Abstract
Clubroot, caused by the obligate parasite Plasmodiophora brassicae, is an economically important disease affecting plants in the family Brassicaceae worldwide. In Canada, it has mainly been a problem on cruciferous vegetables in the traditional production areas of Ontario, Quebec, British Columbia and the Atlantic Provinces. In the Prairie Provinces, clubroot has been reported sporadically in a few home gardens and commercial vegetable fields in Alberta and Manitoba over the past 80 years; however, this situation changed dramatically with the discovery of 12 infected canola (Brassica napus) fields near Edmonton, AB in 2003. Annual surveys carried out since 2003 have revealed that clubroot is a much more widespread and serious disease in Alberta than initially thought. By 2008, it had been detected in about 410 canola, mustard and vegetable fields in central and southern areas of the province. The Alberta Clubroot Management Plan was developed to guide farmers, agribusinesses, oil and gas companies, contractors, and the general public in adopting good growing practices or taking measures to prevent further spread of this disease. An unprecedented research effort is underway in western Canada to develop a better understanding of the biology and management of clubroot, especially in the canola production systems on the Prairies. These studies are broadly based and include activities such as the improvement of diagnostic tests, determination of P. brassicae pathotypes, investigation of modes of seed and soil transmission, evaluation of fungicide efficacy, soil amendments, biological control agents and equipment sanitation protocols, and modelling disease distribution and risk. Through these efforts, new information is being generated that will give the agricultural industry and other stakeholders some new perspectives on this old disease threat.
Résumé: La hernie, causée par le parasite obligatoire Plasmodiophora brassica, est une maladie de grande importance économique, qui s'attaque aux plantes de la famille des Brassicaceae partout dans le monde. Au Canada, le problème s'est manifesté principalement dans les cultures de crucifères des régions traditionnelles de production de l'Ontario, du Québec, de la Colombie-Britannique et des provinces de l'Atlantique. Dans les provinces des Prairies, au cours de 80 dernières années, la hernie a été rapportée sporadiquement dans quelques potagers domestiques et dans certaines cultures maraichères commerciales de l'Alberta et du Manitoba. Toutefois, la situation a changé radicalement lorsqu'on a découvert 12 champs de canola (Brassica napus) infestés près d'Edmonton en 2003, en Alberta. Les études annuelles menées depuis 2003 ont révélé que la hernie est beaucoup plus répandue et beaucoup plus sérieuse en Alberta qu'on le croyait au départ. En 2008, elle avait été détectée dans environ 410 champs de canola, de moutarde et de légumes du centre et du sud de la province. L'Alberta Clubroot Management Plan a été mis en œuvre pour guider les agriculteurs, l'agro-industrie, les sociétés de pétrole et de gaz, les entrepreneurs et le public en général à adopter de saines pratiques de culture ou à prendre les mesures visant à prévenir la plus ample propagation de cette maladie. Des efforts de recherche sans précédent sont déployés dans l'Ouest canadien afin de mieux comprendre la biologie et la gestion de la hernie, particulièrement dans le système de culture du canola dans les Praires. Ces études, qui incluent un large spectre d'activités, visent l'amélioration des tests de diagnostic, l'identification des pathotypes de P. brassica, les recherches quant aux modes de transmission par semence et par le sol, l'évaluation de l'efficacité des fongicides, les amendements du sol, les agents de lutte biologique, les protocoles de nettoyage de la machinerie et la modélisation de la distribution de la maladie et des risques. Grâce à ces efforts, les nouvelles données recueillies offriront à l'industrie agricole ainsi qu'aux autres parties concernées de nouvelles perspectives quant aux menaces qui émanent de cette ancienne maladie.
Introduction
Clubroot is one of the most recognizable plant diseases, and virtually all plant pathology students learn about it in their undergraduate or graduate training. In many areas of the world where cruciferous crops are grown, it represents the most persistent and significant disease threat. Despite the fact that countless scientific papers, theses, factsheets, newsletters and popular articles have been written about clubroot, there are still many mysteries about the origins and global spread of this disease, the biology of the pathogen, Plasmodiophora brassicae Woronin, and the environmental and physiological factors affecting disease development in the many hosts that this disease can affect. It also remains a significant challenge to successfully manage, despite over 100 years of research and the intensive use of a wide variety of cultural, chemical and biological control measures.
The focus of this review is the history, geographical distribution and importance of clubroot on cruciferous vegetables and canola in Canada, and on past and present research aimed at better understanding the nature and control of this disease. The new and serious threat that clubroot currently poses to the canola industry in western Canada has created unprecedented interest amongst producers, agronomists, agribusiness representatives, plant pathologists, and extension specialists. In a curious twist of fate, the oil and gas, transportation and construction industries in Alberta have also been drawn into this situation because of the concern over disease spread via infested soil, especially on heavy equipment that could be moved long distances. All of this has stimulated an extensive research effort aimed at better understanding the biology and management of clubroot, especially in the canola production systems on the Canadian Prairies. Scientists and other investigators are generating new information that will give the agricultural industry and other interested parties new perspectives on this old disease threat.
Historical perspectives on the clubroot disease
Clubroot is one of the oldest known plant diseases. Pallidus described the development of spongy roots on rape, turnip and radishes grown in soil fertilized with manure in Italy in the fourth century AD (Watson & Baker, Citation1969). Turnips were grown and used by the Romans for both human and animal food. This observation is believed to be the first record of clubroot and its transmission by livestock manure. A second probable record of this disease by Diaz de Isla in 1539 describes clubroot-like symptoms on cabbage in Spain. Fuchs illustrated four cultivated Brassica species in 1542, one of which showed a large spherical gall on the roots of Brassica oleracea primum (= Brassica arvensis Kuntze). This may be the first published illustration of clubroot symptoms. These records support the idea that clubroot originated in the Mediterranean area, close to the centre of origin of the genus Brassica. Clubroot was first reported in England on turnip in 1736 by Ellis, and subsequent reports from across Europe in the following 100 years attest to its widespread distribution. In the late 1860s, clubroot caused heavy losses in cabbage fields around St. Petersburg, Russia, and prompted the first detailed description of the disease, including identification of the causal agent, by Michael Woronin in 1878 (Woronin, Citation1878). The first record of clubroot in North America was in the vicinity of New York City in 1853 (Watson & Baker, Citation1969).
It is unclear when clubroot was first introduced to Canada, but it seems likely that it came to this country with fodder turnips used to feed the livestock brought over by early European settlers, following the same pattern by which the disease is believed to have been introduced to Australia, New Zealand and the USA. The earliest records of clubroot in Canada probably exist in the form of notes and diaries kept by early horticulturists and botanists, which were unavailable to the authors of this mini-review. Estey (Citation1994) noted that clubroot research was being conducted by P.J. Shaw at the Nova Scotia Agricultural College, Truro, Nova Scotia in 1916. The most extensive published records of clubroot in vegetables begin in the 1920s and are contained in the Canadian Plant Disease Survey (CPDS). Clubroot was reported almost every year in British Columbia, Quebec, and the Maritime Provinces from the 1920s to the 1950s. The disease was also reported sporadically in Ontario during that same time; however, this is most likely due to a lack of reporting rather than the actual absence of the disease. Clubroot was primarily reported on cabbage, cauliflower and rutabaga or swede turnip. Occasionally, it was also observed on broccoli, Brussels sprouts, Chinese cabbage and kale.
Clubroot appeared in British Columbia for the first time in 1920 in a home garden in Victoria (Rankin & Fraser, Citation1920). By 1928, it had spread to new areas and was present on 90–100% of plants in many infested fields. It was reported on Chinese cabbage in British Columbia in 1928, perhaps the first report on this crop in North America. Clubroot continued to spread and cause serious damage into the early 1930s and eventually made its way into the lower mainland.
During the 1920s and early 1930s, clubroot was widespread in the Maritime Provinces, and by 1923 it had spread through Nova Scotia and was becoming very problematic. In 1926, it was so severe on turnips in Nova Scotia and New Brunswick that it curtailed production to the point of ruining the fatted cattle industry in some areas. The year 1932 was a particularly destructive one for clubroot on cruciferous vegetables in the Maritime Provinces. Over $15 000 in losses was estimated in New Brunswick. In 1931, the turnip variety ‘Bangholm’ was reported to be resistant and could be successfully grown in heavily infested soils in Prince Edward Island; however, it was totally destroyed in test plots when grown in New Brunswick in 1932. It appears that a unique pathotype in New Brunswick was able to overcome the resistance in ‘Bangholm’.
Very little information on the history and spread of the clubroot in Ontario and Quebec appears in the CPDS. Rankin & Fraser (Citation1920) reported clubroot on cabbage in a field near Ottawa in 1920. There were several reports of clubroot on cabbage and turnip in both provinces throughout the 1920s. By 1935, the disease was reported in Quebec on Brussels sprouts, cabbage cauliflower, Chinese cabbage and turnip (Conners, Citation1935).
