ABSTRACT
Objectives
Inpatient mental health beds for people with dementia are a limited resource. Practitioners need an understanding of this population to provide high-quality care and design services. This review examines the characteristics, care, and outcomes of people with dementia admitted to inpatient mental health services.
Methods
Systematic searches of key databases were undertaken up to November 2021. Findings were grouped into categories and then synthesized into a narrative review.
Results
The review identified 36 international papers, the majority of which were retrospective audits. The literature describes significant psychiatric and medical comorbidity and significant risk of change in residence and death associated with admission.
Conclusions
We found a limited literature describing the characteristics, care, and outcomes of people with dementia in inpatient mental health services. The lack of research is striking given the complexity and vulnerability of this client group. More research is needed to describe the needs of this group, current and best practice to optimize care.
Clinical Implications
Professionals working in inpatient mental health services need to be aware of the evidence base available, consider how they evaluate patient outcomes, review their staffing and skills mix, and seek the views of patients and relatives in improving services.
Introduction
Dementia affects 55 million people worldwide and this number will increase as the population ages (World Health Organisation, Citation2021). Changes in behavior and mood are common, with up to 80% of people experiencing these during the course of their illness (Barnes et al., Citation2012; Bucher, Dubuc, von Gunten, Trottier, & Morin, Citation2016). From a psychiatric perspective, these are typically regarded as neuropsychiatric or non-cognitive dementia-related symptoms and from a psychosocial perspective as expressions of psychological distress arising from unmet needs (Wolverson et al., Citation2019). There are a variety of terms used describe changes in behavior in dementia (Wolverson, Dunn, Moniz‐Cook, Gove, & Diaz‐Ponce, Citation2021), in this review the term distress behaviors is adopted.
Specific behaviors that challenge services and families providing care include; agitation, depression, apathy, delusions, hallucinations, aggression, restlessness, sexual disinhibition, anxiety, irritability, euphoria, and sleep disturbances. These experiences are debilitating and distressing for people living with dementia and their families (Black & Almeida, Citation2004) and are associated with earlier institutionalization (de Vugt et al., Citation2005), longer hospital stays (Sampson et al., Citation2014), increased morbidity, mortality, and more rapid progression of dementia (Kales, Gitlin, & Lyketsos, Citation2014). If these behaviors are so severe the person cannot be managed safely in any other setting, an admission to a mental health unit may occur for safety, treatment adjustment, and care planning (National Institute of Health and Clinical Excellence, Citation2016). In the UK, admission may be either to a specialist dementia unit, or to a mixed older adult unit where people with mental health difficulties, such as depression and schizophrenia are also treated (Gondhalekar, Bakkar, Chaplin, Parker, & Low, Citation2021). Availability of these beds is limited and admission relatively uncommon. Different ways of meeting the medical and psychiatric needs of patients are employed in general hospitals, most notably in the UK by the significant expansion of consultation/liaison services for older adults and the use of delirium units or dedicated wards for patients with comorbid dementia. These general hospital models are beyond the scope of this review, which focuses on care for patients with dementia, specifically, in psychiatric hospitals.
Concerns have been reported regarding the quality of general hospital care for people with dementia (Alzheimer’s Disease International, Citation2016) and of the significant risks and poor outcomes associated with hospitalization for people with dementia (Alzheimer’s Society, Citation2016; Royal College of Psychiatrists, Citation2019). Admission to and outcomes of mental health inpatient care is much less well described. To date, there has been no review of the literature exploring the characteristics and needs of people with dementia admitted to inpatient mental health services. The focus of this review is to better understand inpatient geropsychiatric settings namely specialist dementia units and mixed older adult units as these are most common settings were individuals with dementia are hospitalized (Pinner, Hiller, Branton, & Ramakrishnan, Citation2011). These settings provide specialist expertise in the management of distress behaviors with intensive levels of assessment, monitoring, and treatment when it is not possible to provide care elsewhere. This systematic review of people with dementia admitted to inpatient mental health units will examine:
Characteristics – gender, age, martial status, type of dementia and severity of dementia;
Care – reason for admission, medical needs, level of dependency, medication use, and family support;
Outcomes – length of stay, discharge destination, treatment of distress, repeat admissions, and death.
We include a comparison between specialist dementia units and mixed older adult units, which treat both dementia and other mental illnesses.
Methods
Search strategy and selection criteria
Medline, PsycINFO and CINAHL were systematically searched using the following key terms;
old* or elder* or geriatric* or senior* AND (Psychiatr* OR psychogeriatric* OR “mental health”) N2 (inpatient* OR ward* OR unit* OR acute) AND dementia OR alzheimer* OR “cognitive impairment” OR “memory loss”
Search strategies were developed by the project team and tailored to the databases. All databases were searched up to November 2021. Papers were selected if they fulfilled the following criteria:
Inclusion criteria
Mental health units, which provide inpatient care for older people with dementia, either in specialist dementia units or mixed older adult units for both organic and functional diagnoses. Mixed units were only included if it was possible to extract data for people with dementia.
A minimum of two clinical and/or demographic features relating to people with dementia per paper was set for inclusion in the review, as a number of papers simply reported the number of patients with dementia on a unit.
A paper or letter describing original findings and published in a peer-reviewed journal.
Exclusion criteria:
Research concerning long-stay hospitals, dementia units in care homes, general hospitals, adult mental health units, and outpatient clinics was not included. Novel units such as combined medical and mental health inpatient units were not included, as the aim was to focus on usual care. The care of people with dementia in medical units with psychiatric input has been covered by other reviews (Karrer, Schnelli, Zeller, & Mayer, Citation2021).
Studies where it was not possible to extract data solely for patients with dementia – for example, studies that report “organic” diagnoses together not separating dementia and delirium.
Qualitative studies of practices on units.
Studies comparing older adult inpatients with working age adult inpatients where it was not possible to extract data for patients with dementia
Commentaries and editorials where no original findings were reported.
No date limits or limits on geographical location were set on the inclusion of papers. Abstracts of studies were appraised for inclusion by the first author. In cases of uncertainty, papers were independently assessed by another member of the research team. References of selected studies were also hand-searched and a citation search was carried out using Google Scholar.
Data extraction and analysis
Information was extracted from studies using a data extraction template. We examined the description of the site, demographic, clinical factors, and outcomes of patients. Outcomes included were mortality, length of hospital stay, and place of residence on discharge. A narrative synthesis was undertaken which followed guidance from the ESRC methods program (Popay et al., Citation2006).
Results
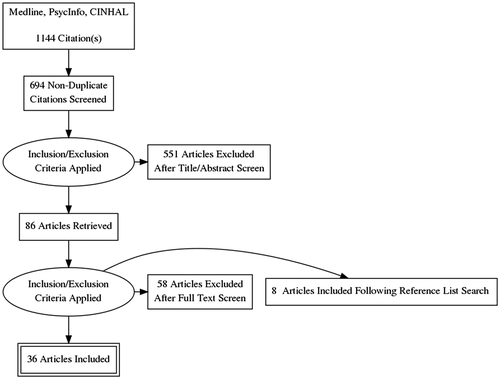
The results of the database searches were manually screened and articles removed that did not fit the selection criteria (see ). At title and abstract screening the most common reason for excluding studies was that they were conducted in general hospital settings. During full-text screening articles were excluded because it was not possible to extract data for people with dementia. Two pilot studies of novel combined medical and mental health inpatient units (Astell, Clark, & Hartley, Citation2008; Goldberg et al., Citation2013) and one study of care in a psychiatric intensive care unit (PICU) were excluded as they did not reflect standard/usual care.
