Abstract
Objective: Our objective was to synthesize both trial and observational studies and undertake a meta-analysis to explore the associations between calcium from dietary and supplemental intakes and cardiovascular disease (CVD) risks.
Methods: Data sources were from PubMed, Cochrane Central, Scopus, and Web of Science, published from the inception dates up to March 2019. Randomized controlled trials (RCTs) and prospective cohort studies with data on dietary or supplemental intake of calcium, with or without vitamin D, and cardiovascular outcomes, were included.
Results: Of the 1,212 identified studies, 26 prospective cohort studies and 16 RCTs were included. Results of cohort studies reveled that dietary calcium intakes (DCIs) ranging from 200 to 1500 mg/d did not affect the risk of CVD, coronary heart disease (CHD), and stroke (relative risk (RR) RR for CVD = 0.96, 95% CI, 0.87–1.05; RR for CHD = 0.98, 95% CI, 0.88–1.08; RR for stroke = 0.94, 95% CI, 0.85–1.04). Pooled RR of RCTs showed that the risk of CHD due to calcium supplements (CSs) increased 8% (RR = 1.08, 95% CI, 1.02–1.22; I2 = 0.0%) and increased 20% allocated to CSs alone (RR = 1.20, 95% CI, 1.08–1.33; I2 = 0.0%). CSs increased the risk of myocardial infarction (MI) by 14% (RR = 1.14, 95% CI, 1.05–1.25; I2 = 0.0%), and CSs alone increased the MI risk 21% (RR = 1.21, 95% CI, 1.08–1.35; I2 = 0.0%).
Conclusions: We concluded that calcium intake from dietary sources do not adequately increase the risk of CVD including CHD and stroke, while calcium supplements might raise CHD risk, especially MI.
Introduction
Calcium is a macro mineral that plays many critical biological roles, especially in bone physiology and the cardiovascular (CV) system (Citation1). The Institute of Medicine (IOM) and the National Institutes of Health (NIH) recommend a daily calcium intake of 1200 mg to 1500 mg for women as well as men older than 65 years (Citation2,Citation3).
The beneficial effects of calcium intake on nonskeletal outcomes, mainly including the incidence and mortality of cardiovascular disease (CVD), have received growing attention. Several prospective cohort and randomized studies have demonstrated that calcium was associated with beneficial cardiovascular effects (Citation4–7), shown to be associated significantly with beneficial effects regarding hypertension (Citation8), coronary heart disease (CHD) (Citation9), vascular disease (Citation10), and stroke (Citation11) by several cohort studies. Consequently, increasing calcium intake has been recommended by many professionals, and calcium supplements (CSs) as are widely used in the elderly population (Citation12). However, many studies have questioned the beneficial cardiovascular effects of calcium intake and have shown that calcium intake is associated with increased mortality or the risk of myocardial infarction (MI) and stroke (Citation13), especially among those taking CSs (Citation2).
Overall, our literature search identified several systematic reviews on this topic, but none synthesized both trials and observational studies. We undertook a meta-analysis to establish the evidence about the associations between calcium from dietary and supplemental intake and the risk of cardiovascular events.
Methods
Search strategy
We followed the Meta-analysis of Observational Studies in Epidemiology (Citation14) and randomized controlled trials (RCTs) in PRISMA method for conducting and reporting the present study.
A meta-analysis of prospective cohort studies was carried out to evaluate the associations between dietary and supplemental calcium intake and the risk or mortality of CVD. In addition, the meta-analysis of RCTs was conducted to evaluate the associations between CSs and the incidence or mortality of CVD.
A literature search was performed in PubMed, Cochrane Central, Scopus, and Institute for Scientific Information from the inception dates to March 2019. Databases were searched systematically by three independent investigators (CY, XLS, DP). The computer-based searches included the keywords “calcium supplements,” “calcium intake,” “calcium supplementation,” “dietary calcium,” and “supplemental calcium” and outcomes terms including “cardiovascular disease,” “angiocardiopathy,” “angina pectoris,” “myocardial infarction,” “coronary disease,” “coronary heart disease,” “stroke,” “cerebrovascular disorders,” “hemorrhagic stroke,” and “ischemic stroke.” Moreover, the reference or citation lists from the retrieved articles, systematic reviews, and meta-analyses were checked to search for further relevant studies. The language was limited to English.
Inclusion criteria and exclusion strategy
Studies were included if they met the following criteria: (1) observational cohorts or RCTs that considered source of calcium intake (dietary or supplemental) and the risk or mortality of CVD; (2) studies providing outcomes including MI, CHD, ischemic heart disease (IHD), stroke, and other secondary outcomes; and (3) publications that provided estimates of relative risks (RRs), odds ratios (ORs), and hazard ratios (HRs) with corresponding 95% confidence intervals (CIs).
When studies reported the relationship of serum calcium of CVD mortality or risk, reported cross-sectional, case-control and animal studies, and reported the effects of dietary intake, not calcium separately, we also excluded them. Additionally, studies without adjustments for potential confounders and studies that did not report ORs, RRs, or HRs and corresponding 95% CIs were also excluded for cohort studies. Articles with CS doses less than 800 mg/d were excluded. Authors consider to exclude data from the same population, but a unified population with different follow-up times is considered to be two studies, for it is very helpful for our subgroup analyses. (details exclusion strategies are reporting in the ).
Two authors (CY and XLS) examined the full-text reports for compliance with eligibility criteria independently. Inconsistencies were resolved by discussion until a consensus was reached.
Ascertainment of cardiovascular events
CHD was considered to have occurred when either of the terms “ischemic heart disease” or “myocardial infarction” or “heart attack” or International Classification of Diseases, Ninth Revision (ICD-9) codes 410-414 and ICD-10 codes I20-I25 were used to describe the events. We considered a stroke to have occurred when any of the terms “stroke,” “cerebral infarction,” “intracerebral hemorrhage,” “subarachnoid hemorrhage,” “cerebrovascular accident,” or any of the ICD-9 codes 430-438 and ICD-10 codes I60-I69 were used to describe the event.
Quality assessment and data extraction
The methodological quality for the included RCTs and cohort studies was assessed independently by two researchers (CY, HCL) based on the Jadad score and the Newcastle-Ottawa Scale (NOS). The Jadad score is based on randomization, concealment of treatment allocation, blinding, completeness of follow-up, and the use of intention-to-treat analysis (Citation15). The NOS assigns a maximum of 9 points to each cohort study: 4 for selection, 2 for comparability, and 3 for assessment of outcomes (9 representing the highest quality). Any discrepancies were resolved by discussion.
Data extraction was conducted using standardized data collection independently by three investigators (CY, XY, HX). The primary exposure variable was dietary and supplemental calcium intake. Outcomes of interest in this study were risk of CVD, including CV secondary outcomes. All outcomes were classified based on the World Health Organization’s International Classification of Disease criteria (Citation16). For the cohort studies, we extracted first author name, baseline patient characteristics (country, gender, age), years of follow-up, assessment method, and the adjusted RRs for the highest versus the lowest calcium intake; the number of deaths and the total number of participants for each category and the mean or median calcium intakes in each category were extracted. For RCTs, we extracted the number of calcium group/control group, the dose of calcium and vitamin D, and serum 25(OH)D, excepting essential information. Disagreements were resolved by discussion.