In 1943, clubroot infection was observed on Iberis sp. L. (candytuft) growing in Prince Edward Island, the first published record of this disease on an ornamental plant in Canada (Conners & Savile, Citation1944). During the 1950s, the disease began to attract increasing national attention. In 1956, clubroot was specifically mentioned in the CPDS (Conners et al., Citation1956) as a ‘noteworthy disease’ for the following reasons:
-
Its effect as the principal disease of cabbage and cauliflower in a number of areas in Quebec and the Maritimes, causing critical production issues in some areas.
-
Its spread into areas where it had not previously occurred such as in the Bradford Marsh area of Ontario. Extensive flooding took place in 1954 due to hurricane Hazel and clubroot became widely disseminated, so that in 1955 it was present in most cabbage fields in the area, more than 8 km from the original 1953 infestation.
-
Its appearance in cabbage and swede turnips in newly cleared fields in Newfoundland.
During this same time, reports stated that clubroot had become a serious problem in many areas of Canada. Additionally, seven to eight year rotations were not sufficient to prevent damaging outbreaks, and cruciferous weeds were implicated in maintaining or increasing P. brassicae inoculum (Creelman, Citation1958).
In the 1960s, clubroot was less serious in Eastern Canada than in previous years for several reasons:
-
Vegetable varieties that were more tolerant to the disease had been identified, e.g. ‘Wilhelmsburger’ cabbage and ‘York’ rutabaga, depending on the pathotypes present.
-
Environmental conditions were unfavourable for disease development, with the exception of 1964 in Newfoundland (Creelman, Citation1965).
-
Growers began having soil assayed for disease potential prior to planting cruciferous crops.
Outbreaks of the disease were often attributed to poor crop husbandry, i.e. insufficient rotation or poor weed management (Creelman, Citation1965). In 1964, an outbreak of clubroot in previously uninfested fields was linked to the spread of manure from animals fed on diseased roots (Creelman, Citation1965).
During the 1970s and 1980s, CPDS reports focused on the identification of prevalent races of clubroot, the development of resistant varieties and chemical control of the disease. In the 1970s, clubroot remained the most important pathogen and limiting factor in production of cabbage and other crucifers in areas where it was well established; however, it was not reported outside of the established vegetable growing areas in British Columbia, central Canada and the Maritimes until unpublished reports of its occurrence in a few home gardens near Edmonton, AB in 1977.
Host range, disease cycle and symptoms
Host range
All 330 genera and 3700 species of the family Brassicaceae are predicted to be potential hosts for P. brassicae; however, few studies on infection have been conducted outside the genera Brassica, Raphanus and Arabidopsis (Dixon, Citation2009). Commonly cultivated Brassica host species include all varieties of Brassica oleracea L. (Brussels sprouts, cabbage, cauliflower, kale and kohlrabi), Brassica rapa L. (turnip, turnip rape and Chinese cabbage), B. napus L. (rutabaga or swede turnip, oil seed rape, mustard and canola), Raphanus sativus L. (radish), and all crops derived from Brassica carinata A. Braun, Brassica nigra L. and Brassica juncea (L.) Czern. Cruciferous weeds such as shepherd's purse (Capsella bursa-pastoris (L.) Medik.) and stinkweed (Thlaspi arvense L.) are also susceptible to infection by P. brassicae (Buczacki & Ockendon, Citation1979).
Disease cycle
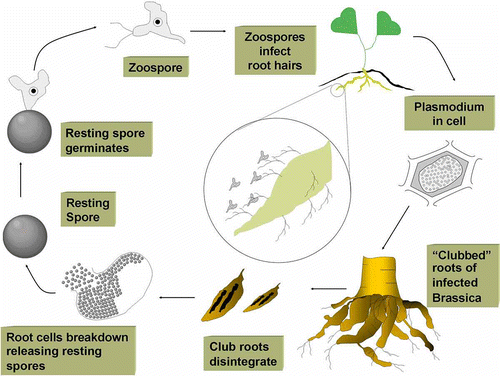
The disease cycle for clubroot is shown in . Resting spores are extremely long-lived, with a half-life of about four years, but can survive in the soil up to 20 years (Wallenhammar, Citation1996). Primary zoospores are formed from resting spores and require water films in the soil in order to swim to and infect root hairs by penetration of the cell wall, causing malformations of the root hairs. This primary infection, or root hair infection stage, is followed by formation of primary plasmodia (multinucleate masses). The primary plasmodia cleave into zoosporangia, each containing 4–16 secondary zoospores, which are released into the soil. Primary infections do not cause macroscopic symptoms and are not responsible for significant yield and quality losses. The secondary zoospores released into the soil from the primary plasmodia penetrate the host, invading cortical tissues of the main roots. Secondary infections lead to the development of secondary plasmodia within cells of the root cortex, resulting in the recognizable symptoms of the disease, i.e. club-shaped malformations of the roots (Kageyama & Asano, Citation2009). A secondary plasmodium will eventually be cleaved into millions of resting spores within the root gall. As the root tissues deteriorate, the resting spores are released into the soil to complete the disease cycle. The pathogen usually completes one cycle per season and cannot spread rapidly in the soil, as zoospore motility is limited. Clubroot spread over significant distances occurs mainly via movement of resting spores in soil or in infected plant material. Therefore, dissemination of the disease is often associated with the spread of infested soil from field to field on farm machinery and equipment. Nevertheless, clubroot can also be spread in manure from livestock fed P. brassicae-infested fodder, through the erosion of infested soil via wind and water, and as a contaminant on seed.
Symptoms
Clubroot gets its name from the tumorous galls that form on the roots of susceptible plants; however, symptoms vary depending on the growth stage of the crop at the time of infection. Seedling infection can result in above-ground wilting, stunting and yellowing symptoms. Infection that occurs at later stages may not result in such pronounced above-ground symptoms, but infected plants will senesce prematurely. Thus, yield and quality losses may still be observed. For example, cauliflower and broccoli with clubroot infections may have smaller or deformed spears or hearts, whilst heavier upright crops such as Brussels sprouts may lodge. All factors of yield and quality can be diminished when a plant's root system is compromised, including oil content, uniformity and storability. Above-ground clubroot symptoms may be confused with drought, nutrient deficiency or other diseases, so suspect plants should be dug from the soil to check for the characteristic gall formation on the roots.
Distribution and economic importance on cole crop vegetables in Canada
Prior to 2003, P. brassicae was considered an established pest in British Columbia, Ontario, Quebec and the Atlantic Provinces in most areas where cruciferous vegetables were produced (Howard et al., Citation1994; Rimmer et al., Citation2003), but it was generally absent from vegetable fields in Alberta, Saskatchewan and Manitoba. Only a few published and unpublished reports of localized, scattered infestations in Alberta and Manitoba exist. Clubroot was reported on Swede turnip and Breadstone green top in Manitoba in 1925 (McCurry & Hicks, Citation1925), and unpublished reports suggest a small, localized outbreak in a market garden in the early 1980s (MAFRI, Citation2009). Two unpublished reports of localized outbreaks of clubroot in Alberta were made, the first on broccoli, cabbage and cauliflower in 1977 (I. R. Evans, personal communication) in home gardens in the Edmonton area, and the second in 2001 (R. J. Howard, unpublished data) on Chinese cabbage in a market garden near Leduc. In 2004, the year after clubroot was found on canola near St. Albert, Alberta (Tewari et al., Citation2005), surveys in vegetables were undertaken and localized occurrences of clubroot were discovered in cabbage, cauliflower and rutabaga in commercial and market gardens (Harding et al., Citation2005, Citation2006, Citation2007, Citation2008, Citation2009). Saskatchewan remains the only province in Canada with no report of a clubroot infestation.
The significance and impact of clubroot in cruciferous vegetables is highest in regions of Canada where vegetable production predominates and has had a long history. Cruciferous vegetables belonging to the Brassica oleracea L. group are distributed across the country; however, the largest hectarages for cabbage (4261 ha), broccoli (3569 ha), cauliflower (1476 ha), and rutabaga and turnip (1675 ha) occur mainly in Ontario and Quebec, with less than 5% of production in the Prairie Provinces () (Howat, Citation2005; Walker Citation2005; Statistics Canada, Citation2009a, Citation2009b). Other cole crop vegetables (Brussels sprouts, Chinese cabbage, kale, kohlrabi, radish,) are not as common, but are grown in concentrated areas in Canada or in market gardens and home gardens across the country. All cole crops are potential hosts for clubroot (Dixon, Citation2009), but economically important outbreaks are mostly limited to areas where production is concentrated or where large commercial hectarages become infested.
Table 1. Production areas of major cole crop vegetables in Canada (Statistics Canada, Citation2009a)
Because the disease is now established in all major cole crop producing areas of Canada, the economic impact of clubroot is determined mainly by the environment and grower management practices. The impact is also greater in areas with climates that favour disease development, such as areas with wet, acidic soils. In years where soil moisture and temperature are favourable for disease development, especially early onset, the resulting infestations can have a severe impact on yield. Additionally, in cases where growers do not sufficiently rotate away from cruciferous crops, or do not control cruciferous weeds, disease pressure and resulting losses can be devastating.
