Abstract
Sooty bark disease is an invasive disease causing significant mortality of sycamore maple (Acer pseudoplatanus L.) in Europe, where it is emerging due to increasing drought and heat events. The causal agent, Cryptostroma corticale (Ellis & Everh.) P. H. Greg. & S. Waller, is a fungus endemic to the Great Lake region in eastern Canada, where it does not appear to cause disease within its natural host range (e.g. sugar maple, Acer saccharum Marshall). Sooty bark disease was reported causing mortality on sycamore maple and additional species within Washington State beginning in 2017. In summer 2022, sooty bark disease was found on a sycamore maple near Vancouver, representing the first report of the disease and causal agent within the province of British Columbia (BC). In this study, we identify the causal agent of sooty bark disease by morphological and molecular methods and confirm its pathogenicity in a controlled growth chamber experiment fulfilling Koch’s postulates on sycamore maple. Cryptostroma corticale has so far been found in BC on sycamore maple, Norway maple (A. platanoides L.) and bigleaf maple (A. macrophyllum Pursh). Sooty bark disease has been previously shown to increase in severity and occurrence under drought and warm conditions; we anticipate rising sooty bark disease cases as BC experiences increasingly frequent and extreme summer droughts and heat events.
Résumé
La maladie de l’écorce fuligineuse est une maladie envahissante qui provoque une mortalité importante chez l’érable sycomore (Acer pseudoplatanus L.) en Europe, où elle émerge en raison de la multiplication des épisodes de sécheresse et de chaleur. L’agent causal, Cryptostroma corticale (Ellis & Everh.) P. H. Greg. & S. Waller, est un champignon endémique de la région des Grands Lacs dans l’est du Canada, où il ne semble pas causer de maladie dans sa gamme d’hôtes naturels (par exemple l’érable à sucre, Acer saccharum Marshall). La maladie de l’écorce fuligineuse a été signalée comme causant la mortalité de l’érable sycomore et d’autres espèces dans l’État de Washington à partir de 2017. Au cours de l’été 2022, la maladie de l’écorce fuligineuse a été trouvée sur un érable sycomore près de Vancouver, ce qui représente le premier signalement de la maladie et de l’agent causal dans la province de la Colombie-Britannique (BC). Dans cette étude, nous identifions l’agent causal de la maladie de l’écorce fuligineuse par des méthodes morphologiques et moléculaires et confirmons sa pathogénicité dans une expérience en chambre de croissance contrôlée répondant aux postulats de Koch sur l’érable sycomore. Cryptostroma corticale a jusqu’à présent été trouvé en B C sur érable sycomore, érable de Norvège (A. platanoides L.) et érable à grandes feuilles (A. macrophyllum Pursh). Il a déjà été démontré que la maladie de l’écorce fuligineuse augmente en gravité et en fréquence en cas de sécheresse et de conditions chaudes; nous prévoyons une augmentation des cas de maladie de l’écorce fuligineuse dans la mesure où la Colombie-Britannique connaît des sécheresses et des chaleurs estivales de plus en plus fréquentes et extrêmes.
Introduction
In 1945, an unidentified fungus was observed sporulating profusely on a sycamore maple (Acer pseudoplatanus L.) snag in Wanstead Park outside of London, UK (Gregory and Waller Citation1951). By 1948, this mycological curiosity was unexpectedly associated with the death of approximately 40 sycamore maple trees and was soon found outside of the confines of the park. Disease symptoms included wilting and dieback, a characteristic greenish-brown to yellow stain of the heartwood that extended longitudinally for long distances and the formation of raised, vertically-elongated blisters, which preceded bark-shedding that exposed large masses of dark asexual spores (conidia) (Gregory et al. Citation1949; Gregory and Waller Citation1951). The conidial masses produced from extensive stromata within the bark gave rise to the name ‘sooty bark disease’. Gregory and Waller (Citation1951) identified the causal agent as Cryptostroma corticale (Ellis & Everh.) P. H. Greg. & S. Waller, an obscure fungus initially described in 1889 as Coniosporium corticale Ellis & Everh. from sugar maple (A. saccharum Marshall) firewood collected in London, Ontario (Ellis and Everhart Citation1889). Consequently, Cryptostroma corticale appears to be endemic to eastern North America, where it occurs on sugar maple presumably as a saprotroph following tree death and has not been associated with disease.
Sooty bark disease (SBD) has since emerged and spread throughout Europe, causing significant mortality as changes in climatic parameters trigger pathogenicity in this latent pathogen. Sooty bark disease outbreaks tend to occur in waves following exceptionally hot and dry summers. The first UK outbreak peaked in 1950, being referred to as ‘a disease in decline’ as early as 1955, and then saw a moderate resurgence in 1956 before again declining (Peace Citation1955; Paviour-Smith Citation1976). Notably, the summers of 1947, 1949 and 1950 were particularly hot in the London, UK area. More recent reports suggest that, following droughts and high summer temperatures, C. corticale expansively colonizes its stressed host, becomes pathogenic and can rapidly cause dieback and death (Dickenson and Wheeler Citation1981; Ogris et al. Citation2021; Muller et al. Citation2023). While first reports of SBD have accelerated in the last decade, for example from Bulgaria (Bencheva Citation2014), Italy (Oliveira Longa et al. Citation2016), Slovenia (Ogris et al. Citation2021) and Switzerland (Cochard et al. Citation2015), its epidemiology suggests that C. corticale was likely established long before it was first detected. Cryptostroma corticale causes prolonged asymptomatic infections as an endophyte within woody tissues (Koukol et al. Citation2015; Schlößer et al. Citation2023). Throughout Europe, SBD causes varying levels of mortality of sycamore maple, which is so far identified as the most susceptible host (Burgdorf et al. Citation2022).
A unique aspect of SBD is its potential impact on human health. Cryptostroma corticale produces vast numbers of spores on infected trees; for example, a stem approximately 5 m high × 32 cm in diameter may produce ca. 50 trillion spores (Gregory and Waller Citation1951). The airborne spores are allergenic and occupational exposure has been linked to hypersensitivity pneumonitis (Emanuel et al. Citation1966; Wenzel and Emanuel Citation1967; Tewksbury et al. Citation1968; Shepherd et al. Citation1989; Braun et al. Citation2021; Kespohl et al. Citation2022). Sooty bark disease therefore presents a possible occupational hazard for arborists and workers removing and handling infected wood. Additional considerations may be warranted for the removal and disposal of infected trees (Braun et al. Citation2021). Sooty bark disease is a rare example of a tree pathogen having direct impacts on human health, aligning with the One Health paradigm (Schulte et al. Citation2023).
Cryptostroma corticale was first reported in the Pacific Northwest (PNW) in 1969, when specimens from a dead sycamore maple were collected in Whitman County, Washington State (Goree Citation1969). The next report in the PNW was from a fallen branch of bigleaf maple (Acer macrophyllum Pursh) collected in 2007 (Brooks et al. Citation2023). In 2017, SBD began emerging in Washington State, causing mortality of sycamore maple planted in urban forests (Forest Health Watch Citation2023). Additional potential hosts have been identified in Washington State including Japanese (A. palmatum Thunb.) and Norway (A. platanoides L.) maple, horse-chestnut (Aesculus hippocastanum L.) and Pacific dogwood (Cornus nuttallii Audubon ex Torr. & A. Gray) (Washington State University Citation2023). Cryptostroma corticale was reported on A. hippocastanum in Germany; however, its pathogenicity was not verified (Brenken et al. Citation2024). Garbelotto et al. (Citation2024) recently provided the first report of SBD in California from three silver maples (Acer saccharinum) and one Norway maple, and subsequently confirmed pathogenicity in silver maple via inoculation experiments.
