Abstract
In August 2006, a ware potato field of 19 ha, in the Saint-Amable region, Quebec, Canada showed patches of poor growth. Roots of infested plants showed golden spherical shaped cysts. Cysts and second stage juveniles were extracted from soil and root samples. The cyst nematodes were subsequently identified as Globodera rostochiensis Wollenweber, 1923 (Behrens, 1975) by morphological and molecular methods. The cysts had the general characteristics of Globodera genus with a Granek's ratio of 4.4 (1.8–6.0). The second stage juvenile stylets were 21 (18–23) μm long with rounded knobs, the dorsal one sloping posteriorly. Polymerase chain reaction of the nematodes with the species-specific primers yielded a fragment of about 315 bp, consistent with the fragment from a known G. rostochiensis. The phylogenetic analysis inferred by the sequence of the rDNA ITS region confirmed the identity of the cyst nematode from Quebec, Canada as G. rostochiensis with 100% match to the G. rostochiensis from Russia, Peru, Japan and UK.
Résumé
Au mois d'août 2006, un champ de pommes de terre de consommation de 19 ha de la région de Saint-Amable au Québec, Canada, affichait des portions où la croissance laissait à désirer. Les racines des plantes infestées étaient couvertes de kystes sphériques dorés. Des kystes et des jeunes de deuxième stade ont été extraits du sol et des échantillons de racines. À la suite d'analyses morphologiques et moléculaires, les kystes des nématodes ont été identifiés en tant que Globodera rostochiensis Wollenweber, 1923 (Behrens, 1975). Les kystes affichaient les caractéristiques du genre Globodera avec un ratio de Granek de 4,4 (1,8–6,0). Les stylets des jeunes de deuxième stade mesuraient 21 μm (18–23) de long et possédaient des tubercules arrondis, le tubercule dorsal étant incliné vers l'arrière. La réaction des nématodes aux amorces spécifiques d'espèces de la PCR a produit un fragment d'environ 315 bp, correspondant au fragment d'un G. rostochiensis connu. L'analyse phylogénétique déduite de la séquence de la région du rADN ITS a confirmé l'identité du nématode à kyste provenant du Québec en tant que G. rostochiensis, et la compatibilité entre celui-ci et les G. rostochiensis de Russie, du Pérou, du Japon et du Royaume-Uni était totale.
Introduction
Two species of the potato cyst nematode (PCN) which include the potato golden cyst nematode, Globodera rostochiensis (Wollenweber 1923) Behren 1975, and the potato pale nematode, G. pallida (Stones 1973) Behren 1975, are serious pests of potatoes worldwide and are quarantined internationally, subject to stringent regulatory measures wherever either one or both occur. Potato cyst nematode can be devastating pests of potatoes in temperate regions if not controlled (Baldwin & Mundo-Ocampo, Citation1991; Marks & Brodie, Citation1998).Footnote †
Globodera rostochiensis has been found in 75 countries with the most recent report from Iran (Gitty & Maafi, Citation2009). In North America, this species occurs in Mexico, USA and Canada (Brodie, Citation1998). In Canada, G. rostochiensis has previously been found in the Saanich Peninsula of Vancouver Island, British Columbia (Orchard, Citation1965), and both G. rostochiensis and G. pallida were found on the island of Newfoundland (Morris, Citation1971). Recently, another species of this genus G. tabaccum (Lownsbery & Lownsbery) was detected in tobacco in Quebec (Bélair & Miller, Citation2006).
In August 2006, potato cyst nematodes were discovered in a potato field in Saint-Amable, Quebec and were diagnosed as G. rostochiensis (Sun et al., Citation2007). After an extensive survey, the infested area was defined and strict regulatory measures were implemented to prevent the further spread of G. rostochiensis. National surveys of PCN and other comprehensive collaborative research projects on pathotypes, genetic populations and integrated management have been implemented by scientists from the Canadian Food Inspection Agency (CFIA), Agriculture and Agri-Food Canada (AAFC) and Le Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (MAPAQ).
