ABSTRACT
Objective: Iron deficiency is the most common nutrient deficiency in the world. While deficiency can often be resolved through dietary supplementation with iron, adverse events are common and frequently preclude compliance. The objective of this study was to determine whether a food-derived dietary supplement containing a low dose of iron and nutrients that increase iron absorption could resolve iron deficiency with fewer adverse events than reported at higher doses.
Methods: A pilot clinical trial (NCT02683369) was conducted among premenopausal women with nonanemic iron deficiency that was verified by blood screening. Participants consumed a dietary supplement (Blood Builder®/Iron Response®) once daily for 8 weeks containing 26 mg of iron, vitamin C, folate, and other food-derived nutrients. Primary outcomes were markers of iron status (serum ferritin, hemoglobin, soluble transferrin receptor, total body iron stores) and secondary outcomes were self-reported fatigue and energy. All outcomes were assessed at baseline and 8 weeks. Adverse events were monitored with questionnaires, daily diaries, and contact with a physician. Dependent samples t test and Wilcoxon signed-rank test were used to analyze outcomes.
Results: Twenty-three participants enrolled in the study. Iron deficiency was resolved in the sample (mean serum ferritin: baseline = 13.9 μg/L, 8 weeks = 21.1 μg/L, p < 0.001). All other markers of iron status, fatigue, and energy also improved during the study (p < 0.04). No adverse events were reported. Conclusions: While larger and controlled studies are needed to confirm these findings, a food-derived dietary supplement with a low dose of iron and absorption-enhancing nutrients resolved iron deficiency and improved all other markers of iron status without any adverse events.
Introduction
Iron is a mineral for which intake is required for proper human physiology. Among other functions, iron plays a key role in energy metabolism, oxygen transport, DNA replication and repair, and the production of neurotransmitters (Citation1–3). Iron can be obtained in the human diet from a variety of food sources. The more bioavailable heme form of iron is contained in meat and poultry and the less bioavailable nonheme form of iron is found in vegetables and legumes.
Despite the abundance of dietary sources of iron, iron deficiency is the most common nutrient deficiency throughout the world. More than 2 billion people worldwide experience some form of iron deficiency (Citation4–6) and the condition is often associated with fatigue, impaired energy metabolism, immune system dysregulation, and decreased cognition, work performance, and overall physical function (Citation4,Citation5,Citation7). Iron deficiency can occur with or without anemia. Nonanemic iron deficiency is classified as having normal hemoglobin (Hgb) levels (Hgb ≥ 12 g/dL) with low serum ferritin (SF) levels (SF < 20 μg/L) (Citation4,Citation7). While resolution of iron deficient anemia is very well studied, not as many studies have focused on resolving nonanemic iron deficiency.
Premenopausal women are at particularly high risk for iron deficiency. Globally, 22% of premenopausal women are iron-deficient Citation(8), and recent data from the National Health and Nutrition Examination Surveys revealed that 8% of premenopausal women in the United States were nonanemic iron-deficient Citation(9). Symptoms of iron deficiency often become apparent only in the more severe stages of the condition (Citation10,Citation11). Thus, there is substantial public health interest in resolving nonanemic iron deficiency due to the deleterious effects that low iron levels can have on human health and the importance of preventing the progression to anemia.
Guidelines to resolve nonanemic iron deficiency offered by the World Health Organization and other entities suggest that daily dietary supplementation with iron is often warranted (Citation6,Citation10). Iron supplementation has consistently been shown to increase SF Citation(12) and protect against progression to iron-deficient anemia Citation(13). Confounding the dietary supplementation guidelines are the many different forms of iron supplements that are commercially available Citation(14). Iron is most commonly contained in dietary supplements as ferrous and ferric iron salts such as ferrous sulfate, ferrous gluconate, ferrous citrate, or ferric sulfate (Citation14–16). The specific iron salts utilized in dietary supplements and their solubility affect their bioavailability (Citation16–19). Interactions with other nutrients can also impact the bioavailability of iron. For example, iron absorption has been shown to be synergistically increased by both vitamin C Citation(18) and folate Citation(20).
Despite differences in solubility and absorption among various iron supplements, guidelines for resolving iron deficiency do not recommend specific iron salts or formulations but rather offer blanket recommended daily iron supplemental dosages of 60 to 120 mg for iron deficiency Citation(11). This is a substantial increase over both the 18-mg recommended dietary allowance for women 19 to 50 years of age and the tolerable upper intake level (UL) of 45 mg Citation(21). The relatively high doses of iron that are recommended to iron-deficient women may contribute to the common incidence of adverse events that often interfere with compliance. The most common adverse events affect the gastrointestinal system and include constipation, nausea, and vomiting (Citation7,Citation12,Citation13,Citation21). The optimal form and dosage of iron supplementation that successfully improves iron status while minimizing the incidence of adverse events has not yet been established.
