ABSTRACT
Objectives
Impaired self-awareness is a common feature of dementia, with considerable clinical impact. Some therapeutic strategies such as cognitive stimulation and psychotherapy have been suggested to mitigate loss of awareness. Nevertheless, evidence of intervention improving awareness of deficits is scarce. The present study aims to explore the impact of a Brazilian adapted version of Cognitive Stimulation Therapy (CST-Brasil), an evidence-based psychosocial intervention for people with dementia (PwD), on the level of awareness, reporting here a secondary outcome of a pilot randomized controlled trial.
Methods
47 people with mild to moderate dementia attending an out-patient unit were randomly allocated to CST (n = 23) or treatment as usual (TAU) (n = 24) across 7 weeks, in a pilot randomized controlled trial. Awareness was measured before and after the intervention.
Results
Results indicated that people in both groups increased in overall awareness of the disease, but only those receiving CST exhibited improvements of awareness of cognitive ability.
Conclusions
These findings suggest that CST may also improve metacognitive abilities in PwD, which could potentially be applied to other settings with beneficial effects.
Clinical Implications
Considering the negative impacts of anosognosia, CST-led improvements in awareness have the potential to benefit PwD and their caregivers.
Introduction
Lack of awareness for cognitive and/or behavioral deficits, also termed anosognosia, is a common characteristic of dementia (Mograbi et al., Citation2012). Both cross-sectional and longitudinal studies showed that awareness of deficits increases with disease severity (Starkstein et al., Citation2006; Turró-Garriga et al., Citation2016). Considering the association between anosognosia and cognitive decline, however, the results are inconsistent, with studies highlighting that awareness declined along with cognition (Turró-Garriga et al., Citation2016) and others showing that, despite increasing cognitive deficits, some individuals showed preserved awareness (Vogel et al., Citation2015). Another important aspect of awareness in dementia is its relationship with mood. Indeed, many studies exploring the clinical correlates of anosognosia in PwD highlighted a negative association between depression and anosognosia, with increased depression associated with better awareness (Clare et al., Citation2012; Harwood et al., Citation2000).
There is variability in the presentation and severity of anosognosia in people with dementia (PwD), with unawareness ranging from slight minimization to complete denial of the condition. These variations might reflect differences in the criteria used to define awareness, the disease severity of the sample and the method of assessing awareness (Clare et al., Citation2005). In the literature, three main methods of awareness assessment are described: clinician rating scales, discrepancy between patient and informant report, discrepancy between patient evaluation and actual performance on a specific task (Cosentino, Citation2014). The multidimensional aspect of awareness might also contribute to variations in its prevalence in dementia (Bertrand et al., Citation2019; Clare et al., Citation2011). The multidimensionality of anosognosia in PwD has been demonstrated by studies showing that awareness differs according to domain assessed. For example, some studies (Kotler-Cope & Camp, Citation1995; Vasterling et al., Citation1995) suggested that people with Alzheimer’s disease present better awareness of behavioral difficulties relative to cognition, while for patients with frontotemporal dementia, reduced awareness is observed for behavioral changes (Salmon et al., Citation2008). Moreover, findings that instruments measuring awareness can be divided into various factors also reflect the multifaceted characteristic of anosognosia (M.C. Dourado et al., Citation2014; Starkstein et al., Citation2006, Citation1996). For example, M.C. Dourado et al. (Citation2014), analyzing the Assessment Scale of Psychosocial Impact of the Diagnosis of Dementia (M. Dourado et al., Citation2007) showed a four-factor division: awareness for activities of daily living, awareness of cognitive functioning, awareness of emotional state, and awareness of social functioning and relationships.
Anosognosia has major psychosocial implications on three spheres (Bertrand et al., Citation2013). First, lack of awareness regarding cognitive and behavioral disturbances negatively influences the course of the illness (Spalletta et al., Citation2012). Indeed, PwD with diminished awareness are more likely to present with poorer quality of life, to engage in high-risk situations, such as driving, and to refuse treatment (Arlt et al., Citation2008; Hurt et al., Citation2010; Seltzer et al., Citation1997). Nevertheless, studies have indicated that reduced awareness is linked to reduced depression (e.g., Bertrand et al., Citation2016; Clare et al., Citation2012), suggesting that some level of unawareness may protect against mood disorder. Second, because anosognosia leads to an increased need for family support, studies have demonstrated a link between the presence of anosognosia and increased caregiver burden (Clare et al., Citation2011; Rymer et al., Citation2002; Turró-Garriga et al., Citation2013). Finally, unawareness of deficits amplifies the cost of dementia for the society, through earlier institutionalization, increased need of care for PwD and their caregivers (Stefanacci, Citation2011; Wimo et al., Citation2013; Zhu et al., Citation2015).