Due to their huge economic and trade impacts, accurate and rapid identification of G. rostochiense and G. pallida is extremely important. Morphological identification based on a few characters of the second stage juvenile (J2) and of the perineal of the cyst have been quite successful but always with some uncertainty, since the morphometrics of these characters are overlapping. Molecular methods such as Polymerase Chain Reaction (PCR), Restriction Fragment Length Polymorphism (RFLP) and DNA sequence comparison have been successfully applied to differentiate cyst nematode species, especially G. rostochiensis and G. pallida. PCR was used for identifying PCN in Australia (Bulman & Marshall, Citation1997) and Ukraine (Pylypenko et al., Citation2005), and species-specific primers for PCR have been developed for identifying G. rostochinesis and G. pallida (Fullaondo et al., Citation1999). Amplified fragment length polymorphism (AFLP) was used for identifying G. tabacum (Marché et al., Citation2001), and DNA sequencing for G. pallida in Idaho, USA (Skantar et al., Citation2007).
The objective of this study was to characterize G. rostochiensis from Quebec, Canada using morphological and molecular methods.
Materials and methods
Nematode samples
Voucher specimens of G. rostochiensis and G. pallida previously collected from infested fields in Newfoundland and deposited in the Canadian National Collection of Nematodes in Ottawa were used as the reference materials for the molecular comparisons. Soil samples were collected from infested fields in Saint-Amabel, Quebec, Canada. The cysts were extracted from the soil samples using the Fenwick Can method (Fenwick, Citation1940). The second stage juveniles (J2) were hatched from the cysts with the presence of potato root exudates (Evans, Citation1983). Eight isolates of G. rostochiensis from Quebec and one isolate of G. pallida from Newfoundland were used for molecular studies.
Morphological characterization
Vulva cones of cysts were cut from cysts and mounted in Canada balsam (Hooper, Citation1970). Juveniles were fixed in TAF (formalin, triethanolamine and distilled water at a ratio of 7:2:92) and processed in glycerin. Specimens were examined using a Leica DM5500 B compound microscope with differential interference contrast and pictures were taken with a Leica DFC 420 digital camera. The observed characters of the cysts and J2 were compared with those reference materials and the description of the Neotypes in the literature (Golden & Ellington, Citation1972; Manduric et al., Citation2004). Measurements were made using a Lexica micro application system on the images, and dimensions are expressed in a formula proposed by de Man (Citation1880).
Nematode DNA
Freshly hatched juveniles were used for DNA extractions. Each juvenile was cut with a sharp forceps and placed into an Eppendorf tube with 20 μL of the prepared lysis buffer (10 mM TRIS pH 8.0, 1mM EDTA, 1% Tergitol-type NP-40 and 100 μg mL−1 proteinase K) and incubated at 55 °C for 1 h. Following the lysis reaction, proteinase K was inactivated by incubating the samples at 95 °C for 10 min. Samples were stored in a −20 °C freezer.
PCR by species-specific primers
Species identification for G. rostochiensis and G. pallida was performed by using the primers and methods developed by Fullaondo et al. (Citation1999). The primers for G. rostochiensis were: 5′-GCAAGCCAGCGTCAGCAA C-3′ and 5′-GAACATCAACCTCCTATCGG-3′. The primers for G. pallida were: 5′-TGTCCATTCCTCTCC ACCAG-3′ and 5′-CCGCTTCCCCATTGCTTTCG-3′.
PCR–RFLP
The rDNA ITS 1 region of the nematodes were PCR amplified using primers: rDNA1 (5′- TTGATTACGTCC CTGCCCTTT-3′) (Vrain et al., Citation1992) and 1.58S (5′-ACGAGCCGAGTCATCCACCG-3′) (Cherry et al., Citation1997). The PCR-amplified products were digested with restriction enzyme BstU I following the protocol provided by the supplier (Sigma, St. Louis, MO). A master mix was prepared for PCR: 1X Native Pfu Ultra Buffer (VWR, Mississauga, ON), 0.2 μM dNTP (Qiagen, Mississauga, ON), 0.6 μM each primer (Invitrogen, Burlington, ON), 0.05 U μL−1 native Pfu ultra high fidelity DNA polymerase (VWR), ultra pure distilled and DNase, RNase free water (Invitrogen). The total reaction volume was 100 μL. The PCR cycling conditions were as follow: 35 cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 2.5 min. The PCN product was digested with restriction enzyme BstU I and revealed by electrophoresis on a 1.5% agarose gel. Ultra pure distilled, water and lysis buffer were used as negative control.
