Abstract
In 2002, poison centers in the US reported 5816 human exposures to ethylene glycol. A guideline that effectively determines the threshold dose for emergency department referral and need for pre-hospital decontamination could potentially avoid unnecessary emergency department visits, reduce health care costs, optimize patient outcome, and reduce life disruption for patients and caregivers. An evidence-based expert consensus process was used to create guideline. Relevant articles were abstracted by a trained physician researcher. The first draft of the guideline was created by the primary author. The entire panel discussed and refined the guideline before distribution to secondary reviewers for comment. The panel then made changes based on the secondary review comments. The objective of this guideline is to assist poison center personnel in the out-of-hospital triage and initial management of patients with a suspected exposure to ethylene glycol by 1) describing the process by which the exposure might be evaluated, 2) identifying the key decision elements in managing the case, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline is based on an assessment of current scientific and clinical information. The panel recognizes that specific patient care decisions may be at variance with this guideline and are the prerogative of the patient and health professionals providing care, considering all of the circumstances involved. Recommendations are in chronological order of likely clinical use. The grade of recommendation is in parentheses. 1) A patient with exposure due to suspected self-harm, misuse, or potentially malicious administration should be referred to an emergency department immediately regardless of the dose reported (Grade D). 2) Patients with inhalation exposures will not develop systemic toxicity and can be managed out-of-hospital if asymptomatic (Grade B). Patients with clinically significant mucous membrane irritation should be referred for evaluation (Grade D). 3) Decontamination of dermal exposures should include routine cleansing with mild soap and water. Removal of contact lenses and immediate irrigation with room temperature tap water is recommended for ocular exposures. All patients with symptoms of eye injury should be referred for an ophthalmologic exam (Grade D). 4) Patients with symptoms of ethylene glycol poisoning should be referred immediately for evaluation regardless of the reported dose (Grade C). 5) The absence of symptoms shortly after ingestion does not exclude a potentially toxic dose and should not be used as a triage criterion (Grade C). 6) Adults who ingest a “swallow” (10–30 mL), children who ingest more than a witnessed taste or lick, or if the amount is unknown of most ethylene glycol products should be referred immediately for evaluation. The potential toxic volume of dilute solutions (e.g., concentration < 20%) is larger and can be estimated by a formula in the text (Grade C). 7) A witnessed taste or lick only by a child, or an adult who unintentionally drinks and then expectorates the product without swallowing, does not need referral (Grade C). 8) Referral is not needed if it has been > 24 hours since a potentially toxic unintentional exposure, the patient has been asymptomatic, and no alcohol was co-ingested (Grade D). 9) Gastrointestinal decontamination with ipecac syrup, gastric lavage or activated charcoal is not recommended. Transportation to an emergency department should not be delayed for any decontamination procedures (Grade D). 10) Patients meeting referral criteria should be evaluated at a hospital emergency department rather than a clinic. A facility that can quickly obtain an ethylene glycol serum concentration and has alcohol or fomepizole therapy available is preferred. This referral should be guided by local poison center procedures and community resources (Grade D). 11) The administration of alcohol, fomepizole, thiamine, or pyridoxine is not recommended in the out-of-hospital setting (Grade D).
Introduction
Scope of the Problem
Ethylene glycol has been utilized in a variety of industrial and home settings since the 1930s. Today it is used primarily as antifreeze for vehicle radiators and some water pipe systems. It is not present in all radiator antifreeze products, however, as some contain propylene glycol as the active ingredient. It is readily available to people of all ages in the home and workplace. Human poisoning has occurred in isolated cases and in epidemics. In 1987, 29 patients had symptoms of toxicity after drinking a beverage unintentionally contaminated with ethylene glycol at a picnic Citation1. A summertime cluster of 22 intentional ingestions occurred in Illinois in 1996 that might have been related to descriptions in the mass media of an index case Citation2.
In 2002, poison centers in the United States reported 5816 human exposures to ethylene glycol. The routes of exposure included ingestion (54%), dermal (19%), inhalation (15%), and ocular (11%). Of these exposures, 4767 (82%) were unintentional and 676 (12%) involved children under 6 years of age. The most common sites of exposure were the home (75%) and workplace (10%). Chronic dermal or inhalation exposures were uncommon; 94% of reported exposures were acute in nature Citation3. Acute unintentional ingestions are associated with less risk of toxicity than intentional ingestions. An analysis of the American Association of Poison Control Centers' Toxic Exposure Surveillance System (TESS) database for ingestion of ethylene glycol by all ages revealed that unintentional ingestions were 37 times more likely than intentional ingestions to have no effect or minor effects (). Nevertheless, unintentional ingestions are still potentially dangerous because 6.3% (129/2041) of them were associated with moderate or severe clinical toxicity Citation3.
Table 1. Known outcome by reason for ingestion of ethylene glycol for all ages 2000–2002
In 2003, 2241 patients were evaluated in healthcare facilities and 23 died according to TESS. Ethylene glycol was the most common chemical agent responsible for deaths reported by US poison centers in 2003 Citation4. These deaths were exclusively in adolescents and adults. No deaths of patients under the age of 12 years or from unintentional exposure were reported by poison centers to TESS from 1985 through 2002 (WA Watson, PharmD, Associate Director, AAPCC, written communication, May 2004).
Substances and Definitions
This guideline is intended to address exposures to ethylene glycol only. Guidance for exposures to other glycols, such as propylene glycol and diethylene glycol, or glycol ethers is not included in this document. The term “out-of-hospital” is defined as the period before a patient reaches a health care facility. An acute ingestion is defined as any number of ingestions that occur within a period of no more than 8 hours. A child is defined as a person less than 6 years of age.
Current Poison Center Practice and Importance of the Guideline
The triage and management of unintentional ethylene glycol ingestion by US poison centers appears to be variable. The guideline consensus panel solicited local referral and management guidelines for ethylene glycol from 64 US poison centers in 2003 and received 13 documents (). One state poison center system (four poison centers) and 12 other individual poison centers submitted guidelines. Eleven centers specifically replied that they did not have guidelines for ethylene glycol. The remaining 40 centers did not respond to the request. Review of the submitted guidelines revealed that there were referral thresholds for ingestion of “any amount,” “a mouthful,” an amount that exceeded a hypothetical calculated serum concentration, and various weight-based formulas (0.05–2 mL/kg of ethylene glycol). Out-of-hospital decontamination was not addressed by most of the guidelines but ipecac-induced emesis was recommended by two guidelines in certain situations. This wide range of approaches suggests the need for a clear, evidence-based guideline. A guideline that effectively determines the threshold dose for emergency department referral and need for pre-hospital decontamination could potentially avoid unnecessary emergency department visits, reduce health care costs, optimize patient outcome, and reduce life disruption for patients and caregivers. In addition, a more consistent approach to this exposure might facilitate research.
Table 2. Summary of US Poison Control Center guidelines 2003
Sources and Physical Properties of Ethylene Glycol
Ethylene glycol (C2H6O2; 1,2-ethanediol; CAS Reg. No. 107-21-1) is a polyhydric alcohol solvent. It is a clear, sweet-tasting, viscous and odorless liquid. Its physical properties include: specific gravity 1.12 g/mL, boiling point 198°C (388°F), vapor pressure 0.092 mmHg at 25°C and evaporation rate less than 0.01 (relative to butyl acetate = 1). Many commercial antifreeze products contain a yellow-green fluorescent dye (sodium fluorescein) to allow for detection of coolant system leaks.
Ethylene glycol is produced commercially in large amounts and is widely used as antifreeze (concentration range, 80–99%) or deicing solutions (concentration range, 3–40%) for cars, boats, and aircraft. It is also used in the chemical synthesis of plastics, films, and solvents. It can be found in many consumer products including solvents, paints, and coolants (concentration range, 20–95%).
Pharmacokinetics and Pathophysiology of Ethylene Glycol
Ethylene glycol has an oral bioavailability in rodents of 92–100% Citation5. It is rapidly absorbed from the gastrointestinal tract in humans as symptoms of intoxication and elevated serum concentrations have occurred 20–30 minutes after ingestion Citation6&7. It has a relatively low volume of distribution (0.5–0.8 L/kg). Twenty percent of the parent compound is eliminated unchanged in the urine. The other 80% is metabolized in the liver by alcohol dehydrogenase to glycoaldehyde and then by aldehyde dehydrogenase to several acid metabolites (glycolic acid, glyoxylic acid, oxalic acid). These metabolites are responsible for the anion gap metabolic acidosis, calcium oxalate crystals, and renal injury that occur with ethylene glycol poisoning Citation8-13. The glycolic acid metabolite is the only metabolite that appreciably accumulates in the blood and it appears to be primarily responsible for the metabolic acidosis Citation11-14. Glycolic acid is converted to glyoxylic acid, which is toxic to renal tubular cells Citation15. Glyoxylic acid is metabolized to oxalic acid, which can combine with calcium to form calcium oxalate crystals and cause subsequent hypocalcemia Citation6Citation16-18. Thiamine and pyridoxine facilitate the conversion of glyoxylic acid to α-hydroxy-β-ketoadipic acid, glycine, and hippuric acid, which are considered less toxic than oxalate. The elimination half-life of the parent compound is 3–8.5 hours Citation11Citation19&20. Fomepizole (4-methylpyrazole) is an inhibitor and alcohol a preferred substrate of alcohol dehydrogenase. They will increase the first-order elimination half-life of ethylene glycol to approximately 17 hours in patients with normal renal function Citation19&20.
