Abstract
Litylenchus crenatae mccannii is a foliar nematode that causes severe damage to beech, known as beech leaf disease (BLD). Previous studies have characterized and identified L. crenataein Japan and a subspecies L. crenatae mccannii in the USA. Litylenchus crenatae mccannii has been found to cause BLD in native and non-native beech hosts in North America. As little is known about the distribution and diversity of the nematode in Ontario, Canada, beech foliage was sampled throughout southcentral Ontario. Incidence and severity maps show that BLD spread from its first detections in 2017, in Elgin and Norfolk counties, to the west and northeast. A gradient in severity and incidence was observed with the highest ratings at the locations where it was originally found. The extent of the nematode’s distribution is much broader than BLD, occurring throughout much of the range of American beech. Sequences of two barcoding loci for the L. crenatae mccannii isolates from 11 locations in Ontario showed 100% similarity within and among these locations. The genotype of L. crenatae mccannii found in Ontario is the same as that identified in the USA. This finding is consistent with the fact that 92% of beech imported into Canada is from the USA and suggests that the USA could be the origin of L. crenatae mccannii nematodes in Ontario or that both countries have imported beech trees from the same source country of BLD. This research provides motivation for further studies to clarify the sources of L. crenatae mccannii.
Résumé
Litylenchus crenatae mccannii est un nématode foliaire qui provoque de graves dégâts sur le hêtre, connus sous le nom de maladie de la feuille de hêtre (BLD). Des études antérieures ont caractérisé et identifié L. crenatae au Japon et une sous-espèce L. crenatae mccannii aux Etats-Unis. Litylenchus crenatae mccannii a été trouvé responsable de la BLD sur des hôtes indigènes et non-indigènes du hêtre en Amérique du Nord. La distribution et la diversité du nématode en Ontario (Canada) étant peu connues, des échantillons de feuillage de hêtre ont été prélevés dans tout le centre-sud de l’Ontario. Les cartes d’incidence et de gravité montrent que la BLD s’est propagée à partir de ses premières détections en 2017, dans les comtés d’Elgin et de Norfolk, vers l’ouest et le nord-est. Un gradient de gravité et d’incidence a été observé, les notes les plus élevées se trouvant aux endroits où il a été trouvé à l’origine. L’étendue de la distribution du nématode est beaucoup plus large que celle de la BLD, puisqu’il est présent dans une grande partie de l’aire de répartition du hêtre américain. Les séquences de deux loci de codage à barres pour les isolats de L. crenatae mccannii provenant de 11 localités de l’Ontario ont montré une similarité de 100 % à l’intérieur de ces localités et entre elles. Le génotype de L. crenatae mccannii trouvé en Ontario est le même que celui identifié aux Etats-Unis. Cette découverte est cohérente avec le fait que 92% des hêtres importés au Canada proviennent des Etats-Unis et suggère que les Etats-Unis pourraient être l’origine des nématodes L. crenatae mccannii en Ontario ou que les deux pays ont importé des hêtres du même pays source de BLD. Cette recherche motive des études supplémentaires pour clarifier les sources de L. crenatae mccannii.
Introduction
Beech trees (Fagus L., Fagaceae) are present in North America, Europe, and Asia. There are nine commonly recognized species. Six species are native to East Asia, two to Europe and West Asia, and one to North America (Denk et al. Citation2005; Fang and Lechowicz Citation2006; Denk and Grimm Citation2009). Species from East Asia are short and shrub-like, and Japanese beech (F. crenata BI.) is planted for bonsai production. Other beech species native to other regions are taller and are often planted as ornamentals in western countries, with the predominant species being European beech (F. sylvatica L.). Wood from beech can be used for furniture, flooring, household items, and firewood. Additionally, beech nuts provide an important source of protein and fat for overwintering wildlife. This versatility makes beech economically and ecologically important.
In recent decades, beech trees in North America have been increasingly threatened due to accidental introductions of pests and pathogens. The beech scale, Cryptococcus fagisuga Lindinger [Hemiptera: Eriococcidae], which reduces tree health and size, was first detected in the 1890s in Nova Scotia after the planting of beech trees from Europe (Fries Citation1832; Hewitt Citation1914). Two fungi, Neonectria faginata (Lohman et al.) Castl. & Rossman and Neonectria ditissima (Tul. & C. Tul.) Samuels & Rossman [Hypocreales: Nectriaceae], colonize feeding wounds made by the beech scale (Gwiazdowski et al. Citation2006). This infection triggers the production of cankers and lesions, which results in beech bark disease. Another major impact on beech trees in North America is caused by the beech leaf-mining weevil, Orchestes fagi (Sweeney et al. Citation2020). The weevil is native to Europe and was first reported in Nova Scotia (Canada) in 2012 and in nearby New Brunswick and Prince Edward Island in 2020 (Klymko and Anderson Citation2022). At one location in Nova Scotia, weevil incidence increased from 48–83% and beech mortality increased from 10–70% between 2016 and 2019 (Sweeney et al. Citation2020).