Despite these well-documented implications of anosognosia in dementia, studies exploring interventions to improve awareness of deficits are scarce, with no known published randomized controlled trials. Based on the Cognitive Awareness Model, Morris and Mograbi (Citation2013) suggested that interventions targeting error monitoring capacity might benefit patients with executive anosognosia, while interventions focusing on memory rehabilitation enhance awareness of PwD with mnemonic anosognosia. Additionally, other therapeutic strategies have been suggested to reduce the level of anosognosia in PwD, such as cognitive behavioral therapy and cognitive stimulation enhancing memory functions (Al-Aloucy et al., Citation2011) or support and psychotherapy groups (Logsdon et al., Citation2010). Given the potential relationship between awareness and depression, interventions for the former should carefully consider potential impacts on mood.
Considering these suggestions, Cognitive Stimulation Therapy (CST) might be a useful intervention to improve awareness in PwD. CST is a psychosocial intervention for PwD (Spector et al., Citation2003) composed of structured group activities, such as word association and current affairs. This 14 session group intervention aims to mentally stimulate people through complex psychological techniques (including implicit learning and multi-sensory stimulation). It has a robust evidence-base, significantly improving functionality, cognition and quality of life (Orrell et al., Citation2014; Spector et al., Citation2003). It is endorsed by Alzheimer’s Disease International and has been adapted in more than 35 countries, including Brazil (CST-Brasil; Bertrand et al., Citation2019). CST-Brasil was adapted according to guidelines from the original CST protocol. Following work with stakeholders to identify barriers and facilitators for CST in the Brazilian context, some minor adjustments were made, such as running the two weekly sessions on the same day and using material reflecting the Brazilian population reality (e.g., replacing traditional English sweets with typical Brazilian ones). A pilot single‐blind randomized controlled trial showed that CST-Brasil was beneficial for activities of daily living and depressive mood (Marinho et al., Citation2021). Reporting here a secondary outcome of this randomized controlled trial, the aim of the present study was to investigate the impact of CST-Brasil on the level of awareness of people with mild to moderate dementia. The Assessment Scale of Psychosocial Impact of the Diagnosis of Dementia (ASPIDD; M.C. Dourado et al., Citation2014), used in the current study, allows us to examine this impact on different domains of awareness. Considering previous findings and the multidimensional nature of awareness, we expected that the participation in CST-Brasil would increase levels of awareness and, additionally, we hypothesized this improvement to differ according to the domains of awareness.
Methods
The present study is reporting a secondary outcome, namely awareness, of a larger pilot randomized controlled trial. For a full description of the methodology and results regarding other outcomes (e.g., cognition, depression, activities of daily living, quality of life and caregiver burden), see, Marinho et al. (Citation2021).
Participants
52 outpatient participants attending the Center for Alzheimer’s Disease of the Federal University of Rio de Janeiro (CDA-UFRJ) were recruited. Inclusion criteria were: clinical diagnosis of dementia according to DSM-IV criteria (American Psychiatric Association & American Psychiatric Association. Task Force on DSM-IV, Citation1994); Mini-Mental State Examination (MMSE) scores between 10 and 24 (mild to moderate dementia; Folstein et al., Citation1975). Exclusion criteria were: presence of any communication (e.g., aphasia), sensorial (e.g., blindness) or physical disability (e.g., locomotor) that could affect their participation in CST. For the purpose of this analysis, considering that discrepancy measures were used to assess awareness (below), absence of an informant was also an exclusion criteria. Inclusion/exclusion criteria mirrored that in the original CST trial (Spector et al., Citation2003).
After collecting informed consent from PwD and their caregivers, individuals were consecutively allocated into groups (treatment as usual [TAU] or CST + TAU [CST]) using a random list generated by a computer program and after stratification for dementia severity (Clinical Dementia Rating [CDR] scores; Maia et al., Citation2006). Blinding participants to the group to which they were allocated was not possible, considering the nature of the treatment. Nonetheless, outcome assessment and data analysis were conducted by researchers (EB and DM, respectively) blind to the group and without direct contact with the outpatient clinic.