In June 2022, the first case of SBD in British Columbia (BC) was discovered in the Greater Vancouver area on a sycamore maple. As of March 2024, more than 30 additional observations of SBD have been found on Southern Vancouver Island and in the Greater Vancouver area. Here we provide a first report of SBD in BC based on observations confirmed by morphology, isolated strains, DNA sequencing and qPCR. We also demonstrate the pathogenicity of a strain of C. corticale isolated from Victoria, BC by fulfilling Koch’s postulates.
Materials and methods
Study sites
Sampling was conducted from 2022–2023 in the Capital Regional District (Southern Vancouver Island) and the Greater Vancouver area. Sampling activities were primarily opportunistic based on reports and observations by arborists, municipal workers and members of the public, as well as visual surveys of sycamore maple trees within the city of Victoria conducted by some of the authors throughout summer and fall 2023. A sooty bark disease project was created on iNaturalist (https://inaturalist.ca/projects/sooty-bark-disease) in collaboration with the South Vancouver Island Mycological Society to publicly track and verify potential cases of sooty bark disease within British Columbia. All sampling was done with the prior permission of the respective municipality.
Fungal microscopy, isolation, identification
Pieces of stromata and surrounding wood were removed from symptomatic trees using a chisel and hammer that were sterilized with 70% ethanol before and between each use. Samples were stored within two Ziploc® freezer bags or sterile 50 mL Falcon™ tubes and processed immediately upon return to the lab and then stored at 4°C or air-dried. Cryptostroma corticale was isolated by aseptically removing exposed conidia from stromata and placing on the agar surface of 9 cm diameter Petri dishes containing acidified potato dextrose [aPDA; 39 g Difco potato dextrose agar (Becton, Dickinson and Company, Sparks, MD, USA), 1 L distilled H2O, 10% HCl to adjust pH to 3.5] and 2% malt extract agar [MEA; 20 g malt extract (Becton, Dickinson and Company), 15 g agar (Becton, Dickinson and Company), 1 L distilled H2O]. In addition to this isolation method, approximately 1 cm2 of stroma was scraped with a sterile knife tip and added to a 2 mL centrifuge tube containing 1 mL of sterile distilled water. This solution was serially diluted in sterile water (1:10, 1:100, 1:1000) and 50 µL of each dilution was spread onto Petri dishes containing aPDA. Petri dishes were incubated at 20°C in darkness and checked daily for growth. Emerging mycelia were subcultured by aseptically excising hyphal tips and placing them on individual Petri dishes containing aPDA or MEA.
Isolated strains were grouped by morphotype and representatives were initially identified by comparing sequences of their internal transcribed spacer (ITS) rDNA region with available reference sequences in NCBI GenBank. Mycelia from 2–4-week-old cultures were harvested using a sterile scalpel and genomic DNA was extracted using the DNeasy UltraClean Microbial Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Four gene regions were amplified by PCR: ITS with the primer pair V9G/LS266 (de Hoog and Gerrits van den Ende Citation1998; Gerrits van den Ende and de Hoog Citation1999), the nuclear 28S large ribosomal subunit (LSU) with the primer pair LR0R/LR5 (Vilgalys and Hester Citation1990; Rehner and Samuels Citation1995), β-tubulin (BenA) with the primer pair Bt2a/Bt2b (Glass and Donaldson Citation1995), and the RNA polymerase II second largest subunit (RPB2) with the primer pair RPB2-5f2/RPB2-7cR (Liu et al. Citation1999; Sung et al. Citation2007). PCR reactions were performed in a 2720 Thermal Cycler (Applied Biosystems Inc. Foster City, CA, USA) in a total volume of 50 µL using Platinum Taq polymerase (Invitrogen, Carlsbad, CA, USA) or AllTaq PCR Core Kit (Qiagen, Hilden, Germany; RPB2 only) and 4 µL of template DNA using the following conditions: an initial denaturation step at 94°C for 2 min (5 min for RPB2), followed by 35 cycles of denaturation (30 s at 94°C; 45 s at 95°C for RPB2), annealing (30 s at 55°C; 80 s at 56°C for RPB2) and extension (1 min at 72°C; 2 min at 72°C for RPB2), and a final extension at 72°C for 10 min. PCR products were visualized on 1% agarose electrophoresis gel; single band PCR products were each sequenced on both strands at the CHU de Québec-Université Laval Research Center SANGER Sequencing platform in Québec, QC, Canada.
A multigene phylogenetic analysis was conducted by populating a dataset with sequences of related taxa from GenBank (). The dataset consisted of nine different strains representing four taxa with Kretzschmaria deusta selected as outgroup based on the results of Li et al. (Citation2021). Sequences of three taxonomically-informative loci (ITS, BenA and RPB2) were aligned using MAFFT (Katoh et al. Citation2019) and concatenated in Geneious Prime 2019 v.2019.0.4 (Biomatters, Auckland, New Zealand). The phylogenetic tree was reconstructed with maximum likelihood (ML) using IQ-TREE v1.6.11 (Trifinopoulos et al. Citation2016), with the best-fit substitution model selected automatically for each partition with ModelFinder (Chernomor et al. Citation2016; Kalyaanamoorthy et al. Citation2017) and node support computed with 10 000 ultrafast bootstraps (Hoang et al. Citation2018), 10 000 SH-aLRT branch tests (Guindon et al. Citation2010) and an approximate Bayes test. Phylogenetic trees were visualized in FigTree 1.4.2 (available at http://tree.bio.ed.ac.uk/software/figtree/) and edited with Adobe Illustrator CC 2019 v.23.0.1 (Adobe Systems, San Jose, CA, USA).
Table 1. Strains used for phylogenetic analysis.
Samples were prepared for microscopy by making hand sections of stromata using a safety razor blade or scalpel and collecting conidia using an insect pin and mounting in tap water or 60% lactic acid. Observations were made using a Leica DM4 B light microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany) and a Leica M165 C stereomicroscope with a TL5000 Ergo light base (Leica Microsystems Ltd., Singapore). Images were captured with Leica DFC450 C (light microscope) and Leica DMC5400 (stereomicroscope) cameras using Leica LAS X 3.7.1 software (Leica Microsystems CMS GmbH). Photographs of stromata and trees were also taken with a Nikon Z 7II camera (Nikon Corporation, Tokyo, Japan) equipped with a Nikon NIKKOR Z 50 mm f/1.8 S lens. The photographic plate was assembled using Adobe Photoshop CC 2019 v.23.0.1 (Adobe Systems, San Jose, California).
To assess diameter growth and colony morphology, three strains (CHEM-JM-1928a, DAOMC 252 738, DAOMC 252 739) were each inoculated via 5-mm mycelial plugs onto 10 cm diameter Petri dishes containing either aPDA or MEA. Plates were incubated at either 20°C or 25°C upside-down in the dark and measured daily for 10 d. Inoculations were conducted in triplicate for each medium and temperature. Differences in growth rates after 7 d under the different temperature and medium treatments were compared using the unpaired Student’s t-test.