Description of included studies
The 36 studies included were from worldwide locations, including; 18 European, nine North American, seven Australasian, one Asian, and one African study (see ). Of the included studies, 33 were described as audits and 27 were retrospective studies using routine data. Only nine included data collected specifically for the study (Alanen, Pitkänen, Suontaka-Jamalainen, Kampman, & Leinonen, Citation2015; Djernes, Gulmann, Abelskov, Juul-Nielsen, & Sørensen, Citation1998; Ekiz, Videler, & Van Alphen, Citation2020; Koskas et al., Citation2011; McMinn & Hinton, Citation2000; Paschali et al., Citation2018; Pongan et al., Citation2017; Rouch et al., Citation2017; Saidlitz, Sourdet, Voisin, & Vellas, Citation2017). One study collected data at one time point (O’Connor et al., Citation2018), three studies compared data extracted at two time points (Aziz, Hill, & Kumar, Citation2018; Haw, Stoffels, Purkayastha, & Sudad, Citation2015; Koskas et al., Citation2011), the remainder collected data over a period of time ranging from one month (McMinn & Hinton, Citation2000) to seven years (Nirodi & Mitchell, Citation2002).
Table 1. Patients in specialist dementia units.
Table 2. Patients with dementia in mixed older adult units.
The charactersitics and outcomes of single units were examined in 17 papers (Akpaffiong, Kunik, Hale, Molinari, & Orengo, Citation1999; Alanen et al., Citation2015; Brackenridge, Courtnay, Jha, & Lawrence, Citation1997; Chan, Chiu, Lam, & Leung, Citation2002; Chan et al., Citation2009; Davis, Holman, & Brameld, Citation2000; Djernes et al., Citation1998; Freyne & Wrigley, Citation1997; Hassett, George, & Harrigan, Citation1999; Koskas et al., Citation2011; McMinn & Hinton, Citation2000; Mei-Tal & Meyers, Citation1986; Nahas, Kunik, Orengo, Molinari, & Workman, Citation1997; Pitkänen, Alanen, Kampman, & Leinonen, Citation2018; Saidlitz et al., Citation2017; Sajatovic, Friedman, Sabharwal, & Bingham, Citation2004; Wilkins, Goldstein, & Forester, Citation2019), 14 papers combined data from a number of units within one region (Aartsma et al., Citation2019; Aziz et al., Citation2018; Ekiz et al., Citation2020; Harrison, Kernutt, & Piperoglou, Citation1988; Haw et al., Citation2015; Ismail et al., Citation2015; Koskas et al., Citation2011; Manu et al., Citation2013; Moss, Wilson, Harrigan, & Ames, Citation1995; Neville, Boyle, & Baillon, Citation1999; Nirodi & Mitchell, Citation2002; Paschali et al., Citation2018; Rockwood, Stolee, & Brahim, Citation1991) and five compared the practice of units across different regions or health-care providers (Edmans et al., Citation2021; Livingston et al., Citation2020; O’Connor et al., Citation2018; Pongan et al., Citation2017; Rouch et al., Citation2017).
Description of units
The characteristics, care and outcomes of mixed older adult units were described in 24 papers, whilst 12 reported data from specialist dementia units. There was little detail provided about the units. Only 15 papers reported the number of beds available. Specialist dementia units ranged in size from 16 beds (Wilkins et al., Citation2019) to 84 beds across three units (Aziz et al., Citation2018). Mixed older adult units ranged in size from seven beds (Brackenridge et al., Citation1997) to 24 beds (Paschali et al., Citation2018). Edmans et al. (Citation2021) which described four units; on average there was one bed for every 250 people with dementia with admission rates of around 1 patient per week (Edmans et al., Citation2021). Three papers described the staffing on the units (S.S. Chan et al., Citation2002; Edmans et al., Citation2021; Rouch et al., Citation2017) and three commented on the built environment (Brackenridge et al., Citation1997; Koskas et al., Citation2011; McMinn & Hinton, Citation2000).
Patient characteristics
The combined data represented 3950 people with dementia in inpatient mental health care.
Gender
The gender of patients was reported in 23 papers. Nine reported a higher percentage of female patients (Alanen et al., Citation2015; Chan et al., Citation2002; Citation2009; Ismail et al., Citation2015; Manu et al., Citation2013; Pongan et al., Citation2017; Rouch et al., Citation2017; Sajatovic et al., Citation2004; Wilkins et al., Citation2019), Six reported a higher percentage of male patients (Akpaffiong et al., Citation1999; Davis et al., Citation2000; Edmans et al., Citation2021; Ekiz et al., Citation2020; McMinn & Hinton, Citation2000; Mei-Tal & Meyers, Citation1986) and eight had an evenly balance sample (Aziz et al., Citation2018; Brackenridge et al., Citation1997; Hassett et al., Citation1999; Nirodi & Mitchell, Citation2002; Paschali et al., Citation2018; Pitkänen et al., Citation2018; Saidlitz et al., Citation2017; Smith, Hassett, Harrigan, & Fortune, Citation2010).
Ethnicity
Four reported details relating to the race or ethnicity of patients. The two papers on specialist dementia units (Akpaffiong et al., Citation1999; Chan et al., Citation2009), both based in the USA, reported a majority 73 to 86% White patient population. The mixed older adult units (Hassett et al., Citation1999; Smith et al., Citation2010) both based in Australia, reported a significant amount of their patient population were either born overseas or were from non-English speaking backgrounds.
Age
The age of patients was reported in 23 papers, with the mean age ranging from 73.8 to 86 years (Akpaffiong et al., Citation1999; Alanen et al., Citation2015; Aziz et al., Citation2018; Brackenridge et al., Citation1997; Chan et al., Citation2002; Citation2009; Ekiz et al., Citation2020; Ismail et al., Citation2015; Koskas et al., Citation2011; Livingston et al., Citation2020; Manu et al., Citation2013; Mei-Tal & Meyers, Citation1986; Nahas et al., Citation1997; Nirodi & Mitchell, Citation2002; Pitkänen et al., Citation2018; Pongan et al., Citation2017; Rouch et al., Citation2017; Saidlitz et al., Citation2017; Smith et al., Citation2010; Wilkins et al., Citation2019).
Marital status
Seven papers reported on the marital status of patients (Akpaffiong et al., Citation1999; Alanen et al., Citation2015; Brackenridge et al., Citation1997; Ekiz et al., Citation2020; Ismail et al., Citation2015; Pitkänen et al., Citation2018; Pongan et al., Citation2017). In five of these studies, the majority of patients were married. Across these seven studies, 24% to 71% of patients were widowed and 5% to 21% were divorced.
Type of dementia
The subtypes of dementia were reported in 23 studies (Aartsma et al., Citation2019; Akpaffiong et al., Citation1999; Alanen et al., Citation2015; Aziz et al., Citation2018; Brackenridge et al., Citation1997; Chan et al., Citation2002; Citation2009; Djernes et al., Citation1998; Edmans et al., Citation2021; Ekiz et al., Citation2020; Harrison et al., Citation1988; Livingston et al., Citation2020; McMinn & Hinton, Citation2000; Mei-Tal & Meyers, Citation1986; Moss et al., Citation1995; Nahas et al., Citation1997; Neville et al., Citation1999; Nirodi & Mitchell, Citation2002; Paschali et al., Citation2018; Pitkänen et al., Citation2018; Rouch et al., Citation2017; Saidlitz et al., Citation2017; Wilkins et al., Citation2019) see .
Table 3. Dementia subtypes reported in patients in inpatient mental health settings.
Severity of dementia
The severity of dementia was reported in 11 studies. Seven studies (Akpaffiong et al., Citation1999; Alanen et al., Citation2015; Brackenridge et al., Citation1997; Chan et al., Citation2009; Djernes et al., Citation1998; Koskas et al., Citation2011; Pitkänen et al., Citation2018) reported this using the Mini Mental State Exam (MMSE (Folstein, Robins, & Helzer, Citation1983)), with average scores ranging from 9.3 to 22.1 points, indicating that inpatient units were caring for people with severe (<10) and mild (21 to 25) dementia. One study (Pongan et al., Citation2017) used the Clinical Dementia Rating (CDR (Morris, Citation1997)), concluding that patients had moderate or severe cognitive impairment.