Treatment conditions
The intervention used in the current study corresponds to the adapted and validated version of CST for the Brazilian population (CST-Brasil; Bertrand et al., Citation2019, Marinho et al., Citation2021). A detailed description of the CST program can be found elsewhere (Spector et al., Citation2003). For the CST group, the program was conducted by three researchers (VM, IB and RN). Groups were composed of 5 to 8 participants. The intervention was conducted over 7 weeks, twice a week, completing a total of 14 sessions. The two weekly sessions (roughly 45 minutes each) were run on the same day, separated by a short break, to facilitate attendance. All sessions began with the group song, followed by a general orientation exercise and a main activity based on that week’s theme (e.g., sounds, foods, childhood, words). Sessions were specifically adapted to the groups’ abilities and to be as inclusive as possible for each member of the groups.
TAU
TAU comprised regular visits every two/three months to a geriatric psychiatrist and cholinesterase inhibitors prescription (AChEI). All patients received AChEI and no changes in prescription were allowed for both groups during the study.
Measurement of awareness
Participants were assessed at baseline (a week before) and follow-up (a week after the treatment). Primary and secondary endpoints were evaluated by a researcher blind to the group (EB), without direct contact with the outpatient clinic. The assessment took place on dates and rooms independent from those were the CST sessions occurred.
The Assessment Scale of Psychosocial Impact of the Diagnosis of Dementia (ASPIDD; M.C. Dourado et al., Citation2014) was used to assess awareness. The scale measures awareness of disease (total scores), cognitive abilities (e.g., “Do you have difficulties in recognizing persons or things?,” “Do you have memory problems?,” “Do you think you have a disease?”), affective changes (e.g., “Are you angrier than before because of health problems?,” “Are you sadder than before because of health problems?”), social abilities (e.g., “Do you think your family gives the attention you need?,” “Has your family recently changed the way they treat you because of a health problem?”), and functional capacity (e.g., “Has your routine changed lately?,” “Do you need help to perform your tasks?”). PwD and their caregivers answered the same questions separately. The awareness scoring was calculated based on the degree of discrepancy between the dyad responses, with one point being scored for each discrepant response. It allows generating scores for the full scale and each domain (cognitive, affective, social, and functional). The ASPIDD is composed of 30 items, with scores in each item being 0 or 1 (0 to 30 for the full scale), with higher scores indicating more impaired awareness.
Statistical analysis
Considering that a per-protocol analysis was performed, participants who did not complete the 14 sessions were excluded from the analysis. Sociodemographic and clinical (CDR) characteristics were compared between groups with independent samples t-tests or chi-square tests according to the variable characteristics (continuous or binary respectively). Differences between groups in the awareness measure were explored with 2 × 2 mixed-design ANOVAs, with group as a between-subjects factor (CST or TAU) and time as a within-subjects factor (pre- and post-treatment). For all analyses, α was set at .05. IBM SPSS Statistics (Version.24) was used for all analyses.
Ethics
The study was approved by a local research ethics committee (CAAE: 57,019,616.5.0000.5263) and all patients and caregivers provided written informed consent to participate.
Results
For the main analysis, one participant from the CST group was excluded for having attended only two of the 14 sessions, with another participant dropping out of the study. Two participants from the control group were excluded due to the absence of an informant and one participant dropped out of the study. The final sample consisted of 47 participants (CST n = 23; TAU n = 24). The informants were all informal caregivers, being a spouse or an adult child of the person with dementia.
Sample characteristics
Sociodemographic and clinical characteristics of the sample can be seen in . There were no significant differences between the CST and TAU groups for age (t [45] = 0.59, p = .558), years of education (t [45] = 1.03, p = .310), sex (χ2 [1] = 1.18, p = .278) or CDR (χ2 [1] = 0.02, p = .882), suggesting that the stratified randomization procedure was effective.
Table 1. Sociodemographic and clinical characteristics by group.
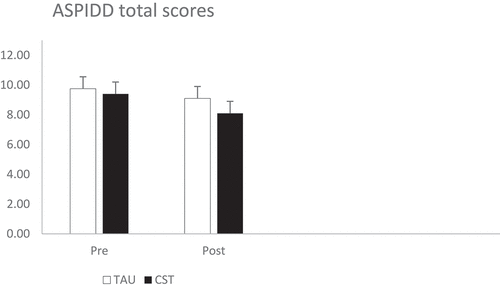
Awareness of disease
There was a significant main effect of time (F [1, 45] = 4.63, p = .037, ηp2 = .09), with an increase in awareness of disease (lower AIPDD scores) post‐treatment. There was no significant group main effect (F [1, 45] = 0.38, p = .542, ηp2 = .01) or interaction (F [1, 45] = 0.44, p = .510, ηp2 = .01). Results can be seen in .