Accordingly, there is growing interest in determining whether lower doses of iron can increase markers of iron status without causing the adverse events that so commonly occur. A 2016 systematic review and meta-analysis of iron supplementation for iron-deficient premenopausal women found an increased risk of adverse events for daily doses greater than 60 mg Citation(13). Another systematic review concluded that weekly, as opposed to daily, doses of 60 to 120 mg may be adequate for nonanemic iron deficiency Citation(22). Direct dose-comparison studies in iron-deficient women have also shown that iron absorption was maximized by providing lower doses (up to 80 mg ferrous sulfate) and avoiding twice-daily doses Citation(23). In addition, a 60-mg dose was shown to be as effective as an 80-mg dose in resolving iron deficiency Citation(7).
Nevertheless, even 60 mg of iron still falls above the UL and may contribute to the occurrence of adverse events that commonly prevent compliance with iron supplementation. Some (Citation24–26) but not all (Citation27,Citation28) lower-dose studies with less than the UL of iron utilizing a mean of 27 mg iron in various forms have demonstrated significant increases in SF. Given these inconsistent results, there is a need for additional clinical research evaluating whether iron supplementation at doses below the UL can effectively resolve iron deficiency without causing adverse events.
The objective of this pilot clinical trial was to assess the efficacy of a low-dose iron dietary supplement (MegaFood Blood Builder®/Innate Response Iron Response®) on raising markers of iron status among premenopausal women with nonanemic iron deficiency. This food-derived supplement contained just 26 mg of iron, along with vitamin C, folate, and other elements in food that enhance iron absorption. Food-derived dietary supplements have become increasingly popular on the premise that lower nutrient doses are required to resolve nutrient deficiency due to synergy in food components, although clinical studies are needed to verify this premise. Considering the suggestive evidence of efficacy of low-dose iron and the absorption-enhancing nutrients contained in this formulation, the investigators hypothesized that this low-dose iron supplement would increase iron levels among premenopausal women with nonanemic iron deficiency with fewer adverse events than typically experienced with higher doses of iron.
Materials and methods
Study design
An 8-week pilot clinical trial assessing the tolerability and efficacy of a dietary supplement containing a low dose of iron and nutrients known to increase iron absorption was conducted among premenopausal women with nonanemic iron deficiency in Baltimore, Maryland, from May 2016 to April 2017. The 8-week duration of the clinical trial was deemed sufficient to detect the potential resolution of iron deficiency, in light of the many previous clinical trials of iron supplements that were 8 weeks in duration or shorter (Citation4,Citation27,Citation29–37), without subjecting our iron-deficient study sample to a low-dose iron intervention of as yet unknown efficacy for an unnecessarily long period of time. Similarly, a control group was not included since the primary goal of the clinical trial was to first establish whether the low-dose of iron with synergistic nutrients was efficacious at resolving iron deficiency prior to conducting any comparisons to other formulations. The clinical trial received ethical approval from both Western Institutional Review Board and the Institutional Review Board (IRB) of the University of Maryland School of Medicine and was registered on ClinicalTrials.gov (NCT02683369).
Participant selection
Premenopausal women in the Baltimore, Maryland, metropolitan area were recruited from the community via flyers and social media outlets. Interested participants contacted a research associate for an initial telephone screening of study eligibility criteria related to demographics, health status, etc. Participants who met the initial eligibility criteria screening were subsequently scheduled for a blood screening at an IRB-approved medical practice located at University of Maryland St. Joseph Medical Center. Blood was collected from each potential participant to assess her levels of Hgb (to determine anemia status) and ferritin (to determine iron deficiency status). Participants whose results demonstrated nonanemic iron deficiency (ferritin < 20 μg/L, Hgb ≥ 12 g/dL) were deemed eligible to participate in the study. Those whose Hgb demonstrated anemia (< 12 g/dL) were referred to medical care.
The main inclusion criteria of the study included premenopausal females at least 18 years of age with nonanemic iron deficiency identified during the blood screening. Eligible participants were also required to be able to understand and write English, agreed to continue with current diet and any dietary supplements, and voluntarily consented to participation in the study and understanding potential risks and adverse events. Exclusion criteria included pregnancy or breastfeeding; daily use of an iron-containing supplement (other than multivitamin) within the past 2 weeks; known allergies to any substance in the study product; taking medication that may interfere with the absorption of iron; history of alcohol, drug, or medication abuse; current tobacco smoking; donation of blood in the past month or plans to do so at any time during the study; and current diagnosis of inflammatory bowel disease (e.g., Crohn's disease or ulcerative colitis).
