
- Vasanthakumar A, McManus PS. 2004. Indole-3-acetic acid-producing bacteria are associated with cranberry stem gall. Phytopathology. 94(11):1156–1275. doi: 10.1094/PHYTO.2004.94.11.1164.
- Vuletin Selak G, Raboteg Božiković M, Abrouk D, Bolčić M, Žanić K, Perica S, Normand P, Pujic P. 2022. Pseudomonas ST1 and Pantoea Paga strains cohabit in olive knots. Microorganisms. 10(8):1529. doi: 10.3390/microorganisms10081529.
- Young JM, Kuykendall LD, Martinez-Romero E, Kerr A, Sawada H. 2001. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int J Syst Evol Microbiol. 51(Pt 1):89–103. doi: 10.1099/00207713-51-1-89.
- Young JM, Kuykendall LD, Martínez-Romero E, Kerr A, Sawada H. 2003. Classification and nomenclature of Agrobacterium and Rhizobium – a reply to Farrand et al. (2003). Int J Syst Evol Microbiol. 53(5):1689–1695. doi: 10.1099/ijs.0.02762-0.
- Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J Comput Biol. 7(1–2):203–214. doi: 10.1089/10665270050081478.
- Zhang X, Xu G, Cheng C, Lei L, Sun J, Xu Y, Deng C, Dai Z, Yang Z, Chen X, et al. 2021. Establishment of an Agrobacterium -mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in hemp (Cannabis Sativa L.). Plant Biotechnol J. 19(10):1979–1987. doi: 10.1111/pbi.13611.