Sub-domain analysis
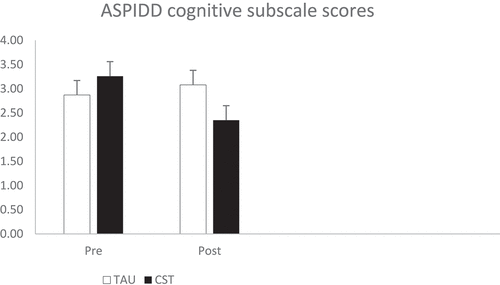
Awareness of cognitive abilities
There was a significant time x group interaction (F [1, 45] = 5.28, p = .026, ηp2 = .10). Pairwise comparisons indicated that the CST group showed lower scores (better awareness) after the intervention (p = .012), while the TAU group had no change in awareness of cognitive difficulties (p = .545). There were no significant main effects of time (F [1, 45] = 2.09, p = .156, ηp2 = .04) or group (F [1, 45] = 0.21, p = .650, ηp2 < 0.01). Results can be seen in .
Awareness of affective changes
There were no significant main effects (time: F [1, 45] = 2.46, p = .124, ηp2 = .05; group: F [1, 45] = 0.83, p = .368, ηp2 = .02) or interaction (F [1, 45] = 0.67, p = .417, ηp2 = .01) for awareness of affective changes.
Awareness of social abilities
There were no significant main effects (time: F [1, 45] < 0.01, p = .983, ηp2 < .01; group: F [1, 45] = 0.06, p = .805, ηp2 < .01) or interaction (F [1, 45] = 1.04, p = .313, ηp2 = .02) for awareness of social changes.
Awareness of functional capacity
There were no significant main effects (time: F [1, 45] = 1.95, p = .169, ηp2 = .04; group: F [1, 45] = 2.34, p = .133, ηp2 = .05) or interaction (F [1, 45] = 0.08, p = .784, ηp2 < .01) for awareness of ADLs capacity.
Discussion
The present study explored the impact of a cognitive stimulation intervention (CST-Brasil) on the level of awareness of PwD, reporting here a secondary outcome of a pilot randomized controlled trial. The results showed that, regardless of treatment, general awareness of disease increased, but only PwD who received CST experienced an improvement regarding their level of awareness for cognitive ability. Levels of awareness regarding affective, social and ADL domains remained the same despite the treatments.
Previous studies showed that unawareness increases over time in PwD as the neurodegenerative disease progresses (Hanseeuw et al., Citation2020; Wilson et al., Citation2015). However, contrary to what has been hypothesized, our results highlight an improvement of general awareness regarding the disease in both the CST and TAU groups. One explanation refers to a possible impact of care in general (e.g., monthly visits to a geriatric psychiatrist, use of a pharmacological treatment) on awareness in PwD. This highlights how access to services and provision of care for PwD and their caregivers may improve awareness, suggesting a link between social and individual levels of awareness. Additionally, it suggests that studies on awareness conducting in clinical settings may be more vulnerable to sampling biases, given that participants are already prompted about their difficulties and clinical condition. Population-based studies (e.g., Mograbi et al., Citation2012, Citation2015) are a potential way to overcome this bias, also avoiding type I error due to underpowered samples. Another possible explanation for awareness improvement in both groups could be related to assessment effect. In fact, being questioned about their abilities might have led PwD and their caregivers to pay more attention to these assessed domains in the awareness measure (cognitive, affective, social, and functional). Future studies should consider using awareness assessment which are not relying on caregivers’ report to avoid, in part, this bias.
Metacognition is defined as the knowledge about one’s own cognitive processes (Flavell, Citation1979), and unawareness in PwD can affect particularly changes in cognition (Bertrand et al., Citation2016; Ernst et al., Citation2015). Current results indicating improvements in awareness of cognitive ability suggest that CST can be seen as a metacognitive intervention, leading participants to reflect on their cognitive capacities. One potential reason for that is the group structure of CST, with performance of activities in a group setting potentially helping PwD to measure more accurately their own ability in relation to others. Indeed, interacting with other group members is one of the key principles of the programme (Spector et al., Citation2006), CST also improves communication skills (Spector et al., Citation2003), and it has been shown how interaction with others and assuming an external perspective may improve awareness (Bertrand et al., Citation2016).