Intervention procedures
Eligible participants were scheduled for a baseline study visit at the medical practice with a research associate. During the baseline visit, the research associate obtained informed consent and instructed the participant to take one tablet of the iron supplement per day in the morning. The participant was also instructed not to change her diet or dietary supplementation during the study. The research associate also administered questionnaires to assess each of the following during the week prior to the baseline visit: consumption of iron-containing foods (e.g., red meat, chicken, lentils, and beans) through a food frequency questionnaire, multivitamin or other dietary supplement usage, fatigue and energy levels on a 1-to-5 Likert scale, and the frequency and severity of gastrointestinal discomfort (constipation, nausea, vomiting, and diarrhea). These assessments enabled the determination of changes in these potential confounders of iron status as well as documentation of any preexisting gastrointestinal discomfort prior to the study.
A blood draw was also performed during the baseline visit to account for any fluctuations in iron levels that might have occurred in the time between the screening assessment and the commencement of supplementation after the baseline visit. The baseline blood draw analyzed an important marker of iron status, soluble transferrin receptor (sTfR), in addition to Hgb and ferritin. The collection of sTfR also allowed for the calculation of total body iron stores. Both of these outcomes are described in a subsequent section.
The baseline visit concluded with the research associate providing the bottle containing the dietary supplement along with instructions to consume the supplement once per day. A daily diary was also provided to the participant to record dietary supplement compliance, any changes in medications, and any adverse events that occurred. Participants were instructed to directly contact a physician co-investigator immediately if there were major adverse events.
A research associate performed telephone follow-ups with each participant after 3 and 6 weeks to obtain ongoing assessments of changes in dietary iron intake via food frequency questionnaire, multivitamin or other dietary supplement usage, gastrointestinal adverse events, and any other adverse events reported by the participant. At the conclusion of the 8-week study, a follow-up visit with a research associate was conducted at the medical office. The follow-up visit involved a blood draw for postintervention evaluation of the iron markers as well as collection of diaries; pill count to further assess compliance with the iron supplement regimen; and administration of questionnaires to assess the consumption of iron-containing foods, energy levels and fatigue, and gastrointestinal discomfort or any other adverse events during the previous week.
Study supplement
The dietary supplement under study (Blood Builder®/Iron Response®) has been commercially available in its current form since 2005 and is commonly used by people experiencing iron deficiency to help increase their iron levels and improve energy. Each tablet contained 26 mg of elemental iron cultured by Saccharomyces cerevisiae. The 26-mg dose of elemental iron was selected for this low-dose clinical trial because it was one of the few commercially available iron dietary supplements that is dosed below the 45-mg UL and it contains numerous nutrients known to increase iron absorption. In addition to the 26 mg of iron, the supplement also contained 125 mg of beetroot, 15 mg of vitamin C (orange), 400 mcg of folate (broccoli), and 6 mcg of vitamin B12 (S cerevisiae).
Study outcomes
Baseline and follow-up blood draws were performed and analyzed in an onsite CLIA-certified laboratory at University of Maryland St. Joseph Medical Center. The markers of iron status in this study were Hgb, SF, and sTfR. Hgb, assessed with Beckman Coulter LH 780 hematology analyzer (Quest Diagnostics), is a protein that carries oxygen though the circulatory system and serves as the primary iron pool in humans. The majority of the remaining storage iron is used to regenerate Hgb and is considered an important indicator of iron status. Key iron storage proteins include SF and sTfR. SF, assessed with Vitros Immunodiagnostic Products Ferritin Reagent Pack (Quest Diagnostics), is the most commonly utilized marker of iron deficiency in clinical practice the levels of which reflect intracellular iron stores. sTfR, assessed with nephelometry (Quest Diagnostics), reflects the number of iron receptors expressed on cellular membranes and it increases as iron stores become lower. All three biomarkers (Hgb, SF, and sTfR) are commonly measured in studies assessing the effect of iron intake on iron status (Citation4,Citation7,Citation13).
The final marker of iron status in this study was total body iron stores. This marker helps detect tissue iron deficiencies by quantifying the amount of SF and sTfR and is calculated as follows:
This method of determining total body iron using stores has been published Citation(38), validated, and utilized as a marker of iron status in many iron interventions (Citation4,Citation39–42).
In addition to the markers of iron status that served as the primary outcomes in this study, secondary outcomes included patient-reported fatigue and energy levels. These outcomes were scored on 1-to-5 Likert scales as follows: frequency of fatigue (1 = never fatigued, 2 = occasionally fatigued, 3 = frequently fatigued, 4 = very frequently fatigued, 5 = constantly fatigued), severity of fatigue (1 = very minor or no fatigue, 2 = minor fatigue, 3 = moderate fatigue, 4 = high fatigue, 5 = very high fatigue), and energy (1 = very low energy, 2 = low energy, 3 = moderate energy, 4 = high energy, 5 = very high energy).