The clinical manifestations of ethylene glycol poisoning range from being initially asymptomatic to seizures, coma, renal failure, and cardiovascular collapse Citation6 Citation18 Citation21. The etiology of the central nervous system, metabolic, cardiopulmonary, and renal toxicities are primarily due to the formation and accumulation of toxic intermediary metabolites, especially aldehyde metabolites Citation9, glycolic acid, and to a lesser extent oxalate production and excretion Citation8 Citation22. Unchanged ethylene glycol is thought to be responsible for the initial signs and symptoms of intoxication, which include altered mental status, slurred speech, and ataxia Citation23. Gastrointestinal symptoms such as nausea, vomiting, and abdominal pain are often present soon after ingestion Citation21. Infants can demonstrate irritability, lethargy, vomiting, pallor, hypotonia, and poor feeding Citation24&25. A patient who presents with coma, hyperkalemia, seizures, and severe acidosis has a poor prognosis.
Considerations for Estimating a Potentially Toxic Ingestion
Determining a Potentially Toxic Serum Concentration as a Basis for Referral
Determining the minimum potentially toxic ethylene glycol serum concentration could help guide poison centers in determining a potentially toxic dose. A peak serum concentration of 20 mg/dL or greater has been cited as potentially toxic and treatment has been recommended at this threshold Citation7 Citation18. The source of this toxic threshold concentration is not clear, as human studies have not specifically addressed this parameter. However, based on the few available case reports (level 4) of patients presenting early with serum concentrations less than this, it seems reasonable. One case report described a 2 1/2-year-old girl who presented 3 hours after ingestion of an unknown amount of antifreeze. Her ethylene glycol serum concentration was 9.3 mg/dL. She developed metabolic acidosis (serum bicarbonate 12.5 mEq/L, anion gap 17) and elevated urine oxalate and glycolate concentrations but no renal injury Citation26. Two adults presented 1.5 hours after ingestion with ethylene glycol concentrations of 13 and 9 mg/dL. These concentrations are well below the traditional toxic serum concentration. The patients developed mild anion gap acidosis (bicarbonate 19 mEq/L, anion gap 18) and no metabolic abnormalities, respectively Citation13. An adult presented 4 hours after ingestion with a serum concentration of 8 mg/dL, mild metabolic acidosis (bicarbonate 17 mEq/L), and no renal failure but with a serum alcohol of 27 mg/dL that might have affected the outcome. Another adult presented 10 hours after ingestion with an ethylene glycol concentration 6 mg/dL, arterial pH 7.35, bicarbonate 16 mEq/L, and elevated serum glycolate (5.1 mmol/L) and no renal failure Citation13. Thus, an estimated peak ethylene glycol serum concentration of less than 20 mg/dL appears relatively safe based on limited case reports. Patients who develop clinically important toxicity such as altered mental status, renal injury, seizures, hypotension, or respiratory depression routinely present either late (e.g., greater than 4–6 hours after ingestion) or early with serum concentrations greater than 20 mg/dL Citation12&13Citation27&28. Data could not be found on the outcome of untreated patients with serum concentrations greater than 20 mg/dL. Whether the threshold peak concentration for potential toxicity is greater than 20 mg/dL is not known. This toxic concentration estimate does not account for possible risk factors such as pre-existing renal failure. Peak ethylene glycol serum concentrations occur early after ingestion (i.e., 30–60 minutes) and this must be taken into account when obtaining serum concentrations in patients with delayed presentation.
Volume of a Swallow for Dose Estimation
Ethylene glycol is a liquid; therefore, the fixed dosage units associated with tablets or capsules do not exist, making dose estimates more difficult and unreliable. The liquid volume in an unmarked container before ingestion is often unknown. The estimated dose is often based on whether more or less than a “swallow” or “mouthful” was ingested. The volume of a swallow of water has been investigated in children, adolescents, and adults Citation29-33. The estimated average volume of a swallow in a child 18–66 months of age is 5–10 mL with a range of 1–29 mL. The average swallow volume in children aged 6–9 years is also approximately 10 mL and increases to 20 mL in 11- to 13-year-old boys. The volume of a swallow in children less than 18 months of age has not been studied. Older males (age greater than 12 years) demonstrate larger swallow volumes compared to females of similar age Citation30. Adolescent males can consume up to 40 mL in a single swallow Citation30. The average volume of a swallow in an adult has been estimated as 25.6 mL Citation32. Along with the concentration of the ethylene glycol product, knowledge of the potential volume of a swallow aids in estimating the dose of ethylene glycol ingested and potential serum concentration as demonstrated in the next section.
Estimating the Peak Ethylene Glycol Serum Concentration from Dose
Based on standard pharmacokinetic principles and assuming complete absorption (F = 1), a hypothetical peak ethylene glycol serum concentration can be conservatively estimated if the volume ingested, ethylene glycol concentration, and patient weight are known. Although not validated in human studies of ethylene glycol ingestion, a common formula Citation34 used is:
Formula 1: Estimated peak ethylene glycol serum concentration (mg/dL) = [volume ingested (mL) × concentration of ethylene glycol (%) × specific gravity (g/mL)]/[volume of distribution (L/kg) × patient weight (kg)]. The Vd is 0.6 L/kg and specific gravity is 1.12 g/mL for ethylene glycol. Use a whole number, not a fraction for product concentration (e.g., 95 not 0.95).
An estimate of the potential peak serum concentration resulting from ingestion can be made using the above formula as in the following examples for a child and an adult:
A 2-year-old, 10-kg child who ingests “a small swallow” (estimated volume 5 mL) of 95% ethylene glycol solution could potentially attain a serum concentration of (5 mL × 95 × 1.12 g/mL)/(0.6 L/kg × 10 kg) or 88 mg/dL. The same volume of a 25% solution could result in a serum concentration of 23 mg/dL.
A 70-kg man who ingests “one swallow” (estimated volume 25 mL) of 95% ethylene glycol solution could potentially attain a serum concentration of (25 mL × 95 × 1.12 g/mL)/(0.6 L/kg × 70 kg) or 63 mg/dL. The same volume of a 25% solution would result in a serum concentration of 17 mg/dL.
It appears that the pharmacokinetics of ethylene glycol and the average swallow volumes of children and adults support the likelihood of “one swallow” and possibly less to produce a potentially toxic serum concentration of 20 mg/dL or greater over a range of product concentrations (e.g., 25–100%).
By rearranging formula 1 and using a potential toxic serum concentration of 20 mg/dL, a toxic dose in mL per kg can be estimated with varying product concentrations:
Formula 2: Potential toxic dose (mL/kg) = (0.6 L/kg × 20 mg/dL)/[product concentration (%) × 1.12] = 10.7/product concentration (%).
Using formula 2, the minimum toxic oral dose of a 99% ethylene glycol solution should be approximately 0.1 mL/kg. If the product is known to be very dilute, such as a 3% deicing solution, then the potential toxic dose would be greater, approximately 3.5 mL/kg. These calculations rely on the accuracy of ingestion history, a single exposure, no complicating co-ingestants (e.g., alcohol), complete absorption, and no elimination or hepatic metabolism. The formula is based on several pharmacokinetic assumptions but can provide a framework for risk estimation until more valid human data are available. Over-reliance on the calculation to the exclusion of other historical or clinical data may lead to erroneous decision-making.
Intended Users of This Guideline
The intended users of this guideline are personnel in US poison centers. This guideline has been developed for the conditions prevalent in the United States. While the toxicity of ethylene glycol is not expected to vary in a clinically significant manner in other nations, the out-of-hospital conditions could be much different. This guideline should not be extrapolated to other settings unless it has been determined that the conditions assumed in this guideline are present.
Objective of This Guideline
The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial management of patients with a suspected exposure to ethylene glycol by 1) describing the process by which an exposure to ethylene glycol might be evaluated, 2) identifying the key decision elements in managing cases of ethylene glycol exposure, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline applies to exposure to ethylene glycol alone. Exposure to additional substances could require different referral and management recommendations depending on the combined toxicities of the substances.
This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions may be at variance with this guideline and are the prerogative of the patient and health professionals providing care, considering all of the circumstances involved.
Methodology
The methodology used for the preparation of this guideline was developed after reviewing the list of key elements of guidelines described by Shaneyfelt et al. Citation35. An expert consensus panel was established to oversee the guideline development process (Appendix 1). The American Association of Poison Control Centers (AAPCC), the American Academy of Clinical Toxicology (AACT), and the American College of Medical Toxicology (ACMT) appointed members of their organizations to serve as panel members. To serve on the expert consensus panel, an individual had to have an exceptional track record in clinical care and scientific research in toxicology, board certification as a clinical or medical toxicologist, significant US poison center experience, and be an opinion leader with broad esteem. Two specialists in poison information were included as full panel members to provide the viewpoint of potential end-users of the guideline.