The relationship between awareness and mood, specifically depression, has been described in the literature, with many studies indicating that PwD presenting more depressive symptoms are also more aware of their condition and/or deficits (Bertrand et al., Citation2016; Clare et al., Citation2012). The direction of the relationship is not clear however, and, as suggested by Mograbi and Morris (Citation2014), confronting PwD to failure should be carried out carefully, as it might lead to higher level of depression. However, Marinho et al. (Citation2021) showed that PwD receiving CST-Brasil exhibited significant improvements in mood. Therefore, the present findings suggest that CST might be a suitable intervention to improve awareness while also showing mood benefits. This could be explained by some of the key principles on which CST relies (Spector et al., Citation2006), such as implicit and errorless learning, and encouragement of opinions rather than facts, leading to adjustments of awareness without excessive exposure to failure. CST activities are tailored to the abilities of participants, which may also provide optimal conditions for improvements in awareness without deleterious mood effects.
Additionally, our results support the view that anosognosia is a multidimensional phenomenon. Indeed, CST only improved awareness regarding cognitive functioning. This is in line with previous studies highlighting the relative independence of awareness considering different domains (e.g., Bertrand et al., Citation2019) and that PwD show varying degrees of awareness in relation to different domains of awareness (Kotler-Cope & Camp, Citation1995; Vasterling et al., Citation1995; Verhülsdonk et al., Citation2013). These results have clinical implications for the evaluation of awareness, as well for interventions aiming to improve awareness, which should therefore consider different domains.
It is necessary to consider some limitations of the present study. The small sample size may affect generalizability of the results and does not allow separate analysis to be carried out for different subtypes of dementia. Further studies are therefore needed to confirm our findings and to explore it in regards to dementia etiology. Another limitation of the study refers to the assessment of awareness relying on patient-informant discrepancy. Indeed, previous studies suggested that informants’ report might be influenced by different factors, such as caregiver burden and depression (Clare et al., Citation2012). Nevertheless, it is important to note that there is no objective measure of anosognosia suited for multiple different domains. In addition to considering the multidimensionality of awareness, future research should include different methods of awareness assessment, such as comparison of patient reports with both informant-reports and objective testing.
In summary, the results of the present study highlight the impact of CST on the level of awareness regarding cognitive deficits in PwD. To the best of our knowledge, this is the first study exploring the efficacy of cognitive stimulation on anosognosia in PwD using a randomized controlled clinical trial design. These findings suggest that CST might be a promising intervention, not only regarding cognitive functioning, as it has already been shown (e.g., Woods et al., Citation2012), but also regarding metacognitive functioning. Considering the negative impact of impaired awareness in PwD and their caregivers, these results have important clinical implications. CST-led improvements in awareness have the potential to benefit PwD, their caregiver and, more broadly, society. Indeed, by improving the level of awareness of PwD, CST might help treatment adherence, reduce engagement in high-risk situations, delay institutionalization, and for their caregivers, lower caregiver burden, and as a result, diminish dementia cost for the society. Future research is needed to replicate these findings, using larger samples, and allowing the comparison of different dementia subtypes, and also combining this with neuroimaging to explore the possible neural correlates of these changes. Randomized controlled trials with active control designs should be considered to explore further the impact of CST on awareness, as well as comparison with other intervention strategies. Finally, considering the different patterns of change in awareness of disease over time in dementia (Mayelle et al., Citation2022), future studies should include longitudinal follow-up to explore trajectories of awareness.
Clinical implications
CST might be used as an intervention improving cognitive and metacognitive functioning in PwD.
CST-led improvements in awareness have the potential to help treatment adherence, delay institutionalization of PwD, and lower caregiver burden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Al-Aloucy, M. J., Cotteret, R., Thomas, P., Volteau, M., Benmaou, I., & Dalla Barba, G. (2011). Unawareness of memory impairment and behavioral abnormalities in patients with Alzheimer’s disease: Relation to professional health care burden. The Journal of Nutrition, Health & Aging, 15(5), 356–360. https://doi.org/10.1007/s12603-011-0045-1
- American Psychiatric Association, & American Psychiatric Association. Task Force on DSM-IV. (1994). Diagnostic and statistical manual of mental disorders: DSM-IV (4th) ed.).
- Arlt, S., Lindner, R., Rösler, A., & von Renteln-Kruse, W. (2008). Adherence to medication in patients with dementia: Predictors and strategies for improvement. Drugs & Aging, 25(12), 1033–1047. https://doi.org/10.2165/0002512-200825120-00005
- Bertrand, E., Dourado, M. C., Laks, J., Morris, R. G., Landeira-Fernandez, J., & Mograbi, D. C. (2016). Mood-congruent recollection and anosognosia in Alzheimer’s disease. Cortex, 84, 55–62. https://doi.org/10.1016/j.cortex.2016.09.001
- Bertrand, E., Landeira-Fernandez, J., & Mograbi, D. C. (2013). L’impact psychosocial de l’anosognosie dans la Démence de Type Alzheimer [The psychosocial impact of anosognosia in Alzheimer’s disease dementia]. Revista Ibero Americana de Gerontologia, 2, 52–69.