If RCTs used more than one control or intervention group and permitted multiple comparisons, we only extracted the data and information of interest reported in the original articles. For studies that reported outcomes in men and women separately, investigators participating in the meta-analysis were asked to consider them as two articles. If we found unpublished data provided by the primary authors in a meta-analysis, we extracted those data from forest plots of the meta-analysis and tried to confirm whether the trials met our inclusion criteria through reviewing original articles. When studies reported not only the risk of CVD but also reported them of secondary outcomes, we only considered total CVD risk to pooled RRs. If a study only reported the risk of a secondary CVD outcome, such as CHD or stroke, we only pooled them into secondary outcomes risk.
Statistical analysis
In our meta-analysis, ORs and HRs were directly considered as RRs, and CVD mortality and incidence both were regarded as CVD risk. A random-effects model was used to summarize the data, and statistical heterogeneity between pooled data was evaluated using the I2 statistic (Citation17).
We used meta-regression to conform the possible sources of heterogeneity in case that studies had significant between-study heterogeneity. Subgroup analyses for prospective cohort studies were performed according to outcomes (incidence or mortality), gender (female or male), location (Europe and America or Asia), duration (≤10 years or >10 years), and adjustments (supplemental calcium intake or supplemental vitamin D intake). For RCTs, we specified subgroups based on dosage of calcium (≤1000 mg/d or >1000 mg/d), gender (female or male), location (Europe and/or America or Asia), dose of vitamin D (≤4,00 IU or >4,00 IU), duration (≤3years or >3 years), trial size (≤1000 participants or >1000 participants). We also performed the sensitivity analysis by considering methodological quality (scores) and dietary assessment tools (for cohort studies) or trial design (for RCTs; ). Publication bias was assessed using Begg’s and Egger’s regression model.
Table 1. Dietary calcium intake and the risk of CVD: An overview of selected studies for meta-analysis.
Table 2. Calcium supplementation with or without vitamin D and the mortality or events of CVD: An overview of selected studies for meta-analysis.
STATA software (version 11.2; Stata Corporation, College Station, Texas, USA) was used to conduct all the statistical evaluation. All tests were two-tailed, and p < 0.05 was considered significant for all included studies.
Results
Studies retrieved and characteristics
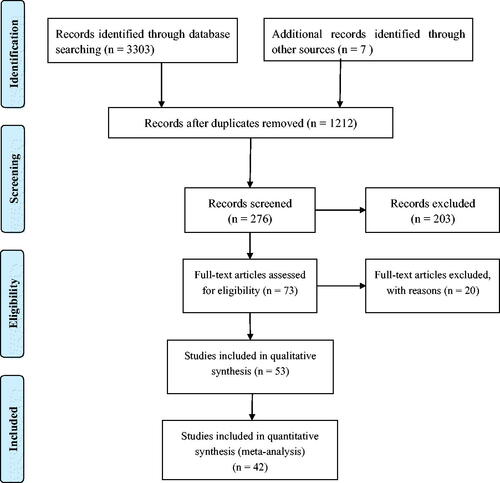
We identified 1212 articles from our initial search, of which 936 were excluded during an initial review (title and abstract). We retrieved the full text for the remaining 276 articles, and 42 articles met the inclusion criteria including 26 prospective cohort studies and 16 RCTs (). and summarize the baseline characteristics of the included studies.
The meta-analysis results of prospective cohort studies
Overall, 1,221,041 participants originated from prospective cohort studies. Fifteen articles (Citation4,Citation9,Citation10,Citation18–29) provided the data on dietary calcium intakes (DCIs) on CVD and its subcategories mortality, whereas 11 studies (Citation4,Citation9,Citation10,Citation18–23,Citation25,Citation26) contributed CHD data, and 8 studies (Citation10,Citation18,Citation21–23,Citation25,Citation26,Citation29) contributed stroke data. As to associations between DCIs and CVD and its subcategories events risk, 12 studies (Citation5,Citation11,Citation13,Citation21,Citation27,Citation30–36) contributed the data, 11 studies (Citation11,Citation13,Citation21,Citation27,Citation30–36) involved stroke data, and 5 (Citation5,Citation11,Citation21,Citation27,Citation34) related to CHD data. Two RR values (Citation4,Citation13) that compared the lowest quartile to the highest quartile (reference) need to change into RRs regarding the lowest quartile as reference. Of these articles that met inclusion criteria, no low-quality assessment occurred in that all NOS scores were higher than 5 (). Thirty-three studies were conducted in America and/or Europe and only 7 in Asia (Citation10,Citation11,Citation18,Citation23,Citation24,Citation30,Citation37). DCIs were assessed by Food Frequency Questionnaire (FFQ) in 24 studies and recall or other ways in 2 studies (Citation4,Citation13). Furthermore, most studies showed that the main sources of dietary calcium were dairy foods and nonalcoholic beverages (shown in Supplemental Table 1).
The association between DCIs and CVD risk
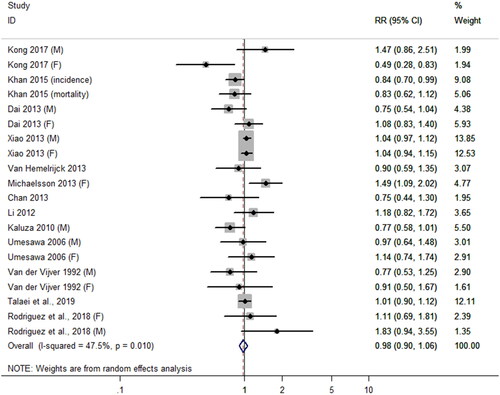
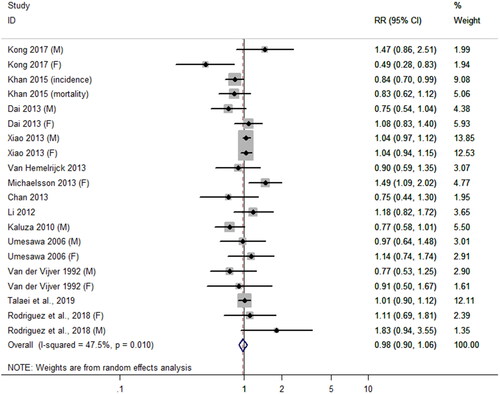
Thirteen articles (Citation4,Citation10,Citation18,Citation20–28,Citation30) contributed CVD risk data, 2 articles (Citation21,Citation30) provided CVD incidence data, and 11 articles (Citation4,Citation10,Citation18,Citation20,Citation22–28) provided CVD mortality data. As shown in . The pooled RR of CVD risk comparing the highest and lowest level of DCIs was 0.98 (95% CI, 0.90–1.06; p = 0.60), with moderate heterogeneity among the studies (I2 = 47.5%; p = 0.01).
Figure 2. The association between dietary calcium intake and the risk of cardiovascular disease (CVD). For Kong 2017, the RR of the CVD risk was reported separately by sex, respectively showed by Kong (M) and Kong (F). Other studies that differentiate gender used the same annotation mentioned above, such as Dai 2013, Xiao 2013, Umesawa 2006, Van der Vijver 1992. For studies involving incidence and mortality, (incidence) and (mortality) annotation were used, respectively, like Khan 2015.
Subgroup analyses were performed by CVD outcomes, gender, study location, duration, adjustments for supplemental calcium intake, and adjustments for supplemental vitamin D intake. The results showed an inverse relationship between DCIs and CVD risk when duration was not more than 10 years (RR = 0.84; 95% CI, 0.72–0.98; ); however, no significant differences were found in other subgroups. In order to further explore the sources of heterogeneity, we performed meta-regression by covariates which was consistent with subgroup factors to quantify the heterogeneity. However, no one factor accounted for the interstudy heterogeneity (p > 0.05). The Begg rank correlation test and Egger linear regression test indicated no significant publication bias (Begg’s test: 0.1, p = 0.92; Egger’s test: –0.37, p = 0.47).