Literature Search
The National Library of Medicine's MEDLINE database was searched (1966–September 2003) using ethylene glycol as a MeSH term with the subheadings poisoning (po) or toxicity (to), limited to humans. The MEDLINE and PreMEDLINE (1966–September 2003) were searched using ethylene glycol as textwords (title, abstract, MeSH term, CAS registry) plus poison** or overdos** or tox**, limited to humans. This same process was repeated in International Pharmaceutical Abstracts (1970–September 2003, excluding abstracts of meeting presentations), Science Citation Index (1977–September 2003), Database of Abstracts of Reviews of Effects (accessed September 2003), Cochrane Database of Systematic Reviews (accessed September 2003), and Cochrane Central Register of Controlled Trials (accessed September 2003). Reactions (1980–September 2003), the ethylene glycol poisoning management in POISINDEX Citation36, and the bibliographies of recovered articles were reviewed to identify previously undiscovered articles. Furthermore, North American Congress of Clinical Toxicology abstracts published in the Journal of Toxicology-Clinical Toxicology (1995–2003) were reviewed for original human data. The chapter bibliographies in four major toxicology textbooks Citation37-40 were reviewed for citations of additional articles with original human data. Finally, The Toxic Exposure Surveillance System (TESS) maintained by the American Association of Poison Control Centers, was searched for deaths resulting from unintentional ethylene glycol poisoning or any deaths from ethylene glycol poisoning in children. These cases were abstracted for use by the panel.
Article Selection
The recovered citations were entered into an EndNote library and duplicate entries were eliminated. The abstracts of these articles were reviewed, looking specifically for those that dealt with estimations of exposure doses with or without subsequent signs or symptoms, time of onset of symptoms, and management techniques that might be suitable for out-of-hospital use (e.g., gastrointestinal decontamination). Articles were excluded that didn't meet the preceding criteria, didn't add new data (e.g., some reviews, editorials), or that exclusively described inpatient-only procedures (e.g., dialysis).
Data Extraction
All articles retrieved by the search were reviewed by a single abstractor. Each article was assigned a level of evidence score from 1 to 6 using the rating scheme developed by the Centre for Evidence-based Medicine at Oxford University (Appendix 2); the complete paper was reviewed for original human data regarding the toxic effects of ethylene glycol or original human data directly relevant to the out-of-hospital management of patients with ethylene glycol toxicity or overdose. Relevant data (e.g., dose of ethylene glycol, resultant effects, time of onset of effects, therapeutic interventions or decontamination measures given, efficacy or results of any interventions, and overall patient outcome) were compiled into a table and a brief summary description of each article was written. This full evidence table is available at http://www.aapcc.org/DiscGuidelines/EGEvidenceTable.pdf. The completed table of all abstracted articles was then forwarded to the panel members for review and consideration in developing the guideline. Every attempt was made to locate significant foreign language articles and have their crucial information translated and tabulated. Copies of all of the articles were made available for reading by the panel members on a secure AAPCC website.
Guideline Writing and Review
A guideline draft was prepared by the primary author. The draft was submitted to the expert consensus panel for comment. Using a modified Delphi process, comments from the expert consensus panel members were collected, copied into a table of comments, and submitted to the primary author for response. The primary author responded to each comment in the table and, when appropriate, the guideline draft was modified to incorporate changes suggested by the panel. The revised guideline draft was again reviewed by the panel and, if there was no strong objection by any panelist to any of the changes made by the primary author, the draft was prepared for the external review process. External review of the second draft was conducted by distributing it electronically to AAPCC, AACT, and ACMT members and the secondary review panel. The secondary review panel consisted of representatives from the federal government, public health, emergency services, pediatrics, pharmacy practice, and consumer organizations (Appendix 3). Comments were submitted via a discussion thread on the AAPCC web site or privately through email communication to AAPCC staff. All submitted comments were stripped of any information that would identify their sources, copied into a table of comments. The primary author responded to each comment in the table and his responses and subsequent changes in the guideline were reviewed and accepted by the panel. Following a meeting of the expert consensus panel, the final revision of the guideline was prepared.
Evaluation of Evidence
Route of Exposure
Inhalation
Exposure to ethylene glycol vapor can occur in the occupational or private setting. One situation might be a leaking automobile radiator with potential vapor exposure to the occupant via the ventilation system. Fifteen percent (n = 911) of ethylene glycol exposures reported to US poison centers in 2002 involved inhalation. There were no moderate or major clinical effects or deaths associated with this route of exposure Citation3.
One controlled study of ethylene glycol vapor exposure in humans has been published (level 2b). Twenty male prisoner volunteers were placed in an enclosed room for 20–22 hours per day for 30 days. Aerosolized ethylene glycol (1–5 μ droplets) was continuously vented into the room. A control group of 14 prison volunteers were kept in another room of the same building. A peak air concentration of 188 mg/m3 was tolerated for only 15 minutes and 244 mg/m3 for only 1–2 minutes due to mucous membrane irritation. An air concentration of 308 mg/m3 was completely intolerable. The mean weekly air concentration of ethylene glycol ranged from 17 to 49 mg/m3. There were no differences in the urine or serum ethylene glycol concentrations, serum bicarbonate concentration, and serum electrolytes between the exposed and control groups. The serum ethylene glycol ranged from 9.4 to 18.2 mg/dL for the exposure group and 8 to 21.0 mg/dL in the control group Citation41.
Increasing the air concentration by almost two-fold was not associated with an increase in serum or urine ethylene glycol concentration in the study group. Symptoms of nose and throat irritation, slight headache, and low backache were reported. No renal impairment or metabolic abnormalities were noted. One possible limitation of the study is the accuracy of the serum and urine ethylene glycol concentrations.
Mice and rats were exposed to ethylene glycol vapor concentration of 400 mg/m3 for 8 hours/day for up to 16 weeks and did not demonstrate any histological evidence of toxicity. Ethylene glycol blood concentrations were not measured Citation42.
Based on pharmacokinetic assumptions, an exposure to an ethylene glycol concentration of 600 mg/m3 for more than 24 hours would be required to achieve a serum concentration of 30 mg/dL in an adult Citation43. Humans do not tolerate this vapor concentration due to mucous membrane irritation Citation41. The panel concluded that exposure to ethylene glycol vapor can result in mucus membrane irritation but has not been reported to cause systemic toxicity.
Dermal
Skin exposure was the route in 19% (n = 1155) of ethylene glycol cases reported to US poison centers in 2002 Citation3. Ethylene glycol has not been reported to be a skin irritant. No human cases of systemic toxicity from dermal absorption of ethylene glycol were identified from the literature review. In vitro experiments suggest that ethylene glycol can be absorbed when left in prolonged contact with human skin. In one study, approximately 27% of the applied dose (8 µg/cm2) was absorbed over a 24-hour period Citation44. The panel concluded that absorption of ethylene glycol through intact skin is minimal and not clinically significant.
Ocular
Ocular exposures comprised 11% of ethylene glycol related calls to US poison centers in 2002 Citation3. The only published report of a human eye exposure occurred in 1951. In that case, a “concentrated” ethylene glycol antifreeze solution splashed into the eye of a gasoline station attendant and resulted in eyelid edema, conjunctival inflammation, chemosis, keratitis, and iritis Citation45. Animal studies have demonstrated that splashes to rabbit eyes result in immediate discomfort and temporary conjunctival inflammation with significant corneal damage. Repeated exposure to concentrations of 20–40% cause corneal injury in rabbit eyes Citation46. The panel concluded that eye exposure to ethylene glycol can result in conjunctival or corneal injury.
Ingestion
Ingestion was the most common route of exposure (54%) for ethylene glycol cases reported to US poison centers in 2002 Citation3. All systemic toxicity and fatalities reported in the literature occurred by this route. The remaining key decision elements all relate to the ingestion of ethylene glycol.
In 1991, the addition of a bittering agent (denatonium benzoate) to consumer automotive products containing more than 10% ethylene glycol or more than 4% methanol was mandated by the Oregon Legislature. A study of the Oregon Poison Center database (level 4) did not demonstrate a decrease in the frequency of unintentional childhood exposures related to this change in product formulation Citation47. The effect on adult exposures was not evaluated in the study.