- Bertrand, E., Landeira-Fernandez, J., & Mograbi, D. C. (2016). Metacognition and perspective-taking in Alzheimer’s disease: A mini-review. Frontiers in Psychology, 7, 1812. https://doi.org/10.3389/fpsyg.2016.01812
- Bertrand, E., Mograbi, D. C., Brown, R. G., Landeira-Fernandez, J., & Morris, R. G. (2019). Heterogeneity of anosognosia in Alzheimer’s disease according to the object of awareness. Psychology & Neuroscience, 12(2), 282. https://doi.org/10.1016/j.cortex.2016.09.001
- Bertrand, E., Naylor, R., Laks, J., Marinho, V., Spector, A., & Mograbi, D. C. (2019). Cognitive stimulation therapy for Brazilian people with dementia: Examination of implementation’ issues and cultural adaptation. Aging & Mental Health, 23(10), 1400–1404. https://doi.org/10.1080/13607863.2018.1488944
- Clare, L., Marková, I., Verhey, F., & Kenny, G. (2005). Awareness in dementia: A review of assessment methods and measures. Aging & Mental Health, 9(5), 394–413. https://doi.org/10.1080/13607860500142903
- Clare, L., Nelis, S. M., Martyr, A., Roberts, J., Whitaker, C. J., Markova, I. S., Roth, I., Woods, R. T., & Morris, R. G. (2012). The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: Testing a biopsychosocial model. International Journal of Geriatric Psychiatry, 27(2), 167–177. https://doi.org/10.1002/gps.2705
- Clare, L., Whitaker, C. J., Nelis, S. M., Martyr, A., Markova, I. S., Roth, I., Woods, R. T., & Morris, R. G. (2011). Multidimensional assessment of awareness in early-stage dementia: A cluster analytic approach. Dementia and Geriatric Cognitive Disorders, 31(5), 317–327. https://doi.org/10.1159/000327356
- Cosentino, S. (2014). Metacognition in Alzheimer’s disease. In S. M. Fleming & C. D. Frith (Eds.), The cognitive neuroscience of metacognition (pp. 389–407). Springer-Verlag Publishing.
- Dourado, M., Marinho, V., Soares, C., Engelhardt, E., & Laks, J. (2007). Awareness of disease in Alzheimer’s dementia: Description of a mild to moderate sample of patient and caregiver dyads in Brazil. International Psychogeriatrics, 19(4), 733–744. https://doi.org/10.1017/S1041610207005492
- Dourado, M. C., Mograbi, D. C., Santos, R. L., Sousa, M. F., Nogueira, M. L., Belfort, T., Landeira-Fernandez, J., & Laks, J. (2014). Awareness of disease in dementia: Factor structure of the assessment scale of psychosocial impact of the diagnosis of dementia. Journal of Alzheimer’s Disease, 41(3), 947–956. https://doi.org/10.3233/JAD-140183
- Ernst, A., Moulin, C., Souchay, C., Mograbi, D. C., & Morris, R. (2015). Anosognosia and metacognition in Alzheimer’s disease: Insights from experimental psychology. In J. Dunlosky & S. U. K. Tauber (Eds.), The Oxford handbook of metamemory (pp. 451–472). Oxford University Press.
