Abstract
Background: It has been hypothesized that Irvingia gabonensis can promote weight loss by increasing fatty acid breakdown and inhibiting fatty acid synthesis.
Objective: We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of Irvingia gabonensis seed extract supplementation on weight-related health outcomes.
Methods: Literature searches were conducted in 4 databases from January 2018 to identify randomized controlled trials (RCTs) investigating the effects of Irvingia gabonensis seed extract supplementation on anthropometric measures and cardiovascular biomarkers. Two investigators independently performed abstract screenings, full-text screenings, data extraction, and risk of bias (ROB) assessments. Random effects meta-analyses were performed when 3 or more RCTs reported the same outcome.
Results: Five RCTs met the eligibility criteria for this systematic review. Four of the 5 RCTs were rated as having a high ROB, and only one RCT was rated as having a low ROB. Random-effects meta-analysis of the 5 RCTs showed that a significant decrease in body weight, body fat, and waist circumference was observed in relation to Irvingia gabonensis seed extract supplementation. However, the only one low-ROB trial did not have significantly different outcomes. Meta-analysis also showed beneficial effects of Irvingia gabonensis seed extract supplementation on total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides. Only the low-ROB trial showed a trend of increasing HDL-cholesterol levels (net percent change = 11.61%; 95% confidence interval (CI: −6.12%, 29.34%) and decreasing triglyceride levels (net percent change = −29%; 95% CI: −76%, 19%). The reported adverse events were minor in these 5 RCTs.
Conclusions: Overall efficacy of Irvingia gabonensis seed extract supplementation on weight loss seems positive but is limited due to poor methodological quality and the insufficient reporting of the clinical trials. Further high quality RCTs are needed to determine the effectiveness of Irvingia gabonensis seed extract supplement on the weight-related health outcomes.
Introduction
The global prevalence of obesity has approximately tripled since 1975 (Citation1). The World Health Organization (WHO) estimated that worldwide, more than one-third of adults were overweight or obese in 2018 (Citation1). Dietary supplements for weight loss in addition to lifestyle changes such as reducing calorie intake and increasing physical activity may help people who are overweight or obese to achieve their weight-loss goals. Almost 15% of U.S. adults have used a weight-loss supplement at least once in their life (Citation2), and the use of natural and plant-based ingredients has been increasing in the global market (Citation3). The global market size of weight-loss dietary supplements is expected to show accelerated growth with a compound annual growth rate of 7.4% from 2015 to 2025 (Citation4).
The seed extract of Irvingia gabonensis, also known as the African mango or wild mango, has become a popular herbal dietary supplements for weight loss (Citation5). It has been hypothesized that Irvingia gabonensis can promote weight loss by increasing fatty acid breakdown and inhibiting fatty acid synthesis. Animal studies have shown that Irvingia gabonensis seed extract can decrease the expression of adipogenic enzymes (i.e., lipoprotein lipase and fatty acid synthase), and it can lead to a decrease in fat accumulation by inhibiting fatty acid synthesis (Citation6–8). In addition, ellagic acid, as a bioactive component of IGOB131, has been reported to decrease adipogenesis by inhibiting of C/EBP expression (Citation9). Supplementation with Irvingia gabonensis seed extract has been shown to increase the expression of adiponectin in obese mice (Citation6). Adiponectin can play a critical role in regulating appetite and fatty acid breakdown (Citation10). Findings from an in vitro study using mouse adipocytes have also shown that Irvingia gabonensis seed extract can inhibit the expression of adipogenic transcription factors (i.e., peroxisome proliferation activated receptor (PPAR-γ) and adipocyte-specific proteins (i.e., leptin) and increase the expression of adiponectin and C/EBP, thereby leading to a reduction in body fat via the control of adipogenesis and adipocyte differentiation in humans (Citation11).