Pathogenicity assay
An inoculation study was conducted to confirm the pathogenicity of C. corticale using the strain DAOMC 252 739, which was isolated from conidia obtained from stroma on a dead sycamore maple tree showing symptoms of SBD in Cordova Bay, Vancouver Island, BC (18 October 2022), and deposited in the Canadian Collection of Fungal Cultures (DAOMC; Ottawa, Ontario, Canada). The strain was incubated for 2 months before being preserved in the first author’s culture collection as described by Wertman et al. (Citation2024). A culture was subsequently revived from a mycelial plug stored in 10% glycerol at −80°C for 4 months and subsequent subcultures were used for inoculum. Sycamore maple trees (3/8” caliper) were obtained from a commercial nursery in Salem, Oregon and repotted in #2 containers containing 2.5 kg of the following potting mix: 3.8 ft3 peat moss, 2 ft3 perlite, 1 ft3 vermiculite, 1 ft3 sand, 750 g dolomite, 250 g Micromax®, and 750 g Osmocote® 18-6-12. Following repotting the trees were watered until field capacity and kept moist in a growth chamber set at 25°C with a 12 h dark, 12 h light cycle. The experimental design consisted of a humid (1 L water twice weekly) or drought-like (200 mL water twice weekly) watering treatment and inoculation treatment by 5 mm diameter MEA plugs (negative control) or 5 mm diameter MEA plugs containing 10 day-old mycelia of DAOMC 252 739. Each treatment consisted of three trees (replicates), i.e., three trees each of humid × DAOMC 252 739, drought × DAOMC 252 739, humid × control inoculation or drought × control inoculation, for a total of 12 trees. Trees were randomly numbered and tagged. Six weeks after repotting the trees were wound-inoculated (DAOMC 252 739 or blank MEA plugs) at three locations along the stem 30, 60 and 90 cm above the root collar. The inoculation points were surface sterilized by wiping with a Kimwipe saturated with 70% ethanol, which was allowed to evaporate, before a disc of bark was removed using a flame-sterilized and cooled 5 mm diameter cork borer. One 5 mm diameter plug of agar (with or without mycelia of DAOMC 252 739) was placed on the wound; plugs containing mycelia were placed so the mycelia were in direct contact with the wound. The inoculation point was covered by wrapping with Parafilm, which was removed 3 weeks after inoculation. Tree height and diameter at root collar were measured 1 week before inoculation and then 4, 12, 20, and 28 weeks after inoculation. The significance of the effects of the watering and inoculation treatments (independent variables) on height and diameter growth (dependent variables) was assessed using two-way analysis of variance (ANOVA) and post hoc Tukey’s significant difference (HSD) test (p < 0.05) with R v.4.3.3 within RStudio 2023.12.1 Build 402.
Trees were regularly assessed for symptoms and the experiment was terminated 28 weeks after inoculation. The whole tree was photographed using a Nikon 7II camera equipped with a Nikon NIKKOR Z 50 mm f/1.8 S lens. Trees were harvested by clipping the stem at the root collar using secateurs; all tools were flame-sterilized and cooled before and after each use. The bark and then cambium surrounding each inoculation point was carefully removed using a scalpel to allow for visualization of any changes to the cambium and underlying wood. The length of bark discolouration was measured using a ruler and inoculation points were photographed using a Nikon 7II camera equipped with a Nikon AF-S Micro NIKKOR 60 mm 1:2.8 G ED lens and a Leica M165 C stereomicroscope.
To attempt to re-isolate C. corticale from trees inoculated with DAOMC 252 739 and the negative control trees, wood was removed using scalpels and/or secateurs. Wood was harvested from necrotic or intact tissue around the 60 cm inoculation point for every tree. For some trees, additional isolates were made from other inoculation points and at, or below, the root collar. Wood was immersed in 70% ethanol for 30 seconds followed by three rinses in sterile distilled water for 1 minute per rinse. After the third rinse, the wood tissue was air-dried under a laminar flow hood. Approximately 2 mm pieces of wood, incorporating a margin of discoloured and healthy tissue whenever possible, were removed using a scalpel. For each sample, ten pieces of wood were evenly distributed and partially embedded in the agar surface on an individual Petri dish containing either MEA or aPDA. Inoculated plates were incubated at 20°C in darkness and checked daily for mycelia emerging from the wood pieces. Emerging mycelia were excised using a sterile insect pin or scalpel and placed on individual labelled Petri dishes containing MEA or aPDA. Resulting cultures resembling C. corticale were identified by ITS sequencing, as described above.
Real-time PCR detection of C. corticale from infected wood tissues
The entire section of stem containing the remaining lesions and discoloured wood, or unaffected wood, at the 30 cm and 90 cm height inoculation points was cut using flame-sterilized and cooled secateurs, placed in a labelled (i.e., tree number, inoculation point, harvest date) 50 mL centrifuge tube, and stored at −20°C. One cm long wood sections were collected approximately 1 cm below the inoculation point. DNA was directly extracted from these wood pieces using the DNeasy® Plant Pro Kit (QIAGEN Inc., Toronto, Canada) with the following protocol modifications: step 2 was replaced with a 15 min incubation at 65°C and centrifugation at 300 rpm followed by two rounds of grinding at 4 m s−1 for 30 s in a MP FastPrep-24TM 5 G (MP Biomedicals, Santa Ana, CA, USA). Samples were then centrifuged at 12 000 g for 2 min before removing the wood piece with sterilized tweezers. The final elution buffer EB was warmed to 65°C and incubated for 5 min on the filtering membrane before elution.
Presence of C. corticale was determined using the real-time PCR TaqMan assay developed by Muller et al. (Citation2023). Real-time PCR reactions were performed with a final concentration of 1× PrimeTimeTM Gene Expression Master Mix and 1× of PrimeTime Mini qPCR assay probe (Integrated DNA Technologies, Coralville, IA, USA) containing primers and the TaqMan probe labelled with fluorescein (6-FAM) at the 5′ end and with the quencher Iowa Black FQ (ZEN-IBFQ). Amplification reactions were completed on a QuantStudio 3 Real-Time PCR System instrument (Thermo Fisher Scientific, Waltham, MA, USA) with the following conditions: 95°C, 15 min enzyme activation step, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. The assay was run in duplicate with the original DNA extraction and, for a subset of four samples, one more time with a new DNA extraction from the same sample to confirm the results.
Results
Identification of C. corticale
The first positive case of SBD was collected on 2 June 2022 from a dead sycamore maple tree planted in Ladner, BC. A specimen was submitted to the British Columbia Ministry of Agriculture and Food (BCMAF) Plant Health Laboratory and identified based on morphological characteristics as described in the CMI description (Sutton and Gibson Citation1977). Briefly, dark brown, immersed septate mycelial growth covered with dark brown powdery mass were situated within the bark. The conidia observed were 4.9–7.3 × 3.5–3.8 µm in size, aseptate, pale brown, mostly globose and smooth with a comparatively thicker and darker cell wall (Joshi et al. Citation2023). We collected additional samples from this tree and verified the identification by Sanger sequencing (ITS) and qPCR and isolated axenic strains from conidia. Samples were collected and confirmed as C. corticale from other symptomatic trees (three A. pseudoplatanus, one A. platanoides) around Greater Victoria in the summer and autumn of 2022. As of March 13, 2024, 33 symptomatic trees showing C. corticale stromata have been found within the Southern Vancouver Island-Greater Vancouver area. These cases consist of 30 sycamore maple, two Norway maple (A. platanoides) and one bigleaf maple (A. macrophyllum) (). Trees ranged from immature to mature trees ranging from 4–60+ cm DBH.