Beech trees in eastern North America are now threatened by a recently described foliar disease, beech leaf disease (BLD), that affects American beech (F. grandifolia Ehrh.) in forests (Ewing et al. Citation2019). In urban areas and arboretums, the disease also affects European (F. sylvatica L.), Oriental (F. orientale Lipsky.) and Chinese beech (F. engleriana Seeman ex Diels.) (Ewing et al. Citation2019; Burke et al. Citation2020; Marra and LaMondia Citation2020). Since 2012, the disease has been detected in the Great Lakes Region (Ontario, CA; Ohio, Pennsylvania, New York, and Michigan, USA) and along the Atlantic Coast (Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Rhode Island, and Virginia, USA) (Ewing et al. Citation2019; Carta et al. Citation2020; Marra and LaMondia Citation2020; Reed et al. Citation2020; Kantor et al. Citation2022; Vieira et al. Citation2022). Disease symptoms first appear as darkened interveinal tissues giving the leaf a striped appearance. Severely affected leaves have thickened, chlorotic tissues that can become necrotic. Early leaf abscission and bud abortions occur when the infection is severe (Carta et al. Citation2020; Reed et al. Citation2020). Damage occurs in leaf buds, and symptoms do not progress after leaf-out (Fearer et al. Citation2022b).
The causal agent of BLD has recently been described as a subspecies of the foliar nematode Litylenchus crenatae, namely Litylenchus crenatae ssp. mccannii (Carta et al. Citation2020). The nematode differs slightly in morphology and tree hosts from L. crenatae, which has itself only recently been described from diseased leaves of Japanese beech trees (Kanzaki et al. Citation2019). The nematodes complete their entire life cycle in leaves and buds, although L. crenatae mccannii has been reported to overwinter in detached leaves on the ground (Reed et al. Citation2020). Furthermore, the nematode occurs both in asymptomatic and symptomatic leaves and buds (Burke et al. Citation2020; Reed et al. Citation2020). Reed et al. (Citation2020) demonstrated that populations of the nematode occur in smaller numbers in asymptomatic tissues than in symptomatic tissues. Some have noted the presence of L. crenatae mccannii in and outside the range of BLD and suggested the nematode spread from eastern USA into Canada (Ewing et al. Citation2019; Fearer et al. Citation2022a). Litylenchus species have only been reported from Korea, Japan, and New Zealand, and consequently it is hypothesized that L. crenatae mccannii originated from the Pacific Rim (Carta et al. Citation2020).
Arthropods can serve as vectors for nematodes. Known examples include the pinewood nematode (Bursaphelenchus xylophilus) vectored by the Japanese pine sawyer (Monochamus alternatus) (Jauharlina et al. Citation2012) or Parasitodiplogaster sp. carried by fig wasps (Elisabethiella baijnathi) (Togashi and Shigesada Citation2006). In both examples, the nematodes enter their vector through body openings such as spiracles (Enda Citation1977). The nematodes then vacate and enter the plant or tree host through wounds caused by their vectors (Mamiya and Enda Citation1972; Morimoto and Iwasaki Citation1972; Martin et al. Citation1973). With respect to L. crenatae mccannii, eggs and juvenile stages of the nematode have been found on the bodies of mites (Popkin Citation2019; Carta et al. Citation2020, Citation2023). Martin et al. (Citation2023) have demonstrated a possible link between L. crenatae mccannii and avian transport as birds are known to feed on beech buds.
A major mode of spread of parasitic nematodes over longer distances is through import of live plant or tree material, seeds or soil (Liebhold et al. Citation2012; Singh et al. Citation2013; Aalders et al. Citation2017). The trade of live tree material unintentionally enables nematodes to survive long distance transport between countries. Beech is an import commodity and especially Japanese and European beech are traded between North America and other regions, as well as within North America. Beech leaf disease and L. crenatae mccannii have been detected in seven of the eight Ontario counties closest to Lake Erie, as well as on a European beech hedge in Toronto (Reed et al. Citation2020). More recently, the disease and nematode have been detected in the counties east and west of Toronto, along Lake Ontario.
In southern Ontario, nurseries and businesses receive, grow, and sell cultivars of European beech. American beech is an important component of the surrounding forests, so much so that Braun (Citation1950) included this region as part of the beech-maple forest type. American beech is sometimes grown and sold in southern Ontario, but production has nearly ceased now that beech bark disease is present. The two most likely sources for global L. crenatae mccannii movement are thought to be through the import of beech from the not-yet-identified native range or from a country already harbouring the nematode. In the context of biosecurity, investigating routes of transport and introduction are important.
The aim of this study was to confirm the identity and diversity of L. crenatae mccannii from 11 locations in Ontario, Canada using two barcoding loci (LSU and ITS rDNA). Also assessed was whether disease severity across these sites correlates with duration of disease presence. Finally, we consider possible sources of introduction of L. crenatae mccannii into Ontario. For this purpose, information was collected from nursery growers and government organizations on the movement of beech into and within Ontario.
Materials and methods
BLD surveillance in Ontario
Set up and visual survey of plots
In 2019, staff of the Ontario Ministry of Natural Resources and Forestry set up a network of 34 plots in 17 locations (Reed et al. Citation2022) to investigate the long-term effects of BLD on forests containing American beech. Thirteen of the locations, representing five counties, had trees with BLD symptoms. All locations were in southwestern Ontario, within 70 km of Lake Erie. These forests are classified as upland tolerant hardwoods (Strobl and Bland Citation2000) and occur in the deciduous forest region (Ontario Citation2023), historically referred to as Carolinian (Waldron Citation2003).
