Abstract
Background
Ketosis has been reported to benefit healthspan and resilience, which has driven considerable interest in development of exogenous ketones to induce ketosis without dietary changes. Bis hexanoyl (R)-1,3-butanediol (BH-BD) is a novel ketone di-ester that can be used as a food ingredient that increases hepatic ketogenesis and blood beta-hydroxybutyrate (BHB) concentrations.
Methods
Here, we provide the first description of blood ketone and metabolite kinetics for up to five hours after consumption of a beverage containing BH-BD by healthy adults (n = 8) at rest in three randomized, cross-over conditions (25 g + Meal (FEDH); 12.5 g + Meal (FEDL) ; 25 g + Fasted (FASTH)).
Results
Consumption of BH-BD effectively raised plasma r-BHB concentrations to 0.8–1.7 mM in all conditions, and both peak r-BHB concentration and r-BHB area under the curve were greater with 25 g versus 12.5 g of BH-BD. Urinary excretion of r-BHB was <1 g. Plasma concentration of the non-physiological isoform s-BHB was increased to 20–60 µM in all conditions. BH-BD consumption decreased plasma glucose and free fatty acid concentrations; insulin was increased when BH-BD was consumed with a meal.
Conclusions
These results demonstrate that consumption of BH-BD effectively induces exogenous ketosis in healthy adults at rest.
Introduction
The ketone bodies beta hydroxybutyrate (BHB), acetoacetate, and acetone are fat-derived endogenous molecules with a dual function. First, ketones act as an energy source that allowed our ancestors to survive periods of starvation by utilizing energy stored in lipids (Citation1). Second, ketones are signaling metabolites that form part of an environmentally responsive network that regulates healthspan and lifespan (Citation2). BHB is a chiral molecule at the 3′ hydroxyl group and this may be an important feature in consideration of BHB signaling and its therapeutic potential. r-beta hydroxybutyrate (r-BHB) is the normal product of mammalian ketogenesis. A state of ketosis is clinically characterized by blood r-BHB > 0.5 mM, and typically occurs when dietary carbohydrate intake is low, such as during fasting (Citation3), and a ketogenic diet (Citation4). The metabolism and function of s-beta hydroxybutyrate (s-BHB) enantiomer is incompletely understood. s-BHB is a common intracellular intermediate of the fatty acid beta-oxidation pathway but is not usually found in the circulation at high concentrations and cannot be oxidized by the same pathway as r-BHB (Citation5, Citation6). Despite this, s-BHB does share some of the signaling activities of r-BHB (Citation7, Citation8) and therefore may have functional effects that are yet to be defined.
Endogenous ketosis involves several metabolic processes: lipolysis to provide the fatty acid substrate to the liver, hepatic ketogenesis, hyperketonemia and increased ketone oxidation in peripheral tissues () (Citation9). Recently, exogenous sources of ketones have been developed for their ability to elevate blood ketone concentration without the need to restrict dietary carbohydrates or fasting. Whether ketosis achieved through exogenous means has similar effects as endogenous ketosis is an active area of research, many pre-clinical studies compare the effects of ketogenic diet consumption to the effects of exogenous ketone administration (Citation7, Citation10–12). Exogenous ketone compounds do not increase lipolysis, but some can increase ketogenesis and when provided in sufficient amounts produce a state of ketosis and elevated ketone oxidation (Citation13, Citation14). It is hypothesized that consumption of exogenous ketones may replicate a subset of the effects of endogenous ketosis (Citation15).
Figure 1. Metabolic pathways involved in endogenous and exogenous ketosis. Endogenous ketosis increases lipolysis, classical hepatic ketogenesis, hyperketonemia and ketone oxidation. Exogenous ketosis with BH-BD consumption involves intestinal hydrolysis of BH-BD, classical and non-classical hepatic ketogenesis, hyperketonemia and ketone oxidation. BHB, beta-hydroxybutyrate. Created with BioRender.com.
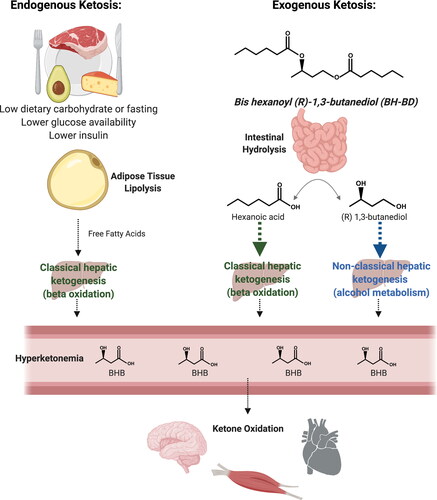
Examples of exogenous ketone compounds include medium chain triglycerides, ketone salts and ketone esters. Many variants of these compounds have been studied for their ability to induce ketosis and their impact on a range of outcomes, including physical performance (Citation16–18), cognitive function (Citation19–22), blood glucose control and appetite regulation (Citation23–25). Ketone esters are of particular translational interest because they elevate blood ketone concentrations without an accompanying acid or mineral load (Citation26). The molecular structure of some ketone esters allows for more complete replication of endogenous ketosis if they provide substrates that elevate blood ketones via the classical process of hepatic ketogenesis, compared to compounds that deliver ketones directly into the circulation, by-passing ketogenesis in the liver.