- Flavell, J. H. (1979). Metacognition and cognitive monitoring: A new area of cognitive–developmental inquiry. American Psychologist, 34(10), 906–911. https://doi.org/10.1037/0003-066X.34.10.906
- Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). ”Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6
- Hanseeuw, B. J., Scott, M. R., Sikkes, S. A., Properzi, M., Gatchel, J. R., Salmon, E., Marshall, G. A., & Vannini, P., & Alzheimer’s Disease Neuroimaging Initiative. (2020). Evolution of anosognosia in Alzheimer’s disease and its relationship to amyloid. Annals of Neurology, 87(2), 267–280. https://doi.org/10.1002/ana.25649
- Harwood, D. G., Sultzer, D. L., & Wheatley, M. V. (2000). Impaired insight in Alzheimer disease: Association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 13(2), 83–88. https://pubmed.ncbi.nlm.nih.gov/10780626/
- Hurt, C., Banerjee, S., Tunnard, C., Whitehead, D., Tsolaki, M., Mecocci, P., Kłoszewska, I., Soininen, H., Vellas, B., & Lovestone, S., & AddNeuroMed Consortium, & onbehalf of the AddNeuroMed Consortium. (2010). Insight, cognition and quality of life in Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 81(3), 331–336. https://doi.org/10.1136/jnnp.2009.184598
- Kotler-Cope, S., & Camp, C. J. (1995). Anosognosia in Alzheimer disease. Alzheimer Disease and Associated Disorders, 9(1), 52–56. https://doi.org/10.1097/00002093-199505000-00010
- Logsdon, R. G., Pike, K. C., McCurry, S. M., Hunter, P., Maher, J., Snyder, L., & Teri, L. (2010). Early-stage memory loss support groups: Outcomes from a randomized controlled clinical trial. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 65(6), 691–697. https://doi.org/10.1093/geronb/gbq054
- Maia, A. L. G., Godinho, C., Ferreira, E. D., Almeida, V., Schuh, A., Kaye, J., & Chaves, M. L. F. (2006). Aplicação da versão brasileira da escala de avaliação clínica da demência (Clinical Dementia Rating-CDR) em amostras de pacientes com demência [Applying the Brazilian version of the Clinical Dementia Rating (CDR) scale in a sample of patients with dementia]. Arquivos de Neuropsiquiatria, 64(2b), 485–489. https://doi.org/10.1590/S0004-282X2006000300025
- Marinho, V., Bertrand, E., Naylor, R., Bomilcar, I., Laks, J., Spector, A., & Mograbi, D. C. (2021). Cognitive stimulation therapy for people with dementia in Brazil (CST-Brasil): Results from a single blind randomized controlled trial. International Journal of Geriatric Psychiatry, 36(2), 286–293. https://doi.org/10.1002/gps.5421
- Mayelle, A., Hazebrouck, C., El Haj, M., Mograbi, D. C., & Antoine, P. (2022). Awareness for people with Alzheimer’s disease: Profiles and weekly trajectories. Frontiers in Aging neuroscience, 13, 781426. https://doi.org/10.3389/fnagi.2021.781426
- Mograbi, D. C., Ferri, C. P., Sosa, A. L., Stewart, R., Laks, J., Brown, R., & Morris, R. G. (2012). Unawareness of memory impairment in dementia: A population-based study. International Psychogeriatrics, 24(6), 931–939. https://doi.org/10.1017/S1041610211002730
- Mograbi, D. C., Ferri, C. P., Stewart, R., Sosa, A. L., Brown, R. G., Laks, J., & Morris, R. G. (2015). Neuropsychological and behavioral disturbance correlates of unawareness of memory impairment in dementia: A population-based study. Journal of Geriatric Psychiatry and Neurology, 28(1), 3–11. https://doi.org/10.1177/0891988714541868
- Mograbi, D., & Morris, R. (2014). On the relation among mood, apathy, and anosognosia in Alzheimer’s disease. Journal of the International Neuropsychological Society, 20(1), 2–7. https://doi.org/10.1017/S1355617713001276
- Morris, R. G., & Mograbi, D. C. (2013). Anosognosia, autobiographical memory and self-knowledge in Alzheimer’s disease. Cortex, 49(6), 1553–1565. https://doi.org/10.1016/j.cortex.2012.09.006
- Orrell, M., Aguirre, E., Spector, A., Hoare, Z., Woods, R. T., Streater, A., Donovan, H., Hoe, J., Knapp, M., Whitaker, C., & Russell, I. (2014). Maintenance cognitive stimulation therapy for dementia: Single-blind, multicentre, pragmatic randomised controlled trial. British Journal of Psychiatry, 204(6), 454–461. https://doi.org/10.1192/bjp.bp.113.137414
- Rymer, S., Salloway, S., Norton, L., Malloy, P., Correia, S., & Monast, D. (2002). Impaired awareness, behavior disturbance, and caregiver burden in Alzheimer disease. Alzheimer Disease and Associated Disorders, 16(4), 248–253. https://doi.org/10.1097/00002093-200210000-00006
- Salmon, E., Perani, D., Collette, F., Feyers, D., Kalbe, E., Holthoff, V., Sorbi, S., & Herholz, K. (2008). A comparison of unawareness in frontotemporal dementia and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 79(2), 176–179. https://doi.org/10.1136/jnnp.2007.122853
- Seltzer, B., Vasterling, J. J., Yoder, J. A., & Thompson, K. A. (1997). Awareness of deficit in Alzheimer’s disease: Relation to caregiver burden. The Gerontologist, 37(1), 20–24. https://doi.org/10.1093/geront/37.1.20
- Spalletta, G., Girardi, P., Caltagirone, C., & Orfei, M. D. (2012). Anosognosia and neuropsychiatric symptoms and disorders in mild Alzheimer disease and mild cognitive impairment. Journal of Alzheimer’s Disease, 29(4), 761–772. https://doi.org/10.3233/JAD-2012-111886
- Spector, A., Thorgrimsen, L., Woods, R. T., & Orrell, M. (2006). Making a difference: An evidence-based group programme to offer Cognitive Stimulation Therapy (CST) to people with dementia. Hawker Publications.