Patient Age and Intent
There were no articles identified that directly addressed the relationship between patient age and intent (e.g., unintentional vs. suicidal ingestion). A review of ethylene glycol ingestions for all ages with known outcomes reported to TESS from 2000–2002 revealed that 6.3% of unintentional and 71% of intentional ingestions resulted in a moderate effect, major effect, or death () Citation3. A review of US poison center fatality data for the 18-year period 1985–2002 did not find any suspected suicides or deaths from ethylene glycol reported in children under the age of 12 years. There were 74 ethylene glycol fatalities reported to the TESS database during 2001–2002. All reported fatalities occurred in patients 17 years of age or older. The intent was suicide in 80%, unknown in 12%, and intentional misuse/abuse in 3% of these cases Citation3. Other published reports of fatalities found in the literature review were primarily adults with suicidal or intentional misuse intent Citation8,Citation16Citation48-50. Use of products contaminated with ethylene glycol Citation1 or mislabeled as other products have also resulted in serious toxicity and death. Twelve adolescents (aged 12–15 years) mistakenly drank antifreeze (range 30–200 mL) thinking it was wine. A range of clinical toxicity occurred, including the death of a 14-year-old boy (level 4) Citation21. Cases of intentional administration to infants resulting in severe toxicity have also been reported Citation24&25. The panel noted that it is widely believed among poison centers that older children and adults are much more likely to have suicidal or homicidal intent. The earliest age at which suicidal intent might first be involved is not known but is very unlikely for children less than 6 years old. In 2003, only 119 of 1,227,381 (0.01%) poison exposures in children less than 6 years old reported to TESS were coded as suicidal intent and it is possible that these were coding errors Citation3.
The panel concluded that ingestions secondary to self-harm, intentional misuse, or malicious intent result in severe outcomes (e.g., moderate, major effect or fatality) much more frequently than unintentional ingestions. This is likely to be a consequence of a larger dose and delayed time to treatment.
Onset of Symptomatic Toxic Effects
The precise time course for the onset of effects after ingestion of ethylene glycol was extremely difficult to determine from the literature. The wide range of doses, effects of co-ingestants, historical inaccuracies in reported dose and time of ingestion, and lack of out-of-hospital monitoring make this assessment difficult. In many articles, only those symptoms that were observed at hospital presentation were noted, but symptom onset likely occurred before presentation. For such cases only an upper time limit for onset of effect can be assigned. For example, patients who presented 12 hours after ingestion with coma would be labeled as having had their effects occur less than 12 hours after ingestion.
No level 1–3 studies were found that investigated the time of onset of effects after acute ingestion. Four case series (level 4) contained onset of effect data but it was not the primary goal of the studies Citation12&13Citation27&28. A retrospective review of 11 patients with ethylene glycol poisoning seen at two hospitals over a 4–5 year period noted both time and the specific clinical effects at presentation to the hospital. The onset of symptoms out-of-hospital was not addressed. Patients were grouped into those presenting less than 12 hours after ingestion (n = 6) and those presenting more than 12 hours after ingestion (n = 5, range 48–120 hours). Thus, the maximum time at which clinical effects could have possibly developed after ingestion was 120 hours. Patients who presented within 10 hours of exposure had fewer complications than those who presented 12 or more hours after exposure Citation28. A retrospective case series of seven patients noted that patients presenting 12 hours or longer after ingestion developed renal failure and required more prolonged hospitalization Citation27. In a prospective case series, seven patients presented between 3.5 and 21.5 hours and three at unknown times after ingestion, suggesting a maximum time of 21.5 hours to symptom onset Citation12. A retrospective review of 39 patients found that the longer the delay to presentation, the more severe the metabolic acidosis and outcome. The time to presentation ranged from 3 to 30 hours Citation13.
There were seven patients described in seven articles (level 4) in which the exact time of effect onset was documented Citation6&7Citation9Citation51-54. None of these patients had ingested alcohol and only one had taken co-ingestants (diazepam and caffeine) Citation52. Clinical effects began within 30 minutes to 6 hours for six of the seven cases. Symptoms began 2 days after ingestion in one case Citation54.
Two teenaged boys drank 1–2 ounces of antifreeze at an evening party and “remained well until the morning” when they complained of dizziness, poor coordination, confusion, and urinary difficulty. They developed calcium oxalate crystalluria, metabolic acidosis, and renal impairment over the next 3 days and recovered after 7–10 days Citation55. Five adult male soldiers drank an antifreeze and water mixture and developed symptoms of vomiting, stupor, and respiratory distress within 8 hours. No antidote was administered and all died 18–32 hours after ingestion Citation48.
Occasionally, patients developed effects over the course of several days. One patient did not present to a medical facility for up to 11 days after the acute exposure but the time of onset of symptoms was not documented Citation56. Whether symptoms actually developed earlier in some patients but were not severe enough to prompt them to seek medical care is unknown.
No studies were identified that evaluated the correlation of serum ethylene glycol concentration with symptoms. Adult and pediatric patients with relative lack of central nervous system symptoms and documented serum ethylene glycol concentrations are listed in Tables and , respectively. These cases indicate that patients may have elevated serum ethylene glycol concentrations and not manifest concurrent neurological symptoms suggestive of the severity of the intoxication.
Table 3. Reported cases of adult ethylene glycol toxicity with lack of CNS effects
Table 4. Ethylene glycol toxicity and CNS symptoms reported in children
The expert consensus panel concluded that after an acute ingestion most clinical effects develop within hours but can be delayed for days, particularly if alcohol is also ingested. The absence of symptoms in the hours following a potentially toxic dose does not preclude toxicity. Referral for evaluation should not be withheld based on the absence of symptoms unless it has been more than 24 hours after an unintentional exposure. All patients with symptoms attributed to ethylene glycol should be referred to an emergency department for evaluation.
Chronic Dosing
There were no level 1–3 studies of ethylene glycol toxicity from chronic or repeated ingestion. In one case report, a patient was given “repeated” doses of an ethylene glycol-contaminated beverage and died (level 4) Citation13. There is insufficient evidence to distinguish the toxicity of chronic versus acute ingestion. The expert consensus panel concluded that all chronic or repeated ingestions of ethylene glycol should be evaluated in an emergency department.
Toxic Dose of Ethylene Glycol by Ingestion
Evidence regarding the relationship between ethylene glycol dose and toxicity is limited to one prospective cohort study (level 2b), one case-control study (level 3b), many case reports, and a few case series (level 4). The minimal toxic dose is difficult to evaluate from the literature for several reasons. Patient histories regarding the dose ingested can be unreliable and are often obtained during a period of extreme emotional stress for the patients and their family. Often, the exact product and ethylene glycol concentration are not known or documented. The co-ingestion of alcohol might have affected the outcome or affected the time to presentation. In addition, there are often confounding factors such as co-ingestion of drugs that affect the central nervous system. In most reports, the accuracy of the history was not addressed and the history was not confirmed by outside sources (e.g., family members) or objective evidence (e.g., empty product containers).
Acute Ingestion by Children
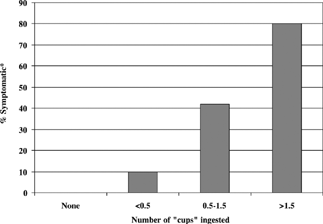
No level 1 or 2 studies were found specifically investigating a threshold dose for the development of toxicity or containing specific dose-response information for children. One case-control study (level 3b) of 19 children under 10 years of age demonstrated a dose-response effect. The children drank a beverage made from a powdered soft drink mixture that was reconstituted with water containing 9% ethylene glycol. Ten percent of children ingesting up to 1/2 cup, 42% consuming 1/2 to 1 1/2 cups, and 80% consuming more than 1 1/2 cups of the beverage became symptomatic with fatigue or ataxia (). Serum ethylene glycol concentrations and the volume of the cup were not reported Citation1.
Figure 1. Dose-response for 19 patients less than 10 years of age who drank an ethylene glycol-contaminated soft drink mixture (9% ethylene glycol) Citation1. **Subjects complaining of fatigue or ataxia were considered symptomatic.
There were an additional eight pediatric cases (level 4) reported with dose-response information () Citation57-59. The lowest published toxic doses of ethylene glycol occurred in two siblings in 1954. A 6-year-old boy drank 2–3 ounces of a 1:1 aqueous antifreeze solution (< 50% ethylene glycol) and became critically ill with central nervous system depression, muscle spasms, metabolic acidosis (serum bicarbonate 3.0 mEq/L), and bilateral plantar extension response. He was treated with intravenous infusion of sodium lactate and calcium gluconate. His 4-year-old brother drank “less than an ounce” of the same diluted ethylene glycol solution, developed drowsiness and metabolic acidosis (serum bicarbonate 10 mEq/L), and recovered with sodium lactate infusion. Serum ethylene glycol concentrations were not reported. Both boys recovered without signs of renal injury Citation58. Four children drank 30–100 mL of antifreeze and developed serum concentrations ranging from 113 to 270 mg/dL. All four recovered after treatment with fomepizole Citation59. An 8-month-old infant drank “up to 120 mL” of a 95% ethylene glycol solution and developed lethargy and a serum ethylene glycol concentration of 384 mg/dL Citation57.
Table 5. Reported doses in acute ingestion of ethylene glycol by children
Estimating doses in children is often unreliable, as parents might over- or underestimate the actual ingested dose depending on the circumstances. Since the ethylene glycol concentration of a “diluted” solution is usually unknown, it should be assumed to be a high concentration product (e.g., greater than 20%) unless credible evidence suggests otherwise.
The expert consensus panel concluded that ingestions by children of an unknown amount or more than a witnessed taste or lick of concentrated (> 20%) ethylene glycol solution should be considered potentially toxic.
Acute Ingestion by Older Children, Adolescents and Adults
No level 1–3 studies were found specifically investigating a threshold dose for the development of toxicity or containing specific dose-response information in older children, adolescents, or adults.
