Abstract
Personalized nutrition holds tremendous potential to improve human health. Despite exponential growth, the field has yet to be clearly delineated and a consensus definition of the term “personalized nutrition” (PN) has not been developed. Defining and delineating the field will foster standardization and scalability in research, data, training, products, services, and clinical practice; and assist in driving favorable policy. Building on the seminal work of pioneering thought leaders across disciplines, we propose that personalized nutrition be defined as: a field that leverages human individuality to drive nutrition strategies that prevent, manage, and treat disease and optimize health, and be delineated by three synergistic elements: PN science and data, PN professional education and training, and PN guidance and therapeutics. Herein we describe the application of PN in these areas and discuss challenges and solutions that the field faces as it evolves. This and future work will contribute to the continued refinement and growth of the field of PN.
PN approaches can be most effective when there is consensus regarding its definition and applications.
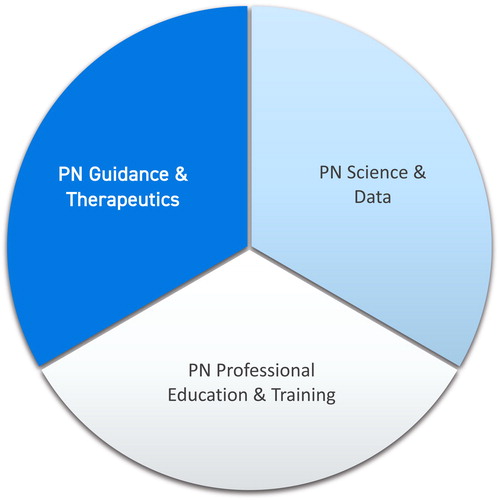
PN can be delineated into three main areas of application: PN science and data, PN education and training, PN guidance and therapeutics.
PN science and data foster understanding about the impact of genetic, phenotypic, biochemical and nutritional inputs on an individual’s health.
PN education and training equip a variety of healthcare professionals to apply PN strategies in many healthcare settings.
PN professionals have greater ability to tailor interventions via PN guidance and therapeutics.
Favorable policy allows PN to be more fully integrated into the healthcare system.
Teaching points
Introduction
Personalized nutrition (PN) is rooted in the concept that one size does not fit all; differences in biochemistry, metabolism, genetics, and microbiota contribute to the dramatic inter-individual differences observed in response to nutrition, nutrient status, dietary patterns, timing of eating, and environmental exposures. PN has been described in a variety of ways, and other terms such as “precision nutrition,” “individualized nutrition,” and “nutritional genomics” have similar, sometimes overlapping, meanings in the literature. Boorsma et al. state that “Personalized nutrition tailors dietary recommendations to specific biological requirements on the basis of a person's health status and goals,” focusing on the clinical and biological aspects of nutrition practice (Citation1). This conception of PN as related to nutrition recommendations or advice for disease management and/or health optimization is echoed by many (Citation1–7). A 2018 paper by Ordovás et al. describes PN as an “approach that uses information on individual characteristics to develop targeted nutritional advice, products, or services,” expanding the scope beyond clinical interventions (Citation5). While these definitions vary somewhat, experts agree that the goal of PN is to advance human health and wellbeing by tailoring nutrition recommendations and interventions to individuals or groups of individuals with similar traits; it may integrate a variety of inputs including clinical assessments, biomarkers of physiological function and pathological processes, genetic information, data from biosensors such as activity trackers, and other available data derived from advanced technologies (Citation1, Citation2, Citation4, Citation5, Citation8, Citation9).
Several distinguishing characteristics describe the discipline of PN: (Citation1) PN has emerged relatively recently; (Citation2) PN is rooted in scientific evidence; (Citation3) PN relies on analytical technologies as well as the coaching of trained practitioners; (Citation4) PN is multidisciplinary, drawing on knowledge from other fields such as genomics, epigenetics, systems biology, medicine, and behavioral sciences in addition to traditional nutrition science and clinical practice; and PN enables further tailoring of interventions to meet the needs of individuals or specific groups of people (Citation1, Citation10).
In its 2016 position paper by Ferguson et al., the International Society of Nutrigenetics/Nutrigenomics (ISNN) proposed that tailoring nutrition recommendations to individuals or groups of people in this way “should be more effective at preventing chronic diseases than general recommendations about diet…Recognition of diverse individual nutritional needs and responses to diet are changing standards of nutritional care, creating new possibilities for this field” (Citation11). Given the considerable potential of PN to contribute to health and wellness, a consensus definition and delineation of the field is imperative to promoting efforts in research, education, clinical practice, and policy that may be applied in a variety of settings for the betterment of human health and wellbeing (Citation5). This paper describes the need for PN, proposes a consensus definition of the term, and delineates three elements of PN as a novel, multi-disciplinary framework for the field: PN science and data, PN professional education and training, and PN guidance and therapeutics. Further, we outline the areas of research informing PN, its therapeutic potential, and the challenges inherent to this emerging field.
Why is PN needed?
PN addresses the chronic disease crisis
The U.S. is gripped by a crisis of chronic disease. Seven of the top ten leading causes of death in the U.S. are chronic diseases, most of which are considered preventable (Citation12). In 2014, about 60% of American adults suffered from at least one chronic disease (in addition to 27% of children) and about a quarter of Americans had multiple chronic conditions (Citation13–15). It is well established that poor nutrition is a primary driver of chronic disease, particularly cardiometabolic conditions and diet-related cancers. These conditions are also among the leading causes of death in the United States and globally (Citation16, Citation17). A recent systematic analysis for the Global Burden of Disease (GBD) sought to discern the disease-specific burden attributable to a variety of dietary risk factors; in 2017 approximately 11 million global deaths and 255 million disability-adjusted life years (DALYs) could be attributed to dietary risk factors (Citation17). A study in the same year estimated that nearly half of all deaths due to cardiometabolic diseases in the U.S. can be attributed to poor diet (Citation18). Mounting evidence continues to suggest that diets rich in vegetables, fruits, whole grains, legumes, and nuts are associated with lower risk of cardiometabolic diseases across various adult subgroups (Citation19, Citation20).