In each of the 17 forest locations, two circular 0.04 ha plots were established to assess disease in overstory trees. Each plot contained a minimum of three overstory (≥10 cm diameter) at breast height (DBH) or understory trees (<10 cm DBH, >1 metre tall). A smaller 0.004 ha plot was established in the centre of each overstory plot to assess understory beech trees. The crown of each beech tree was visually assessed for BLD using binoculars. More details about the pest assessment can be found in Reed et al. (Citation2022). Briefly, surveyors visually assessed a tree crown to determine the percentage of leaves with no symptoms, low severity symptoms (bands on less than 2/3 of leaf) and high severity symptoms (>2/3 of leaf affected, tissue thickening, chlorosis). Categories for the leaves affected were (1) 0%, (2) 1–10%, (3) 10–25%, (4) 25–50%, (5) 50–75% and (6) 75–100%.
Calculation of disease severity
To calculate severity for each tree, the midpoint for each category was first determined. The midpoint for the percentage of normal, low severity and high severity leaves in the crown were adjusted relative to one another so their total was 100%. Symptom severity for a tree was the summation of the adjusted percentages for the low and high severity classes.
L. crenatae mccannii surveillance in Ontario
Sample collection
In 2022, forest health technicians and researchers from the Ontario Ministry of Natural Resources and Forestry submitted leaf samples from eastern Ontario as part of a survey for L. crenatae mccannii. Samples were collected between June and September and were made whenever American beech trees were encountered in a forest. Forests with and without BLD were sampled. Leaf samples consisted of 25 beech leaves placed in a sealable plastic food storage bag. The sample was collected from saplings and seedlings in a 25-metre radius. Leaf samples were refrigerated until shipment in an insulated cooler with ice packs.
Leaf DNA extraction and nematode DNA amplification and sequencing
Five leaves were selected per sample and 100 mg tissue was cut from each leaf and minced. The total 500 mg minced sample was thoroughly mixed, and three 100 mg samples were each transferred to a 2 mL tube with a 4 mm stainless steel bead and stored at − 80°C until needed. Total DNA was isolated from one of the three 100 mg samples using the Qiagen DNeasy Plant Mini kit. The leaf material was first ground into a fine powder using the Qiagen TissueLyzer II and DNA extraction was performed using the QIAcube according to the manufacturer’s instructions.
A PCR assay was performed on the Eppendorf Mastercycler X50a instrument. The PCR reaction mixture consisted of the following final concentrations: 1× Thermo Scientific DreamTaq Buffer (Waltham, Massachusetts, USA), 0.625 units of Thermo Scientific DreamTaq DNA polymerase, 0.2 mm dNTP mix, 0.4 µM each of 33F and 234 R primers (Burke et al. Citation2023), 1 µL of DNA template, and nuclease-free water to 25 µL. Cycling conditions consisted of an initial denaturation at 95°C for 1 minut followed by 40 cycles of 95°C for 15 sec, 58°C for 10 sec, 72°C for 30 sec and a final extension at 72°C for 5 min. PCR amplicons were analyzed by gel electrophoresis on a 1% agarose gel. Amplicons of 32 selected samples with expected sizes (~200 bp) were sequenced with the 33F primer at Laval University (Quebec) using a Sanger ABI3730×l sequencer. DNA sequences were trimmed and edited using the Geneious software v2022.1 and subjected to GenBank BLASTn search on the NCBI website.
Beech imports into and within Canada
A request for records of imports pertaining to beech into Canada between the years 2010 to 2019 was submitted to the Canadian Food Inspection Agency (CFIA). Beech imports included live trees, logs, chips, foliage, or other products associated with beech. For each year, the data was categorized into four groups: live material, logs, chips, and other materials. We then extracted data specific to Ontario. Finally, the countries from which Canada imported beech were investigated. A map was drawn to show the import movement of beech into Canada.
In addition, a survey on the movement of live beech trees in Ontario nurseries and out of Ontario was conducted via email. Survey respondents were asked (1) if they purchased American, European, Oriental, or Chinese beech; (2) if they propagated or purchased their beech trees; (3) which state or province they purchased beech from; (4) if they purchased beech from a country other than the USA or Canada; and (5) if their nursery shipped beech trees outside of Ontario or sold them locally.
Assessing the diversity of L. crenatae mccannii from Ontario
Nematode extraction
Leaf samples were collected for nematode extraction from four trees at 11 of 17 locations surveyed for BLD (). Five symptomatic leaves were collected from each tree. Leaves were kept cool during transport and refrigerated until nematodes were extracted.