Patient needs
Reason for admission
In describing units and their function, papers made reference to the treatment of distress behaviors (Aartsma et al., Citation2019; Freyne & Wrigley, Citation1997; Moss et al., Citation1995), where these symptoms had “significant consequences for both the patient and his family environment” (Pongan et al., Citation2014). Behavioral reasons for admission were investigated in nine papers (25%) (Alanen et al., Citation2015; Ismail et al., Citation2015; Koskas et al., Citation2011; McMinn & Hinton, Citation2000; Neville et al., Citation1999; Paschali et al., Citation2018; Pitkänen et al., Citation2018; Rockwood et al., Citation1991; Saidlitz et al., Citation2017). The frequency and severity of distress behaviors was rated in nine studies; six of these (Alanen et al., Citation2015; Koskas et al., Citation2011; Pitkänen et al., Citation2018; Pongan et al., Citation2017; Rouch et al., Citation2017; Saidlitz et al., Citation2017) used the Neuropsychiatric Inventory (NPI; (Cummings et al., Citation1994)) with average global scores across studies from 33.9 (Pitkänen et al., Citation2018) to 40.5 (Rouch et al., Citation2017) where the scale is scored with a range of 0 to 144. Two studies (Akpaffiong et al., Citation1999; Nahas et al., Citation1997) used the Cohen-Mansfield Agitation Inventory (Cohen-Mansfield, Marx, & Rosenthal, Citation1989) and one (Paschali et al., Citation2018) used Staff Observation Aggression Scale-Revision (Palmstierna & Wistedt, Citation1987).
We can get an idea of the distress behaviors being treated on admission from the nine studies examining reasons for admission and the studies reporting distress measures, behaviors documented as a reason for admission include aggression (Akpaffiong et al., Citation1999; Harrison et al., Citation1988; Haw et al., Citation2015; Koskas et al., Citation2011; Neville et al., Citation1999; Rockwood et al., Citation1991; Wilkins et al., Citation2019) and agitation (Haw et al., Citation2015; Koskas et al., Citation2011; Mei-Tal & Meyers, Citation1986; Wilkins et al., Citation2019), sexual disinhibition (Harrison et al., Citation1988; Haw et al., Citation2015), self-neglect (Freyne & Wrigley, Citation1997; Ismail et al., Citation2015), paranoid delusions (Freyne & Wrigley, Citation1997; Mei-Tal & Meyers, Citation1986), euphoria and agitation (Mei-Tal & Meyers, Citation1986), wandering (Koskas et al., Citation2011; Rockwood et al., Citation1991), problems with addiction/dependency (Ismail et al., Citation2015) and involvement in the criminal justice system (Ismail et al., Citation2015). Ismail et al. (Citation2015) reported that 46% of patients were a threat or danger to others and 89% a danger to themselves. Suicidal ideation and suicidal behavior were not listed as a reason for admission in any study. One study described 27% of patients with three or more distress behaviors on admission (Neville et al., Citation1999) and another reported an average of 3.6 distress behaviors per patient (Nirodi & Mitchell, Citation2002). High rates depression comorbid with dementia were also described as a reason for admission (Mei-Tal & Meyers, Citation1986). One study reported that 77.5% of patients with dementia also had “comorbid maladaptive personality traits” (Ekiz et al., Citation2020).
Family intervention and support
In explaining the role and function of units, carer distress (Freyne & Wrigley, Citation1997; Pongan et al., Citation2017; Rockwood et al., Citation1991; Wilkins et al., Citation2019) and illness (Freyne & Wrigley, Citation1997; Neville et al., Citation1999) were described as common reasons for admission. One study reported measures of caregiver distress (Pongan et al., Citation2017), which they found did not predict discharge destination. No studies reported family carer interventions, although four (11%) (Akpaffiong et al., Citation1999; Nahas et al., Citation1997; Rouch et al., Citation2017; Sajatovic et al., Citation2004) referred to family education and psychological support as part of their usual treatment.
Active medical problems
Medical comorbidity was reported in 11 papers (31%). The most frequently recorded health conditions were cerebrovascular disease, hypertension, chronic cardiac disease, diabetes, chronic kidney disease, folate deficiency, and anemia. The average number of comorbidities reported per person ranged from 1.3 (Djernes et al., Citation1998) to 6.23 (Aziz et al., Citation2018). Transfer to acute medical wards for treatment was common with one study reporting that 42.3% of patients had a significant medical deterioration during their admission requiring emergency transfer (Manu et al., Citation2013). Transfer to the acute hospital was associated with a significantly longer length of stay (Neville et al., Citation1999). Two papers reported on data from the EVITAL study where 41.1% of patients were diagnosed with an acute illness during their admission (Rouch et al., Citation2017). Higher rates of acute somatic diseases were present in people discharged into nursing care (67.9%) than in those discharged home (55.1%) (Pongan et al., Citation2017).
Features of inpatient care
Medication management
Medication use was reported in 13 studies (43%). The mean number of repeat daily medications (all medications not just dementia related) reported per patient ranged from 7.8 (Chan et al., Citation2009) to 10.88 (Aziz et al., Citation2018). The average number of medications prescribed per patient for all conditions increased from admission to discharge (Chan et al., Citation2009). In the study by Aziz et al. (Citation2018), p. 95% of patients had received medication with a high risk of an adverse drug reaction (defined as events leading to admission to hospital, death, and drug-to-drug interactions).
Nirodi and Mitchell (Citation2002) reported that 98% of patients were prescribed a psychotropic mediation and Saidlitz et al. (Citation2017) found that 52.3% had received four or more psychotropic mediations on admission (typically antipsychotics, benzodiazepines, and carbamates). On mixed older adult units nearly all patients with dementia (98%) were prescribed psychotropic medications during their period of hospitalization (Nirodi & Mitchell, Citation2002), with a mean of 2.2 psychotropic medications a day reported (Nahas et al., Citation1997).
Three studies demonstrated an increase in the number of prescribed psychotropic mediations from admission to discharge (sChan et al., Citation2009; Nirodi & Mitchell, Citation2002; Rouch et al., Citation2017), with one paper reporting a mean of 2.74 new psychotropic drugs prescribed to each patient during their admission (Nirodi & Mitchell, Citation2002). One study reported a significant decrease in the prescriptions of antipsychotics, benzodiazepines, and carbamates; however, prescriptions for antidepressants, hypnotics, and antiepileptic medications were significantly higher at discharge (Saidlitz et al., Citation2017). The mean number of prescribed psychotropic mediations that patients were discharged with (2.28) remained largely the same 12 months after discharge in one study (2.22) (Rouch et al., Citation2017).
Haw et al. (Citation2015) reported that 76% of patients were prescribed an antipsychotic (Haw et al., Citation2015). The most commonly prescribed antipsychotics were Risperidone (Edmans et al., Citation2021) and Olanzapine (Haw et al., Citation2015). A significant number of people were admitted to inpatient units (38.9% to 57.3%) having been already prescribed antipsychotic medication (Edmans et al., Citation2021). Rates of antipsychotic prescribing and dosages increased from admission to discharge in two studies (Alanen et al., Citation2015; Pitkänen et al., Citation2018). Antipsychotic polypharmacy (haloperidol and olanzapine, zuclopenthixol and quetiapine and haloperidol and zuclopenthixol) increased from 2007 to 2012 in one audit and was thought to reflect the increasing complexity of patient needs (Haw et al., Citation2015).