Nutrition-related chronic disease is a global problem, and the effects are not restricted to adults or those nearing the end of life. The “first 1000 days” concept points to the critical period of physiological and brain development from conception to 2 years of age. Early life exposures to poor nutrition may program children for long-term health effects including obesity, metabolic conditions, and cardiovascular diseases – those responsible for the most deaths globally (Citation21, Citation22). Such findings have placed nutrition and lifestyle interventions to modify disease risk across the life cycle at the center of much contemporary research.
Despite the evidence that it is core to a comprehensive approach to addressing complex chronic disease and promoting human health, personalized nutrition is largely absent from our healthcare culture and system. The current model for treating chronic disease is a result of breakthroughs during the last century, primarily targeting acute conditions, wherein single-agent causes of illness were identified and single-agent pharmacologic treatments were developed. However, chronic diseases are now the major causes of death and disability in both developed and developing countries, outpacing the rates of acute diseases in many nations (Citation23, Citation24). Notwithstanding, medical research, practitioner education, clinical care, public and private health policy continue to skew toward application of the acute-care model to complex, multifactorial conditions that develop over time. A disease-centered, acute care approach is ill-suited to chronic conditions that have multiple causes and impact multiple biological systems, as they typically have no single agent of action indicating a clear single intervention (Citation25). These factors, often hidden from view, must be uncovered and approached comprehensively, from the standpoint of the individual’s unique circumstances. Thus, PN seeks to elucidate and beneficially influence how diet shapes an individual’s response to nutrients and, reciprocally, how genetic makeup impacts nutrient metabolism and nutrient requirements in service of optimizing health and function.
Implications for PN policy framework and healthcare costs
Treating NCDs within the current healthcare model accounts for a staggering 90% of our nation’s $3.3 trillion healthcare costs (Citation26). In fact, over 90% of total health care spending in the United States is attributed to care for Americans with one or more chronic condition(s) (Citation13). As noted, many national chronic disease organizations, as well as the U.S. Department of Agriculture, have posited and promoted nutrition recommendations as preventive and adjunctive measures to reduce the incidence of chronic disease and the cost of treating these conditions. Yet, steady and increasing rates of most chronic lifestyle diseases and skyrocketing healthcare spending reveal that that translation and implementation continue to be major hurdles, and that the current model is unsustainable.
A 2010 National Alliance for Nutrition and Activity report aiming to quantify the benefit of healthy nutrition suggested that eating healthfully could save at least $87 billion per year in medical costs, lost productivity and lost lives (Citation27). Thus, there is great need for a policy framework to support nutritional approaches to health care that are driven by the science of PN. At the county, municipal, or community level, policy can be applied to create health initiatives that focus on the health and wellbeing of individuals within their communities by considering the local environment, demographics, social determinants of health, community culture, and resources. PN science and data can inform policymakers and community decisionmakers in crafting initiatives that promote and improve access to health-promoting resources including greater availability of healthy food and the ability to access affordable, culturally congruent nutrition education and counseling in their communities.
Toward greater personalization in public health guidelines
Why have nutrition guidelines thus far failed to slow the epidemic of the chronic disease epidemic? Surveys suggest that 80% of Americans encounter conflicting nutrition information, and that 59% doubt their nutritional choices (Citation28). A study by the International Food Information Council Foundations entitled “Views Toward Nutrition and Healthful Eating Among Millennials” found that most young Americans get their nutrition and health information from social media or news sources (primarily online, television, or print media). Furthermore, millennials are skeptical of nutrition information, distrusting the credibility of any source based on a perception that nutrition recommendations are often promulgated by industry groups who stand to profit from them (Citation29). With limited access to clear, science-based, unbiased nutrition information, public trust in generalized nutrition guidelines is compromised.
By definition, national recommendations such as Reference Dietary Intakes (RDIs) and Dietary Reference Intakes (DRIs) established by government agencies for particular nutrients are broadly stratified by demographics (stages of the life cycle, age, and sex). Various public health organizations go a step further than general population or public nutrition guidelines, incorporating a degree of customization into nutritional guidelines and recommendations for the treatment and recovery from the chronic conditions they seek to remediate (Citation30–32). However, these guidelines have only limited ability to address the myriad inputs that influence the unique manifestation of an individual’s health or disease status (Citation4).
Meaningful changes can be made in the lives of individuals and communities with small, targeted changes to diet, even in the absence of extensive data and one-on-one nutrition counseling. For example, a growing body of research points to the protective nature of plant foods against NCDs (Citation33). Thus, generalizable nutrition recommendations have been, and can be, drawn from the existing evidence base to guide public policy; however, “eat your fruits and vegetables” messaging does not sufficiently address inter-individual variability and has proven to be of limited utility in stemming the tide of chronic diseases.
Proposed definition and delineation of personalized nutrition
Given the need for PN in health care and its potential impact on human health, we propose the following definition:
Personalized nutrition is a field that leverages human individuality to drive nutrition strategies that prevent, manage, and treat disease and optimize health.
There are three elements, or areas of application, that delineate the field of PN: PN science and data, PN professional education and training, and PN guidance and therapeutics.
These elements are further described below as discrete areas of application, and a discussion of their interdependence follows.
The elements of PN
PN is delineated by three elements that form and inform one another (); advances in any element initiate and necessitate advances in the others to fully equip thought leaders, scientists, and practitioners. Each offers a unique set of tools and perspectives that, together, position PN to contribute to improved health care and its delivery.
PN Science and Data builds upon knowledge gleaned from traditional research methods such as observational studies and randomized controlled trials (RCTs), along with other human interventions and “citizen science” or crowdsourced data projects (Citation9, Citation34). Blumberg et al., assert that “advancing evidence-based nutrition will depend upon research approaches that include RCTs but go beyond them.” (Citation9) Emerging and advanced omics technologies also contribute to the robust PN knowledge base. Marrying methods and technologies enables better understanding of the potential impact of nutrition interventions on individuals and groups of people based on pertinent inputs and variables.
