Abstract
The incidence of Hop latent viroid (HLVd) affecting cannabis plants in licenced production facilities in Canada during 2020–2023 was determined by RT-PCR analyses of 15 947 samples from nine provinces. Positive detection ranged from 5.3% to 92% of samples submitted, depending on province and year of sampling. The average country-wide HLVd infection incidence was 25.6%. Symptoms on affected plants varied with plant growth stage, and ranged from asymptomatic to mild leaf curl and mottling on stock (mother) plants and on vegetative plants, to extreme stunting and reduced inflorescence development on flowering plants. All symptomatic and some asymptomatic plants contained a 256 nucleotide RNA fragment with 100% sequence homology to HLVd from GenBank. The viroid was detected in various tissues of stock, vegetative, and flowering plants, including the uppermost leaves, at middle and lower positions in the canopy, and in the roots. The incidence of infection varied with the cannabis genotype. Flowering plants displayed yellowing or darkening of inflorescence leaves surrounding the pistillate flowers. Reductions of 12–42% in inflorescence stem lengths, fresh weights, and plant heights were observed in infected plants compared to noninfected plants, depending on the genotype. Levels of tetrahydrocannabinol (THC) and terpenes in diseased inflorescences were also significantly lower. The development of glandular trichomes which produce and store cannabinoids and terpenes was greatly reduced by HLVd infection. The affected trichomes had shorter stalk lengths and smaller glandular head sizes, and appeared shrivelled upon drying of the inflorescences. Hop latent viroid poses a significant economic threat to the cannabis industry.
Résumé
L’incidence du viroïde latent du houblon (VdLH) s’attaquant aux plants de cannabis dans les installations de production autorisées au Canada de 2020 à 2023 a été déterminée par des analyses RT-PCR de 15 947 échantillons provenant de neuf provinces. Chez les échantillons soumis, la détection positive a varié de 5,3 % à 92 %, selon la province et l’année d’échantillonnage. La moyenne pancanadienne de l’incidence de l’infection par le VdLH était de 25,6 %. Les symptômes associés aux plants touchés différaient en fonction du stade de croissance, variant d’une frisolée légère sur les feuilles ainsi que de marbrures sur le matériel de départ (plantes mères) et les plantes végétatives, jusqu’à un rabougrissement extrême et à une réduction du développement des inflorescences chez les plants en fleurs. Tous les plants symptomatiques et certains des plants asymptomatiques contenaient un fragment d’ARN de 256 bp possédant une homologie de séquence de 100 % avec le VdLH de la GenBank. Le viroïde a été détecté dans divers tissus du matériel de départ, des plantes végétatives et des plants en fleurs, y compris sur les feuilles les plus hautes, dans les portions médianes et inférieures de la canopée, et sur les racines. L’incidence de l’infection variait en fonction du génotype du cannabis. Les plants en fleurs affichaient un jaunissement ou un noircissement des feuilles de l’inflorescence entourant les fleurs pistillées. Des réductions de 12 % à 42 % des longueurs de la tige de l’inflorescence, des poids frais et des hauteurs des plants ont été observées chez les plants infectés, comparativement aux plants sains, selon le génotype. Les taux de
Introduction
Hop latent viroid (HLVd) is an emerging disease threat to cannabis (Cannabis sativa L., high-THC containing strains or marijuana) production in Canada and the USA. HLVd is a single-stranded RNA molecule, 256 nucleotides in length, that belongs to the genus Cocadviroid in the family Pospiviroidae, which also includes Potato spindle tuber viroid (PSTVd) (Takeda and Ding Citation2009; Sano Citation2021). The pathogen is reported to infect hop plants (Humulus lupulus L., a member of the Cannabaceae family to which cannabis also belongs) and is widespread in most hop yards worldwide (Eastwell and Nelson Citation2007; Pethybridge et al. Citation2008). Symptoms may not be apparent on infected hop plants (latent or asymptomatic) but a reduction in yield and quality of hop cones has been reported on certain cultivars (Barbara et al. Citation1990; Adams et al. Citation1992). The natural host range of HLVd is currently limited to H. lupulus and H. japonicus but there may be other as-yet undetermined hosts of this viroid.
The first published reports of HLVd infecting cannabis (marijuana) plants were from California in 2019 (Bektaş et al. Citation2019; Warren et al. Citation2019). A report suggested that the pathogen might have originated from a 20-year-old hop plant in 2011 and subsequently been spread to cannabis, possibly through mechanical transmission (Scheck Citation2020), which is its primary means of dissemination within hop yards (Adams et al. Citation1992; Pethybridge et al. Citation2008). Since it was first officially reported in 2019, the viroid appears to have spread to other regions of the USA and Canada where cannabis is cultivated due to the distribution of infected plant materials. However, published reports confirming its range of distribution are lacking and the frequency of HLVd occurrence on cannabis plants in Canada or the USA is unknown. The first report of HLVd on cannabis in Canada was from a plant sample originating from British Columbia in 2020 (K. Wang, personal communication); however, the pathogen was likely already widely distributed but remained undetected before then. The extent to which HLVd is currently distributed in other provinces in Canada where cannabis is cultivated is unknown. HLVd has been recently reported from outdoor hemp fields in Colorado (Chiginsky et al. Citation2021) and Washington (Jarugula et al. Citation2023) in mixed infections with other viral pathogens and is very prevalent on hops in Washington state (Eastwell and Nelson Citation2007).
Symptoms of infection by HLVd on cannabis plants first reported from California included stunted plants, brittle stems, reduced inflorescence size, and lower trichome numbers on affected plants (Bektaş et al. Citation2019; Warren et al. Citation2019). The terms ‘dudding’ and ‘stunting disease’ were used to describe HLVd symptoms on cannabis plants. These reports indicate that HLVd has the potential to similarly impact cannabis production in Canada, but no prior studies have been conducted to establish the extent to which HLVd is present, nor the corresponding symptoms that are produced on infected plants.
Due to the lack of previous reports on the epidemiology and symptomology of HLVd on cannabis in Canada, this study was conducted with the following objectives: (i) to establish the distribution of HLVd in all provinces of Canada in which cultivation of cannabis occurs from samples collected over 3 years (2020–2022); (ii) to describe the symptoms of HLVd infection in multiple cannabis genotypes grown under greenhouse conditions; (iii) to conduct RT-PCR to detect HLVd in tissues of different genotypes of cannabis; (iv) to quantify the impact of HLVd on growth and inflorescence development in four cannabis genotypes; and (v) to describe the impact of HLVd infection on trichome development, morphology and production of tetrahydrocannabinol (THC) and terpenes. Preliminary results from this study have been previously published (Punja et al. Citation2023b).
Materials and methods
Survey of Hop latent viroid distribution in Canada
During the period January 2020 to December 2022, the cumulative number of plant samples submitted by anonymous cannabis producers to a diagnostic laboratory (A and L Laboratories, London, ON, www.alcanada.com) for analysis for the presence of Hop latent viroid (HLVd) using an in-house RT-PCR assay was summarized. The samples were mostly comprised of leaves submitted from nine provinces in Canada. The number of samples that tested positive for HLVd from the total number submitted was analyzed by province and year of submission. The cumulative number of samples tested over 3 years was 15 947 ().
Table 1. Incidence of Hop latent viroid in cannabis plant samples submitted to a diagnostic laboratory from provinces in Canada during 2020–2022.
Observations of disease development and symptomology
Plant growth conditions
Observations of symptom development on cannabis plants were made in a Health Canada approved facility in which plants were grown under greenhouse conditions using a hydroponic method of cultivation with cocofibre or rockwool as a substrate. A range of different genotypes (strains) were grown during the fall-winter months (October-April) and spring-summer months (May-September) during 2021–2022, which generally included 3–4 cropping cycles per year. In the winter period, plants were provided with supplementary lighting to achieve 1,600 uEin m−2 of photosynthetically active radiation (PAR) using sodium lamps emitting a wavelength centred at 590 nm. During the summer period, supplementary lighting was provided where needed on overcast days to achieve the required PAR.
Plant propagation
All cannabis plants were initiated from vegetative cuttings taken from stock (mother) plants of various genotypes, which were first rooted in a propagation room for 2 weeks under 80–85% relative humidity, and then grown in a greenhouse nursery area with a temperature range of 23–28°C and relative humidity of 60–70%. After 3 weeks of vegetative growth, the plants were transferred to a large flowering room where the photoperiod was adjusted to 12 hr light and 12 hr dark to induce flowering. The plants were grown under these conditions for 7–8 weeks.
Observations of disease symptoms and sampling
At weekly intervals over the period 2021–2022, cannabis plants that were grown as stock plants, as vegetative plants, and following transfer to the flowering room, were visually assessed for symptoms of stunting, leaf curl, mosaic patterns, reduced development of inflorescences (flower heads), and unusual growth habits (). The distribution of symptomatic plants (mostly showing stunted growth and reduced inflorescence development) within the rows in the flowering room was recorded for four genotypes in one crop cycle during 2022. Photographs were taken and symptoms were recorded, which was followed by molecular confirmation of the presence of HLVd (see below).