However, there are a limited number of human clinical trials of the use of Irvingia gabonensis seed extract for weight loss. A 2013 systematic review identified only three randomized controlled trials (RCTs) and concluded that Irvingia gabonensis cannot be recommended for use as a weight-loss aid (Citation12). Although the findings from these RCTs showed a significant decrease in body weight and waist circumference after Irvingia gabonensis seed supplementation compared to the effects of the placebo, the 2013 systematic review did not draw a conclusion on the efficacy of Irvingia gabonensis seed extract for weight loss due to the poor reporting and methodological quality of the existing RCTs (Citation12). The present systematic review and meta-analysis was conducted to reevaluate the functionality of the Irvingia gabonensis seed extract supplement as a functional food commissioned by the Korean Ministry of Food and Drug Safety (KMFDS). In this study, we aimed to evaluate the efficacy and safety of Irvingia gabonensis seed extract supplementation on anthropometric outcomes and cardiovascular biomarkers.
Methods
We followed the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Citation13) and reported the results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline (Citation14).
Literature search
The search strategy was developed by the National Evidence-based Healthcare Collaborating Agency in Korea (Citation15) and was implemented using the following 4 databases from inception to January 2018: PubMed, EMBASE, the Web of Science, and the Cochrane Central Register of Controlled Trials. No language restriction was added. Search terms included “gabonensis*”, “mango”, “bread tree*”, “dikanut*”, “dika nut*”, “IGOB131”, “ogbono”, and “dika”. Terms for clinical trials were also added. The complete search strategy is presented in Supplemental Table 1.
Study selection process and selection criteria
The abstracts and titles of all citations were independently screened by two investigators according to the a priori study eligibility criteria using Abstrackr (Citation16). Full-text screening was performed independently by two investigators based on the eligibility criteria. Conflicts were resolved by group consensus. Studies were included if they 1) focused on generally healthy people, with < 20% of participants having known diseases (e.g., diabetes or cardiovascular disease) or individuals who were overweight or obese (which was not considered as a disease in this systematic review); 2) used oral intake of IGOB131 (a proprietary patented form of Irvingia gabonensis seed extract) or any other preparation of Irvingia gabonensis seed extract; 3) reported outcomes related to weight, such as body weight, body fat, and waist circumference; cardiovascular biomarkers, such as HDL-cholesterol, LDL-cholesterol, total cholesterol, and triglycerides; or blood pressure; and 4) were parallel or crossover RCTs or quasi-RCTs. Observational studies and review articles were excluded.
Data extraction and risk-of-bias assessment
Data extraction was independently conducted by two investigators using standardized data extraction forms. The discrepancies were resolved by discussion between the two investigators. The ROB assessment was performed independently by two investigators using the Cochrane risk of bias 2.0 tool for parallel RCTs (Citation17). Disagreements were resolved by consensus between the two investigators.
One study did not clearly report which part (e.g., fruit, seed, or other) of Irvingia gabonensis was used (Citation18) for preparation of the Irvingia gabonensis capsules. Therefore, the corresponding author was contacted to obtain the information.
Meta-analysis
In light of clinical heterogeneity (e.g., different doses, different combinations of Irvingia gabonensis seed extracts), random effects meta-analyses were conducted for outcomes with at least 3 RCTs reporting the same outcome. The reported or calculated net percentage change between the intervention arm and control arm was used as the effect size measure for all outcomes since most studies reported within-group percentage changes. The mean within-group percentage change, if not reported, was calculated by using the postintervention mean minus the baseline mean, dividing that value by the baseline mean, and multiplying the value by 100%. The standard deviation (SD) was estimated by dividing the SD of the mean change by the baseline mean. The net percentage change was the difference between the 2 within-group percentage changes; thus, a negative net percentage change indicates an effect (i.e., more weight loss) favoring the Irvingia gabonensis supplementation arm. The SD of the net percentage change was the pooled SD of the 2 SDs of the mean change: SDpooled = √{[(n1 − 1) × SD1 + (n2 − 1) × SD2]/(n1 + n2 − 2)}, where n1 and n2 are the sample sizes and SD1 and SD2 are the SDs of the mean within-group percentage changes of the intervention and control arms, respectively. The missing SDs of the within-group changes were imputed by employing the following formula: SDdiff = √SDB2 + SDF2 – 2 x Corr (0.5) x SDB x SDF, where SDB is the SD of baseline and SDF is the SD of the final measures in the study (Citation19). To impute the missing SDs of the mean within-group change, a correlation coefficient (Corr) value of 0.50 was used. Both the Q statistic (considered significant when the P-value was less than 0.10) and the I-square index were used to quantify the extent of statistical heterogeneity. I- squared values of 25%, 50%, and 75% were defined as low, moderate, and high heterogeneity, respectively. These cutoffs were arbitrary and were used for descriptive purposes only (Citation20).