PCR, cloning and DNA sequencing
The segment of rDNA comprised of partial 18S, ITS 1, 5.8S, ITS 2 and partial 28S of the nematode was amplified by PCR. The primers were rDNA1 (5′-TTGAT TACGTCCCTGCCCTTT -3′) and rDNA2 (5′-TTTCAC TCGCCGTTACTAAGG -3′), and the PCR conditions were as specified by Subbotin et al. (Citation2000). The amplified products on the gels were cut out, extracted, and purified using the GenElute Gel Extraction kit (Sigma-Aldrich, Oakville, ON), and cloned directly into the pST Blue-1 Blunt vector (Novagen, San Diego, CA) following the protocol provided by the supplies. Fragments were sequenced using Applied Biosystems 3730 DNA Analyzer by the Ottawa Health Research Institute, StemCore Laboratories, DNA Sequencing Facility (Ottawa, ON).
Phylogenetic analysis
DNA sequences were aligned by Clustal W (http://workbench.sdsc.edu, Bioinformatics and Computational Biology Group, Dept. Bioengineering, UC San Diego, CA). The model of base substitution was evaluated using MODELTEST (Posada & Crandall, Citation1998; Huelsenbeck & Ronquist, Citation2001). The Akaike-supported model, the base frequencies, the proportion of invariable sites, and the gamma distribution shape parameters and substitution rates were used in phylogenetic analyses. Bayesian analysis was performed to confirm the tree topology for each gene separately using MrBayes 3.1.0 (Huelsenbeck & Ronquist, Citation2001) running the chain for 1 × 106 generations and setting the “burnin” at 1000. The MCMC (Markov Chain Monte Carlo) method was used within a Bayesian framework to estimate the posterior probabilities of the phylogenetic trees (Larget & Simon, Citation1999) using 50% majority-rule.
Results
Morphology and morphometrics
The morphological characters of the cysts and J2 of the nematodes from Quebec populations matched the descriptions of those of the neotypes of G. rostochiensis. The morphometrics were in the range of those of the neotypes ().
Table 1. Morphometrics of Globodera rostochiensis associated with potato in Quebec, Canada compared with neotypes
Second stage juvenile
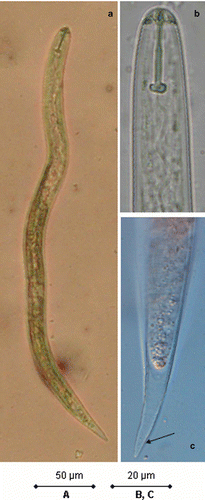
The body tapered off at both ends but much more posteriorly. There was lateral field with four lines for most of the body. The head was offset slightly with five annules. The stylet was well-developed with round knobs, the dorsal knob sloping posteriorly. The excretory pore was posterior and almost adjacent to the hemizonid. The tail tapered to a small, rounded terminus ().
Cysts
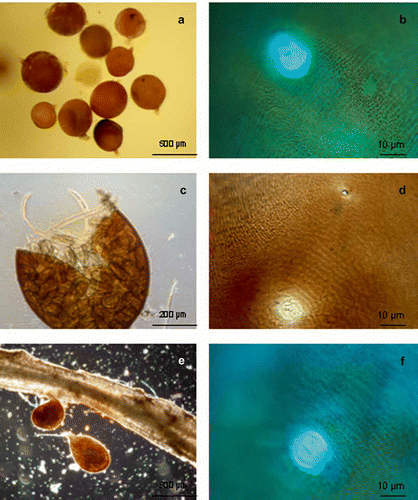
Cysts were brown in colour, spherical in shape, with protruding necks. Vulva were fenestrate, much larger than the small but distinct, V-shaped anus. The cyst wall pattern was prominent especially near the mid-body ().