PN Professional Education and Training integrate established nutrition science with PN science and data and advanced research methods. It incorporates traditional clinical care concepts with current, advanced PN interventions for health promotion and disease management. The clinical efficacy of PN requires that practitioners are adequately trained to apply this knowledge in practice. Laddu and Hauser note that while PN research continues to expand, the translation into clinical action and its utilization by healthcare practitioners lags behind. They recommend that general nutrition education be improved at all levels, including health professional training programs and continuing education (Citation2). PN should be utilized by a variety of healthcare professionals and, thus, training and education should be appropriate to the level of application.
PN Guidance and Therapeutics are clinical approaches in which the individual client or patient is central to the care process and the development of meaningful recommendations, mirroring the emerging model of personalized medicine. Interventions are designed based on the fullness of available objective data including anthropomorphic, biochemical, genetic, microbial and/or omic; in addition to socio-behavioral and subjective factors such as personal and family history, cultural background and personal beliefs and preferences. In the PN paradigm, health and disease are not viewed as binary, but as existing along a continuum of function. Systems are not viewed in isolation, but in relationship to one another. The PN practitioner can map areas of greatest importance across function and systems in order to more fully understand an individual’s phenotype and nutritional needs and advise accordingly.
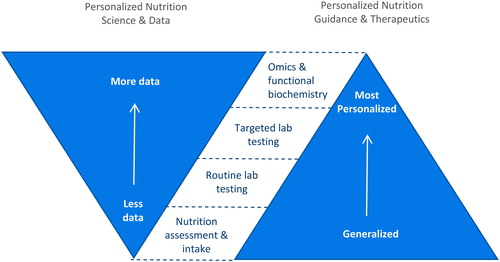
Richer and more robust data can lead to more targeted recommendations and interventions ().* Results can be achieved to enhance quality of life and health outcomes with protocols developed based on standard nutrition intakes and assessments. When the practitioner has access to additional data for those with specific traits or health conditions, the practitioner can further tailor evidence-based strategies and interventions for individuals or subgroups with certain attributes such as insulin resistance or impaired immunity. Tools such as genomics and functional testing may be utilized for further impact on the health and behavior of individuals now and in the future (Citation3, Citation35–37). This is important as growing evidence suggests that personalizing nutrition guidance and recommendations leads to more sustainable and effective behavior change on an individual level (Citation38, Citation39).
The PN care model
An individual’s health status is not simply the presence or absence of a diagnosable disease, but rather the culmination of the interplay of systems and inputs - some inherited, though the majority a result of environmental exposures, diet, and lifestyle. By understanding data that reflects the entirety of a patient’s unique circumstances, health history, and functional imbalances, the practitioner is able to intervene at any stage: disease prevention, subclinical symptom management, disease manifestation and progression, health optimization, and performance enhancement.
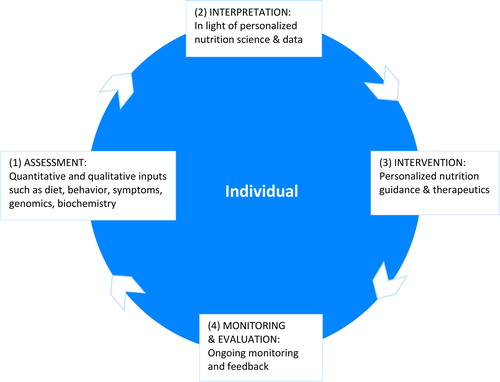
The proposed definition of PN points to the formulation of strategies that, when applied, prevent, manage, and treat disease and optimize health. A supportive care model creates a framework enabling PN practitioners to achieve a greater level of personalization, and allows individuals and groups of people to benefit from strategies based on their unique traits. This care model reflects aspects of accepted care models utilized across healthcare professions: (Citation1) assessment, (Citation2) interpretation, (Citation3) intervention, and (Citation4) monitoring and evaluation, and is adapted to meet the need for emerging personalized care incorporating new technologies that expand clinical tools in PN. As in other care models, the PN Care Model () is cyclical, allowing for continual refinement of interventions to achieve the desired outcomes. More research is needed before PN will take its place as a centerpiece of our healthcare system (Citation5). However, as PN science and data allow us to understand more about the impact of genetic, phenotypic, biochemical, and nutritional inputs on an individual’s health, PN professionals will have greater ability to tailor interventions (Citation5). A personalized model of care is the hallmark of this clinical approach.
Assessment
Extensive assessment and ongoing patient input and feedback provide quantitative and qualitative information that enable the PN practitioner to gain greater understanding of the unique landscape of the individual. Readiness, motivation, behavior, sociocultural preferences, symptoms and manifestations of dysfunction are considered alongside results of objective assessments. These include, but are not limited to, biochemical assays, advanced analyses of inflammation, oxidation and environmental contaminants, nutritional genomic reports, and microbial panels. Consideration of both subjective and objective input empowers the practitioner to create a framework for care and identify the most fruitful areas of focus and intervention.
Interpretation
The PN assessment is evaluated through the lens of the rapidly evolving body of PN scientific evidence. The PN professional considers the fullness of data gathered to create a roadmap of an individual’s function or dysfunction and repletion or depletion across body systems and biochemical pathways.
Intervention
The PN practitioner uses the data and roadmap developed to design actionable interventions, education, counseling and ongoing care to address manifestations of dysfunction (i.e. symptoms) as well as the underlying root causes of imbalance. Interventions can include changes to diet; targeted nutraceuticals; lifestyle factors such as movement, sleep, and stress management; and food-related behaviors such as timing of eating, eating environment, fasting, food selection, food storage, and food preparation.
Monitoring and evaluation
Ongoing monitoring and evaluation are crucial to a robust care model, as they enable further personalization of interventions throughout the duration of the care process. The PN practitioner regularly assesses subjective input and collects objective data in order to hone and refine therapeutic intervention strategies to build self-efficacy and behavior change in the individual, thereby optimizing quantitative and qualitative measures of an individual’s health.