All calculations and meta-analyses were conducted in Stata SE 14 (Stata Corp). Two-tailed P-values less than 0.05 were considered statistically significant.
Results
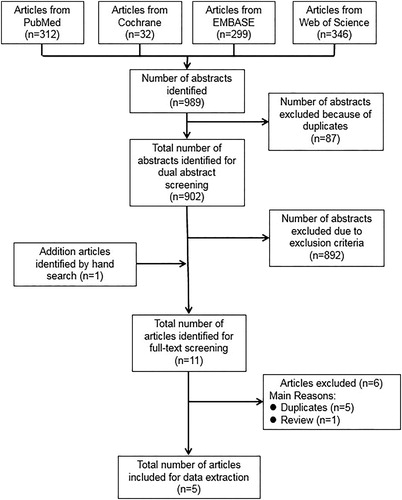
The literature search yielded a total of 989 citations. After removing duplicates, 902 abstracts were identified for the dual abstract screening. One additional article was identified by the manual search. Then, we conducted full-text screening of 11 articles and finally included 5 RCT studies (Citation8,Citation18,Citation21–23). The process of the literature search and paper selection is summarized in .
Each included RCT reported more than one outcome of interest (). The ROB assessments for individual studies are presented in . Two out of the five included RCTs were commercially funded (Citation22,Citation23), while one RCT was supported by an academic institution (Citation21). The remaining two RCTs did not report the source of funding (Citation8,Citation18).
Table 1. Characteristics of the included RCT studies.Table Footnotea
Table 2. Risk of bias assessment of the included RCT studies1.
Four RCTs were conducted in Cameroon (2 RCTs were from the same group of investigators) with adults who were overweight or obese (Citation8,Citation21–23). All four RCTs were rated as having a high ROB. Limitations of these four RCTs include no specific information about the randomization process or baseline differences between groups, no information of washout time for one crossover RCT, and no compliance information (). One RCT was conducted in Mexico with patients with metabolic syndrome defined by the International Diabetes Federation (IDF) diagnostic criteria (Citation18). According to the IDF definition, patients with metabolic syndrome must have central obesity as well as two of the following factors: (1) high triglyceride levels (> 150 mg/dL); (2) low HDL-cholesterol levels (< 40 mg/dL); (3) elevated blood pressure (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg); and (4) elevated fasting plasma glucose levels (≥ 100 mg/dL) (Citation24). This trial was rated as having a low ROB ().
The intervention durations ranged from 4 to 13 weeks (Citation8,Citation18,Citation21–23). The daily doses of Irvingia gabonensis seed extracts ranged from 300 mg to 1,050 mg. The participants in four studies were overweight or obese (Citation8,Citation21–23) and those in one study had metabolic syndrome (Citation18). The control groups received either oat bran, maltodextrin, calcined magnesia, or placebo capsules without detailed information on the formulation. The types of anthropometric measurements included the following: (1) body weight in five studies (Citation8,Citation18,Citation21–23); (2) percent body fat in four studies (Citation8,Citation21–23); (3) waist circumference in five studies (Citation8,Citation18,Citation21–23); (4) body mass index in 2 studies (Citation18,Citation21); (5) fat mass in one study (Citation18); and (6) hip circumference in two studies (Citation8,Citation21). The results were synthesized and reported outcome-by-outcome as follows.