Antidepressants were prescribed to between 24.6% (Nirodi & Mitchell, Citation2002) and 72% of patients (Akpaffiong et al., Citation1999). The number of patients prescribed antidepressants reduced from admission to discharge in one study (Pitkänen et al., Citation2018). ECT was used to treat depression in two patients with dementia in one study (Nahas et al., Citation1997).
The use of when required (PRN) psychotropic medication was reported in three (8.3%) studies (McMinn & Hinton, Citation2000; Nirodi & Mitchell, Citation2002; O’Connor et al., Citation2018). McMinn and Hinton (Citation2000) found PRN was used on average 3.62 times a day per person, with higher rates of PRN use among men than women. Nearly double the number of PRN medications were administered (6.05) when patients did not have access to outdoor areas. Nirodi and Mitchell (Citation2002) report a mean a 0.9 PRN drug prescriptions per patient with dementia. O’Connor et al. (Citation2018) reviewed 239 PRN prescriptions and found the medication indication was only specified in 63.6% of prescriptions.
Prescribing was described in four (11%) international papers (UK, USA, and Australia) (Aziz et al., Citation2018; Chan et al., Citation2009; Nirodi & Mitchell, Citation2002; O’Connor et al., Citation2018). One medication audit (Aziz et al., Citation2018) found that 25% drugs were inappropriately prescribed and could be stopped as defined by the STOP/START criteria (O’Mahony et al., Citation2014). The most common drugs inappropriately prescribed were benzodiazepines, antipsychotics, and opiates. On a mixed older adult unit, errors were found to be common in prescribing, particularly for psychotropic PRN medication and were more common for patients with dementia (Nirodi & Mitchell, Citation2002). Nirodi and Mitchell (Citation2002) reported that 20% of prescriptions for patients with dementia were illegible and among prescriptions of regular medication, 33% contained missing information (dose, frequency, or, indications for use). In a review of prescribing across units in one region of Australia, problematic areas concerned the gathering of information at admission about patients’ psychotropic prescribing histories and the handing over of information at discharge concerning newly prescribed medications and the reasons for stopping medications, including any adverse reactions (O’Connor et al., Citation2018). Two studies outlined audits that successfully improved prescribing practices (Aziz et al., Citation2018; Chan et al., Citation2009), but noted that sustaining these changes would require continued efforts.
High levels of dependency
Five studies (Alanen et al., Citation2015; Brackenridge et al., Citation1997; Chan et al., Citation2009; Djernes et al., Citation1998; Pitkänen et al., Citation2018) explored the daily functioning of patients. The research exploring the impact of admission on activities of daily living (ADL) and levels of dependency is conflicting. Four studies reported no evidence of a reduction in levels of functioning and autonomy following admission (Alanen et al., Citation2015; Brackenridge et al., Citation1997; Chan et al., Citation2009; Djernes et al., Citation1998), while one reported a significant decline (Pitkänen et al., Citation2018).
Outcomes
Length of stay
Average length of stay was reported in 13 papers (Akpaffiong et al., Citation1999; Brackenridge et al., Citation1997; Chan et al., Citation2009; Edmans et al., Citation2021; Freyne & Wrigley, Citation1997; Ismail et al., Citation2015; Nahas et al., Citation1997; Neville et al., Citation1999; Paschali et al., Citation2018; Pitkänen et al., Citation2018; Saidlitz et al., Citation2017; Sajatovic et al., Citation2004; Wilkins et al., Citation2019). Overall, a mean admission duration of 41.3 days was reported, with a range of admission lengths from 1 to 795 days. Papers from the USA generally reported much shorter admissions (see table one and two). There appears to be a cohort of patients who had much longer admissions: 2.5% of people in four UK units had admissions of over a year in duration (Edmans et al., Citation2021). One paper reported longer admission durations when patients were not being discharged to their usual address (Patterson & Compton, Citation1989). Similar average length of stay was reported for White patients (34 days) and Black patients (32 days) (Akpaffiong et al., Citation1999).
Treatment of distress behaviors
Eight papers compared measures of behavior at admission and discharge and reported a reduction in distress behaviors on standardized scales (Akpaffiong et al., Citation1999; Alanen et al., Citation2015; Djernes et al., Citation1998; Nahas et al., Citation1997; Pitkänen et al., Citation2018; Rockwood et al., Citation1991; Rouch et al., Citation2017; Saidlitz et al., Citation2017). This improvement was reported in both White and Black patients (Akpaffiong et al., Citation1999). Distress behaviors remained improved following discharge at 21 day follow-up (Saidlitz et al., Citation2017) and one year post discharge (Rouch et al., Citation2017). A reduction in measures of depression from admission to discharge was reported by two studies (Akpaffiong et al., Citation1999; Nahas et al., Citation1997), while another reported no improvement (Djernes et al., Citation1998).
Discharge destination
Discharge locations were reported by 10 papers (Brackenridge et al., Citation1997; Edmans et al., Citation2021; Koskas et al., Citation2011; McMinn & Hinton, Citation2000; Neville et al., Citation1999; Pitkänen et al., Citation2018; Pongan et al., Citation2017; Rouch et al., Citation2017; Saidlitz et al., Citation2017; Wilkins et al., Citation2019). Rates of discharge home varied considerably with 10.6% to 50% of patients returning home (Edmans et al., Citation2021). Of those patients returning home, the majority had a new or increased package of care (Neville et al., Citation1999). Entry to or return to long-term institutional care following inpatient care was common with reports of between 14.4% (Pitkänen et al., Citation2018) to 67.2.% (Edmans et al., Citation2021) of patients entering care settings. Delayed discharges as a result of lack of suitable placements were reported as an issue (Neville et al., Citation1999). A smaller group of patients were discharged to long-stay mental health settings (McMinn & Hinton, Citation2000; Sajatovic et al., Citation2004). Discharge to a general hospital (Edmans et al., Citation2021; Pitkänen et al., Citation2018; Saidlitz et al., Citation2017) was also reported (8% to 19.8% of patients).
Repeat admissions
Readmission rates following discharge for people with dementia were reported in two papers. One paper reported that patients who were discharged home were more likely to be hospitalized again over the next three months (26.7%) compared to those who were discharged to nursing care (3.7%) and that re-admissions for distress behaviors was seen in 11.6% of people who returned home (Pongan et al., Citation2017). In examining the readmission rate to their specialist dementia unit of 8.5% after one month and 20.1% after three months (Saidlitz et al., Citation2017), one paper found no patient factors (length of stay, measure of behavior severity, measures of dependence in activities of daily living, or number of medications taken) at admission or discharge were found to predict readmission.
Death
The number of people who died in inpatient units during their admission ranged from 2% (Saidlitz et al., Citation2017) to 8% (Edmans et al., Citation2021) with higher rates reported during the COVID-19 pandemic (Livingston et al., Citation2020). In examining deaths following discharge, one study reported that 5.9% of people died before three month follow-up; 4.5% of people died between three and six month follow-up and 6.3% of people died between six and 12 month follow-up (Rouch et al., Citation2017).
Discussion
We found a limited literature describing the characteristics, care, and outcomes of people with dementia in inpatient mental health services. The literature was mixed in methods, size, and outcomes described, however we were able to synthesize the available evidence describing the characteristics of people admitted, the types of treatment received, and broad outcomes on discharge.
Characteristics of admission and discharge
The primary reason for admission was the management of distress behaviors in dementia, posing significant risks and leading to distress for people with dementia and their families. The majority of patients were over 70, with dementia of Alzheimer’s type being the most common diagnosis. Admission length of several weeks was common, and in some cases much longer. Repeat admission was rare. Discharge was commonly a point of transition from independent living to long-term care, often at the end of an unplanned admission. The literature did not describe whether alternatives, for example, planned transition to institutional care directly from home requiring only one change of environment rather than two, were possible or would improve outcomes. Higher rates of readmission were noted when patients were discharged to independent living. Further research is needed to explore whether improving behavioral care at home and during transitions between settings (home to residential care) may be a way to prevent the need for inpatient psychiatric care.