Integration of PN into clinical practice
In the PN model, more targeted interventions and more effective prevention and treatment may be possible as PN practitioners are equipped to assess multiple inputs and determine their distinct and collective roles in an individual’s health trajectory. For instance, as genetic testing becomes more affordable, accessible, and popular, practitioners and the public have increased awareness of and access to information about gene variants that may play a role in health and disease. Genetic reports often include nutritional recommendations based on an individual’s genome. Nielsen and El-Sohemy found that “DNA-based dietary advice results in greater changes in intake for some dietary components compared to population-based dietary advice,” results that suggest potential for the impact of gene-based testing on tailoring recommendations to individuals (Citation34).
To this end, the important role of the nutrition professional should be considered. Zubair et al. conducted an observational study in 2531 participants enrolled in a program that combined multiomic data with personalized coaching via phone. They looked at the program’s impact on 55 clinical markers and the impact of genetic predisposition on changes to those markers. The results revealed “sustained improvements” in anthropometric, nutrient, inflammatory and cardiometabolic risk markers in particular, improvements to hemoglobin A1c. Their results also demonstrated that genetic markers could be associated with longitudinal changes to these clinical markers. They concluded that: “Overall, these results suggest that a program combining multi-omic data with lifestyle coaching produces clinically meaningful improvements, and that genetic predisposition impacts clinical responses to lifestyle change” (Citation40).
Similar findings emerged from a study by Araujo Almeida et al., of 478 people who received the results of a genetic test with or without practitioner-facilitated education and interventions. The group that received practitioner-facilitated nutrition interventions had “greater improvements in diet quality…when compared with receiving a standard gene test report” (Citation36). Studies such as these suggest that the optimal path to achieving long-term goals and outcomes may be a collaborative approach leveraging available technologies that analyze data and assist with tailoring recommendations, facilitated by trained professionals.
Advances in science & technology enabling PN
The omics sciences
The “omics sciences” - nutritional genomics, epigenomics, transcriptomics, metabolomics, proteomics, microbiomics, and others - inform the elements of PN: PN research, PN education and PN practice. PN considers omics analyses that identify relevant molecules (metabolites, proteins, microbes, genes) in conjunction with analyses of body system function, nutritional, and environmental inputs, allowing for a more comprehensive understanding of an individual’s health circumstances and needs (Citation10, Citation41, Citation42). Bland, Minich, and Eck describe how these omics sciences and technologies inform personalized practice by allowing PN practitioners to evaluate, track and map complex gene expression, proteins, and metabolites noting that, while promising, our ability to translate omics data into relevant, personalized guidance and clinical interventions is still nascent and both the data and any resulting recommendations require scrutiny (Citation10).
Nutritional genomics
Nutritional genomics is a specific area of research exploring the interaction between genes, nutritional components, and health outcomes (Citation43). Nutritional genomics research informs a broadening and deepening understanding of the interactions of genes, nutritional components, and health toward the application of personalized approaches, particularly within certain populations with similar traits (Citation43). Many variants are being prioritized for future research of genotype-directed population-specific nutrition strategies.
Microbiomics
Highly individualized microbial communities residing in the gut exert influence on digestion and assimilation, thereby impacting the nutrients derived from food. These microbes also work to shape human metabolism by contributing their own exogenous enzymatic functions (Citation44, Citation45). The interplay of food and nutrients with the microbiome and our genetic material influences the systems and biological processes that lead us toward health resilience or dysfunction and disease. In addition to risk for diabetes and obesity, the microbiome has been implicated in a variety of other biological processes such as modulation of host immune function and modulation of neuroinflammation impacting brain function (Citation42, Citation46). Interactions between food, genomics, and microbiomics “could become the new challenge for the future in preventive medicine” (Citation42). Thus PN will play an important role in elucidating these interactions and developing tools and strategies for the prevention, management, and treatment of a range of chronic diseases.
Promising clinical applications for PN
Approaches to therapeutic diets
Although therapeutic dietary patterns are often embedded into PN plans, they must be individualized in order to exert greatest positive health outcomes. Two examples are discussed here along with PN perspectives on modifications to tailor these therapeutic dietary patterns to subgroups or individuals.
Allergen-free diets have the common aim of removing immunological triggers from an individual’s diet. These plans may be indicated for the treatment of known allergies, intolerances and sensitivities or for the identification of unknown triggers via elimination diet. An allergen-free diet is typically tailored to the individual by a PN practitioner based on the specifics of their response, the type of immune reaction (ex. IgE or non-IgE mediated), and other contributing causes such as lack of enzymes or gastrointestinal factors. Some examples of allergen-free diets include peanut free, tree nut-free, low FODMAP, gluten-free, and casein- or dairy-free (Citation47–49). Tree nut and peanut allergies are some of the most severe IgE mediated food allergies, and avoidance is considered the most effective clinical strategy. However, alternatives to complete avoidance of tree nuts are being proposed. By employing “selective avoidance” for those with tree nut allergies who are clinically tolerant to only some tree nuts, personalized approaches can expand the diet and mitigate the development of additional allergies (Citation50). Furthermore, food allergy prevention is being recommended, with early introduction of allergenic food in infants to build immune tolerance (Citation51).
Some of these diets are being considered in the treatment of non-allergic conditions. For example, gluten-free diets are being explored for the treatment of a diverse range of conditions for which gluten has been implicated in the pathology, such as gluten-related gastrointestinal, autoimmune, skin, nervous system, psychiatric, and neurological conditions (Citation52–57). Researchers continue to explore predictive factors for successful application of these and other therapeutic diets based on genetic, clinical, biochemical and demographic inputs.
Ketogenic diets are designed to drive metabolism into a state of ketosis, providing ketones as a primary source of cellular fuel. The hallmarks of ketogenic diets are low-carbohydrate and high-fat; however, the macronutrient ratios needed to promote and maintain ketosis varies from person to person. Additionally, ketogenic diets can be tailored to the specific lifestyle and preferences of an individual, such as a vegan ketogenic plan. Thus, personalization is involved in the clinical decision to select a ketogenic diet, the identification of the most appropriate ketogenic diet for that individual, and the most effective duration of the diet, among other considerations. As noted by Paoli et al., ketogenic diet therapy is the focus of ongoing research for many conditions “…such as diabetes, polycystic ovary syndrome, acne, neurological diseases, cancer and the amelioration of respiratory and cardiovascular disease risk factors. The possibility that modifying food intake can be useful for reducing or eliminating pharmaceutical methods of treatment, which are often lifelong with significant side effects, calls for serious investigation” (Citation58).