Assessment of adverse events
In light of the high incidence of adverse events in previous clinical trials of iron supplementation, the assessment of adverse events was an area of focus in this study. Participants were specifically asked about the frequency and severity of gastrointestinal discomfort (constipation, nausea, vomiting, and diarrhea) during the past week at baseline, 3 weeks, 6 weeks, and follow-up concluding the 8-week study. In addition, the participant was provided a daily diary and was instructed to record any adverse events that occurred in their diary and to immediately contact a physician co-investigator on the research team for any major adverse events.
Statistical methods
Continuous data were reported as mean and standard deviation or median and interquartile range, depending on normality assumptions. Categorical data were reported as counts and percentages. Changes in Hgb, SF, total body iron stores, fatigue, and energy over the 8-week treatment period were assessed by dependent samples t test. Values for sTfR were nonnormally distributed and were analyzed using Wilcoxon signed-rank test. Statistical significance was defined as p < 0.05. Statistical analyses were performed using Predictive Analytics Software (v. 22; IBM, Inc.).
Results
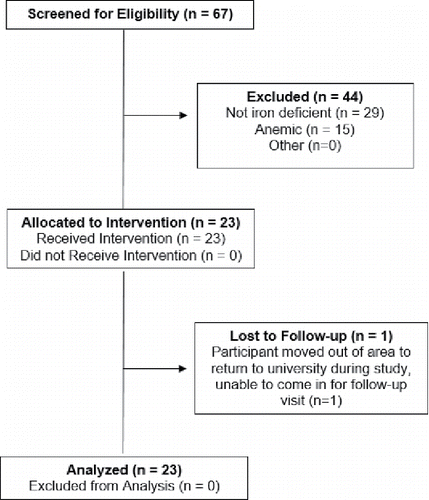
As revealed in , a total of 67 premenopausal women participated in blood screening to enable determination of nonanemic iron deficiency eligibility status. Twenty-nine of those screened were not iron-deficient (SF > 20 μg/L), and 15 were anemic (Hgb < 12 g/dL) and referred to medical care. The remaining 23 eligible participants provided informed consented, completed a baseline study visit, and were allocated to the dietary supplement intervention. Of the 23 participants who enrolled, 22 participants (95.6%) completed the study. The sole dropout in the study moved from the area to return to university and was unable to travel to the follow-up visit.
characterizes the study population at baseline. In brief, the study sample was approximately 30 years of age, normal weight (mean body mass index = 24.4 kg/m2), racially diverse (52% white, 48% black or other), and highly educated (87% had at least a college degree).
Table 1. Baseline Participant Characteristics (n = 23).
The changes in the markers of status from baseline to the conclusion of the study are provided in . All markers of iron status showed statistically significant improvements from baseline to the 8-week follow-up visit (p < 0.04). Most notably, the sample experienced a mean increase of 7.2 μg/L in SF. This increase represented resolution of iron deficiency back to normal range (mean baseline SF = 13.9 μg/L, mean follow-up SF = 21.1 μg/L, p < 0.001). Similar improvements were also noted for Hgb, sTfR, and total body iron stores (p < 0.04).
Table 2. Markers of Iron Status.
The changes in fatigue and energy levels from baseline to the conclusion of the study are provided in . There were modest but statistically significant reductions (p < 0.001) in fatigue frequency, fatigue severity, and composite fatigue score (frequency × severity). Energy also increased (p < 0.001) among the study sample.
Table 3. Fatigue and Energy During the Previous Week.
While there was a slight decrease in red meat intake from the week before the baseline visit (mean = 1.3 servings) to the week before the 8-week follow-up visit (mean = 0.9 servings), this decrease was not significant (p = 0.1) and there were no changes in intake of any other foods containing high amounts of iron during the study. Only three participants were consuming a multivitamin at baseline, and there were no changes in multivitamin or other dietary supplement usage (neither beginning nor stopping) among any participants during the study.
There were no changes in frequency, severity, or composite score (frequency × severity) of any of the symptoms of gastrointestinal distress (constipation, nausea, vomiting, and diarrhea) from baseline to the conclusion of the study (p > 0.3). Moreover, there were no adverse events of any sort reported during the course of the study.
Discussion
The dietary supplement under study (Blood Builder®/Iron Response®) restored mean SF levels among a sample of nonanemic iron-deficient women back into normal range during this 8-week pilot clinical trial. All other markers of iron status (Hgb, sTfR, total body iron stores) also demonstrated improvements from baseline to the conclusion of this brief study (p < 0.04). Alongside the improvements noted in all of the markers of iron status, there were modest increases noted in energy and reductions in fatigue (p < 0.001).