The novel ketone di-ester, bis-hexanoyl (R)-1,3-butanediol (BH-BD) is an example of a ketone ester that strongly induces hepatic ketogenesis. BH-BD was recently developed for use as a food ingredient to induce ketosis in humans. BH-BD is rapidly metabolized by enzymes in the small intestine to form ketogenic precursors: the medium chain fatty acid hexanoic acid, and the ketogenic alcohol (R)-1,3-butanediol (Citation27) (). These hydrolysis products are thought to enter the portal circulation and pass from there into the liver. In the liver, hexanoic acid acts as a constitutive substrate for classical hepatic ketogenesis resulting in release of r-BHB and acetoacetate, whereas (R)-1,3-butanediol is converted to r-BHB via a non-classical hepatic ketogenic pathway. Both of these hepatic ketogenic pathways occur largely independently of dietary carbohydrate intake or circulating insulin concentrations. Notably, BH-BD does not contain s-BHB or obvious metabolic precursors to s-BHB, such as (R, S)-1,3-butanediol. Oral administration of BH-BD in rats and mice produced rapid and robust elevations in blood r-BHB concentrations (Citation27). The first human study of BH-BD described its’ safety and tolerability when 25 g was taken daily for 28 days by healthy adults, and found that consumption of 25 g of this ketone ester elevated blood r-BHB measured 1 hour following consumption (Citation28). However, the full-time course of blood ketone response to consumption of BH-BD as well as the possible presence of its hydrolysis products in the systemic circulation is unknown. It is also unknown if BH-BD alters circulating concentrations of metabolites such as glucose and non-esterified free fatty acids (NEFA) in a similar manner to other exogenous ketone compounds. Therefore, we undertook a randomized, cross-over study in healthy adults to investigate the changes in r- and s-BHB, and in glucose, NEFA and insulin following consumption of BH-BD at two serving sizes and under different feeding conditions. This work extends our limited knowledge of BH-BD metabolism in humans and will inform the future use of BH-BD to generate a repeatable state of exogenous ketosis.
Materials and methods
Study overview
Healthy adults (n = 8) without prior history of chronic illness who regularly undergo recreational exercise, took part in a randomized three-arm cross-over study, in which blood r- and s-BHB and metabolite concentrations were measured for 5 hours following ingestion of beverages containing the novel ketone ester, BH-BD (C6 Ketone Di-ester) (). Conditions studied were 25 g of BH-BD taken with a meal (FEDH), 25 g taken whilst fasted (FASTH) and 12.5 g taken with a meal (FEDL). This study was approved by the Institutional Review Board at the Ohio State University (#2020H0005, approved on 13th March 2020) and was conducted in accordance with the Declaration of Helsinki (2004) (Citation29).
Participants and screening
Participants provided written informed consent prior to inclusion, and completed a confidential medical history questionnaire to determine eligibility. Participants included healthy adult men (n = 5) and women (n = 3). Females were non-pregnant, non-lactating and using birth control during and 30 days after the study. Exclusion criteria included current illness, allergies to ingredients in the test product, history of alcoholism, prescriptive or over-the-counter medications, or excessive smoking habits (>5 cigarettes a day). Seven of the participants habitually consumed a non-carbohydrate restricted diet, while one subject had consumed a ketogenic diet for a period of months. Anthropometric characteristics were measured in the laboratory (height and weight) and are shown in . The sample size selected was based on other pharmacokinetic studies of ketone esters that were similar in nature (Citation30, Citation31) and on past work which tested the effect of meal feeding on ketone ester responses and found a 1 mM (standard deviation 0.6 mM) decrease in peak BHB concentration when ketone ester was taken with a meal versus fasted (Citation32). A sample size calculation found that 6 participants were required to detect a 1 mM difference in peak BHB concentration with a power of 0.8 and alpha of 0.05. We enrolled 8 participants to account for potential drop outs and provide enhanced statistical power for ketones and other metabolic data.
Table 1. Anthropometric characteristics of study participants.
Ketone ester beverage
The ketone ester, BH-BD, was manufactured by Abitec Corporation (WI, USA). The ketone ester was formulated into a chocolate flavored beverage matrix (water, whey protein concentrate, modified gum acacia, natural and artificial flavors, cocoa powder) under aseptic conditions by The National Food Laboratory (NY, USA). It was aliquoted into bottles containing 25 g of BH-BD in a total volume of 75 mL. In the FEDH and FASTH condition, participants consumed one full bottle (25 g BH-BD in 75 mL), in the FEDL condition participants consumed half of one bottle (12.5 g BH-BD in 37.5 mL). Nutritional information is shown in .