Anthropometric outcomes
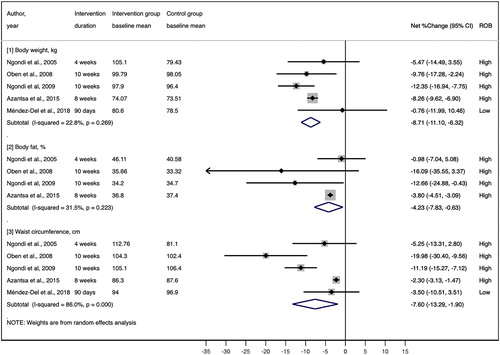
All five RCTs (four parallel RCTs and one crossover RCT) investigated the effects of Irvingia gabonensis seed extracts on anthropometric outcomes. The effects of Irvingia gabonensis seed extracts on body weight and waist circumference were inconsistent across the five RCTs (Citation8,Citation18,Citation21–23). Specifically, three RCTs showed that the body weight and waist circumferences of the Irvingia gabonensis seed extract supplementation group decreased significantly compared with the same parameters in the control groups (Citation21–23), whereas 2 RCTs did not show significant differences (Citation8,Citation18) (). Our random effects meta-analysis of 5 RCTs showed that the Irvingia gabonensis seed extract supplementation decreased body weight by an average of 8.71% compared with the body weight of the controls, with low statistical heterogeneity (pooled mean net percent change = −8.71%; 95% CI: −11.10%, −6.32%; I2 = 22.8%) and decreased waist circumference by an average of 7.6% compared with the values of the controls, with high statistical heterogeneity (pooled mean net percent change = −7.60%; 95% CI: −13.29%, −1.90%; I2 = 86.0%) (). However, the only RCT with a low ROB did not find significant differences in body weight or waist circumference outcomes between the groups (Citation18).
Four of the five RCTs (Citation8,Citation21–23) also investigated percent body fat. Our random effects meta-analysis of these four RCTs (all rated as having a high ROB) showed that the percent body fat of Irvingia gabonensis seed extract supplementation group decreased significantly compared with that of the control group, with moderate statistical heterogeneity (pooled mean net percent change = −4.23%; 95% CI: −7.83%, −0.63%; I2 = 31.5%) (). Only one RCT (low ROB) investigated the effects of Irvingia gabonensis seed extract supplementation on fat mass, and reported no significant difference between the intervention and control groups over the 90 days of the intervention (Citation18).
Due to the small number of studies, we did not conduct a meta-analysis of the studies reporting body mass index (BMI) and hip circumference outcomes. Of the two RCTs that examined the effects of Irvingia gabonensis seed extract supplementation on BMI (Citation18,Citation21), one found that Irvingia gabonensis seed extract supplementation significantly decreased BMI by 2.7 kg/m2 after 8 weeks (p < 0.05), while the placebo group showed a nonsignificant reduction in BMI of 0.5 kg/m2 (Citation21). In contrast, the other RCT did not find any significant difference in BMI between the Irvingia gabonensis seed extract supplementation group and the control group over the 90-day treatment period (Citation18). Only one RCT investigated the effects of Irvingia gabonensis seed extract supplementation on hip circumference (Citation21). The results showed that 300 mg of Irvingia gabonensis seed extract supplementation daily significantly decreased hip circumference by 6.3 cm over the 8-week treatment period (p < 0.01), while nonsignificant change of 2 cm in hip circumference was observed in the placebo group.
Cardiovascular biomarkers
All five RCTs also investigated total cholesterol and LDL-cholesterol outcomes (Citation8,Citation18,Citation21–23). For both outcomes, the results were mostly consistent. Our random effects meta-analysis of 5 RCTs showed that compared with the control group, the Irvingia gabonensis seed extract supplementation group exhibited a significant total cholesterol reduction of 24% (pooled mean net percent change = −24.01%; 95% CI: −37.53%, −10.50%; I2 = 96.0%) and an LDL-cholesterol reduction of 27% (pooled mean net percent change: −27.08%; 95% CI: −38.12%, −16.05%; I2 = 93.8%) (). However, statistical heterogeneity was very high in both meta-analyses, and the only low-ROB trial (Méndez-Del et al. 2018 (Citation18)) did not show significant differences among patients with metabolic syndrome.
Figure 3. Effects of wild mango seed extracts on total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides.