Table 3. Subgroup analysis and meta-regression on calcium intake and CVD risk.
Secondary outcomes
The association between DCIs and the risk of CHD
Of the 26 articles included in the prospective cohort studies, 15 articles (Citation4,Citation5,Citation9–11,Citation18–23,Citation25–27,Citation34) reported the association between DCIs and the risk of CHD. In total, 900,832 subjects (aged ≥ 17 years) were included in 15 studies.
The summary relative risk of CHD comparing the highest and lowest level of DCIs was 1.10 (95% CI, 0.96–1.06; p = 0.77), with moderate heterogeneity among selected studies (I2 = 31.1%; p = 0.09; ). There were no significant associations between DCIs with CHD mortality or incidence equally (for incidence: RR = 0.93, 95% CI, 0.83–1.05, I2 = 0.0%; for mortality: RR = 1.00, 95% CI, 0.91–1.11, I2 = 42.5%, p interactive = 0.36). There were no significant differences in other subgroups (). The meta-regression was performed by covariates to quantify the heterogeneity for further exploring the sources of heterogeneity. Duration seemingly accounted for the interstudy heterogeneity (p = 0.496). No evidence for publication bias was found (Begg’s test: 0.15; p = 0.88; Egger’s test: –0.97; p = 0.34).
Figure 3. The association between dietary calcium intake and the risk of coronary heart disease. Different annotations were explained above. (M) and (F) represent different sexes. For studies involving incidence and mortality, (incidence) and (mortality) annotation were used, respectively.
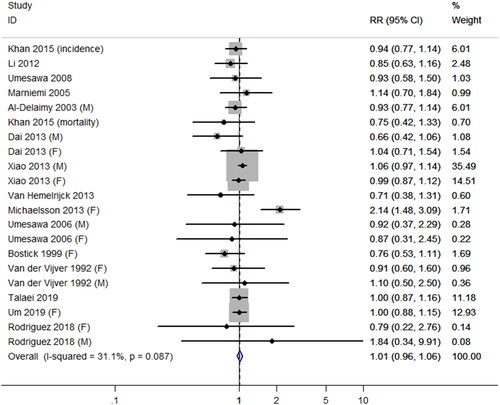
Table 4. Subgroup analysis and meta-regression on calcium intake and secondary outcomes.
The association between DCIs and the risk of stroke
Of the 26 articles included in the prospective cohort studies, 17 articles (Citation10,Citation11,Citation13,Citation18,Citation21–23,Citation25–27,Citation29–36) reported the association between DCI and the risk of stroke, which included 984,562 subjects.
The pooled RR of the risk of stroke comparing the highest and lowest level of DCIs was 0.94 (95% CI, 0.86–1.03; p = 0.21). A significant between-study heterogeneity was shown among selected studies (I2 = 48.0%; p = 0.01; ). Subgroup analyses by stroke outcomes showed no significance (incidence: RR = 0.94, 95% CI, 0.82–1.07, I2 = 59.9%; mortality: RR = 0.99, 95% CI, 0.89–1.10, I2 = 15.1%). Subgroup analyses displayed that DCIs may be associated with the lower risk of stroke in three stratifications (RRfemale and male = 0.85; 95% CI, 0.76–0.96; I2 = 44.4%, RRunadjusted calcium supplements = 0.83; 95% CI, 0.69–1.00; I2=46.4%, RRadjusted calcium supplements = 0.90; 95% CI, 0.81–0.99; I2 = 21.0%).
Figure 4. The association between dietary calcium intake and the risk of stroke. Different annotations were explained above. (M) and (F) represent different sexes. For studies involving incidence and mortality, (incidence) and (mortality) annotation were used, respectively.
Meta-regression by covariates prespecified above was performed and found that two factors that adjusted for supplemental calcium (p = 0.014) and vitamin D (p = 0.045) intake accounted for the interstudy heterogeneity, which was consistent with the result of subgroup analysis (). The Begg’s test and Egger’s test indicated no significant publication bias (Begg’s test: 1.24; p = 0.22; Egger’s test: −0.96; p = 0.08).
The meta-analysis results of RCTs
For RCTs from which 138,188 participants were retrieved, there were 16 eligible studies (Citation6,Citation37–51) reporting the effects of CSs (alone or with vitamin D) on CVD and its subcategories risk. Nine studies (Citation6,Citation40–47) contributed data from effects of CSs alone on CVD and its subcategories events risk. Seven trials (Citation37–39,Citation48–51) provided combination use of calcium and vitamin D data, including 4 (Citation38,Citation39,Citation49,Citation59) about CHD risk and 4 (Citation37,Citation38,Citation48,Citation51) about stroke risk.
Four trials (Citation39,Citation42,Citation47,Citation50) were evaluated with the score of 5, seven trials (Citation38,Citation40,Citation41,Citation43,Citation45,Citation49,Citation51) scored 4, two (Citation6,Citation37) scored 3, two (Citation44,Citation48) scored 2, and one article scored 1 (Citation46) (). Three trials were conducted in Asia (Citation6,Citation36,Citation43), and the other thirteen studies were in America and Europe. The control group of 14 trials was placebo, that control of another two trials was usual care [36] or vitamin D (Citation42). We obtained the data for 7 trials (Citation40,Citation42–47) from 3 meta-analyses (Citation52–54). Hsia et al. (Citation49) and Jackson et al. (Citation55) had analyzed the effects of calcium and vitamin D co-supplementation on the risk of CVD events from the same research in America, and we selected the data of the first article (Citation49). Prentice et al. (Citation38) and two other articles (Citation49,Citation55) mentioned above contributed the same MI and stroke data, and we selected the data of the first article (Citation38). For Grant et al. (Citation42), events were reported in those who received calcium vs placebo and calcium plus vitamin D vs vitamin D only, and we regarded the article as two studies.
Calcium was administered as calcium carbonate, calcium citrate, and element calcium or unclear form. Vitamin D was administered as vitamin D3 or unclear vitamin D form. We only extracted baseline 25[OH]D concentration in serum from one trial (Citation37).
CSs with or without vitamin D and the risk of CVD
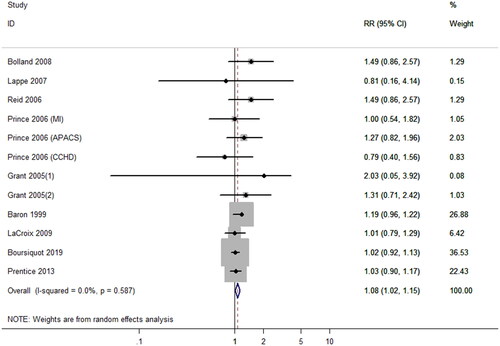
Seven articles (Citation6,Citation38,Citation40,Citation41,Citation46,Citation48,Citation49) contributed data on CVD risk of CSs including 13,315 participants. As shown in , there was no significant association between CSs and the risk of CVD (RR = 0.99; 95% CI, 0.93–1.05; p = 0.74), with a mild heterogeneity (I2 = 41.1%; p = 0.12). Prespecified subgroup analyses showed that calcium co-supplementation with vitamin D or without did not increase the risk of CVD (RRco-supplementation = 0.99, 95% CI, 0.93–1.15; p = 0.77, I2 = 0.0%; RRsupplementation alone = 0.91, 95% CI, 0.56–1.47; p = 0.69, I2 = 67.8%). Furthermore, the results of subgroup analyses by factors including gender, duration, and outcomes had no statistical significance ().