Fig. 1 (Colour online) (A–C) First sooty bark disease detection in BC (Ladner, BC) with exposed stromata showing dark brown conidia (B, C). (D–F) Extensive stromata developed on dead sycamore maple trees in Victoria, BC, with D and E showing the younger greyish-white stromata. (G) Stromata at base of dead Norway maple tree in Saanich, BC. (H–K) Stromata on dead sycamore maple trees in Langford, BC on planted trees (H–J) and natural regeneration (K); note the teeth marks on stroma (J). (L, M) Large, greyish-white stroma at base of a dead sycamore maple in Victoria, BC, with bark stripped off and scattered at the base of the tree (L) and prominent teeth marks (M). (N) Cross-section of tree depicted in H showing characteristic greenish-brown stain, which quickly oxidizes to brown. (O, P) Cryptostroma corticale conidia obtained from stroma and mounted in 60% lactic acid; scale bars = 10 µm.

The three strains of C. corticale included in this study (CHEM-JM-1928a, DAOMC 252 738, DAOMC 252 739) showed significantly more growth after 7 d on MEA versus aPDA at 20°C (p = 0.0014) and 25°C (p = 0.0083). However, the differences in growth after 7 d at 20°C versus 25°C were not statistically significant on MEA (p = 0.057) and aPDA (p = 0.335). After 7 d on aPDA, colonies reached an average diameter of 58 mm (SD = 15.9 mm) at 20°C and an average diameter of 66 mm (SD = 18.5 mm) at 25°C. After 7 d on MEA, colonies reached an average diameter of 30.3 mm (SD = 14.6 mm) at 20°C and an average diameter of 43.5 mm (SD = 12.8 mm) at 25°C. Colonies on MEA after 7 d were hyaline to white, reverse same, moderate cottony aerial mycelia, growth flat, margins filamentous, no exudates or soluble pigments. Colonies on aPDA at 20°C were white and often honey to cinnamon towards centre, reverse buff to honey to saffron and darker towards centre, sometimes with abundant very dark brown submerged hyphae, abundant cottony aerial mycelia, often with characteristic bluish exudates from aerial mycelia (). The bluish exudates formed on aPDA appeared to be a good diagnostic character for distinguishing C. corticale from other fungi isolated from sycamore maple wood.
Fig. 2 (Colour online) Results from pathogenicity assay involving wound inoculation of sycamore maple trees with Cryptostroma corticale DAOMC 252 739 or 2% malt extract agar plug (negative control) and subjected to humid or drought watering treatment, after 28 weeks. (A) Negative control tree subjected to humid watering treatment. (B) Negative control tree subjected to drought treatment. (C, D) Trees inoculated with C. corticale and subjected to humid treatment. (E, F) Trees inoculated with C. corticale and subjected to drought treatment. (G–J) Inoculation site at 60-cm with bark intact (left) and then bark peeled (right) for negative control tree subjected to humid watering treatment (G), negative control tree subjected to drought treatment (H), tree inoculated with C. corticale and subjected to humid watering treatment (I), and tree inoculated with C. corticale and subjected to drought treatment (J). (K–M) Inoculation site at 60-cm for tree inoculated with C. corticale and subjected to drought treatment with bark peeled back at inoculation point (K), 10 cm from inoculation point, and a cross-section 6 cm from inoculation point (M); note the strong wood discolouration. (N) Examples of C. corticale emerging from 10 wood chips harvested from inoculated trees and placed on acidified potato dextrose agar (aPDA) plates; the plate on the far right is an axenic subculture showing the characteristic blue exudates when cultivated on aPDA. Scale bars: G–J = 1 mm; K–L = 5 mm; M = 2 mm.

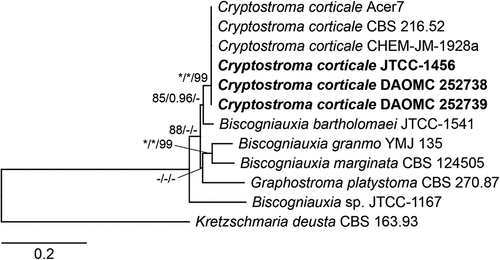
The sequence alignment consisted of seven taxa with concatenated ITS, BenA and RPB2 sequences forming three partitions that totalled 2405 columns, 452 distinct patterns, 236 parsimony-informative and 1595 constant sites. The best-fit models selected using ModelFinder were TIM2e+I (ITS partition), K2P+G4 (BenA partition) and TNe+G4 (RPB2 partition). The reconstructed phylogeny placed all C. corticale strains within a single strongly-supported clade sister to Biscogniauxia bartholomaei (). Sequences from the strains from BC clustered with sequences from strains from the natural range (JTCC-1453; Ottawa, ON) and initial UK invasion (CBS 216.52; Essex, UK), e.g., between DAOMC 252 739 and CBS 216.52: 99% similarity for ITS [identities = 611/612 (99%). 0 gaps], 100% similarity for BenA [identities = 464/464 (100%), 0 gaps], and 99% similarity for RPB2 [identities = 1053/1054 (99%), 0 gaps] in a group with strong statistical support (BS = 99%; SH-aLRT branch tests = 100%).
Fig. 3 Maximum likelihood (ML) phylogenetic tree obtained from an IQ-TREE analysis of Cryptostroma corticale and related Biscogniauxia species based on ITS, BenA and RPB2 sequences. Sequences obtained from C. corticale strains isolated from British Columbia are shown in bold. Numbers at the nodes correspond to SH-aLRT (≥80%), aBayes (≥0.95) and ultrafast bootstrap (≥95%) support values; an asterisk (*) indicates full support (100% or 1.0) and a hyphen (-) indicates support lower than the respective significant values. The scale bar shows the expected number of nucleotide substitutions per site.
Pathogenicity assay
Of the three trees inoculated with DAOMC 252 739 and subjected to the humid watering treatment, one tree (#22) died within 12 weeks of inoculation after showing top-down wilting and developing basal epicormic shoots. The other two trees showed some mottled necrosis and wilting of the leaves but otherwise had a full crown and were still alive at the end of the experiment (28 weeks post-inoculation) (). Of the three trees inoculated with DAOMC 252 739 and subjected to the drought watering treatment, one tree (#88) showed top-down wilting and death of the stem except for two epicormic shoots 15 cm above the root collar and the other two trees exhibited very thin crowns with leaves that showed mottled necrosis, scorched margins and wilting. All inoculated trees showed extensive brown discolouration of the wood extending longitudinally from the inoculation points. From the 60-cm inoculation point, the discolouration was between 17–81 cm in length and often coalesced with other inoculation points. For one tree under the drought treatment the wood discolouration extended below the root collar into the main taproot. DAOMC 252 739 inoculation points appeared swollen with developing callus tissue; this callus tissue was less developed in trees subjected to the drought versus humid watering treatment. The inoculation points for the negative control trees were healed although the callus tissue was less developed in the drought treatment. Negative control trees were alive with full crowns at the end of the experiment, however the drought treatment resulted in leaf scorch at the margins of some leaves. No wood discolouration was observed in the negative control trees. There were statistically-significant differences in height growth by inoculum treatment (p = 0.0206) based on the ANOVA but no significance was observed with Tukey’s HSD test ().