Table 2. Nutritional information of study beverage and standard meal.
Standard meal
The standardized meal in the FEDH and FEDL conditions was a smoothie that provided 336 kcal as 40/30/30 (%kCal carb/fat/protein) as determined by nutrition software (Nutritionist Pro, Axxya Systems, Redmond, WA). The meal consisted of blended banana, frozen strawberries, unsweetened almond milk, peanut butter, baby spinach, collagen powder (Primal Kitchen, CA, USA), and unflavored whey protein (biPro, MN, USA). Nutritional information is shown in .
Study visit procedures
Participants reported to the OSU PAES Building having complied with pretest requirements: fasted (10 h ± 2 h), no alcohol for 24 h, no caffeine for 10 h, no exercise for 10 ± 2 h and adequate hydration (confirmed by urine specific gravity testing (< 1.025)). Visit order was randomized based on sequences from an online random sequence generator, allocated by order of enrollment. The participants randomized to the FEDH and FEDL conditions consumed a 25 g or 12.5 g serving of BH-BD formulated into a beverage and the standardized meal at 0 h, whereas the participants in the FASTH condition consumed a 25 g serving of BH-BD formulated into a beverage only. Participants were instructed to consume the BH-BD beverage and meal in less than 0.25 h. Venous blood samples were collected at −0.5, 0.5, 1, 2, 3, 4 and 5 h, where t = 0 is the time of BH-BD beverage consumption (). Participants remained sedentary throughout the duration of the study and were instructed to make minimal ambulatory movements.
Biological sampling and analysis
Venous blood samples (2–3 mL) were obtained during all visits through venepuncture at the antecubital fossa. Samples were stored on ice, centrifuged at 4 °C and 1200 g for 10 minutes, and duplicate plasma aliquots stored at −80 °C. All urine passed during the visit was collected, the total volume recorded, and 1 mL aliquots were frozen and retained for analysis. Samples were analyzed at The Buck Institute for Research on Aging. Commercially available enzymatic assay kits were used to determine plasma and urinary r-BHB (StanBio, TX, USA), plasma glucose (Sigma Aldrich, MO, USA), NEFA (Randox, WV, USA) and insulin (Crystal Chem, IL, USA). Plasma concentration of s-BHB was determined by Liquid Chromatography—Mass Spectrometry (see supplementary material for details). Samples from one condition (FEDH) were analyzed for the hydrolysis products of BH-BD ((R)-1,3 butanediol and hexanoic acid) by Liquid Chromatography-Mass Spectrometry (see supplementary material for details).
Statistical methods
Statistics were performed using Prism 9 (GraphPad, CA, USA) with two-tail α significance set at p < 0.05. All ketone and glucose values were normalized to each individual’s baseline value for each test visit and were compared across testing conditions to determine significant differences using a 2-way (condition × time) repeated measures analysis of variance (ANOVA). Area under the curve (AUC) was calculated from the normalized BHB and glucose measurements using the trapezoidal method (GraphPad Prism, Ver. 9). Between-condition significance in total AUC and time-to-peak shift was calculated by one-way ANOVA. Significant post-hoc interactions were analyzed with the Least Square Differences correction. Simple linear regression was performed to calculate the R squared value for the relationship between body weight and peak BHB concentration achieved and to determine if the slope significantly deviated from zero.
Results
Plasma and urine r- and s-BHB
Participants varied in fasting plasma r-BHB from 0.1 to >1 mM, primarily reflecting differences in habitual carbohydrate intake. For that reason, we analyzed r-BHB responses normalized to each participant’s baseline value (△r-BHB). As expected, consumption of BH-BD effectively elevated circulating plasma r-BHB concentrations over baseline values in all conditions. There were significant differences between conditions in mean plasma r-BHB concentrations over the 5 h time course with significant time (p < 0.0001), condition (p = 0.0014), and interaction effects (p < 0.0001) observed (). Mean peak plasma △r-BHB occurred after 0.5 h in the FEDL condition (p vs. FEDH and FASTH < 0.0001), 1 h in the FEDH condition (p vs. FEDL < 0.0001; p vs. FASTH = 0.029) and 2 h in the FASTH condition (p vs. FEDL < 0.0001; p vs. FEDH = 0.7975). Plasma r-BHB remained significantly elevated over baseline for 1 h in the FEDL condition, 2 h in the FEDH condition and for the duration of the 5 h experiment in the FASTH condition. There was a main effect of condition on the peak △r-BHB concentration (Cmax) measured at any time for each participant (p = 0.004). △r-BHB Cmax values were not significantly different between FEDH (1.7 ± 0.6 mM) and FASTH (1.4 ± 0.4 mM) conditions, but both were significantly greater than those during FEDL (0.8 ± 0.2 mM, p vs. FEDH = 0.012; p vs. FEDL = 0.01) (). Similarly, there was a main effect of condition (p = 0.0057) on incremental △ r-BHB area under the curve, which was similar between FEDH (3.97 ± 2.3 mM × h) and FASTH (3.96 ± 1.06 mM × h), but was significantly lesser in FEDL than the other two conditions (1.29 ± 0.34 mM × h, p vs. FEDH = 0.031; p vs. FASTH = 0.0004) (). One participant followed a ketogenic diet, this individual had the greatest △ r-BHB Cmax and AUC observed during the study during the FEDH condition (△r-BHB Cmax FEDH = 2.7 mM, AUC = 9.03 mM × h). We repeated the statistical analysis of △r-BHB Cmax (our primary outcome measure) excluding this participant and all between group differences remained significant. There was no correlation between body weight adjusted dose and r-BHB Cmax in the FEDH and FEDL conditions (R2 = 0.1229, p = 0.1831) (), only the two fed conditions were included to standardize the possible effect of meal consumption. The amount of r-BHB excreted in the urine was low, being <1 g for all conditions, but was significantly lower in the FEDL condition compared to the FEDH condition ().