Figure 5. Effect of calcium supplementation (alone or co-supplementation with vitamin D) on the risk of cardiovascular disease.
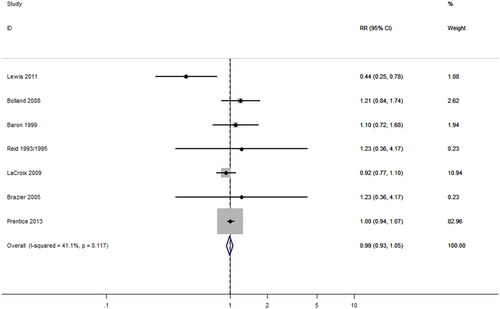
We performed the meta-regression by the same covariates above to quantify the heterogeneity for further exploring the sources of heterogeneity. No factor accounted for the interstudy heterogeneity (p > 0.05) which was consistent with the result of subgroup analyses ().
No evidence for publication bias was found (Begg’s test: 0.75; p = 0.45; Egger’s test: −0.25; p = 0.74).
Secondary outcomes
CS with or without vitamin D and the risk of CHD
Pooled RR of RCTs showed that the risk of CHD due to CS increased 8% (RR = 1.08, 95% CI, 1.02–1.22; I2 = 0.0%; ) and increased 20% due to CS alone (RR = 1.20, 95% CI, 1.08–1.33; I2 = 0.0%). The effect of CSs (alone or with vitamin D) on the risk of CHD was investigated in 9 studies (Citation38–44,Citation47,Citation50) involving a total 100,042 subjects. There was no heterogeneity with I2 equal to 0.0% among studies, so we just made subgroup analyses. Subgroup analyses showed that CSs less than 1000 mg/d would have no obvious association with CHD risk (RR = 1.04; 95% CI, 0.97–1.12; p = 0.22; I2 = 0.0%). Also, no significant difference was detected in female stratification and time of follow-up greater than 4 years stratification (female: RR = 1.04; 95% CI, 0.97–1.12; follow-up > 4 years: RR = 1.04, 95% CI, 0.97–1.12; ). Other results of subgroup analyses showed significant associations between CSs with the risk of CHD. In addition, we considered the outcome of MI especially and found that there was a clear association between CSs and MI risk (RR = 1.14, 95% CI, 1.05–1.25; I2 = 0.0%). MI risk increased 21% with taking CSs alone (RR = 1.21, 95% CI, 1.08–1.35; I2 = 0.0%; Supplemental Figure 1). No evidence for publication bias was found (Begg’s test: 0.55; p = 0.58; Egger’s test: −0.33; p = 0.50).
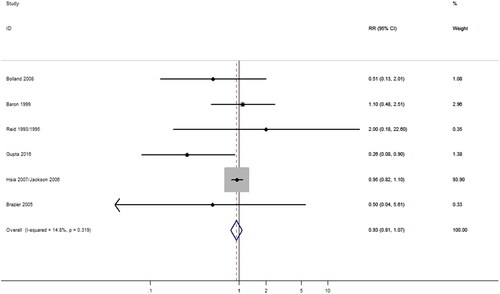
CS with or without vitamin D and the risk of stroke
The effect of CS on stroke risk was investigated in 6 studies (Citation37,Citation40,Citation41,Citation46,Citation48,Citation49) involving a total of 39,063 subjects. RR value had not reached levels of significance (p > 0.05) for the risk of stroke with taking CSs (alone or co-supplementation with vitamin D; RR = 0.85; 95% CI, 0.61–1.20) (). The summary results showed that there was slight heterogeneity among studies, but the difference was not significant statistically (I2 = 14.8%; p = 0.32). Prespecified subgroup analysis was conducted by covariates including dose of calcium intake, outcomes, gender, study location, duration, and whether co-supplementation with vitamin D, no any factors accounted for the interstudy heterogeneity which was consistent with the result of meta-regression (p > 0.05; ).<TQ: Please clarify “and whether co-supplementation with vitamin D, no any factors accounted for the interstudy heterogeneity which was consistent with the result of meta-regression”> Furthermore, stratified results of all subgroups showed no significant difference between CSs and the risk of stroke ().
Sensitivity analysis
Sensitivity analysis was conducted by considering the methodological quality and the assessment tools in prospective cohort study. The sensitivity analysis was performed in the meta-analysis on associations between DCIs and the risk of CVD; we excluded one study by Van der Vijver et al. (Citation4) which was with the low-quality score, the result also kept stable (RR = 0.98; 95% CI, 0.90 to 1.07; p = 0.65; I2 = 49.9%). In the sensitivity analysis on associations between DCIs with the risk of CHD, we excluded two articles by Marniemi et al. (Citation34) and Van der Vijver et al. (Citation4) that used non-FFQ assessment tools, and the result also kept stable (RR = 0.99; 95% CI, 0.87–1.09; p = 0.59; I2 = 53.4%). After excluding a study with a low-quality score by Van der Vijver et al. (Citation4), the result remained unchanged obviously (RR = 0.97; 95% CI, 0.92–1.07; p = 0.78; I2 = 34.7%). For studies on the association between DCIs with the risk of stroke, we made a sensitivity analysis by excluding studies conducted by Marniemi et al. (Citation34) and van der Pols et al. (Citation13), whose assessment tools were not FFQs. We also found no sensitivity existed in the meta-analysis (RR = 0.95; 95% CI, 0.86–1.04; p = 0.21; I2 = 45.1%).
Similarly, we performed sensitivity analysis by excluding the low methodological quality or analyzing trial designs in randomized controlled trials. The results of sensitivity analysis were not altered in CSs alone or with vitamin D studies after excluding the trials by Prince et al. (Citation44), Reid et al. (Citation46), and Brazier et al. (Citation48) which were in low quality score.
Discussion
In this meta-analysis of observational studies, there was no associations between DCIs and the risk of CVD, including its secondary outcomes, in a certain range (200 mg/d to 1500 mg/d). Consistent with our results, two meta-analysis by Asemi et al. (Citation56) and Wang et al. (Citation57) showed similar associations between DCIs and CVD mortality. We found that dairy foods and nonalcoholic beverages were the main sources of dietary calcium, and the former occupies a large proportion especially in Europe and America; the study by Li et al. (Citation27) reported dairy foods providing 39.9% of the DCI (shown in Supplemental Table 1). Several explanations for our findings are possible. Clinical trials have shown that consumption of dairy foods has beneficial effects on levels of some cardiometabolic risk factors, including lipid profiles (Citation58), hypertension, and insulin resistance (Citation59,Citation60). In addition, some beneficial health effects on blood cholesterol, blood pressure, and immune function may provide prophylactic protection against heart disease and stroke (Citation61,Citation62). Dietary calcium mainly from dairy products does not increase cardiovascular risk, and we assumed that dairy food matrix accompanied by several other nutrients may contribute to lower CVD risk. Astrup et al. (Citation63) suggested that observational studies are also useful for examining the foods consumed in people’s diets rather than examining individual nutrients. An expert consensus panel concluded that the food matrix is more important for predicting the effect of a food on risk of CHD (Citation64). As far as the role of dairy and its saturated fat composition, its link to CVD has been analyzed in some studies (Citation65,Citation66), suggesting a beneficial action of this food group on reducing cardiovascular risk in high-risk groups (Citation67,Citation68).