Multiple case reports and case series (level 4) were found with dose-response information (). There were two case series that contained some dose-response data but establishing a toxic threshold was not the primary goal of the studies. A retrospective review of 11 patients compared patient outcome between different types of treatment and times to presentation Citation28. Partial dose information was available for only one patient who ingested 60 mL of an ethylene glycol solution (unknown strength) and subsequently developed severe toxicity. Factors contributing to lethal outcomes were evaluated in a retrospective review of 17 cases of acute poisoning Citation50. Doses were known for eight of the patients and were presented as a range of 250–800 mL. Unfortunately, the authors did not correlate these doses with specific individual outcomes and, therefore, a dose-response relationship could not be established.
Table 6. Minimum reported toxic doses in acute ethylene glycol ingestion by patients 10 years of age and older
The lowest amount reported to cause toxicity was a case report of an adult ingestion of 10 mL of an unspecified antifreeze solution. It had been taken along with cough syrup and led to acute renal failure requiring dialysis Citation61. The history of exposure was obtained retrospectively from the patient without corroboration. The next lowest toxic dose was 20 mL of an unknown ethylene glycol product that resulted in somnolence in one patient Citation49. Four adult patients ingested 20–30 mL of “pure” antifreeze and developed somnolence; one developed anuria Citation49. Finally, there were several published cases of adults ingesting 30 mL of antifreeze or other ethylene glycol products resulting in clinical effects ranging from moderate to severe ().
Most fatalities occurred as a result of suspected suicide or misuse of ethylene glycol Citation3,Citation64. In 1932, Hunt Citation65 suggested that a lethal adult dose would be approximately 100 mL based on data extrapolated from animal experiments. This estimate has been borne out in a few reported human fatalities. Ingestion of 50–100 mL resulted in death in two cases Citation49.
The expert consensus panel concluded that ingestion of 10–30 mL of a highly concentrated (80–99%) ethylene glycol solution is potentially toxic and 50–100 mL is potentially lethal to an older child, adolescent, or adult. There were no reports of toxicity resulting from ingestion of less than 10 mL in this age group.
Out-of-Hospital Detection of Ethylene Glycol by Urine Fluorescence
Some commercial antifreeze products contain a fluorescent dye, such as sodium fluorescein, in order to facilitate detection of leaks by using an ultraviolet light. Adult urine can fluoresce under ultraviolet light (Wood's lamp) for up to 2 hours after ingesting an amount of sodium fluorescein (0.6 mg) that is present in 30 mL of antifreeze Citation66. However, false positives can occur, particularly in children Citation67. Plastic urine specimen containers have a high native fluorescence Citation66. A blinded study (level 3b) of examination of urine samples with a Wood's lamp (ultraviolet light) for fluorescence before and after ingestion 0.6 mg of fluoroscein had an optimal sensitivity 42%, specificity 75%, and accuracy 50% Citation68.
The expert consensus panel concluded that the out-of-hospital use of ultraviolet light to diagnosis ethylene glycol ingestion by urine fluorescence is unreliable and should not be performed. The definitive diagnosis of ethylene glycol poisoning in patients with a recent history of ingestion can only be made by measuring the serum ethylene glycol concentration Citation18.
Potential Out-of-Hospital Management to Prevent or Ameliorate Ethylene Glycol Toxicity
The expert consensus panel identified potential methods for reducing ethylene glycol toxicity in the out-of-hospital setting. These were reducing gastrointestinal absorption and inhibiting metabolism of absorbed ethylene glycol. Absorption could theoretically be decreased by early gastrointestinal decontamination, such as emesis, gastric aspiration, or administration of activated charcoal soon after ingestion of large amounts. Drugs such as alcohol or fomepizole can inhibit ethylene glycol metabolism. No studies were found that specifically addressed out-of-hospital decontamination or use of antidotes or treatments.
Decontamination
Inhalation
There were no reports of significant absorption from vapor exposure or of bystanders or rescue personnel being exposed to “off-gassing” from patients exposed to ethylene glycol vapors. The panel concluded that routine decontamination of patients exposed to ethylene glycol vapors is not warranted.
Dermal
There are no reports of significant cutaneous effects or systemic absorption of ethylene glycol when applied to intact skin. The panel concluded that routine cleansing with mild soap and water should be performed after skin or hair exposure to ethylene glycol.
Ocular
Concentrated ethylene glycol exposure to the eye can result in conjunctival irritation and corneal injury Citation45. There are no studies of eye decontamination after exposure. The panel concluded that ethylene glycol eye exposures should be treated as other irritating chemical ocular exposures. Removal of contact lenses and immediate irrigation with room temperature tap water is recommended. All patients with symptoms of eye injury (e.g., eye pain or foreign body sensation) after irrigation should be referred for an ophthalmologic exam.
Ingestion
There were no level 1–3 studies that investigated the effects of decontamination measures on ethylene glycol absorption in the out-of-hospital or in-hospital settings. Various decontamination measures such as activated charcoal, gastric lavage, and gastric aspiration were reported in a number of case reports, case series, and abstracts but their efficacy was impossible to assess. There were no human studies located that assessed the binding capacity of activated charcoal for ethylene glycol. However, an in vitro stomach model demonstrated that 68% of ethylene glycol was adsorbed by a 5:1 ratio of activated charcoal to ethylene glycol Citation69. There were no reports on the use of ipecac syrup after ethylene glycol ingestion.
The panel concluded that there was no evidence that out-of-hospital gastrointestinal decontamination offered benefit to the patient. Induction of emesis with ipecac syrup is not recommended because of the potential risk of pulmonary aspiration of gastric contents if the patient subsequently loses consciousness. The efficacy of oral activated charcoal in preventing the absorption of ethylene glycol has not been adequately studied and thus is not recommended for routine administration. Transportation to an emergency department should not be delayed in order to attempt activated charcoal administration.
Alteration of Ethylene Glycol Metabolism: Alcohol and Fomepizole
Alcohol and fomepizole have been frequently used as antidotes for ethylene glycol poisoning Citation17-19Citation57 Citation59. Fomepizole is approved by the Food and Drug Administration for ethylene glycol toxicity and is administered intravenously. There were no studies that evaluated the use of fomepizole in the pre-hospital environment.
Alcohol has the advantage of being available without prescription and can be administered orally. There were no studies evaluating the out-of-hospital use of alcohol as an antidote. However, it has been suggested that alcohol be administered orally for the treatment of ethylene glycol poisoning Citation19. One patient reportedly ingested alcohol and antifreeze concurrently and presented 6 hours later with a serum alcohol concentration 116 mg/dL, ethylene glycol 150 mg/dL, and no signs of acidosis Citation70. However, several patients have consumed alcohol concurrently with ethylene glycol and presented to emergency departments with evidence of metabolic acidosis Citation14 Citation49Citation71&72. A randomized cross-over trial (level 1b) of oral versus intravenous alcohol in 20 human adult male volunteers found that the mean time to peak alcohol concentration was approximately 105 minutes after ingesting a 20% alcohol solution (700 mg/kg) in orange juice over 10 minutes. This was about 1 hour later than the peak achieved with intravenous administration Citation73. Although no subject had clinical manifestations of hypoglycemia, it is notable that 90% (18/20) had at least one episode of blood glucose less than 80 mg/dL and 65% (13/20) had blood glucose less than 59 mg/dL following alcohol administration. Most of the oral group failed to reach the target concentration of 100 mg/dL (mean peak concentration 71 mg/dL, range 39–119 mg/dL), whereas the mean peak concentration for the intravenous group was 104 mg/dL. Risks of oral alcohol include vomiting, hypoglycemia, altered mental status, lethargy, and electrolyte disturbances, particularly in children Citation74.
The expert consensus panel concluded that the risks of out-of-hospital administration of alcohol outweigh any demonstrated benefits and does not recommend its routine use in this setting. The risks and benefits of out-of-hospital use of fomepizole are not known.
Detoxification of Ethylene Glycol Metabolites (Glyoxylic Acid)
Thiamine and pyridoxine are cofactors that theoretically facilitate the conversion of glyoxylic acid to metabolites less toxic than oxalate (level 5) Citation9&10Citation17&18. Thiamine and pyridoxine are available as over-the-counter dietary supplements and as parenteral solutions. There were no level 1–3 studies evaluating the efficacy of these co-factors in the out-of-hospital or hospital setting. There are many reports of patients receiving cofactor supplementation during hospitalization but no outcome analysis has been performed. There is no evidence that cofactor administration alters patient outcome and the expert consensus panel does not recommend its use in the out-of-hospital setting.
Poison Center Referral of a Patient to an Emergency Department
Time of Referral
Three retrospective case series (level 4) found that earlier presentation times to the hospital were associated with a better outcome Citation13Citation27&28. Whether this difference was due to more rapid institution of decontamination measures, treatment measures, or some other factor is not clear. However, another case series (level 4) found no association between time to presentation or treatment and clinical outcome Citation50. Peak serum ethylene glycol concentrations occur early in the intoxication Citation6&7.