Fig. 1 Symptoms associated with Hop latent viroid infection on stock (mother) plants of cannabis. (a, b) curling of leaves at the top of the plant on the younger leaves. (c) mosaic and streaking on young developing leaves. (d) stunted growth of a diseased plant (right) compared to a healthy plant (left). (e, f) sideways (lateral) growth of bottom stems on a diseased plant. (g) inward curling of leaf margins on diseased plants. (h) three-month-old stock plant from which leaf samples were collected at various positions in the canopy, labelled 1–4. (i) RT-PCR gel based on samples of leaves from (h) showing multiple bands corresponding to HLVd. The positive control and negative control are shown. MW markers are indicated on the 1 kb ladder.

Fig. 2 (a) Disease incidence (percentage of infected plants) on cannabis stock plants sampled over a 6–8-month period representing 23 genotypes. All genotypes were confirmed to have HLVd present in root tissue samples by RT-PCR. The range of infected plants varied from 5% to 40%. (b) distribution of cannabis plants with visible symptoms of stunting and reduced inflorescence growth (plants marked with) in two flowering rooms represented by four different genotypes. The (x) represents asymptomatic plants.
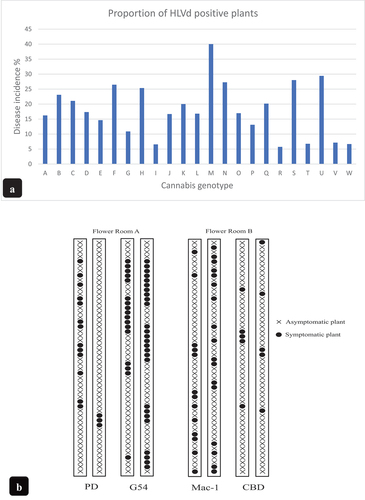
Fig. 3 Symptoms due to Hop latent viroid infection on flowering plants of cannabis. (a) comparison of symptomatic plant (left) with asymptomatic plant (right) after two weeks into the flowering period. Leaf samples were collected for analysis at the positions indicated by (1–4). (b) RT-PCR gel showing bands characteristic of HLVd present in the symptomatic plants shown in (a). The asymptomatic plant showed very faint bands only in the roots. (c-f) comparison of symptomatic plants (arrows) with asymptomatic plants at 3–4 weeks into flower development (c, d) and at 7–8 weeks (e, f) of development. (g, h) reduction in root volume and root mass as a result of HLVd infection in cannabis genotypes ‘Powdered Donuts’ (g) and genotype ‘Mac-1’ (h). Arrows in all photos show the infected plants.

Molecular confirmation of Hop latent viroid presence
Sample collection
From stock plants in the nursery area, the percentage of infected plants was estimated on a total of 23 genotypes grown under similar conditions over a period of 6–8 months and confirmed using molecular analysis. The stock plants were sampled weekly at random and leaf and root tissues were sent to a commercial laboratory for confirmation of HLVd presence using RT-qPCR (3 Rivers Biotech., www.3riversbiotech.com). Following that, from a select number of stock plants confirmed to be infected with HLVd, as well as visibly symptomatic plants in the first 2–3 weeks of the flowering period, more extensive sampling was conducted by removing leaves from specific locations on the plant (top, middle and bottom) within the canopy. On flowering plants, samples of leaves, inflorescences, and roots were obtained from symptomatic and asymptomatic plants and verified to contain HLVd by molecular analysis. For root sampling, fine roots emerging from the rockwool block or the bottom of the cocofibre block were removed with forceps to minimize damage and disturbance to the remainder of the root system. Tissue samples representing leaves, roots and inflorescences were placed in Ziploc bags and transported to the laboratory where they were placed at 4°C for 1–2 days prior to analysis or frozen at −80°C for later analysis.
Molecular analysis
Samples of ca. 50 mg of plant tissue were used for nucleic acid extraction. They were ground to a powder with liquid nitrogen and processed using the Qiagen RNeasy Plant Mini Kit (cat. #74904) according to the manufacturer’s instructions. The final RNA product was eluted with 52 µL nuclease-free H2O. The QIAGEN OneStep RT-PCR Kit (cat. #210212) was used for reverse transcription and PCR amplification. The reaction mixture contained 14 µL of water, 5 µL of 5× reaction buffer, 1 µL of dNTPs (10 mM), 1.5 µL each of HLVd primers (Eastwell and Nelson Citation2007): F- 5’-ATACAACTCTTGAGCGCCGA-3’, R 5’-CCACCGGGTAGTTTCCCAACT-3’, 1 µL of RNA template, and 1 µL of enzyme mix, resulting in a total volume of 25 µL. All PCR amplifications were performed in a MyCycler thermocycler (BIORAD) with the following program: 30 min at 50°C, 15 min at 95°C, followed by 35 cycles of 30 sec at 94°C, 30 sec at 58°C, 60 sec at 72°C, and final extension at 72°C for 10 min. The resulting PCR products were run on a 1% TAE agarose gel and images were captured with E-gel imager (Life Technologies). Bands of the expected size (ca. 256 and 512 bp) were purified with QIAquick Gel Extraction Kit and sent to Eurofins Genomics (Eurofins MWG Operon LLC 2016, Louisville, KY) for sequencing. The resulting sequences were compared to the corresponding HLVd sequences from the National Centre for Biotechnology Information (NCBI) GenBank database to confirm identity.
Impact of Hop latent viroid on plant growth, inflorescence development and THC content
Plants of four cannabis genotypes approaching harvest (seven weeks after the initiation of the flowering period) and displaying symptoms of HLVd infection (reduced inflorescence growth and stunting) were selected for sampling during February-April 2022. The plants were confirmed to contain the viroid by RT-PCR analysis. The plants were located in rows adjacent to each other and five replicate plants were selected on which the following measurements were made. Plant height (cm) from the bottom to the highest leading inflorescence stem was recorded; the height of five leading inflorescence stems from the top to the junction of the first secondary branch was measured and averaged; the uppermost 15 cm of five of these stems were cut and weighed to obtain fresh weight; inflorescence leaves were removed and the average length was recorded; the five inflorescence stem segments measuring 15 cm were dried in a drying room to 12–14% moisture content and sent to a commercial laboratory for THC and terpene analysis (Caro Analytical Services, Richmond, BC, www.caro.ca). The means and standard errors (n = 5) were calculated from these data.
Comparative trichome development on infected and healthy plants
Visibly symptomatic plants of genotypes ‘Headband’ and ‘Mac-1’ confirmed to be HLVd positive by RT-PCR at harvest time were analyzed for trichome development by light and scanning electron microscopy. Small segments of bract tissues were gently removed from inflorescences using a pair of forceps. For light microscopy, bract segments of both genotypes were examined under a dissecting microscope at 50× magnification and photographed. For scanning microscopy, tissue pieces measuring 0.5 × 0.5 cm were processed as follows. Tissue segments were adhered to a stub using a graphite-water colloidal mixture (G303 Colloidal Graphite, Agar Scientific, UK) and Tissue-Tek (O.C.T. Compound, Sakura Finetek, NL). The samples were submerged in a nitrogen slush for 10–20 s to rapidly freeze them. After freezing, the sample was placed in the preparation chamber of a Quorum PP3010T cryosystem attached to a FEI Helios NanoLab 650 scanning electron microscope (4D Labs., Simon Fraser University). The frozen samples were sublimed for 5 min at −80°C, after which a thin layer of platinum (10 nm thickness) was sputter-coated onto the samples for 30 s at a current of 10 mA. The samples were moved into the SEM chamber and the electron beam was set to a current of 50 pA at 3 kV. Images were captured at a working distance of 4 mm, at a scanning resolution of 3072 × 2207 collected over 128 low-dose scanning passes with drift correction.
Observations of trichome morphology and development
Measurements of trichome stalk length and glandular head size and density per mm2 were determined by examining and making measurements from 25 SEM images of genotypes ‘Mac-1’ and ‘Headband’. The inflorescence samples from the same plant were then subjected to commercial drying conditions (4–5 days in a drying room at 21–23 C and 50–55% relative humidity) and representative samples were sent for analysis of THC and terpene content to Caro Analytical Services.
To further determine the impact of HLVd on trichome density, images taken of infected and healthy cannabis inflorescences of genotype ‘Headband’ were obtained as described by Sutton et al. (Citation2023). Magnified images were then subjected to the deep learning neural network ‘DO-U-Net’ designed for instant segmentation of repeated convex shapes in 2D microscopy and aerial images, where a dual-decoder U-Net architecture predicts a semantic mask describing foreground membership (trichome glands), and the edges of each trichome gland (size) in the image (Sutton et al. Citation2023). This method provided a comparative spatial distribution pattern of trichomes and gland size in HLVd-infected and healthy plants.