Table 2. Height and diameter growth of trees from pathogenicity assay. Growth is the difference between the measurements taken immediately before inoculation (Ti) and before harvesting (Th). for treatments: + = inoculation with DAOMC 252 739, – = inoculation with MEA plug (negative control); D = drought, H = humid.
Attempts to re-isolate C. corticale from DAOMC 252 739-inoculated trees were successful: every inoculated tree yielded C. corticale colonies from incubated wood pieces along with less frequent colonies of Chaetomium, Cladosporium, Penicillium, Trichoderma and an unidentified sterile white morphotype. Isolation attempts from negative control trees resulted in colonies of Chaetomium, Paecilomyces, Xylaria and unknown sterile white morphotypes, but not C. corticale.
Real-time PCR results
Wood samples from trees inoculated with C. corticale had an average real-time PCR detection threshold significantly earlier (Cq-value <25.32; average = 20.96 ± 2.99) than for samples from the negative control trees (Cq > 29.99; 32.83 ± 1.67) (F = 185.79; p < 0.0001). As all control samples returned a Cq-value under 40 cycles, we tested the hypothesis of cross-contaminations with C. corticale with the following two approaches. Firstly, the real-time PCR product obtained for each control tree was sequenced with Sanger technology and returned either a sequence with no similarity in the GenBank database or a sequence with similarity to a short region of the Acer campestre genome (GenBank accession number GCA_954870605); for example, 89% similarity to an 84 bp region of chromosome 12 [OX940810; identities = 75/85 (89%), 0 gaps]. Secondly, the internal transcribed spacer regions of DNA samples from six trees inoculated with C. corticale and six control trees were identified using the DNA-barcoding protocol described in Feau et al. (Citation2023). No PCR product was obtained for the control seedlings while those inoculated with DAOMC 252 739 returned an ITS sequence 100% identical to that of C. corticale.
Discussion
In this study we confirmed the presence of SBD and the pathogenicity of C. corticale on sycamore maple in BC (Natural Resources Canada Citation2024). Koch’s postulates were satisfied with our pathogenicity assay, and we demonstrated that C. corticale can extensively colonize the wood of infected but asymptomatic trees. The ability to persist as an endophyte in woody tissues has been recently confirmed and suggests that, as in other localities, C. corticale asymptomatic infections in BC are likely more widespread than realized (Schlößer et al. Citation2023). The fungus probably colonizes the wood extensively over prolonged periods of time and is positioned to invade the phloem and cambium soon after the host tree is weakened by drought and heat stress. Several residents in Victoria shared observations that sycamore maple trees killed by SBD had leafed out and appeared healthy in the spring but declined rapidly within the following summer season (unpublished observation). The experimental design of our pathogenicity assay was focused on establishing the pathogenicity of C. corticale isolated from BC and not quantifying the overall impact on tree growth. Therefore, tree growth results are not robust given the small number of replicates per treatment, especially with one tree dying early in the inoculated-humid treatment and one tree experiencing extensive dieback of the shoot in the inoculated-drought treatment. The diameter measurement was taken at the root collar, i.e., below the inoculation point; it is possible that diameter growth could be affected above the inoculation point, which was not measured. However, there was evidence showing a statistically-significant effect of C. corticale on height growth. Anecdotally, some symptomatic sycamore maple trees observed within even-aged boulevards in Greater Victoria were noticeably shorter and had smaller crowns than neighbouring trees, suggesting a chronic reduction in growth and vigour (unpublished observation). The impact of C. corticale on tree growth should be further assessed because this may provide a means for non-invasive assessment of infection in the presymptomatic phase.
We speculate that the initial SBD cases identified in Greater Victoria and the Greater Vancouver area in 2022 were exacerbated by the extreme weather events of the preceding 2021 summer, including 53 consecutive days without rain and the western heat dome that resulted in record high temperatures (Environment and Climate Change Canada Citation2021, Citation2024a). The summer of 2022 was also characterized by an extreme prolonged drought of 94 days (Environment and Climate Change Canada Citation2024a), which probably precipitated the rise in SBD cases identified in 2023. A modelling study conducted in France and Switzerland indicated that the best variable to explain an SBD outbreak was a negative water balance (i.e., drought) during the preceding growing season (April–August) (Muller et al. Citation2023). Additionally, SBD reports were predicted to increase when, during the 2 years preceding disease, at least 33 days per year had a temperature higher than 25°C (Muller et al. Citation2023). Trees in urban areas are also typically exposed to more stress and abiotic agents, such as drought, the heat island effect and poor soil conditions, than trees in natural environments, which predisposes trees to disease and insect attack. Consequently, urban forests may act as sentinels for not only biological invasions, but also opportunistic pathogens and additional abiotic factors exacerbated by a changing climate (Paap et al. Citation2017). For example, Kelnarová et al. (Citation2017) found that the incidence of SBD in Prague increased with heavier NOx pollution combined with the presence of paths and roads. British Columbia has recently experienced its four most severe wildfire seasons (2017, 2018, 2021 and 2023) of the last 50 years, which resulted in dense and persistent smoke cover (Parisien et al. Citation2023). The effect of airborne pollutants, such as those contained in wildfire smoke, on the incidence of forest pathogens and the dispersal of their propagules has not been extensively investigated (Parmeter and Uhrenholdt Citation1976; Kobziar et al. Citation2018, Citation2022).
Sycamore maple is not a significant species within the urban forests of Victoria and Vancouver, where they represent 0.8% (271 trees) and 1.36% (2059 trees) of the trees managed by these cities, respectively (City of Victoria Tree Species Citation2024; City of Vancouver Citation2024). However, a major concern is the potential for SBD to manifest in endemic maple species, such as bigleaf maple and vine maple (A. circinatum Pursh). In Washington State, bigleaf maple decline was recently identified and is suggested to be the result of abiotic conditions including warm temperatures and local site conditions such as hotter urban sites (Betzen et al. Citation2021). Under such conditions, bigleaf maple may be primed for SBD. Brooks et al. (Citation2023) presented evidence of C. corticale being widespread on bigleaf maple in Washington State; however, it has not been shown if the fungus is uniquely an endophyte or if it is pathogenic. So far, C. corticale was identified on only one dead bigleaf maple in BC found east of Nanaimo on Vancouver Island; a diagnosis was confirmed with the real-time PCR assay described in this study, but has yet to be confirmed with Koch’s postulates. Additionally, the specificity of both the real-time PCR assay and the nested PCR assay used by Brooks et al. (Citation2023) should be further verified by testing it against phylogenetically-related taxa that commonly form black crust-like stromata on bigleaf maple bark, for example Kretzschmaria deusta (Hoffm.) P. M. D. Martin and an undescribed Biscogniauxia sp. (; in preparation). We are currently monitoring a greenhouse experiment involving the inoculation of sycamore, bigleaf and vine maple with C. corticale under humid and drought watering treatments and expect to shed light on the ability of C. corticale to infect and cause disease in bigleaf and vine maple (unpublished data). A population genomics study could potentially elucidate the population structure of C. corticale across its endemic and introduced ranges and provide evidence on its introduction in the PNW.