Figure 3. Plasma and urine r and sBHB concentrations in n = 8 healthy adults following consumption of 25 g or 12.5 g of BH-BD with or without a meal. (A) change in mean plasma rBHB normalized to each individual’s baseline (−0.5 h) value, (B) peak rBHB concentration for each subject, (C) rBHB AUC, (D) relationship between body weight adjusted dose and rBHB concentration in FEDL and FEDH conditions, (E) urine rBHB excretion, (F) mean plasma sBHB. AUC; area under the curve, BHB; beta hydroxybutyrate, BH-BD; bis hexanoyl (R)1,3-butanediol, Cmax; peak concentration, FEDH; 25 g BH-BD when fed, FEDL; 12.5 g BH-BD when fed, FASTH; 25 g BH-BD when fasted. Data are mean ± SD. *p < 0.05 between group, ***p < 0.001 between group, †p < 0.05 vs. baseline, ‘a’ = significant difference between both FEDH and FASTH vs. FEDL at indicated time point, ‘b’ = significant difference between FASTH vs. FEDL at indicated timepoint, ns = not significant.
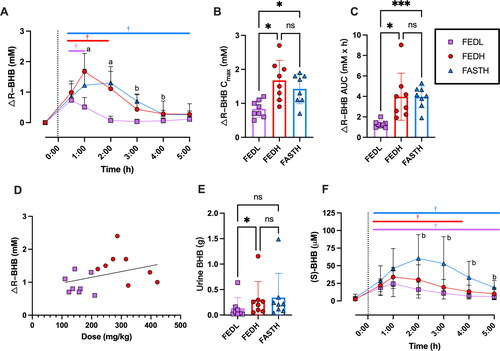
As expected, plasma s-BHB concentrations were negligible at baseline. Interestingly, despite BH-BD not directly delivering a source of s-BHB, concentrations of s-BHB significantly increased following BH-BD consumption in all conditions to 20–60 µM (time effect, p < 0.0001, condition effect, p = 0.0095), being generally highest in the FASTH condition ().
BH-BD hydrolysis products
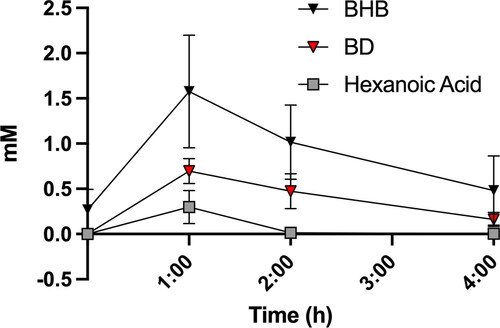
To determine if the immediate hydrolysis products of BH-BD appear in the circulation, we measured plasma (R)-1,3 butanediol and hexanoic acid concentrations in the FEDH condition for 4 h following ingestion of BH-BD, and compared it to the concentration of BHB (). Plasma concentration of both hydrolysis products were increased following BH-BD ingestion but were substantially lower than BHB concentrations at multiple time points due to effective conversion to BHB, with the peak concentration of (R)-1,3 butanediol and hexanoic acid being 44% (0.70 ± 0.15 mM, p = 0.0003 vs. BHB Cmax) and 19% (0.30 ± 0.18 mM, p < 0.0001 vs. BHB Cmax) respectively of BHB Cmax (1.59 ± 0.6 mM). Hexanoic acid concentrations returned to basal values after 2 h, whereas (R)-1,3 butanediol was cleared more slowly, persisting after 4 h.
Figure 4. Plasma concentrations of (R)-1,3 butanediol and hexanoic acid in n = 8 healthy adults following consumption of 25 g of BH-BD with a standard meal (FEDH). BHB; beta hydroxybutyrate, BD; butanediol Data are mean ± SD. *p < 0.05 between both BD and hexanoic acid vs. BHB, †p < 0.05 between hexanoic acid vs. BHB only.