A few studies on the economic impact of clubroot in areas of Canada have been done. One study reported that in British Columbia, the disease was continually a problem in the lower Fraser Valley in the mid-1960s, causing 2–8% losses in broccoli, cabbage and cauliflower and over $30 000 in lost revenue each year (Toms, Citation1966, Citation1968). On a worldwide scale, clubroot incidences have been estimated at greater than 10% (Crête, Citation1981; Dixon, Citation2009). Historic estimates of financial losses in fields with significant clubroot infestations indicate a reduction of about 50% in overall returns (Dixon, Citation2009). This estimate takes into account losses due to decreased yield in crops such as cabbage, cauliflower and broccoli, and reductions in yield and marketability for root crops such as rutabaga, turnip, radish and kohlrabi. However, such estimates do not reflect losses due to land taken out of cruciferous crop production, which may be a significant dollar value depending on the area and crops in rotation. Some soils may become unfit for cruciferous crop production for many years. At best, recommendations for clubroot control often suggest rotations of more than four years, sometimes up to eight years, to manage the disease. Additionally, estimates do not account for the complications and expenses associated with the increased management required to plan lengthy rotations and to prevent movement of infested soils on equipment and machinery (Dixon, Citation2009).
Distribution and economic importance on canola and other cruciferous field crops in Canada
The first report of clubroot on non-vegetable cruciferous crops in Canada appears to be on rape in Newfoundland in 1965 (Creelman, Citation1967). This was followed by a report of the disease on canola in Quebec in 1997 by Morasse et al. (Citation1997). Pageau et al. (Citation2006) conducted an extensive study on the impact of growing 31 varieties of B. napus in soil infested with clubroot at Normandin, Quebec in 1998–99.
In 2003, Tewari et al. (Citation2005) reported finding 12 infested canola (B. napus) fields in Sturgeon County northwest of Edmonton, Alberta. Annual surveys carried out since 2003 revealed that clubroot was a much more widespread and serious disease in Alberta than initially thought. By 2008, it had been detected in at least 405 canola fields in central and southern areas of the province (Cao et al., Citationin press). Infestation levels ranged from negligible to very high, with resulting yield reductions in many cases. A few canola fields were not harvested because of extremely low yield potentials. Although clubroot was more prevalent in crops grown on acidic soils, it was also detected in a few fields with pH ratings ≥ 7.5, which were significantly higher than had previously been considered suitable for clubroot development. The ability of the clubroot pathogen to survive and infect canola crops grown in alkaline soils suggests that the disease could spread beyond the traditional production areas in central and northern Alberta, where acidic soils are common, into southern areas where alkaline soils prevail. Additionally, this finding suggests that some traditional management strategies, such as liming of soils, may be unsuitable in such areas. A small infestation of clubroot was reported in an experimental plot of canola in southern Manitoba in 2005 (MAFRI, Citation2009; Cao et al., Citationin press). As of 2008, however, clubroot-infected plants had not been found in commercial canola fields in Saskatchewan, Manitoba or British Columbia. Unlike the relatively scattered distribution and smaller sizes of commercial cole crop vegetable production areas, canola can be found in large contiguous areas centred in the Prairies and covering millions of hectares (). Whereas the Prairie Provinces produce less than 5% of the cruciferous vegetables in Canada (), they produce more than 95% of the canola and mustard (). Thus, while clubroot on the Prairies will have little effect on Canadian vegetable production, its impact on canola and mustard production could be substantial.
Table 2. Production areas of canola and mustard in Canada (Statistics Canada, Citation2009b)
Disease management strategies
Efforts to successfully manage clubroot depend on integrating available cultural and chemical control strategies, with emphasis on prevention. Disease management strategies for clubroot in vegetables are discussed in Howard et al. (Citation1994), MAFRI (Citation2009), Rimmer et al. (Citation2007), Tremblay et al. (Citation1999) and many other publications. A summary of some of the most commonly used practices follows.
Field selection
The selection of fields with well-drained soils free of clubroot contamination, where possible, is recommended. Allowing four to five years between successive plantings of cruciferous crops will minimize the risk of disease build-up. Longer intervals may be necessary if inoculum levels are very high. Spread of the disease from infested to non-infested fields can be restricted by following practices such as equipment sanitation, controlling soil and water erosion, and working disease-free fields before infested fields.
Crop and variety selection
Susceptibility to clubroot varies widely between different types of cruciferous vegetables. Asian vegetables (Chinese cabbage, bok choy, suey choy and pak choy), cabbage and Brussels sprouts are amongst the most susceptible. Broccoli, cauliflower, collards, kale, kohlrabi, rutabaga and turnip are considered moderately resistant to clubroot, while radish is perhaps the most tolerant. Some progress has been made in breeding vegetable varieties with resistance to clubroot, but their durability may depend on prevalent pathotypes. Some examples of resistant varieties that have been used in Canada include ‘Kingston’ and ‘York’ rutabaga and ‘Richelain’ cabbage. Growers are advised to check with seed suppliers on the availability of adapted varieties with resistance to local pathotypes. Overuse of such varieties under conditions of high disease pressure may lead to a breakdown of resistance.
Transplants and seed
Seedlings that have been grown in clubroot-free plant beds or growing media should be used for transplanting. It is easy to inadvertently spread clubroot to previously non-infested fields via infected transplants. They are also a means by which the disease can be moved long distances and foreign pathotypes can be introduced. Transplant beds should be isolated from production fields to minimize the risk of becoming infested. Cruciferous weeds in plant beds should be controlled and clean water used for irrigation. Infested beds should be fumigated, where practical, or preferably relocated. Seed crops should not be located in fields with clubroot because of the risk of contaminating the seed with soil dust tags containing resting spores.
Contaminated soil and equipment
Resting spores present in soil can readily contaminate transplant trays, harvest containers, vehicles, tools and field equipment; therefore, they should be cleaned and sanitized prior to use. Sanitation involves three key steps: first, removing bulk soil and crop debris; second, pressure washing, scrubbing or using compressed air to remove any remaining residues; and third, applying a recommended disinfectant to the clean surfaces and allowing at least 20 minutes of contact time to insure that any remaining spores are killed. Clubroot-contaminated soil should never be used for transplant production and steps should be taken to prevent the movement of infested soil from field to field on footwear, machinery, vehicles, tools and equipment.
Manure application and cull vegetable disposal
Clubroot spores can survive passage through livestock, so the use of raw manure on vegetable fields from animals that have been fed or pastured on clubroot-infected fodder should be avoided. Likewise, spreading cull vegetables from clubroot-infected crops onto fields could introduce the disease to new areas or augment existing inoculum levels in the soil. Although research on the effect of the composting process is limited, temperature and moisture content were found to be important for the successful eradication of P. brassicae resting spores from composted residues infested with clubroot (Fayolle et al., Citation2006).
Irrigation
Irrigation water contaminated with resting spores could potentially infest large areas of fields. Water should not be applied to plantings of cruciferous vegetables if it has been drawn from ponds or creeks contaminated with clubroot spores resulting from runoff from infested fields.
Crop rotation
Rotating susceptible cruciferous vegetables with non-hosts has the potential of reducing P. brassicae inoculum levels in the soil. Some examples of non-host crops are cereal grains, alfalfa, onions, peas, beans and carrots. Some non-Brassica species have been shown to be hosts of P. brassicae under experimental conditions and, where possible, should be avoided in rotations with cruciferous vegetables. These include Agrostis stolonifera L. (creeping bentgrass), Dactylis glomerata L. (orchardgrass), Fragaria spp. L. (strawberry), Lolium perenne L. (perennial ryegrass), Papaver rhoeas L. (corn poppy) and Trifolium pratense L. (red clover).
Soil amendments and fertilizers
Materials that raise the pH of soil or growing media, such as lime and wood ash, may create conditions that are unfavourable for clubroot infection and development. These products are most effective where inoculum levels are relatively low, and their use should be preceded by a soil test to determine if treatment is agronomically desirable. Lime amendments are available in various forms, e.g. agricultural lime (calcium carbonate and calcitic lime), dolomitic lime (calcium and magnesium carbonate), hydrated lime (calcium hydroxide), and quicklime (calcium oxide). Agricultural and dolomitic limes are relatively slow acting, whereas hydrated lime and quicklime are more reactive. Application of slow-acting limes may have to be made in the fall to allow enough time for the amendments to work prior to spring planting, and considerable quantities may have to be applied. Fast-acting limes are more suitable for spring application. Finely ground amendments are likely to be more reactive than coarse formulations and should alter the pH more rapidly. Annual applications of soil amendments may be required to raise the pH and to maintain it at desirable levels. A pH of 7.2 or above is considered optimal for clubroot control, but raising it this high may not be feasible or economical in highly acidic soils. Where clubroot infestations are localized, spot treatments with amendments may be used to suppress disease development and also lessen the cost of treatment compared with broadcast applications. Massive or repeated applications of lime may reduce the availability of nutrients, such as phosphorus, boron, magnesium, manganese and zinc. If so, supplementary treatments for deficient nutrients may be needed. Calcium cyanamide (Perlka) has been successfully used to combat clubroot in Europe, Australia and New Zealand, but has not been widely used in Canada. It breaks down into calcium oxide and urea, thus increasing the soil pH and providing a source of nitrogen for the plants.