PCR by species-specific primers
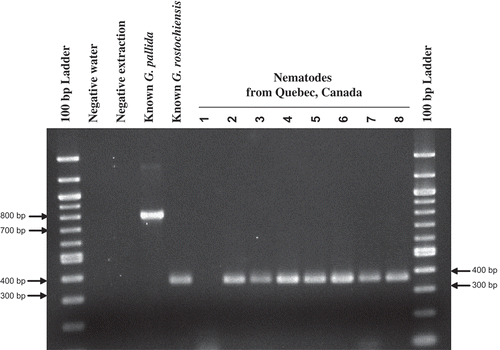
All the selected Quebec samples, except one, yielded a single PCR product of about 315 bp, the same size as the one from the known G. rostochiensis standard, and the G. pallida standard yielded a band at the size of about 800 bp ().
PCR–RFLP
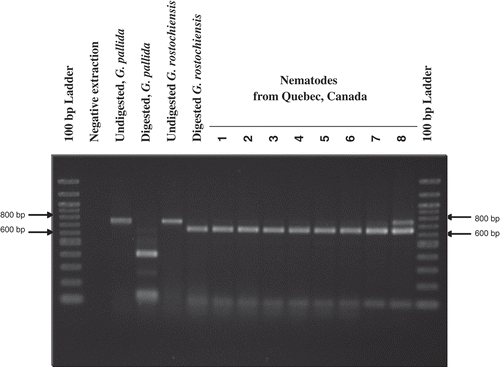
The profiles of the restriction digestion products of the PCR products with BstU I of the Quebec samples were consistent with G. rostochiensis. All of them had two bands (103, 650 bp), whereas the G. pallida had four bands (103, 116, 135, 399 bp) ().
DNA sequencing
Four cloned sequences from the Quebec isolate of G. rostochiensis were obtained. One overall consensus sequence was derived from these four cloned sequences, and was used for further phylogenetic analysis. The rDNA sequence of G. rostochiensis from Quebec and G. pallida from Newfoundland were deposited in Genbank with the accession members EU517119 and EU517120, respectively.
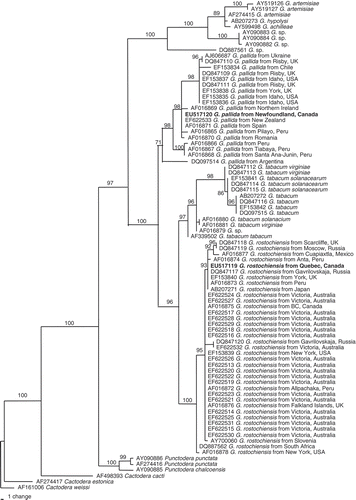
Phylogenetic analysis
presents a phylogenetic tree based on the rDNA ITS 1 from a multiple alignment of 612 total characters. This dataset has 455 constant characters (74.35%). The average nucleotide composition was as follows: 18.95% A, 25.57% C, 29.33% G and 26.14% T. Using Cactodera weissi as an out-group taxon, this tree inferred many highly supported monophyletic groups. All species of Globodera are in a clade with 97% support, clearly separated from all other genera. All 37 populations of G. rostochiensis from different regions of the world were in a 100% supported monophyletic clade, with G. tabacum as the closest sister clade. The sequence of the Quebec population was identical to those populations from Gasvarilovakaja, Russia (DQ847117); from York, UK (EF153840); from Peru (AF016873); and from Japan (AB207271). This population has only three nucleotide differences from those populations from BC, Canada (AF016875); from Peru (AF016872); from UK (AF016876); and Victoria, Australia (EF622513 – EF622530). The 19 populations of G. pallida from different regions of the world were in one clade, a sister clade to the clade comprised of G. tabaccum clade and G. rostochiensis clade.
Fig. 5. The 10001st Bayesian tree inferred from ITS1 rDNA sequence under GTR+G model (lnL = 2329.3086; freqA = 0.1895; freqC = 0.2557; freqG = 0.2933; freqT = 0.2614; R(a) = 0.6018; R(b) = 4.0381; R(c) = 1.6271; R(d) = 0.3558; R(e) = 2.5265; R(f) = 1; Pinva = 0; Shape = 0.4198). Posterior probability values exceeding 50% are given on appropriate clades.