Given the endophytic capability of C. corticale, it may be assumed that susceptible trees are asymptomatically infected where local cases are observed (Koukol et al. Citation2015; Schlößer et al. Citation2023). Following hot and dry summers, urban forest management plans should anticipate removal of dead and dying trees and subsequent replacement with non-host species. Furthermore, removal and disposal of infected trees with stromata require additional considerations given the potential for human health effects from chronic exposure to conidia. For example, arborists may wear personal protective equipment while felling trees and removal should occur in the winter months when sporulation is lowest and precipitation can mitigate conidia becoming airborne (Braun et al. Citation2021). Infected trees should not be used for firewood, as stromata can develop in drying pieces of wood (Ogris et al. Citation2021; Kespohl et al. Citation2022). We do not recommend chipping and using the resulting woodchips as mulch because this may cause a mass release of conidia that will serve as potential inoculum and increase exposure risk to workers and pedestrians. After removal, infected trees can be transported in closed containers for burial or incineration (Kespohl et al. Citation2022).
Cryptostroma corticale is presumably spread by airborne conidia infecting wounds, but animal vectors may also play a role. We observed many stromata displaying characteristic teeth marks (J, M) and symptomatic sycamore maple trees with strips of bark strewn on the ground (L). Squirrels are renowned mycophagists and observations support their scratching and chewing of stromata produced by various fungi, including Biscogniauxia spp (Spooner Citation2007; Fukasawa Citation2021). The teeth marks that we observed were similar to those reported from C. corticale stromata caused by invasive eastern grey squirrel (Sciurus carolinensis Gmelin) in the UK (Abbott et al. Citation1977). Grey squirrels are also invasive in Greater Victoria and the Greater Vancouver area. Notably, grey squirrels and C. corticale are sympatric within their endemic range. Their bark-stripping behaviour can create wounds and girdle maple trees (Robinson and Cowan Citation1954; Bruemmer et al. Citation1999; Gonzales Citation2005). Abbott et al. (Citation1977) reported that 100% of C. corticale spores isolated from the buccal cavity and claws of harvested grey squirrel germinated within 24 h on 3% malt extract agar at 25°C; 30% of spores from the hindgut germinated under the same conditions. Grey squirrels may therefore vector C. corticale by stripping bark to expose stromata, capturing viable conidia in their fur, claws, buccal cavity, and gut, and then dispersing the conidia to new hosts by creating entry wounds. This might represent a tripartite relationship between a changing climate, an invasive mammal, and a putatively invasive fungus. Xylariales species are renowned for their production of diverse and biologically active secondary metabolites (Becker and Stadler Citation2021); it is conceivable that C. corticale stromata produce volatile organic compounds that attract squirrels to aid in dispersal.
Ongoing climate change may result in increasingly hot and dry summers in the PNW (Case et al. Citation2021). Planting sycamore maple, the most susceptible host of SBD, should be avoided, and the species removed from planting lists of suitable species due to the increasing likelihood of mortality from SBD and the potential increased costs in tree removal and disposal. Within the urban forest, SBD may be managed by planting non-hosts, practicing proper site selection (e.g., moist or irrigated sites) when planting potential hosts, scheduling pruning when C. corticale aerial spore loads are lowest (i.e., winter and early spring; Burgdorf et al. Citation2022) and providing irrigation where disease risk is high among important, susceptible hosts during periods of drought and high temperatures. Western Canada is experiencing emerging diseases (e.g., Swiss needle cast, Dothistroma needle blight) and dieback (e.g., western redcedar) that have been linked to a changing climate (Woods et al. Citation2010; Herpin-Saunier et al. Citation2022). Sooty bark disease is one more climate change-associated challenge to tree health in BC and its impact on endemic species, especially bigleaf maple, should be assessed.
Acknowledgements
We thank the City of Victoria (Parks Division) for providing sampling permission and valuable communication, and the Capital Regional District, the District of Saanich (Parks Division), the City of Langford and Dave Parsons (Eager Beaver Tree Service) for providing samples. Kem Luther and the South Vancouver Island Mycological Society assisted in setting up the iNaturalist project. Cameron D’Amours (University of Victoria) assisted with the inoculation experiment and other laboratory duties and Shayla Thom (University of Victoria) provided assistance with harvesting the sycamore maple. We thank the Forest Health Program (Stefan Zeglen), BC Ministry of Forests for the purchase of sycamore maple trees. This work was funded by the CFS Pest Risk Management Program.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abbott RJ, Bevercombe GP, Rayner ADM. 1977. Sooty bark disease of sycamore and the grey squirrel. Trans Br Mycol Soc. 69(3):507–508. doi: 10.1016/S0007-1536(77)80095-8.
- Becker K, Stadler M. 2021. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J Antibiot (Tokyo). 74(1):1–23. doi: 10.1038/s41429-020-00376-0.
- Bencheva S. 2014. First report of Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller on Acer platanoides L. in Bulgaria. Silva Balcania. 15:101–104.
- Betzen JJ, Ramsey A, Omdal D, Ettl GJ, Tobin PC. 2021. Bigleaf maple, Acer macrophyllum Pursh, decline in western Washington, USA. For Ecol Manag. 501:119681. doi: 10.1016/j.foreco.2021.119681.
- Braun M, Klingelhöfer D, Groneberg DA. 2021. Sooty bark disease of maples: the risk for hypersensitivity pneumonitis by fungal spores not only for woodman. J Occup Med Toxicol. 16(1):1–7. doi: 10.1186/s12995-021-00292-5.
- Brenken AC, Kehr R, Riebesehl J, Esch J, Enderle R. 2024. First report of Cryptostroma corticale on Aesculus hippocastanum causing sooty bark disease in Germany. J Plant Prot Res. 10(3):1087–1092. doi: 10.1007/s41348-024-00891-4.
- Brooks RK, Omdal D, Brown S, Marshall CJ, Hulbert JM, Elliott M, Chastagner G. 2023. Cryptostroma corticale, the causal agent of sooty bark disease of maple, appears widespread in western Washington State, USA. For Pathol. 53(6):e12835. doi: 10.1111/efp.12835.
- Bruemmer C, Lurz P, Larsen K, Gurnell J. 1999. Impacts and management of the alien eastern grey squirrel in Great Britain and Italy: lessons for British Columbia. In: Darling L editor. Proceedings of the Conference on the Biology and Management of Species and Habitats at Risk, Kamloops, BC; Kamloops, B.C.; B.C. Ministry of Environment, Lands and Parks, Victoria, B.C. and University College of the Cariboo. p. 15–19.
- Burgdorf N, Härtl L, Hahn WA. 2022. Sooty bark disease in sycamore: seasonal and vertical variation in spore release of Cryptostroma corticale. Forests. 13(11):1956. doi: 10.3390/f13111956.