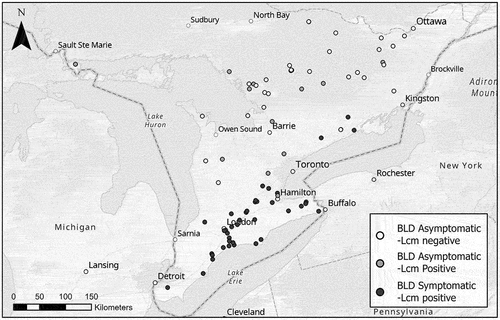
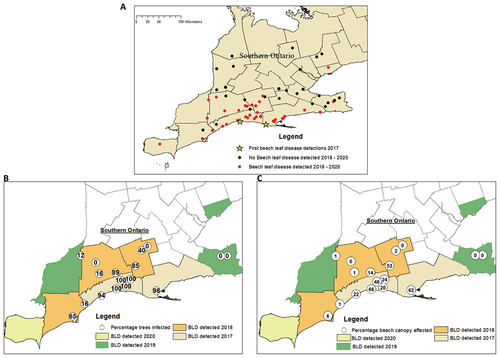
Fig. 1 (Colour online) Beech leaf disease incidence and severity: (A) locations where the nematode Litylenchus crenatae mccanii was detected in southern Ontario from 2017 to 2020, (B) percentage of trees infected, and (C) percentage of beech canopy affected in the same period.
Nematodes were extracted using a modified-pan method (Townshend Citation1963; Reed et al. Citation2020). One half of three randomly selected leaves were cut into strips and each leaf half was placed into its own Petri plate with 50 mL deionized water for 24 hours. The water was transferred to test tubes. After the nematodes settled to the bottom, all but the bottom 1 mL of water was siphoned off. Nematodes from each of the three leaf halves were combined and the settling and siphoning procedure repeated so that 1 mL remained. The concentrated nematode solution was transferred to a 1.5 mL microcentrifuge tube. After the nematodes had settled, most of the water was siphoned off, and the tube was filled with 99% ethanol. Preserved nematodes were refrigerated until further processing.
DNA extraction, amplification and sequencing
Single nematodes from the water suspension were removed for DNA extraction. DNA was extracted from a single nematode for each tree from all the locations (). The NucleoSpin Tissue XS kit (Macherey-Nagel) was used to extract DNA from each nematode following the manufacturer’s methodology.
Two universal nematode barcoding regions, the large subunit region (LSU rDNA, Nunn Citation1992) and the internal transcribed spacer region (ITS rDNA, Subbotin et al. Citation2001), were amplified using PCR with the primer pairs D2A (5’-ACAAGTACCGTGAGGGAAAGT-3’) and D3B (5’-TGCGAAGGAACCAGCTACTA-3’), and TW81 (5’-GTTTCCGTAGGTGAACCTGC-3’) and AB28 (5’-ATATGCTTAAGTTCAGCGGGT-3’), respectively. The total volume of the PCR reaction for both loci was 25 µL and consisted of 5 µL of 5× MyTaqTM Reaction Buffer (Bioline USA, Taunton, Massachusetts), 0.1 µM of each primer, 2.5 units MyTaqTM DNA polymerase (Bioline USA) and about 100 ng DNA template. The PCR conditions were as follows: initial denaturation of 95°C for 3 min, 35 cycles of 95°C for 45 sec, 58°C for 30 sec, 72°C for 1 min, and a final extension of 72°C for 10 min. The PCR products were purified using the Monarch® DNA Clean-up kit (New England Biolabs, Ipswich, MA) according to the manufacturer’s protocol. The same primers were used to sequence the ITS and LSU gene regions. Sequencing was performed at Laval University using a Sanger ABI3730×l sequencer.
Confirmation of nematode identity and genetic diversity analysis
Representative sequences of Litylenchus spp. were downloaded from GenBank for both the ITS and LSU gene regions (Supplementary Table S1). Alignment was performed on the entire data set using MAFFT v. 7 (Katoh and Standley Citation2013) and verified using ClustalW in MEGA v. 10 (Tamura et al. Citation2011). For each gene region the best fit model was separately determined using jModelTest v2.1.3 (Darriba et al. Citation2012). The best fit model was then applied to construct the Maximum Likelihood tree (ML) using PhyML v3.1. Additionally, Maximum Parsimony phylogenetic analysis was conducted in PAUP v4.0 (Swofford Citation2003).
Results
Beech leaf disease surveillance in Ontario
Beech leaf disease incidence was highest at sites in Elgin and Norfolk counties where the disease was first reported (). Incidence was less, but still greater than 80% at sites in counties closest to Elgin and Norfolk County (Middlesex and Oxford). The lowest BLD incidence scores occurred at sites farthest from the first confirmed detections.
Beech leaf disease severity values were less than incidence values and were below 73% (). The overall pattern for disease severity was similar to that for disease incidence, with higher scores at sites in Elgin and Norfolk counties and scores decreasing farther away from those sites.
L. crenatae mccannii surveillance in Ontario
Samples were received from as far west and north as St. Joseph Island, as far east as Lanark, and as far south as Windsor, Ontario (). Of the 168 samples (80 symptomatic and 88 asymptomatic) tested for nematode DNA, 115 (68%) were positive and 53 (32%) were negative for L. crenatae mccannii DNA. The 115 positive samples consisted of all 80 symptomatic and 35 of the 88 (~40%) asymptomatic samples. DNA sequencing was conducted on PCR amplicons of 32 of the 35 asymptomatic samples that tested positive for nematode DNA. A BLASTn search on GenBank indicated that all DNA sequences from the 32 samples matched L. crenatae mccannii DNA at 96–100% identity and 97–100% coverage.