Discussion
The results in this study clearly demonstrated that the cyst nematode associated with potato in Quebec, Canada is the golden cyst nematode, G. rostochiensis. The results also demonstrated that the species-specific primers for G. rostochiensis for PCR are consistent and reliable. The results revealed that the phylogenetic analysis based on rDNA ITS1 was highly useful for identifying species and examining their phylogenetic relationships from all species/populations around the world. This analysis resolved the key monophyletic groups, including Globodera, G. rostochiensis, G. pallida and G. tabaccum. The DNA sequencing is proved to be an accurate tool to identify species with highly similar morphology and morphometrics of the closely related species. The results were in agreement with the results achieved in similar studies by Skantar et al. (Citation2007) and Subbotin et al. (Citation2000).
The lower level of genetic variation of ITS rDNA of the isolates of G. rostochiensis over the genetic variation of ITS rDNA of the isolates of G. pallida revealed in this study is in agreement with the finding by Zaheer et al. (Citation1996). The higher genetic variation of G. pallida over G. rostochiensis may suggest the possibility that the former one is a more recently evolved species. Although the ITS sequence of the G. rostochiensis from Quebec was identical to those from Russia, Peru, Japan and UK, this does not necessarily mean that the nematode in Quebec originated from these locations.
Cyst-forming nematodes from Heteroderinae are some of the most important nematode pathogens worldwide (Baldwin & Mundo-Ocampo, Citation1991). Most species are restricted to cool climates. In Canada, most genera of the family are present, including species of Dolichodera, Globodera, Punctodera, Heterodera and Cactodera (Ebsary, Citation1986). In the genus Globodera, the three major species are recorded: G. rostochiensis, G. pallida and G. tabacum. As globalization expands, and climate change continues, more resources will be required to combat alien invasive species such as G. rostochiensis and G. pallida from threatening agriculture in Canada.
Acknowledgements
We thank Steven Wood for providing PCN cysts from Newfoundland. Appreciation also goes to Dr John Potter for his critical comments on the manuscript.
Notes
†AAFC Contribution number: #09–002.
References
- Baldwin , J.G. and Mundo-Ocampo , M. 1991 . “ Heteroderinae, cyst- and non-cyst-forming nematodes ” . In Manual of Agricultural Nematology , Edited by: Nickle , W.R. 275 – 362 . New York : Marcel Dekker Inc . InEd.pp.
- Bélair , G. and Miller , S. 2006 . First report of Globodera tabacum infesting tobacco plants in Quebec, Canada . Plant Dis. , 90 : 527
- Brodie , B.B. 1998 . “ Potato cyst nematodes (Globodera species) in Central and North America ” . In Potato Cyst Nematodes: Biology, Distribution and Control , Edited by: Marks , R.J. and Brodie , B.B. 317 – 332 . Wallingford : CAB International . InEds.pp.
- Bulman , S.R. and Marshall , J.W. 1997 . Differentiation of Australasian potato cyst nematode (PCN) populations using the polymerase chain reaction (PCR) . N. Z. J. Crop Hort. Sci. , 25 : 123 – 129 .
- Cherry , T. , Szalanski , A.L. , Todd , T.C. and Powers , T.O. 1997 . The internal transcribed spacer region of Belonolaimus (Nemata: Belonolaimidae) . J. Nematol. , 29 : 23 – 29 .
- De Man , J.G. 1880 . Die Einheimischen, frei in der reinen Erde und im sussen Wasser lebenden Nematoden. Vorlaufiger Bericht und descriptiv-systematischer Theil . Tijdschr. ned. dierk. Vereen , 5 : 1 – 104 .
- Ebsary , B.A. 1986 . Species and distribution of Heteroderidae and Meloidogynidae (Nematoda: Tylenchida) in Canada . Can. J. Plant Pathol. , 8 : 170 – 184 .
- Evans , K. 1983 . Hatching of potato cyst nematodes in root diffusates collected from 25 potato cultivars . Crop Prot. , 2 : 97 – 103 .