Fungicides and fumigants
In Canada, pentachloronitrobenzene (Adobe® 75WP, Crusoe® 75WP and Quintozene 75WP) and fluazinam (Allegro 500F) are registered on cruciferous vegetables and can be applied as pre- or post-planting drenches on transplants. Propagation beds used to produce transplants can be fumigated with metam sodium (Vapam HL®). Several biological control products are being evaluated as soil treatments for clubroot control (Gary Peng, personal communication). Some soil-applied surfactants have demonstrated efficacy against clubroot (Hildebrand & McRae, Citation1998); however, phytotoxicity has been noted and none is presently registered in Canada.
Weed control
Cruciferous weeds may act as alternative hosts for the clubroot pathogen and should be controlled within the cropping season, as well as within the break crops used in rotations with susceptible vegetables. Some examples of potential weed hosts for P. brassicae include Armoracia rusticana P.G. Gaertn., B. Mey. & Scherb (1800) (horseradish, red cole), Brassica hirta Moench (white mustard), Brassica kaber (DC.) L.C. Wheeler (wild mustard), Camelina sativa L. Crantz (camelina, false flax), Camelina microcarpa Andrz. (small-seeded false flax), Capsella bursa-pastoris (L.) Medik. (shepherd's purse), Erysimum asperum (Nutt.) DC. (western wallflower), Lepidium campestre (L.) W.T. Aiton (pepperwort), Rorippa islandica (Oeder) Borbás (marsh cress), Rorippa sylvestris (L.) Besser (creeping yellow cress, yellow field cress), Sisymbrium altissimum L. (tumbling mustard), Sisymbrium officinale (L.) Scop. (hedge mustard), Thlaspi arvense L. (stinkweed), Rumex spp. L. (dock), Barbarea vulgaris R. Br. (wintercress, yellow rocket) and Hesperis matronalis L. (dame's rocket, dame's violet).
Crop scouting
Regular examination of cruciferous vegetables for clubroot symptoms may help to pinpoint new field infestations or to monitor disease spread within or between fields. Scouting should begin in transplant beds and continue through to harvest. Plants should be inspected for both foliar and root symptoms. Some root rot diseases may produce foliar symptoms, e.g. stunting, yellowing, wilting that mimic those of clubroot, so plants should be examined carefully for characteristic root galls containing resting spores. In addition, a genetic disorder known as ‘hybridization nodules’ wherein roots develop hard, spherical swellings can also be mistaken for clubroot.
Strategies for managing clubroot in field crops, such as canola and mustard, have not been as well researched or tested in on-farm situations as compared with cruciferous vegetables; however, many of the basic principles of good crop husbandry and disease prevention described above would apply for these two crops as well. The Alberta Clubroot Management Plan (Alberta Clubroot Management Committee, Citation2008), developed mostly for canola, outlines nine key strategies:
-
Use long crop rotations between successive canola crops, e.g. ≥3 years for light infestations and ≥ 5 years for moderate to heavy infestations.
-
Control volunteer canola, mustard and cruciferous weeds in infested fields.
-
Follow recommended sanitation measures, i.e. clean, wash and disinfect to reduce the risk of spreading clubroot on infested machinery, equipment and vehicles.
-
Use direct seeding and other soil conservation practices to reduce the number of tillage operations in fields.
-
Minimize machinery and vehicle traffic to and from infested fields.
-
If fields are infested only at the entrance, create a new access well away from the original point of entry.
-
Scout canola and mustard fields regularly and carefully.
-
Restrict the use of straw, hay, green feed, silage and manure from infested farms.
-
Avoid the use of common seed (e.g. cereals, oilseed and pulses) from infested fields which could introduce resting spores present in earth to non-infested fields.
Overview of new research initiatives on vegetables
A number of successful research initiatives aimed at improving management of clubroot in vegetables have come from Canada. For example, regional populations of P. brassicae were characterized to establish pathotype (‘race’) distribution in eastern Canada (Ayers, Citation1957, Citation1972; Reyes et al., Citation1974). Subsequently, cruciferous vegetables were screened for clubroot resistance (Crête & Chiang, Citation1967, Citation1980; Chiang & Crête, Citation1972), and breeding programs were established to develop resistant cultivars with agronomically suitable traits (Chiang & Crête, Citation1970, Citation1985a; Chiang et al., Citation1977; Chong et al., Citation1985; Vigier et al., Citation1989). Two clubroot-resistant cabbage cultivars were released: ‘Acadie’ and ‘Richelain’ (Chiang & Crête, Citation1985b, Citation1989). The chemical control of clubroot on vegetables has also been studied in Canada, including the use of lime and calcium cyanamide (Bélec et al., Citation2004; McDonald et al., Citation2004; Tremblay et al., Citation2005), surfactants (Hildebrand & McRae, Citation1998), phosphonate fungicide (Abbasi & Lazarovits, Citation2006), and other chemical fungicides (Crête et al., Citation1963; Finlayson & Campbell, Citation1969, Citation1971). In some regions, timing of seeding has been demonstrated to play a major role in clubroot management, such that seeding crops later in the season (i.e. August) is sufficient to avoid clubroot development (McDonald et al., Citation2004).
New clubroot research initiatives involving cruciferous vegetables continue to emerge in Canada as a result of the continuing economic impact of this disease on crop production. For example, screening of new fungicides and soil amendments for efficacy against clubroot have been conducted in British Columbia (J. Elmhirst, unpublished data) and Alberta (R.J. Howard, unpublished data). These studies have included products such as Allegro (fluazinam), Ranman (cyazofamid), Blinix (rhamnolipid) and calcium nitrate. These types of studies often support registrations for new product chemistries or formulations, providing growers with new options for clubroot control. An example is Allegro 500F, which was registered in 2008 for use on leafy Brassica vegetables for clubroot control (British Columbia Ministry of Lands, Citation2009). Other fungicides recently registered on vegetables for clubroot control include Crusoe® 75WP (quintozene) and Vapam HL® (metam sodium). Chemical disinfectants used to sanitize equipment and machinery are also being tested for efficacy against P. brassicae resting spores, as described below (R.J. Howard and S.E. Strelkov, unpublished data).
A resurgence of clubroot research has recently occurred as a result of its appearance in canola (B. napus) fields in Canada. As canola is a relatively new host for P. brassicae in Canada, some basic biological information was needed. For example, what is the distribution of the disease incidence in canola and what effect may clubroot in canola have on vegetable production? Is the disease spreading and, if so, how fast and how far could it spread? Which pathotype(s) is (are) present in infested canola regions and are these isolates virulent on vegetables? What was the source of the original infestation(s)?
One of the first initiatives resulting from the discovery of clubroot on canola in Alberta was the seasonal disease surveillance effort aimed at establishing the effects of clubroot in canola on disease prevalence on vegetables in Alberta (Harding et al., Citation2009). Clubroot has been found in a few Alberta vegetable fields, but it has not generated the same attention as it has in the canola industry because of its limited occurrence on vegetables and the existence of a relatively small cruciferous vegetable industry in the province. Furthermore, the occurrence of clubroot on vegetables was not novel, as P. brassicae has been reported on cruciferous vegetables in Canada for nearly a century and rare outbreaks of the disease have occurred in Alberta since the 1970s. Current research strategies on the Canadian Prairies focus mainly on the disease in canola and are discussed below. Nevertheless, information obtained from the canola system may, in many cases, be of great value to all Brassica-crop production systems.
Overview of new research initiatives on canola
Designing effective diagnostic tests
The ability to detect and accurately diagnose clubroot is important for effective management of this disease on canola and other host plants. Only after a crop or field is identified as clubroot-infested can proper cropping restrictions and containment strategies be imposed. Detection of P. brassicae, however, is hampered by the fact that this organism is an obligate parasite that cannot be cultured axenically. Historically, clubroot infestation was detected by means of a bioassay, in which highly susceptible bait plants were grown in the suspect soil (Faggian & Strelkov, Citation2009). While bioassays represent a reliable method to detect viable P. brassicae inoculum, they are time-consuming, since the bait plants must be grown in the soil for a period of five to six weeks to allow for gall development. Moreover, bioassays are labour-intensive and may require large amounts of greenhouse space. As a consequence, numerous other approaches have been developed to detect and diagnose clubroot in plant and soil samples.