Impact of Hop latent viroid infection on macro- and micro-nutrients levels in foliage and terpene levels in inflorescences
Plants of two cannabis genotypes confirmed to be HLVd positive by RT-PCR analysis (G-54 and ‘Powdered Donuts’) were selected for macro- and micro-nutrient analysis. Foliage tissues from flowering plants in week 7 of the flowering period (one week prior to harvest) were collected in duplicate from the two genotypes and sent to A & L Laboratories for a complete tissue nutrient analysis. Inflorescence samples were forwarded to Caro Analytical Services following harvest and drying under standard commercial conditions for an analysis of terpene compounds. A comparison was made of the nutrient and terpene profiles in healthy and HLVd-infected plants.
Results
Survey of Hop latent viroid distribution in Canada
In the first year of sampling (2020) following the detection of HLVd in British Columbia, the highest number of samples (2,092) were submitted from that province, with the remainder of the provinces in Canada submitting a total of only 75 samples (). The percent infection frequency was 92% in BC, and Alberta, Quebec, and Saskatchewan were the only other provinces where HLVd was detected. The percent infection frequency country-wide was 88.9% from a total of 2,167 samples. In 2021, cannabis producers in Ontario submitted the highest number of samples (total of 6,815), followed by 696 samples from BC (). HLVd was detected in all submitting provinces except Manitoba, New Brunswick, and Nova Scotia in 2021. The percent infection frequencies ranged from 13.3% to 34.7% for these provinces, with a country-wide average of 16.2% from a total of 8,331 samples submitted. In 2022, the highest number of samples were submitted from Ontario (2,135), and samples were received from each of the other eight submitting provinces () for a total of 5,449 samples. HLVd was detected in every province except Manitoba, with an average infection frequency country-wide of 14.8%. The provinces with the highest incidence of HLVd in 2022 were Alberta, Ontario and BC (). Over the 3-year study, a total of 15 947 samples were submitted from nine provinces, with an average percent infection frequency of 25.6% country-wide. The frequency of HLVd declined over the 3-year study from 88.9% in 2020 to 14.8% in 2022 () while the sample numbers submitted increased from 2,167 in 2020 to 5,449 in 2022.
Observations of disease development and symptomology
The symptoms attributed to HLVd infection are described according to the growth stages of the plants within the cannabis production facility. These sequential stages are as follows: vegetative growth stage, early flowering stage, late flowering stage, and mother (stock) plants. Vegetatively propagated clones in the rooting room which originated as cuttings taken from stock plants were not examined due to the high planting density that made observations of any symptoms on individual cuttings difficult to discern.
On vegetative plants that developed from rooted cuttings originating from stock plants, symptoms were first seen on young leaves, and included curling and twisting; in some cases, a mild mosaic and striping of the leaves or interveinal chlorosis was also observed (). The average incidence of plants showing these symptoms from > 500 plants examined at this stage of growth was < 5%. Some affected plants showed visibly stunted growth, with shortened internodes and smaller leaf area, sometimes appearing slightly darker green (). Many of these symptoms were genotype-dependent, i.e. some genotypes expressed these symptoms, while others did not. Some genotypes showed a lateral branching and sideways growth of stems that normally grew upright (), and the branches drooped, particularly during flowering, due to the weight of the developing inflorescence stems. Occasionally (<1% of plants), some plants of certain genotypes showed inward curling of the leaflet margins, which felt stiff to the touch (). Overall, >80% of the plants in the vegetative stage showed no obvious symptoms and were considered to be asymptomatic. In all instances where symptoms were apparent on affected plants, the presence of HLVd was confirmed by RT-PCR analysis. There were no symptomatic plants that did not contain HLVd.
On stock plants that were 2–4 months old, very few symptoms were observed that could be attributed directly to HLVd infection when compared to those seen during vegetative growth. At most, affected plants may develop smaller-sized leaves than normal (). Samples of leaves taken from the upper, middle, and bottom positions within the canopy of stock plants followed by RT-PCR analysis showed that HLVd was present in all of these tissues (). Over the course of this two-year study, approximately 24.7% of the stock plants were found to be HLVd positive (data not shown). Root samples from these plants also tested positive for HLVd. Multiple bands on the PCR gel that were presumed to be dimers (512 bp) and trimers (768 bp) of the 256 bp band characteristic of HLVd (monomer) were confirmed through sequencing. The multiple bands were diagnostic for HLVd, i.e. they were consistently seen under the PCR conditions used in this study (). Sequences of the 256 bp bands showed 100% homology in GenBank to an isolate from hemp in Colorado (MZ090890.1) and an isolate from hops in China (EF613183). The complete sequence of hop latent viroid from this study has been deposited in GenBank (accession no. OQ420426).
Regular sampling of stock plants representing 23 genotypes grown under the same greenhouse conditions over a 6–8-month period to detect the presence of HLVd showed that all genotypes were affected by the viroid to some extent, i.e. none of them were free of HLVd. The frequency of infected plants out of the total sampled, expressed as disease incidence, ranged from 5% to 40%, depending on the genotype (). There were 5 genotypes that showed a low frequency of diseased plants (<10%). These may be postulated to have a higher tolerance to infection but further research is needed to confirm this.
During the early stages of flowering (first two weeks after the ambient photoperiod was reduced from 18 hr light:6 hr dark to 12 hr light:12 hr dark), pronounced stunting was observed on some cannabis genotypes, with up to a 30–40% reduction in plant height; these plants had shortened internodes and smaller, darker green leaves (). Leaf samples taken from the upper, middle and lower parts of the canopy of these flowering symptomatic plants, as well as root samples, showed the multiple banding pattern characteristic for HLVd as previously observed in the stock plants (). Plants that were not displaying these symptoms and considered to be healthy (asymptomatic) lacked the presence of these multiple bands except in some instances, where faint bands were seen only in root tissues (). Progressive development of symptoms over time on flowering plants confirmed to contain HLVd included stunting, reduced inflorescence development, and an overall reduction in plant stature (). These plants could be identified within the flowering room by their distinctly stunted growth (). In addition, the root systems of these plants were visibly reduced in volume and root mass by as much as 50% (). The presence of HLVd was confirmed in these roots (). The distribution of symptomatic plants of four genotypes in two flowering rooms is shown in . There were both clusters of infected plants as well as randomly distributed plants, depending on the genotype. In G54–2, clusters of up to 12 symptomatic plants in a row were observed, while in ‘Mac-1’, symptomatic plants were distributed seemingly at random.
Fig. 4 Symptoms of hop latent viroid infection on inflorescences of flowering plants of several genotypes of cannabis. (a, b) reduced inflorescence stem growth and size in symptomatic genotype ‘black Cherry’ (arrows) compared to asymptomatic plants. (c, d) reduced inflorescence stem growth and size in symptomatic genotype ‘CBD’ (arrows) compared to asymptomatic plants. (e-h) reductions in inflorescence size and colour as a result of HLVd infection on genotype ‘Powdered Donuts’. (e) smaller inflorescence on symptomatic (right) compared to asymptomatic (left) plant. (f-h) development of yellowing symptoms on inflorescences as a result of reduced chlorophyll production in infected plants. (i) yellowing symptom on inflorescence of genotype ‘Mac-1’ due to HLVd infection (right) compared to asymptomatic plant. (j) confirmation of the presence of HLVd in seven cannabis genotypes on which inflorescence symptoms were present. Asymptomatic plants of genotypes PK and SD did not contain the characteristic bands for HLVd. Root samples of ‘Mac-1’ are shown to be positive for HLVd.

Inflorescence development continued to be markedly reduced at 4–5 weeks into the flowering period, and affected plants of several different genotypes produced markedly smaller inflorescences (). In some genotypes, these inflorescences developed a light yellow colour on the bracts (unifoliate leaves surrounding the inflorescence) (). The yellowing symptom was very pronounced in week 5 of the flowering period, and on certain genotypes, e.g. ‘Powdered Donuts’, all inflorescences on infected plants appeared a bright yellow instead of the normal green colour (). At harvest (weeks 7–8 of the flowering period), symptoms of HLVd were very apparent on genotypes such as ‘Mac-1’ (), which showed pronounced stunting with a visible reduction in the height of flowering stems. As well, there was a pronounced reduction in the overall size of the inflorescence, both in width and height (). In general, up to 25% of the flowering plants showed some symptoms attributed to HLVd. Tissue samples from affected plants representing a range of genotypes subjected to RT-PCR all showed positive bands for the presence of HLVd (). There were no cases where symptomatic flowering plants did not contain HLVd; however, the opposite was true, i.e. some asymptomatic plants sampled at random were observed to be infected with HLVd at the flowering stage, producing intense or faint bands in the PCR gels, depending on the sample (data not shown).