Since the primary objective is to prevent metabolism of ethylene glycol to its toxic metabolites, the expert consensus panel concluded that patients who require health care evaluation should be referred to an emergency department immediately after exposure.
Type of Healthcare Facility and Mode of Travel
There were no studies that addressed the type of healthcare facility to which patients should be referred or how they should be transported. Ethylene glycol ingestion can result in severe toxicity that requires sophisticated laboratory, clinical assessment, and management resources.
The expert consensus panel concluded that patients should be referred to facilities that have the ability to assess and manage altered mental status, metabolic abnormalities, and renal injury and to measure blood and urine laboratory parameters in a timely manner. This requires referral to a hospital emergency department rather than an outpatient clinic. Facilities that can obtain ethylene glycol serum concentrations, perform hemodialysis, and have alcohol or fomepizole therapy available are preferred but not required. The patient's clinical condition, local protocols, and transportation resources should dictate the mode of transportation.
Conclusions
Key Decision Elements
The expert consensus panel identified the patient's age, weight, intent, route of exposure, estimated dose of ethylene glycol, time since exposure, and symptoms as critical information needed in order to make a sound triage decision.
Patient Intent
All patients in whom suicidal or malicious intent (e.g., child abuse, neglect, or homicide) is known or suspected should be referred to an emergency department for medical evaluation regardless of the dose ingested. Unintentional exposures have a low rate of associated clinical toxicity but this reason alone cannot be used to exclude potential adverse clinical effects.
Route of Exposure
Exposure to ethylene glycol can occur by inhalation, ingestion, and dermal or eye contact. Systemic toxicity and death has only been documented by ingestion. Inhalation of vapors or liquid contact with the skin is very unlikely to result in significant toxicity other than local irritation. Eye exposure can cause localized tissue irritation or injury.
Dose of Ethylene Glycol
The minimum toxic dose has not been established for children or adults. Limited evidence (level 4) supports ingestion of as little as 10 to 30 mL, or a “swallow”, of concentrated (80–99%) ethylene glycol solution as potentially toxic to an adult. Likewise, children have manifested toxic effects with ingestion of less than 30 mL of dilute (< 50%) solutions.
Time of Onset of Toxicity After Overdose
Ethylene glycol is rapidly absorbed from the gastrointestinal tract and patients have become symptomatic as soon as 30–60 minutes after ingestion Citation6,Citation51. There are many case reports of delayed presentation to the hospital but assessment of onset of symptoms before presentation was not possible. It is possible that some patients might not become symptomatic from metabolic acidosis or renal failure until many hours or days after ingestion.
Presence of Symptoms
Potentially toxic serum concentrations of ethylene glycol (e.g., 20–30 mg/dL) do not appear to reliably produce early symptoms in children or adults. Therefore, the lack of symptoms does not exclude a potentially toxic ingestion and should not be used as a triage criterion unless it has been more than 24 hours after an unintentional exposure.
Potential Out-of-Hospital Management Techniques
Decontamination
Routine decontamination of inhalation exposures is not warranted. Dermal and ocular exposures should be decontaminated according to local protocols for chemical exposures. There is no evidence that out-of-hospital induction of emesis, use of gastric aspiration, or administration of activated charcoal will improve outcome and are therefore not recommended. Their risk-benefit ratios are unknown and an effective antidote is available. Induction of emesis is not advised due to the possibility of the onset of decreased mental status resulting in the inability to protect the airway and possible aspiration of gastric contents. Delay in transportation to the emergency department should not occur in order to institute any gastrointestinal decontamination.
Alteration of Ethylene Glycol Metabolism
The out-of-hospital uses of alcohol or fomepizole as an antidote have not been studied. Their routine use in this setting can not be advocated at this time.
Cofactor Therapy
There are no published data evaluating the efficacy of supplemental thiamine or pyridoxine in ethylene glycol poisoned patients. The panel does not recommend administration of these cofactors in the out-of-hospital setting.
Limitations of the Published Data
The strength of evidence for this guideline is limited to case series, case reports (level 4), one cohort inhalation study (level 2b) and one case-control study of ingestion (level 3b). Level 4 data do not provide a sound basis for toxic dose estimation or triage recommendations. The case reports and case series varied widely in the level of clinical detail presented, severity of clinical effects of the poisoning, timing of interventions, co-ingestants, estimated dose, and treatments administered.
The lack of precision in dose measurement is a major limitation of this literature analysis. The estimates are subject to many assumptions. Data for amount ingested are often inaccurate or incomplete. The history might be obtained from an intoxicated patient or an emotionally stressed friend or relative. Parents might under- or overestimate the ingested dose because of denial or anxiety. Poison center staffs often record the dose taken as the worst case scenario in order to provide a wide margin of safety. Estimating the volume ingested from examining most containers is unreliable. In most case reports and case series the estimates of exposure were not independently verified.
In most of the reports the exact time of ingestion was not reported or was not known. The time of onset of toxicity could only be estimated as occurring within a range of hours after the suspected ingestion in the majority of cases.
Recommendations
Recommendations are in chronological order of likely clinical use. The grade of recommendation is in parentheses.
Patients with exposure due to suspected self-harm, misuse, or potentially malicious administration should be referred to an emergency department immediately regardless of the doses reported (Grade D).
Patients with inhalation exposures will not develop systemic toxicity and can be managed out-of-hospital if asymptomatic (Grade B). Patients with clinically significant mucous membrane irritation should be referred for evaluation (Grade D).
Decontamination of dermal exposures should include routine cleansing with mild soap and water. Removal of contact lenses and immediate irrigation with room temperature tap water is recommended for ocular exposures. All patients with symptoms of eye injury should be referred for an ophthalmologic exam (Grade D).
Patients with symptoms of ethylene glycol poisoning (e.g., vomiting, slurred speech, ataxia, altered mental status) should be referred immediately for evaluation regardless of the reported doses (Grade C).
The absence of symptoms shortly after ingestion does not exclude a potentially toxic dose and should not be used as a triage criterion (Grade C).
Adults who ingest a “swallow” (10–30 mL), children who ingest more than a witnessed taste or lick, or if the amount is unknown of most ethylene glycol products should be referred immediately for evaluation. The potential toxic volume of very dilute solutions (e.g., product concentration known to be < 20%) is larger and can be estimated by the formula (Formula 2) in the text. If the concentration of the product is not known, it should be assumed to be a concentrated (> 20%) product (Grade C).
A witnessed “taste or lick” only in a child, or an adult who unintentionally drinks and then expectorates all of a concentrated product without swallowing, does not need referral (Grade C).
Referral is not needed if it has been more than 24 hours since a potentially toxic unintentional exposure, the patient has been asymptomatic, and no alcohol was co-ingested (Grade D).
Gastrointestinal decontamination in the out-of-hospital setting with ipecac syrup, gastric lavage, or activated charcoal is not recommended. Transportation to an emergency department should not be delayed for any decontamination procedures (Grade D).
Patients meeting referral criteria should be evaluated at a hospital emergency department rather than a clinic. A facility that can quickly obtain an ethylene glycol serum concentration and has alcohol or fomepizole therapy available is preferred. This referral should be guided by local poison center procedures and community resources (Grade D).
The administration of alcohol, fomepizole, thiamine, or pyridoxine is not recommended in the out-of-hospital setting (Grade D).
These recommendations are summarized in Appendix 4.
Implications for Research
The expert consensus panel identified the following topics where additional research is needed or analysis of existing databases might be useful.
Determine how well symptoms correlate with serum ethylene glycol concentrations.
Determine whether any subgroup of adult or pediatric patients has increased susceptibility to ethylene glycol toxicity.
Evaluate the feasibility, effectiveness, and safety of the pre-hospital use of alcohol and fomepizole for patients with prolonged transportation times.
Evaluate the pharmacokinetics and adverse effects of orally administered fomepizole.
Further research on the efficacy of adsorbents, such as activated charcoal, as decontaminants for ethylene glycol.
Evaluate the effectiveness of thiamine and pyridoxine administration on clinical outcome.
Evaluate the effectiveness of adding bittering agents to ethylene glycol products in order to limit ingestion.
Evaluate the utility of gastric aspiration by nasogastric tube soon after ingestion to prevent absorption.
Evaluate the relationship between the dose ingested and subsequent serum concentration in children and adults.
Evaluate the sources of exposure in children (e.g., open containers, not original containers).
Study the role of pre-existing renal disease on the risk for renal toxicity.
Evaluate the efficacy of regulations promoting the substitution of equally effective but less toxic glycols in commercial products in reducing the incidence of ethylene glycol poisonings.
Disclosures
There are no potential conflicts of interest reported by the expert consensus panel or project staff regarding this guideline.
References
- Ethylene glycol intoxication due to contamination of water systems.MMWR, Morb Mortal Wkly Rep 1987; 36:611–614.
- Leikin J B, Toerne T, Burda A, McAllister K, Erickson T. Summertime cluster of intentional ethylene glycol ingestions.JAMA 1997; 278:1406., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Toxic Exposure Surveillance System (TESS). Washington, DC: American Association of Poison Control Centers, 2004. , Database accessed May 2004.