Alternative diagnostic methods have included microscopic examination of the root hairs for signs of infection (MacFarlane, Citation1952) or staining of the P. brassicae resting spores with fluorochromes to distinguish them from soil particles (Takahashi & Yamaguchi, Citation1988, Citation1989). However, these techniques can also be time-consuming and must be conducted by highly trained personnel. Serological detection methods have also been employed (Lange et al., Citation1989; Wakeham & White, Citation1996), but these have been based on polyclonal antiserum which is of limited quantity and may vary with respect to specificity and sensitivity. The development of serological assays based on monoclonal antibodies, however, could represent a significant advance in clubroot detection since monoclonal antibodies exhibit improved specificity and are available in infinite quantities (Faggian & Strelkov, Citation2009). Currently, however, most diagnostic tests are based on the polymerase chain reaction (PCR). PCR-based tests are fast, sensitive, generally reliable and have been employed for the detection of other plant pathogens (McCartney et al., Citation2003).
Most PCR-based protocols for detection of P. brassicae have targeted the ribosomal genes (rDNA) and internal transcribed spacer (ITS) regions of the pathogen genome (Chee et al., Citation1998; Faggian et al., Citation1999; Wallenhammar & Arwidsson, Citation2001; Cao et al., Citation2007), although one test was based on amplification of an isopentyltransferase-like gene (Ito et al., Citation1999). In Alberta, several private diagnostic laboratories offer commercial versions of the PCR test developed by Cao et al. (Citation2007). Commercial testing services are heavily subscribed to by municipalities, as well as by the agricultural and oil and gas industries, which usually obtain independent verification of a clubroot-diagnosis through the PCR-based assays. Regardless of diagnostic method, confirmation of fields as clubroot-positive may be complicated by the often patchy distribution of this disease (Cao et al., Citation2007). Thus, the use of global positioning systems (Faggian & Strelkov, Citation2009) and strategic sampling based on likely clubroot distribution (Faggian et al., Citation2001) may be helpful in refining surveillance strategies.
Clubroot surveillance
Initial surveys for clubroot were focused on the Edmonton region as this is where the disease was first identified (Strelkov et al., Citation2005). However, as clubroot has become more prevalent, surveillance activities have expanded over a much larger geographical area. In 2008, surveys conducted by the University of Alberta, Alberta Agriculture and Rural Development, and local municipalities encompassed most of the canola and mustard-growing regions of Alberta. More than 5000 canola fields have been scouted for clubroot in this province since 2005 (Cao et al., Citationin press). Moreover, a clubroot-targeted survey of 30 fields was conducted in Saskatchewan in 2008, and a larger survey of canola fields in Saskatchewan and Manitoba is planned for 2009 (R. Kutcher, personal communication). In Alberta, surveillance activities are expected to continue over the next few years, particularly as clubroot-resistant canola cultivars will soon be introduced (Pioneer Hi-Bred, Citation2009). Hence, continued surveying will play in an important role in clubroot resistance stewardship by helping to monitor changes in the virulence of P. brassicae populations.
Pathotype determination
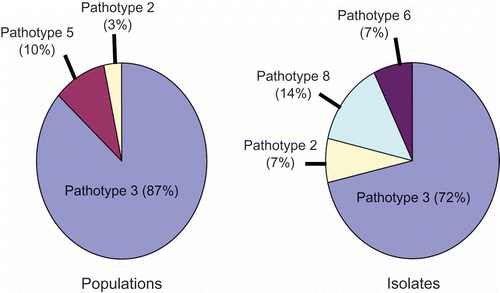
Physiological specialization has long been known in P. brassicae (Honig, Citation1931) and represents a challenge to the development of clubroot-resistant canola cultivars, since pathogen strains differ in their ability to infect specific host genotypes. As such, breeding efforts must be guided by an understanding of the pathogenic diversity of P. brassicae populations in the region(s) intended for cultivar deployment. There has been a strong effort to characterize the virulence of P. brassicae field populations (Strelkov et al., Citation2006, Citation2007; Cao et al., 2009) and single-spore isolates (Xue et al., Citation2008) from Canadian canola. This work has revealed that pathotype 3, P2 or ECD 16/15/12, as classified on the differentials of Williams (Citation1966), Somé et al. (Citation1996), and the European Clubroot Differential (ECD) set (Buczacki et al., Citation1975), respectively, is predominant in Alberta (Strelkov et al., Citation2006, Citation2007; Xue et al., Citation2008).
Other pathotypes of P. brassicae have also been detected, particularly when single-spore isolates rather than populations of the pathogen were examined (Strelkov et al., Citation2006; Xue et al., Citation2008). Therefore, while pathotype 3 represents 87% of the populations and 72% of the single-spore isolates characterized from Alberta thus far, pathotypes 2, 5, 6 and 8 have also been found () (Strelkov et al., Citation2006, Citation2007; Xue et al., Citation2008; Cao et al., Citationin press). This suggests that clubroot-resistant canola germplasm will have to be well-managed, since the pathotype composition of P. brassicae populations can shift rapidly in response to selection pressure (Seaman et al., Citation1963). It is also likely that the current differential sets do not reflect the full pathogenic diversity of P. brassicae on Canadian canola, since these differentials were developed to study pathogen populations from Europe or vegetable Brassicas. As such, the development of a differential system for the Canadian context may be of great value in resistance breeding and management efforts.
Fig. 2. Pathotype composition of Plasmodiophora brassicae field populations and single-spore isolates collected from canola in Alberta, as defined on the differential set of Williams (Citation1966).
Evaluation and modes of seed and soil transmission
Detailed surveying within P. brassicae-infested canola fields revealed that the frequency of clubroot infection was highest at the field entrances (90%) and dropped sharply at distances 150 m and 300 m from the entrance (Cao et al., Citationin press). This finding strongly suggests that the primary mechanism of clubroot spread between fields is via the movement of infested soil on farm or other machinery, which is consistent with the soilborne nature of P. brassicae. Nevertheless, the occasional identification of isolated cases of clubroot, or of fields in which diseased patches are far from the entrance or any known inoculum source, suggests the possibility of a secondary form of spread. Research is underway to determine whether this secondary spread involves transmission of P. brassicae resting spores as external contaminants on seeds, presumably in dust or soil tags. Testing of seedlots harvested from clubroot-infested fields using a PCR-based method (Cao et al., Citation2007) revealed the occurrence of P. brassicae DNA in numerous samples, indicating that pathogen resting spores were present (D. Rennie and S.E. Strelkov, unpublished data). The capacity of this inoculum to initiate new infections and to survive fungicidal seed treatments is still under investigation.
Soil-applied fungicides and amendments
Given the persistence of P. brassicae resting spores in the soil, clubroot will represent an ongoing challenge to canola production in infested areas. While a number of treatments are registered in Canada for clubroot-control on cruciferous vegetables, no such tools are currently available for canola. Moreover, traditional management strategies recommended for clubroot on vegetables, such as soil liming (Murakami et al., Citation2002), may be prohibitively expensive for canola given the lower value of this crop and the large size of the fields in which it is grown. In order to evaluate the efficacy of various soil-applied fungicides and amendments in reducing clubroot severity in canola, experiments were established at two naturally infested field locations in central Alberta (Hwang et al., Citation2008). Relative to non-treated controls, root symptom severity was significantly lower in plants grown in soils treated with Terraclor 75% WP (PCNB, pentachloronitrobenzene) resulting in reduced seedling mortality and increased plant cover and height. In less heavily infested soils, treatment with Ranman (cyazofamid) also had a positive effect on these parameters. Similarly, soil amendment with calcium carbonate at 5.0 or 7.5 t ha−1 or with wood ash at 7.5 t ha−1 resulted in increased plant cover and height, even under heavy disease pressure (Hwang et al., Citation2008). These results indicate that treatment with Terraclor 75% WP or high levels of calcium carbonate or wood ash may reduce the impact of clubroot on canola, but further work is required to optimize rates and application strategies. Additional research is aimed at integrating the use of soil fungicides and amendments with cultural control strategies such as crop rotation and early seeding (S.F. Hwang et al., unpublished data).
Biological control
Biological control of P. brassicae has revolved around two principal strategies: (1) the cropping of host or non-host bait crops that induce resting spore germination in the soil, and (2) the use of soil microorganisms as biofungicides. Although spontaneous germination of P. brassicae resting spores can occur in the absence of a host plant, germination appears to be stimulated in the presence of a host (MacFarlane, Citation1970; Takahashi, Citation1994) or in some cases, a non-host species (Friberg et al., Citation2005). Zoospores released from germinated resting spores do not survive long in the absence of a living host. Therefore, inclusion of a non-host bait crop, or of a host bait crop that is destroyed after resting spore germination but prior to completion of the pathogen life cycle, may help to decrease inoculum levels by reducing resting spore persistence in the soil (Friberg et al., Citation2006). Harling & Kennedy (Citation1991) evaluated the efficacy of oilseed rape as a bait crop, and found that a baiting period of four to five weeks reduced disease severity and increased yields in the subsequent calabrese (B. oleracea var. italica Plenck) crop. Non-host species that stimulate P. brassicae resting spore germination could also be desirable as bait crops, since these would have no risk of increasing inoculum levels if the resting spores are produced earlier than expected (Robak, Citation1996; Friberg et al., Citation2006). However, while evaluation of a number of non-hosts of P. brassicae revealed that they could stimulate resting spore germination and reduce clubroot severity in subsequent greenhouse bioassays, none of these species reduced the spore concentration in field experiments (Friberg et al., Citation2005, Citation2006). In Alberta, research aimed at evaluating the effectiveness of various host and non-host bait crops for reducing clubroot severity in canola is currently underway, and includes both field and greenhouse components (S.F. Hwang et al., unpublished data).