The improvements in the markers of iron status among this iron-deficient study sample are particularly noteworthy in light of both the very low dose of iron (26 mg) contained in the dietary supplement and the complete absence of adverse events reported during this study. The investigators believe that the absence of adverse events may have been due largely to the much lower dose of iron in this dietary supplement compared to doses that have typically been studied. While some recent studies have shown that 60 mg of iron may ameliorate iron deficiency as efficaciously as higher doses of between 80 and 120 mg, adverse events associated with iron supplementation are still quite common even at 60-mg doses. In fact, the mean incidence of adverse events in previous clinical trials of iron supplementation among other samples of iron-deficient premenopausal women is approximately 34% (Citation7,Citation13,Citation30,Citation32,Citation33,Citation43–45). The relatively high incidence of adverse events noted previously is not terribly surprising, as most of these studies utilized doses above the tolerable UL of 45 mg of iron. Consequently, the investigators conducted a rigorous assessment of adverse events, including probing by a research associate throughout the study for gastrointestinal (constipation, nausea, vomiting, and diarrhea) and any other types of distress, which makes the lack of adverse events in this study compelling.
The investigators offer several hypotheses as to how this dietary supplement was able to restore iron levels at such a low dose. The inclusion of synergistic nutrients in this dietary supplement along with the iron—most notably folate and vitamin C, which have been previously shown to increase iron absorption (Citation18,Citation20)—may have resulted in its efficacy at a low dose. While less is currently known about the impact of beetroot and vitamin B12 on iron absorption, our findings suggest that they might merit future study as adjuvant ingredients in iron supplements. However, the addition of novel dietary ingredients to iron formulas must be selected and studied carefully, as some dietary polyphenols have been shown to decrease iron absorption (Citation46,Citation47).
In addition to the synergistic nutrients included in this supplement, the food-derived process through which the iron was obtained might have also contributed to its efficacy at a low dose. The form of iron and binding salts contained in dietary supplements have previously been shown to impact the solubility and bioavailability in humans. For instance, ferrous iron has been shown to be more soluble and with higher bioavailability than ferric iron (Citation16,Citation18). It is possible that the iron attained from S cerevisiae culturing of whole-food sources may have resulted in a more soluble and bioavailable form of iron that ultimately lent itself to enhanced efficacy in our study sample. Comparative studies of the solubility and bioavailability of this food-derived form of iron versus other forms would allow for a more conclusive determination of this potential mechanism. Food-derived dietary supplements in general have become increasingly popular in recent years and more studies are needed to determine whether there is indeed additional synergy among nutrients in these types of supplements as compared to synthetic versions.
The accompanying synergistic nutrients and food-derived form of iron in this dietary supplement might have also contributed to the absence of adverse events noted in this study. Clinical trials of iron supplementation with co-administration of vitamin C or citrus juice containing high levels of vitamin C have reported markedly lower rates of adverse events as compared to iron supplementation alone (Citation25,Citation34,Citation44). Thus, the vitamin C and other accompanying dietary ingredients in this supplement might have provided some protection against the incidence of adverse events in this study. Adverse events have also varied by the form of the iron supplement in previous studies, with the highest mean incidence of adverse events approximately 91% for carbonyl iron Citation(33) and the lowest mean incidence of adverse events of approximately 17% for both ferrous gluconate (Citation48,Citation49) and ferrous sulfate (Citation4,Citation7,Citation28,Citation30,Citation32,Citation45,Citation49–51). Future studies would be needed to elucidate the specific mechanisms underlying the lack of adverse events for the dietary supplement utilized in this study.
While the findings in this study are promising, there were several notable limitations that are worthy of consideration. As an uncontrolled pilot trial aimed at assessing the tolerability and preliminary efficacy of this low-dose iron-containing dietary supplement, the inference is limited until larger, randomized, and placebo-controlled studies can be conducted. As there is no endogenous production of iron in human beings, very little placebo effect was believed to have occurred with respect to the improvements noted across the comprehensive set of blood-based biomarkers of iron status that served as the primary outcomes in this study. However, while the increases in energy and reductions in fatigue are notable in light of the limited room for improvement from baseline (participants were neither markedly low in energy nor fatigued at baseline), these participant-reported outcomes are far more prone to the placebo effect and inference should be viewed in that limited context. Controlled studies comparing this efficacious dietary supplement to equal doses of other forms of iron in two-arm clinical trials, as well as to equal doses of food-derived iron without synergistic nutrients in three-arm clinical trials, appear warranted to establish the comparative efficacy of this unique iron formulation.