Glycemic response
The personalized approach has been explored with regard to post-prandial glycemic response (PPGR). For example, Zeevi et al. monitored 800 individuals with type 2 diabetes for one week, and found widely varied post-meal blood glucose changes in response to identical meals. They reported that “Personalized diets created with the help of an accurate predictor of blood glucose response that integrates parameters such as dietary habits, physical activity, and gut microbiota may successfully lower postmeal blood glucose and its long-term metabolic consequences” (Citation59). Mendes-Soares et al. conducted a similar study in 327 non-diabetic individuals indicating that a personalized predictive model may enable individuals to better manage PPGR (Citation60). Further research in this area will contribute to a growing understanding of the role of personalized assessment in creating tailored recommendations to improve glycemic responses and reduce the incidence and consequences of long-term hyperglycemia, as well as other cardio-metabolic processes and conditions.
Meeting the challenges of PN
While the number of PN practitioners is growing, considerable obstacles must be overcome before PN can be fully actualized. Several challenges are discussed below and potential solutions and strategies are presented.
Challenge – nutrition research: data management, study design & translation
Research is continuously identifying additional potential for PN application, and large datasets are being collected. With vast information at our fingertips, collecting, organizing and analyzing these data can be daunting, costly, and labor-intensive. Results of nutrition trials may be called into question due to lack of reproducibility, lack of statistical power and methodological issues (Citation7). The multivariate nature of inter-relationships between genetic factors, biosystems, biomarkers and nutrients are complex and are not fully understood; moreover, the conclusions drawn from the data can be contentious (Citation10).
Harnessing the available research and translating it into actionable recommendations presents another challenge. For example, since the mapping of the human genome, many have discussed how genetic information might impact clinical practice, but Murgia and Adamski note that “…fifteen years and hundreds of publications later, the gap between the experimental and epidemiologic evidence and health practice is not yet closed.” (Citation44) Clinicians and scientists have and always will be challenged to translate the growing evidence base into meaningful clinical interventions (Citation44).
Meeting the challenge – PN research & data
Personalized approaches have brought to light concerns regarding the RCT as the “gold standard” of evidence (Citation9, Citation61, Citation62). When interventions are chosen based an individual’s health data and circumstances, population-based evidence may not be sufficient for determining the best intervention (Citation61). Leveraging the wealth of available tools may be the most effective way forward for PN research. These tools may include evidence from RCTs, as well as other types of research approaches with varying degrees of certainty, such as small human studies based on sub-populations, and even n-of-1 data using biomarkers, genetic variants, functional markers, technology platforms, and data from biosensors.
As technology advances, data collection will become increasingly affordable and much more accessible. Already, wearable devices and mobile apps enable individuals to collect real-time information of activity, diet and fluid intake, and other parameters of health. Wang and Hu suggest that integration of these types of tracking technologies with “big data analytics” will enable ever more personalized guidance (Citation7). Integrating technologies, advanced computational methods, artificial intelligence, and systems approaches to analyzing a broad range of data from various inputs will likely enable more targeted, personalized guidance (Citation6, Citation10, Citation63, Citation64).
Interdisciplinary knowledge platforms will ultimately allow for the necessary creation and adoption of newer standards of nutrition science investigation (Citation62, Citation64). With robust repositories of more precise data and greater understanding of the relationships between biomarkers, genetics, and health outcomes, researchers may be able to improve hypothesis generation for future research initiatives (Citation64, Citation65). Growing understanding of these complexities contributes to a robust and well-rounded evidence base, which provides the foundation for the development of evidence-based clinical strategies and therapeutic interventions, informs education curricula and training programs, and lays the groundwork for effective policy and public health initiatives (Citation9).
Challenge - equipping practitioners
Despite the complexities inherent to PN, practitioners have a mandate to provide timely, science-based recommendations to their clients and patients. As Laddu and Hauser note, “it could be argued that it is an impossible task for clinicians to add the significant volume of new information needed to personalize recommendations at the omics level to the already large and ever-increasing amount of content that they are expected to know” (Citation2). For example, nutrition professionals are considered a primary resource for actionable nutrition recommendations based on genetic individuality. However, Murgia and Adamski note that professionals are lacking evidence summaries and guidelines to effectively make such recommendations. While clinicians are challenged to manage the rapidly evolving world of genetic testing, omic technology, and emerging research to support their patients and clients, their training does not often equip them to do so (Citation44).
For example, nutrition is within the scope of practice of a range of healthcare providers, including physicians, however nutrition is underutilized largely due to insufficient nutrition education in medical training and a resulting lack of confidence implementing nutrition strategies in practice (Citation66–68). As such, Barnard suggests that a prudent and immediate step for physicians is to recognize the importance of nutrition counseling, communicate with the patient, and refer as appropriate (Citation69).
Meeting the challenge – PN professional education and training
PN can be incorporated more fully into health care by improving nutrition education for all healthcare practitioners (Citation2, Citation70). Academic institutions and training associations should thoughtfully design nutrition education curricula to equip clinicians with leading-edge science and clinical tools relevant to the type of practitioner. As PN science continues to develop, university curricula, certification standards, training programs, and continuing education courses must continue to evolve and provide dynamic and up-to-date offerings. Third-party accrediting bodies can serve in this effort, as they ensure the high quality of nutrition education programs through periodic monitoring and evaluation of programs (Citation66, Citation71). Additionally, accredited certifications that set standards for practicing professionals will support efforts to equip qualified practitioners by ensuring programmatic rigor, a robust practice experience, and a solid knowledge and skill base (Citation72). Academic institutions and training organizations are developing and implementing programs based on standards set by nationally recognized accreditation and certification bodies. As more update their curricula, additional programs will become available across the healthcare profession to further equip practitioners and advance PN in practice.