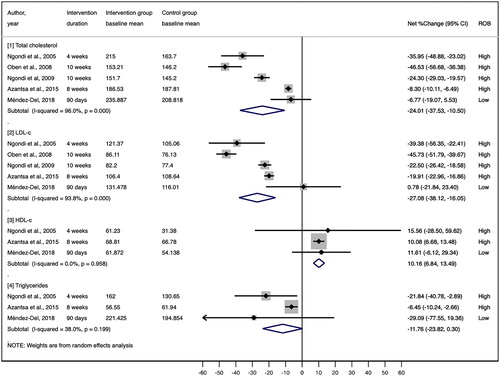
Three of the five RCTs reported HDL-cholesterol and triglyceride outcomes (Citation8,Citation18,Citation21). Our random effects meta-analysis of these 3 RCTs showed that compared to the control group, Irvingia gabonensis seed extract supplementation group exhibited a significant HDL-cholesterol increase of 10% with no statistical heterogeneity (pooled mean net percent change = 10.16%; 95% CI: 6.84%, 13.49%; I2 = 0.0%) and a triglyceride decrease of 12% with moderate heterogeneity (pooled mean net percent change = 11.76%; 95% CI: −23.82%, 0.30%; I2 = 38.0%) (). The only low-ROB trial (Méndez-Del et al. 2018 (Citation18)) showed a trend toward increased HDL-cholesterol levels (net percent change = 11.61%; 95% CI: −6.12%, 29.34%) and decreased triglyceride levels (net percent change = −29%; 95% CI: −76%, 19%) among patients with metabolic syndrome.
Two RCTs examined systolic blood pressure (SBP) and diastolic blood pressure (DBP) (Citation8,Citation18). One RCT (high ROB) reported a significant net change of 6.6 mmHg in SBP by comparing the Irvingia gabonensis seed extract supplementation group to the placebo group (decreased level, 3.6 vs. 10.2 mmHg) after 4 weeks of treatment (p < 0.001) (Citation8). However, the other RCT (low ROB) did not find any significant difference in SBP pressure over the 90-day trial period between the Irvingia gabonensis seed extract supplementation group and the placebo group (Citation18). Both RCTs indicated no significant net change in DBP between the Irvingia gabonensis seed extract supplementation group and the placebo group (Citation8,Citation18).
Adverse events
Three RCTs reported adverse events, including headache, sleep difficulty, gas, intestinal flatulence, and intestinal constipation (Citation18,Citation22,Citation23) (). The incidence of these adverse events was similar between the Irvingia gabonensis seed extract supplementation group and the control group. One study reported no adverse events during the study (Citation21), and the last RCT did not report any information regarding adverse events (Citation8) ().
Table 3. Adverse events.
Discussion
Current evidence on the efficacy and safety of Irvingia gabonensis seed extract supplements is limited due to poor methodological quality and poor reporting of the clinical trials. Random effects meta-analysis results from all available RCTs indicated that Irvingia gabonensis seed extract supplementation led to a significant decrease in anthropometric outcomes (i.e., body weight, body fat, and waist circumference) and beneficial effects on cardiovascular risk measures (i.e., total cholesterol, LDL-cholesterol, and HDL-cholesterol), compared to the effects associated with the placebo. However, the current evidence mostly came from the studies with poor reporting and high ROB.
The KMFDS has two tracks to regulate health/functional foods (Citation25). The track is determined by the inclusion of either generic or product-specific functional ingredients in health/functional foods. To obtain product-specific certification, the manufacturer has to submit documents regarding 1) special characteristics of the functional ingredient; 2) safety; 3) efficacy; and 4) the method used to analyze the functional ingredient. These documents are evaluated by government officials; members of consumer unions; and experts in the field of nutrition, food science, and medicines. Finally, the KMFDS decides whether the product is approved as a health/functional food.
The KMFDS approved wild mango seed extract (IGOB131) as a health/functional food in 2015 (Citation26). The Korean government considered ellagic acid, which is found in wild mango seed extract, to be the functional component, permitting the claim “reduction in body fat” to be used for wild mango seed extract. The National Evidence-based Healthcare Collaborating Agency (NECA) conducted a systematic review of the safety and efficacy of Irvingia gabonensis seed extract on weight-related outcomes in 2016 (Citation15). The NECA reported the poor quality of the included studies and several adverse effects of Irvingia gabonensis seed extract supplementation, such as chronic renal failure, liver toxicity, headache, sleep disorder, and intestinal gas. According to the Health/Functional Foods Act, the KFMDS would reexamine the issues regarding the efficacy and safety of the functional foods publicly noted (Citation27). Due to the recognition of the poor study quality and safety issues of Irvingia gabonensis seed extract, the efficacy and safety of Irvingia gabonensis was reevaluated by the health/functional review committee of the KMFDS in 2017. The KMFDS did not change the status of Irvingia gabonensis seed extract from being a functional food because they did not have conclusive studies to deny the previous health claims for functional food on weight loss.