Beech imports into and within Canada
During 2010–2019, approximately 47% of the total beech products imported into Canada were live trees for propagation, 45% were wood chips, and 8% were logs. Foliage and wire baskets made up less than 1% of the beech products imported into Canada. Products imported into Canada were from five countries including the USA, Italy, Germany, the Netherlands, and Poland. More than 90% of beech products imported into Canada were from the USA (). Imports from the four other countries were 5% or less.
Fig. 3 Beech import into Canada. Arrows indicate routes of beech trade. Solid arrows show beech trade other than live beech trees, such as logs and wood chips; dashed lines represent routes of live beech tree trade. Percentages represent beech import from the total into Canada and the percentage of live beech trees is in italics.
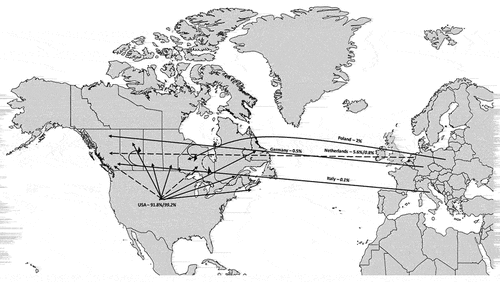
Live beech trees were imported into Canada from three countries, namely the USA (88.63%), the Netherlands (11.36%), and Italy (0.014%). Ontario imported live beech trees from two of these countries, the USA (88.64%) and the Netherlands (11.36%). British Columbia was the only other Canadian province to import live beech trees.
Six responses to the survey were received from Ontario nurseries. All six nurseries purchase or sell European or American Beech. Only one nursery propagated its own American beech seedlings. The remainder purchased 1-year old beech seedlings from nurseries in western North America, including British Columbia, Washington, and Oregon. Of these five nurseries, four listed Oregon as a source, two listed British Columbia as a source, and one listed Washington as a source for 1-year old seedlings. These seedlings are then grown in Ontario nurseries until they can be sold for local use. One respondent stated that European beech had been purchased from the Netherlands but not for many years. None of the respondents sell beech outside Ontario.
Confirmation of nematode identity and genetic diversity analysis
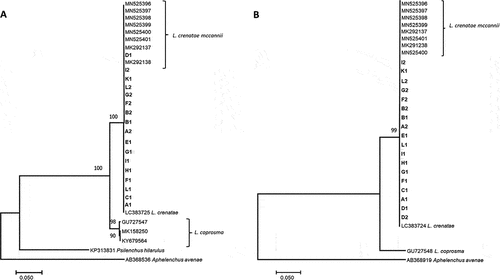
PCR fragments of 719 bp for LSU and 900 bp for ITS were amplified and sequenced for L. crenatae mccannii from Canada. No sequence diversity was found for the LSU (NCBI GenBank Database MW295607–MW295624) or ITS loci (MW295557–MW295573) of L. crenatae mccannii among samples from this study or the reference samples from the USA. Comparing the sequences from this study to that of L. crenatae from Japan only revealed differences, one insertion and two deletions, in the ITS gene region (, Supplementary Fig. S1).
Fig. 4 Sequence diversity of Litylenchus crenatae mccanii in Canada. Phylogenetic analysis using maximum likelihood (ML) of (A) 719 bp of the large subunit region (LSU) and (B) 900 bp of the internal transcribed spacer region (ITS-rDNA). The trees are rooted to Aphelenchoides sp. The isolates from this study are in bold.
Discussion
The presence of L. crenatae mccannii in Ontario was confirmed in this study using two gene regions. The overall pattern of disease incidence and severity supports the idea that BLD is spreading in Ontario. Disease severity and incidence values were highest in Elgin and Norfolk counties, the two counties where the disease was first detected, and decreased with increasing distance from these sites. It is unlikely that environmental factors are responsible for the large differences in incidence and severity since all 17 sampling sites are in the same ecoregion. At larger geographic scales, the risk of beech leaf disease is most strongly correlated with the difference between the mean temperature of the warmest and coldest months (24–26°C), relative humidity, and distance from water bodies or riverbeds (Selvi Citation2023). Geographic patterns of incidence and severity in Ontario suggest a relationship between time since detection and disease severity. Locations where BLD was first detected in 2017 and nearby locations had 100% tree infection and the highest numbers for percentage beech canopy infection. Incidence and severity decreased radially from the initial points of detection. The factors causing this gradient still need direct investigation and likely are multiple. These factors likely include the movement of beech internationally and regionally as well as vectors distributing the nematode locally. Furthermore, with time the number of nematodes in an already infected tree is expected to increase, as will the potential for local spread.
The wide distribution of L. crenatae mccannii across Ontario may contribute to the rapid expansion of the range of BLD in both Ontario and the USA. It appears that nematode populations may be present beyond the range where BLD symptoms are now evident, but at low numbers. The numbers are evidently too low to elicit noticeable symptoms, particularly symptoms severe enough to be detected through visual surveys. The incidence, severity, and spread of nematode-induced diseases are strongly influenced by population size (Lucas et al. Citation1985; Kohl et al. Citation2010; Khan and Ahamad Citation2020). Therefore, long-term monitoring of L. crenatae mccannii populations outside the range of BLD is necessary to fully understand the development of disease and the risk it poses to American beech in Canada. The less frequent detection of the nematode in more northern regions, where BLD is not yet present, provides additional evidence for the northward spread of the disease from sites along Lake Erie.