Another related limitation was the lack of standardized diets provided to participants to control for any changes in dietary iron intake during the study. A standardized diet was viewed as infeasible for the participants in this community-based study and, to the authors' knowledge, has never been implemented in previous clinical studies of this nature. In order to control for potential confounding introduced by changes in iron-containing foods during the study, the investigators administered food frequency questionnaires including intake of a variety of iron-containing foods at all time points in the study (baseline, 3 weeks, 6 weeks, and 8-week follow-up) and there were no statistically significant changes in dietary intake noted over the course of the study (p ≥ 0.1). The only dimension of dietary intake that even trended toward a change throughout the study was red meat, which decreased from 1.3 servings in the week prior to the baseline visit to 0.9 servings per week in the week prior to the 8-week visit (p = 0.1). Red meat is one of the richest sources of bioavailable heme iron in the diet and, as such, the improvements in the markers of iron status noted in this study might have been slightly underestimated by the slight decrease in mean red meat intake that occurred during the study. There were also no changes reported in multivitamin usage or any other forms of dietary supplementation during the study. Nevertheless, dietary intake assessment is imperfect and it is possible that unreported changes in iron intake during the study might have influenced the observed efficacy of the dietary supplement on improving markers of iron status.
Menstruation can also impact markers of iron status. While the study sample was limited to premenopausal women on this basis to reduce the potential for confounding within a mixed pre- and postmenopausal sample, the timing of menstrual cycles during the study was not assessed and this might have had an impact on some participants. Last, while data are conflicting, inflammation may also impact some markers of iron status Citation(52) and changes in inflammation were not assessed in this study. In order to more directly account for the potential impact of inflammation on the iron status of our sample, the investigators included sTfR among the markers of iron status assessed in this study as it is robust to changes in inflammation Citation(53). The improvements in sTfR among our sample suggest that inflammation did not have an influence on the resolution of iron deficiency in this study, although future studies might include markers of inflammation alongside sTfR to allow for direct and indirect assessment of the potential impact of inflammation on iron status. Larger and controlled trials in the future would help overcome the primary limitations of this pilot study.
The findings in this study raise intriguing possibilities regarding the potential for nutrient synergy in iron dietary supplement formulation, which may enable lower doses of iron than are typically recommended to resolve iron deficiency while simultaneously reducing the high incidence of adverse events that often interfere with compliance at higher doses. Nutrient synergy is common in calcium dietary supplements, which often contain vitamin D and other nutrients known to increase calcium absorption, and most commercially available iron dietary supplements have not yet espoused this approach. While the findings in this pilot study are promising, larger and controlled studies are needed to more conclusively determine the efficacy of the approach of low-dose iron with synergistic nutrients to resolve iron deficiency.
Conclusion
The resolution of iron deficiency and improvements in all other markers of iron status with just 26 mg of supplemental iron in this iron-deficient study sample, without the occurrence of any adverse events, is highly encouraging. Future mechanistic and clinical studies comparing this low-dose iron supplement with synergistic food-derived nutrients to synthetically isolated and higher-dosage iron supplements known to have high rates of adverse events appear warranted.
Disclosure
The authors have no personal financial interest in the work or with a commercial sponsor.
Additional information
Funding
References
- Huebers H. Iron metabolism: iron transport and cellular uptake mechanisms. In: Lonnerdal B, editor. Iron metabolism in infants. Boca Raton (FL): CRC Press; 1989. p. 163.
- Anderson G, Vulpe C. The cellular physiology of iron. In: Yehuda S, Mostofsky DI editors. Iron deficiency and overload: from basic biology to clinical medicine. Totowa, NJ: Humana Press; 2010. p. 3–29.
- Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem. 2014;25:1101–1107. doi:10.1016/j.jnutbio.2014.07.003. PMID:25154570.
- DellaValle DM, Haas JD. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med Sci Sports Exerc. 2014;46:1204–1215. doi:10.1249/MSS.0000000000000208. PMID:24195864.
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi:10.1001/jama.1997.03540360041028. PMID:9091669.
- Stoltzfus RJ, Dreyfuss ML. Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Washington (DC): ILSI Press; 1998.
- Leonard AJ, Chalmers KA, Collins CE, Patterson AJ. Comparison of two doses of elemental iron in the treatment of latent iron deficiency: efficacy, side effects and blinding capabilities. Nutrients. 2014;6:1394–1405. doi:10.3390/nu6041394. PMID:24714351.
- Galan P, Yoon HC, Preziosi P, Viteri F, Valeix P, Fieux B, Briancon S, Malvy D, Roussel AM, Favier A, Hercberg S. Determining factors in the iron status of adult women in the SU.VI.MAX study. Eur J Clin Nutr. 1998;52:383–388. doi:10.1038/sj.ejcn.1600561. PMID:9683388.
- Sekhar DL, Murray-Kolb LE, Kunselman AR, Weisman CS, Paul IM. Association between menarche and iron deficiency in non-anemic young women. PLoS One. 2017;12:e0177183. doi:10.1371/journal.pone.0177183. PMID:28486542.
- World Health Organization. Iron deficiency anaemia: Assessment, prevention and control. A guide for programme managers. Geneva (Switzerland): World Health Organization; 2001.
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47:1–29.
- Smith GA, Fisher SA, Doree C, Di Angelantonio E, Roberts DJ. Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors. Cochrane Database Syst Rev. 2014; 7(Jul): 1–153.