The strength of this systematic review is that we employed the rigorous methods presented in the Cochrane handbook for systematic reviews. Although we employed rigorous methods for the systematic review, we could not overcome the generally high ROB in the included studies. The primary studies included in this systematic review had several problems, such as poor reporting and poor methodological quality. In addition, the ethnicity of the participants of the included studies was mainly African, limiting the applicability of the findings of this systematic review. All 5 included studies were conducted in developing countries (4 in Cameroon & 1 in Mexico), and the effects of Irvingia gabonensis seed extract supplementation might not be applicable to Caucasians or Asians in developed countries. Three RCTs out of the included five RCTs were conducted by the same group of authors in the same laboratory (Laboratory of Nutrition and Nutritional Biochemistry, University of Yaounde I, Cameroon) and published in the same journal (i.e., Lipids in Health and Disease) (Citation8,Citation22,Citation23). Another limitation of this systematic review was poor reporting. The results of one crossover RCT were reported as a paralleled trial (Citation8). Statistical analysis did not test or adjust for carry-over effects. Therefore, we entered the data for the meta-analysis as a paralleled trial of two independent groups. One included study reported only total sample size but did not report the sample size in each group (Citation21).
Given the limitations in the existing literature, we have several suggestions for future studies. First, rigorous RCTs are needed to confirm the effects observed in the existing RCTs and to evaluate whether the effects can be replicated in different populations such as Caucasians and Asians. Second, we would suggest that the authors follow the reporting guidelines for clinical trials such as the CONSORT statement (Citation28). Moreover, the studies should improve their methodological quality to minimize the risk of bias, especially regarding selection bias, performance bias, attrition bias, and reporting bias.
Disclaimer
The viewpoints expressed in the manuscripts by the authors should not be construed as an official endorsement by the Ministry of Food and Drug Safety, Republic of Korea.
Supplemental Material
Download PDF (1.6 MB)Acknowledgements
JL, MC, and HL designed research; JL, ZF, and JC conducted research; JL, MC, and ZF analyzed data; MC performed statistical analysis; JL, MC, and HL had primary responsibility for final content; JL, MC, ZF, JC, and HL wrote the paper. All authors have read and approved the final manuscript.
Additional information
Funding
References
- WHO. Obesity and overweight. Geneva: World Health Organization; 2018 [accessed 2018 Aug 6]. http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Blanck HM, Serdula MK, Gillespie C, Galuska DA, Sharpe PA, Conway JM, Khan LK, Ainsworth BE. Use of nonprescription dietary supplements for weight loss is common among Americans. J Am Diet Assoc. 2007;107(3):441–447. doi:10.1016/j.jada.2006.12.009.
- Persistence Market Research. North America to witness strong growth in the global weight loss dietary supplements market during the forecast period 2017–2026. New York (NY): Persistence Market Research; 2017 [accessed 2018 Aug 6]. https://www.persistencemarketresearch.com/mediarelease/weight-loss-dietary-supplements-market.asp.
- Grand View Research. Dietary supplements market size, share & trend analysis report by ingredient (botanicals, vitamins, minerals, amino acids, enzymes), by product, by application, by end-use, and segment forecasts, 2018–2024. San Francisco (CA): Grand View Research; 2018.
- Sun J, Chen P. UHPLC/HRMS analysis of African mango (Irvingia gabonensis) seeds, extract and related dietary supplements. J Agric Food Chem. 2012;60(35):8703–8709. doi:10.1055/s-0032-1320571.
- Lee MH, Nam D-E, Kyung KO, Shim TJ, Kim JH, Lee JM. Anti-obesity effects of African mango (irvingia gabonesis, IGOB 131™) extract in leptin-deficient obese mice. J Korean Soc Food Sci Nutr. 2014;43(10):1477–1483. doi:10.3746/jkfn.2014.43.10.1477.
- Nangue TJ, Womeni HM, Mbiapo FT, Fanni J, Michel L. Irvingia gabonensis fat: nutritional properties and effect of increasing amounts on the growth and lipid metabolism of young rats wistar sp. Lipids Health Dis. 2011;10(1):43. doi:10.1186/1476-511X-10-43.