Impact of Hop latent viroid on plant growth, inflorescence development and THC content
A comparison of the impact of HLVd infection on plant height, inflorescence stem length, inflorescence fresh weight, inflorescence leaf length, and total THC (%) in the inflorescence tissues of four cannabis genotypes is shown in . The average reduction in these growth parameters ranged from 28% to 34%, and was significantly affected by genotype. In ‘Mac 1’ and ‘Powdered Donuts’, both considered to be highly susceptible to HLVd, growth reductions of up to 48% were observed (). The growth parameter that was most significantly affected across all cannabis genotypes was plant height and inflorescence stem length. On the stems of some genotypes at the flowering stage, e.g. CBD, branches grew hemi-spherically sideways instead of upright, giving the plant a bushy appearance, and the leaves had a slightly darker green colour (). When these plants had fully developed inflorescences, the stems were bent downwards by the weight of the flowering structure (). To confirm that these plants were infected by HLVd, leaf and stem tissues were taken from asymptomatic and symptomatic plants (, d). The diagnostic bands for HLVd following PCR were present in the latter and absent in the former (). When free-hand sections were cut through these stems and examined under a scanning electron microscope, visible differences in the extent of development of cortical and xylem tissues were seen (, g). In asymptomatic plants (), the cortical tissues formed a narrow layer below the epidermis, followed by an expansive layer of xylem tissues below. In symptomatic plants, the cortical tissue was seen as a much wider layer which resulted in a much narrower zone of underlying xylem tissues ().
Table 2. Impact of Hop latent viroid on growth characteristics of four cannabis genotypes in a commercial greenhouse environment.
Fig. 5 Changes to stem growth in flowering cannabis plants of genotype ‘CBD’ resulting from infection by HLVd. (a) comparison of healthy plant (left) with infected plant (right) which shows a short, bushy appearance as a result of reduced stem elongation and lateral stem development. (b) infected flowering plant close to harvest showing the bending of the branches due to the weight of the inflorescence. (c, d) comparison of stem growth in infected (c) and healthy (d) plant and the positions from which stem samples were taken (1, 2, H) for confirmation of HLVd presence. (e) RT-PCR gel of samples 1, 2, H from (c) and (d) showing HLVd presence in symptomatic but not healthy stems. Positive (+ve) and negative (−ve) control samples are also shown. (f, g) sections cut through stems of healthy (f) and HLVd infected (g) stems and examined under the scanning electron microscope. In (f), the arrow points to the normal width of the cortical tissues and the underlying xylem tissues. In (g), the arrow points to a much wider zone of cortical tissues and a reduced layer of xylem tissues.

In several cannabis genotypes, infection by HLVd resulted in reduced chlorophyll accumulation in the inflorescence leaves that led to a visible yellow colour, e.g. in ‘Powdered Donuts’, or a purple and yellow pigmentation developed, e.g. in ‘Black Cherry’ (, c). In ‘Mac 1’, a distinct purple colour reminiscent of anthocyanin production was seen (), as well as yellowing in other plants (). The corresponding dry weights of these inflorescences were visibly reduced (, d, g), reflecting the impact of HLVd on total yield of the plants (dry matter per unit area). RT-PCR analysis of dried inflorescence samples obtained from six different batches showed 50% to be HLVd-positive ().
Fig. 6 Symptoms of Hop latent viroid infection on inflorescences of flowering plants of several genotypes of cannabis. (a) on ‘Powdered Donuts’, the infected inflorescence (arrow) is much smaller in size compared to the healthy sample (left) and has developed distinct yellowing of the inflorescence leaves. (b) dried inflorescence stems of ‘Powdered Donuts” showing a significant reduction in size and density as a result of HLVd infection (arrow) compared to the healthy sample (left). (c) on genotype ‘black Cherry’, the infected inflorescence (arrow) develops an intense yellow colour on the inflorescence leaves with purple pigmentation on the most inner leaves compared to a healthy sample (left). (d) dried inflorescence stems of ‘black Cherry’ showing a significant reduction in the size and density as a result of HLVd infection (arrow) compared to a healthy sample (left). (e) on genotype ‘Mac-1’, the infected inflorescence (arrow) is reduced in size and has developed a purple pigmentation on the inflorescence leaves compared to a healthy sample (left). (f) reduced size of the inflorescence of ‘Mac-1’ due to infection (arrow) and yellowing of the inflorescence leaves compared to a healthy sample (right). (g) the appearance of whole dried cannabis plants harvested and hung upside down showing all inflorescence stems produced from a healthy plant of genotype ‘Mac-1’ (left) and a plant infected by HLVd (right). The stem lengths are reduced and the tissues are slightly darker green on the infected plant (arrow). (h) RT-PCR gel showing the presence of the characteristic bands of HLVd in dried cannabis inflorescence samples. Three out of 6 samples were shown to contain the viroid (lanes 2, 5, 6). A very faint band was seen in lane 3.

Comparative trichome development on infected and healthy plants
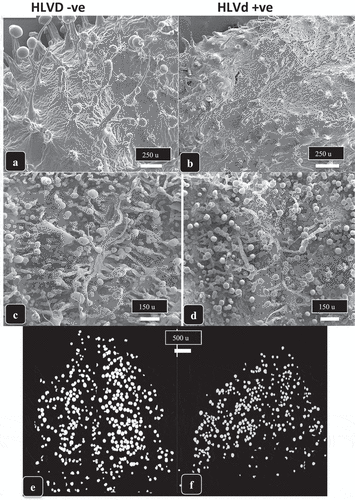
Inflorescence leaves (bracts) on cannabis plants surrounding the calyx contain pistillate structures that are covered by microscopic glandular trichomes, which impart a silvery appearance to the inflorescences when viewed with the naked eye. In comparing asymptomatic (confirmed to be HLVd free by RT-PCR) vs. HLVd-infected inflorescences, the latter displayed a reduction in the glossy shine characteristic of mature trichomes (). Under the magnification of a dissecting microscope (50×), the significantly-reduced development of glandular trichomes in HLVd-infected inflorescences could readily be seen compared to asymptomatic bracts, on which long, stalked trichomes with globular heads were produced (). When these bracts were examined under the higher magnification of a scanning electron microscope, both the upper and lower bract surfaces showed a marked difference in the appearance of the trichomes resulting from HLVd infection (). On the upper bract surface, trichome stalk lengths and glandular head size were considerably reduced on HLVd-infected plants compared to asymptomatic plants (). On the lower bract surface, the difference was more pronounced, with HLVd-infected bracts bearing shorter and poorly developed glandular trichome heads compared to the long-stalked and larger glandular heads visible on asymptomatic plants (confirmed to be HLVd-free) ().
Fig. 7 The impact of Hop latent viroid infection on development of trichomes on inflorescence leaves and bracts. a) genotype ‘chocolate fondue’ shows a silvery appearance on the inflorescence leaves and bracts (arrow) on a healthy plant (left) reflecting the normal development of trichome glands compared to an HLVd-infected plant (right) in which trichome development is considerably reduced. (b) same as (a) but on genotype ‘Headband’ in which trichome development on the bracts of a healthy inflorescence (left) is much greater than on an infected inflorescence (right). (c) magnified view of an inflorescence bract of genotype ‘Mac-1’ under a dissecting microscope (50×) showing the healthy trichomes with long stalks and fully developed trichome heads (left) compared to the much reduced trichome stalks and smaller heads on a bract from an HLVd-infected plant (right).

Fig. 8 Scanning electron microscopic images of bracts of cannabis genotype ‘Headband’ from a healthy plant (a, c, e) compared to an HLVd-infected plant (b, d, f). In (a, b) the upper bract surface shows well developed stalked trichomes with large heads (a) compared to the stunted development of trichomes on a diseased bract (b). In (c, d), higher magnification views of the lower bract surface of healthy (c) and diseased (dt) plants shows the reduced stalk length and head size resulting from viroid infection. (e, f) Computer-derived image analysis in which trichome segmentation was performed to represent the glandular heads by white circles, showing that the size of glandular heads but not the density of trichomes was reduced as a result of HLVd infection (f) compared to a healthy sample (e).
Magnified views of trichome heads on asymptomatic and HLVd-infected inflorescence bracts which had been subjected to commercial drying conditions are shown in . In the former, the normal spherical glandular heads measuring approximately 80–110 µm in diameter could be seen (). In contrast, trichome heads on HLVd-infected inflorescences appeared shrivelled and were much smaller in size, measuring 40–50 µm in diameter (). Much higher magnified views of these trichomes under the scanning microscope showed the large, fully-formed trichome heads in asymptomatic inflorescences (, e) with a fully-formed cuticle surrounding the heads. In contrast, trichome heads on HLVd-infected inflorescences were collapsed and the cuticle was shrivelled, resulting in much smaller-sized trichome heads ( d, f). The impact of these changes in trichome size on levels of THC and terpenes is illustrated in . In this example, a plant which bore two main branches was shown by RT-PCR to be HLVd-positive in the bottom branch and was HLVd-negative in the upper branch. This allowed a direct comparison to be made of trichome development, THC, and terpene levels in the same plant. On the upper healthy branch bearing asymptomatic inflorescences, the THC and terpene levels were 27.8% and 1.3%, respectively, in dried inflorescence samples. On the lower branch that bore symptomatic inflorescences, the THC and terpene levels were reduced to 15.9% and 1.1%, respectively (). Differences in trichome development were observed as shown in . By comparison, a noninfected plant of the same strain would normally show a THC range of 26.5–29% and 1.2–1.3% terpenes in dried inflorescences (unpublished observations).