These findings suggested that adequate DCIs are beneficial to CV protection. Our results also supported these views. Results showed that DCIs were associated with a 16% lower risk of CVD when duration was not more than 10 years (). Results showed inverse relationships between DCIs and stroke risk in subgroups that a 15% lower risk in male and female, a 17% lower risk in no adjustment for supplemental calcium intake group, and a 10% lower risk in adjustment for supplemental vitamin D intake group. We believe that doses of DCIs can explain these results. One study reported that the mortality rate decreased at calcium intake concentrations of 600 to 1400 mg/d and increased at > 1400 mg/d (Citation26). Considering that the mean DCIs were only 49 to 1200 mg/d in this study—adequate, but not excessive—DCIs could be associated with a reduced risk of stroke (Citation69). A meta-analysis suggested a U-shaped association between DCIs and cardiovascular mortality, with lowest mortality at about 800 mg/d (Citation57). Another study also showed that the CVD risk and DCIs showed a U-shaped association in women by Kong et al. (Citation30). But our dose–response meta-regressions did not detect statistically significant linear or nonlinear relationships between levels of DCIs and the risk of CVD, CHD, or stroke. In our nonlinear dose–response analysis, the result suggested an approximate U-shaped association between DCIs and the risk of CVD, with no increase of risk within 1000 mg/d, but the result had no significant difference (p > 0.05) (see Supplemental Material 1). Differences in the data synthesis methods may account for the apparent discordant results and conclusions.
As what results displayed, there was no association between DCIs within 1500 mg/d and the risk of CVD. A study directly supported our viewpoint. Chrysant et al. (Citation70) suggested that low calcium intake is associated with lower risk of CVD and stroke, and patients with low calcium intake should be encouraged to increase their calcium intake through dietary means to a daily dose of 1200 mg to 1500 mg, as recommended. Also, no another adverse effect on body health was found by DCIs. Some studies showed that a high DCI sustainably resulted in reducing efficiency of intestinal absorption and increasing urinary calcium excretion, so that the long-term effect of dietary calcium on blood calcium and bone density was negligible (Citation71,Citation72).
In fact, very high calcium intake is difficult if not impossible to achieve by dietary sources alone. Therefore, the concerns regarding potential adverse CV risks are related to the use of calcium supplements. Seven trials examined the effects of CSs on CVD risk, nine on CHD risk and six on stroke risk (doses ranging from 1000 to 1400 mg/d). Results showed that CSs did not increase the risk of CVD and stroke. However, the risk of CHD in those allocated to CSs increased by 8% (p = 0.02; I2 = 0.0%), and the risk of CHD increased by 20% in oral CSs alone (p = 0.001; I2 = 0.0%). In addition, there was a clear effect between CSs and the risk of MI (p = 0.002; I2 = 0.0%). The risk of MI was increased by 21% with taking CSs alone (p = 0.001; I2 = 0.0%). The study by Li et al. (Citation27) made the similar conclusion that CSs might raise the risk of MI. Interestingly, subgroup analyses revealed that CSs with vitamin D had no association with the risk of CHD in our meta-analysis. In line with our results, a recent meta-analysis by Lewis et al. (Citation52) found that there were consistently null effects of the combination of calcium and vitamin D supplements on any verified coronary artery disease manifestations, including MI, before and after excluding participants taking personal CSs at baseline in the Women’s Health Initiative (WHI) study. Vitamin D functions in many body systems, but perhaps the best attested of the nutrient's actions is its role in transferring calcium (and phosphorus) from ingested food into body fluids. In this capacity, vitamin D (works in concert with parathyroid hormone) operates to maintain constancy of the calcium ion concentrations in the extracellular fluid against the demands of obligatory excretory losses and skeletal mineralization (Citation73).
Our results also revealed that CSs had no significant effect on CVD risk in females (RR = 0.99; 95% CI, 0.93–1.05). It differed to a previous meta-analysis (Citation74) which reported a 21% increase in the risk of MI. That study was based on mix genders, and only three trials’ data were included, with fewer participants. Male data included seemed to increase the risk weight in that article male data included seemed to increase the risk weight in that article. A study (Citation54) reported CS increasing the risk of CVD death in men, but not women. Furthermore, a recent meta‐analysis of RCTs of CSs reported that men may experience more harmful effects of CSs than women (Citation75).
For the inconsistencies in findings from the meta-analysis of observational studies and RCTs, the reasons can be explained by considering the differences in the amount of calcium intake, the duration of intervention and follow-up, as well as the existence of several confounders. The dosage of calcium intake in cohort studies has a lower level than in RCTs, in which most subjects were taking 1,000 mg/d or more. The duration of intervention in RCTs is always less than the time of follow-up in cohort studies, mainly inducing subjects taking higher accumulated doses of calcium. We have reviewed some articles explaining the reason; CSs acutely increased serum calcium levels (Citation76,Citation77), and serum calcium substantially increased the risk of MI, which has already been proven in several large observational studies (Citation78,Citation79). Ionized calcium concentration increased from a mean of 1.22 to 1.30 mmol/L following supplementation with 1 g of calcium, either as the citrate or as the lactate-gluconate (Citation76). It is biologically plausible that increasing serum calcium concentrations might alter the balance of this system in favor of calcification (Citation76). Circulating concentrations of calcium are tightly regulated by homeostatic mechanisms involving the calcium-sensing receptor, parathyroid hormone, and 1,25-dihydroxyvitamin D, and disorders of calcium homeostasis are associated with a range of adverse clinical outcomes, including CVD (Citation80). There were significant positive relationships between plasma calcium and each of body mass index, systolic and diastolic blood pressure, and glucose and total cholesterol. Plasma calcium is a predictor of CV events (Citation81). The difference could also be caused by dietary magnesium intake in cohort studies. Some studies showed that dietary magnesium intake was associated with reduce of risk of CVD (Citation82,Citation83). Magnesium was possible counters that magnified the effects of calcium on risk of CVD. None of the included studies adjusted for dietary magnesium intake.
The limitations of our study are as follows: One limitation of the study was that it was based on self-reports of calcium intake, which may result in overestimates of calcium intake. A secondary limitation was that our study included some original data extracted from other meta-analyses with potential second-extraction error. Third, the outcomes of included studies designed to evaluate effect of calcium intake were not mainly CV outcomes, rather bone density or fracture, in which cases may existed inaccuracy.
Conclusions
In summary, DCIs adequately do not increase the risk of CVD, including CHD, and stroke. Our results provide support for public health recommendations on encouraging residents to take calcium from dietary food. Extra CSs may bring potential CHD risk, especially MI. Thus, intake recommendations for calcium should consider individual characteristics and should focus on people with low intake levels of calcium, rather than increasing the intake of those with adequate amounts of calcium. The intake dosage of CS should be less than 1000 mg/d, and combining vitamin D supplementation may avoid the harmful effects of CS alone.
Contributors
CY, XLS, and DP systematically searched databases independently. CY and HCL assessed independently the methodological quality for the included RCTs and cohort studies. CY and XY examined the full-text reports for compliance with eligibility criteria independently. Data extraction was conducted using a standardized data collection independently by three investigators (CY, XY, HX). CY wrote the first draft of the original manuscript. Professor GJS has revised the manuscript critically. All authors have read and approved the final manuscript. CY is guarantor and had full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical approval
Not required.
Supplemental Material
Download PDF (479.7 KB)Supplemental Material
Download PDF (241.2 KB)Supplemental Material
Download PDF (129.5 KB)Supplemental Material
Download MS Power Point (76.4 KB)Acknowledgements
We thank the systematic literature review team at Southeast University for their contributions to the cardiovascular disease database. I would like to express my heartfelt thanks to the working group of the Chinese Nutrition Society on “consensus of experts on vitamins and mineral supplements” for its support.