- Ngondi JL, Oben JE, Minka SR. The effect of irvingia gabonensis seeds on body weight and blood lipids of obese subjects in Cameroon. Lipids Health Dis. 2005;4(1):12.
- Wang L, Li L, Ran X, Long M, Zhang M, Tao Y, Luo X, Wang Y, Ma X, Halmurati U, et al. Ellagic acid reduces adipogenesis through inhibition of differentiation-prevention of the Induction of rb phosphorylation in 3T3-L1 adipocytes. Evid Based Complement Alternat Med. 2013;2013:1–11. doi:10.55/2013/287534.
- Goldfine AB, Kahn CR. Adiponectin: linking the fat cell to insulin sensitivity. Lancet. 2003;362(9394):1431–1432. doi:10.1016/S0140-6736(03)14727-7.
- Oben JE, Ngondi JL, Blum K. Inhibition of Irvingia gabonensis seed extract (OB131) on adipogenesis as mediated via down regulation of the PPARgamma and leptin genes and up-regulation of the adiponectin gene. Lipids Health Dis. 2008;7(1):44. doi:10.1186/1476-511X-7-44.
- Onakpoya I, Davies L, Posadzki P, Ernst E. The efficacy of Irvingia gabonensis supplementation in the management of overweight and obesity: a systematic review of randomized controlled trials. J Diet Suppl. 2013;10(1):29–38. doi:10.3109/19390211.2012.760508.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Chichester (UK): The Cochrane Collaboration; 2011.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi:10.7326/0003-4819-151-4-200908180-00135.
- Shin CM, Park JY, Park JJ, Seo SW, Shim JI. Assessment of clinical safety and efficacy for weight loss supplements (Garcinia cambogia extract, Irvingia gabonensis seed extract) in humans. Seoul: National Evidence-based Healthcare Collaborating Agency; 2017.
- Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA, editors. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium; 2012 Jan 28–30; Miami (FL): Association for Computing Machinery; 2012. p. 819–24.
- Cochrane. RoB 2: a revised cochrane risk-of-bias tool for randomized trials. Odense: Cochrane Methods Bias [accessed 2019 May 13]. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials.
- Méndez-del Villar M, González-Ortiz M, Martínez-Abundis E, Pérez-Rubio KG, Cortez-Navarrete M. Effect of Irvingia gabonensis on metabolic syndrome, insulin sensitivity, and insulin secretion. J Med Food. 2018;21(6):568–574. doi:10.1089/jmf.2017.0092.
- Green S, Higgins J. Cochrane handbook for systematic reviews of interventions. London: The Cochrane Collaboration; 2011.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557.
- Azantsa B, Kuate D, Chakokam R, Paka G, Bartholomew B, Nash R. The effect of extracts of Irvingia gabonensis (IGOB131) and dichrostachys glomerata (Dyglomera™) on body weight and lipid parameters of healthy overweight participants. Funct Foods Health Dis. 2015;5(6):200–208.
- Ngondi JL, Etoundi BC, Nyangono CB, Mbofung CM, Oben JE. IGOB131, a novel seed extract of the West African plant Irvingia gabonensis, significantly reduces body weight and improves metabolic parameters in overweight humans in a randomized double-blind placebo controlled investigation. Lipids Health Dis. 2009;8(1):7. doi:10.1186/1476-511X-8-7.
- Oben JE, Ngondi JL, Momo CN, Agbor GA, Sobgui C. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double-blind placebo-controlled study. Lipids Health Dis. 2008;7(1):12. doi:10.1186/1476-511X-7-12.
- Alberti SG, Zimmet P, Shaw J, Grundy SM. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels: International Diabetes Federation; 2005.
- Kim JY, Kim DB, Lee HJ. Regulations on health/functional foods in Korea. Toxicology. 2006;221(1):112–118. doi:10.1016/j.tox.2006.01.016.
- Korean Ministry of Food and Drug Safety. Handbook of functional foods - body fat loss. Osong: Korean Ministry of Food and Drug Safety; 2012.
- Korean Ministry of Food and Drug Safety. Health Functional Foods Act, Act No. 13300 (2015).
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Open Med 2010;4(1):60–68.