The northern spread of symptoms in Ontario appears to conflict with the strong west to east pattern observed in the USA. There, BLD incidence is greatest between Cleveland, Ohio, and New York, New York. Disease development is occurring in the other cardinal directions but appears slower (Selvi Citation2023). Ontario is north of the initial detections which would support the idea that nematode populations are still increasing to the north, west, and south of the initial detections. A better understanding of the current range of the nematode in North America will help in understanding the direction and rate of nematode spread and disease development.
The sequence data from this study confirmed the presence of the subspecies L. crenatae mccannii in Ontario in all sampled sites. Litylenchus crenatae mccannii was first described and reported in North America (Carta et al. Citation2020; Marra and LaMondia Citation2020). The authors highlighted morphological and host range differences compared to the described L. crenatae nematode species from Japan (Kanzaki et al. Citation2019). In the current study, we were also able to distinguish between L. crenatae and L. crenatae mccannii based on a nuclear insertion and two deletions in the ITS region, as was also reported by Carta et al. (Citation2020). Additional molecular markers such as microsatellite markers or more variable sequence makers are needed to not only differentiate between L. crenatae and L. crenatae mccannii but also to facilitate investigations into the geographic structure in nematode populations.
All isolates of L. crenatae mccannii obtained in Ontario represented a single sequence genotype of the LSU and ITS. This genotype is identical to that found in the USA, suggesting a link between these populations. The currently known areas infested with BLD in the USA are all represented by one genotype (Carta et al. Citation2020; Fearer Citation2022). These data suggest a recent and limited introduction. It is possible that there was a single introduction into the USA (where the disease first appeared), which then spread into Canada. This spread into Canada could have resulted from wind, birds, or arthropod dispersal or importation of infected nursery stock. It is also possible that Canada and the USA imported infected nursery stock from the same country. The possibility of multiple introductions of the nematode is not excluded, but they would likely have come from a common origin. Further research using more diverse markers and more comprehensive sampling will be needed to obtain a deeper understanding of the invasion history.
The beech import data collected during this study aligns with the conclusions about the invasion suggested by the molecular data. Imports of beech into Canada originate from five countries (Italy, Germany, the Netherlands, Poland, and the USA) but are dominated by the USA. Live beech trees imported into Canada come from Italy, the Netherlands, and the USA, with most from the latter. These three countries are of concern with respect to L. crenatae mccannii movement across countries and being the possible route of introduction into Canada. Surveys from Ontario nurseries reported importation and movement patterns of beech products consistent with those observed in the CFIA data, including the movement of small beech trees from the western USA and Canada to Ontario.
Given the continued import of beech from various countries, continued monitoring of L. crenatae mccannii and its population genetics is warranted. The current study can serve as a baseline for that work. Continued trade and import of beech could introduce other L. crenatae mccannii genotypes into Canada/Ontario. Such new genotypes may have no effect or may increase disease severity or facilitate adaptation for a broader geographic or host range. Ultimately, the presence of multiple genotypes could make management and treatment of BLD more difficult and efforts should be taken to prevent this from happening.
Future studies may involve more powerful tools such as microsatellite markers or even genome wide single nucleotide polymorphisms (SNP) analysis to assess the genetic diversity, structure across the distribution, possible origin, and sources of spread. This approach will require greater sampling efforts locally from nursery stocks (Canada, USA), as well as globally. Such efforts can and should be focused on the main trade routes of beech trees and products. Furthermore, breeding studies are needed to clarify the species status of L. crenatae mccannii (Carta et al. Citation2020). Such knowledge will aid in managing the import and export of beech, as well as guide the development and improvement of BLD management strategies.
Supp. Table 1.docx
Download MS Word (28.9 KB)Supplementary figure1v1.tif
Download TIFF Image (631.1 KB)Acknowledgments
We thank Rebecca Lidster, Vanessa Chaimbrone, Paulette Hebert, Tess Ward, Susan McGowan, Ariel Ilic, Amy Cocksedge, Sylvia Greifenhagen, Jessica Walker, and Christine St. Jules of the Ontario Ministry of Natural Resources and Forestry (MNRF) for collecting samples and performing visual surveys. Thank you to Marc Ouellette (MNRF) for creating maps. We appreciate Ausable Bayfield Conservation Authority (CA), Catfish Creek CA, Grand River CA, Lower Thames CA, Kettle Creek CA, Upper Thames CA, Thames Talbot Land Trust, Nature Conservancy Canada, Ontario Nature, and Ontario Parks for allowing plot establishment on their properties.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2024.2312150.
Additional information
Funding
References
- Aalders LT, McNeill MR, Bell NL, Cameron C. 2017. Plant parasitic nematode survival and detection to inform biosecurity risk assessment. NeoBiota. 36:1–16. doi: 10.3897/neobiota.36.11418.
- Braun EL. 1950. Deciduous forests of eastern North America. Philadelphia (PA): Blakiston Company.
- Burke DJ, Hoke AJ, Koch J. 2020. The emergence of beech leaf disease in Ohio: probing the plant microbiome in search of the cause. Forest Pathol. 50(2):e12579. doi: 10.1111/efp.12579.