- Case MJ, Johnson BG, Bartowitz KJ, Hudiburg TW. 2021. Forests of the future: climate change impacts and implications for carbon storage in the Pacific Northwest, USA. For Ecol Manag. 482:118886. doi: 10.1016/j.foreco.2020.118886.
- Chernomor O, Von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol. 65(6):997–1008. doi: 10.1093/sysbio/syw037.
- City of Vancouver. 2024. Vancouver (BC): City of Vancouver Open Data Portal; [accessed 2024 Jan 20]. https://opendata.vancouver.ca/explore/dataset/street-trees/.
- City of Victoria Tree Species. 2024. Victora (BC): City of Victoria Open Data Portal; [accessed 2024 Jan 20]. https://opendata.victoria.ca/datasets/tree-species.
- Cochard B, Crovadore J, Bovigny PY, Chablais R, Lefort F. 2015. First reports of Cryptostroma corticale causing sooty bark disease in Acer sp. in Canton Geneva, Switzerland. New Dis Rep. 31(1):1–8. doi: 10.5197/j.2044-0588.2015.031.008.
- de Hoog GS, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses. 41(5–6):183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x.
- Dickenson S, Wheeler BEJ. 1981. Effects of temperature, and water stress in sycamore, on growth of Cryptostroma corticale. Trans Br Mycol Soc. 76(2):181–185. doi: 10.1016/S0007-1536(81)80136-2.
- Ellis JB, Everhart BM. 1889. New species of hyphomycetous fungi. J Mycol. 5(2):68–72. doi: 10.2307/3752309.
- Emanuel DA, Wenzel FJ, Lawton BR. 1966. Pneumonitis due to Cryptostroma corticale (maple-bark disease). N Engl J Med. 274(25):1413–1418. doi: 10.1056/NEJM196606232742504.
- Environment and Climate Change Canada. 2021. Canada’s top 10 weather stories of 2021 Ottawa (ON): Government of Canada; [accessed 2024 Mar 6]. https://www.canada.ca/en/environment-climate-change/services/top-ten-weather-stories/2021.html.
- Environment and Climate Change Canada. 2024a. Historical data: daily data report for July-October 2022 VICTORIA GONZALES CS BRITISH COLUMBIA Ottawa (ON): Government of Canada; [accessed 2024 Mar 6]. https://climate.weather.gc.ca/climate_data/daily_data_e.html?hlyRange=1994-02-01%7C2024-03-05&dlyRange=1973-01-01%7C2024-03-05&mlyRange=1972-01-01%7C2007-02-01&StationID=114&Prov=BC&urlExtension=_e.html&searchType=stnProv&optLimit=yearRange&StartYear=2021&EndYear=2021&selRowPerPage=25&Line=259&lstProvince=BC&timeframe=2&Day=5&Year=2022&Month=7.
- Environment and Climate Change Canada. 2024b. Historical data: daily data report for june-august 2021 VICTORIA GONZALES CS BRITISH COLUMBIA Ottawa (ON): Government of Canada; [accessed 2024 Mar 6]. https://climate.weather.gc.ca/climate_data/daily_data_e.html?hlyRange=1994-02-01%7C2024-03-05&dlyRange=1973-01-01%7C2024-03-05&mlyRange=1972-01-01%7C2007-02-01&StationID=114&Prov=BC&urlExtension=_e.html&searchType=stnProv&optLimit=yearRange&StartYear=2021&EndYear=2021&selRowPerPage=25&Line=259&Month=6&Day=5&lstProvince=BC&timeframe=2&Year=2021.
- Feau N, Herath P, Hamelin RC. 2023. DNA-barcoding identification of plant pathogens for disease diagnostics. In: Foroud N Neilson J, editors. Plant-pathogen interactions. Methods in molecular biology Vol. 2659, New york (NY): Springer US; p. 37–49.
- Forest Health Watch. 2023. Puyallup (WA): Washington State University; [accessed 2023 Dec 28]. https://foresthealth.org/sbd/.
- Fukasawa Y. 2021. Invertebrate assemblages on Biscogniauxia sporocarps on oak dead wood: an observation aided by squirrels. Forests. 12(8):1124. doi: 10.3390/f12081124.
- Garbelotto M, Schmidt D, Popenuck T, Rooney-Latham S, Ewing C, Smith T. 2024. First report of Cryptostroma corticale causing sooty bark disease in California and first worldwide report of silver maple as a host. Plant Dis. 108(5):1395. doi: 10.1094/PDIS-12-23-2734-PDN.
- Gerrits van den Ende AHG, de Hoog GS. 1999. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud Mycol. 43:151–162.
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 61(4):1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995.
- Gonzales EK. 2005. The distribution and habitat selection of introduced Eastern Grey Squirrels, Sciurus carolinensis, in British Columbia. Can Field-Nat. 119(3):343–350. doi: 10.22621/cfn.v119i3.143.
- Goree H. 1969. Occurrence of Cryptostroma corticale in northwestern United States. Plant Dis Rep. 53(1):87.
- Gregory PH, Peace TR, Waller S. 1949. Death of sycamore trees associated with an unidentified fungus. Nature. 164(4163):275–275. doi: 10.1038/164275a0.
- Gregory PH, Waller S. 1951. Cryptostroma corticale and sooty bark disease of sycamore (Acer pseudoplatanus). Trans Br Mycol Soc. 34(4):579–597. doi: 10.1016/S0007-1536(51)80043-3.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59(3):307–321. doi: 10.1093/sysbio/syq010.
- Herpin-Saunier NY, Sambaraju KR, Yin X, Feau N, Zeglen S, Ritokova G, Omdal D, Côté C, Hamelin RC. 2022. Genetic lineage distribution modeling to predict epidemics of a conifer disease. Front For Glob Change. 25(4):756678. doi: 10.3389/ffgc.2021.756678.
- Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 35(2):518–522. doi: 10.1093/molbev/msx281.
- Hsieh HM, Ju YM, Rogers JD. 2005. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia. 97(4):844–865. doi: 10.1080/15572536.2006.11832776.
- Joshi V, Burlakoti P, Babu B. 2023. Diseases/symptoms diagnosed on commercial crop samples submitted to the British Columbia Ministry of Agriculture and Food (BCMAF), Plant Health Laboratory in 2022. In: Elmhirst J, editor. Canadian Plant Disease Survey 2023 Volume 103: Disease Highlights 2022, Can J Plant Pathol. 45(sup 1):7–12. doi: 10.1080/07060661.2023.2222486.
- Kalyaanamoorthy S, Minh BQ, Wong TK, Von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589. doi: 10.1038/nmeth.4285.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166. doi: 10.1093/bib/bbx108.
- Kelnarová I, Černý K, Zahradník D, Koukol O, Sieber T. 2017. Widespread latent infection of Cryptostroma corticale in asymptomatic Acer pseudoplatanus as a risk for urban plantations. For Pathol. 47(4):e12344. doi: 10.1111/efp.12344.
- Kespohl S, Riebesehl J, Grüner J, Raulf M. 2022. Impact of climate change on wood and woodworkers—cryptostroma corticale (sooty bark disease): a risk factor for trees and exposed employees. Front Public Health. 10:973686. doi: 10.3389/fpubh.2022.973686.