- Low MS, Speedy J, Styles CE, De-Regil LM, Pasricha SR. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst Rev. 2016; 4(Apr): 1–225. PMID:27087396.
- National Institutes of Health, Office of Dietary Supplements: Iron: dietary supplement fact sheet [Internet]. Available from: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/#en5.
- Manoguerra AS, Erdman AR, Booze LL, Christianson G, Wax PM, Scharman EJ, Woolf AD, Chyka PA, Keyes DC, Olson KR, Caravati EM, Troutman WG. Iron ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2005;43:553–570. doi:10.1081/CLT-200068842. PMID:16255338.
- Murray-Kolbe LE, Beard J. Iron, 2nd edition. New York: Informa Healthcare; 2010.
- Yetley EA. Multivitamin and multimineral dietary supplements: definitions, characterization, bioavailability, and drug interactions. Am J Clin Nutr. 2007;85:269S–276S. PMID:17209208.
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S–1467S. doi:10.3945/ajcn.2010.28674F. PMID:20200263.
- Bueno L, Pizzo JC, Marchini JS, Dutra-de-Oliveira JE, Dos Santos JE, Barbosa Junior F. Iron (FeSo4) bioavailability in obese subjects submitted to bariatric surgery. Nutr Hosp. 2013;28:100–104. PMID:23808436.
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, Katz J, West KP. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. J Nutr. 2003;133:3492–3498. PMID:14608063.
- Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): The National Academies Press; 2001.
- Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Intermittent iron supplementation for reducing anaemia in US preschool children and nonpregnant females of childbearing age: National health and nutrition examination survey 2003–2006. Am J Clin Nutr. 2009;89:1334–1342. doi:10.3945/ajcn.2008.27151. PMID:19357218.
- Moretti D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H, Melse-Boonstra A, Brittenham G, Swinkels DW, Zimmermann MB. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981–1989. doi:10.1182/blood-2015-05-642223. PMID:26289639.
- Hoppe M, Brun B, Larsson MP, Moraeus L, Hulthen L. Heme iron-based dietary intervention for improvement of iron status in young women. Nutrition. 2013;29:89–95. doi:10.1016/j.nut.2012.04.013. PMID:22951158.
- Taniguchi M, Imamura H, Shirota T, Okamatsu H, Fujii Y, Toba M, Hashimoto F. Improvement in iron deficiency anemia through therapy with ferric ammonium citrate and vitamin C and the effects of aerobic exercise. J Nutr Sci Vitaminol (Tokyo). 1991;37:161–171. doi:10.3177/jnsv.37.161. PMID:1919803.
- Fogelholm M, Suominen M, Rita H. Effects of low-dose iron supplementation in women with low serum ferritin concentration. Eur J Clin Nutr. 1994;48:753–756. PMID:7835330.
- Marks DC, Speedy J, Robinson KL, Brama T, Capper HR, Mondy P, Keller AJ. An 8-week course of 45 mg of carbonyl iron daily reduces iron deficiency in female whole blood donors aged 18 to 45 years: Results of a prospective randomized controlled trial. Transfusion. 2014;54:780–788. doi:10.1111/trf.12464. PMID:24660763.
- Mujica-Coopman MF, Borja A, Pizarro F, Olivares M. Effect of daily supplementation with iron and zinc on iron status of childbearing age women. Biol Trace Elem Res. 2015;165:10–17. doi:10.1007/s12011-014-0226-y. PMID:25582309.
- Ballin A, Berar M, Rubinstein U, Kleter Y, Hershkovitz A, Meytes D. Iron state in female adolescents. Am J Dis Child. 1992;146:803–805. PMID:1496946.
- Maghsudlu M, Nasizadeh S, Toogeh GR, Zandieh T, Parandoush S, Rezayani M. Short-term ferrous sulfate supplementation in female blood donors. Transfusion. 2008;48:1192–1197. doi:10.1111/j.1537-2995.2007.01671.x. PMID:18363581.
- Pereira DI, Couto Irving SS, Lomer MC, Powell JJ. A rapid, simple questionnaire to assess gastrointestinal symptoms after oral ferrous sulphate supplementation. BMC Gastroenterol. 2014;14:103. doi:10.1186/1471-230X-14-103. PMID:24899360.
- Waldvogel S, Pedrazzini B, Vaucher P, Bize R, Cornuz J, Tissot JD, Favrat B. Clinical evaluation of iron treatment efficiency among non-anemic but iron-deficient female blood donors: A randomized controlled trial. BMC Med. 2012;10:8. doi:10.1186/1741-7015-10-8. PMID:22272750.
- Gordeuk VR, Brittenham GM, Hughes MA, Keating LJ. Carbonyl iron for short-term supplementation in female blood donors. Transfusion. 1987;27:80–85. doi:10.1046/j.1537-2995.1987.27187121482.x. PMID:3810831.