The biological control of P. brassicae with other soil microorganisms has also shown some promise. Narisawa et al. (Citation2000) found that the fungal endophyte Heteroconium chaetospira (Grove) M.B. Ellis reduced clubroot severity by 52–97% in field-grown Chinese cabbage transplants. Recent studies from western Canada have examined the efficacy of several microbial fungicides for the control of clubroot on canola and found that Serenade® (Bacillus subtilis (Ehrenberg 1835) Cohn 1872 QRD 137), Prestop® (Gliocladium catenulatum Gillman & Abbott J1446), and Mycostop® (Streptomyces griseoviridis F.H.-S. 1832 strain K61) reduced disease by 91%, 81% and 61%, respectively (Agriculture and Agri-Food Canada, Citation2009). These latter studies were conducted under greenhouse conditions and the results must still be validated in the field. Nevertheless, it appears that biological controls may have some utility in controlling clubroot on canola, most likely in combination with other management strategies. It remains to be seen, however, whether these would represent an economic alternative to chemical controls and host resistance.
Disinfectants for equipment, machinery and storage
A main strategy for controlling the spread of clubroot in Alberta has focused on the adoption of proper sanitation methods by growers and the agricultural and oil and gas industries (Alberta Clubroot Management Committee, Citation2008). Without proper sanitation, infested soil will continue to spread, resulting in new infestations and reduced effectiveness of other management practices. While chlorine has been shown to be useful for disinfesting water containing P. brassicae resting spores (Datnoff et al., Citation1987), this compound can be corrosive on equipment and is therefore not desirable for routine application. Research from Australia revealed that of nine commercial disinfectants evaluated, none completely eliminated clubroot (Donald et al., Citation2002). Nevertheless, given the intensive use of machinery in Canadian canola production, the identification of effective and practical disinfestation methods is highly desirable for clubroot containment. A research project is now underway to compare the relative effectiveness of selective physical (dry heat, hot water, steam, freezing, scraping, brushing and compressed air) and chemical (bleach, peroxide, quaternary ammonia, electrolyzed water, ozone, acetic acid, peracetic acid, potassium peroxomonosulphate, oxidized silver nitrate and industrial detergents) methods in removing soil and plant residues from equipment and other kinds of hard surfaces, and for killing resting spores of P. brassicae (R.J. Howard and S.E. Strelkov, unpublished data). The data generated will help to guide clubroot management recommendations in Alberta and western Canada, and should serve to slow the spread of the disease.
Modelling of disease distribution and risk
The spread of clubroot on canola in Alberta has caused concern regarding its possible impact on canola crops in Saskatchewan and Manitoba, if and when the disease reaches these two provinces. As such, preliminary forecasts of the potential distribution and severity of the disease in Canada have been developed using CLIMEX V2.0 software (Hearne Scientific Software Pty. Ltd., Melbourne, Australia). These models are based on temperature and moisture parameters obtained from previous reports, the occurrence of clubroot in epidemic areas, and an iterative process (Turkington et al., Citation2004). The resulting forecasts have predicted the occurrence of clubroot in regions where it is already a problem on cruciferous vegetables. They have also indicated the potential for losses in southeastern Manitoba and in central Alberta (Turkington et al., Citation2004). Adjustment of climatic data to account for above-average rainfall indicates that, in wetter years, clubroot could also be a problem over a wider region of the Prairies, including parts of Saskatchewan (T.K. Turkington, personal communication). Further refinement of the modelling parameters is ongoing and the risk maps produced may eventually serve as important tools in clubroot management.
Conclusions
Clubroot has had a long history of threatening cruciferous vegetable production in Europe and has steadily spread worldwide to become a disease of global importance. Its occurrence on vegetables in many parts of Canada and recent introduction into canola fields in Alberta have led to increased awareness and interest in this disease. The pathogen's ability to survive for long periods in the soil, reproduce on a wide variety of host plants under different ecoclimatic regimes, and its genetic diversity, pose significant challenges in successfully managing clubroot. Unprecedented efforts are now underway to determine the geographical distribution and impact of the disease on the Prairies and to investigate its biology and control in canola production systems. These investigations have already generated new information on the areas infested, yield impacts, methods of disease spread, prevalent pathotypes and the relative efficacy of selected control measures. In the coming years, it is expected that many more questions will be answered through research. The Canola Council of Canada launched a Clubroot Mitigation Initiative in 2009 that will provide leadership and focus to the efforts of scientists and other stakeholders as they attempt to combat the spread and minimize the economic impact of clubroot.
Notes
†Contribution to the symposium “Root Diseases; Challenges and Perspectives” held during the Canadian Phytopathological Society Annual Meeting, 22–25 June 2009, Winnipeg, MB.
References
- Abbasi , P.A. and Lazarovits , G. 2006 . Effect of soil application of AG3 phosphonate on the severity of clubroot of bok choy and cabbage caused by Plasmodiophora brassicae . Plant Dis. , 90 : 1517 – 1522 .
- Agriculture and Agri-Food Canada (2009). Biocontrol of clubroot on canola – a new initiative. AAFC No. 10876, Cat. No. A52-140/2009E-PDF. p. 2.
- Alberta Clubroot Management Committee (2008). Alberta clubroot management plan. AGDEX 140/638-2. Alberta Agriculture and Rural Development. 8 pp. Retrieved from http//www1.agric.gov.ab.ca/$Department/deptdocs.nsf/all/agdex11519 (Accessed: 4 May 2009 ).
- Ayers , G.W. 1957 . Races of Plasmodiophora brassicae . Can. J. Bot. , 35 : 923 – 932 .
- Ayers , G.W. 1972 . Races of Plasmodiophora brassicae infecting crucifer crops in Canada . Can. Plant Dis. Surv. , 62 : 77 – 81 .
- Bélec , C. , Tremblay , N. and Coulombe , J. 2004 . Liming and calcium cyanamide for clubroot control in cauliflower . Acta Hort. , 635 : 41 – 46 .
- British Columbia Ministry of Lands (2009). Pesticides – new registrations. Retrieved from http://www.al.gov.bc.ca/pesticides/j_3.htm
- Buczacki , S.T. and Ockendon , J.G. 1979 . Preliminary observations on variation in susceptibility to clubroot among collections of some wild crucifers . Ann. Appl. Biol. , 92 : 113 – 118 .
- Buczacki , S.T. , Toxopeus , H. , Mattusch , P. , Johnston , T.D. , Dixon , G.R. and Hobolth , L.A. 1975 . Study of physiologic specialization in Plasmodiophora brassicae: Proposals for attempted rationalization through an international approach . Trans. Br. Mycol. Soc. , 65 : 295 – 303 .
- Cao , T. , Manolii , V.P. , Hwang , S.F. , Howard , R.J. and Strelkov , S.E. in press . Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada . Can. J. Plant Pathol. ,
- Cao , T. , Tewari , J. and Strelkov , S.E. 2007 . Molecular detection of Plasmodiophora brassicae, causal agent of clubroot of crucifers, in plant and soil . Plant Dis. , 91 : 60 – 87 .
- Chee , H.Y. , Kim , W.G. , Cho , W.D. , Jee , H.J. and Choi , Y.C. 1998 . Detection of Plasmodiophora brassicae Woron . Kor. J. Plant Pathol. , 14 : 589 – 593 .
- Chiang , M.S. , Chiang , B.Y. and Grant , W.F. 1977 . Transfer of resistance to race 2 of Plasmodiophora brassicae from Brassica napus to cabbage (B. oleracea var. capitata). I. Interspecific hybridization between B. napus and B. oleracea var. capitata . Euphytica , 26 : 319 – 336 .
- Chiang , M.S. and Crête , R. 1970 . Inheritance of clubroot resistance in cabbage (Brassica oleracea L. var. capitata L.) . Genome , 12 : 253 – 256 .
- Chiang , M.S. and Crête , R. 1972 . Screening crucifers for germplasm resistance to clubroot Plasmodiophora brassicae . Can. Plant Dis. Surv. , 52 : 46 – 50 .
- Chiang , M.S. and Crête , R. 1985a . Male fertile and male sterile cabbage, broccoli, and cauliflower clubroot resistant breeding lines . HortScience , 20 : 457 – 458 .
- Chiang , M.S. and Crête , R. 1985b . Acadie: A clubroot resistant cabbage cultivar . Can. J. Plant Sci. , 65 : 233 – 235 .