Disclosure statement
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: (1) no support from any organization for the submitted work; (2) no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years; and (3) no other relationships or activities that could appear to have influenced the submitted work.
Data sharing
No additional data available.
Additional information
Funding
References
- Cano A , Chedraui P , Goulis DG , Lopes P , Mishra G , Mueck A , Senturk LM , Simoncini T , Stevenson JC , Stute P , et al. Calcium in the prevention of postmenopausal osteoporosis: emas clinical guide. Maturitas. 2018;107:7–12. doi:10.1016/j.maturitas.2017.10.004.
- Pentti K , Tuppurainen MT , Honkanen R , Sandini L , Kroger H , Alhava E , Saarikoski S . Use of calcium supplements and the risk of coronary heart disease in 52-62-year-old women: the kuopio osteoporosis risk factor and prevention study. Maturitas. 2009;63(1):73–78. doi:10.1016/j.maturitas.2009.03.006.
- Ross AC , Manson JE , Abrams SA , Aloia JF , Brannon PM , Clinton SK , Durazo-Arvizu RA , Gallagher JC , Gallo RL , Jones G , et al. The 2011 report on dietary reference intakes for calcium and vitamin d from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi:10.1210/jc.2010-2704.
- Vandervijver LPL , Vanderwaal MAE , Weterings KGC , Dekker JM , Schouten EG , Kok FJ . Calcium intake and 28-year cardiovascular and coronary heart-disease mortality in dutch civil-servants. Int J Epidemiol. 1992;21(1):36–39. doi:10.1093/ije/21.1.36.
- Al-Delaimy WK , Rimm E , Willett WC , Stampfer MJ , Hu FB . A prospective study of calcium intake from diet and supplements and risk of ischemic heart disease among men. Am J Clin Nutr. 2003;77(4):814–818. doi:10.1093/ajcn/77.4.814.
- Lewis JR , Calver J , Zhu K , Flicker L , Prince RL . Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year rct and a 4.5-year follow-up. J Bone Miner Res. 2011;26(1):35–41. doi:10.1002/jbmr.176.
- Wang L , Manson JE , Sesso HD . Calcium intake and risk of cardiovascular disease: a review of prospective studies and randomized clinical trials. Am J Cardiovasc Drugs. 2012;12(2):105–116. doi:10.2165/11595400-000000000-00000.
- Resnick LM . The role of dietary calcium in hypertension - a hierarchal overview. American Journal of Hypertension. 1999;12(1):99–112. doi:10.1016/S0895-7061(98)00275-1.
- Bostick RM , Kushi LH , Wu Y , Meyer KA , Sellers TA , Folsom AR . Relation of calcium, vitamin d, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149(2):151–161. doi:10.1093/oxfordjournals.aje.a009781.
- Umesawa M , Iso H , Date C , Yamamoto A , Toyoshima H , Watanabe Y , Kikuchi S , Koizumi A , Kondo T , Inaba Y , et al. Dietary intake of calcium in relation to mortality from cardiovascular disease - the jacc study. Stroke. 2006;37(1):20–26. doi:10.1161/01.STR.0000195155.21143.38.
- Umesawa M , Iso H , Ishihara J , Saito I , Kokubo Y , Inoue M , Tsugane S , Grp JS . Dietary calcium intake and risks of stroke, its subtypes, and coronary heart disease in japanese - the jphc study cohort i. Stroke. 2008;39(9):2449–2456. doi:10.1161/STROKEAHA.107.512236.
- Bailey RL , Dodd KW , Goldman JA , Gahche JJ , Dwyer JT , Moshfegh AJ , Sempos CT , Picciano MF . Estimation of total usual calcium and vitamin d intakes in the united states. J Nutr. 2010;140(4):817–822. doi:10.3945/jn.109.118539.
- Abbott RD , Curb JD , Rodriguez BL , Sharp DS , Burchfiel CM , Yano K . Effect of dietary calcium and milk consumption on risk of thromboembolic stroke in older middle-aged men. The Honolulu Heart Program. Stroke. 1996;27(5):813–818.
- Stroup DF , Berlin JA , Morton SC , Olkin I , Williamson GD , Rennie D , Moher D , Becker BJ , Sipe TA , Thacker SB , Grp M . Meta-analysis of observational studies in epidemiology - a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi:10.1001/jama.283.15.2008.
- Jadad AR , Moore RA , Carroll D , Jenkinson C , Reynolds DJM , Gavaghan DJ , McQuay HJ . Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4.
- World Health Organization’s International Classification of Disease criteria. ICD Revision Topic Advisory Groups, World Health Organization. Available from: http://www.who.int/classifications/icd/TAGs/en. [accessed 2016 April 20].
- Higgins JPT , Thompson SG , Deeks JJ , Altman DG . Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557.
- Talaei M , Koh W-P , Yuan J-M , van Dam RM . Dash dietary pattern, mediation by mineral intakes, and the risk of coronary artery disease and stroke mortality. JAHA. 2019;8(5):e011054. doi:10.1161/JAHA.118.011054.
- Um CY , Prizment A , Hong C-P , Lazovich D , Bostick RM . Associations of calcium and dairy product intakes with all-cause, all-cancer, colorectal cancer, and coronary heart disease mortality among older women in the iowa women's health study. Br J Nutr. 2019;121(10):1188–1200. doi:10.1017/S000711451900045X.
- Rodriguez AJ , Scott D , Khan B , Hodge A , English DR , Giles GG , Abrahamsen B , Ebeling PR . High calcium intake in men not women is associated with all-cause mortality risk: Melbourne collaborative cohort study. Arch Osteoporos. 2018;13(1):101. doi:10.1007/s11657-018-0518-5.
- Khan B , Nowson CA , Daly RM , English DR , Hodge AM , Giles GG , Ebeling PR . Higher dietary calcium intakes are associated with reduced risks of fractures, cardiovascular events, and mortality: a prospective cohort study of older men and women. J Bone Miner Res. 2015;30(10):1758–1766. doi:10.1002/jbmr.2515.
- Xiao Q , Murphy RA , Houston DK , Harris TB , Chow W-H , Park Y . Dietary and supplemental calcium intake and cardiovascular disease mortality: the National Institutes of Health-AARP diet and health study. Jama Intern Med. 2013;173(8):639–646. doi:10.1001/jamainternmed.2013.3283.
- Dai Q , Shu X-O , Deng X , Xiang Y-B , Li H , Yang G , Shrubsole MJ , Ji B , Cai H , Chow W-H , et al. Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study (vol 3, e002111, 2013). Bmj Open. 2013;3(2):e002111., doi:10.1136/bmjopen-2012-002111.
- Chan R , Leung J , Woo J . A prospective cohort study examining the associations of dietary calcium intake with all-cause and cardiovascular mortality in older chinese community-dwelling people. Plos One. 2013;8(11):e80895. doi:10.1371/journal.pone.0080895.
- Van Hemelrijck M , Michaelsson K , Linseisen J , Rohrmann S . Calcium intake and serum concentration in relation to risk of cardiovascular death in nhanes iii. Plos One. 2013;8(4):e61037. doi:10.1371/journal.pone.0061037.
- Michaelsson K , Melhus H , Warensjo E , Wolk A , Byberg L . Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. Br Med J. 2013;346: f228. doi:10.1136/bmj.f228.