Medical comorbidity is common in this population. This appears to lead to high levels of clinical need resulting in transfer of patients to medical wards. Transfer was a common occurrence, and was associated with longer admissions. The population admitted to these units also includes a significant number who are in the last year of their life, and indeed for between 2% and 8% of people these units are their place of death. This indicates there are substantial medical and palliative needs which should be reflected in the competencies and training of staff on these units (Burton et al., Citation2016; Patrick, Donaldson, & Short, Citation2020). The geographical and organizational isolation of mental health units and acute medical care facilities, may make it more likely that access to such skills is restricted.
Service model
We identified two common service designs, specialist dementia units, and mixed older age units (Gondhalekar et al.). We found only one study, which compared these settings (Koskas et al., Citation2011). This small pilot project found no evidence to support one setting being more effective than the other. The patient needs and staff skills required to effectively support the two patient groups would appear different however. For example, patients with severe dementia and distress behaviors in secondary care have specific care needs (feeding, dressing, tailored care approaches, etc.) that require staff with specific dementia training, expertise, mentoring, and time to deliver care (Handley, Bunn, & Goodman, Citation2017). These needs differ somewhat from patients on mental health inpatient units, where common diagnoses include depression, schizophrenia, or mania each; of which need a different nursing approach and environment.
A further alternative model is caring for this patient group in a general hospital setting with psychiatric support and this approach has been subject to previous systematic review. This suggested advantage over care being delivered in a general hospital setting, but also that evidence was limited (McCausland et al., Citation2019). We found no work comparing outcomes from this setting with outcomes from specialist mental health units.
Treatment
While the findings offer some encouraging evidence that these facilities are successful in achieving their goal of treating distress behaviors in dementia, they are limited in their ability to comment on how this was achieved due to lack of information on interventions and outcomes and studies looking at their interaction. That dedicated units, such as those described here could be therapeutic in itself is an idea first mooted nearly 30 years ago and though more units now exist as we demonstrate in this review, their evaluation has been limited (Lewis et al., Citation1993). Similarly, small studies looking at specific interventions such as Snoezelen rooms have been published but not followed by larger trials or incorporation in to best practice (Staal et al., Citation2007).
Medication-based approaches were commonly described, particularly the use of antipsychotics. The finding that risperidone and olanzapine were the most commonly prescribed antipsychotics should be of some reassurance, as these have the best evidence for effectiveness (National Institute for Health and Clinical Excellence, Citation2018; Schneider et al., Citation2006). However, the prevalence of prescribing in other psychotropic categories, and overall accumulation of psychotropic prescriptions during admissions, is a concern, given the more limited evidence of effectiveness of these other medication classes, and the vulnerability of this group of patients to adverse effects (Tampi, Tampi, Balachandran, & Srinivasan, Citation2016). The observed heterogeneity in treatment approaches is likely to be a result of the lack of specific guidelines to clinicians in this area. The patients admitted to inpatient mental health care are likely to have complex needs, and by the time of admission there may have been many unsuccessful attempts to manage the situation. Such guidance does exist (Alzheimer’s Society, Citation2011) but may have been exhausted by this point, leaving clinicians to choose treatment approaches based on their own preference and influences.
Another striking finding, given the universal instruction in guidelines on treatment of distress behaviors to adopt non-pharmacological approaches (James & Moniz-Cook, Citation2018; National Institue for Health and Care Excellence, Citation2019), is the absence of any comment on this aspect of management in any of the studies. Environmental factors and staff skills and training are vital to creating a unit milieu that promotes adaptive behaviors, and there has been much research into this in long-term care settings, and evidence that interventions based on these components can be effective (Moniz-Cook et al., Citation2017). A complex intervention of medication review and person-centered care training for staff can improve quality of life, reduce antipsychotic prescribing and agitation in care home residents in a cost-effective way (Ballard et al., Citation2018). Whether similar approaches translate to inpatient mental health units, where medication is reviewed at least weekly and staff may have higher baseline levels of training and staff-to-patient ratios, is unknown. However, descriptions of settings in the studies reviewed were largely limited.
It may be that people in these units are too distressed to take part in traditional non-pharmacological interventions, such as cognitive stimulation therapy or music therapy. Indeed, the majority of psychosocial interventions have been designed to prevent distress occurring. When someone is extremely distressed psychosocial interventions are likely to centered around skilled communication strategies and de-escalation skills, which are much harder to define. The lack of research into psychosocial interventions in these settings may also reflect the challenges in conduct research in such an acute setting and the associated ethical issues.
Outcomes
The facilities appear to be effective in their primary purpose; reducing the level of distress behaviors. However, indicators of quality were notably absent in most studies. While improvement in scales measuring the severity of distress behaviors was noted, there was often little comment on the potential for adverse consequences of the chosen treatment approaches. Some studies examined measures of prescribing quality, frequent errors, and inappropriate medication use were found to be common. Only one study (Edmans et al., Citation2021) reported adverse incidents, such as assaults and falls. Given the apparent reliance on pharmacological treatment strategies, their limited effectiveness, and tangible associated risks (side effects, falls, stroke, increased mortality), there is a clear need for better evidence to support clinicians’ decision-making, and greater monitoring for negative outcomes. Outcome measurement is hampered by a lack of agreed outcomes for both benefit and harm. Work with patients, carers, and professionals to determine what constitutes measurable outcomes indicating benefit and harm would seem a priority to allow any further future evaluation.
Limitations
Most of the articles reviewed were service evaluations, which were limited in their scope. These evaluations did not look to compare practice to externally validated standards of care, but simply described aspects of the setting or patient population that was accessing it.
The lack of a single agreed nomenclature to describe inpatient units that cater for patients with dementia, makes a fully inclusive search challenging. We kept our focus to units that specialized in distress behaviors in dementia. Given the limited literature base, we did not set a time period on our searches and consequently some of the papers included are old and may not reflect current practice.
Most of the articles included in this review were audits. While these are a useful source of information they are subject to less rigorous governance potentially increasing the risk of bias. In particular, audits tend to be conducted in units where a particular concern about an outcome has been identified, and therefore they may not represent the wider/national picture. Many of the articles were of small samples, and many reflected practice on a single units or single region.
The articles come from a number of countries operating differing health-care models and with different cultures toward older people, mental illness, and managing dementia. It would be informative to compare and contrast practices in different countries, however the included papers provided very few details about their units and there models of care, future work should try to set the context for their service more clearly. There is significant heterogeneity between the data collection methods employed and outcomes presented from one article to another, which limits our ability to draw conclusions from the data. Overall, these factors limit the generalizability of the data presented. For some of the findings, there was consistency across different studies covering a wide geographical range, which should give some reassurance as to their wider relevance. The studies also cover several decades and in this time there have been significant changes in care provision, for example, the reduction in inpatient beds and the increase of community-intensive support teams in the UK, which means findings from older studies may no longer be applicable.
We found no study that considered patient and family experiences of the care received, which maybe reflective of the outcomes considered important at the time of publication. Patient and family feedback has become an increasingly important metric of care quality that supports organizational and individual learning (National Institute for Health Research, Citation2019) and should not be over looked in the future.
Conclusions
We present a systematic review of the literature describing the characteristics, care, and outcomes of people with dementia in inpatient mental health care. The limited amount and quality of evidence available is in direct contrast to the prevalence and severity of need in this vulnerable population.