Plasma glucose, insulin and non-esterified fatty acid concentrations
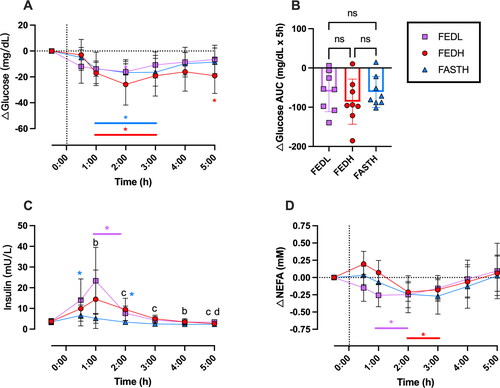
Due to variability between participants in baseline glucose concentrations, we focused on glucose changes normalized to each individual’s baseline. In all study conditions, ingestion of BH-BD with or without a meal decreased normalized plasma glucose concentrations with significant time (p < 0.001) and time × condition interaction effects (p = 0.0102) (). The glucose lowering effect was seen only at 2 h in FEDL but across 1–3 h for both FEDH and FASTH conditions, despite the large difference in carbohydrate intake occurring due to meal ingestion in the FEDH arm. The normalized glucose AUC was not significantly different between conditions (), despite significant differences in insulin between conditions (p = 0.015) (). In both conditions where the standard meal was consumed (FEDH and FEDL) insulin was increased for up to 2 h, in the FASTH where BH-BD was consumed alone there was no significant increase in insulin concentration. There was a main effect of time on plasma NEFA concentrations (p < 0.0001) following BH-BD consumption (). Plasma NEFA concentrations were significantly lower than baseline following BH-BD ingestion in both FEDL (between 1–2 h) and FEDH (between 2–3 h) conditions, but not in FASTH despite a similar magnitude of change from baseline (FEDL = 0.21 mM, FEDH = 0.24 mM, FASTH = 0.27 mM). NEFA concentrations tended to rise in all conditions in the final 2 h of the study.
Figure 5. Plasma glucose, insulin and NEFA concentrations in n = 8 healthy adults following consumption of 25 g or 12.5 g of BH-BD. (A) change in mean plasma glucose normalized to each individual’s baseline (−0.5 h) value, (B) glucose AUC, (C) insulin, (D) change in mean normalized NEFA. AUC; area under the curve, BH-BD; bis hexanoyl (R)1,3-butanediol, FEDH; 25 g BH-BD when fed, FEDL; 12.5 g BH-BD when fed, FAST; 25 g BH-BD when fasted, NEFA; non esterified free fatty acid. Data are mean ± SD. *p < 0.05 vs. baseline values, ‘b’ = significant difference between FASTH vs. FEDL at indicated timepoint, ‘c’ = significant difference between both FEDH and FEDL vs. FAST, ‘d’ = significant difference between FEDL and FEDH.
Discussion
The present study provides the first data demonstrating the time course of increased circulating BHB concentrations following ingestion of the novel ketone ester, BH-BD, in healthy adults. This data also illustrates the low presence of BH-BD hydrolysis products, as well as changes in other metabolic parameters: plasma glucose, insulin and NEFA.
As we had previously found in mice and rats (Citation27), BH-BD rapidly induces exogenous ketosis lasting several hours in a dose-dependent manner in humans. Because BH-BD does not contain r-BHB, the low, transient circulating concentrations of the hydrolysis products, (R)-1,3 butanediol and hexanoic acid, in comparison to r-BHB demonstrate an effective conversion of the precursors into ketone bodies. Concentrations of hexanoic acid in the plasma seen here are consistent with studies showing that medium chain triglyceride ingestion (20 g) in humans, resulted in detection of the corresponding free fatty acids (i.e., caprylic and capric acid) in the plasma at amounts corresponding to a small fraction (1.2–2.2%) of the total ingested (Citation33, Citation34). The plasma kinetics of 1,3 butanediol following ingestion of 1,3 butanediol itself or other ketone esters containing 1,3 butanediol are unknown.
The r-BHB Cmax following in the FEDH condition (1.7 mM) was approximately double the FEDL condition (0.8 mM), demonstrating a relationship between BH-BD dose and BHB Cmax response at the levels studied here, similar to work with other ketone esters (Citation31). Notably, we did not employ body weight adjusted dosing such as is commonly used in research studies of ketone esters (Citation32), resulting in a weight adjusted dose range of 110–210 mg/kg for the 12.5 g serving and 220–421 mg/kg for the 25 g serving. This design was chosen to increase the translatability of the data, as real-world consumption of ketone esters in consumer products is typically in standard serving sizes ranging from 10–30 g. We found no significant correlation between body weight and plasma ketone increase as has been seen with other ketone ester compounds (Citation31); increasing the sample size and number of doses studied would be necessary to determine if this is a real effect or an artifact. We can conclude from our data that BH-BD effectively achieves exogenous ketosis (BHB > 0.5 mM) in subjects with a range of body weight.