- Watson W A, Litovitz T L, Klein-Schwartz W, Rodgers G C Jr, Youniss J, Reid N, Rouse W G, Rembert R S, Borys D. 2003 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System.Am J Emerg Med 2004; 22:335–404., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Frantz S W, Beskitt J L, Grosse C M, Tallant M J, Dietz F K, Ballantyne B. Pharmacokinetics of ethylene glycol. I. Plasma disposition after single intravenous, peroral, or percutaneous doses in female Sprague-Dawley rats and CD-1 mice.Drug Metab Dispos 1996; 24:911–921., [PUBMED], [INFOTRIEVE], [CSA]
- Berman L B, Schreiner G E, Feys J. The nephrotic lesion of ethylene glycol.Ann Intern Med 1957; 46:611–619., [PUBMED], [INFOTRIEVE], [CSA]
- Boyer E W, Mejia M, Woolf A, Shannon M. Severe ethylene glycol ingestion treated without hemodialysis.Pediatrics 2001; 107:172–173., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pons C A, Custer R P. Acute ethylene glycol poisoning: a clinico-pathologic report of eighteen fatal cases.Am J Med Sci 1946; 211:544–552., [CSA]
- Parry M F, Wallach R. Ethylene glycol poisoning.Am J Med 1974; 57:143–150., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gabow P A, Clay K, Sullivan J B, Lepoff R. Organic acids in ethylene glycol intoxication.Ann Intern Med 1986; 105:16–20., [PUBMED], [INFOTRIEVE], [CSA]
- Jacobsen D, Hewlett T P, Webb R, Brown S T, McMartin K E. Ethylene glycol intoxication: evaluation of kinetics and crystalluria.Am J Med 1988; 84:145–152., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Moreau C L, Kerns W II, Tomaszewski C A, McMartin K E, Rose S R, Ford M D, Brent J. Glycolate kinetics and hemodialysis clearance in ethylene glycol poisoning. META Study Group.J Toxicol Clin Toxicol 1998; 36:659–666., [PUBMED], [INFOTRIEVE], [CSA]
- Porter W H, Rutter P W, Bush B A, Pappas A A, Dunnington J E. Ethylene glycol toxicity: the role of serum glycolic acid in hemodialysis.J Toxicol Clin Toxicol 2001; 39:607–615., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Jacobsen D, Ovrebo S, Ostborg J, Sejersted O M. Glycolate causes the acidosis in ethylene glycol poisoning and is effectively removed by hemodialysis.Acta Med Scand 1984; 216:409–416., [PUBMED], [INFOTRIEVE], [CSA]
- Poldelski V, Johnson A, Wright S, Rosa V D, Zager R A. Ethylene glycol-mediated tubular injury: identification of critical metabolites and injury pathways.Am J Kidney Dis 2001; 38:339–348., [PUBMED], [INFOTRIEVE], [CSA]
- Karlson-Stiber C, Persson H. Ethylene glycol poisoning: experiences from an epidemic in Sweden.J Toxicol Clin Toxicol 1992; 30:565–574., [PUBMED], [INFOTRIEVE], [CSA]
- Jacobsen D, McMartin K E. Antidotes for methanol and ethylene glycol poisoning.J Toxicol Clin Toxicol 1997; 35:127–143., [PUBMED], [INFOTRIEVE], [CSA]
- Barceloux D G, Krenzelok E P, Olson K, Watson W. American Academy of Clinical Toxicology practice guidelines on the treatment of ethylene glycol poisoning. Ad hoc Committee.J Toxicol Clin Toxicol 1999; 37:537–560., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Peterson C D, Collins A J, Himes J M, Bullock M L, Keane W F. Ethylene glycol poisoning: pharmacokinetics during therapy with ethanol and hemodialysis.N Engl J Med 1981; 304:21–23., [PUBMED], [INFOTRIEVE], [CSA]
- Sivilotti M L, Burns M J, McMartin K E, Brent J. Toxicokinetics of ethylene glycol during fomepizole therapy: implications for management. For the Methylpyrazole for Toxic Alcohols Study Group.Ann Emerg Med 2000; 36:114–125., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Moriarty R W, McDonald R H Jr. The spectrum of ethylene glycol poisoning.Clin Toxicol 1974; 7:583–596., [PUBMED], [INFOTRIEVE], [CSA]
- Clay K L, Murphy R C. On the metabolic acidosis of ethylene glycol intoxication.Toxicol Appl Pharmacol 1977; 39:39–49., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Levy R I. Renal failure secondary to ethylene glycol intoxication.JAMA 1960; 173:1210–1213., [PUBMED], [INFOTRIEVE], [CSA]
- Saladino R, Shannon M. Accidental and intentional poisonings with ethylene glycol in infancy: diagnostic clues and management.Pediatr Emerg Care 1991; 7:93–96., [PUBMED], [INFOTRIEVE], [CSA]
- Woolf A D, Wynshaw-Boris A, Rinaldo P, Levy H L. Intentional infantile ethylene glycol poisoning presenting as an inherited metabolic disorder.J Pediatr 1992; 120:414–421., [CSA]
- De Leacy E A, Moxon L N, Ellis V M, Van Dongen J M, Johnson L P, Doddrell D M, Cowley D M. A report of accidental ethylene glycol ingestion in 2 siblings.Pathology (Phila) 1995; 27:273–276., [CSA], [CROSSREF]
- Momont S L, Dahlberg P J. Ethylene glycol poisoning.Wisc Med J 1989; 88:16–20., [CSA]
- Rydel J J, Carlson A, Sharma J, Leikin J. An approach to dialysis for ethylene glycol intoxication.Vet Hum Toxicol 2002; 44:36–39., [PUBMED], [INFOTRIEVE], [CSA]
- Jones D V, Work C E. Volume of a swallow.Am J Dis Child 1961; 102:427., [PUBMED], [INFOTRIEVE], [CSA]
- Watson W A, Bradford D C, Veltri J C. The volume of a swallow: correlation of deglutition with patient and container parameters.Am J Emerg Med 1983; 1:278–281., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Saylor J H. Volume of a swallow: role of orifice size and viscosity.Vet Hum Toxicol 1987; 29:79–83., [PUBMED], [INFOTRIEVE], [CSA]
- Nilsson H, Ekberg O, Olsson R, Kjellin O, Hindfelt B. Quantitative assessment of swallowing in healthy adults.Dysphagia 1996; 11:110–116., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ratnapalan S, Potylitsina Y, Tan L H, Roifman M, Koren G. Measuring a toddler's mouthful: toxicologic considerations.J Pediatr 2003; 142:729–730., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Winter M E. Basic Clinical Pharmacokinetics. 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2004.
- Shaneyfelt T M, Mayo-Smith M F, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature.JAMA 1999; 281:1900–1905., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Poisindex System. Klasco R K, ed. Greenwood Village, CO: Thomson Micromedex, , edition expires September 2003.
- In: Ellenhorn M J, ed. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore: Williams & Wilkins, 1997.
- Ford M D, Delaney K A, Ling J, Erickson T. Clinical Toxicology. WB Saunders: Philadelphia, 2000.
- In: Goldfrank L R, Flomenbaum N E, Lewin N A, Howland M A, Hoffman R S, Nelson L S, eds. Goldfrank's Toxicologic Emergencies. 7th ed. New York: McGraw-Hill, 2002.
- In: Haddad L M, Shannon M W, Winchester J F, eds. Clinical Management of Poisoning and Drug Overdose. 3rd ed. Philadelphia: WB Saunders, 1998.
- Wills J H, Coulston F, Harris E S, McChesney E W, Russell J C, Serrone D M. Inhalation of aerosolized ethylene glycol by man.Clin Toxicol 1974; 7:463–476., [PUBMED], [INFOTRIEVE], [CSA]
- Wiley J H, Hueper W C, von Oettingen W F. The toxicity and potential dangers of ethylene glycol.J Ind Hyg Toxicol 1936; 18:123–126., [CSA]
- Hodgman M J, Wezorek C, Krenzelok E. Toxic inhalation of ethylene glycol: a pharmacological improbability.J Toxicol Clin Toxicol 1997; 35:109–111., [PUBMED], [INFOTRIEVE], [CSA]
- Driver J, Tardiff R G, Sedik L, Wester R C, Maibach H I. In vitro percutaneous absorption of [14C] ethylene glycol.J Expo Anal Environ Epidemiol 1993; 3:277–284., [PUBMED], [INFOTRIEVE], [CSA]
- Sykowski P. Ethylene glycol toxicity.Am J Ophthalmol 1951; 34:1599–1600., [PUBMED], [INFOTRIEVE], [CSA]
- Grant W M, Schulman J S. Toxicology of the Eye: Effects on the Eyes and Visual System from Chemicals, Drugs, Metals and Minerals, Plants, Toxins and Venoms: also Systemic Side Effects from Eye Medications. 4th ed. Springfield, IL: Thomas, 1993.