- Chiang , M.S. and Crête , R. 1989 . Richelain: A clubroot resistant cabbage cultivar . Can. J. Plant Sci. , 69 : 337 – 340 .
- Chong , C. , Chiang , M.S. and Crête , R. 1985 . Studies on glucosinolates in clubroot resistant selections and susceptible commercial cultivars of cabbages . Euphytica , 34 : 65 – 73 .
- Conners , I.L. 1935 . Fifteenth annual report of the Canadian plant disease survey . Can. Plant Dis. Surv. , 15 : 24 – 44 .
- Conners , I.L. and Savile , D.B.O. 1944 . Twenty-fourth annual report of the Canadian plant disease survey . Can. Plant Dis. Surv. , 24 : i – v .
- Conners , I.L. , Shoemaker , R.A. and Creelman , D.W. 1956 . Thirty-sixth annual report of the Canadian plant disease survey . Can. Plant Dis. Surv. , 36 : ii – v .
- Creelman , D.W. 1958 . Thirty-eighth annual report of the Canadian plant disease survey . Can. Plant Dis. Surv. , 38 : 48 – 84 .
- Creelman , D.W. 1965 . Diseases of vegetable crops . Can. Plant Dis. Surv. , 45 : 52 – 63 .
- Creelman , D.W. 1967 . Summary of the prevalence of plant diseases in Canada in 1965. A Compilation . Can. Plant Dis. Surv. , 47 : 31 – 71 .
- Crête , R. 1981 . Worldwide importance of clubroot [Brassica] . Clubroot Newsl. , 11 : 6 – 7 .
- Crête , R. and Chiang , M.S. 1967 . Screening tests of crucifers for resistance to clubroot in organic soils of Quebec . Plant Dis. Rep. , 51 : 991 – 1002 .
- Crete , R. and Chiang , M.S. 1980 . Screening Brassicas for resistance to clubroot, Plasmodiophora brassicae Wor . Can. Plant Dis. Surv. , 60 : 17 – 19 .
- Crête , R. , Lalibertg , J. and Jasmin , J.J. 1963 . Lutte chimique contre la hernie, Plasmodiophora brassicae Wor., des crucifere: En sols minkal et organique . Can. J. Plant Sci. , 43 : 349 – 354 .
- Datnoff , L.E. , Kroll , T.K. and Lacy , G.H. 1987 . Efficacy of chlorine for decontaminating water infested with resting spores of Plasmodiophora brassicae . Plant Dis. , 71 : 734 – 736 .
- Dixon , G.R. 2009 . The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease . J. Plant Growth Regul. , 28 : 194 – 202 .
- Donald , E.C. , Lawrence , J.M. and Porter , I.J. 2002 . Evaluation of a fluorescent staining technique as an indicator of pathogenicity of resting spores of Plasmodiophora brassicae . Austral. Plant Pathol. , 31 : 373 – 379 .
- Estey , R.H. 1994 . Essays on the early history of plant pathology and mycology in Canada , Montreal, Québec and Kingston, Ontario : McGill–Queen's University Press .
- Faggian , R. , Bulman , S.R. , Lawrie , A.C. and Porter , I.J. 1999 . Specific polymerase reaction primers for the detection of Plasmodiophora brassicae in soil and water . Phytopathology , 89 : 392 – 397 .
- Faggian , R. , Parsons , S. , Porter , I.J. and Lawrie , A.C. 2001 . “ Detection of clubroot in Australia: Progress towards a reliable molecular diagnostic assay ” . In Proceedings of the 2nd Australasian Soilborne Diseases Symposium Edited by: Porter , I.J. Lorne, , Australia
- Faggian , R. and Strelkov , S.E. 2009 . Detection and measurement of Plasmodiophora brassicae . J. Plant Growth Regul. , 28 : 282 – 288 .
- Fayolle , L. , Nobel , R. , Coventry , E. , Aime , S. and Alabouvette , C. 2006 . Eradication of Plasmodiophora brassicae during composting of wastes . Plant Pathol. , 55 : 553 – 558 .
- Finlayson , D.G. and Campbell , C.J. 1969 . Insecticides, fungicides, and lime combined for control of cabbage maggots, clubroot, and wire stem . J. Entomol. Soc. Brit. Columbia , 66 : 14 – 18 .
- Finlayson , D.G. and Campbell , C.J. 1971 . Fungicides for preventing clubroot of cauliflower in loam and peat soils . Can. Plant Dis. Surv. , 61 : 122 – 126 .
- Friberg , H. , Lagerlöf , J. and Rämert , B. 2005 . Germination of Plasmodiophora brassicae resting spores stimulated by a non-host plant . Eur. J. Plant Pathol. , 113 : 275 – 281 .
- Friberg , H. , Lagerlöf , J. and Rämert , B. 2006 . Usefulness of nonhost plants in managing Plasmodiophora brassicae . Plant Pathol. , 55 : 690 – 695 .
- Harding , M.W. , Howard , R.J. , Manolii , V.P. , Manolii , A.V. , Strelkov , S.E. , Basu , K. and Spencer , R.C.J. 2008 . Incidence of clubroot on cruciferous vegetables in Alberta in 2007 . Can. Plant Dis. Surv. , 88 : 126 – 128 .
- Harding , M.W. , Howard , R.J. , Neeser , C. , Strelkov , S.E. , Tewari , J.P. , Lisowski , S.L.I. , Slomp , D.L. , Xue , S. and Spencer , R.C.J. 2005 . Incidence of clubroot on cruciferous vegetables in Alberta in 2004 . Can. Plant Dis. Surv. , 85 : 98 – 99 .
- Harding , M.W. , Howard , R.J. , Pugh , C.A. , Snider , M.D. , Daniels , G.C. , Strelkov , S.E. and Spencer , R.C.J. 2009 . Incidence of clubroot on cruciferous vegetables in Alberta in 2008 . Can. Plant Dis. Surv. , 89 : 136 – 139 .
- Harding , M.W. , Howard , R.J. , Xue , S. , Strelkov , S.E. , Chang , K.F. , Lisowski , S.L.I. , Pugh , S.L. and Spencer , R.C.J. 2006 . Incidence of clubroot on cruciferous vegetables in central Alberta in 2005 . Can. Plant Dis. Surv. , 86 : 116 – 117 .
- Harding , M.W. , Howard , R.J. , Xue , S. , Strelkov , S.E. , Chang , K.F. and Spencer , R.C.J. 2007 . Incidence of clubroot on cruciferous vegetables in central Alberta in 2006 . Can. Plant Dis. Surv. , 87 : 132 – 134 .
- Harling , R. and Kennedy , S.H. 1991 . Biological control of Plasmodiophora brassicae using a bait crop . Rijksuniversiteit Faculteit Landbouwwetenschappen Gent , 43 : 159 – 170 .
- Hildebrand , P.D. and McRae , K.D. 1998 . Control of clubroot caused by Plasmodiophora brassicae with nonionic surfactants . Can. J. Plant Pathol. , 20 : 1 – 11 .
- Honig , F. 1931 . Der Kohlkropferreger (Plasmodiophora brassicae Wor.) . Gartenbauwissenschaft , 5 : 116 – 225 .
- Howard , R.J. , Garland , J.A. and Seaman , W.L. 1994 . Diseases and pests of vegetable crops in Canada , The Canadian Phytopathological Society and the Entomological Society of Canada .
- Howat , S. 2005 . Crop profile for rutabaga in Canada , Ottawa, Ontario : Pest Management Centre, Agriculture and Agri-Food Canada .
- Hwang , S.F. , Strelkov , S.E. , Turnbull , G.D. , Manolii , V.P. , Howard , R.J. , Hartman , M. and Laflamme , P. 2008 . Soil treatments and amendments for management of clubroot on canola in Alberta . [Abstract]. J. Plant Pathol. , 90 S2.410
- Ito , S. , Maehara , T. , Maruno , E. , Tanaka , S. , Kameya-Iwaki , M. and Kishi , F. 1999 . Development of a PCR-based assay for the detection of Plasmodiophora brassicae in soil . J. Phytopathol. , 147 : 83 – 88 .
- Kageyama , K. and Asano , T. 2009 . Life cycle of Plasmodiophora brassicae . J. Plant Growth Regul. , doi: 10.1007/s00344-009-9101-z
- Lange , L. , Heide , M. , Hobolth , L. and Olson , L.W. 1989 . Serological detection of Plasmodiophora brassicae by dot immunobinding and visualization of the serological reaction by scanning electron microscopy . Phytopathology , 79 : 1066 – 1071 .
- MacFarlane , I. 1952 . Factors affecting the survival of Plasmodiophora brassicae Wor. in the soil and its assessment by a host test . Ann. Appl. Biol. , 39 : 239 – 256 .
- Macfarlane , I. 1970 . Germination of resting spores of Plasmodiophora brassicae . Trans. Br. Mycol. Soc. , 55 : 97 – 112 .