- Burke D, Hoke A, Reed S, Martin D, Kyker S, Pitts M, Battigan S. 2023. Development of primers specific for detection of Litylenchus crenatae, the causal agent of beech leaf disease, in plant tissue. Plant Dis. doi: 10.1094/PDIS-12-22-2911-SR.
- Carta LK, Handoo ZA, Li S, Kantor M, Bauchan G, McCann D, Gabriel CK, Yu Q, Reed S, Koch J. 2020. Beech leaf disease symptoms caused by newly recognized nematode subspecies Litylenchus crenatae mccannii (Anguinata) described from Fagus grandifolia in North America. Forest Pathol. 50(2):e12580. doi: 10.1111/efp.12580.
- Carta LK, Li S, Mowery J. 2023. Beech leaf disease (BLD), Litylenchus crenatae and its potential microbial virulence factors. In: Asiegbus F, Kovalchuk A, editors. Forest microbiology: tree diseases and pests. Vol. 3. Cambridge (MA): Elsevier Academic Press; p. 183–192.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772–772. doi: 10.1038/nmeth.2109.
- Denk T, Grimm GW. 2009. The biogeographic history of beech trees. Rev Palaeobot Palyno. 158(1–2):83–100. doi: 10.1016/j.revpalbo.2009.08.007.
- Denk T, Grimm GW, Hemleben V. 2005. Patterns of molecular and morphological differentiation in Fagus (Fagaceae): phylogenetic implications. Am J Bot. 92(6):1006–1016. doi: 10.3732/ajb.92.6.1006.
- Enda N. 1977. Boarding of Bursaphelenchus xylophilus on Monochamus alternatus and its departure from vectors. Studies on the control of pine wilt disease. Tokyo: Secretariat of Agriculture, Forestry and Fisheries Research Council, Ministry of Agriculture, Forestry and Fisheries; p. 83–85.
- Ewing CJ, Hausman CE, Pogacnik J, Slot J, Bonello P. 2019. Beech leaf disease: an emerging forest epidemic. Forest Pathol. 49(2):e12488. doi: 10.1111/efp.12488.
- Fang J, Lechowicz MJ. 2006. Climatic limits for the present distribution of beech (Fagus L.) species in the world. J Biogeogr. 33(10):1804–1819. doi: 10.1111/j.1365-2699.2006.01533.x.
- Fearer CJE 2022. Investigating the Fagus grandifolia-Beech leaf disease pathosystem using metabarcoding, phenological observations, and near-infrared spectroscopy [ dissertation]. Columbus (OH): The Ohio State University. http://rave.ohiolink.edu/etdc/view?acc_num=osu1650572129664455.
- Fearer CJ, Conrad AO, Marra RE, Georsk Ey C, Villari C, Slot J, Bonello P. 2022a. A combined approach for early in-field detection of beech leaf disease using near-infrared spectroscopy and machine learning. Front Forests Glob Change. 5:934545. doi: 10.3389/ffgc.2022.934545.
- Fearer CJ, Volk D, Hausman CE, Bonello P. 2022b. Monitoring foliar symptom expression in beech leaf disease through time. Forest Pathol. 52(1):e12725. doi: 10.1111/efp.12725.
- Fries E. 1832. Systema mycologicum, sistens fungorum ordines generum, et species. Vol. 3. Gryphiswaldae: Subtibus Ernesti Mauritii.
- Gwiazdowski RA, Van Driesche RG, Desnoyers A, Lyon S, S-A W, Kamata N, Normark BB. 2006. Possible geographic origin of beech scale, Cryptococcus fagisuga (Hemiptera: Eriococcidae), an invasive pest in North America. Biol Control. 39(1):9–18. doi: 10.1016/j.biocontrol.2006.04.009.
- Hewitt CG. 1914. Note on the occurrence of the felted beech coccus Cryptococcus fagi (Baerens) Dougl. in Nova Scotia. Can Entomol. 46(1):15–16. doi: 10.4039/Ent4615-1.
- Jauharlina J, Lindquist E, Quinnell R, Robertson H, Compton S. 2012. Vectors wasps as vectors of mites and nematodes. Afr Entomol. 20(1):101–110. doi: 10.4001/003.020.0113.
- Kantor M, Handoo Z, Carta L, Li S. 2022. First report of beech leaf disease, caused by Litylenchus crenatae mccannii, on American beech (Fagus grandifolia) in Virginia. Plant Dis. 106(6):1764. doi: 10.1094/PDIS-08-21-1713-PDN.
- Kanzaki N, Ichihara Y, Aikawa T, Ekino T, Masuya H. 2019. Litylenchus crenatae n. sp. (Tylenchomorpha: Anguinidae), a leaf gall nematode parasitising Fagus crenata Blume. Nematology. 21(1):5–22. doi: 10.1163/15685411-00003190.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. doi: 10.1093/molbev/mst010.
- Khan MR, Ahamad F. 2020. Incidence of root-knot nematode (Meloidogyne graminicola) and resulting crop losses in paddy rice in northern India. Plant Dis. 104(1):186–193. doi: 10.1094/PDIS-12-18-2154-RE.
- Klymko J, Anderson K. 2022. First records of the invasive beech leaf-mining weevil (Orchestes fagi) in New Brunswick and Prince Edward Island, Canada. J Acad Entomol Soc. 18:23–25.