- Li K , Kaaks R , Linseisen J , Rohrmann S . Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European prospective investigation into cancer and nutrition study (epic-Heidelberg). Heart. 2012;98(12):920–925.
- Kaluza J , Orsini N , Levitan EB , Brzozowska A , Roszkowski W , Wolk A . Dietary calcium and magnesium intake and mortality: a prospective study of men. Am J Epidemiol. 2010;171(7):801–807. doi:10.1093/aje/kwp467.
- van der Pols JC , Gunnell D , Williams GM , Holly JMP , Bain C , Martin RM . Childhood dairy and calcium intake and cardiovascular mortality in adulthood: 65-year follow-up of the boyd orr cohort. Heart. 2009;95(19):1600–1606. doi:10.1136/hrt.2009.168716.
- Kong SH , Kim JH , Hong AR , Cho NH , Shin CS . Dietary calcium intake and risk of cardiovascular disease, stroke, and fracture in a population with low calcium intake. Am J Clin Nutr. 2017;106(1):27–34. doi:10.3945/ajcn.116.148171.
- Adebamowo SN , Spiegelman D , Flint AJ , Willett WC , Rexrode KM . Intakes of magnesium, potassium, and calcium and the risk of stroke among men. Int J Stroke. 2015;10(7):1093–1100. doi:10.1111/ijs.12516.
- Larsson SC , Virtamo J , Wolk A . Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol. 2011;174(1):35–43. doi:10.1093/aje/kwr051.
- Larsson SC , Virtanen MJ , Mars M , Mannisto S , Pietinen P , Albanes D , Virtamo J . Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch Intern Med. 2008;168(5):459–465. doi:10.1001/archinte.168.5.459.
- Marniemi J , Alanen E , Impivaara O , Seppanen R , Hakala P , Rajala T , Ronnemaa T . Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15(3):188–197. doi:10.1016/j.numecd.2005.01.001.
- Iso H , Stampfer MJ , Manson JE , Rexrode K , Hennekens CH , Colditz GA , Speizer FE , Willett WC . Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30(9):1772–1779.
- Ascherio A , Rimm EB , Hernan MA , Giovannucci EL , Kawachi I , Stampfer MJ , Willett WC . Intake of potassium, magnesium, calcium, and fiber and risk of stroke among us men. Circulation. 1998;98(12):1198–1204. doi:10.1161/01.cir.98.12.1198.
- Gupta A , Prabhakar S , Modi M , Bhadada SK , Kalaivani M , Lal V , Khurana D . Effect of vitamin d and calcium supplementation on ischaemic stroke outcome: a randomised controlled open-label trial. Int J Clin Pract. 2016;70(9):764–770. doi:10.1111/ijcp.12866.
- Prentice RL , Pettinger MB , Jackson RD , Wactawski-Wende J , LaCroix AZ , Anderson GL , Chlebowski RT , Manson JE , Van Horn L , Vitolins MZ , et al. Health risks and benefits from calcium and vitamin d supplementation: women's health initiative clinical trial and cohort study. Osteoporos Int. 2013;24(2):567–580. doi:10.1007/s00198-012-2224-2.
- Boursiquot BC , Larson JC , Shalash OA , Vitolins MZ , Soliman EZ , Perez MV . Vitamin d with calcium supplementation and risk of atrial fibrillation in postmenopausal women. Am Heart J. 2019;209:68–78. doi:10.1016/j.ahj.2018.12.006.
- Baron JA , Beach M , Mandel JS , van Stolk RU , Haile RW , Sandler RS , Rothstein R , Summers RW , Snover DC , Beck GJ , Calcium Polyp Prevention Study G, et al. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340(2):101–107. doi:10.1056/NEJM199901143400204.
- Bolland MJ , Barber PA , Doughty RN , Mason B , Horne A , Ames R , Gamble GD , Grey A , Reid IR . Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336(7638):262–266. doi:10.1136/bmj.39440.525752.BE.
- Grant AM , Anderson FH , Avenell A , Campbell MK , Cooper C , Donaldson C , Francis RM , Gillespie WJ , Robinson CM , Torgerson DJ , et al. Oral vitamin d3 and calcium for secondary prevention of low-trauma fractures in elderly people (randomised evaluation of calcium or vitamin d, record): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–1628.
- Lappe JM , Travers-Gustafson D , Davies KM , Recker RR , Heaney RP . Vitamin d and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi:10.1093/ajcn/85.6.1586.
- Prince RL , Devine A , Dhaliwal SS , Dick IM . Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women . Arch Intern Med. 2006;166(8):869–875. doi:10.1001/archinte.166.8.869.
- Reid IR , Ames R , Mason B , Reid HE , Bacon CJ , Bolland MJ , Gamble GD , Grey A , Horne A . Randomized controlled trial of calcium supplementation in healthy, nonosteoporotic, older men. Arch Intern Med. 2008;168(20):2276–2282. doi:10.1001/archinte.168.20.2276.
- Reid IR , Ames RW , Evans MC , Gamble GD , Sharpe SJ . Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal women - a randomized controlled trial. Am J Med. 1995;98(4):331–335. doi:10.1016/S0002-9343(99)80310-6.
- Reid IR , Mason B , Horne A , Ames R , Reid HE , Bava U , Bolland MJ , Gamble GD . Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119(9):777–785. doi:10.1016/j.amjmed.2006.02.038.
- Brazier M , Grados F , Kamel SI , Mathieu M , Morel A , Maamer M , Sebert JL , Fardellone P . Clinical and laboratory safety of one year's use of a combination calcium plus vitamin d tablet in ambulatory elderly women with vitamin d insufficiency: results of a multicenter, randomized, double-blind, placebo-controlled study. Clin Therapeutics. 2005;27(12):1885–1893. doi:10.1016/j.clinthera.2005.12.010.
- Hsia J , Heiss G , Ren H , Allison M , Dolan NC , Greenland P , Heckbert SR , Johnson KC , Manson JE , Sidney S , et al. Calcium/vitamin d supplementation and cardiovascular events. Circulation. 2007;115(7):846–854. doi:10.1161/CIRCULATIONAHA.106.673491.
- LaCroix AZ , Kotchen J , Anderson G , Brzyski R , Cauley JA , Cummings SR , Gass M , Johnson KC , Ko M , Larson J , et al. Calcium plus vitamin d supplementation and mortality in postmenopausal women: the women's health initiative calcium-vitamin d randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64(5):559–567. doi:10.1093/gerona/glp006.
- Donneyong MM , Hornung CA , Taylor KC , Baumgartner RN , Myers JA , Eaton CB , Gorodeski EZ , Klein L , Martin LW , Shikany JM , et al. Risk of heart failure among postmenopausal women: a secondary analysis of the randomized trial of vitamin d plus calcium of the women's health initiative. Circ Heart Fail. 2015;8(1):49–56. doi:10.1161/CIRCHEARTFAILURE.114.001738.
- Lewis JR , Radavelli-Bagatini S , Rejnmark L , Chen JS , Simpson JM , Lappe JM , Mosekilde L , Prentice RL , Prince RL . The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res. 2015;30(1):165–175. doi:10.1002/jbmr.2311.
- Bolland MJ , Avenell A , Baron JA , Grey A , MacLennan GS , Gamble GD , Reid IR . Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. Br Med J. 2010;341(jul29 1):c3691. doi:10.1136/bmj.c3691.
- Mao P-J , Zhang C , Tang L , Xian Y-Q , Li Y-S , Wang W-D , Zhu X-H , Qiu H-L , He J , Zhou Y-H . Effect of calcium or vitamin d supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol. 2013;169(2):106–111. doi:10.1016/j.ijcard.2013.08.055.