Fig. 9 Scanning electron microscopic images of trichomes of cannabis genotype ‘Mac-1’ from a healthy plant (a, c, e) compared to an HLVd-infected plant (b, d, f). In (a, b) the lower bract surface shows well developed stalked trichomes with large heads (a) compared to the stunted development of trichomes on a diseased bract (b). In (c, d), higher magnification views of healthy (c) and diseased (d) bracts show the much larger trichome heads that are fully developed (c) compared to smaller under-developed and shrunken heads resulting from viroid infection (d). (e, f) a highly magnified view of an intact fully developed turgid trichome head surrounded by a cuticle on a healthy bract (e) compared to a shrunken trichome head on an infected bract (f). Note the cuticle is wrinkled due to the reduced cannabinoid content that caused the fully formed head to deflate. The trichome head is also considerably reduced in size as a result of HLVd infection.
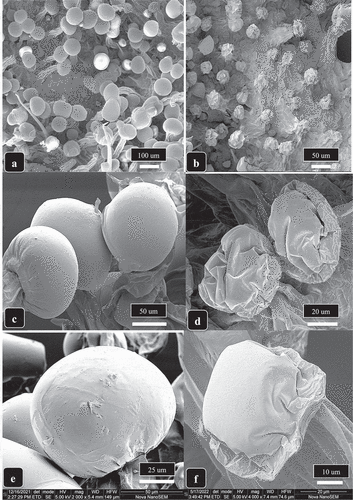
Fig. 10 A direct comparison of the impact of Hop latent viroid infection on two different branches of the same cannabis plant genotype ‘Powdered Donuts’. On the branch on the bottom left of the plant, symptoms of infection include reduced inflorescence stem growth and yellowing of the inflorescence leaves, as well as reduced inflorescence size. These tissues were confirmed to contain HLVd by RT-PCR. On the upper right branch, normal development includes longer inflorescence stems with fully developed inflorescences. The tissues were confirmed to be negative for HLVd by RT-PCR. The numbers in boxes denote the THC and terpene levels in dried inflorescence samples taken from each respective branch. The insets show scanning electron microscope images of the lower bract surface on inflorescences from HLVd-negative (top) and HLVd-positive (bottom) branches of the plant. The differences in trichome development can be seen.

Observations of trichome morphology and development
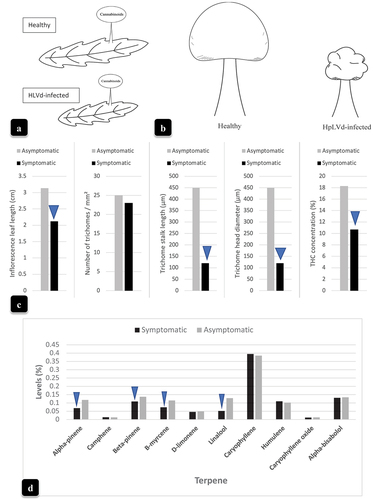
With the aid of a computer-based image analysis in which trichome segmentation was performed (Sutton et al. Citation2023), the glandular heads were represented by white circles which showed that the size but not the density of trichomes was reduced as a result of HLVd infection (). Schematic illustrations of the inflorescence bracts bearing trichomes are shown in . In addition to the smaller bract size in HLVd-infected plants, trichome size was also significantly reduced (). The accompanying data are presented in , which shows the significantly reduced inflorescence leaf length, trichome stalk length, trichome head diameter, and final THC levels in HLVd-infected compared to HLVd-negative plants. Trichome density was not affected. The impact of HLVd infection on overall terpene levels is shown in . Among 10 terpene compounds, there was a reduction in alpha- and beta-pinene, B-myrecene, and linalool. The remainder of the compounds were unaffected ().
Fig. 11 The impact of Hop latent viroid infection on cannabis inflorescence bract size, trichome development, and THC and terpene content. (a, b) Comparative bract and trichome gland development in HLVd-negative and HLVd-positive inflorescences. (c) measurements of inflorescence bract length, numbers of trichomes, trichome stalk length, trichome head diameter, and THC content made from HLVd-negative and HLVd-positive inflorescence samples. (d) levels of the major terpene compounds present in HLVd-negative and HLVd-positive inflorescences. For (c), data were obtained from 25 measurements. For (d), data represent the average measurements from two samples conducted by a commercial laboratory. Blue arrows in (c) and (d) indicate significant differences between HLVd-negative and HLVd-positive samples according to a Chi-square analysis (P = 0.05).
Discussion
The results from this study demonstrate that the incidence of HLVd on cannabis plants in Canada is widespread, and the pathogen was detected in eight out of nine provinces from a total of 15 947 samples submitted, at an overall country-wide frequency of 25.6%. The provinces with the highest numbers of positive samples were British Columbia and Ontario, which represent the two regions with the greatest cannabis production acreage and from which the highest numbers of samples were received. This was followed by Alberta and Quebec, which showed infection frequencies ranging from 11.4% to 34.7%, depending on the year. None of the samples submitted from Manitoba were positive for HLVd, but the sample size was small (n = 6 for all three years). It is unclear if the samples submitted by anonymous growers were symptomatic, but in the present study, HLVd was detected in both symptomatic and asymptomatic stock plants, vegetative plants, and flowering plants by RT-PCR. The presence of asymptomatic infected plants, particularly stock plants from which cuttings are routinely taken for propagation, presents a challenge for detection of HLVd in commercial cannabis facilities. Accurate confirmation would require routine testing of all planting stock and vegetative plants using established molecular methods (Eastwell and Nelson Citation2007; Bektaş et al. Citation2019; Warren et al. Citation2019; this study). Many stock plants that were positive for HLVd in the present study and from which cuttings were obtained for propagation only displayed symptoms occasionally, sometimes producing smaller leaves and slightly stunted growth, depending on the genotype. Up to 24.7% of these stock plants were confirmed to be infected with HLVd to varying degrees based on RT-PCR tests conducted on leaf and/or root samples. The varied responses to infection by HLVd among cannabis genotypes that were cultivated in the same greenhouse suggests that some genotypes may display as-yet-undetermined mechanisms that can mitigate replication or spread of the viroid. These varied responses, however, can also result from differences in viroid titre, depending on when infection first occurred, as well as environmental conditions that could influence viroid replication, which are as yet unknown. Since HLVd is a recently discovered pathogen of cannabis, there is a considerable amount of information that is lacking regarding its epidemiology. However, previous studies conducted on viroids infecting other plant species can provide a useful basis for comparison.
In general, viroid infections cause a range of symptoms in diseased plants, including stunting, leaf chlorosis and mosaic, leaf curl, wood and bark alterations, reduction in size as well as deformation of fruits, tubers and flowers (Flores et al. Citation2005; Di Serio et al. Citation2012; Sano Citation2021). Furthermore, viroid infections may be latent or asymptomatic, which is predominantly the case for HLVd infection in hop plants (Puchta et al. Citation1988; Eastwell and Nelson Citation2007; Pethybridge et al. Citation2008). On vegetative cannabis plants of some genotypes, a unique symptom seen was a sideways (lateral) growth of stems compared to the mostly upright growth in healthy plants. This may be the result of disrupted meristematic growth. The stems on these infected plants were supple and frequently snapped; on flowering plants, the stems were bent downwards due to the weight of the inflorescences. A preliminary microscopic study showed the cortical zone had expanded relative to the underlying xylem tissues in the stems of infected plants, suggesting that xylem development was compromised and structural support was reduced in these stems. The changes in cellular composition in infected stems resulting in the observed fragility of the stems may be the result of plant growth regulator disruptions caused by viroid infection, but little is currently known about the basis for symptom development in the cannabis-HLVd pathosystem. In chrysanthemum plants infected with Chrysanthemum stunt viroid, genes involved in the biosynthesis of gibberellic acid and cytokinin were down-regulated, which explained the stunting symptom seen in these plants (Takino et al. Citation2019). Additional symptoms observed on certain cannabis genotypes in the present study were yellowing of bract leaves surrounding the inflorescences, and occasionally a darker pigmentation reflective of anthocyanin production, as well as shorter internode lengths. These symptoms are described for the first time on cannabis plants grown under greenhouse conditions and establish the range of phenotypic effects resulting from HLVd infection on this host. The most distinctive symptoms were observed on flowering plants and included reduced inflorescence stem growth and weight, as well as overall stunted growth of the plants. Reduced chlorophyll production and reduced root development were also symptoms associated with HLVd infection on flowering plants.