Challenge – access to PN services
Despite evidence that such services may support better health outcomes and lower the cost of healthcare, PN services are unavailable in most healthcare settings. Improving access to PN care requires addressing two systemic issues: limited access to nutrition providers and lack of insurance reimbursement. Outdated state laws regulating the practice of nutrition have made PN services out of reach for most Americans. Many state practice laws place outdated restrictions on which professionals can deliver clinical nutrition services, preventing healthcare practitioners from offering services they are trained to provide. Importantly, PN services are not yet widely covered by insurance plans. This leaves residents of many states with limited access and little choice when seeking PN services.
Meeting the challenge – expanded nutrition policy & access to PN care
In order to embed nutrition in the healthcare system and the toolset of healthcare professionals, it is important to advocate for laws that authorize all practitioners to practice to the level of their training. Furthermore, recognition of and opportunities for nutrition practitioners at various skill levels will expand access to care by promoting a diverse workforce, ensuring high-quality services and market competition, and supporting the creation and implementation of favorable policy. An example is a 2018 North Carolina state law change which authorizes a broader array of nutrition professionals to practice than previously allowed (Citation73). Another is the 2014 Centers for Medicare & Medicaid Services (CMS) rule authorizing clinically qualified nutrition professionals to order therapeutic diets in hospitals and other institutional facilities (Citation74).
Despite these inroads, the cost of PN services remains a significant barrier. While some insurers cover nutrition counseling, reimbursement is typically inadequate. It is common for insurers to only enroll certain types of nutrition providers; only pay for care for individuals with certain medical conditions; and provide little to no reimbursement of functional laboratory tests, dietary supplements, and medical foods which are frequently utilized in PN guidance and therapeutics. Federal and state health insurance laws which are favorable to nutrition can help to ensure that patients who want or need access to PN care can afford to receive it. Additionally, effective public health initiatives remain an untapped strategy for maintaining and improving the health of society at large, and there is an urgent need for a policy framework to support national public health messaging and more effective approaches to health care driven by the science of PN.
Conclusion
PN is a field with great potential to address chronic disease and optimize human health and performance. An agreed-upon definition and clear delineation of PN is imperative for its acceptance, utilization, and expansion.
Herein we propose the definition of PN as: a field that leverages human individuality to drive nutrition strategies that prevent, manage, and treat disease and optimize health. PN is delineated by three elements: PN science and data, PN professional education and training, and PN guidance and therapeutics. Novel research methods and the continued development of innovative technology solutions will lead to increasingly individualized nutrition guidance, products and services. Enhanced education and training will equip a generation of practitioners who can apply personalized models of care to better support the health and wellbeing of individuals and communities.
Solutions to complex problems require creativity, collaboration and big-picture thinking. The interdisciplinary nature of PN requires that stakeholders act in concert, participating as allies toward a paradigm shift in the healthcare landscape (Citation10). With consensus and collaboration among scientists, experts, clinicians, food and health industry leaders, and policymakers, we can advance PN science, train PN practitioners, and enhance access to PN care. Building on the seminal work done by pioneers across many disciplines, this definition can serve as a springboard to embed PN in the healthcare system to prevent, treat, and manage disease, and optimize human health.
Acknowledgments
JMO would like to thank the US Department of Agriculture, Agriculture Research Service (8050–51000-098-00D).
Disclosure statement
AES is the founder of and holds shares in Nutrigenomix, Inc. DMM is a consultant for Metagenics, Inc.
References
- Boorsma A, van Someren E, de Hoogh I, Hogenelst K, van Erk M, Wopereis S, Rouhani-Rankouhi T, van den Broek T, Pasman W, van Ommen B, Anthony JC. Systems biology of personalized nutrition. Nutr Rev. 2017;75(8):579–599. doi:10.1093/nutrit/nux029.
- Laddu D, Hauser M. Addressing the nutritional phenotype through personalized nutrition for chronic disease prevention and management. Prog Cardiovasc Dis. 2019;62(1):9–14.
- Guest NS, Horne J, Vanderhout SM, El-Sohemy A. El-Sohemy a: sport nutrigenomics: personalized nutrition for athletic performance. Front Nutr. 2019;6:8. doi:10.3389/fnut.2019.00008.
- Michel M, Burbidge A. Nutrition in the digital age - how digital tools can help to solve the personalized nutrition conundrum. Trends Food Sci Technol. 2019;90194–200. doi:10.1016/j.tifs.2019.02.018.
- Ordovas JM, Ferguson LR, Tai ES, Mathers JC. Personalised nutrition and health. BMJ. 2018;361:bmj.k2173.
- Westerman K, Reaver A, Roy C, Ploch M, Sharoni E, Nogal B, Sinclair DA, Katz DL, Blumberg JB, Blander G. Longitudinal analysis of biomarker data from a personalized nutrition platform in healthy subjects. Sci Rep. 2018;8(1):14685.
- Wang DD, Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6(5):416–426.
- de Toro-Martin J, Arsenault BJ, Despres JP, Vohl MC. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients. 2017;9(8):913. doi:10.3390/nu9080913.
- Blumberg JB, Heaney RP, Huncharek M, Scholl T, Stampfer M, Vieth R, Weaver CM, Zeisel SH. Evidence-based criteria in the nutritional context. Nutr Rev. 2010;68(8):478–484. doi:10.1111/j.1753-4887.2010.00307.x.
- Bland JS, Minich DM, Eck BM. A systems medicine approach: translating emerging science into individualized wellness. Adv Med. 2017;2017:1. doi:10.1155/2017/1718957.
- Ferguson LR, De Caterina R, Gorman U, Allayee H, Kohlmeier M, Prasad C, Choi MS, Curi R, de Luis DA, Gil A, et al. Guide and position of the international society of nutrigenetics/nutrigenomics on personalised nutrition: part 1 - fields of precision nutrition. J Nutrigenet Nutrigenomics. 2016;9(1):12–27. doi:10.1159/000445350.
- National Center for Chronic Disease Prevention and Health Promotion. The power of prevention chronic disease: the public health challenge of the 21st century. 2009.
- Buttorff C, Ruder T, Bauman M. Multiple chronic conditions in the United States. Santa Monica, CA: Rand Corporation, 2017.