- Fenwick , D.W. 1940 . Methods for the recovery and counting of cysts of Heterodera schachtii from soil . J. Helminth. , 21 : 37 – 41 .
- Fullaondo , A. , Barrena , E. , Viribay , M. , Barrena , I. , Salazar , A. and Ritter , E. 1999 . Identification of potato cyst nematode species Globodera rostochiensis and G. pallida by PCR using specific primer combinations . Nematology , 1 : 157 – 163 .
- Gitty, M., & Maafi, Z.T. (2009). First report of a potato cyst nematode, Globodera rostochiensis, on potato, in Iran. New Dis. Rep., 19. http://www.bspp.org.uk/publications/new-disease-reports/ndr.php?id=019038
- Golden , A.M. and Ellington , D.M.S. 1972 . Redescription of Heterodera rostochiensis (Nematoda: Heteroderidae) with a key and notes on closely related species . Proc. Helminth. Soc. Wash. , 39 : 64 – 78 .
- Hooper , D.J. 1970 . “ Handling, fixing, staining and mounting nematodes ” . In Laboratory Methods for Work with Plant and Soil Nematodes , 5th , Edited by: Southey , J.F. 59 – 80 . London : HMSO .
- Huelsenbeck , J.P. and Ronquist , F. 2001 . MR BAYES: Bayesian inference of phylogenetic trees . Bioinformatics , 17 : 1754 – 1755 .
- Larget , B. and Simon , D. 1999 . Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees . Mol. Biol. Evol. , 16 : 750 – 759 .
- Manduric , S. , Olsson , E. , Englund , J.E. and Andersson , S. 2004 . Separation of Globodera rostochiensis and G. pallida (Tylenchida: Heteroderidae) using morphology and morphometrics . Nematology , 6 : 171 – 182 .
- Marché , L. , Valette , S. , Grenier , E. and Mugniéry , D. 2001 . Interspecies DNA polymorphism in the tobacco cyst–nematode complex (Globodera tabacum) using AFLP . Genome , 44 : 941 – 946 .
- Marks , R.J. and Brodie , B.B. , eds. 1998 . Potato Cyst Nematodes: Biology, Distribution and Control , Wallingford : CAB International .
- Morris , R.F. 1971 . Distribution and biology of the golden nematode, Heterodera rostochiensis in Newfoundland . Nematologica , 17 : 370 – 376 .
- Orchard , W.R. 1965 . Occurrence of the golden nematodes on Vancouver Island, British Columbia . Can. Plant Dis. Surv. , 45 : 89
- Posada , D. and Crandall , K.A. 1998 . MODELTEST: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Pylypenko , L.A. , Uehara , T. , Phillips , M.S. , Sigareva , D.D. and Blok , V.C. 2005 . Identification of Globodera rostochiensis and G. pallida in the Ukraine by PCR . Eur. J. Plant Pathol. , 111 : 39 – 46 .
- Skantar , A.M. , Handoo , Z.A , Carta , L. K. and Chitwood , D.J. 2007 . Morphological and molecular identification of Globodera pallida associated with potato in Idaho . J. Nematol. , 39 : 133 – 144 .
- Subbotin , S. , Halford , P.D. , Warry , A. and Perry , R. 2000 . Variations in ribosomal DNA sequences and phylogeny of Globodera parasitising solanaceous plants . Nematology , 2 : 591 – 604 .
- Sun , F. , Miller , S. , Wood , S. and Cote , M.J. 2007 . Occurrence of golden cyst nematode Globodera rostochiensis in Saint Amable region, Quebec, Canada . Plant Dis. , 91 : 908
- Vrain , T.C. , Wakarchuk , D.A. , Lévesque , C.A. and Hamilton , R.I. 1992 . Intraspecific rDNA restriction fragments length polymorphisms in the Xiphinema americanum group . Fund. Appl. Nematol. , 15 : 563 – 573 .
- Zaheer , K.C. , Fleming , C. and Turner , S.J. 1996 . Genetic variation in potato cyst nematodes in Northern Ireland . Biochem. Syst. Ecol. , 24 : 509 – 519 .