- Mullins M E, Zane Horowitz B. Was it necessary to add Bitrex (denatonium benzoate) to automotive products?. Vet Hum Toxicol 2004; 46:150–152., [PUBMED], [INFOTRIEVE], [CSA]
- Smith D E. Morphologic lesions due to acute and subacute poisoning with antifreeze (ethylene glycol).AMA Arch Pathol 1951; 51:423–433., [PUBMED], [INFOTRIEVE], [CSA]
- Gaultier M, Conso F, Rudler M, Leclerc J P, Mellerio F. Intoxication aiguë par l'ethylène-glycol acute.Eur J Toxicol Environ Hyg 1976; 9:373–379., [PUBMED], [INFOTRIEVE], [CSA]
- Hylander B, Kjellstrand C M. Prognostic factors and treatment of severe ethylene glycol intoxication.Intensive Care Med 1996; 22:546–552., [PUBMED], [INFOTRIEVE], [CSA]
- Stokes J B. Prevention of organ damage in massive ethylene glycol ingestion.JAMA 1980; 243:2065–2066., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Curtin L, Kraner J, Wine H, Savitt D, Abuelo J G. Complete recovery after massive ethylene glycol ingestion.Arch Intern Med 1992; 152:1311–1313., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Baud F J, Bismuth C, Garnier R, Galliot M, Astier A, Maistre G, Soffer M. 4-Methylpyrazole may be an alternative to ethanol therapy for ethylene glycol intoxication in man.J Toxicol Clin Toxicol 1986; 24:463–483., [PUBMED], [INFOTRIEVE], [CSA]
- Spillane L, Roberts J R, Meyer A E. Multiple cranial nerve deficits after ethylene glycol poisoning.Ann Emerg Med 1991; 20:208–210., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Friedman E A, Greenberg J B, Merrill J P, Dammin G J. Consequences of ethylene glycol poisoning.Am J Med 1962; 32:891–902., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mallya K B, Mendis T, Guberman A. Bilateral facial paralysis following ethylene glycol ingestion.Can J Neurol Sci 1986; 13:340–341., [PUBMED], [INFOTRIEVE], [CSA]
- Baum C R, Langman C B, Oker E E, Goldstein C A, Aviles S R, Makar J K. Fomepizole treatment of ethylene glycol poisoning in an infant.Pediatrics 2000; 106:1489–1491., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Nadeau G, Cote R, Delaney F J. Two cases of ethylene glycol poisoning.Can Med Assoc J 1954; 70:69–70., [PUBMED], [INFOTRIEVE], [CSA]
- Caravati E M, Heileson H L, Jones M. Treatment of severe pediatric ethylene glycol intoxication without hemodialysis.J Toxicol Clin Toxicol 2004; 42:255–259., [PUBMED], [INFOTRIEVE], [CSA]
- Gillet Y, Gaulier J, Floret D, Cochat P. Ethylene-glycol poisoning in a child.Arch Pediatr 1996; 3:612–613., [PUBMED], [INFOTRIEVE], [CSA]
- Jaffery J B, Aggarwal A, Ades P A, Weise W J. A long sweet sleep with sour consequences.Lancet 2001; 358:1236., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Theurl A, Battista H J, Fritzer W. Akzidentelle glykolvergiftung.Wien Med Wochenschr 1989; 139:390–395., [PUBMED], [INFOTRIEVE], [CSA]
- Hagstam K E, Ingvar D H, Paatela M, Tallqvist H. Ethylene-glycol poisoning treated by haemodialysis.Acta Med Scand 1965; 178:599–606., [PUBMED], [INFOTRIEVE], [CSA]
- Litovitz T L, Klein-Schwartz W, Rodgers G C Jr, Cobaugh D J, Youniss J, Omslaer J C, May M E, Woolf A D, Benson B E. 2001 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System.Am J Emerg Med 2002; 20:391–452., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hunt R. Toxicity of ethylene and propylene glycols.Ind Eng Chem 1932; 24:361., [CSA], [CROSSREF]
- Winter M L, Ellis M D, Snodgrass W R. Urine fluorescence using a Wood's lamp to detect the antifreeze additive sodium fluorescein: a qualitative adjunctive test in suspected ethylene glycol ingestions.Ann Emerg Med 1990; 19:663–667., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Casavant M J, Shah M N, Battels R. Does fluorescent urine indicate antifreeze ingestion by children?. Pediatrics 2001; 107:113–114., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wallace K L, Suchard J R, Curry S C, Reagan C. Diagnostic use of physicians' detection of urine fluorescence in a simulated ingestion of sodium fluorescein-containing antifreeze.Ann Emerg Med 2001; 38:49–54., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Decker W J, Corby D G, Hilburn R E, Lynch R E. Adsorption of solvents by activated charcoal, polymers, and mineral sorbents.Vet Hum Toxicol 1981; 23:44–46., [PUBMED], [INFOTRIEVE], [CSA]
- DaRoza R, Henning R J, Sunshine I, Sutheimer C. Acute ethylene glycol poisoning.Crit Care Med 1984; 12:1003–1005., [PUBMED], [INFOTRIEVE], [CSA]
- Davis D P, Bramwell K J, Hamilton R S, Williams S R. Ethylene glycol poisoning: case report of a record-high level and a review.J Emerg Med 1997; 15:653–667., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vasavada N, Williams C, Hellman R N. Ethylene glycol intoxication: case report and pharmacokinetic perspectives.Pharmacotherapy 2003; 23:1652–1658., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cobaugh D J, Gibbs M, Shapiro D E, Krenzelok E P, Schneider S M. A comparison of the bioavailabilities of oral and intravenous ethanol in healthy male volunteers.Acad Emerg Med 1999; 6:984–988., [PUBMED], [INFOTRIEVE], [CSA]
- Lamminpaa A. Acute alcohol intoxication among children and adolescents.Eur J Pediatr 1994; 153:868–872., [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
Appendix 1
Expert Consensus Panel Members
Lisa L. Booze, Pharm.D.
Certified Specialist in Poison Information
Maryland Poison Center
University of Maryland School of Pharmacy
Baltimore, Maryland
E. Martin Caravati, M.D., M.P.H., F.A.C.M.T., F.A.C.E.P.
Professor of Surgery (Emergency Medicine)
University of Utah
Medical Director
Utah Poison Center
Salt Lake City, Utah
Gwenn Christianson, R.N., M.S.N.
Certified Specialist in Poison Information
Indiana Poison Center
Indianapolis, Indiana
Peter A. Chyka, Pharm.D., F.A.A.C.T., D.A.B.A.T.
Professor, Department of Pharmacy
University of Tennessee Health Science Center
Memphis, Tennessee
Daniel C. Keyes, M.D., M.P.H.
Medical Director
Pine Bluff Chemical Demilitarization Facility
Associate Professor, Southwestern Toxicology Training Program
Dallas, Texas
Anthony S. Manoguerra, Pharm.D., D.A.B.A.T., F.A.A.C.T.
Professor of Clinical Pharmacy and Associate Dean
School of Pharmacy and Pharmaceutical Sciences
University of California San Diego
Former Director, California Poison Control System, San Diego Division
San Diego, California
Kent R. Olson, M.D., F.A.C.E.P., F.A.A.C.T., F.A.C.M.T.
Medical Director
California Poison Control System, San Francisco Division
Clinical Professor of Medicine and Pharmacy
University of California, San Francisco
San Francisco, California
Elizabeth J. Scharman, Pharm.D., D.A.B.A.T., B.C.P.S., F.A.A.C.T.
Director, West Virginia Poison Center
Professor, West Virginia University School of Pharmacy, Department of Clinical Pharmacy
Charleston, West Virginia
Paul M. Wax, M.D., F.A.C.M.T.
Managing Director
Banner Poison Center
Professor of Clinical Emergency Medicine
University of Arizona School of Medicine
Phoenix, Arizona
Alan D. Woolf, M.D., M.P.H., F.A.C.M.T.
Director, Program in Environmental Medicine
Children's Hospital, Boston
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Appendix 2
Appendix 3
Secondary Review Panel Organizations
Ambulatory Pediatric Association
American Academy of Breastfeeding Medicine
American Academy of Emergency Medicine
American Academy of Pediatrics
American Association for Health Education
American College of Clinical Pharmacy
American College of Emergency Physicians
American College of Occupational and Environmental Medicine
American Public Health association
American Society of Health System Pharmacists
Association of Maternal and Child Health Programs
Association of Occupational and Environmental Clinics
Association of State and Territorial Health Officials
Canadian Association of Poison Control Centres
Centers for Disease Control and Prevention-National Center for Injury Prevention and Control
Consumer Federation of America
Consumer Product Safety Commission
Department of Transportation
Emergency Medical Services for Children
Emergency Nurses Association
Environmental Protection Agency
European Association of Poisons Control Centres and Clinical Toxicologists
Food and Drug Administration
National Association of Children's Hospitals and Related Institutions
National Association of Emergency Medical Services Physicians
National Association of Emergency Medical Technicians
National Association of School Nurses
National Association of State Emergency Medical Services Directors
National Safe Kids Campaign
Teratology Society
World Health Organization International Programme on Chemical Safety