The units presented are generally successful in their primary purpose: admitting and treating people with distress behaviors of dementia prior to a successful discharge. Evidence of the quality of care provided is less clear. Pharmacological treatment approaches are common, with some evidence for concerns over the quality and appropriateness of medication choices. We found evidence that this was a population with complex needs arising from medical comorbidity, including palliative care needs, and little assurance that settings were suitably skilled to manage this. We found two common models of care delivery, specialist dementia units, and mixed older adult units, but no evidence to guide, which may be more effective. We found no evidence that the experiences or concerns of patients and their families has been collected and acted upon in service design or delivery. These findings establish that the lack of evidence to inform best practice in caring for this extremely vulnerable group of patients.
Clinical implications
Clinicians should be aware of the current evidence regarding these units and its limitations
For those working on these units consideration should be given to the routine collection of outcome data
More data is needed to establish an evidence base for the use of a range of team-based environmental and psychosocial interventions on inpatient psychiatric units
Staffing and skill mix should be reviewed to make sure that the complex needs of these patients can be met. In particular, consideration should be given to the complexity of medical and psychiatric problems where access to a range of staff with specialist skills is likely to be needed.
Patients and carers should be consulted and included in service improvement
This review has highlighted the lack of research concerning these services. From our review important questions for further examination would include, what are the most appropriate outcome measures to evaluate quality of care in this setting and what are the interventions, which maximize quality and what are the resources needed to deliver these? More specifically, we have identified consistent mortality on these units and attention might be given specifically to the delivery and quality of palliative care. It is important these issues are considered, added to and prioritized by engaging with patients, carers, and professional staff.
Description of authors’ roles
The research team designed the review and agreed the review question together. E. Wolverson and R. Dunning carried out the search, selected the papers, conducted the data extraction, and drafted the paper. The analysis was undertaken by E. Wolverson and verified by R. Dunning, with any discrepancies being verified by the rest of the team. All authors contributed to the drafting of the paper and agreed on the final paper for publication.
Acknowledgments
The authors would like to thank the Library and Knowledge Services at Hull University Teaching Hospitals for their support in retrieving articles. BRU’s post is part funded by a donation from Gnodde Goldman Sachs giving.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Aartsma, D. P., Groenewald, E., Koen, L., Potocnik, F., & Niehaus, D. (2019). A clinical profile of inpatient admissions to the psychogeriatric unit at Stikland hospital. South African Journal of Psychiatry, 25(1), 1–5. doi:10.4102/sajpsychiatry.v25i0.1344
- Akpaffiong, M., Kunik, M. E., Hale, D., Molinari, V., & Orengo, C. (1999). Cross‐cultural differences in demented geropsychiatric inpatients with behavioral disturbances. International Journal of Geriatric Psychiatry, 14(10), 845–850. doi:10.1002/(SICI)1099-1166(199910)14:10<845::AID-GPS34>3.0.CO;2-H
- Alanen, H.-M., Pitkänen, A., Suontaka-Jamalainen, K., Kampman, O., & Leinonen, E. (2015). Acute psychogeriatric inpatient treatment improves neuropsychiatric symptoms but impairs the level of functioning in patients with dementia. Dementia and Geriatric Cognitive Disorders, 40(5–6), 290–296. doi:10.1159/000431087
- Alzheimer’s Disease International. (2016). World alzheimer report 2016: Improving healthcare for people living with dementia.
- Alzheimer’s Society. (2011). Optimising treatment and care for people with behavioural and psychological symptoms of dementia. A best practice guide for health and social care professionals. A. s. Society. https://www.alzheimers.org.uk/sites/default/files/2018-08/Optimising%20treatment%20and%20care%20-%20best%20practice%20guide.pdf?downloadID=609
- Alzheimer’s Society. (2016). Fix dementia care: Hospitals https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_-_hospitals.pdf
- Astell, A. J., Clark, S. A., & Hartley, N. T. (2008). Predictors of discharge destination for 234 patients admitted to a combined geriatric medicine/old age psychiatry unit. International Journal of Geriatric Psychiatry: A Journal of the Psychiatry of Late Life and Allied Sciences, 23(9), 903–908. doi:10.1002/gps.2002
- Aziz, V. M., Hill, N., & Kumar, S. (2018). Completed audit cycle to explore the use of the STOPP/START toolkit to optimise medication in psychiatric in-patients with dementia. BJPsych Bulletin, 42(1), 37–41. doi:10.1192/bjb.2017.10
- Ballard, C., Corbett, A., Orrell, M., Williams, G., Moniz-Cook, E., Romeo, R., … Woodward-Carlton, B. (2018). Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: A cluster-randomised controlled trial. PLoS medicine, 15(2), e1002500. doi:10.1371/journal.pmed.1002500
- Barnes, T. R., Banerjee, S., Collins, N., Treloar, A., McIntyre, S. M., & Paton, C. (2012). Antipsychotics in dementia: Prevalence and quality of antipsychotic drug prescribing in UK mental health services. The British Journal of Psychiatry, 201(3), 221–226. doi:10.1192/bjp.bp.111.107631
- Black, W., & Almeida, O. P. (2004). A systematic review of the association between the behavioral and psychological symptoms of dementia and burden of care. International Psychogeriatrics, 16(3), 295–315. doi:10.1017/S1041610204000468
- Brackenridge, P., Courtnay, K., Jha, A., & Lawrence, R. (1997). Does hospital admission affect dependency levels of elderly psychiatric patients? International Journal of Geriatric Psychiatry, 12(7), 750–752. doi:10.1002/(SICI)1099-1166(199707)12:7<750::AID-GPS627>3.0.CO;2-O
- Bucher, C. O., Dubuc, N., von Gunten, A., Trottier, L., & Morin, D. (2016). Development and validation of clinical profiles of patients hospitalized due to behavioral and psychological symptoms of dementia. BMC Psychiatry, 16(1), 1–12. doi:10.1186/s12888-015-0706-4
- Burton, M. C., Warren, M., Cha, S. S., Stevens, M., Blommer, M., Kung, S., & Lapid, M. I. (2016). Identifying patients in the acute psychiatric hospital who may benefit from a palliative care approach. American Journal of Hospice and Palliative Medicine®, 33(3), 228–232. doi:10.1177/1049909114554795
- Chan, S. S., Chiu, H. F., Lam, L. C., & Leung, V. P. (2002). Prevalence of dementia with Lewy bodies in an inpatient psychogeriatric population in Hong Kong Chinese. International Journal of Geriatric Psychiatry, 17(9), 847–850. doi:10.1002/gps.716
- Chan, V. T., Woo, B. K., Sewell, D. D., Allen, E. C., Golshan, S., Rice, V., … Daly, J. W. (2009). Reduction of suboptimal prescribing and clinical outcome for dementia patients in a senior behavioral health inpatient unit. International Psychogeriatrics, 21(1), 195–199. doi:10.1017/S104161020800803X
- Cohen-Mansfield, J., Marx, M. S., & Rosenthal, A. S. (1989). A description of agitation in a nursing home. Journal of Gerontology, 44(3), M77–M84. doi:10.1093/geronj/44.3.M77
- Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., & Gornbein, J. (1994). The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308. doi:10.1212/WNL.44.12.2308
- Davis, P. S., Holman, C. D. A. J., & Brameld, K. (2000). Gender differences in survival of 234 patients referred to a psychogeriatric service. International Journal of Geriatric Psychiatry, 15(11), 1061–1069. doi:10.1002/1099-1166(200011)15:11<1061::AID-GPS280>3.0.CO;2-9
- de Vugt, M. E., Stevens, F., Aalten, P., Lousberg, R., Jaspers, N., & Verhey, F. R. (2005). A prospective study of the effects of behavioral symptoms on the institutionalization of patients with dementia. International Psychogeriatrics, 17(4), 577–589. doi:10.1017/S1041610205002292
- Djernes, J. K., Gulmann, N. C., Abelskov, K. E., Juul-Nielsen, S., & Sørensen, L. (1998). Psychopathologic and functional outcome in the treatment of elderly inpatients with depressive disorders, dementia, delirium, and psychoses. International Psychogeriatrics, 10(1), 71–83. doi:10.1017/S104161029800516X
- Edmans, B. G., Wolverson, E., Dunning, R., Slann, M., Russell, G., Crowther, G., … Underwood, B. R. (2021). Inpatient psychiatric care for patients with dementia at four sites in the United Kingdom. International Journal of Geriatric Psychiatry, 37(2), 1-4.