Replication of members of the Pospiviroidae, which includes HLVd, occurs in the nucleus of plant cells. As with other viroids, the RNAs do not encode for any proteins, and replication occurs using host RNA polymerases and other intermediaries in an asymmetric rolling circle mechanism to produce linear strands (Flores et al. Citation2005; Steger and Riesner Citation2018; Venkataraman et al. Citation2021). The strands develop as multimers of 256 nucleotide-size fragments from the continuously replicating circular RNA molecule, which can result in the formation of dimers and trimers in the absence of ribozyme activity (Venkataraman et al. Citation2021). These multimeric bands were identified by their varying MW sizes on gels in this study and were reported earlier by Eastwell and Nelson (Citation2007). Cannabis plants containing a higher titre of the viroid (as determined by RT-qPCR) more commonly showed the multimeric banding pattern compared to plants which had a lower titre, which was reflected in a single band observed at 256 bp (data not shown). This implies that the RT-PCR results from the present study may be reflective of active multiplication which produces multiple bands vs. lower rates of multiplication resulting in a single 256 nucleotide band. In PSTVd-infected plants, longer-than-unit strands were proposed to be replicative intermediates representative of the anti-genomic (-) strands as expected for the rolling-circle model of replication (Sano Citation2021), which are then further transcribed into positive (+) strands. To avoid degradation of the strands by cellular RNases, complementary base-pairing occurs to produce double-stranded secondary structures with stem loops, forming a rod-like configuration. This makes viroids very resilient to degradation by nucleases and makes them heat-stable (Sanger et al. Citation1976; Owens Citation2007; Steger and Riesner Citation2018; Sano Citation2021). In some hosts, viroid-infected tissues, such as seeds and tubers, can harbour viable RNA for many years (Singh Citation2014).
The viroid RNAs interact with and redirect host gene expression through several mechanisms, one of which is host gene silencing through RNA-interference (RNAi) (Flores et al. Citation2005; Di Serio et al. Citation2012; Pokorn et al. Citation2017; Sano Citation2021), thereby significantly altering the physiology and metabolism of the host plant (Koeppe et al. Citation2023). Movement of PSTVd (and presumably HLVd) RNA from cell to cell occurs via plasmodesmata and systemic spread occurs through movement in the phloem tissues, usually following a source-sink direction of photo-assimilates (Schaffer et al. Citation1996), i.e. metabolically-active tissues would tend to accumulate more viroid (Flores et al. Citation2005; Takeda and Ding Citation2009). This is similar to the systemic movement pattern for many plant viruses (Hipper et al. Citation2013). Interestingly, phloem sieve tubes have not been found to contain RNases that could potentially destroy mRNAs present in plants (Sasaki et al. Citation1998; Doering-Saad et al. Citation2002), thereby also ensuring survival and spread of viruses and viroids in these tissues. In the present study, HLVd was detected in the roots of vegetative plants, stock plants and flowering plants. A number of viroids and plant viruses have been shown to be present in the roots of their hosts, and following replication, their spread occurs systemically in the phloem concurrently with photo-assimilate movement (Rajamaki and Valkonen Citation2002; Lunello et al. Citation2007; Gosalvez-Bernal et al. Citation2008). This ensures rapid spread to actively growing tissues, including the inflorescences. In the present study, HLVd was confirmed to be present in cannabis inflorescences, representing the first report of its detection in floral tissues. Furthermore, harvested and dried inflorescences were also found to contain HLVd in up to 50% of the samples tested (data not shown). The abundant glandular trichomes produced in the floral tissues of cannabis are reported to act as metabolic carbon sinks to which photosynthates are distributed from adjacent tissues (Conneely et al. Citation2021). This could explain the movement and accumulation of HLVd in infected inflorescences observed in this study. A number of plant viruses have been shown to enter and accumulate in the trichomes of systemically infected plants (Angell and Baulcombe Citation1995; Waigmann and Zambryski Citation1995; Christensen et al. Citation2009; Kogovšek et al. Citation2011). The extent to which HLVd can enter the trichome gland cells found in infected inflorescence tissues remains to be determined.
The rate at which HLVd spreads internally within cannabis plants from the initial point of infection is unknown and this movement is likely to be influenced by the plant genotype and external environmental conditions, such as light and temperature, as well as the initial viroid titre within the plant. Rapid plant-to-plant spread, however, does occur through vegetative propagation, i.e. the obtaining and rooting of clones from infected stock plants, and through pruning activities that can spread sap from infected plants, similar to that reported for PSTVd in potato and tomato crops (Li et al. Citation2015; Mackie et al. Citation2015). Given the high percentage of stock plants that were shown to be infected by HLVd in this study, spread of the viroid through clonal propagation is likely to be an important route for dissemination. This was confirmed by detection of HLVd in early flowering plants which were derived directly from vegetatively propagated clones that originated from infected stock plants. The extent to which HLVd spreads from plant to plant in the flowering rooms is not known. The distribution of symptomatic flowering plants occurred at random in some genotypes and in other genotypes showed a clustered distribution. Transmission through seed or pollen has not yet been verified for HLVd in cannabis, but it is likely to occur given the high incidence of viroid detected in floral tissues which contain large numbers of pistils that should give rise to infected seeds if pollinated. Seed transmission has previously been reported for several viroids, including PSTVd (Singh Citation2014; Matsushita et al. Citation2018).
In hop yards, HLVd can spread from plant to plant through mechanical means, such as during pruning and harvesting activities, as well as through vegetative propagation and dissemination of diseased planting materials (Pethybridge et al. Citation2008; Adams et al. Citation1996). There is no evidence of transmission by insect vectors, such as aphids, and the transmission efficiency through pollen and seed has been reported to be low (Matousek and Patzak Citation2000). No symptoms are generally visible on infected hop plants, hence the term ‘latent’ was included in the original description (Puchta et al. Citation1988). However, in certain infected hop cultivars, such as ‘Omega’, symptoms of chlorosis, slower growth, and production of fewer laterals and smaller cones were reported (Adams et al. Citation1996; Pethybridge et al. Citation2008). In addition, a build-up of viroid titres in affected tissues can be influenced by hop cultivar susceptibility, which in turn can impact symptom development. Symptoms can also be influenced by interactions between the host genotype, viroid genetic composition (strain) and environmental factors, in particular temperature and light (Flores et al. Citation2005). For example, higher temperatures and light intensity were reported to promote greater symptom development following infection by viroids (Flores et al. Citation2005). The genotype × environment interactions in cannabis that may influence symptom development due to HLVd are presently unknown. Unlike hop plants, however, many cannabis genotypes were symptomatic in this study, especially during the onset of flowering, suggesting potentially enhanced susceptibility of this host to HLVd. A significant reduction in the growth and size of inflorescences, as well as in root development, was observed in many genotypes. This led to reduced harvestable yields (dry weight of cannabis flowers per m2 of production area). In addition, levels of all macro- and micro-nutrients were reduced in diseased leaves compared to healthy leaves (Table S1). The impact of HLVd infection on plant growth is therefore wide-ranging, impacting root, shoot and inflorescence development as well as nutrient uptake in cannabis plants.
The greatest economic threat to cannabis producers from HLVd is the significant decrease in the production of cannabinoids, in particular tetrahydrocannabinol and cannabidiol, as well as specific terpene compounds, in diseased plants as demonstrated in the present study. This reduction was observed to different extents in four cannabis genotypes included in this study. We are the first to demonstrate that a reduction in cannabinoid and terpene accumulation resulting from infection by HLVd was correlated with a significant reduction in the development and maturation of the stalked glandular trichomes that manufacture these compounds, but not in their density. These trichomes had smaller glandular heads and shorter stalks, both of which would lead to lower cannabinoid accumulation at maturity (Livingston et al. Citation2020; Punja et al. Citation2023a). The underlying mechanisms by which trichome development and cannabinoid levels are reduced as a result of HLVd infection are unknown. In HLVd-infected hop cones, down-regulation of genes involved in secondary metabolism pathways caused a reduction in a range of biochemical compounds (Patzak et al. Citation2021). Using a transcriptomic approach, down-regulation of additional genes and up-regulation of pathogenesis-related protein genes were reported in HLVd-infected hop plants (Pokom et al. Citation2017). Similar transcriptomic and metabolomic changes are likely occurring in HLVd-infected cannabis plants. Further studies will shed light on the multitude of changes that are likely taking place as a result of HLVd infection in cannabis plants, many of which will affect different metabolic pathways in the host plant.
In general, management options for viroid pathogens, such as HLVd affecting hops, include developing a pathogen-free certification program in which growers are required to start new plantings with materials originating from certified sources (Adams et al. Citation1996; Pethybridge et al. Citation2008). A similar approach led to the successful eradication of PSTVd in potatoes in Canada (Singh Citation2014). A certification program requires availability of a tissue culture system from which regeneration of viroid-free plants is possible, ideally starting from meristem tips as explants. Although tissue culture approaches for cannabis micropropagation have been described (Holmes et al. Citation2021; Monthony et al. Citation2021), the successful application of these methods to obtain HLVd-free planting materials has yet to be confirmed. If successful, additional certification of these plants would be required to confirm they are pathogen-free prior to being distributed to producers. In hops, studies have shown that pathogen-free plants could be obtained using meristem tip culture (0.2 to 0.5 mm in size) if the viroid titre was first significantly reduced by cold pre-treatment of plants (Adams et al. Citation1996; Grudzińska et al. Citation2006; Pethybridge et al. Citation2008). However, the response was cultivar-dependent and some plants still showed viroid presence after an extended period of growth (Grudzińska et al. Citation2006). A similar approach was used to eliminate PSTVd in potato plants (Lizarraga et al. Citation1980). Thermotherapy (35–40°C) has not provided long-lasting removal of viroids from plant tissues since it is known that viroids such as PSTVd and HLVd are heat-stable (Grudzińska et al. Citation2006). Various approaches that are used to eliminate virus/viroid infections in plant tissues, including the use of chemicals that can reduce virus replication (Wang et al. Citation2018) could potentially also be applied to cannabis and this area of research deserves attention.