We found no differences between r-BHB Cmax or AUC when 25 g of BH-BD was consumed whilst fasted or with 35 g of carbohydrate, however previous research that investigated the effect of meal feeding on ketone ester induced ketosis found that a meal lowered r-BHB Cmax by 1 mM and AUC by 27% given matched doses of ketone ester (Citation32). Therefore, BH-BD can be used to induce several hours of exogenous ketosis without the need for fasting or low dietary carbohydrate intake. Interestingly, the individual who followed a ketogenic diet had the greatest BHB increase following BH-BD consumption. Future work should extend the dose-response range, and investigate sources of intra- and inter individual differences, the effects of exercise (Citation18), habitual dietary carbohydrate intake, and metabolic health (Citation24, Citation25) on ketone kinetics following BH-BD ingestion.
To our knowledge, this is the first study to measure plasma s-BHB concentrations following ketone ester ingestion; previous studies have only examined s-BHB concentrations following ingestion of racemic (i.e., mixture of r- and s-BHB) ketone salts (Citation32, Citation35). The finding that plasma s-BHB increases following ingestion of an ester that hydrolyzes to form (R)-1,3-butanediol and hexanoic acid was surprising, and led us to form the hypothesis that the observed s-BHB is released as a result of hexanoic acid availability causing increased flux through the beta-oxidation pathway of fatty acid metabolism, in which s-BHB is a normal intermediate. Our previous observation that basal s-BHB concentrations are slightly elevated in keto-adapted individuals, representing 2–3% of total BHB (Citation35) provides support for this hypothesis. Along with its relative scarcity compared to r-BHB and uncertain role in metabolism and signaling activities of ketone bodies, s-BHB is more difficult to quantify than r-BHB and thus has not been included in studies of ketogenic diet or exogenous ketones, hence low levels of circulating s-BHB could be a normal but previously undescribed phenomena during endogenous ketosis. Whilst this observation is interesting, the highest absolute s-BHB concentration of ∼60 µM is ∼28-fold lower than that of r-BHB, so it is unclear if concentrations this low would have a physiologically relevant effect. Given that the sparse literature to date suggests that s-BHB has lower affinity for known targets of r-BHB, including the receptor HCAR2 (Citation8) and the NLRP3 inflammasome (Citation7), it seems unlikely that 60 µM s-BHB would have an effect. The ‘threshold’ for different functional effects of ketone bodies as r- or s-BHB is poorly defined and is an important topic for future research.
Ketone esters can acutely decrease plasma glucose whilst fasted or in response to a glucose load (Citation24, Citation25, Citation32, Citation36) and daily ingestion for 2 weeks improves glucose control (Citation23). Here, we saw decreases in plasma glucose in all conditions despite ingestion of 35 g of carbohydrate in the meal in the FEDH and FEDL conditions, and significant differences in insulin release between conditions. Notably in the FASTH arm where insulin release was minimal, a reduction in plasma glucose was still observed suggesting a possible role for exogenous ketosis via BH-BD in lowering plasma glucose. However, without a meal-only control arm, only limited conclusions can be drawn on the effects of BH-BD on glucose metabolism. Similarly, the decrease in NEFA observed here is consistent with the known effect of ketone esters to acutely decrease circulating NEFA concentrations via BHB binding to G-protein coupled receptors leading to inhibition of lipolysis (Citation8). Lipolysis is exquisitely sensitive to insulin, hence insulin likely also contributed to the observed changes in NEFA concentrations. Whilst some data suggests that ketones can potentiate or increase insulin secretion in the presence of high glucose concentrations (Citation37, Citation38), this study and others (Citation24, Citation25, Citation32, Citation36, Citation39) have found that insulin responses are unchanged in the presence of exogenous ketosis, indeed, in this study insulin concentrations were generally higher given the lower dose of BH-BD (FEDL condition) compared to the same meal given with a higher dose of BH-BD (FEDH). Overall, our findings suggest that BH-BD has similar effects on glucose and NEFA metabolism to other ketone esters, but this requires further exploration in a placebo-controlled study.
This work has several limitations that should be considered. As mentioned above, the absence of a meal-only placebo arm means that limited conclusions on the metabolic effect of BH-BD can be drawn from the glucose, NEFA and insulin data. Whilst we measured plasma r- and s-BHB and urine r-BHB, we did not measure breath acetone or plasma acetoacetate which would have provided a more complete picture of the ketogenic effect of BH-BD. Finally, although the sample size was adequate to demonstrate significant differences in the kinetic properties of BH-BD under different conditions, factors such as age, sex-differences, physical fitness, habitual diet, metabolic health status and ethnicity could all affect metabolism of ketone esters such as BH-BD and so these results may not be generalizable to all populations.