- Spector, A., Thorgrimsen, L., Woods, B., Royan, L., Davies, S., Butterworth, M., & Orrell, M. (2003). Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: Randomised controlled trial. British Journal of Psychiatry, 183(3), 248–254. https://doi.org/10.1192/bjp.183.3.248
- Starkstein, S. E., Jorge, R., Mizrahi, R., & Robinson, R. G. (2006). A diagnostic formulation for anosognosia in Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 77(6), 719–725. http://dx.doi.org/10.1136/jnnp.2005.085373
- Starkstein, S. E., Sabe, L., Chemerinski, E., Jason, L., & Leiguarda, R. (1996). Two domains of anosognosia in Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 61(5), 485–490. https://doi.org/10.1136/jnnp.61.5.485
- Stefanacci, R. G. (2011). The costs of Alzheimer’s disease and the value of effective therapies. The American Journal of Managed Care, 17(Suppl 13), S356–S362. https://pubmed.ncbi.nlm.nih.gov/22214393/
- Turró-Garriga, O., Garre-Olmo, J., Calvó-Perxas, L., Reñé-Ramírez, R., Gascón-Bayarri, J., & Conde-Sala, J. L. (2016). Course and determinants of Anosognosia in Alzheimer’s Disease: A 12-Month Follow-up. Journal of Alzheimer’s Disease: JAD, 51(2), 357–366. https://doi.org/10.3233/JAD-150706
- Turró-Garriga, O., Garre-Olmo, J., Vilalta-Franch, J., Conde-Sala, J. L., de Gracia Blanco, M., & López-Pousa, S. (2013). Burden associated with the presence of anosognosia in Alzheimer’s disease. International Journal of Geriatric Psychiatry, 28(3), 291–297. https://doi.org/10.1002/gps.3824
- Vasterling, J. J., Seltzer, B., Foss, J. W., & Vanderbrook, V. (1995). Unawareness of deficit in Alzheimer’s disease: Domain-specific differences and disease correlates. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 8(1), 26–32. https://psycnet.apa.org/record/1995-41126-001
- Verhülsdonk, S., Quack, R., Höft, B., Lange-Asschenfeldt, C., & Supprian, T. (2013). Anosognosia and depression in patients with Alzheimer’s dementia. Archives of Gerontology and Geriatrics, 57(3), 282–287. https://doi.org/10.1016/j.archger.2013.03.012
- Vogel, A., Waldorff, F. B., & Waldemar, G. (2015). Longitudinal changes in awareness over 36 months in patients with mild Alzheimer’s disease. International Psychogeriatrics, 27(1), 95–102. https://doi.org/10.1017/S1041610214001562
- Wilson, R. S., Boyle, P. A., Yu, L., Barnes, L. L., Sytsma, J., Buchman, A. S., Bennett, D. A., & Schneider, J. A. (2015). Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology, 85(11), 984–991. https://doi.org/10.1212/WNL.0000000000001935
- Wimo, A., Jönsson, L., Bond, J., Prince, M., & Winblad, B., Alzheimer Disease International. (2013). The worldwide economic impact of dementia 2010. Alzheimer’s & Dementia, 9(1), 1–11. https://doi.org/10.1016/j.jalz.2012.11.006
- Woods, B., Aguirre, E., Spector, A. E., & Orrell, M. (2012). Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database of Systematic Reviews, 2, CD005562–CD005562. https://doi.org/10.1002/14651858.CD005562.pub2
- Zhu, C. W., Scarmeas, N., Ornstein, K., Albert, M., Brandt, J., Blacker, D., Sano, M., & Stern, Y. (2015). Health-care use and cost in dementia caregivers: Longitudinal results from the predictors caregiver study. Alzheimer’s & Dementia, 11(4), 444–454. https://doi.org/10.1016/j.jalz.2013.12.018