- National Center for Chronic Disease Prevention and Health Promotion. About chronic diseases. [accessed 2019 May 1]. https://www.cdc.gov/chronicdisease/about/index.htm.
- Focus for Health. Chronic illness and the state of our children’s health. [accessed 2019 May 1]. https://www.focusforhealth.org/chronic-illnesses-and-the-state-of-our-childrens-health/.
- Heron M. National vital statistics reports, 2019. https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_06-508.pdf.
- Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, Mullany EC, Abate KH, Abbafati C, Abebe Z, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958. doi:10.1016/S0140-6736(19)30041-8.
- Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912–924. doi:10.1001/jama.2017.0947.
- Qian F, Liu G, Hu F, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(10):1335–1344. doi:10.1001/jamainternmed.2019.2195.
- Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med. 2018;28(7):437–441. doi:10.1016/j.tcm.2018.02.004.
- Mameli C, Mazzantini S, Zuccotti GV. Nutrition in the first 1000 days: the origin of childhood obesity. IJERPH. 2016;13(9):838. doi:10.3390/ijerph13090838.
- Moreno Villares JM. Nutrition in early life and the programming of adult disease: the first 1000 days. Nutr Hosp. 2016;33(Suppl 4):337. doi:10.20960/nh.337.
- U.S. Department of State: Infections Disease and Non-Infectious Disease. 2009. [accessed 2019 May 20]. https://2001-2009.state.gov/g/oes/id/.
- The U.S. Government and Global Non-Communicable Disease Efforts. 2019. [accessed 2019 May 20]. https://www.kff.org/global-health-policy/fact-sheet/the-u-s-government-and-global-non-communicable-diseases/.
- Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals–directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1(1):9–10. doi:10.1001/jamacardio.2015.0248.
- National Center for Chronic Disease Prevention and Health Promotion: Health and Economic Costs of Chronic Diseases. [accessed 2019 May 1]. https://www.cdc.gov/chronicdisease/about/costs/index.htm.
- Pokress BH. National health priorities: reducing obesity, heart disease, cancer, diabetes, and other diet- and inactivity-related diseases, costs, and disabilities. 2010. https://cspinet.org/sites/default/files/attachment/cdc_briefing_book_fy10.pdf.
- International Food Information Council Foundation: 2018 Food and Health Survey. 2018. [accessed 2019 May 1]. https://foodinsight.org/wp-content/uploads/2018/05/2018-FHS-Report-FINAL.pdf.
- International Food Information Council Foundation: Views Toward Nutrition and Healthful Eating Among Millennials. 2013. https://foodinsight.org/wp-content/uploads/2014/10/Report-IFIC-Foundation-Millennial-Focus-Groups-11-20-2013.pdf
- American Diabetes Association: Food. [accessed 2019 May 20]. http://www.diabetes.org/food-and-fitness/food/?loc=ff-slabnav.
- American Heart Association: Nutrition Basics. 2018. [accessed 2019 May 20]. https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutritionbasics.
- American Cancer Society: Eat Healthy and Get Active. [accessed 2019 May 20]. https://www.cancer.org/healthy/eat-healthy-get-active.html.
- Fardet A, Boirie Y. Associations between food and beverage groups and major diet-related chronic diseases: An exhaustive review of pooled/meta-analyses and systematic reviews. Nutr Rev. 2014;72(12):741–762. doi:10.1111/nure.12153.
- Lichten CA, Ioppolo B, d'Angelo C, Simmons RK, Jones MM. Citizen science: crowdsourcing for research. The Healthcare Improvement Studies Institute, 2018. https://www.thisinstitute.cam.ac.uk/wp-content/uploads/2018/05/THIS-Institute-Crowdsourcing-for-research-978-1-9996539-0-3.pdf
- Nielsen DE, El-Sohemy A. Disclosure of genetic information and change in dietary intake: a randomized controlled trial. Plos One. 2014;9(11):e112665. doi:10.1371/journal.pone.0112665.
- Picó C, Serra F, Rodríguez AM, Keijer J, Palou A. Biomarkers of nutrition and health: new tools for new approaches. Nutrients. 2019;11(5):1092. doi:10.3390/nu11051092.
- Araujo Almeid V, Littlejohn P, Cop I, Brown E, Afroze R, Davison KM. Comparison of nutrigenomics technology interface tools for consumers and health professionals: a sequential explanatory mixed methods investigation. J Med Internet Res. 2019;21(6):e12580. doi:10.2196/12580.
- Celis-Morales C, Livingstone KM, Mathers JC, …Drevon CA. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2016;46(2):578–588.
- Horne J, Madill J, O’Connor C, Shelley J, Gilliland J. A systematic review of genetic testing and lifestyle behaviour change: are we using high-quality genetic interventions and considering behaviour change theory? Lifestyle Genom. 2018;11(1):49–63. doi:10.1159/000488086.
- Zubair N, Conomos MP, Hood L, Omenn GS, Price ND, Spring BJ, Magis AT, Lovejoy JC. Genetic predisposition impacts clinical changes in a lifestyle coaching program. Sci Rep. 2019;9(1):6805. doi:10.1038/s41598-019-43058-0.
- Di Renzo L, Gualtieri P, Romano L, Marrone G, Noce A, Pujia A, Perrone MA, Aiello V, Colica C, De Lorenzo A. Role of personalized nutrition in chronic-degenerative diseases. Nutrients. 2019;11(8):1707. doi:10.3390/nu11081707.
- Nilsson PD, Newsome JM, Santos HM, Schiller MR. Prioritization of variants for investigation of genotype-directed nutrition in human superpopulations. IJMS. 2019;20(14):3516. doi:10.3390/ijms20143516.
- Loos RJF. From nutrigenomics to personalizing diets: are we ready for precision medicine? Am J Clin Nutr. 2019;109(1):1–2. doi:10.1093/ajcn/nqy364.
- Murgia C, Adamski MM. Translation of nutritional genomics into nutrition practice: the next step. Nutrients. 2017;9(4):366. doi:10.3390/nu9040366.