- Kohl LM, Warfield CY, Benson DM. 2010. Population dynamics and dispersal of Aphelenchoides fragariae in nursery-grown Lantana. J Nematol. 42(4):332–341.
- Liebhold AM, Brockerhoff EG, Garrett LJ, Parke JL, Britton KO. 2012. Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Front Ecol Environ. 10(3):135–143. doi: 10.1890/110198.
- Lucas G, Campbell C, Lucas L. 1985. Introduction to plant diseases, identification and management. West Port (CT): The AVI Publishing Company Inc; p. 313.
- Mamiya Y, Enda N. 1972. Transmission of Bursaphelenchus lignicolus (nematoda: Aphelenchoididae) by Monochamus alternatus (coleoptera: Cerambycidae). Nematologica. 18(2):159–162. doi: 10.1163/187529272X00395.
- Marra RE, LaMondia J. 2020. First report of beech leaf disease, caused by the foliar nematode, Litylenchus crenatae mccannii, on American beech (Fagus grandifolia) in Connecticut. Plant Dis. 104(9):2527. doi: 10.1094/PDIS-02-20-0442-PDN.
- Martin GC, Owen AM, Way JI. 1973. Nematodes, wasps and wasps. J Nematol. 5(1):77.
- Martin DKH, Parkinson SR, Stoleson SH, Burke DJ, Lituma CM. 2023. Exploration of avian vectors of Litylenchus crenatae mccannii, the nematode associated with beech leaf disease. Poster session presented at: 31st USDA interagency research forum on invasive species; Jan 10–13. Annapolis, MD.
- Morimoto K, Iwasaki A. 1972. Role of Monochamus alternatus (Coleoptera: Cerambycidae) as a vector of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae). J Jpn For Soc. 54(6):177–183.
- Nunn GB 1992. Nematode molecular evolution: an investigation of evolutionary patterns among nematodes based upon DNA sequences [ dissertation]. Nottingham (UK): University of Nottingham.
- Ontario. 2023. Ontario’s forest regions. [ accessed 2023 Apr]. https://www.ontario.ca/page/forest-regions.
- Popkin G. 2019. A mysterious disease is striking American beech trees. Science. 366(6467):786. doi: 10.1126/science.366.6467.786.
- Reed SE, Greifenhagen S, Yu Q, Hoke A, Burke DJ, Carta LK, Handoo ZA, Kantor MR, Koch J. 2020. Foliar nematode, Litylenchus crenatae ssp. mccannii, population dynamics in leaves and buds of beech leaf disease‐affected trees in Canada and the US. Forest Pathol. 50(3):e12599. doi:10.1111/efp.12599.
- Reed SE, Volk D, Martin DK, Hausman CE, Macy T, Tomon T, Cousins S. 2022. The distribution of beech leaf disease and the causal agents of beech bark disease (Cryptoccocus fagisuga, Neonectria faginata, N. ditissima) in forests surrounding Lake Erie and future implications. For Ecol Manag. 503:119753. doi: 10.1016/j.foreco.2021.119753.
- Selvi E 2023. Assessing the risk of beech leaf disease in a changing climate [ master’s thesis]. Columbus (OH): The Ohio State University.
- Singh SK, Hodda M, Ash G, Banks N. 2013. Plant‐parasitic nematodes as invasive species: characteristics, uncertainty and biosecurity implications. Ann Appl Biol. 163(3):323–350. doi: 10.1111/aab.12065.
- Strobl S, Bland D. 2000. A silvicultural guide to managing Southern Ontario forests, version 1.1. Toronto (ON): Ontario Ministry of Natural Resources.
- Subbotin SA, Vierstraete A, De Ley P, Rowe J, Waeyenberge L, Moens M, Vanfleteren JR. 2001. Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Molecular Phylogenet Evol. 21(1):1–16. doi: 10.1006/mpev.2001.0998.
- Sweeney JD, Hughes C, Zhang H, Hillier NK, Morrison A, Johns R. 2020. Impact of the invasive beech leaf-mining weevil, Orchestes fagi, on American beech in Nova Scotia, Canada. Front For Glob Change. 3:46. doi: 10.3389/ffgc.2020.00046.
- Swofford D. 2003. Inferring evolutionary trees with PAUP*. Curr Protoc Bioinform. Chapter 6. doi: 10.1002/0471250953.bi0604s00.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molec Biol Evol. 28(10):2731–2739. doi: 10.1093/molbev/msr121.
- Togashi K, Shigesada N. 2006. Spread of the pinewood nematode vectored by the Japanese pine sawyer: modeling and analytical approaches. Popul Ecol. 48(4):271–283. doi: 10.1007/s10144-006-0011-7.
- Townshend J. 1963. A modification and evaluation of the apparatus for the Oostenbrink direct cottonwool filter extraction method. Nematologica. 9(1):106–110. doi: 10.1163/187529263X00205.
- Vieira P, Kantor M, Medina-Mora C, Sakalidis ML, Handoo Z. 2022. First report of the beech leaf disease nematode Litylenchus crenatae mccannii (Nematoda: Anguinidae) in Michigan. Plant Dis. 107(7):2266. doi: 10.1094/PDIS-10-22-2468-PDN.
- Waldron GE. 2003. Trees of the carolinian forest. Erin (ON): Boston Mills Press.