- Ekiz, E., Videler, A. C., & Van Alphen, S. P. (2020). Feasibility of the cognitive model for behavioral interventions in older adults with behavioral and psychological symptoms of dementia. Clinical Gerontologist, 45(4), 1–12.
- Folstein, M. F., Robins, L. N., & Helzer, J. E. (1983). The mini-mental state examination. Archives of General Psychiatry, 40(7), 812. doi:10.1001/archpsyc.1983.01790060110016
- Freyne, A., & Wrigley, M. (1997). Acute inpatient admissions in a community oriented old age psychiatry service. Irish Journal of Psychological Medicine, 14(1), 4–7. doi:10.1017/S0790966700002810
- Goldberg, S. E., Bradshaw, L. E., Kearney, F. C., Russell, C., Whittamore, K. H., Foster, P. E., … Lewis, S. A. (2013). Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: Randomised controlled trial (NIHR TEAM trial). Bmj, 347(jul02 1), f4132–f4132. doi:10.1136/bmj.f4132
- Gondhalekar, M. R., Bakkar, A., Chaplin, R., Parker, E., & Low, R. (2021). Organic versus mixed specialist older adult inpatient mental health units for people with dementia. Nursing Older People, 33(4). https://journals.rcni.com/nursing-older-people/evidence-and-practice/organic-versus-mixed-specialist-older-adult-inpatient-mental-health-units-for-people-with-dementia-nop.2021.e1353/abs
- Handley, M., Bunn, F., & Goodman, C. (2017). Dementia-friendly interventions to improve the care of people living with dementia admitted to hospitals: A realist review. BMJ open, 7(7), e015257. doi:10.1136/bmjopen-2016-015257
- Harrison, A. W., Kernutt, G. J., & Piperoglou, M. V. (1988). A survey of patients in a regional geriatric psychiatry inpatient unit. Australian & New Zealand Journal of Psychiatry, 22(4), 412–417. doi:10.3109/00048678809161350
- Hassett, A., George, K., & Harrigan, S. (1999). Admissions of elderly patients from English-speaking and non-English-speaking backgrounds to an inpatient psychogeriatric unit. Australian & New Zealand Journal of Psychiatry, 33(4), 576–582. doi:10.1080/j.1440-1614.1999.00537.x
- Haw, C., Stoffels, M., Purkayastha, D. D., & Sudad, S. (2015). Antipsychotics for behavioral and psychiatric symptoms of dementia: A reaudit in a specialist inpatient service. International Psychogeriatrics, 27(1), 171–172. doi:10.1017/S1041610214001288
- Ismail, Z., Arenovich, T., Granger, R., Grieve, C., Willett, P., Patten, S., & Mulsant, B. H. (2015). Associations of medical comorbidity, psychosis, pain, and capacity with psychiatric hospital length of stay in geriatric inpatients with and without dementia. International Psychogeriatrics, 27(2), 313–321. doi:10.1017/S1041610214002002
- James, I., & Moniz-Cook, E. (2018). Evidence briefing: Behaviour that challenges in dementia. British Psychological Society. www.bps.org.uk
- Kales, H. C., Gitlin, L. N., & Lyketsos, C. G.; Assessment t. D. E. P. o. t., & Dementia, M. o. t. N. S. o. (2014). Management of neuropsychiatric symptoms of dementia in clinical settings: Recommendations from a multidisciplinary expert panel. Journal of the American Geriatrics Society, 62(4), 762–769.
- Karrer, M., Schnelli, A., Zeller, A., & Mayer, H. (2021). A systematic review of interventions to improve acute hospital care for people with dementia. Geriatric Nursing, 42(3), 657–673. doi:10.1016/j.gerinurse.2021.03.006
- Koskas, P., Feugeas, M. C. H., Saad, S., Belqadi, S., Daraux, J., & Drunat, O. (2011). Management of behavioral and psychological symptoms of dementia in a dedicated psychogeriatric unit: A pilot experience. Alzheimer Disease & Associated Disorders, 25(2), 184–186. doi:10.1097/WAD.0b013e3181fa2f81
- Lewis, L., Pennypaker, L., Simpson, W., Bachman, D., Wohlreich, G., Meeks, A., … Sampson, R. (1993). Behavioral intensive care unit (BICU): A new concept in the management of acute agitated behavior in elderly demented patients. The Gerontologist, 33(6), 801–806. doi:10.1093/geront/33.6.801
- Livingston, G., Rostamipour, H., Gallagher, P., Kalafatis, C., Shastri, A., Huzzey, L., … Marston, L. (2020). Prevalence, management, and outcomes of SARS-CoV-2 infections in older people and those with dementia in mental health wards in London, UK: A retrospective observational study. The Lancet Psychiatry, 7(12), 1054–1063. doi:10.1016/S2215-0366(20)30434-X
- Manu, P., Grudnikoff, E., Khan, S., Kremen, N. J., Greenwald, B. S., Kane, J. M., & Correll, C. U. (2013). Medical outcome of patients with dementia in a free-standing psychiatric hospital. Journal of Geriatric Psychiatry and Neurology, 26(1), 29–33. doi:10.1177/0891988712473799
- McCausland, B., Patel, H., Amin, J., Baldwin, D., Loughran, K., & Osman-Hicks, V. (2019). A systematic review of specialist inpatient dementia care services versus standard inpatient dementia care in acute hospitals. Aging Clinical and Experimental Research, 31(5), 595–610. doi:10.1007/s40520-018-1021-y
- McMinn, B. G., & Hinton, L. (2000). Confined to barracks: The effects of indoor confinement on aggressive behavior among inpatients of an acute psychogeriatric unit. American Journal of Alzheimer’s Disease, 15(1), 36–41. doi:10.1177/153331750001500106
- Mei-Tal, V., & Meyers, B. S. (1986). Empirical study on an inpatient psychogeriatric unit: Diagnostic complexities. The International Journal of Psychiatry in Medicine, 15(2), 91–109. doi:10.2190/APUG-89N0-X5F6-6032
- Moniz-Cook, E., Hart, C., Woods, R., Whitaker, C., James, I., Russell, I., … Campion, P. (2017). Challenge demcare: Management of challenging behaviour in dementia at home and in care homes–development, evaluation and implementation of an online individualised intervention for care homes; and a cohort study of specialist community mental health care for families. Programme Grants for Applied Research, 5(15). https://www.journalslibrary.nihr.ac.uk/pgfar/pgfar05150/#/abstract
- Morris, J. C. (1997). Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the alzheimer type. International Psychogeriatrics, 9(S1), 173–176. doi:10.1017/S1041610297004870
- Moss, F., Wilson, B., Harrigan, S., & Ames, D. (1995). Psychiatric diagnoses, outcomes and lengths of stay of patients admitted to an acute psychogeriatric unit. International Journal of Geriatric Psychiatry, 10(10), 849–854. doi:10.1002/gps.930101006
- Nahas, Z., Kunik, M. E., Orengo, C. A., Molinari, V., & Workman, R. (1997). Depression in male geropsychiatric inpatients with and without dementia: A naturalistic study. Journal of Affective Disorders, 46(3), 243–246. doi:10.1016/S0165-0327(97)01430-4
- National Institue for Health and Care Excellence. (2019). Dementia quality standard [QS184] https://www.nice.org.uk/guidance/qs184
- National Institute for Health and Clinical Excellence. (2018). Dementia: Assessment, management and support for people living with dementia and their carers. www.nice.org.uk/guidance/ng97