In conclusion, exogenous ketones represent a practical method to achieve ketosis independently of the ketogenic diet or fasting; the results of this study provide critical data to support the future use of BH-BD to enable ketosis. Ingestion of 25 g of BH-BD elevates circulating r-BHB concentrations by ∼1.7 mM, maintains ketosis > 0.5 mM for 3 h independently of body weight and leads to small increases in plasma s-BHB. BH-BD does not directly deliver these ketone bodies to the circulation, instead relying on use of the ester hydrolysis products as an efficient substrate for endogenous hepatic ketone production independent of nutritional state. This characteristic could have implications for the functional effect of BH-BD and its possible use to optimize metabolic function.
Authors’ contribution
Conceptualization, B.J.S., P.N.H., J.C.N., J.S.V.; methodology, B.J.S., P.N.H., O.P., T.N.S.; formal analysis, B.J.S.; investigation, C.D.C., O.P., T.B., S.R.-D., A.B., M.L.K.; data curation, B.J.S., C.D.C.; writing—original draft preparation, B.J.S., C.D.C.; writing—review and editing, all authors; visualization, B.J.S.; supervision, B.J.S., J.S.V.; project administration, B.J.S., P.N.H., C.D.C.; funding acquisition, B.J.S., J.C.N. All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
This study was conducted according to the guidelines of the Declaration of Helsinki (2004), and approved by the Ohio State University Institutional Review Board (13th March 2020, 2020H0005).
Disclosures statement
BHB Therapeutics provided the study beverages used in this work. J.C.N is a co-founder with equity interest in BHB Therapeutics Ltd. B.J.S. has stock options in BHB Therapeutics Ltd., and Juvenescence Ltd. J.C.N and B.J.S. are inventors on patents related to the use of ketone bodies. J.C.A. holds stock options in Juvenescence Limited and Juvenescence Life Sciences Limited and serves as Chief Scientific Officer of the JuvLife Division.
Supplemental Material
Download ()Acknowledgments
Thank you to the study participants for their willingness to help with our research. John Ma at WuXiAppTec (NJ) performed the analysis for hexanoic acid and 1,3 butanediol.
Data availability statement
The data presented here may be available upon reasonable request from the corresponding author with intellectual property limitations.
Additional information
Funding
References
- Cahill GF, Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi:10.1146/annurev.nutr.26.061505.111258.
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25(1):42–52. doi:10.1016/j.tem.2013.09.002.
- Cahill GF, Jr. Starvation in man. N Engl J Med. 1970;282(12):668–75. doi:10.1056/NEJM197003192821209.
- Hallberg SJ, McKenzie AL, Williams PT, Bhanpuri NH, Peters AL, Campbell WW, Hazbun TL, Volk BM, McCarter JP, Phinney SD, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583–612. doi:10.1007/s13300-018-0373-9.
- Webber RJ, Edmond J. Utilization of L(+)-3-hydroxybutyrate, D(-)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid-synthesis in 18-day-old rat. J Biol Chem. 1977;252(15):5222–6. doi:10.1016/S0021-9258(19)63335-1.
- Reed WD, Ozand PT. Enzymes of L-(+)-3-hydroxybutyrate metabolism in the rat. Arch Biochem Biophys. 1980;205(1):94–103. doi:10.1016/0003-9861(80)90087-9.
- Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–9. doi:10.1038/nm.3804.
- Taggart AKP, Kero J, Gan X, Cai T-Q, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu T-J, et al. (D)-beta-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280(29):26649–52. doi:10.1074/jbc.C500213200.
- Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60(1):143–87. doi:10.1152/physrev.1980.60.1.143.
- Koronowski KB, Greco CM, Huang H, Kim J-K, Fribourgh JL, Crosby P, Mathur L, Ren X, Partch CL, Jang C, et al. Ketogenesis impact on liver metabolism revealed by proteomics of lysine β-hydroxybutyrylation. Cell Rep. 2021;36(5):109487. doi:10.1016/j.celrep.2021.109487.
- Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, Turnbaugh JA, Verdin E, Hall KD, Leibel RL, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263–75.e16. doi:10.1016/j.cell.2020.04.027.
- Cheng C-W, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, Tripathi S, Calibasi-Kocal G, Rickelt S, Butty VL, et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. 2019;178(5):1115–31.e15. doi:10.1016/j.cell.2019.07.048.
- Dearlove DJ, Harrison OK, Hodson L, Jefferson A, Clarke K, Cox PJ. The effect of blood ketone concentration and exercise intensity on exogenous ketone oxidation rates in athletes. Med Sci Sports Exerc. 2021;53(3):505–16. doi:10.1249/MSS.0000000000002502.
- Mikkelsen KH, Seifert T, Secher NH, Grøndal T, van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab. 2015;100(2):636–43. doi:10.1210/jc.2014-2608.
- Poff AM, Koutnik AP, Egan B. Nutritional ketosis with ketogenic diets or exogenous ketones: features, convergence, and divergence. Curr Sports Med Rep. 2020;19(7):251–9. doi:10.1249/JSR.0000000000000732.
- Poffe C, et al. Bicarbonate unlocks the ergogenic action of ketone monoester intake in endurance exercise. Med Sci Sports Exerc. 2021;53(2):431–41.