Since HLVd infection of cannabis plants has the potential to severely and negatively impact commercial producers in Canada and elsewhere, concerted measures need to be put into place to reduce pathogen spread, both within and between production facilities. These can include the use of certified pathogen-free planting materials (currently unavailable in the cannabis industry) and implementing strict sanitation measures within affected growing facilities, which includes destroying diseased plants to reduce pathogen spread. Rouging and destroying affected plants is recommended to contain the spread of viroid pathogens, accompanied by frequent testing to confirm absence of the viroid (Hammond and Owens Citation2006; Eastwell and Nelson Citation2007; Pethybridge et al. Citation2008). The overall frequency of detection of HLVd Canada-wide was observed to decline over the 3 years of sampling in this study despite an increase in sampling frequency, presumably due to destruction of diseased plants and monitoring conducted by producers in affected growing facilities. The effectiveness of these various management approaches in curtailing spread of HLVd on cannabis plants awaits further research.
The greatest potential for HLVd reduction lies in the identification of cannabis genotypes with potential resistance or tolerance to infection and obtaining a better understanding of the intricacies of the host-pathogen interactions and factors affecting replication and pathogen spread within these plants. Our results demonstrate that the broad genetic diversity presently available among cannabis genotypes can potentially lead to genetic selections with enhanced HLVd resistance/tolerance. This, coupled with better information on the efficacy of sanitizing products which have been shown to reduce transmission of PSTVd in potatoes and tomatoes (Li et al. Citation2015; Mackie et al. Citation2015), can lead to better management of this emerging pathogen. However, genetic changes occurring within HLVd genomes could impact the durability of any identified resistance. Viroids are reported to have a high rate of mutation, leading to the development of a mixture of strains (quasispecies) during infection (Steger and Riesner Citation2018; Adkar-Purushothama et al. Citation2020; Venkataraman et al. Citation2021). Such variants can vary in virulence from highly virulent to weakly virulent (Sano Citation2021). For example, a single nucleotide change can affect the pathogenicity of a viroid such as PSTVd (Wassenegger et al. Citation1996). The current isolates of HLVd affecting cannabis in British Columbia show complete sequence homology to strains from hemp in Colorado (Chiginsky et al. Citation2021) and hops in China, suggesting there is currently limited genetic variation in the pathogen. The availability of a symptomatic host species, such as tomato, which rapidly expresses symptoms due to PSTVd (Di Serio et al. Citation2012; Mackie et al. Citation2015), would greatly aid HLVd research. The impact of HLVd on outdoor hemp and cannabis production in Canada is unknown and requires more research.
In conclusion, following the first published reports of HLVd infecting cannabis in 2019, current evidence suggests it has spread extensively within cannabis production facilities in Canada at a rate that has outpaced research efforts to understand its epidemiology and management. Areas of research that are critical to provide knowledge to cannabis producers have been identified in this study and the need for concerted efforts to develop a sustainable disease management program is emphasized. It is unlikely there are any regions of cannabis production world-wide that are free of HLVd, given its widespread occurrence on hops, and concerted testing is needed to establish the extent to which it has spread in other countries. Collaborative research between academic, government and industry personnel would greatly aid in developing strategies to minimize the impact of this important pathogen to the cannabis industry.
Supplementary Fig. 1. CJPP.docx
Download MS Word (5.5 MB)Acknowledgments
We thank several licenced producers for providing access to the growing facilities for monitoring and surveillance purposes. Technical assistance provided by Li Ni, Janesse Holmes, and Cameron Scott is gratefully acknowledged. This research was funded through an NSERC Alliance grant (ALLRP 571270-21) and jointly funded by Pure Sunfarms. Additional funding was provided by the B.C. Ministry of Agriculture/Agriculture and Agri-Food Canada through the Canadian Agricultural Partnership (CAP) Program Project No. URACP 19-212.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2023.2279184.
Additional information
Funding
References
- Adams AN, Barbara DJ, Morton A, Darby P. 1996. The experimental transmission of Hop latent viroid and its elimination by low temperature treatment and meristem culture. Ann Appl Biol. 128(1):37–44. doi: 10.1111/j.1744-7348.1996.tb07087.x.
- Adams AN, Morton A, Barbara DJ, Ridout MS. 1992. The distribution and spread of Hop latent viroid within two commercial plantings of hop (Humulus lupulus). Ann Appl Biol. 121(3):585–592. doi: 10.1111/j.1744-7348.1992.tb03468.x.
- Adkar-Purushothama CR, Bolduc F, Bru P, Perreault J-P. 2020. Insights into potato spindle tuber viroid quasi-species from infection to disease. Front Microbiol. 11:1235. doi: 10.3389/fmicb.2020.01235.
- Angell SM, Baulcombe DC. 1995. Cell-to-cell movement of Potato virus X revealed by micro-injection viral vector tagged with the beta-glucuronidase gene. Plant J. 7:135–140. doi: 10.1046/j.1365-313X.1995.07010135.x.
- Barbara DJ, Morton A, Adams AN, Green CP. 1990. Some effects of Hop latent viroid on two cultivars of hop (Humulus lupulus) in the UK. Ann Appl Biol. 117(2):359–366. doi: 10.1111/j.1744-7348.1990.tb04222.x.
- Bektaş A, Hardwick KM, Waterman K, Kristof J. 2019. Occurrence of Hop latent viroid in Cannabis sativa with symptoms of cannabis stunting disease in California. Plant Dis. 103(10):2699. doi: 10.1094/PDIS-03-19-0459-PDN.
- Chiginsky J, Langemeier K, MacWilliams J, Albrecht T, Cranshaw W, Fulladolsa AC, Kapuscinski M, Stenglein M, Nachappa P. 2021. First insights into the virus and viroid communities in hemp (Cannabis sativa). Front Agron. 3:778433. doi: 10.3389/fagro.2021.778433.
- Christensen NM, Faulkner C, Oparka K. 2009. Evidence for unidirectional flow through plasmodesmata. Plant Physiol. 150(1):96–104. doi: 10.1104/pp.109.137083.
- Conneely LJ, Mauleon R, Mieog J, Barkla BJ, Kretzschmar T, Nagegowda D. 2021. Characterization of the Cannabis sativa glandular trichome proteome. PloS One. 16(4):e0242633. doi: 10.1371/journal.pone.0242633.
- Di Serio F, Torchetti EM, Flores R, Navarro B. 2012. The role of plant viroids in diseases – new developments. CAB Rev. 7:006. doi: 10.1079/PAVSNNR20127006.
- Doering-Saad C, Newbury HJ, Bale JS, Pritchard J. 2002. Use of aphid stylectomy and RT-PCR for the detection of transporter mRNAs in sieve elements. J Exp Bot. 53(369):631–637. doi: 10.1093/jexbot/53.369.631.
- Eastwell KC, Nelson ME. 2007. Occurrence of viroids in commercial hop (Humulus lupulus L.) production areas of Washington State. Plant Health Progr. 8(1):1. doi: 10.1094/PHP-2007-1127-01-RS.
- Flores R, Hernandez C, Martinez de Alba AE, Daros J-A, Di Serio F. 2005. Viroids and viroid-host interactions. Annu Rev Phytopathol. 43:117–139. doi: 10.1146/annurev.phyto.43.040204.140243.
- Gosalvez-Bernal B, Genoves A, Navarro JA, Pallas V, Sanchez-Pina M. 2008. Distribution and pathway for phloem-dependent movement of Melon necrotic spot virus in melon plants. Mol Plant Pathol. 9(4):447–461. doi: 10.1111/j.1364-3703.2008.00474.x.
- Grudzińska M, Solarska E, Czubacka A, Przybyś M, Fajbuś A. 2006. Elimination of Hop latent viroid from hop plants by cold treatment and meristem tip culture. Phytopathol Pol. 40:21–30.
- Hammond RW, Owens RA. 2006. Viroids: new and continuing risks for horticultural and agricultural crops. APSnet Feat. doi: 10.1094/APSnetFeature-2006-1106. ( on-line).
- Hipper C, Brault V, Ziegler-Graff V, Revers F. 2013. Viral and cellular factors involved in phloem transport of plant viruses. Front Plant Sci. 4:154. doi: 10.3389/fpls.2013.00154.