- National Institute for Health Research. (2019). Improving care by using patient feedback
- National Institute of Health and Clinical Excellence. (2016). Dementia: Supporting people with dementia and their carers in health and social care
- Neville, P., Boyle, A., & Baillon, S. (1999). A descriptive survey of acute bed usage for dementia care in old age psychiatry. International Journal of Geriatric Psychiatry, 14(5), 348–354. doi:10.1002/(SICI)1099-1166(199905)14:5<348::AID-GPS915>3.0.CO;2-B
- Nirodi, P., & Mitchell, A. (2002). The quality of psychotropic drug prescribing in patients in psychiatric units for the elderly. Aging & mental health, 6(2), 191–196. doi:10.1080/13607860220126817
- O’Connor, D. W., D’Cunha, C., Clifton, T., Huppert, D., Lowy, H., Macfarlane, S., … Tsanglis, M. (2018). Quality indicators of psychotropic prescribing to people with dementia in aged psychiatry inpatient units. Aging & mental health, 22(11), 1432–1437. doi:10.1080/13607863.2017.1366418
- O’Mahony, D., O’Sullivan, D., Byrne, S., O’Connor, M. N., Ryan, C., & Gallagher, P. (2014). STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age and Ageing, 44(2), 213–218. doi:10.1093/ageing/afu145
- Palmstierna, T., & Wistedt, B. (1987). Staff observation aggression scale, SOAS: Presentation and evaluation. Acta Psychiatrica Scandinavica, 76(6), 657–663. doi:10.1111/j.1600-0447.1987.tb02936.x
- Paschali, M., Kamp, D., Reichmann, C., Jaenner, M., Lange-Asschenfeldt, C., & Supprian, T. (2018). A systematic evaluation of impulsive–aggressive behavior in psychogeriatric inpatients using the staff observation aggression scale-revision (SOAS-R). International Psychogeriatrics, 30(1), 61–68. doi:10.1017/S1041610217001600
- Patrick, C., Donaldson, A., & Short, L. (2020). Letter to Golüke et al in response to” Risk factors of mortality in older patients with dementia in psychiatric care”. International Journal of Geriatric Psychiatry, 35(9), 1079–1080. doi:10.1002/gps.5338
- Patterson, T., & Compton, S. (1989). Mortality rates of patients admitted to a psychogeriatric assessment unit. The Ulster Medical Journal, 58(2), 134.
- Pinner, G., Hiller, J., Branton, T., & Ramakrishnan, A. (2011). In-patient care for older people within mental health services. London: Royal College of Psychiatry.
- Pitkänen, A., Alanen, H.-M., Kampman, O., & Leinonen, E. (2018). Outcome of neuropsychiatric symptoms and daily functioning of patients with dementia treated on an acute psychogeriatric ward. Nordic Journal of Psychiatry, 72(7), 521–525. doi:10.1080/08039488.2018.1532021
- Pongan, E., Freulon, M., Delphin-Combe, F., Dibie-Racoupeau, F., Martin-Gaujard, G., Federico, D., … Fabre, F. (2014). Initial and long-term evaluation of patients with alzheimer’s after hospitalization in cognitive and behavioural units: The EVITAL study design. BMC Psychiatry, 14(1), 1–6. doi:10.1186/s12888-014-0308-6
- Pongan, E., Dorey, J.-M., Krolak-Salmon, P., Federico, D., Sellier, C., Auguste, N., … Rouch, I. (2017). Predictors of discharge destinations and three-month evolution of patients initially hospitalized in a cognitive behavioral unit. Journal of Alzheimer’s Disease, 60(4), 1259–1266. doi:10.3233/JAD-170419
- Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, M., … Duffy, S. (2006). Guidance on the conduct of narrative synthesis in systematic reviews. A Product from the ESRC Methods Programme Version, 1, b92.
- Rockwood, K., Stolee, P., & Brahim, A. (1991). Outcomes of admission to a psychogeriatric service. The Canadian Journal of Psychiatry, 36(4), 275–279. doi:10.1177/070674379103600407
- Rouch, I., Pongan, E., Trombert, B., Fabre, F., Auguste, N., Sellier, C., … Mouchoux, C. (2017). One-year evolution of behavioral and psychological symptoms of dementia in patients initially hospitalized in cognitive behavioral units: The EVITAL prospective cohort. Journal of Alzheimer’s Disease, 57(1), 147–155. doi:10.3233/JAD-161023
- Royal College of Psychiatrists. (2019). National audit of dementia care in general hospitals 2018–19: Round four audit report.
- Saidlitz, P., Sourdet, S., Voisin, T., & Vellas, B. (2017). Management of behavioural symptoms of dementia in a specialized unit care. Psychogeriatrics, 17(2), 81–88. doi:10.1111/psyg.12193
- Sajatovic, M., Friedman, S. H., Sabharwal, J., & Bingham, C. R. (2004). Clinical characteristics and length of hospital stay among older adults with bipolar disorder, schizophrenia or schizoaffective disorder, depression, and dementia. Journal of Geriatric Psychiatry and Neurology, 17(1), 3–8. doi:10.1177/0891988703258821
- Sampson, E. L., White, N., Leurent, B., Scott, S., Lord, K., Round, J., & Jones, L. (2014). Behavioural and psychiatric symptoms in people with dementia admitted to the acute hospital: Prospective cohort study. The British Journal of Psychiatry, 205(3), 189–196. doi:10.1192/bjp.bp.113.130948
- Schneider, L. S., Tariot, P. N., Dagerman, K. S., Davis, S. M., Hsiao, J. K., Ismail, M. S., … Stroup, T. S. (2006). Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New England Journal of Medicine, 355(15), 1525–1538. doi:10.1056/NEJMoa061240
- Smith, B., Hassett, A., Harrigan, S., & Fortune, T. (2010). A profile of inpatient admissions to an aged psychiatry service in Victoria. Australasian Psychiatry, 18(2), 146–151. doi:10.3109/10398560903314112
- Staal, J. A., Amanda, S., Matheis, R., Collier, L., Calia, T., Hanif, H., & Kofman, E. S. (2007). The effects of snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. The International Journal of Psychiatry in Medicine, 37(4), 357–370. doi:10.2190/PM.37.4.a
- Tampi, R. R., Tampi, D. J., Balachandran, S., & Srinivasan, S. (2016). Antipsychotic use in dementia: A systematic review of benefits and risks from meta-analyses. Therapeutic Advances in Chronic Disease, 7(5), 229–245. doi:10.1177/2040622316658463
- Wilkins, J. M., Goldstein, E. K., & Forester, B. P. (2019). Characteristics of hospice referrals from an inpatient geriatric psychiatry unit. The American Journal of Geriatric Psychiatry, 27(2), 162–166. doi:10.1016/j.jagp.2018.11.011
- Wolverson, E., Birtles, H., Moniz-Cook, E., James, I., Brooker, D., & Duffy, F. (2019). Naming and framing the behavioural and psychological symptoms of dementia (BPSD) paradigm: Professional stakeholder perspectives. OBM Geriatrics, 3(4), 1–19. doi:10.21926/obm.geriatr.1904080
- Wolverson, E., Dunn, R., Moniz‐Cook, E., Gove, D., & Diaz‐Ponce, A. (2021). The language of behaviour changes in dementia: A mixed methods survey exploring the perspectives of people with dementia. Journal of Advanced Nursing, 77(4), 1992–2001. doi:10.1111/jan.14787
- World Health Organisation. (2021). Dementia https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Worldwide%2C%20around%2055%20million%20people,and%20139%20million%20in%202050.