- Kobziar LN, Pingree MR, Larson H, Dreaden TJ, Green S, Smith JA. 2018. Pyroaerobiology: the aerosolization and transport of viable microbial life by wildland fire. Ecosphere. 9(11):e02507. doi: 10.1002/ecs2.2507.
- Kobziar LN, Vuono D, Moore R, Christner BC, Dean T, Betancourt D, Watts AC, Aurell J, Gullett B. 2022. Wildland fire smoke alters the composition, diversity, and potential atmospheric function of microbial life in the aerobiome. ISME Commun. 2(1):8. doi: 10.1038/s43705-022-00089-5.
- Koukol O, Kelnarová I, Černý K. 2015. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For Pathol. 45(1):21–27. doi: 10.1111/efp.12129.
- Li Q, Gong X, Zhang X, Pi Y, Long S, Wu Y, Shen X, Kang Y, Kang J. 2021. Phylogeny of Graphostromatacea with two new species (Biscogniauxia glaucae sp. nov. And Graphostroma guizhouensis sp. nov.) and new record of Camillea broomeana isolated in China. Arch Microbiol. 203(10):6119–6129. doi: 10.1007/s00203-021-02574-2.
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol. 16(12):1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092.
- Mirabolfathy M, Ju YM, Hsieh HM, Rogers JD. 2013. Obolarina persica sp. nov. associated with dying Quercus in Iran. Mycoscience. 54(5):315–320. doi: 10.1016/j.myc.2012.11.003.
- Muller E, Dvořák M, Marçais B, Caeiro E, Clot B, Desprez-Loustau ML, Gedda B, Lundén K, Migliorini D, Oliver G, et al. 2023. Conditions of emergence of the sooty bark disease and aerobiology of Cryptostroma corticale in Europe. NeoBiota. 84:319–347. doi: 10.3897/neobiota.84.90549.
- Natural Resources Canada. 2024. Sooty bark disease Ottawa (ON), Canada): Government of Canada; [accessed 2024 Jan 20]. https://natural-resources.canada.ca/our-natural-resources/forests/insects-disturbances/top-forest-insects-and-diseases-canada/sooty-bark-disease/25599.
- Ogris N, Brglez A, Piškur B. 2021. Drought stress can induce the pathogenicity of Cryptostroma corticale, the causal agent of sooty bark disease of sycamore maple. Forests. 12(3):377. doi: 10.3390/f12030377.
- Oliveira Longa CM, Vai N, Maresi G. 2016. Cryptostroma corticale in the northern Apennines (Italy). Phytopathol Mediterr. 55(1): 136–138.
- Paap T, Burgess TI, Wingfield MJ. 2017. Urban trees: bridge-heads for forest pest invasions and sentinels for early detection. Biol Invasions. 19(12):3515–3526. doi: 10.1007/s10530-017-1595-x.
- Parisien MA, Barber QE, Bourbonnais ML, Daniels LD, Flannigan MD, Gray RW, Hoffman KM, Jain P, Stephens SL, Taylor SW, et al. 2023. Abrupt, climate-induced increase in wildfires in British Columbia since the mid-2000s. Commun Earth Environ. 4(1):309. doi: 10.1038/s43247-023-00977-1.
- Parmeter JR Jr, Uhrenholdt B. 1976. Effects of smoke on pathogens and other fungi. Proceedings of the Tall Timbers Fire Ecology and Fire Land Management Conference, No. 14. Tallahassee, Fla: Tall Timbers Research Station. p. 299–304.
- Paviour-Smith K. 1976. How far has Cryptostroma corticale spread in Britain? Bull Br Mycol Soc. 10(1):16–19. doi: 10.1016/S0007-1528(76)80008-9.
- Peace TR. 1955. Sooty bark disease of sycamore – a disease in eclipse. Q J For. 49:197–204.
- Rehner SA, Samuels GJ. 1995. Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Can J Bot. 73(S1):816–823. doi: 10.1139/b95-327.
- Robinson DJ, Cowan IM. 1954. An introduced population of the gray squirrel (Sciurus carolinensis Gmelin) in British Columbia. Can J Zool. 32(3):261–282. doi: 10.1139/z54-026.
- Schlößer R, Bien S, Langer GJ, Langer EJ. 2023. Fungi associated with woody tissues of Acer pseudoplatanus in forest stands with different health status concerning sooty bark disease (Cryptostroma corticale). Mycol Prog. 22(2):13. doi: 10.1007/s11557-022-01861-6.
- Schulte PA, Jacklitsch BL, Bhattacharya A, Chun H, Edwards N, Elliott KC, Flynn MA, Guerin R, Hodson L, Lincoln JM, et al. 2023. Updated assessment of occupational safety and health hazards of climate change. J Occup Environ Hyg. 20(5–6):183–206. doi: 10.1080/15459624.2023.2205468.
- Shepherd GM, Michelis MA, Macris NT, Smith JP. 1989. Hypersensitivity pneumonitis in an orchid grower associated with sensitivity to the fungus Cryptostroma corticale. Ann Allergy. 62(6):522–525.
- Spooner B. 2007. Gnawing by small mammals: on effused stromatic Ascomycetes. Field Mycol. 8(2):63–65. doi: 10.1016/S1468-1641(10)60455-0.
- Stadler M, Kuhnert E, Peršoh D, Fournier J. 2013. The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) concept. Mycology. 4(1):5–21. doi: 10.1080/21501203.2013.782478.
- Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW. 2007. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol. 44(3):1204–1223. doi: 10.1016/j.ympev.2007.03.011.
- Sutton BC, Gibson IAS. 1977. Cryptostroma corticale. CMI Descriptions of Pathogenic Fungi and Bacteria No. 539.
- Tewksbury DA, Wenzel FJ, Emanuel DA. 1968. An immunological study of maple bark disease. Clin Exp Immunol. 3(8):857–863.
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44(W1):W232–W235. doi: 10.1093/nar/gkw256.
- U’Ren JM, Miadlikowska J, Zimmerman NB, Lutzoni F, Stajich JE, Arnold AE. 2016. Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Mol Phylogenet Evol. 98:210–232. doi: 10.1016/j.ympev.2016.02.010.
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 172(8):4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990.
- Washington State University. 2023. Sooty bark disease. Puyallup (WA): Washington State University. [accessed 2023 May 5]. https://ppo.puyallup.wsu.edu/sbd/.
- Wendt L, Sir EB, Kuhnert E, Heitkämper S, Lambert C, Hladki AI, Romero AI, Luangsa-Ard JJ, Srikitikulchai P, Peršoh D, et al. 2018. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol Prog. 17:115–154. doi: 10.1007/s11557-017-1311-3.
- Wenzel FJ, Emanuel DA. 1967. The epidemiology of maple bark disease. Arch Environ Health. 14(3):385–389. doi: 10.1080/00039896.1967.10664759.
- Wertman D, Tanney JB, Hamelin RC, Carroll AL. 2024. Neonectria bordenii sp. nov. a potential symbiote of the alder bark beetle, and its detection by quantitative PCR. Fungal Syst Evol. 13:15–28. doi: 10.3114/fuse.2024.13.02.
- Woods AJ, Heppner D, Kope HH, Burleigh J, Maclauchlan L. 2010. Forest health and climate change: a British Columbia perspective. For Chron. 86(4):412–422. doi: 10.5558/tfc86412-4.