- Poffé C, Ramaekers M, Bogaerts S, Hespel P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J Appl Physiol. 2020;128(6):1643–53. doi:10.1152/japplphysiol.00092.2020.
- Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256–68. doi:10.1016/j.cmet.2016.07.010.
- Waldman HS, Basham SA, Price FG, Smith JW, Chander H, Knight AC, Krings BM, McAllister MJ. Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl Physiol Nutr Metab. 2018;43(7):711–7. doi:10.1139/apnm-2017-0724.
- Mujica-Parodi LR, Amgalan A, Sultan SF, Antal B, Sun X, Skiena S, Lithen A, Adra N, Ratai E-M, Weistuch C, et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc Natl Acad Sci USA. 2020;117(11):6170–7. doi:10.1073/pnas.1913042117.
- Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exerc. 2018;50(11):2330–8. doi:10.1249/MSS.0000000000001700.
- Avgerinos KI, Egan JM, Mattson MP, Kapogiannis D. Medium Chain Triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res Rev. 2020;58:101001.
- Walsh JJ, Neudorf H, Little JP. 14-day ketone supplementation lowers glucose and improves vascular function in obesity: a randomized crossover trial. J Clin Endocrinol Metab. 2021;106(4):e1738–e1754. doi:10.1210/clinem/dgaa925.
- Myette-Cote E, et al. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol. 2018;596(8):1385–95.
- Myette-Côté É, Caldwell HG, Ainslie PN, Clarke K, Little JP. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am J Clin Nutr. 2019;110(6):1491–501. doi:10.1093/ajcn/nqz232.
- Soto-Mota A, Norwitz NG, Clarke K. Why a d-β-hydroxybutyrate monoester? Biochem Soc Trans. 2020;48(1):51–9. doi:10.1042/BST20190240.
- Stubbs BJ, Blade T, Mills S, Thomas J, Yufei X, Nelson FR, Higley N, Nikiforov AI, Rhiner MO, Verdin E, et al. In vitro stability and in vivo pharmacokinetics of the novel ketogenic ester, bis hexanoyl (R)-1,3-butanediol. Food Chem Toxicol. 2021;147:111859. doi:10.1016/j.fct.2020.111859.
- Chen O, Blonquist T, Mah E, Sanoshy K, Beckman D, Nieman K, Winters B, Anthony J, Verdin E, Newman J, et al. Tolerability and safety of a novel ketogenic ester, bis-hexanoyl (R)-1,3-butanediol: a randomized controlled trial in healthy adults. Nutrients. 2021;13(6):2066. doi:10.3390/nu13062066.
- Assembly WG. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the: 56th WMA General Assembly, Tokyo, October 2004. Ferney-Voltaire, France: World Medical Association.
- Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, Ho M, Roberts A, Robertson J, Vanitallie TB, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63(3):401–8. doi:10.1016/j.yrtph.2012.04.008.
- Shivva V, Cox PJ, Clarke K, Veech RL, Tucker IG, Duffull SB. The population pharmacokinetics of d-β-hydroxybutyrate following administration of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Aaps J. 2016;18(3):678–11. doi:10.1208/s12248-016-9879-0.
- Stubbs BJ, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848.
- Fortier M, Castellano C-A, St-Pierre V, Myette-Côté É, Langlois F, Roy M, Morin M-C, Bocti C, Fulop T, Godin J-P, et al. A ketogenic drink improves cognition in mild cognitive impairment: results of a 6-month RCT. Alzheimers Dement. 2021;17(3):543–52. doi:10.1002/alz.12206.
- St-Pierre V, et al. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front Nutr. 2019;6(46):46.
- Kackley ML, Short JA, Hyde PN, LaFountain RA, Buga A, Miller VJ, Dickerson RM, Sapper TN, Barnhart EC, Krishnan D, et al. A pre-workout supplement of ketone salts, caffeine, and amino acids improves high-intensity exercise performance in keto-naïve and keto-adapted individuals. J Am Coll Nutr. 2020;39(4):290–300. doi:10.1080/07315724.2020.1752846.
- Greaves G, Xiang R, Rafiei H, Malas A, Little JP. Prior ingestion of a ketone monoester supplement reduces postprandial glycemic responses in young healthy-weight individuals. Appl Physiol Nutr Metab. 2021; 46(4):309–317.
- Madison LL, Mebane D, Unger RH, Lochner A. The hypoglycemic action of ketones. II. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J Clin Invest. 1964;43:408–15. doi:10.1172/JCI104925.
- Holdsworth DA, Cox PJ, Kirk TOM, Stradling HUW, Impey SG, Clarke K. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sports Exerc. 2017;49(9):1789–95. doi:10.1249/MSS.0000000000001292.
- Balasse E, Ooms HA. Changes in the concentrations of glucose, free fatty acids, insulin and ketone bodies in the blood during sodium beta-hydroxybutyrate infusions in man. Diabetologia. 1968;4(3):133–5. doi:10.1007/BF01219433.