- Davis CD. The gut microbiome and its role in obesity. Nutr Today. 2016;51(4):167–174. doi:10.1097/NT.0000000000000167.
- Janakiraman M, Krishnamoorthy G. Emerging role of diet and microbiota interactions in neuroinflammation. Front Immunol.. 2018;92067–2067. doi:10.3389/fimmu.2018.02067.
- Hochwallner H, Schulmeister U, Swoboda I, Spitzauer S, Valenta R. Cow's milk allergy: from allergens to new forms of diagnosis, therapy and prevention. Methods (San Diego, Calif.). 2014;66(1):22–33. doi:10.1016/j.ymeth.2013.08.005.
- Fedewa A, Rao SS. Dietary fructose intolerance, fructan intolerance and FODMAPs. Curr Gastroenterol Rep. 2014;16(1):370. doi:10.1007/s11894-013-0370-0.
- Rostami K, Bold J, Parr A, Johnson MW. Gluten-free diet indications, safety, quality, labels, and challenges. Nutrients. 2017;9(8):846. doi:10.3390/nu9080846.
- Weinberger T, Sicherer S. Current perspectives on tree nut allergy: a review. JAA.. 2018;Volume 1141–51. doi:10.2147/JAA.S141636.
- Chan ES, Abrams EM, Hildebrand KJ, Watson W. Early introduction of foods to prevent food allergy. Allergy Asthma Clin Immunol. 2018;14(2):57–57.
- Hadjivassiliou M, Sanders DD, Aeschlimann DP. Gluten-related disorders: gluten ataxia. Dig Dis. 2015;33(2):264–268. doi:10.1159/000369509.
- Hollon J, Puppa EL, Greenwald B, Goldberg E, Guerrerio A, Fasano A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. 2015;7(3):1565–1576. doi:10.3390/nu7031565.
- Bhatia BK, Millsop JW, Debbaneh M, Koo J, Linos E, Liao W. Diet and psoriasis, part II: Celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71(2):350–358. doi:10.1016/j.jaad.2014.03.017.
- Slim M, Rico-Villademoros F, Calandre EP. Psychiatric comorbidity in children and adults with gluten-related disorders: a narrative review. Nutrients. 2018;10(7):875. doi:10.3390/nu10070875.
- Shahbazkhani B, Sadeghi A, Malekzadeh R, Khatavi F, Etemadi M, Kalantri E, Rostami-Nejad M, Rostami K. Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: a double-blind randomized placebo-controlled trial. Nutrients. 2015;7(6):4542–4554. doi:10.3390/nu7064542.
- Catassi C, Fasano A. Tempters and gluten-free diet. Nutrients. 2016;8(12):786. doi:10.3390/nu8120786.
- Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–796. doi:10.1038/ejcn.2013.116.
- Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094. doi:10.1016/j.cell.2015.11.001.
- Mendes-Soares H, Raveh-Sadka T, Azulay S, Edens K, Ben-Shlomo Y, Cohen Y, Ofek T, Bachrach D, Stevens J, Colibaseanu D, et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw Open. 2019;2(2):e188102–e188102. : doi:10.1001/jamanetworkopen.2018.8102.
- Schork NJ. Randomized clinical trials and personalized medicine: a commentary on Deaton and Cartwright. Social Sci Med. 2018;21071–73. doi:10.1016/j.socscimed.2018.04.033.
- Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. 2014;72(1):48–54. doi:10.1111/nure.12090.
- Williams AM, Liu Y, Regner KR, Jotterand F, Liu P, Liang M. Artificial intelligence, physiological genomics, and precision medicine. Physiol Genom. 2018;50(4):237–243. doi:10.1152/physiolgenomics.00119.2017.
- Hood L. Systems biology and p4 medicine: past, present, and future. RMMJ. 2013;4(2):e0012. doi:10.5041/RMMJ.10112.
- Andraos S, Wake M, Saffery R, Burgner D, Kussmann M, O'Sullivan J. Perspective: advancing understanding of population nutrient–health relations via metabolomics and precision phenotypes. Adv Nutr. 2019. Published online May 16, 2019. doi:10.1093/advances/nmz045.
- Khandelwal SS, Zemore E, Hemmerling A. Nutrition education in internal medicine residency programs and predictors of residents’ dietary counseling practices. J Med Educ Curr Develop. 2018;5:1–10. doi:10.1177/2382120518763360.
- Adams KM, Butsch WS, Kohlmeier M. The state of nutrition education at US medical schools. J Biomed Educ. 2015;2015:7. doi:10.1155/2015/357627.
- Adams KM, Kohlmeier M, Powell M, Zeisel SH. Nutrition in medicine: nutrition education for medical students and residents. Nutr Clin Pract. 2010;25(5):471–480. doi:10.1177/0884533610379606.
- Barnard ND. Ignorance of Nutrition Is No Longer Defensible. JAMA Intern Med. 2019;179(8):1021–1022. doi:10.1001/jamainternmed.2019.2273.
- Cresci G, Beidelschies M, Tebo J, Hull A. Educating future physicians in nutritional science and practice: the time is now. J Am Coll Nutr. 2019;38(5):387–394. doi:10.1080/07315724.2018.1551158.
- Handbook of Accreditation for Master’s Degree Clinical Programs in Advanced Nutrition. 2019. [accessed 2019 Jul 29]. https://acnpe.org/accreditation/handbook.
- CNS Handbook. 2019. [accessed 2019 Jul 29]. https://nutritionspecialists.org/sites/default/files/CNS%20Handbook%202019.pdf.
- North Carolina Session Law 2018-91 2018, State of North Carolina. [accessed 2019 Jul 29]. https://www.ncleg.net/EnactedLegislation/SessionLaws/HTML/2017-2018/SL2018-91.html
- Centers for Medicare & Medicaid Services (CMS): Medicare and Medicaid Programs; Regulatory Provisions to Promote Program Efficiency, Transparency, and Burden Reduction; Part II. 2014. https://www.federalregister.gov/articles/2014/05/12/2014-10687/medicare-and-medicaid-programs-regulatory-provisions-to-promote-program-efficiency-transparency-and