- Manitoba Agriculture, Food and Rural Initiatives (mafri) (2009). Clubroot of brassica crops. Retrieved from http://www.gov.mb.ca/agriculture/crops/diseases/fac63s00.html
- McCartney , H.A. , Foster , S.J. , Fraaije , B.A. and Ward , E. 2003 . Molecular diagnosis of fungal plant pathogens . Pest Management Sci. , 59 : 129 – 142 .
- McCurry , J.B. and Hicks , A.J. 1925 . Fifth annual report on the prevalence of plant diseases in the Dominion of Canada , Dominion of Canada Department of Agriculture .
- McDonald , M.R. , Kornatowska , B. and McKeown , A.W. 2004 . Management of clubroot of Asian Brassica crops grown in organic soils . Acta Hort. , 635 : 25 – 30 .
- Morasse , I. , Pageau , D. and Lafond , J. 1997 . Attention à la hernie des crucifères dans le canola . Grandes cultures , 7 ( 4 ) : 22 – 23 .
- Murakami , H. , Tsushima , S. , Kuroyanagi , Y. and Shishido , Y. 2002 . Reduction of resting spore density of Plasmodiophora brassicae and clubroot disease severity by liming . Soil Sci. Plant Nutr. , 48 : 685 – 691 .
- Narisawa , K. , Ohki , T. and Hashiba , T. 2000 . Suppression of clubroot and Verticillium yellows in Chinese cabbage in the field by the endophytic fungus, Heteroconium chaetospira . Plant Pathol. , 49 : 141 – 146 .
- Pageau , D. , Lajeunesse , J. and Lafond , J. 2006 . Impact de l'hernie des crucifères [Plasmodiophora brassicae] sur la productivité et la qualité du canola . Can. J. Plant Pathol. , 28 : 137 – 143 .
- Pioneer Hi-Bred (2009). Breakthrough genetic solution for clubroot registered. Retrieved from http://www.pioneer.com/web/site/portal/template.PRINT?print=true (Accessed: 4 May 2009 ).
- Rankin , W.H. and Fraser , W.P. 1920 . Survey of the prevalence of common plant diseases in the Dominion of Canada, 1920, First Annual Report , Ottawa, Ontario : Dominion of Canada Department of Agriculture .
- Reyes , A.A. , Davidson , R.T. and Marks , C.F. 1974 . Races, pathogenicity and chemical control of Plasmodiophora brassicae in Ontario . Phytopathology , 64 : 173 – 177 .
- Rimmer , S.R. , Kutcher , H.R. and Morrall , R.A.A. 2003 . “ Diseases of canola and mustard ” . In Diseases of field crops in Canada , Edited by: Bailey , K.L. , Gossen , B.D. , Gugel , R.K. and Morrall , R.A.A. 129 – 146 . Saskatoon, Saskatchewan : Canadian Phytopathological Society .
- Rimmer , S.R. , Shattuck , V.I. and Buchwaldt , L. 2007 . Compendium of Brassica diseases , St. Paul, MN : APS Press .
- Robak , J. 1996 . “ The effect of some crop rotations on decrease of clubroot, Plasmodiophora brassicae, in soils ” . In Brighton Crop Protection Conference: Pests and Diseases, 1996 , 647 – 651 . Farnham, , UK : British Crop Protection Council .
- Seaman , W.L. , Walker , J.C. and Larson , R.H. 1963 . A new race of Plasmodiophora brassicae affecting Badger Shipper cabbage . Phytopathology , 53 : 1426 – 1429 .
- Somé , A. , Manzanares , M.J. , Laurens , F. , Baron , F. , Thomas , G. and Rouxel , F. 1996 . Variation for virulence on Brassica napus L. amongst Plasmodiophora brassicae collections from France and derived single-spore isolates . Plant Pathol. , 45 : 432 – 439 .
- Strelkov , S.E. , Manolii , V.P. , Cao , T. , Xue , S. and Hwang , S.F. 2007 . Pathotype classification of Plasmodiophora brassicae and its occurrence in Brassica napus in Alberta, Canada . J. Phytopathol. , 105 : 706 – 712 .
- Strelkov , S.E. , Tewari , J.P. , Hartman , M. and Orchard , D. 2005 . Clubroot on canola in Alberta in 2003 and 2004 . Can. Plant Dis. Surv. , 85 : 72 – 73 .
- Strelkov , S.E. , Tewari , J.P. and Smith-Degenhardt , E. 2006 . Characterization of Plasmodiophora brassicae populations from Alberta, Canada . Can. J. Plant Pathol. , 28 : 467 – 474 .
- Statistics Canada . 2009a . Fruit and vegetable production – June 2009 . Catalogue 22-003-X , 78 ( 1 )
- Statistics Canada . 2009b . Field crop reporting series – September estimate of production of principal field crops . Catalogue 22-002-X , 88 ( 7 )
- Takahashi , K. 1994 . Influences of some environmental factors on the viability of resting spores of Plasmodiophora brassicae . Ann. Phytopathol. Soc. Japan , 60 : 658 – 666 .
- Takahashi , K. and Yamaguchi , T. 1988 . A method for assessing the pathogenic activity of resting spores of Plasmodiophora brassicae by fluorescence microscopy . Ann. Phytopathol. Soc. Japan , 54 : 466 – 475 .
- Takahashi , K. and Yamaguchi , T. 1989 . Assessment of pathogenicity of resting spores of Plasmodiophora brassicae in soil by fluorescence microscopy . Ann. Phytopathol. Soc. Japan , 55 : 621 – 628 .
- Tewari , J.P. , Strelkov , S.E. , Orchard , D. , Hartman , M. , Lange , R.M. and Turkington , T.K. 2005 . Identification of clubroot of crucifers on canola (Brassica napus) in Alberta . Can. J. Plant Pathol. , 27 : 143 – 144 .
- Toms , H.N.W. 1966 . Estimates of crop losses in the Lower Fraser Valley of British Columbia in 1965 . Can. Plant Dis. Surv. , 46 : 112 – 114 .
- Toms , H.N.W. 1968 . Estimates of crop losses in the Lower Fraser Valley of British Columbia, 1966 . Can. Plant Dis. Surv. , 48 : 28 – 31 .
- Tremblay , N. , Belec , C. , Coulombe , J. and Godin , C. 2005 . Evaluation of calcium cyanamide and liming for control of clubroot disease in cauliflower . Crop Protection , 24 : 798 – 803 .
- Tremblay , N. , Bélec , C. , Lawrence , H. and Carisse , O. 1999 . Clubroot of crucifers – control strategies , Saint-Jean-sur Richelieu, PQ : Agriculture and Agri-Food Canada .
- Turkington, T.K., Olfert, O.O., Weiss, R.M., Clear, R.M., Xi, K., Tewari, J.P., & Strelkov, S.E. (2004). Forecasting the potential distribution and abundance of plant diseases using CLIMEXTM modeling with historical and potential weather scenarios associated with climate change. In Manitoba Agron. Conf. 2004 Proceedings (pp. 99–110). Retrieved from http://www.umanitoba.ca/afs/agronomists_conf/proceedings/2004/turkington_forecasting_potential.pdf (Accessed: 4 May 2009 ).
- Vigier , B. , Chiang , M.S. and Hume , D.J. 1989 . Source of resistance to clubroot (Plasmodiophora brassicae Wor.) in triazine-resistant spring canola (rapeseed) . Can. Plant Dis. Surv. , 69 : 113 – 115 .
- Wakeham , A.J. and White , J.G. 1996 . Serological detection in soil of Plasmodiophora brassicae resting spores . Physiol. Mol. Plant Pathol. , 48 : 289 – 303 .
- Walker , G. 2005 . Crop profile for cabbage and broccoli in Canada , Ottawa, Ontario : Pest Management Centre, Agriculture and Agri-Food Canada .
- Wallenhammar , A.-C. 1996 . Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels . Plant Pathol. , 45 : 710 – 719 .
- Wallenhammar , A.-C. and Arwidsson , O. 2001 . Detection of Plasmodiophora brassicae by PCR in naturally infested soils . Eur. J. Plant Pathol. , 107 : 313 – 321 .
- Watson , A.G. and Baker , K.F. 1969 . Possible gene centers for resistance in the genus Brassica to Plasmodiophora brassicae. . Econ. Bot , 23 : 245 – 252 .
- Williams , P.H. 1966 . A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga . Phytopathology , 56 : 624 – 626 .
- Woronin , M.S. 1878 . “ Plasmodiophora brassicae, the cause of cabbage hernia ” . In Phytopathological Classic No. 4 , Edited by: Chupp , C. St. Paul, MN : American Phytopathological Society . 1934
- Xue , S. , Cao , T. , Howard , R.J. , Hwang , S.F. and Strelkov , S.E. 2008 . Isolation and variation in virulence of single-spore isolates of Plasmodiophora brassicae from Canada . Plant Dis. , 92 : 456 – 462 .