- Holmes JE, Lung S, Collyer D, Punja ZK. 2021. Variables affecting shoot growth and plantlet recovery in tissue cultures of Drug-type cannabis sativa L. Front Plant Sci. 12:12. doi: 10.3389/fpls.2021.732344.
- Jarugula S, Wagstaff C, Mitra A, Crowder DW, Gang DR, Naidu RA. 2023. First reports of beet curly top virus, citrus yellow vein-associated virus, and Hop latent viroid in industrial hemp (Cannabis sativa) in Washington State. Plant Dis. 107. (in press). doi: 10.1094/PDIS-12-22-2981-PDN.
- Koeppe S, Kawchuk L, Kalischuk M. 2023. RNA interference : past and future applications in plants. Int J Mol Sci. 24:9755. doi: 10.3390/ijms24119755.
- Kogovšek P, Kladnik A, Mlakar J, Tušek Žnidarič M, Dermastia M, Ravnikar M, Pompe-Novak M. 2011. Distribution of Potato virus Y in potato plant organs, tissues, and cells. Phytopathology. 101:1292–1300. doi: 10.1094/PHYTO-01-11-0020.
- Li R, Baysal-Gurel F, Abdo Z, Miller SA, Ling K-S. 2015. Evaluation of disinfectants to prevent mechanical transmission of viruses and a viroid in greenhouse tomato production. Virol J. 12:5. doi: 10.1186/s12985-014-0237-5.
- Livingston SJ, Quilichini TD, Booth JK, Wong DCJ, Rensing KH, Laflamme-Yonkman J, Castellarin SD, Bohlmann J, Page JE, Samuels AL. 2020. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 101(1):37–56. doi: 10.1111/tpj.14516.
- Lizarraga RE, Salazar LF, Roca WM, Schilde-Rentschler L. 1980. Elimination of potato spindle tuber viroid by low temperature and meristem culture. Phytopathology. 70:754–755. doi: 10.1094/Phyto-70-754.
- Lunello P, Mansilla C, Sánchez F, Ponz F. 2007. A developmentally linked, dramatic, and transient loss of virus from roots of Arabidopsis thaliana plants infected by either of two RNA viruses. Mol Plant–Microbe Interact. 20:1589–1595. doi: 10.1094/MPMI-20-12-1589.
- Mackie AE, Coutts BA, Barbetti MJ, Rodoni BC, McKirdy SJ, Jones RAC. 2015. Potato spindle tuber viroid: stability on common surfaces and inactivation with disinfectants. Plant Dis. 99:770–775. doi: 10.1094/PDIS-09-14-0929-RE.
- Matousek J, Patzak J. 2000. A low transmissibility of Hop latent viroid through a generative phase of Humulus lupulus L. Biol Plant. 43(1):145–148. doi: 10.1023/A:1026531819806.
- Matsushita Y, Yanagisawa H, Sano T. 2018. Vertical and horizontal transmission of pospiviroids. Viruses. 10(12):706. doi: 10.3390/v10120706.
- Monthony AS, Kyne ST, Grainger CM, Jones AMP. 2021. Recalcitrance of Cannabis sativa to de novo regeneration- a multi-genotype replication study. PloS One. 16(8):e0235525. doi: 10.1371/journal.pone.0235525.
- Owens RA. 2007. Potato spindle tuber viroid: the simplicity paradox resolved? Mol Plant Pathol. 8(5):549–560. doi: 10.1111/j.1364-3703.2007.00418.x.
- Patzak J, Henychová A, Krofta K, Svoboda P, Malírová I. 2021. The influence of Hop latent viroid (HLVd) infection on gene expression and secondary metabolite contents in hop (Humulus lupulus L.) glandular trichomes. Plants. 10:2297. doi: 10.3390/plants10112297.
- Pethybridge SJ, Hay FS, Barbara DJ, Eastwell KC, Wilson CR. 2008. Viruses and viroids infecting hop significance, epidemiology, and management. Plant Dis. 92(3):324–338. doi: 10.1094/PDIS-92-3-0324.
- Pokorn T, Radišek S, Javornik B, Štajner N, Jakše, J. 2017. Development of hop transcriptome to support research into host-viroid interactions. PLoS One. 12(9):e0184528. doi: 10.1371/journal.pone.0184528.
- Puchta H, Ramm K, Sanger HL. 1988. The molecular structure of Hop latent viroid (HLV), a new viroid occurring worldwide in hops. Nucleic Acids Res. 16(10):4197–4216. doi: 10.1093/nar/16.10.4197.
- Punja ZK, Sutton DS, Kim T. 2023a. Glandular trichome development, morphology, and maturation are influenced by plant age and genotype in high THC-containing cannabis (Cannabis sativa L.) inflorescences. J Cannabis Res. 5:12. doi: 10.1186/s42238-023-00178-9.
- Punja ZK, Wang K, Ni L, Lung S, Buirs L. 2023b. Symptomology and prevalence of Hop latent viroid on greenhouse cannabis (Cannabis sativa L.) plants. Can J Plant Pathol. 45(3):227. ( Abstr).
- Rajamaki ML, Valkonen JPT. 2002. Viral genome-linked protein (vpg) controls accumulation and phloem-loading of a potyvirus in inoculated potato leaves. Mol Plant–Microbe Inter. 15:138–149. doi: 10.1094/MPMI.2002.15.2.138.
- Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. 1976. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 73(11):3852–3856. doi: 10.1073/pnas.73.11.3852.
- Sano T. 2021. Progress in 50 years of viroid research – molecular structure, pathogenicity, and host adaptation. Proc Jpn Acad Ser B. 97:371–401. doi: 10.2183/pjab.97.020.
- Sasaki T, Chino M, Hayashi H, Fujiwara T. 1998. Detection of several mRNA species in rice phloem sap. Plant Cell Physiol. 39(8):895–897. doi: 10.1093/oxfordjournals.pcp.a029451.
- Schaffer AA, Pharr DM, Madore MA. 1996. Cucurbits. In: Zamsky E., Schaffer AA, editors Photoassimilate distribution in plants and crops. Source-sink relationships. New York: Marcel Dekker, Inc; p. 729–758.
- Scheck HJ 2020. California pest rating proposal for Hop latent viroid. California department of food and Agriculture. [accessed 2023 Mar 27]. https://blogs.cdfa.ca.gov/Section3162/?p=7262.
- Singh RP. 2014. The discovery and eradication of potato spindle tuber viroid in Canada. Virus Dis. 25(4):515–424. doi: 10.1007/s13337-014-0225-9.
- Steger G, Riesner D. 2018. Viroid research and its significance for RNA technology and basic biochemistry. Nucleic Acids Res. 46(20):10563–10576. doi: 10.1093/nar/gky903.
- Sutton DB, Punja ZK, Hamarneh G. 2023. Characterization of trichome phenotypes to assess maturation and flower development in Cannabis sativa L. (cannabis) by automatic gland analysis. Smart Agric Tech. 3:100111. doi: 10.1016/j.atech.2022.100111.
- Takeda R, Ding B. 2009. Viroid intercellular trafficking : RNA motifs, cellular factors, and broad impacts. Viruses. 1:210–221. doi: 10.3390/v1020210.
- Takino H, Kitajima S, Hirano S, Oka M, Matsuura T, Ikeda Y, Kojima M, Takebayashi Y, Sakakibara H, Mino M, et al. 2019. Global transcriptome analyses reveal that infection with chrysanthemum stunt viroid (CSVd) affects gene expression profile of chrysanthemum plants, but the genes involved in plant hormone metabolism and signaling may not be silencing target of CSVd-siRnas. Plant Gene. 18:100181. doi: 10.1016/j.plgene.2019.100181.
- Venkataraman S, Badar U, Shoeb E, Hashim G, AbouHaidar M, Hefferon K. 2021. An inside look into biological miniatures: molecular mechanisms of viroids. Int J Mol Sci. 22:2795. doi: 10.3390/ijms22062795.
- Waigmann E, Zambryski P. 1995. Tobacco mosaic virus movement protein-mediated protein transport between trichome cells. Plant Cell. 7(12):2069–2079. doi: 10.1105/tpc.7.12.2069.
- Wang M-R, Cui Z-H, Li J-W, Hao X-Y, Zhao L, Wang Q-C. 2018. In vitro thermotherapy-based methods for plant virus eradication. Plant Methods. 14:87. doi: 10.1186/s13007-018-0355-y.
- Warren JG, Mercado J, Grace D. 2019. Occurrence of Hop latent viroid causing disease in Cannabis sativa in California. Plant Dis. 103(10):2699–2699. doi: 10.1094/PDIS-03-19-0530-PDN.
- Wassenegger M, Spieker RL, Thalmeir S, Gast F-U, Riedel L, Sänger HL. 1996. A single nucleotide substitution converts potato spindle tuber viroid (PSTVd) from a noninfectious to an infectious RNA for Nicotiana tabacum. Virology. 226(2):191–197. doi: 10.1006/viro.1996.0646.