- Zhu YI, Haas JD. Response of serum transferrin receptor to iron supplementation in iron-depleted, nonanemic women. Am J Clin Nutr. 1998;67:271–275. doi:10.1093/ajcn/67.2.271. PMID:9459375.
- Brownlie Tt, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–443. PMID:14985219.
- Binkoski AE, Kris-Etherton PM, Beard JL. Iron supplementation does not affect the susceptibility of LDL to oxidative modification in women with low iron status. J Nutr. 2004;134:99–103. PMID:14704300.
- Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol. 2000;88:1103–1111, Online publication. doi:10.1152/jappl.2000.88.3.1103. PMID:10710409.
- Cook J, Flowers CF, Skikne B. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. doi:10.1182/blood-2002-10-3071. PMID:12521995.
- Biebinger R, Zimmermann MB, Al-Hooti SN, Al-Hamed N, Al-Salem E, Zafar T, Kabir Y, Al-Obaid I, Petry N, Hurrell RF. Efficacy of wheat-based biscuits fortified with microcapsules containing ferrous sulfate and potassium iodate or a new hydrogen-reduced elemental iron: a randomised, double-blind, controlled trial in Kuwaiti women. Br J Nutr. 2009;102:1362–1369. doi:10.1017/S0007114509990353. PMID:19653920.
- Haas JD, Beard JL, Murray-Kolb LE, del Mundo AM, Felix A, Gregorio GB. Iron-biofortified rice improves the iron stores of nonanemic Filipino women. J Nutr. 2005;135:2823–2830. PMID:16317127.
- Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–787. PMID:17344500.
- Zimmermann MB, Winichagoon P, Gowachirapant S, Hess SY, Harrington M, Chavasit V, Lynch SR, Hurrell RF. Comparison of the efficacy of wheat-based snacks fortified with ferrous sulfate, electrolytic iron, or hydrogen-reduced elemental iron: Randomized, double-blind, controlled trial in Thai women. Am J Clin Nutr. 2005;82:1276–1282. PMID:16332661.
- Rybo E, Bengtsson C, Hallberg L, Oden A. Effect of iron supplementation to women with iron deficiency. Scand J Haematol Suppl. 1985;43:103–114. PMID:3863237.
- Bryson D. Iron and anaemia in adolescent girls. A double-blind trial. The Practitioner. 1968;200:694–697. PMID:4870203.
- Flink H, Tegelberg A, Thorn M, Lagerlof F. Effect of oral iron supplementation on unstimulated salivary flow rate: A randomized, double-blind, placebo-controlled trial. J Oral Pathol Med. 2006;35:540–547. doi:10.1111/j.1600-0714.2006.00450.x. PMID:16968234.
- Rutzke CJ, Glahn RP, Rutzke MA, Welch RM, Langhans RW, Albright LD, Combs GF, Wheeler RM. Bioavailability of iron from spinach using an in vitro/human Caco-2 cell bioassay model. Habitation (Elmsford). 2004;10:7–14. doi:10.3727/154296604774808900. PMID:15880905.
- Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR, Mills W, Charlton RW, Mayet F. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr. 1983;49:331–342. doi:10.1079/BJN19830042. PMID:6860621.
- Kiss JE, Brambilla D, Glynn SA, Mast AE, Spencer BR, Stone M, Kleinman SH, Cable RG, for the National Heart L, Blood Institute Recipient Epidemiology and Donor Evaluation Study-III. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313:575–583. doi:10.1001/jama.2015.119. PMID:25668261.
- Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, Haya-Palazuelos J, Ciria-Recasens M, Manasanch J, Perez-Edo L. Tolerability of different oral iron supplements: A systematic review. Curr Med Res Opin. 2013;29:291–303. doi:10.1185/03007995.2012.761599. PMID:23252877.
- Pulani L, Sunethra A, Geethanjali dS, Swarnamali S, Lakshmi D. Evaluation of nutrition education for improving iron status in combination with daily iron supplementation. Food Nutr Bull. 2000;21:259–269. doi:10.1177/156482650002100302.
- Viteri FE, Ali F, Tujague J. Long-term weekly iron supplementation improves and sustains nonpregnant women's iron status as well or better than currently recommended short-term daily supplementation. J Nutr. 1999;129:2013–2020. PMID:10539778.
- Ross AC. Impact of chronic and acute inflammation on extra- and intracellular iron homeostasis. Am J Clin Nutr. 2017;106(Suppl 6):1581S–1587S. doi:10.3945/ajcn.117.155838.
- Al-Saqladi AWM, Bin-Gadeem HA, Brabin BJ. Utility of plasma transferrin receptor, ferritin and inflammatory markers in children with sickle cell disease. Paediatr Int Child Health. 2012;32:27–34. doi:10.1179/2046905511Y.0000000009. PMID:22525445.