- Jackson RD , LaCroix AZ , Gass M , Wallace RB , Robbins J , Lewis CE , Bassford T , Beresford SAA , Black HR , Blanchette P , Womens Hlth Initiative I, et al. Calcium plus vitamin d supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683., doi:10.1056/NEJMoa055218.
- Asemi Z , Saneei P , Sabihi SS , Feizi A , Esmaillzadeh A . Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2015;25(7):623–634. doi:10.1016/j.numecd.2015.03.008.
- Wang X , Chen H , Ouyang Y , Liu J , Zhao G , Bao W , Yan M . Dietary calcium intake and mortality risk from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Bmc Med. 2014;12(1):158. doi:10.1186/s12916-014-0158-6.
- Kai SHY , Bongard V , Simon C , Ruidavets J-B , Arveiler D , Dallongeville J , Wagner A , Amouyel P , Ferrieres J . Low-fat and high-fat dairy products are differently related to blood lipids and cardiovascular risk score. Eur J Prev Cardiolog. 2014;21(12):1557–1567. doi:10.1177/2047487313503283.
- Drouin-Chartier J-P , Gagnon J , Labonte M-E , Desroches S , Charest A , Grenier G , Dodin S , Lemieux S , Couture P , Lamarche B . Impact of milk consumption on cardiometabolic risk in postmenopausal women with abdominal obesity. Nutr J. 2015;14(1):12. doi:10.1186/1475-2891-14-12.
- Conway V , Couture P , Gauthier S , Pouliot Y , Lamarche B . Effect of buttermilk consumption on blood pressure in moderately hypercholesterolemic men and women. Nutrition. 2014;30(1):116–119. doi:10.1016/j.nut.2013.07.021.
- Parvez S , Malik KA , Kang SA , Kim HY . Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100(6):1171–1185. doi:10.1111/j.1365-2672.2006.02963.x.
- Hu D , Huang J , Wang Y , Zhang D , Qu Y . Dairy foods and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24(5):460–469. doi:10.1016/j.numecd.2013.12.006.
- Astrup A , Bertram HC , Bonjour J-P , de Groot LC , de Oliveira Otto MC , Feeney EL , Garg ML , Givens I , Kok FJ , Krauss RM , et al. Who draft guidelines on dietary saturated and trans fatty acids: Time for a new approach? BMJ (Clinical Research ed). 2019;366: l4137–l4137.
- Astrup A , Dyerberg J , Elwood P , Hermansen K , Hu FB , Jakobsen MU , Kok FJ , Krauss RM , Lecerf JM , LeGrand P , et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr. 2011;93(4):684–688. doi:10.3945/ajcn.110.004622.
- Butler TJ , Davies I , Mushtaq S . Full fat cheese intake and cardiovascular health: a randomised control trial. Proc Nutr Soc. 2017;76(OCE4):E223–E223. doi:10.1017/S0029665117003858.
- Lovegrove JA , Hobbs DA . New perspectives on dairy and cardiovascular health. Proc Nutr Soc. 2016;75(3):247–258. doi:10.1017/S002966511600001X.
- Diaz-Lopez A , Bullo M , Martinez-Gonzalez MA , Corella D , Estruch R , Fito M , Gomez-Gracia E , Fiol M , Garcia de la Corte FJ , Ros E , et al. Dairy product consumption and risk of type 2 diabetes in an elderly spanish mediterranean population at high cardiovascular risk. Eur J Nutr. 2016;55(1):349–360. doi:10.1007/s00394-015-0855-8.
- Nilsen R , Hostmark AT , Haug A , Skeie S . Effect of a high intake of cheese on cholesterol and metabolic syndrome: results of a randomized trial. Food Nutr Res. 2015;59: 27651. doi:10.3402/fnr.v59.27651.
- Shin CS , Kim KM . Calcium, is it better to have Less?-Global Health Perspectives. J Cell Biochem. 2015;116(8):1513–1521. doi:10.1002/jcb.25119.
- Chrysant SG , Chrysant GS . Controversy regarding the association of high calcium intake and increased risk for cardiovascular disease. J Clin Hypertens (Greenwich). 2014;16(8):545–550. doi:10.1111/jch.12347.
- Reid IR , Birstow SM , Bolland MJ . Calcium and cardiovascular disease. Endocrinol Metab. 2017;32(3):339–349. doi:10.3803/EnM.2017.32.3.339.
- Tai V , Leung W , Grey A , Reid IR , Bolland MJ . Calcium intake and bone mineral density: systematic review and meta-analysis. Br Med J. 2015;351 h4183.
- Bolland MJ , Grey A , Avenell A , Gamble GD , Reid IR . Calcium supplements with or without vitamin d and risk of cardiovascular events: reanalysis of the women's health initiative limited access dataset and meta-analysis. Br Med J. 2011;342(apr19 1):d2040. doi:10.1136/bmj.d2040.
- Bolland MJ , Grey A . Calcium supplements associated with increased risk of cardiovascular death in men but not women. Evid Based Nurs. 2014;17(3):90–90. doi:10.1136/eb-2013-101460.
- Masson S , Agabiti N , Vago T , Miceli M , Mayer F , Letizia T , Wienhues-Thelen U , Mureddu GF , Davoli M , Boccanelli A , et al. The fibroblast growth factor-23 and vitamin d emerge as nontraditional risk factors and may affect cardiovascular risk. J Intern Med. 2015;277(3):318–330. doi:10.1111/joim.12232.
- Reid IR , Bolland MJ , Grey A . Does calcium supplementation increase cardiovascular risk? Clin Endocrinol (Oxf). 2010;73(6):689–695. doi:10.1111/j.1365-2265.2010.03792.x.
- Rubin MR , Rundek T , McMahon DJ , Lee H-S , Sacco RL , Silverberg SJ . Carotid artery plaque thickness is associated with increased serum calcium levels: The northern manhattan study. Atherosclerosis. 2007;194(2):426–432. doi:10.1016/j.atherosclerosis.2006.08.027.
- Foley RN , Collins AJ , Ishani A , Kalra PA . Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the atherosclerosis risk in communities (aric) study. Am Heart J. 2008;156(3):556–563. doi:10.1016/j.ahj.2008.05.016.
- Jorde R , Sundsfjord J , Fitzgerald P , Bonaa KH . Serum calcium and cardiovascular risk factors and diseases - the tromso study. Hypertension. 1999;34(3):484–490.
- Ward BK , Magno AL , Walsh JP , Ratajczak T . The role of the calcium-sensing receptor in human disease. Clin Biochem. 2012;45(12):943–953. doi:10.1016/j.clinbiochem.2012.03.034.
- Walsh JP , Divitini ML , Knuiman MW . Plasma calcium as a predictor of cardiovascular disease in a community-based cohort. Clin Endocrinol. 2013;78(6):852–857. doi:10.1111/cen.12081.
- Larsson SC , Burgess S , Michaelsson K . Serum magnesium levels and risk of coronary artery disease: Mendelian randomisation study. BMC Med. 2018;16(1):68–68. doi:10.1186/s12916-018-1065-z.
- Hisamatsu T , Miura K , Fujiyoshi A , Kadota A , Miyagawa N , Satoh A , Zaid M , Yamamoto T , Horie M , Ueshima H , Grp SR . Serum magnesium, phosphorus, and calcium levels and subclinical calcific aortic valve disease: A population-based study. Atherosclerosis. 2018;273 145–152. doi:10.1016/j.atherosclerosis.2018.03.035.